Abstract
This study aimed to evaluate the effectiveness and safety of an oral sequential triple combination therapy with selexipag after dual combination therapy with endothelin receptor antagonist (ERA) and phosphodiesterase‐5 inhibitor (PDE5I)/riociguat in pulmonary arterial hypertension (PAH) patients. A total of 192 PAH patients from 10 centers had received oral sequential selexipag therapy after being on dual‐combination therapy with ERA and PDE5i/riociguat for a minimum of 3 months. Clinical data were collected at baseline and after 6 months of treatment. The study analyzed the event‐free survival at 6 months and all‐cause death over 2 years. At baseline, the distribution of patients among the risk groups was as follows: 22 in the low‐risk group, 35 in the intermediate‐low‐risk group, 91 in the intermediate‐high‐risk group, and 44 in the high‐risk group. After 6 months of treatment, the oral sequential triple combination therapy resulted in reduced NT‐proBNP levels (media from 1604 to 678 pg/mL), a decline in the percentage of WHO‐FC III/IV (from 79.2% to 60.4%), an increased in the 6MWD (from 325 ± 147 to 378 ± 143 m) and a rise in the percentage of patients with three low‐risk criteria (from 5.7% to 13.5%). Among the low‐risk group, there was an improvement in the right heart remodeling, marked by a decrease in right atrium area and eccentricity index. The intermediate‐low‐risk group exhibited significant enhancements in WHO‐FC and tricuspid annular plane systolic excursion. For those in the intermediate‐high and high‐risk groups, there were marked improvements in activity tolerance, as reflected by WHO‐FC and 6MWD. The event‐free survival rate at 6 months stood at 88%. Over the long‐term follow‐up, the survival rates at 1 and 2 years were 86.5% and 86.0%, respectively. In conclusion, the oral sequential triple combination therapy enhanced both exercise capacity and cardiac remodeling across PAH patients of different risk stratifications.
Keywords: event, oral sequential triple combination therapy, pulmonary arterial hypertension, risk stratification, survival
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare and progressive disease characterized by heightened pulmonary vascular resistance, eventually culminating in right heart failure and death. 1 According to the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of pulmonary hypertension, the estimated 1‐year mortality rates for PAH patients categorized as low, intermediate, and high‐risk are less than <5%, 5%–20%, and >20%, respectively. 2 Over the past two decades, the survival rate of patients with PAH has significantly improved due to the introduction of three classic targeted therapy pathways. 3 , 4 These therapies include prostacyclin analogs, endothelin‐receptor antagonist (ERA), phosphodiesterase type 5 inhibitor (PDE‐5i), and soluble guanylate cyclase (sGC) stimulators. The effectiveness of triple combination therapy has also been reported. 2 However, most such studies analyzed patients who were treated with oral drugs and parenteral administration of prostanoids, 5 , 6 , 7 while others had small sample sizes and were single‐center studies. 8 , 9 Therefore, evidence regarding the efficacy and safety of oral triple combination therapy remains scarce.
The current guidelines primarily recommend selexipag‐based oral triple combination therapy for PAH patients who are classified as intermediate‐low risk during follow‐up. However, in actual clinical settings, this oral sequential triple combination therapy has been employed for patients who were at intermediate‐high or high risk, especially when the introduction of intravenous or subcutaneous prostacyclin analogs isn't feasible. Moreover, patients with World Health Organization (WHO) I/II classification have shown potential benefits from this oral triple combination regimen. Yet, there is limited knowledge regarding the efficacy of this therapy across diverse risk stratifications. Consequently, there's a pressing need for more evidence to support the broader application of oral triple therapy. 10 To this end, we embarked on a multicenter observational study aiming to assess the efficacy and safety of this treatment approach for PAH patients.
METHODS
Study population and design
This multicenter retrospective study was spearheaded by the Shanghai Pulmonary Hospital, with participation from nine other hospitals across China. We assessed patients with PAH who underwent treatment with an oral sequential triple combination based on prostacyclin‐receptor agonist (PRA). The evaluation encompassed all consecutive patients referred to the ten hospitals between January, 2019 and June, 2022.
The study population comprised patients who met the following criteria: (1) aged between 16 and 80 years with a confirmed diagnosis of group 1 PAH as established by right heart catheterization 11 (mean pulmonary arterial pressure ≥25 mmHg, pulmonary arterial wedge pressure ≤15 mmHg, and pulmonary vascular resistance [PVR] ≥3 Wood units; (2) Those who had been on a dual‐combination therapy of ERA and PDE5i/riociguat for a minimum of 3 months; and (3) those who underwent regular follow‐up evaluations. Patients presenting with severe cardiopulmonary complications, untreated congenital heart disease, or portal hypertension were excluded from the study.
Parameters and outcomes assessment
All participating clinical centers utilized a standardized evaluation form to gather patient data. The baseline assessment at the time of selexipag initiation encompassed medical history, physical examination, 6‐min walking distance (6MWD), WHO functional class (WHO‐FC), levels of N‐terminal fragment of probrain natriuretic peptide (NT‐proBNP), and parameters from echocardiography. After 6 months of treatment, patients underwent a subsequent evaluation that included a physical examination, 6MWD, WHO‐FC, NT‐proBNP, and echocardiographic assessment. This study adhered to the guidelines set out in the Declaration of Helsinki and received approval from the Ethics Committee of Shanghai Pulmonary Hospital (approval number: L21‐222). All participants provided their written informed consent.
Echocardiographic data were obtained using standard views with commercially available devices. The measured echocardiographic parameters, which are commonly utilized in daily clinical practice, encompassed the right atrial (RA) area, right ventricular middle diameter (RVMD), RA pressure, left ventricular end‐diastolic diameter (LVEDD), left ventricular end‐diastolic eccentricity index (EI), pulmonary arterial systolic pressure (PASP), tricuspid annular plane systolic excursion (TAPSE), and presence of pericardial effusion.
Monitored events included all‐cause mortality, heart failure hospitalizations, the start of prostacyclin infusion, and patients withdrawals from selexipag during the 6‐month observation period. For patients who experienced multiple events, only the initial event was recorded.
Defination of four‐strata risk stratification
The risk assessment was streamlined based on the 2022 ESC/ERS guidelines for PAH. 2 This risk stratification was conducted both at the outset and at the 6‐month mark. For this, the WHO‐FC, 6MWD, and NT‐proBNP were utilized to determine the simplified four‐strata risk assessment. Each of these parameters was scored on a scale of 1–4: where 1 = low risk, 2 = intermediate‐low risk, 3 = intermediate‐high risk, and 4 = high risk. The average score was calculated by summing up the individual grades and dividing by the total number of assessed variables for each patient. This average was then rounded to the closest whole number to determine the patient's risk category.
Long‐term follow‐up visit
Patients were evaluated in out‐patient clinics every 3 months. All patient data was extracted from electronic medical records. The primary endpoint was all‐cause mortality. For those who were lost to follow‐up, phone calls were made to ascertain their survival status, with the data censored as of November 28, 2022.
Statistical analysis
Data were stored in a personal computer‐based data spreadsheet. Analysis was conducted using the Statistical Package for the Social Sciences (version 21.0; GraphPad Prism, version 9) and Dishu Tubiao (https://dycharts.com). Categorical data were presented as counts or percentages. The Kolmogorov–Smirnov test assess the normality of the data. Measurement data use the mean ± standard deviation or median and interquartile, count data use rate or composition. Comparisons of the 6MWD and hemodynamic variables from baseline and at 6 months utilized a one‐way analysis of variance for paired groups for normally distributed data and the nonparametric Friedman test for nonnormally distributed data. The Chi‐square test for independence was used to compare differences in categorical variables between baseline and 6 months.
Differences in the clinical parameters at baseline and during follow‐up were examined using the Wilcoxon signed‐rank test. Event‐free survival was estimated using the Kaplan–Meier method. A p‐value less than 0.05 was considered statistically significant.
RESULTS
Baseline clinical characteristics of patients
Of the 192 patients enrolled in the study, 148 (77.1%) were female, and 44 (22.9%) were male. The mean age was 43 ± 14.7 years. Every participant was diagnosed with WHO group 1 PAH. Idiopathic PAH (IPAH) was the predominant diagnosis at 50.5%, followed closely by PAH linked with connective tissue disease (CTD) at 24% and PAH due to corrected congenital heart defects at 24%. A small percentage, 1.6% (or three patients), were identified with hereditary hemorrhagic telangiectasia‐PAH. The median 6MWD stood at 305 meters. Regarding the WHO‐FC at baseline, class III dominated at 65.6%, trailed by class II at 20.8%. Combination therapy with ERA and PDE5i was given to 155 patients (80.7%), while 37 patients (19.3%) were administered ERA in conjunction with riociguat.
Risk stratification revealed that 22 patients (11.5%) fell into the low‐risk category, 35 (18.2%) were intermediate‐low risk, 91 (47.4%) were intermediate‐high risk, and 44 (22.9%) were categorized as high‐risk. The intermediate‐high/high‐risk group were typically older than those in the low‐risk group. As the risk stratification rose, the 6MWD showed a decreasing trend, while NT‐proBNP levels elevated. All low‐risk patients were identified under WHO‐FC II. In the intermediate‐low‐risk group, a majority (34.3%) belonged to WHO‐FC III. The intermediate‐high and high‐risk categories largely consisted of patients with WHO‐FC III/IV (95.6%), with a small fraction (4.4%) in WHO‐FC II. The baseline clinical features of the patients with PAH are summarized in Table 1.
Table 1.
Baseline clinical characteristics.
| Characteristics | All (n = 192) |
|---|---|
| Age, years | 43.0 ± 14.7 |
| Female, n (%) | 101 (74.8) |
| BMI, kg/m2 | 22.7 ± 4.3 |
| HR, bpm | 82.7 ± 17.7 |
| SBP, mmHg | 111.9 ± 14.4 |
| DBP, mmHg | 68.5 ± 12.3 |
| Classification | |
| IPAH, n (%) | 97 (50.5) |
| CHD‐PAH, n (%) | 46 (24.0) |
| CTD‐PAH, n (%) | 46 (24.0) |
| HHT, n (%) | 3 (1.6) |
| WHO‐FC II/III/IV, n | 40/126/26 |
| 6MWD, m | 305.4 ± 152.7 |
| NT‐proBNP, pg/mL | 1604 (542–3174) |
| Risk stratifications, n | |
| Low/Intermediate‐low/Intermediate‐high/high risk | 22/35/91/44 |
| Hemodynamics characteristics | |
| mRAP, mmHg | 8.2 ± 7.3 |
| mPAP, mmHg | 62.9 ± 16.9 |
| mPAWP, mmHg | 9.2 ± 4.4 |
| CO, L/min | 4.2 ± 1.4 |
| CI, L/min/m2 | 2.5 ± 0.8 |
| PVR, WU | 13.8 ± 6.9 |
| SVO2, % | 61.1 ± 10.7 |
| Sequential triple combination therapies | |
| ERA + sGC stimulator + PRA | |
| Ambrisentan + Riociguat + Selexipag, n (%) | 10 (5.2) |
| Macitentan + Riociguat + Selexipag, n (%) | 27 (14.1) |
| ERA + PDE5i + PRA | |
| Ambrisentan + Sildenafil + Selexipag, n (%) | 39 (20.3) |
| Ambrisentan + Tadalafil + Selexipag, n (%) | 17 (8.9) |
| Macitentan + Sildenafil + Selexipag, n (%) | 49 (25.5) |
| Macitentan + Tadalafil + Selexipag, n (%) | 50 (26.0) |
Note: data are presented as n (%), mean ± SD, and interquartile range.
Abbreviations: 6MWD, 6‐min walking distance; BMI, body mass index; CO, cardiac output; DBP, diastolic blood pressure; ERA, endothelin receptor antagonists; GC, soluble guanylate cyclase stimulator; HR, heart rate; IPAH, CHD‐PAH,CTD‐PAH; mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure; mRAP, mean right atrial pressure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PDE5i, phosphodiesterase type 5 inhibitor; PRA, prostacyclin receptor agonist; PVR, pulmonary vascular resistance; SBP, systolic blood pressure; SVO2, mixed venous oxygen saturation; WHO‐FC, World Health Organization functional classification.
Changes and outcomes after treatment in the entire cohort
Following 6 months of treatment, when compared to the baseline, the percentage of patients in WHO‐FC III/IV declined from 79.2% to 60.4% (p < 0.0001). The median 6MWD witnessed an improvement of 52.7 m, moving from 325 ± 147 to 378 ± 143 months (p < 0.0001). NT‐proBNP levels saw a significant reduction (media from 1604 to 678 pg/mL, p < 0.001). A noticeable improvement was observed in the number of low‐risk criteria, as illustrated in Figure 1.
Figure 1.
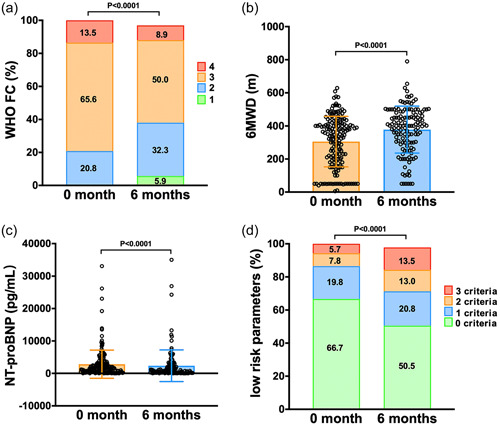
Comparison of clinical parameters before and after 6 months of treatment in the entire cohort. (a) WHO functional class (WHO‐FC); (b) 6‐min walk distance (6MWD); (c) N‐terminal fragment of probrain natriuretic peptide (NT‐proBNP); (d) The number of low risk criteria. WHO, World Health Organization.
Out of the group, 92 patients had echocardiographic evaluations at both the beginning and end of the study to examine the impact of selexipag on right heart remodeling (Table 2). While the RA area (28.4 ± 14.8 to 27.1 ± 16.1 cm2, p = 0.076) and RVMD (from 5.14 ± 1.35 to 5.0 ± 1.37 cm, p = 0.084) showed a declining trend, these changes weren't statistically significant. In contrast, LVEDD experienced an increased of 1.6 mm, transitioning from 35.3 ± 7.2 to 36.9 ± 6.6 mm, (p = 0.007). EI saw a reduction by 0.1 mm, shifting from 1.89 ± 0.67 to 1.78 ± 0.62 mm, (p = 0.001). Significant improvement was evident in right ventricular systolic function. Specifically, TAPSE enhanced by 0.1 cm, moving from 1.63 ± 0.42 to 1.73 ± 0.39 cm (p = 0.006).
Table 2.
Changes in clinical parameters.
| Variable | Baseline | Follow‐up | Change (%) | p‐value |
|---|---|---|---|---|
| PASP, mmHg | 92.6 ± 30.8 | 88.3 ± 30.0 | −4.3 (−5) | 0.050 |
| TR | 4.8 ± 0.8 | 4.6 ± 0.7 | −0.2 (−4) | 0.450 |
| RA area, cm2 | 28.4 ± 14.8 | 27.1 ± 16.1 | −1.3 (−5) | 0.076 |
| RAP, mmHg | 12.1 ± 10.3 | 9.3 ± 5.0 | −2.8 (−23) | 0.013 |
| RV diameter, cm | 5.1 ± 1.4 | 5.0 ± 1.4 | −0.1 (−2) | 0.084 |
| Sm, cm/s | 10.5 ± 2.8 | 10.7 ± 2.3 | 0.2 (2) | 0.236 |
| LVEDD, mm | 35.3 ± 7.2 | 36.9 ± 6.6 | 1.6 (5) | 0.007 |
| LVEF (%) | 79.8 ± 6.1 | 79.8 ± 8.6 | 0 (0) | 1.000 |
| EI | 1.9 ± 0.7 | 1.8 ± 0.6 | −0.1 (−5) | 0.001 |
| TAPSE, cm | 1.6 ± 0.4 | 1.7 ± 0.4 | 0.1 (−6) | 0.006 |
| PE, n (%) | 65 (39.2) | 22 (23.4) | – | 0.013 |
Note: data are presented as n (%), mean ± SD, and interquartile range.
Abbreviations: EI, eccentricity index; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; PASP, pulmonary arterial systolic pressure; PE, pericardial effusion; RA area, right atrium area; RAP, right atrial pressure; RV, right ventricle; Sm, peak systolic velocity of tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
We performed a sub‐analysis to elucidate the outcomes of patients stratified by disease etiology. Specifically, for IPAH, CHD‐PAH, and CTD‐PAH, we compiled data on these three distinct patient cohorts (as presented in Table S1). Notably, each group demonstrated improvements across several metrics, including heart functional classification, 6‐min walk distance (6MWD), NT‐proBNP levels, and echocardiographic findings.
Changes and outcomes after treatment in different risk groups
In the low‐risk group, there was no significant improvement in WHO‐FC, 6MWD, and NT‐proBNP compared to baseline. However, posttreatment, there was a significant reduction in the RA area (22.6 ± 6.0 vs. 19.8 ± 6.0 mm2, p < 0.05) and EI (1.8 ± 0.8 vs. 1.7 ± 0.7, p < 0.05). In the intermediate‐low‐risk group, there was a marked improvement in WHO‐FC, with the percentage of patients in WHO‐FC III/IV dropping from 65.7% to 48.5% (p < 0.05) after treatment. Furthermore, TAPSE also improved (1.9 ± 0.5 vs. 2.0 ± 0.5 cm, p < 0.05). In the intermediate‐high‐risk group, patients showed a significant improvement in activity tolerance, as observed in the WHO‐FC (p < 0.001) and 6MWD (p < 0.05). In terms of ventricular remodeling, there were notable improvement in LVEDD (32.6 ± 6.7 vs. 35.3 ± 6.5 mm, p < 0.01), EI (1.9 ± 0.6 vs. 1.8 ± 0.6, p < 0.01), TAPSE (1.5 ± 0.4 vs. 1.6 ± 0.4 cm, p < 0.05), and the proportion of pericardial effusion (50.6% vs. 31.9%, p < 0.05) after undergoing the triple combination therapy. In addition, in the high‐risk group, patients displayed significant improvements in activity tolerance metrics such as WHO‐FC (p < 0.01) and 6MWD (p < 0.001). Additionally, the RVMD decreased posttreatment (5.4 ± 0.8 vs. 5.0 ± 1.0 cm, p < 0.05). Baseline and follow‐up parameters were detailed in Table 3.
Table 3.
The comparation of clinical characteristics between baseline and 6 months in different risk groups.
| Characteristics | Low (n = 22) | Intermediate‐low (n = 35) | Intermediate‐high (n = 91) | High (n = 44) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | Baseline | Follow‐up | Baseline | Follow‐up | |
| WHO‐FC I/II/III/IV, n | 0/22/0/0 | 3/19/0/0 | 0/12/23/0 | 2/16/17/0* | 0/5/78/8 | 5/24/52/5*** | 0/1/25/18 | 1/3/27/12** |
| 6MWD, m | 498.9 ± 73.8 | 511.2 ± 80.8 | 395.9 ± 81.1 | 421.1±106.1 | 322.8 ± 101.1 | 365.5 ± 130.0* | 102.0 ± 57.3 | 248.7±143.3*** |
| NT‐proBNP, pg/mL | 97.9 (29.3–239.6) | 106.7 (22.1–229.9) | 334.8 (106.1–770.5) | 201.0 (110.7–620.0) | 2009.0 (1132.5–3842.5) | 1473.5 (442.5–3159.0)** | 2940.5 (1759.3–5868.0) | 1770.0 (884.2–5677.5) |
| PASP, mmHg | 71.8 ± 19.7 | 72.7 ± 26.1 | 87.1 ± 35.5 | 84.7 ± 25.1 | 100.6 ± 30.3 | 94.3 ± 32.7 | 97.3 ± 22.6 | 90.7 ± 27.4 |
| RA area, cm2 | 22.6 ± 6.0 | 19.8 ± 6.0* | 20.8 ± 8.0 | 20.9 ± 9.6 | 30.6 ± 15.2 | 28.6 ± 16.0 | 36.4 ± 20.6 | 37.5 ± 23.5 |
| RAP, mmHg | 13.3 ± 14.6 | 7.5 ± 3.8 | 10.0 ± 5.2 | 8.3 ± 4.9 | 11.1 ± 5.1 | 10.5 ± 4.9 | 16.1 ± 17.9 | 8.9 ± 6.1 |
| RV diameter, cm | 5.2 ± 1.7 | 5.1 ± 1.4 | 4.8 ± 1.5 | 4.7 ± 1.4 | 5.2 ± 1.3 | 5.1 ± 1.4 | 5.4 ± 0.8 | 5.0 ± 1.0* |
| Sm, cm/s | 10.3 ± 3.4 | 9.9 ± 3.4 | 11.5 ± 2.0 | 11.4 ± 1.8 | 10.1 ± 2.7 | 10.6 ± 2.2 | 10.5 ± 3.3 | 11.0 ± 2.0 |
| LVEDD, mm | 39.4 ± 4.0 | 41.0 ± 4.8 | 38.9 ± 8.5 | 39.4 ± 6.2 | 32.6 ± 6.7 | 35.3 ± 6.5** | 35.4 ± 5.9 | 34.9 ± 6.6 |
| EI | 1.8 ± 0.8 | 1.7 ± 0.7* | 1.8 ± 0.8 | 1.7 ± 0.7 | 1.9 ± 0.6 | 1.8 ± 0.6** | 1.9 ± 0.6 | 1.9 ± 0.6 |
| TAPSE, cm | 1.8 ± 0.4 | 1.8 ± 0.2 | 1.9 ± 0.5 | 2.0 ± 0.5* | 1.5 ± 0.4 | 1.6 ± 0.4* | 1.5 ± 0.3 | 1.6 ± 0.3 |
| PE, % | 10 | 6.7 | 21.4 | 15.8 | 50.6 | 31.9* | 43.6 | 23.1 |
Note: Data are presented as n (%), mean ± SD, and interquartile range. *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: 6MWD, 6‐min walking distance; EI, eccentricity index; LVEDD, left ventricular end‐diastolic dimension; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PASP, pulmonary arterial systolic pressure; PE, pericardial effusion; RA area, right atrium area; RAP, right atrial pressure; RV, right ventricle; Sm, peak systolic velocity of tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion; WHO‐FC, World Health Organization functional classification.
The changes in the clinical parameters following oral triple combination therapy across the different risk groups are shown in Figure 2. Notably, the improvement in 6MWD was more pronounce in the high‐risk group compared to the low‐risk, intermediate‐low‐risk, and intermediate‐risk groups (Figure 2a). However, no significant variances were observed in the changes in NT‐proBNP and WHO‐FC among the different risk stratification (Figure 2b,c). Changes in specific echocardiographic parameters of patients treated with 6 months of oral triple combination therapy are shown in Figure 2d–g. It is worth noting that no significant differences were discerned in the changes in parameters like PASP, LVEDD, EI, and TAPSE among the different risk groups.
Figure 2.
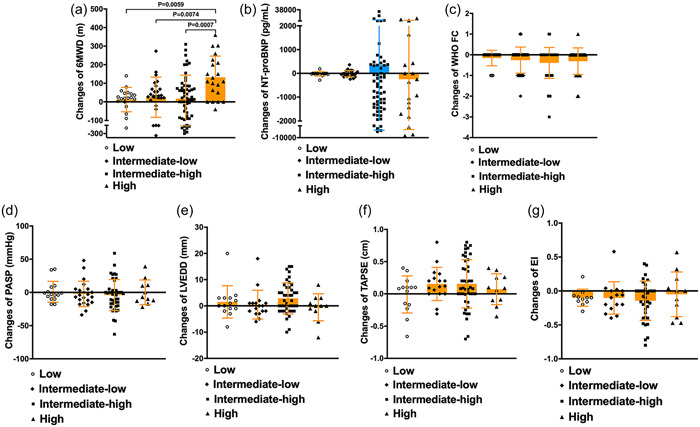
Comparison of changes before and after 6 months of treatment in different risk groups. (a) Change of 6‐min walk distance (6MWD); (b) Change of N‐terminal fragment of probrain natriuretic peptide (NT‐proBNP); (c) Change of WHO functional class (WHO‐FC); (d) Change of pulmonary artery systolic pressure (PASP); (e) Change of left ventricular end diastolic diameter (LVEDD); (f) Change of tricuspid annular plane systolic excursion (TAPSE); (g) Change of eccentricity index (EI).
The Cox proportional hazards analysis of outcomes
In the univariate Cox proportional hazards analysis, factors such as age, WHO‐FC,6MWD, risk stratifications, mean pulmonary artery pressure (mPAP), RA area, and RAP were associated with the survival rate of patients treated with triple sequential combination therapy. However, the multivariate forward stepwise Cox proportional hazatds analysis identified only the 6MWD and mPAP levels as independent predictor of survival in theses patients (Figure 3).
Figure 3.
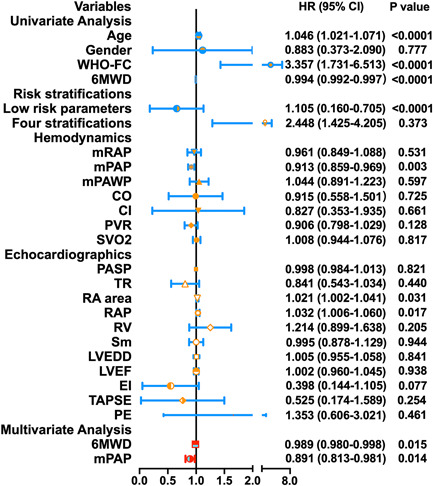
Cox regression analysis of clinical parameters and survival in the entire cohort. 6MWD, 6‐min walking distance; CO, cardiac output; EI, eccentricity index; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure; mRAP, mean right atrial pressure; PASP, pulmonary arterial systolic pressure; PE, pericardial effusion; PVR, pulmonary vascular resistance; RA area, right atrium area; RAP, right atrial pressure; RV, right ventricle; Sm, peak systolic velocity of tricuspid annulus; SVO2, mixed venous oxygen saturation; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; WHO‐FC, World Health Organization functional classification.
Survival and event‐free survival analysis
Over the course of a 6‐month follow‐up, 23 patients encounter adverse clinical events, inculding 10 died and 13 were hospitalized due to heart failure, resulting in event‐free survival rates were 88% (Figure 4a). The majority of those who faced adverse events belonged to the intermediate‐high risk and high risk groups. After a median long‐term follow‐up of 2 years, 50 patients had adverse clinical events, of which 27 succumbed to PAH, while 23 were hospitalized for right‐sided heart failure. The survival rates for the 1st and 2rd year stood at 86.5% and 86.0%, respectively (Figure 4b). We further assessed the 6 months event‐free survival and long‐term survival across different risk stratification groups. Notably, the event‐free survival between the low‐risk and high‐risk groups also showed significant differences (Figure 4c). The findings indicated pronounced disparities in the survival rates among these groups (Figure 4d).
Figure 4.
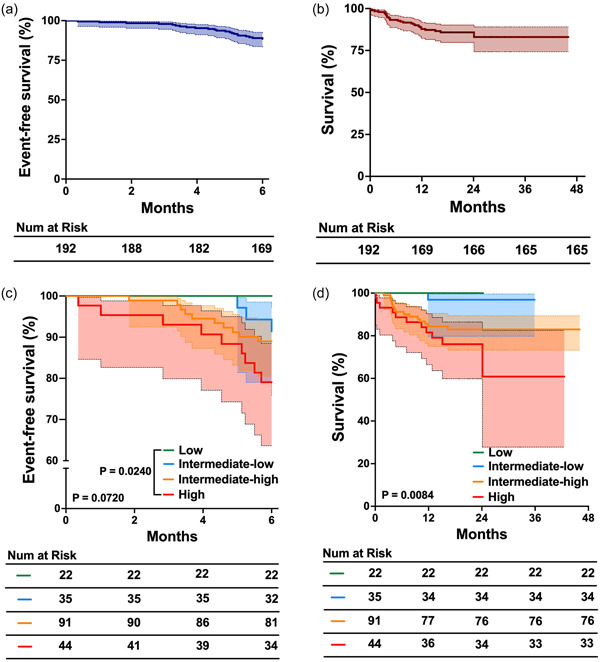
The 6‐month event‐free survival and 2‐year survival of patients undergoing oral triple combination therapy. (a) Six‐month event‐free survival in the entire corhot; (b) 2‐year survival rates in the entire corhot; (c) Comparison of the 6‐month event‐free survival in different risk groups; (d) Comparison of 2‐year survival rates in different risk groups.
Selexipag dosing and side effects
The median maintenance dosage of selexipag was 800 µg administered twice daily. Of the cohort, 92 patients (47.9%) were on a medium‐dose (600–1000 µg twice daily). High‐dose selexipag (1200–1600 µg twice daily) was administered to 47 (24.5%) patients, while 53 (27.6%) patients were on a low‐dose selexipag (200–400 µg twice daily). Notably, only 9.9% of the patients maintained a selexipag dose of 1600 µg twice daily.
During the titration period, headache was the predominant side effect, reported by 49.4% of patients. This was followed by diarrhea in 31.3% of patients and nausea in 14.5%. During the maintenance phase, 78 patients (40.6%) experienced side effects that were deemed tolerable. Notably, there have been no reports of hepatotoxicity or other unidentified side effects.
DISCUSSION
In our multicenter retrospective study, we observed that selexipag‐based sequential oral triple therapy markedly enhanced exercise capacity, NT‐proBNP levels, and right ventricular remodeling. Moreover, oral triple therapy was well‐tolerated by patients with PAH. These findings align with those of earlier studies. Numerous investigations have underscored that an sequential combination therapy of selexipag with ERA and PDE5i is recommended for patients presenting with intermediate‐low risk. This approach has emerged as the predominant clinical strategy. 2 , 11 Furthermore, our study suggests that selexipag‐based sequential oral triple therapy is efficacious across all risk stratification groups. It is particularly potent in improving right ventricular remodeling. Notably, there was a significant enhancement in activity tolerance among the intermediate‐high and high‐risk groups.
Multiple research studies have pinpointed WHO‐FC, 6MWD, and BNP/NT‐proBNP as the most robust predictors of prognosis. 12 , 13 , 14 Consequently, the four‐strata model is advocated as a fundamental risk assessment tool for posttreatment follow‐up in the 2022 ESC guidelines. This four‐strata risk evaluation model effectively projected survival rates across patients with IPAH, hereditary PAH, drug‐induced PAH, and PAH associated with CTD, as well as those with portopulmoanry hypertension. Throughout the follow‐up period, it is advisable to maintain the initial therapy for PAH patients who assessed to have a low risk of mortality. 2 Nevertheless, the guidelines underscore the importance of taking into account supplementary factors, with a particular emphasis on right heart imaging and hemodynamic evaluations. In clinical practice, it's not uncommon to encounter patients with an large right heart, even when their risk is low. Nonetheless, the potential benefits if triple therapy for these patients remains uncertain. Our study revealed that within the low‐risk group, even though there were no significant improvement in WHO‐FC, 6MWD, and NT‐proBNP levels compared to baseline, positive changes were observed in right heart remodeling. Specifically, the right atrium (RA) area and eccentricity index (EI) exhibited significant reductions following the reatment. It is widely acknowledged that alterations in the structure of the right ventricular serves as the primary indicators for predicting clinical outcomes in PAH. 15 We devised a model of attenuated RH remodeling (ARHR), predicated on a diminution in RA area, RVMD, and LV‐EId and established that ARHR serves as an independent mortality predictor. 16 Thus, noninvasive imaging techniques evaluating right ventricular dimensions and functionality are vital, both for PAH patient monitoring and to fathom the right ventricular's response to pulmonary vascular remodeling. 17 Our findings underscore that even low‐risk patients can substantially benefit from sequential oral triple therapy, chiefly through the reversal of right ventricular remodeling.
Selexipag stands as the inaugural non‐prostanoid agonist of the prostacyclin receptor, credited with delaying PAH progression and reducing the combined morbidity/mortality outcome, as highlighted in the GRIPHON study. Contemporary analyses propose that the efficacy of selexipag may be accentuated when initiated early. 18 Although the TRITON study found that initial triple combination therapy didn't present any superior benefit over double combination therapy in terms of hemodynamic and functional metrics by week 26, 19 it's hypothesized that this could be due to a ceiling effect, where maximum enhancement is achieved with dual treatment. Neverthelss, there's emerging evidence suggesting that early selexipag administration might curtail the risks associated with PAH disease progression. 18 A subsequent analysis, drawing from pooled data of both GRIPHON and TRITON trials, demonstrated that introducing selexipag within 6‐month postdiagnosis significantly diminishes the risk of disease progression compared to those only a dual regimen of ERA and PDE‐5i. 20 Essentially, selexipag slashed the progression risk by half, and this efficacy was consistently evident when integrated into a triple therapy approach within the 6‐month diagnostic window. Mirroring this trend, our study's findings underscore that sequential oral triple therapy, even in low‐risk patients, yields commendable results, aligning seamlessly with the prevailing research direction.
Current PAH guidelines advocate the addition of intravenous (IV) epoprostenol or IV/subcutaneoustreprostinil for patients displaying intermediate‐high or high risk during subsequent evaluation. 2 Yet, in our study, 135 intermediate‐high or high risk PAH patients did not undergo IV or subcutaneous prostacyclin analog treatment due to financial constraints or severe side effects. After comprehensive oral dual therapy with ERA and PDE5i/sGC stimulator, these patients continued to demonstrate unsatisfactory cardiac function, elevated NTproBNP levels, and limited exercise tolerance. Introducing selexipag sequentially may offer an alternative for such intermediate‐high or high‐risk patients. Earlier research primarily centered on the triple combination of prostacyclin infusion, oral ERA, and PDE‐5i for acute PAH cases. 5 , 7 However, information on the triple oral combination therapy remains sparse. A pertinent study regarding this was a subgroup analysis from the GRIPHON study, which encompassed nearly half of the WHO‐FC III patients. 21 The TRITON study, another randomized controlled trial, explored the effectiveness and safety of the initial triple oral combination therapy and also included a significant portion of WHO‐FC III/IV patients with elevated NT‐proBNP levels. 19 In our findings,a majority of intermediate‐high and high‐risk patients responded positively to triple oral combination therapy. Notably, the most profound enhancement in the 6MWD was evident among high‐risk patients following this therapy. Our study also highlighted a favorable long‐term prognosis with the triple oral regimen, aligning with prior research. 18 , 21 We observed 1‐ and 2‐year survival rates at 86.5% and 86.0%, respectively. Such findings bolster the potential of sequential triple oral therapy for intermediate‐high and high‐risk patients. They also suggest the viability of introducing selexipag post dual oral therapy when IV/subcutaneous prostacyclin analog are impractical. While selexipag doesn't supplant parenteral treatments, it could serve as a viable option for those patients reluctant or unable to undertake them.
Our study prossesses several limitations. First, the modest sample size inherently limits the scope of our findings. Second, the hospital cases predominantly originated from East China, possibly not reflecting trends across the entire contury. Furthermore, our follow‐up duration was relatively brief, and catheterization data wasn't always timely collected, though we made efforts to gather ultrasound data where feasible.
In summation, our findings indicate that oral sequential triple combination therapy with selexipag significantly enhances exercise capacity, risk assessment, and right ventricular remodeling in PAH patients, all while maintaining good tolerability.
AUTHOR CONTRIBUTIONS
Qin‐Hua Zhao, Su‐Gang Gong, and Lan Wang were directly involved in the patients’ recruitment and care, contributed to the study design, study conduct and supervision, scientific overview, data analysis, and editing of the manuscript. Jun Chen, Fa‐Dong Chen, Hong‐Yun Ruan, Yan‐Li Zhou, Qi‐Qi Wang, Xiao‐Ling Xu, Ke‐Fu Feng, Jian‐Zhou Guo, and Rui‐Feng Zhang contributed to patient enrollment, data analysis, scientific interpretation, drafting, and editing the original manuscript. Wei Zhang contributed to data collection and curation, and formal analysis. All authors have reviewed the manuscript and approved the final version for submission.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Shanghai Pulmonary Hospital (number: L21‐222). Written informed consent was obtained from all patients.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This work was supported by funding from the project of Shanghai pulmonary hospital clinical research in 2020 (FKLY20005 and FKLY20011) and Shanghai Pujiang Talent Plan (21PJD060).
Zhao Q‐H, Chen J, Chen F‐D, Ruan H‐Y, Zhang W, Zhou Y‐L, Wang Q‐Q, Xu X‐L, Feng K‐F, Guo J‐Z, Gong S‐G, Zhang R‐F, Wang L. Evaluating the efficacy and safety of oral triple sequential combination therapy for treating patients with pulmonary arterial hypertension: A multicenter retrospective study. Pulm Circ. 2024;14:e12351. 10.1002/pul2.12351
Qin‐Hua Zhao and Jun Chen contributed equally to this work and share first authorship.
Contributor Information
Su‐Gang Gong, Email: gongsugang@tongji.edu.cn.
Rui‐Feng Zhang, Email: zrf1977313@yeah.net.
Lan Wang, Email: lanwang@tongji.edu.cn.
REFERENCES
- 1. Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. [DOI] [PubMed] [Google Scholar]
- 3. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long‐term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest. 2012;142(2):448–456. [DOI] [PubMed] [Google Scholar]
- 4. Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The Giessen pulmonary hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36(9):957–967. [DOI] [PubMed] [Google Scholar]
- 5. D'Alto M, Badagliacca R, Argiento P, Romeo E, Farro A, Papa S, Sarubbi B, Russo MG, Vizza CD, Golino P, Naeije R. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest. 2020;157(2):376–383. [DOI] [PubMed] [Google Scholar]
- 6. Boucly A, Savale L, Jaïs X, Bauer F, Bergot E, Bertoletti L, Beurnier A, Bourdin A, Bouvaist H, Bulifon S, Chabanne C, Chaouat A, Cottin V, Dauphin C, Degano B, De Groote P, Favrolt N, Feng Y, Horeau‐Langlard D, Jevnikar M, Jutant EM, Liang Z, Magro P, Mauran P, Moceri P, Mornex JF, Palat S, Parent F, Picard F, Pichon J, Poubeau P, Prévot G, Renard S, Reynaud‐Gaubert M, Riou M, Roblot P, Sanchez O, Seferian A, Tromeur C, Weatherald J, Simonneau G, Montani D, Humbert M, Sitbon O. Association between initial treatment strategy and long‐term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;204(7):842–854. [DOI] [PubMed] [Google Scholar]
- 7. Sitbon O, Jais X, Savale L, Cottin V, Bergot E, Macari EA, Bouvaist H, Dauphin C, Picard F, Bulifon S, Montani D, Humbert M, Simonneau G. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014;43(6):1691–1697. [DOI] [PubMed] [Google Scholar]
- 8. Momoi M, Hiraide T, Shinya Y, Momota H, Fukui S, Kawakami M, Itabashi Y, Fukuda K, Kataoka M. Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension. Ther Adv Respir Dis. 2021;15:175346662199504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui X, Lu W, Zhang D, Qie L, Li H, Li X, Liu H, Ji Q. Selexipag‐based triple combination therapy improves prognosis in Chinese pulmonary arterial hypertension patients. Front Cardiovasc Med. 2022;9:991586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun X, Chen R, Yao X, Zheng Z, Wang M, Wang C, Cheng J. Treatment of pulmonary hypertension: is triple therapy necessarily better than monotherapy? Am J Respir Crit Care Med. 2021;204(12):1492–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document G. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 12. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, Elliott CG, Farber HW. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159(1):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk‐Noordegraaf A, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grünig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. [DOI] [PubMed] [Google Scholar]
- 14. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. [DOI] [PubMed] [Google Scholar]
- 15. Cassady SJ, Ramani GV. Right heart failure in pulmonary hypertension. Cardiol Clin. 2020;38(2):243–255. [DOI] [PubMed] [Google Scholar]
- 16. Zhao QH, Gong SG, Jiang R, Li C, Chen GF, Luo CJ, Qiu HL, Liu JM, Wang L, Zhang R. Echocardiographic prognosis relevance of attenuated right heart remodeling in idiopathic pulmonary arterial hypertension. Front Cardiovasc Med. 2021;8:650848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrison A, Hatton N, Ryan JJ. The right ventricle under pressure: evaluating the adaptive and maladaptive changes in the right ventricle in pulmonary arterial hypertension using echocardiography (2013 Grover Conference series). Pulm Circ. 2015;5(1):29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaine S, Sitbon O, Channick RN, Chin KM, Sauter R, Galiè N, Hoeper MM, McLaughlin VV, Preiss R, Rubin LJ, Simonneau G, Tapson V, Ghofrani HA, Lang I. Relationship between time from diagnosis and morbidity/mortality in pulmonary arterial hypertension. Chest. 2021;160(1):277–286. [DOI] [PubMed] [Google Scholar]
- 19. Chin KM, Sitbon O, Doelberg M, Feldman J, Gibbs JSR, Grünig E, Hoeper MM, Martin N, Mathai SC, McLaughlin VV, Perchenet L, Poch D, Saggar R, Simonneau G, Galiè N. Three‐ versus two‐drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021;78(14):1393–1403. [DOI] [PubMed] [Google Scholar]
- 20. Coghlan JG, Gaine S, Channick R, Chin KM, du Roure C, Gibbs JSR, Hoeper MM, Lang IM, Mathai SC, McLaughlin VV, Mitchell L, Simonneau G, Sitbon O, Tapson VF, Galiè N. Early selexipag initiation and long‐term outcomes: insights from randomised controlled trials in pulmonary arterial hypertension. ERJ Open Res. 2023;9(1):00456‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coghlan JG, Channick R, Chin K, Di Scala L, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, McLaughlin V, Preiss R, Rubin LJ, Simonneau G, Sitbon O, Tapson VF, Gaine S. Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs. 2018;18(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.


