Abstract
Inhaled nitric oxide (iNO) is a potent and selective pulmonary vasodilator with a safety concern due to rebound pulmonary hypertension (PH) associated with its withdrawal. We report short‐term pulsed iNO in patients with severe pulmonary arterial hypertension (PAH) and nonoperable chronic thromboembolic PH (nCTEPH). This is a retrospective analysis of 33 patients: 22 with PAH and 11 with nCTEPH. We assessed hemodynamic, echocardiographic, and other noninvasive variables to evaluate safety and efficacy of iNO. We performed an iNO withdrawal test during right heart catheterization and after 3 days of iNO treatment. iNO significantly improved all variables examined in 22 patients with PAH and 11 with nCTEPH. Two patterns of response were observed after sudden iNO withdrawal. Twenty‐nine patients (88%) showed minimal hemodynamic, oxygenation and clinical changes. Four patients (12%) had a reduction in cardiac index ≥20% and PaO2 ≥ 5%, three patients did not show clinical deterioration, and one patient developed hemodynamic collapse that needed iNO administration. This retrospective study suggests that short‐term iNO improves hemodynamics and clinical conditions in some patients with PAH an nCTPEH. However, pulsed iNO withdrawal PH rebound could be a serious concern in these patients. Given the lack of evidence, we do not recommend the use of pulsed iNO in the treatment of patients with chronic PH.
Keywords: chronic thromboembolic pulmonary hypertension, echocardiographyinhaled nitric oxide, pulmonary artery hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are rare clinical condition characterized by the presence of precapillary pulmonary hypertension (PH). 1 Inhaled nitric oxide (iNO), approved for the treatment of persistent PH in newborns (PPHN), 2 has been proposed as a long‐term therapy for PAH and other types of PH. 3 , 4 , 5 Moreover, iNO is occasionally used as a rescue therapy for severely hypoxemic patients both with and without an established diagnosis of PH. 6 Currently, the use of iNO in PAH and CTEPH is not supported by clinical practice guidelines due to the lack of evidence regarding its safety and effectiveness.
Although iNO is generally considered safe, there are some safety concerns, such as rebound of PH. Abrupt discontinuation of iNO can precipitate rapid worsening of ventilation‐perfusion mismatching and/or PH, which typically manifests as hypoxemia and/or hemodynamic compromise. 7 PH rebound has been observed in PPHN, 7 , 8 adult respiratory distress syndrome (ARDS), 6 postoperative cardiac surgery 9 and lung transplantation. 10 However, information on PAH and nonoperable CTEPH (nCTEPH) is scarce. 5
We have examined the efficacy and safety of short‐term iNO in a retrospective analysis of medical records of patients with severe PAH who could not be treated with parenteral prostacyclins. Despite absence of vasodilator indications, patients with nCTEPH were included due to the severe deterioration of their clinical condition and the absence of a specific treatment at that time.
METHODS
Patients
This is a retrospective study, including 33 naive patients (22 with PAH and 11 CTEPH) with high‐risk status who had serious challenges in handling the infusion pump, intravenous access, and medication, due to joint problems in their hands, lack of dexterity, absence of a capable caregiver, or simply a reluctance to assume the responsibilities of epoprostenol treatment at home (the only prostacyclin available in our center at that time). For this reason, on a compassionate basis, iNO safety was assessed for each of these patients in the hospital setting during the main diagnosis process, for possible domiciliary use. During this short (3 days) iNO evaluation, patients were not on specific pulmonary vascular approved therapy. After this initial diagnostic assessment, which included hemodynamic and clinical iNO evaluation, all patients started oral combination therapy (sildenafil, bosentan, and tadalafil), according to clinical practice guidelines. Inhaled NO remained as a possible add on treatment after reevaluation in cases of persistent high‐risk status. In relation to patients with nCTEPH, no targeted therapy had been validated when the study was performed.
Patients were diagnosed in the multidisciplinary Pulmonary Vascular Unit at the University Hospital Universitario Dr Negrín (Las Palmas de Gran Canaria, Spain) between 2005 and 2012. Safety and short‐term effectiveness of iNO were evaluated in each patient according to a protocol approved by the ethics committee (CEIC) of our hospital (#160003) before starting any therapy. Informed consent was obtained from each patient before onset of study and iNO treatment.
Enrolled patients had World Health Organization functional class (WHO‐FC) III and IV, severe hemodynamic impairment (mean pulmonary artery pressure [mPAP] ≥ 40 mmHg and/or pulmonary vascular resistance [RVP] ≥ 400 dyne.s.cm−5) and a high‐risk status of unfavorable outcome or death. 1 All patients, except for two with PAH associated with connective tissue disease, had a negative acute vasodilator test with iNO. In patients with CTEPH, pulmonary endarterectomy was ruled out by a multidisciplinary team due to the presence of vascular lesions not accessible to surgery, hemodynamic disproportionate to vascular obstruction or the presence of various comorbidities. Those patients who met criteria for lung transplantation were included on the corresponding waiting list. Patients receiving iNO did not show pulmonary artery wedge pressure >15 mmHg during right heart catheterization (RHC) nor significant left heart disease (systolic dysfunction defined as a left ventricular ejection fraction <50%, diastolic dysfunction greater than stage 1, cardiomyopathy, or pericarditis). In addition, these patients did not have a significant restrictive (total lung capacity or forced vital capacity <70% of predicted value), or obstructive pulmonary disease (forced expiratory volume in the first second <60% of predicted value).
Inhaled NO protocol
Patients were evaluated according to the HP clinical practice guidelines for PAH and CTEPH. 1 The following variables were collected: WHO‐FC, Borg dyspnea score, 6‐min walking test (6MWT), arterial blood gases, methemoglobin, pulmonary function tests (forced spirometry, whole‐body plethysmography, and diffusing capacity for carbon monoxide), Doppler echocardiography and NT‐pro‐brain natriuretic peptide (NT‐proBNP). Every patient underwent RHC for hemodynamic evaluation, iNO AVT, iNO optimal flow rate, and iNO hemodynamic withdrawal test.
We considered the AVT to be positive when there was a reduction in mPAP of at least 10 mmHg, to achieve an absolute value ≤40 mmHg, with an increase or no change in cardiac output. 1 Inhaled NO flow rate for the ambulatory device was established as causing the greatest decrease in PVR without change in arterial partial pressure of oxygen (PaO2). Hemodynamic iNO withdrawal test was assessed to treat the patients with iNO optimal flow rate for at least 2 h and then abruptly withdrawing it to detect PH rebound. After RHC, patients restarted iNO at the Intermediate Respiratory Care Unit for 3 days. Inhaled NO withdrawal test was performed again under medical supervision and echocardiographic and noninvasive monitoring. Inhaled NO PH rebound was defined in the catheterization lab according to the following criteria: increase in mPAP ≥ 20%, decrease of cardiac index (CI) and mean systemic arterial pressure (mSAP) ≥ 20%, and severe clinical deterioration (dyspnea, thoracic pain, dizziness, tachycardia, or hypoxemia) needed immediate iNO to be restarted.
PH rebound was considered in the Intermediate Respiratory Care Unit when noninvasive mSAP decreased ≥ 20%, echocardiogram showed an increased in systolic pulmonary arterial pressure (sPAP) ≥ 20% and symptoms suddenly worsened, requiring immediate iNO administration. The presence of PH rebound or other side effects excluded ambulatory iNO use. Inhaled NO device consisted of an NO demand valve connected with a nasal cannula (Demand Flow‐62; Air Products and Chemical) to 200 ppm of NO in N2 cylinder. The system was activated on demand at −1.5 cm H2O inspiratory pressure to deliver pulsed doses of gas at the beginning of each inspiration. Environmental alarms for NO and NO2 (portable NO and NO2 environmental monitors; Micro Medical) were installed in the hemodynamic lab and hospital ward.
Statistical analysis
Data are reported as mean and standard deviations (SD). Comparisons between groups of patients with PH and nCTEPH were performed using Mann−Whitney nonparametric test. Results of iNO abrupt withdrawal and short‐term hospital treatment with iNO were carried out using Wilcoxon nonparametric test. Statistical significance was set at p < 0.05, using a SPSS version 20 software (SPSS Inc.).
RESULTS
Patients
A total of 33 patients (55% female) were evaluated for initiation of compassionate use treatment with iNO. Twenty‐two were diagnosed with PAH and 11 with nCTEPH. Patients with PAH were included in following clinical subgroups: idiopathic, drug‐toxic, connective tissue disease and congenital heart disease (Table 1). All patients had WHO‐FC III/IV (52% III/48% IV) and severe deterioration in hemodynamics, exercise capacity and biological markers (Table 1). Patients with nCTEPH showed a significant higher age and lower CI than those with PAH (Table 1).
Table 1.
Demographic, clinical, hemodynamic, and inhaled nitric oxide data.
| Parameters | PAH (n = 22) | nCTEPH (n = 11) | Total (n = 33) |
|---|---|---|---|
| Age, years (mean ± SD) | 53 ± 4 | 66 ± 5a | 57 ± 18 |
| Sex, female (%) | 13 (59) | 5 (45) | 18 (55) |
| WHO Functional class IV, n (%) | 10 (45) | 6 (55) | 16 (48) |
| Idiopathic PAH, n (%) | 11 (50) | ||
| Anorexigenic/toxic PAH, n (%) | 2 (9) | ||
| Connective tissue PAH, n (%) | 5 (23) | ||
| Congenital heart disease PAH, n (%) | 4 (18) | ||
| NT‐proBNP pgr/ml (mean ± SD) | 1611 ± 1785 | 2920 ± 2517 | 2078 ± 2128 |
| 6MWT meters (mean ± SD) | 255 ± 43 | 273 ± 36 | 267 ± 158 |
| Hemodynamics (mean ± SD) | |||
| RAP mmHg | 9 ± 1 | 11 ± 1 | 10 ± 5 |
| mPAP mmHg | 51 ± 3 | 45 ± 4 | 51 ± 13 |
| CI L. min−1.m−2 | 2.7 ± 0.1 | 2.1 ± 0.3a | 2.4 ± 0.7 |
| PaO2 mmHg | 69 ± 2 | 62 ± 4 | 67 ± 8 |
| SVmO2 (%) | 67 ± 2 | 61 ± 2 | 66 ± 8 |
| Acute vasodilatation test (mPAP%) | 18 ± 2 | 10 ± 4 | 14 ± 12 |
| iNO titration (lpm) | 0.8 (±0,2) | 0.75 (±0,3) | 0.8 (±2) |
Abbreviations: 6MWT, 6‐min walking test; Acute vasodilatation test, % in relation to baseline mPAP; CI, cardiac index; HR, heart rate; iNO, inhaled nitric oxide; mPAP, mean pulmonary arterial pressure; nCTEPH, nonoperable chronic thromboembolic pulmonary hypertension; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; RAP, right atrium pressure; SVmO2, mixed venous oxygen saturation; SVR, systemic vascular resistance.
Significant changes between PAH and nCTEPH, p < 0.01.
Hemodynamics
Baseline hemodynamic data showed severe PH, negative AVT and moderate hypoxemia (Tables 1 and 2). Inhaled NO sudden withdrawal after >2 h of exposure during RHC did not cause clinical deterioration suggestive of PH rebound (Table 2). Most patients (29/33, 88%) showed minimal changes in hemodynamic and oxygenation. However, four patients (12%), three with PAH and one with nCTEPH, exhibited a hemodynamic profile suggesting PH rebound: decrease of at least 20% in CI and 5% in PaO2 and, increase between 5 and 12% in mPAP and at least 30% in PVR (Table 3). Of these four subjects, patient # 33, showed 11% and 32% increase in mPAP and PVR respectively, and 20%, 16%, and 10% decrease in CI, mSAP, and PaO2, respectively. This patient stood up for 32% CI increase and, 26% and 43% decrease in mPAP and PVR, respectively in the AVT (Table 3).
Table 2.
Baseline hemodynamics, iNO acute vasodilatation test and iNO withdrawal in the 33 patients of the study.
| Parameters | Basal | iNO (20 ppm) | Post‐iNO (2 h) | p |
|---|---|---|---|---|
| mPAP mmHg | 51 ± 13 | 44 ± 13a | 52 ± 14 | <0.001 |
| RAP mmHg | 9 ± 1 | 9 ± 5 | 10 ± 8 | |
| PAWP mmHg | 11 ± 2.6 | 11 ± 2.7 | 11 ± 3.8 | |
| CI L/min/m2 | 2.4 ± 0.7 | 2.6 ± 0.8a | 2.5 ± 0.9 | 0.03 |
| PVR dy.s/cm5 | 874 ± 484 | 671 ± 388a | 889 ± 501 | <0.001 |
| SVmO2% | 66 ± 8 | 67 ± 7a | 64 ± 8 | 0.02 |
| HR bpm | 75 ± 11 | 73 ± 12 | 75 ± 13 | |
| mSAP mmHg | 89 ± 14 | 91 ± 14 | 89 ± 16 | |
| SVR dy.s/cm−5 | 1557 ± 522 | 1458 ± 397 | 1598 ± 559 | |
| PaO2 mmHg | 67 ± 8 | 71 ± 18 | 69 ± 13 | |
| PaCO2 mmHg | 34 ± 6 | 35 ± 5 | 33 ± 5 | |
| Met‐hb % | 0.5 ± 0.2 | 0.7 ± 0.2a | 0.7 ± 2b | 0.03/0.02 |
Note: Data are presented as mean ± SD.
Abbreviations: CI, cardiac index; HR, heart rate; iNO, inhaled NO; Met‐hb%, percentage of methemoglobin; Met‐hb, methemoglobin; mPAP, mean pulmonary arterial pressure; mSAP, mean systemic arterial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrium pressure; SVmO2, mixed venous oxygen saturation; SVR, systemic vascular resistance.
Significant changes between baseline and iNO acute vasodilatation test;
Significant changes between baseline and iNO withdrawal test.
Table 3.
Hemodynamic profile suggestive of rebound pulmonary hypertension after iNO withdrawal (2 h) in four patients.
| Patients | # 5 | # 11 | # 19 | # 33 |
|---|---|---|---|---|
| Gender/Age (years) | M/72 | M/56 | M/73 | M/65 |
| Diagnosis | nCTEPH | IPAH | IPAH | IPAH |
| WHO‐FC | IV | III | IV | IV |
| mPAP (mmHg) pre/iNO/post (%) | 45/44/48 (7) | 79/62/83 (5) | 45/42/49 (9) | 57/42/64 (11) |
| CI (L/min/m2) pre/iNO/post (%) | 1.5/1.6/1.2 (−20) | 2.7/2.3/2.1 (−22) | 2.9/3.2/2.3 (−21) | 1.5/2.2/1.2 (−20) |
| PVR (dyn.s/cm5) pre/iNO/post (%) | 1322/1169/1811 (37) | 912/869/1188 (30) | 478/386/680 (30) | 1500/853/2189 (32) |
| mSAP (mmHg) pre/iNO/post (%) | 74/67/70 (−5) | 79/66/80 (1) | 85/101/93 (9) | 89/93/75 (−16) |
| PaO2 (mmHg) pre/iNO/post (%) | 77/75/72 (−7) | 66/75/63 (−5) | 60/61/55 (−8) | 61/85/55 (−10) |
Note: Variables recorded during baseline hemodynamics (pre), acute vasodilation test with inhaled NO (iNO), and after sudden withdrawal of iNO (post).
Abbreviations: %, percentage of change between baseline and withdrawal iNO; CI, cardiac index; M, male; IPAH, idiopathic pulmonary arterial hypertension; mPAP, mean pulmonary arterial pressure; mSAP, mean systemic arterial pressure; nCTEPH, nonoperable chronic thromboembolic pulmonary hypertension; PVR, pulmonary vascular resistance; WHO‐FC, world health organization functional class.
Short‐term iNO treatment
All 33 patients were treated with pulsed iNO during 24 h per day, at least 3 days without adverse events or complications. The alarms system did not signal high levels of NO and/or environmental NO2 at any time. Methemoglobin levels were within safety limits (Table 4) and there were no significant variations in pulmonary function tests. After 3 days of initiation of treatment, a new evaluation revealed a significant improvement in exercise dyspnea (Borg dyspnea score), 6MWT, baseline PaO2 and NT‐proBNP (Figure 1 and Table 4). Pulsed iNO sudden withdrawal was not followed by any clinical deterioration in symptoms, heart rate, mSAP, sPAP or gas exchange in 32 patients (Table 4). However, only the patient # 33, with idiopathic PAH, experienced worsening of dyspnea (Borg dyspnea score from 3 to 6), heart rate (75 to 125 bpm), mSAP (84 to 57 mmHg) and gas exchange (PaO2 72 to 49 mmHg, PaCO2 35 to 26 mmHg) 2 min after abrupt iNO suppression, requiring immediate iNO restart. Echocardiographic revealed increase in systolic PAP (75 mmHg up to 110 mmHg) and right ventricle (RV) dilatation and dysfunction (tricuspid annular plane systolic excursion [TAPSE] from 16 to 10 mm and eccentricity index from 1.3 to 1.6) (Figure 2). At baseline clinical evaluation, this patient showed a high risk of deterioration, WHO‐FC (IV), 6MWT (135 m), NT‐proBNP (4232 pgr/mL), and echocardiographic (TAPSE 14 mm) and hemodynamic markers (CI 1.5 L/min/m2) indicating RV deteriorated function. Coronary angiography did not reveal any significant findings.
Table 4.
Data from 32 patients who did not present iNO pulmonary hypertensive rebound after three days of iNO treatment: At the beginning of treatment (Pre‐iNO), 30 min before withdrawal (3 days iNO) and 5 min after withdrawal (Post‐iNO).
| Parameters | Pre‐iNO | 3 days iNO | Post‐iNO | p |
|---|---|---|---|---|
| Dyspnea Borg scale | 3.7 ± 2 | 3.1 ± 2* | 3.2 ± 2** | 0.02/0.03 |
| mSAP mmHg | 82 ± 11 | 86 ± 11* | 82 ± 18 | 0.03 |
| sPAP mmHg | 86 ± 22 | 83 ± 19 | 87 ± 18 | |
| HR bpm | 76 ± 12 | 78 ± 12 | 75 ± 11 | |
| PaO2 mmHg | 69 ± 10 | 72 ± 12* | 70 ± 13 | 0.01 |
| PaCO2 mmHg | 36 ± 6 | 35 ± 5 | 33 ± 9 | |
| FVC L (%pred) | 2.9 ± 1 (92 ± 13) | 2.9 ± 1 (93 ± 12) | ||
| FEV1 L (%pred) | 2.2 ± 0.9 (87 ± 16) | 2.1 ± 0.8 (86 ± 15) | ||
| Met‐hb % | 0.5 ± 0.2 | 0.8 ± 0.2* | 0.7 ± 0.1** | 0.002 |
Note: Data are presented as mean ± SD. * and **Statistical significance between pre‐iNO and 3 days iNO, and Pre‐iNO and Post‐iNO.
Abbreviations: %Pred, percentage of predicted value; FEV1, forced expiratory volume in the first second; FVC, force vital capacity; HR, heart rate; Met‐hb%: percentage of methemoglobin; mSAP, mean systemic arterial pressure; sPAP: systolic pulmonary arterial pressure.
Figure 1.
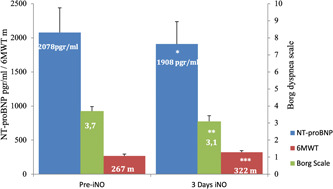
Relevant variables pre and after 3 days of treatment with inhaled Nitric Oxide (meantSEM). *p < 0.001, **p < 0.02, ***p < 0.001. m, meters.
Figure 2.
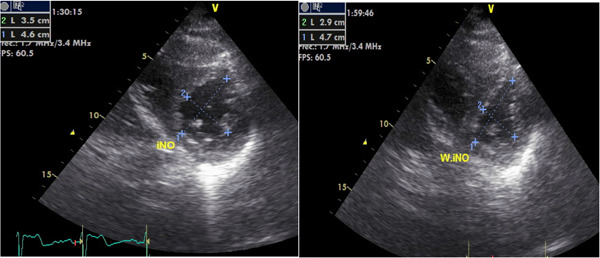
Left ventricle eccentricity index during inhaled Nitric Oxide treatment and 2 min after sudden withdrawal in patient # 33. iNO, inhaled NO; W. iNO, without inhaled NO.
DISCUSSION
This study retrospectively evaluates short‐term pulsed iNO monotherapy in 33 patients with PAH and nCTPEH. We found that short‐term iNO caused an improvement in hemodinamycs and clinical conditions in our series, however, some patients showed PH rebound. We emphasized in our study the absence of other complication associated with iNO treatment. In fact, hospital use of pulsed iNO with available systems has demonstrated environmental safety, 4 , 11 , 12 , 13 , 14 as well as the absence of methemoglobinemia 11 , 15 , 16 , 17 and cytotoxicity at concentrations <100 ppm. 11 , 18
This is the first study to address PH rebound after pulsed iNO withdrawal in adults with severe PAH and nCTEPH. We described two hemodynamics patterns of response after sudden iNO withdrawal. The vast majority showed minimal changes in hemodynamic and oxygenation, and very few patients had a reduction in CI and PaO2. Only one patient of this last group suffered hemodynamic collapse that required iNO reinstitution; this patient was characterized by a worse RV function, large vasoreactivity, and greater decrease in CI after iNO discontinuation. Therefore, hemodynamic profile could be useful as screening of patients at risk of PH rebound. We did not find any other differential characteristic in this study probably due to the small number of patients included in our series. A logistic regression analysis in patients with ARDS found that advanced age, multisystem organ failure, and increase in systemic blood pressure along with an improvement in pulmonary pressure/flow ratio at initiation of iNO were independent factors of PH rebound. 6
To date, only a case of PH rebound has been described in a patient with idiopathic PAH, in whom iNO was used compassionately as a bridge for lung transplantation. 19 No case has been reported in patients with nCTEPH. 20 The percentage of patients with severe PAH and nCTPEH experiencing rebound seems much lower than PPHN (28%) 7 and ARDS (37%). 6 This difference could be related to limited vasoreactivity in patients with severe PAH and nCTEPH, due to remodeling of the small pulmonary vessels. 20 , 21 Although during RHC we observed hemodynamic risk profiles, we did not see PH rebound after 2 h of pulsed iNO. To date, we do not know the doses and duration of treatment with iNO that are at risk for PH rebound development. After iNO withdrawal an increase in RVP greater than 10%, with only 5 min of iNO (80 ppm) exposition, was described in 28% of patients with PAH associated with congenital heart disease. 22 However, more than 10 h of iNO treatment were necessary for the onset of PH rebound in patients with ARDS and PPHN. 6 , 23
This study represents a large series of PAH and nCTEPH patients treated only with short‐term iNO (3 days). Inhaled NO resulted in significant changes in symptoms measured by the Borg dyspnea score, meters walked in 6MWT, NT‐proBNP and PaO2 levels in a 3‐day interval. We emphasize the short time to achieve these findings, probably because careful dose titration is not required. On the other hand, pulsed iNO causes vasodilation in the pulmonary circulation mainly in the well‐ventilated areas. 24 The absence of deterioration in gas exchange under iNO was expected. Recent reviews 25 are reminders of the knowledge that VA/Q in PAH and CTEPH is only mildly altered and that hypoxemia in these patients is essentially related to a low cardiac output. In addition, it does not usually require inotropic support in cases with severe RV dysfunction due to its pulmonary selectivity. We believe that those could be the reasons why iNO has been successfully used in patients with hemodynamic compromise due to severe acute PH or exacerbation of chronic PH, although with limited quality of data. 24 , 26 , 27 , 28 However, pulsed iNO (Bellerophon, NCT02725372, phase 3 trial with iNO as add‐on therapy) in PAH who remain symptomatic on approved PAH monotherapy or combination approved therapy and long‐term oxygen therapy, although it showed tolerability, safety, and hemodynamic improvement, it was closed prematurely (16 weeks) due to poor improvement in the 6‐min walk test. We therefore emphasize the lack of evidence in favor of the use of iNO as a therapy to be added to other treatments already accepted in the clinical practice guidelines. On the other hand, in studies with prevalent patients already treated, more cases, longer evolution time and other outcomes such as time to death or a complication related to PAH (selexipag trial) are necessary. 29 Another outcome useful in a clinical trial with pulsed iNO in patients with pulmonary fibrosis on oxygen therapy and at risk of PH was actigraphy. In a recent trial, it was reported an improvement in moderate/vigorous physical activity and remained stable in overall activity. 30
Among the limitations of our study, we acknowledge its non‐randomized design, the scarce sample of patients treated in the short‐term and the existence of two different groups of patients with PH. Another concern is the restriction that the delivery system imposes on patient's mobility and quality of life. Specific questionnaires assessing quality of life would provide more information on this topic.
In conclusion, this retrospective study suggests that short‐term pulsed iNO monotherapy improves hemodynamics and clinical conditions in some patients with PAH an nCTPEH. However, this study warns us about the risk of iNO PH rebound. 2 h of pulsed iNO treatment during RHC could be hepful to identify patients at hazard for this complication. It is important to stress that this observational study involves a drug not included in PH guidelines and used on a compassionate basis. Therefore, at present, we do not recommend the use of iNO in the treatment of patients with chronic PH.
AUTHOR CONTRIBUTIONS
Gregorio Miguel Pérez‐Peñate, Gabriel Juliá‐Serdá and Miguel Ángel Gómez‐Sánchez have designed and supervised the study. Gregorio Miguel Pérez Peñate and Antonio Garcia‐Quintana performed right heart catheterizations. Gabriel Juliá‐Serdá and Desireé Alemán‐Segura supervised the performance of lung function test, exercise test and Borg dysnea scale. Antonio García‐Quintana and José Ramón Ortega‐Trujillo performed the echocardiography. Gregorio Miguel Pérez‐Peñate, Fernando León‐Marrero, Helena Galván‐Fernández and Iñigo Rúa‐Fernández de Larrinoa evaluated all patients included in this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
iNO protocol was approved by the ethics committee (CEIC) of our hospital (160003). Informed consent was obtained from each patient prior to onset of study. Confidentiality was respected through the anonymization of data.
ACKNOWLEDGMENTS
The authors thank Vicente Morales Garcia and Jose Andrés Ortiz Santana for their technical support and editorial assistance. The authors did not at any time receive at any time payment or services from a third party (government, commercial, private foundation, etc.) for any aspect of the submitted work (including but not limited to grants, data monitoring board, study design, manuscript preparation, statistical analysis, etc.). Moreover, the authors do not have no other relationships or activities that readers could perceive as having influenced us, or that give the appearance of having potentially influenced us. We have received funding solely for publication in the Pulmonary Circulation Journal from the Canarian Foundation for Health Research of the Canary Islands, University Hospital of Gran Canaria Doctor Negrín. Address: C. Pl. Barranco de la Ballena, 35012 Las Palmas de Gran Canaria, Las Palmas. Spain.
Pérez‐Peñate GM, Juliá‐Serdá G, Galván‐Fernández H, Alemán‐Segura D, León‐Marrero F, Garcia‐Quintana A, Larrinoa I‐F, Ortega‐Trujillo JR, Gómez‐Sánchez MÁ. Safety of inhaled nitric oxide withdrawal in severe chronic pulmonary hypertension. Pulm Circ. 2024;14:e12344. 10.1002/pul2.12344
REFERENCES
- 1. Humbert M, Kovacs G, Hoeper MM. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:1–114. [DOI] [PubMed] [Google Scholar]
- 2. Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low‐Dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med. 2000;342:469–474. [DOI] [PubMed] [Google Scholar]
- 3. Channick RN, Newhart JW, Johnson FW, Williams PJ, Auger WR, Fedullo PF, Moser KM. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension. Chest. 1996;109:1545–1549. [DOI] [PubMed] [Google Scholar]
- 4. Pérez‐Peñate G, Julià Serdà G, Cabrera‐Navarro P, Pulido‐Duque JM, Górriz‐Gómez E. One‐year continuous inhaled nitric oxide for primary pulmonary hypertension. Chest. 2001;119:970–973. [DOI] [PubMed] [Google Scholar]
- 5. Pérez‐Peñate GM, Juliá‐Serdà G, Ojeda‐Betancort N, García‐Quintana A, Pulido‐Duque J, Rodríguez‐Pérez A, Cabrera‐Navarro P, Gómez‐Sánchez MA. Long‐term inhaled nitric oxide plus phosphodiesterase 5 inhibitors for severe pulmonary hypertension. J Heart Lung Transplant. 2008;27:1326–1332. [DOI] [PubMed] [Google Scholar]
- 6. Christenson J, Lavoie A, O'CONNOR M, BHORADE S, POHLMAN A, HALL JB. The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med. 2000;161:1443–1449. [DOI] [PubMed] [Google Scholar]
- 7. Miller OI, Tang SF, Keech A, Celermajer DS. Rebound pulmonary hypertension on withdrawal from inhaled nitric oxide. The Lancet. 1995;346:51–52. [DOI] [PubMed] [Google Scholar]
- 8. Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–1047. [DOI] [PubMed] [Google Scholar]
- 9. Ivy DD, Kinsella JP, Ziegler JW, Abman SH. Dipyridamole attenuates rebound pulmonary hypertension after inhaled nitric oxide withdrawal in postoperative congenital heart disease. J Thorac Cardiovasc Surg. 1998;115:875–882. [DOI] [PubMed] [Google Scholar]
- 10. Della Rocca G, Coccia C. Nitric oxide in thoracic surgery. Minerva Anestesiol. 2005;71:313–318. [PubMed] [Google Scholar]
- 11. Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2000;2:CD000399. [DOI] [PubMed] [Google Scholar]
- 12. Ivy DD, Parker D, Doran A, Parker D, Kinsella JP, Abman SH. Acute hemodynamic effects, and home therapy using a novel pulsed nasal nitric oxide delivery system in children and young adults with pulmonary hypertension. Am J Cardiol. 2003;92:886–890. [DOI] [PubMed] [Google Scholar]
- 13. Cuthbertson BH, Dellinger P, Dyar OJ. UK guidelines for the use of inhaled nitric oxide therapy in adult ICUs: American‐European Consensus Conference on ALI/ARDS. Intensive Care Med. 1997;41:266‐73. [DOI] [PubMed] [Google Scholar]
- 14. Executive HS. Occupational exposure limits 1996. London: HMSO; 1996. [Google Scholar]
- 15. Young JD, Dyar O, Xiong L, Howell S. Methaemoglobin production in normal adults inhaling low concentrations of nitric oxide. Intensive Care Med. 1994;20:581–584. [DOI] [PubMed] [Google Scholar]
- 16. Roberts JD, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM, Thusu KG, Zellers TM, Wylam ME, Zaslavsky A. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med. 1997;336:605–610. [DOI] [PubMed] [Google Scholar]
- 17. Journois D, Baufreton C, Mauriat P, Pouard P, Vouheí P, Safran D. Effects of inhaled nitric oxide administration on early postoperative mortality in patients operated for correction of atrioventricular canal defects. Chest. 2005;128:3537–3544. [DOI] [PubMed] [Google Scholar]
- 18. Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, Rhines J, Chang CT. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, Double‐Masked, Placebo‐Controlled, Dose‐Response, multicenter study. Pediatrics. 1998;101:325–334. [DOI] [PubMed] [Google Scholar]
- 19. Snell GI, Salamonsen RF, Bergin P, Esmore DS, Khan S, Williams TJ, Snell GI, Salamonsen RF, Bergin P, Esmore DS, Khan S, Williams TJ. Inhaled nitric oxide used as a bridge to heart‐lung transplantation in a patient with end‐stage pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1263–1266. [DOI] [PubMed] [Google Scholar]
- 20. Imanaka H, Miyano H, Takeuchi M, Kumon K, Ando M. Effects of nitric oxide inhalation after pulmonary thromboendarterectomy for chronic pulmonary thromboembolism. Chest. 2000;118:39–46. [DOI] [PubMed] [Google Scholar]
- 21. Ulrich S, Fischler M, Speich R, Popov V, Maggiorini M. Chronic thromboembolic and pulmonary arterial hypertension share acute vasoreactivity properties. Chest. 2006;130:841–846. [DOI] [PubMed] [Google Scholar]
- 22. Post M, Janssens S, Van de Werf F, BUDTS W. Responsiveness to inhaled nitric oxide is a predictor for mid‐term survival in adult patients with congenital heart defects and pulmonary arterial hypertension. Eur Heart J. 2004;25:1651–1656. [DOI] [PubMed] [Google Scholar]
- 23. Lum LCS, Tan PSK, Saville A, Venkataraman ST, Pinsky MR. Occult nitric oxide inhalation improves oxygenation in mechanically ventilated children. J Pediatr. 1998;133:613–616. [DOI] [PubMed] [Google Scholar]
- 24. Hajian B, De Backer J, Vos W, Van Holsbeke C, Ferreira F, Quinn DA, Hufkens A, Claes R, De Backer W. Pulmonary vascular effects of pulsed inhaled nitric oxide in COPD patients with pulmonary hypertension. Int J Chronic Obstruct Pulm Dis. 2016;11:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naeije R, Richter MJ, Rubin LJ. The physiological basis of pulmonary arterial hypertension. Eur Respir J. 2022;59:2102334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeffery M, Taichman DB. In: Disease P, Mandel J, Taichman DB Eds Management of the acutely ill patient with pulmonary arterial hypertension. Philadelphia: Elsevier Science; 2006. p. 254. [Google Scholar]
- 27. Cornfield DN, Milla CE, Haddad IY, Barbato JE, Park SJ. Safety of inhaled nitric oxide after lung transplantation. J Heart Lung Transplant. 2003;22:903–907. [DOI] [PubMed] [Google Scholar]
- 28. Takaba K, Aota M, Nonaka M, Sugimoto A, Konishi Y. Successful treatment of chronic thromboembolic pulmonary hypertension with inhaled nitric oxide after right ventricular thrombectomy. Jpn J Thorac Cardiovasc Surg. 2004;52:257–260. [DOI] [PubMed] [Google Scholar]
- 29. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, Rubin LJ, Di Scala L, Tapson V, Adzerikho I, Liu J, Moiseeva O, Zeng X, Simonneau G, McLaughlin VV. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. [DOI] [PubMed] [Google Scholar]
- 30. Nathan SD, Flaherty KR, Glassberg MK, Raghu G, Swigris J, Alvarez R, Ettinger N, Loyd J, Fernandes P, Gillies H, Kim B, Shah P, Lancaster L. A randomized, double‐blind, placebo‐controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis. Chest. 2020;158:637–645. [DOI] [PubMed] [Google Scholar]


