Abstract
This retrospective cohort study established malnutrition’s impact on mortality and neurological recovery of older patients with cervical spinal cord injury (SCI). It included patients aged ≥ 65 years with traumatic cervical SCI treated conservatively or surgically. The Geriatric Nutritional Risk Index was calculated to assess nutritional-related risk. Overall, 789 patients (mean follow-up: 20.1 months) were examined and 47 had major nutritional-related risks on admission. One-year mortality rate, median survival time, neurological recovery, and activities of daily living (ADL) at 1 year post-injury were compared between patients with major nutrition-related risk and matched controls selected using 1:2 propensity score matching to adjust for age, pre-traumatic neurological impairment, and activity. In the Kaplan–Meier analysis, the median survival times were 44.9 and 76.5 months for patients with major nutrition-related risk and matched controls, respectively (p = 0.015). Matched controls had more individuals with a neurological improvement of American Spinal Injury Association Impairment Scale ≥ 1 grade (p = 0.039) and independence in ADL at 1 year post-injury than patients with major nutrition-related risk (p < 0.05). In conclusion, 6% of older patients with cervical SCI had major nutrition-related risks; they showed a significantly higher 1 year mortality rate, shorter survival time, poorer neurological improvement, and lower ADL at 1 year post-injury than matched controls.
Subject terms: Trauma, Outcomes research
Introduction
The incidence of spinal cord injury (SCI) is currently reported to be as high as 3.6 to 195.4 per million worldwide. Notably, the direct and indirect costs of SCI are higher than those of similar conditions, such as dementia, multiple sclerosis, and cerebral palsy1,2. Furthermore, the current global aging is spurring the burden of SCI on the population and social economy3–5. The proportion of the world’s population aged > 60 years is expected to nearly double from 12 to 22% between 2015 and 20506. The older population is known to have high rates of osteoporosis, degenerative changes in the spine, and falls due to declines in functional ability7,8. In fact, a high increase rate in SCI incidence from 84 to 131 cases/million (average annual percentage change: 2.7%) was observed in the older population in the United States between 1993 and 20129. For such a population, minor traumas, including low-velocity falls, are becoming the major mechanism of underlying traumatic SCI3–5,10. Due to its characteristic injury pattern and background, 90% of SCIs reportedly occur in the cervical spine rather than in the thoracic spine among the older population5.
Malnutrition among older individuals is a major social concern worldwide. A recent large-scale survey conducted by the Japanese government revealed that 17% of the older population in Japan experienced malnutrition11. Therefore, since nutritional status is a critical factor for patients with SCI, understanding the influence of malnutrition on the outcomes after SCI is necessary. Previous studies have elucidated the relationship between malnutrition in the general population with SCI and clinical complications/mortality12–14. However, the influences of malnutrition in older populations with SCI on clinical outcomes, including mortality and neurological recovery, remains unclear.
Furthermore, a concern exists that the parameters of nutritional status are not unified throughout these studies and that this causes difficulty in interpreting and comparing results11–14. The Geriatric Nutrition Risk Index (GNRI) is a simple index of nutrition-related risk that focuses on the evaluation of older patients15. This index has recently been shown to be an objective and reliable tool for assessing nutrition-related risk in various pathological conditions in the older population16–19. Therefore, this study aimed to determine nutrition-related risk and its impact on mortality and neurological recovery of older patients with cervical SCI using GNRI.
Methods
Study design and ethical considerations
This study retrospectively analyzed multicenter registry data collected by the Japan Association of Spine Surgeons with Ambition (JASA)20–22. All study participants provided written informed consent. The Institutional Review Board of the representative facility reviewed and approved this study (Approval No.: 3352-1). All methods were performed in accordance with the principle of the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. No funding was received for this study.
JASA database
The JASA members, who are spine surgeons from 78 institutions in Japan, reviewed the medical records of their institutions and retrospectively registered the cases in the JASA database based on the following inclusion and exclusion criteria20–22. The following patients were included those aged ≥ 65 years with traumatic cervical SCI, those treated conservatively or surgically between 2010 and 2020 at one’s institution, and those followed up for at least 3 months post-injury. The exclusion criteria were cervical metastases and missing data.
Data collection
Patient background data
Data regarding age at injury, sex, height, weight, body mass index (BMI), number of patients with diabetes, pre-injury ADL (independent, able to walk with assistance, or wheelchair/bedridden), number of ventilator dependents due to SCI-induced respiratory dysfunction, blood examination results at the first visit, and survival data at 1-year post-injury were collected. Blood tests included those for hemoglobin (g/dL), total protein (g/dL), and Alb (g/dL). ADL data on admission and at 1 year post-injury were extracted from the database.
Neurological impairment scale
American Spinal Injury Association Impairment Scale (ASIA) Impairment Scale (AIS) which is International standards of neurologic classification of SCI was used as a parameter of neurological impairment23: Grade A: complete impairment; Grade B: sensory incomplete impairment; Grade C: motor incomplete impairment; Grade D: motor incomplete impairment; and Grade E = no neurological impairment. AIS data on admission and at 1 year post-injury were extracted from the database.
Therapeutic data
Treatment strategies for patients, including conservative or surgical treatment, were recorded. All treatments were determined on a case-by-case basis by each attending physician.
Geriatric Nutrition Risk Index
The GNRI was calculated from the patient’s serum Alb, weight, and ideal weight using the following formula: GNRI = [1.489 × Alb (g/L)] + [41.7 × (body weight/ideal body weight)]15. The ideal body weight was defined as the value calculated from height and BMI of 22 kg/m216 Body weight/ideal body weight was set to 1 if the body weight exceeded the ideal body weight15. The GNRI has the following grading system: > 98 = absence of nutrition-related risk; 92 to ≤ 98 = low risk; 82 to < 92 = moderate risk; and < 82 = major risk.
Statistical analysis
Overview analysis
The overall distribution of nutrition-related risk among patients was narratively described. Subsequently, patients were categorized into two groups using the GNRI data. GNRI was calculated from the data on admission, and patients with GRNI < 82 were defined as those with major nutrition-related risks. All other patients were allocated to the control group. Patient demographics between the groups were compared using the Mann–Whitney U or Chi-square test as appropriate. The results of the residual analysis were considered significant at p values < 0.05 when the variable showed |r|> 1.96, in accordance with the Haberman method24.
Propensity score matching
A matched control group was created using propensity score matching. Therefore, to estimate the propensity score, we fitted a logistic regression model using age, sex, pre-injury ADL, pre-injury AIS grade, and treatment. The nearest-neighbor 1:2 matching procedure was used, restricting the matched propensities to be within 0.01 units of each other.
Survival time analysis
The mortality rate within 1 year was compared between patients with major nutrition-related risk and matched controls using the chi-square test. Kaplan–Meyer analysis was used to calculate the survival curve and median survival time with 95% CI post-trauma. Finally, the log-rank test was used to compare the results between patients with major nutrition-related risks and matched controls.
Neurological improvement and ADL analysis
The number of patients whose neurological symptoms improved by ≥ 1 AIS grade was compared between patients with major nutrition-related risk and matched controls. In this analysis, we compared the numbers after stratification according to the AIS grade on admission20, and the ADL at 1 year post-injury were also compared between the groups.
Settings
Continuous variables are presented with a mean ± 1.0 standard deviation. All analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, N.Y., USA). Statistical significance was set at p values < 0.05.
Ethical approval
The institutional review board of the representative facility reviewed and approved this study. (Kanazawa University, No. 3352-1).
Results
Overview
In total, 789 patients were enrolled in this study (mean age: 75.2 ± 6.6 years; 567 males and 225 females; mean follow-up: 20.1 ± 21.7 months). Among all patients, 47 (6.0%), 226 (28.7%), and 516 (65.4%) had a GNRI of < 82 (indicating major nutrition-related risk), 82–98 (indicating moderate to low risk), and > 98 (indicating no risk), respectively (Table 1). When comparing patients with major nutrition-related risk and controls, the proportion of females was significantly higher, BMI was significantly lower, pre-traumatic ADL was significantly lower, and all blood test results were significantly lower in patients with major nutrition-related risk than in controls (Table 2, p = 0.016, < 0.001, 0.023, and < 0.001, respectively).
Table 1.
Nutrition-related risk of all patients.
| Nutrition-related risk | GNRI | Numbers (%) |
|---|---|---|
| Major risk | < 82 | 47 (6.0) |
| Moderate risk | 82 to < 92 | 107 (13.6) |
| Low risk | 92 to < 98 | 119 (15.1) |
| No risk | ≥ 98 | 516 (65.4) |
GNRI Geriatric Nutritional Risk Index.
Table 2.
Overall comparisons of demographics between patients with and those without major nutrition-related risk.
| Patients with major nutrition-related risk | Patients without major nutrition-related risk | p value | |
|---|---|---|---|
| Numbers | 47 | 742 | |
| Age (years) ± SD | 76.5 ± 7.8 | 75.1 ± 6.6 | 0.242* |
| Female/male | 15/32 | 107/535 | 0.016# |
| BMI (kg/m2) ± SD | 17.8 ± 2.1 | 22.5 ± 3.5 | < 0.001* |
| Pre-trauma ADL (%) | 0.023# | ||
| Independent | 36 (76.6) | 660 (88.9) | < 0.05‡ |
| Walk with assistance | 10 (21.3) | 67 (9.0) | < 0.05‡ |
| Wheelchair/bedridden | 1 (2.1) | 15 (2.0) | |
| Blood test data | |||
| TP (g/dL) ± SD | 5.6 ± 0.5 | 6.6 ± 0.6 | < 0.001* |
| Alb (g/dL) ± SD | 2.6 ± 0.4 | 3.8 ± 0.6 | < 0.001* |
| Hb (g/dL) ± SD | 10.7 ± 2.3 | 12.9 ± 1.8 | < 0.001* |
| AIS grade | |||
| A | 7 (14.9) | 85 (11.5) | 0.436# |
| B | 5 (10.6) | 49 (6.7) | |
| C | 17 (36.2) | 239 (32.5) | |
| D | 18 (38.3) | 363 (49.3) | |
| Treatment | |||
| Conservative | 16 (34.0) | 256 (34.5) | 1.000# |
| Surgical | 31 (66.0) | 486 (65.4) | |
SD standard deviation, BMI body mass index, ADL activities of daily living, TP total protein, Alb albumin, Hb hemoglobin, AIS American spinal injury association impairment scale.
*Mann–Whitney U test, #Chi-square test, ‡residual analysis.
Matching
After 1:2 propensity score matching, 94 patients were selected as matched controls. No significant differences were found in age, sex ratio, number of patients with diabetes, pre-traumatic ADL, AIS grade, or number of ventilator-dependent or treatment procedures between patients with major nutrition-related risk and matched controls (Table 3, p = 0.818, 0.858, 0.421, 0.980, 1.000, and 0.534 respectively).
Table 3.
Comparisons of demographics after matching procedures.
| Patients with major nutrition-related risk | Matched control | p value | |
|---|---|---|---|
| Numbers | 47 | 94 | |
| Age (years) ± SD | 76.5 ± 7.8 | 76.9 ± 7.4 | 0.818* |
| Female/male | 15/32 | 32/62 | 0.851# |
| Diabetes | 10 (21.3) | 26 (27.7) | 0.539# |
| Pre-trauma ADL (%) | |||
| Independent | 36 (76.6) | 80 (85.1) | 0.421# |
| Walk with assistance | 10 (21.3) | 12 (12.8) | |
| Wheelchair/bedridden | 1 (2.1) | 2 (2.1) | |
| Follow-up (month) ± SD | 16.6 ± 12.0 | 22.1 ± 12.1 | 0.099 |
| AIS grade | |||
| A | 7 (14.9) | 12 (12.8) | 0.980# |
| B | 5 (10.6) | 9 (9.6) | |
| C | 17 (36.2) | 35 (37.2) | |
| D | 18 (38.3) | 38 (40.4) | |
| Ventilator dependent | 5 (10.6) | 7 (7.4) | 0.534 |
| Treatment | |||
| Conservative | 16 (34.0) | 32 (34.0) | 1.000# |
| Surgical | 31 (66.0) | 62 (66.0) | |
| BMI (kg/m2) ± SD | 17.8 ± 2.1 | 23.1 ± 4.2 | < 0.001* |
| Blood test data | |||
| TP (g/dL) ± SD | 5.6 ± 0.6 | 6.5 ± 0.7 | < 0.001* |
| Alb (g/dL) ± SD | 2.6 ± 0.4 | 3.7 ± 0.6 | < 0.001* |
| Hb (g/dL) ± SD | 10.7 ± 2.3 | 12.7 ± 1.7 | < 0.001* |
SD standard deviation, BMI body mass index, ADL activities of daily living, TP total protein, Alb albumin, Hb hemoglobin, AIS American spinal injury association impairment scale.
*Mann–Whitney U test; #Chi-square test.
Survival analysis
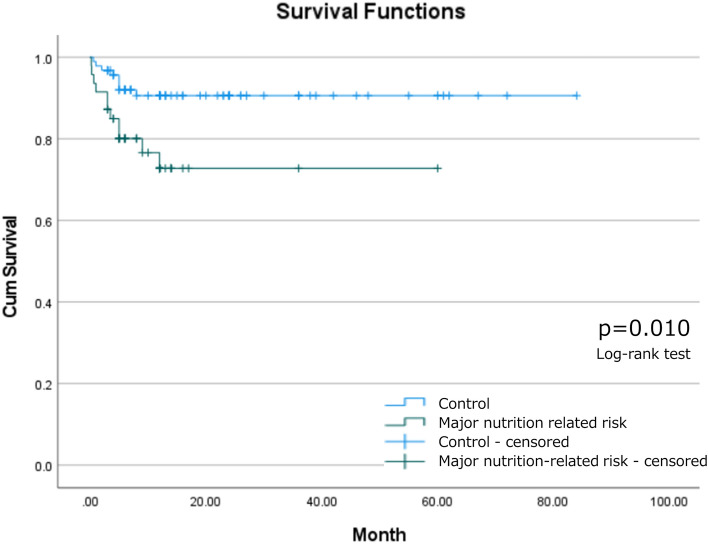
Among patients with major nutrition-related risks and matched controls, 11 (23.4%) and 8 (8.5%) patients died within 1-year post-trauma, respectively. The 1 year mortality ratio was significantly higher among patients with major nutrition-related risk than among matched controls (p = 0.019, Fig. 1). Per the Kaplan–Meier analysis, the median survival time was 44.9 [95% confidence interval (CI) 37.1–52.8] and 76.5 (95% CI 71.5–81.5) months for patients with major nutrition-related risk and matched controls, respectively. Patients with major nutrition-related risk survived for a significantly shorter period after experiencing cervical SCI than matched controls (Fig. 2, p = 0.015).
Figure 1.
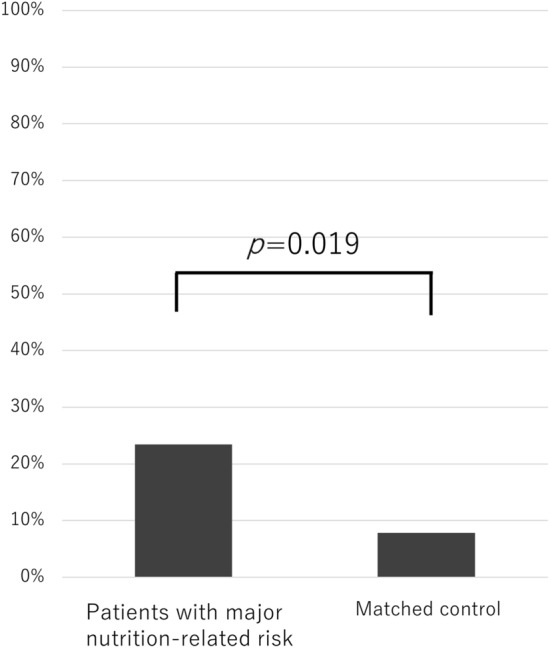
Mortality rate within 1 year post-injury.
Figure 2.
Survival analysis of patients with and those without major nutrition-related risk.
Neurological improvement and ADL
In the subgroups of patients with AIS grades A–C on admission, the matched controls had a significantly higher proportion of patients who achieved a neurological improvement of at least one AIS grade than patients with major nutrition-related risk (57.1% vs. 34.5%, p = 0.039, Table 4). Specifically, 6, 3, and 1 patient among individuals with major nutrition-related risk and 22, 4, and 6 individuals among matched controls improved their neurological impairment from AIS grades from A to B, from B to C, and from C to E, respectively. Meanwhile, no significant differences were observed between patients with major nutrition-related risk and matched controls in the subgroup of patients with AIS grade D on admission (11.1% vs. 18.4%, p = 0.393). Regarding the ADL at 1 year post-injury, the ratios of the independent patient and wheelchair/bedridden individual were significantly lower and higher, respectively, in patients with major nutrition-related risk than in those of matched controls. (overall: p = 0.016, residual analysis: p < 0.05 respectively).
Table 4.
Comparisons of neurological improvement and ADL at 1 year post-injury.
| Patients with major nutrition-related risk | Matched control | p value | |
|---|---|---|---|
| AIS grade A, B, or C on admission | 0.039# | ||
| Improved ≥ 1 grade | 10 (34.5) | 32 (57.1) | |
| Not improved or dead | 19 (65.5) | 24 (42.9) | |
| AIS grade D on admission | 0.393# | ||
| Improved ≥ 1 grade | 2 (11.1) | 7 (18.4) | |
| Not improved or dead | 16 (88.9) | 31 (81.6) | |
| ADL at 1-year post-injury | 0.016# | ||
| Independent | 6 (15.0) | 32 (39.0) | < 0.05‡ |
| Walk with assistance | 16 (40.0) | 29 (35.4) | |
| Wheelchair/bedridden | 18 (45.0) | 21 (25.6) | < 0.05‡ |
ADL activities of daily living, AIS American spinal injury association impairment scale.
#Chi-square test (one-sided), ‡residual analysis.
Discussion
In this study, approximately 35% of the older patients with cervical SCI had nutrition-related risk. Particularly, 6% of these patients had severe malnutrition, which could have major nutrition-related risks. Such patients showed substantially higher mortality rates within 1 year, shorter survival times, poorer neurological improvement, and lower levels of ADL at 1 year post-injury than matched controls.
We used GNRI in this study, rather than a single BMI or serum albumin (Alb) level, to assess the nutritional status15,16. Although GNRI has not previously been reported for SCI, the index was used in nutrition and other areas, such as urology, geriatrics, and cardiology17–19. For example, Kobayashi et al. showed that the GNRI is a substantial predictor for mortality among patients undergoing hemodialysis17, while Ruan et al. reported that the GNRI can serve as an independent prognostic factor for the overall survival of older patients with cancer cachexia18. Additionally, Kawakubo et al. revealed that malnutrition assessed using the GNRI predicts long-term adverse outcomes among hospitalized patients with heart failure with reduced ejection fraction19. Following these studies, we revealed that the GNRI would be useful in evaluating older patients with traumatic SCI. Our study results also revealed that 35% of the enrolled participants had nutrition-related risk, which was clearly higher than that in the general older population11. Therefore, these results could encourage physicians to assess the GNRI of older patients with SCI on admission.
Regarding the nutritional status of patients with SCI, some studies have elucidated the relationship between malnutrition and clinical complications in this cohort12. Hypoproteinemia and malnutrition might be indicators of mortality in patients with SCI13, and BMI is correlated with the recovery of ADL after experiencing SCI14. In addition to these studies, our study demonstrated that malnutrition in older patients with SCI was considerably associated with increased mortality within 1 year and a poorer improvement of neurological impairment. The mortality rate was not unexpected because the GNRI was designed to identify nutrition-related risk, including survival rate, as was validated in many other pathologies described above. However, our study results might add to the knowledge that the GNRI would be useful for predicting mortality in older patients with SCI.
Additionally, this report might be the first to demonstrate the relationship between nutrition-related risk and improvement in neurological impairment. There are three postulated reasons for this result. First, malnutrition might prevent neurological improvement at the cellular level. Previous studies have demonstrated that patients with SCI do not need only macronutrients that produce energy but also micronutrients, such as vitamins and minerals, to ensure proper cellular health, water and nutrient transport, and acid–base balance25. Second, evidence shows that the older population with severe malnutrition tends to have a lower motivation for every activity26. Consequently, the lower motivation to improve neurological symptoms in the older population with malnutrition would prevent vigorous rehabilitation, resulting in poor clinical outcomes. Third, patients with malnutrition tended to have sarcopenia in the chronic phase, which resulted in poor musculoskeletal functional improvement and accelerated musculoskeletal atrophy27. Such reasons or other factors might be entangled with each other, resulting in poor neurological improvement and lower levels of ADL at 1 year post-injury in older patients with malnutrition after SCI.
Gater et al. published guidelines for providing appropriate diet and nutrition after SCI28. The summarized points are as follows. (1) Resting energy expenditure was determined every 1–3 years to ensure an accurate assessment of energy balance. (2) Body fat was annually assessed with an obesity surrogate of BMI ≥ 22.3 kg/m2. (3) Review of negative energy balance, targeted diets, and exercises needed for fat loss; lipid management; and target glycated hemoglobin (HbA1c). (4) The fasting lipid profile was assessed annually to achieve target levels of triglycerides of ≤ 150 mg/dL and high-density lipoprotein cholesterol of ≥ 40 mg/dL. (5) Assess fasting blood glucose and HbA1c levels every 3 years to achieve a target HbA1c level of < 7%. As the number of older people with malnutrition increases, assessing the nutritional status on admission becomes critically important11. Furthermore, SPINE20, which is the advocacy group to bring global attention to the burden of disability caused by spinal disorders, released a recommendation in 2022 stating, “SPINE20 calls upon G20 countries to create a competent workforce and improve the health care infrastructure/facilities, including equipment to provide evidence-based inter-professional services to patients with spinal cord injury throughout their continuum of care”29. Our study results validated the significance of their guide and statements that a multidisciplinary approach with a long-term aspect is essential for older patients with malnutrition and SCI to decrease the mortality rate and improve neurological recovery.
This study had some limitations that should be addressed. First, all data were retrospectively collected. Although patients without missing major data were registered in our database, we did not have information about the exact number of cases excluded from the database. Additionally, the treatment strategy was determined by each physician and may not have been consistent among patients. Second, the study population included only Japanese and was heterogeneous. Third, we did not evaluate nutritional intervention, which limits us from reaching a definitive conclusion on this topic. Fourth, in this study, we did not consider several potentially important factors for outcomes, such as the length of rehabilitation and patients’ comorbidities, except for diabetes. Therefore, based on our results, a prospective international multicenter study should be conducted to test the evidence-based clinical effectiveness and cost-effectiveness of nutritional intervention for older patients with SCI and malnutrition.
In conclusion, approximately 35% of the older patients with cervical SCI in our study had nutrition-related risks. Particularly, 6% of these patients had severe malnutrition with major nutrition-related event risks. They also exhibited considerably shorter survival times, higher 1 year mortality rates, poorer neurological improvement, and lower levels of ADL at 1 year post-injury than matched controls. Therefore, assessing nutrition-related risk for older patients with SCI, as well as a multidisciplinary approach with long-term follow-up for such patients with nutrition-related risk, will be essential to decrease the mortality rate and improve neurological recovery and ADL.
Author contributions
All authors contributed to the collection of clinical data. KT and HT led the drafting of this manuscript in collaboration with other authors. SK, GI, KA, HN, TK, TF, and KW closely revised many sections. All authors contributed to all sections of the manuscript and edited it for key intellectual content. All other authors have read and provided substantive intellectual comments to the draft and have approved the final version of the paper.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: A systematic review. Eur. Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 2.World_Health_Organization. Spinal Cord Injury.https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury Accessed 9 March 2024 (2013).
- 3.Lenehan B, et al. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine (Phila Pa 1976) 2012;37:321–329. doi: 10.1097/BRS.0b013e31822e5ff8. [DOI] [PubMed] [Google Scholar]
- 4.DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch. Phys. Med. Rehabil. 2011;92:332–338. doi: 10.1016/j.apmr.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Miyakoshi N, et al. A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord. 2021;59:626–634. doi: 10.1038/s41393-020-00533-0. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Vol. 2016. 7–11 World Health Statistic 2016. (World Health Organization, 2016). https://www.who.int/docs/defaultsource/gho-documents/world-health-statistic-reports/world-heatlth-statistics-2016.pdf.
- 7.AlEissa SI, et al. SPINE20 A global advocacy group promoting evidence-based spine care of value. Eur. Spine J. 2021;30:2091–2101. doi: 10.1007/s00586-021-06890-5. [DOI] [PubMed] [Google Scholar]
- 8.Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 9.Jain NB, et al. Traumatic spinal cord injury in the United States, 1993–2012. JAMA. 2015;313:2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehlings MG, Pedro K, Hejrati N. Management of acute spinal cord injury: Where have we been? Where are we now? Where are we going? J. Neurotrauma. 2022;39:1591–1602. doi: 10.1089/neu.2022.0009. [DOI] [PubMed] [Google Scholar]
- 11.Ministry_of_Health_Labour_and_Welfare. The Results of National Health and Nutrition Survey 2018 (written in Japanese).https://www.mhlw.go.jp/stf/newpage_14156.html. Accessed 9 March 2024 (2020).
- 12.Wong S, Derry F, Jamous A, Hirani SP, Forbes A. Is undernutrition risk associated with an adverse clinical outcome in spinal cord-injured patients admitted to a spinal centre? Eur. J. Clin. Nutr. 2014;68:125–130. doi: 10.1038/ejcn.2013.238. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Liu Z, Sun T, Ren J, Wang X. Relationship between nutritional status and mortality during the first 2 weeks following treatment for cervical spinal cord injury. J. Spinal Cord Med. 2014;37:72–78. doi: 10.1179/2045772313Y.0000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M, Momosaki R, Wakabayashi H, Kikura T, Maeda K. Relationship between nutritional status and improved ADL in individuals with cervical spinal cord injury in a convalescent rehabilitation ward. Spinal Cord. 2019;57:501–508. doi: 10.1038/s41393-019-0245-9. [DOI] [PubMed] [Google Scholar]
- 15.Bouillanne O, et al. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008;87:106–113. doi: 10.1093/ajcn/87.1.106. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi I, et al. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol. Dial. Transplant. 2010;25:3361–3365. doi: 10.1093/ndt/gfq211. [DOI] [PubMed] [Google Scholar]
- 18.Ruan GT, et al. Geriatric nutrition risk index: Prognostic factor related to inflammation in elderly patients with cancer cachexia. J. Cachexia Sarcopenia Muscle. 2021;12:1969–1982. doi: 10.1002/jcsm.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakubo Y, et al. Potential association with malnutrition and allocation of combination medical therapies in hospitalized heart failure patients with reduced ejection fraction. Sci. Rep. 2022;12:8318. doi: 10.1038/s41598-022-12357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima H, et al. Prognostic factors for cervical spinal cord injury without major bone injury in elderly patients. J. Neurotrauma. 2022;39:658–666. doi: 10.1089/neu.2021.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokogawa N, et al. Differences in clinical characteristics of cervical spine injuries in older adults by external causes: A multicenter study of 1512 cases. Sci. Rep. 2022;12:15867. doi: 10.1038/s41598-022-19789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota R, et al. Prognostic factors for respiratory dysfunction for cervical spinal cord injury and/or cervical fractures in elderly patients: A multicenter survey. Glob. Spine J. 2022;14:101–112. doi: 10.1177/21925682221095470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirshblum SC, et al. International standards for neurological classification of spinal cord injury (revised 2011) J. Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haberman SJ. The analysis of residuals in cross-classified tables. Biometrics. 1973;29:205–220. doi: 10.2307/2529686. [DOI] [Google Scholar]
- 25.Farkas GJ, Pitot MA, Berg AS, Gater DR. Nutritional status in chronic spinal cord injury: A systematic review and meta-analysis. Spinal Cord. 2019;57:3–17. doi: 10.1038/s41393-018-0218-4. [DOI] [PubMed] [Google Scholar]
- 26.Payne L, et al. Beliefs about inevitable decline among home-living older adults at risk of malnutrition: A qualitative study. J. Hum. Nutr. Diet. 2020;33:841–851. doi: 10.1111/jhn.12807. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez G, et al. Musculoskeletal morbidity following spinal cord injury: A longitudinal cohort study of privately-insured beneficiaries. Bone. 2021;142:115700. doi: 10.1016/j.bone.2020.115700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gater DR, Bauman C, Cowan R. A primary care provider's guide to diet and nutrition after spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2020;26:197–202. doi: 10.46292/sci2603-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darwono B, et al. SPINE20 recommendations 2022: Spine care-working together to recover stronger. Eur. Spine J. 2022;31:3262–3273. doi: 10.1007/s00586-022-07432-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.