Abstract
The circadian clock system orchestrates daily behavioral and physiological rhythms, facilitating adaptation to environmental and internal oscillations. Disruptions in circadian rhythms have been linked to increased susceptibility to various diseases and can exacerbate existing conditions. This review delves into the intricate regulation of diurnal gene expression and cell function by circadian clocks across diverse tissues. . Specifically, we explore the rhythmicity of gene expressions, behaviors, and functions in both immune and non‐immune cells, elucidating the regulatory effects and mechanisms imposed by circadian clocks. A detailed discussion is centered on elucidating the complex functions of circadian clocks in regulating key cellular signaling pathways. We further review the circadian regulation in diverse diseases, with a focus on inflammatory diseases, cancers, and systemic diseases. By highlighting the intimate interplay between circadian clocks and diseases, especially through clock‐controlled cell function, this review contributes to the development of novel disease intervention strategies. This enhanced understanding holds significant promise for the design of targeted therapies that can exploit the circadian regulation mechanisms for improved treatment efficacy.
Keywords: cancers, cell function, circadian clock, immune cells, inflammatory diseases, signaling pathway, systemic diseases
Circadian clocks regulate diurnal variations in an array of genes, delineating the roles and intracellular pathways of cells. Disruption in circadian orchestrations compromises the immune system, precipitating disturbances in cell coordination and signaling pathways, thus promoting the progression of diseases. This review indicates the rhythmicity of gene expressions, behaviors, and functions in cells and elucidates the regulatory mechanisms of cell function by the circadian clock. A detailed discussion is devoted to unraveling the multifaceted roles of circadian clocks in governing pivotal signaling pathways. We further provide a comprehensive discussion on circadian regulation across a spectrum of diseases, with a spotlight on inflammatory diseases, cancers, and systemic diseases.
1. INTRODUCTION
The diurnal light/dark cycles resulting from the earth's rotation are responsible for the establishment of circadian rhythms in mammals. 1 A wide array of behavioral phenomena, including the sleep–wake cycle, feeding behaviors, thermoregulation, cardiovascular dynamics, and hormonal secretion, exhibit a circadian rhythm with an approximately 24‐h cycle. 2 These rhythmic patterns are orchestrated by intrinsic biological clocks. At the core of these circadian systems is a central pacemaker located in the suprachiasmatic nucleus (SCN) of the brain, complemented by subsidiary clocks in peripheral tissues. 3 The rhythmicity is transmitted from the central to peripheral clocks via neural or hormonal signals. 4 Intertissue communications, exemplified by the gut–brain axis, brain–heart axis, and gut–liver axis, are instrumental in maintaining circadian rhythms in gene expressions and physiological processes. 5 , 6 , 7 , 8 , 9 For example, the intestinal clock orchestrates the hepatic rhythmic transcriptome and diurnal metabolism, thereby underpinning hepatic metabolic homeostasis. 10 Various factors, such as brain injuries, diseases, sleep deprivation, and trans‐meridian travel result in disrupted circadian rhythm. 11 , 12
The circadian clock system is structured in a hierarchical manner and functions through self‐regulating feedback loops. In the main loop, the Brain and Muscle ARNT‐Like 1 (BMAL1) protein forms a complex with the circadian locomotor output cycles kaput (CLOCK) protein, creating a heterodimer. 13 This BMAL1/CLOCK heterodimer facilitates the transcription of clock‐controlled genes (CCGs), including Periods (PERs) and Cryptochromes (CRYs), by binding to E‐box elements in their promoters. 14 Following their synthesis, PERs and CRYs proteins accumulate and eventually exert an inhibitory effect on the BMAL1/CLOCK complex. 15 When PERs and CRYs protein levels are reduced due to protein degradation, PERs and CRYs are dissociated from the BMAL1/CLOCK complex and a new cycle of transcription is started. 16 Degradation of PERs and CRYs proteins is performed by casein kinases and adenosine monophosphate kinase through phosphorylation for ubiquitination and proteasome degradation. 17 Additional feedback loops involve crucial transcriptional factors such as reverse erythroblastosis virus heme receptors (REV‐REBs), RAR‐related orphan receptors (RORs), and D‐box acting proteins, including albumin D‐site‐binding protein (DBP) and E4 promoter‐binding protein 4 (E4BP4), which contribute to the stability and robustness of the circadian clock system. 18 , 19 Specifically, RORα and REV‐REBα play opposing roles and finely regulate the transcription of Bmal1 and E4bp4, by binding to ROR responsive elements that are located in the promoters of these genes. 20 REV‐ERBs repress the transcription of Bmal1, while RORs activate their transcription. 21 RORs activate the expression of E4bp4, whereas REV‐REBs inhibit its transcription. 22 These intricate regulatory mechanisms ensure the precise control and maintenance of the circadian clock system.
The circadian clock orchestrates a multitude of physiological functions through the modulation of cell functions and signaling pathways. This temporal governance extends to the behaviors, differentiation, and functions of immune cells, integral to both adaptive and innate immunity systems. 23 , 24 , 25 , 26 , 27 , 28 , 29 Research has increasingly focused on the circadian regulation of transcriptional processes that determine gene expressions in key signaling pathways, such as nuclear factor‐κB (NF‐κB), mitogen‐activated protein kinase (MAPK), and Janus kinase/signal transducer and activator of transcription (JAK‐STAT). 30 , 31 , 32 , 33 , 34 , 35 , 36 Disruptions in circadian rhythms can precipitate the malfunctioning of these regulatory pathways, potentially leading to uncontrollable inflammatory responses and diseases. 37 , 38 , 39 , 40 , 41 , 42
Disruption of the circadian clock enhances the susceptibility to various diseases such as inflammatory diseases, cancers, and systemic diseases. 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 For example, circadian oscillations significantly influence the onset and progression of various malignancies. The susceptibility to cancers induced by circadian disruption is closely associated with cell functions and behaviors. 53 , 54 , 55 The temporal coordination of immune cell functions by circadian rhythms, encompassing cell functions and behaviors such as trafficking, pathogen recognition, phagocytosis, and the secretion of inflammatory cytokines, is critical in determining the diurnal variation in cancer severity. 26 , 56 , 57 , 58 , 59 , 60 , 61 , 62
In this review, we highlight the link between circadian disruptions and increased disease susceptibility, focusing on the circadian control of gene expressions and signaling pathways in various cells. The review delves into how disturbances in circadian rhythms impair cell functions and exacerbate disease progression, particularly in inflammatory diseases, cancers, and systemic diseases. It emphasizes the potential of targeted drug interventions that harness circadian regulation, offering novel insights into disease management and therapeutic development.
2. CIRCADIAN REGULATION OF IMMUNE CELL FUNCTION
The biological clocks located in SCN and peripheral tissues (i.e., liver, heart, lung, kidney, stomach, and intestine) consist of several positive and negative molecular feedback loops (Figure 1). The rhythmicity is transmitted from the central to peripheral clocks via neural or hormonal signals (Figure 1). 4 Most immune cells express circadian clock genes and present a wide array of genes expressed with a 24‐h rhythm, thus generating the rhythmicity of various cellular immune functions. 63 These cell‐autonomous rhythms encompass cytokine production, trafficking, and phagocytosis. 64 , 65 A wide range of immune cells including macrophages, neutrophils, eosinophils, basophils, mast cells, monocytes, dendritic cells, and lymphocytes (B cells, T cells, and innate lymphoid cell [ILCs]) in diverse tissues are under the control of circadian clock (Figure 2). Circadian regulation of immune activity involves the daily fluctuation of circulating leukocyte counts, levels of secreted cytokines, tissue infiltration of immune cells, innate and adaptive immune responses, and inflammatory signaling pathways (Figure 2).
FIGURE 1.
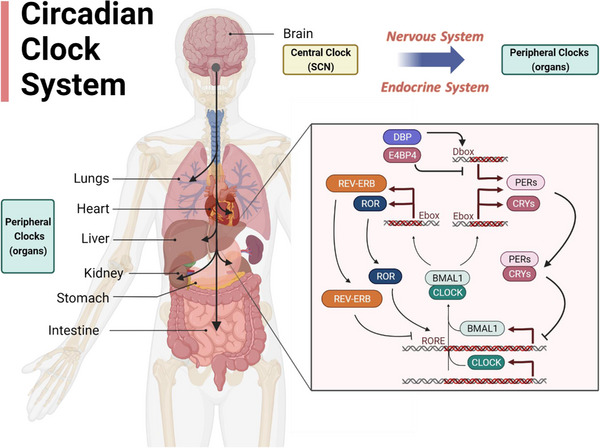
The molecular mechanism of the circadian clock system. Circadian clocks are composed of a central clock located in the SCN of the brain and peripheral clocks situated in various peripheral organs (i.e., lung, heart, liver, kidney, stomach, and intestine). Proteins encoded by clock genes CLOCK, BMAL1, CRYs, PERs, REV‐ERB, and ROR interact to create transcription‐translation feedback loops. BMAL1 interacts with CLOCK to form a heterodimer and transcriptionally regulates the expressions of CCGs (CRYs, PERs, ROR, and REV‐ERB) via E‐box. PERs and CRYs proteins accumulate and subsequently inhibit the activity of the BMAL1/CLOCK complex. RORα and REV‐REBα play opposing roles in regulating the transcription of Bmal1 through RORE elements. DBP and E4BP4 also have inverse effects on the transcription of PERs through D‐box elements. BMAL1, Brain and muscle ARNT‐like 1; CCGs, clock‐controlled genes; CLOCK, circadian locomotor output cycles kaput; CRYs, Cryptochromes; DBP, albumin D‐site‐binding protein; E4BP4, E4 promoter‐binding protein 4; PERs, Periods; REV‐ERB, reverse erythroblastosis virus heme receptors; ROR, retinoic acid‐related orphan receptor; RORE, ROR‐responsive element; SCN, suprachiasmatic nucleus.
FIGURE 2.
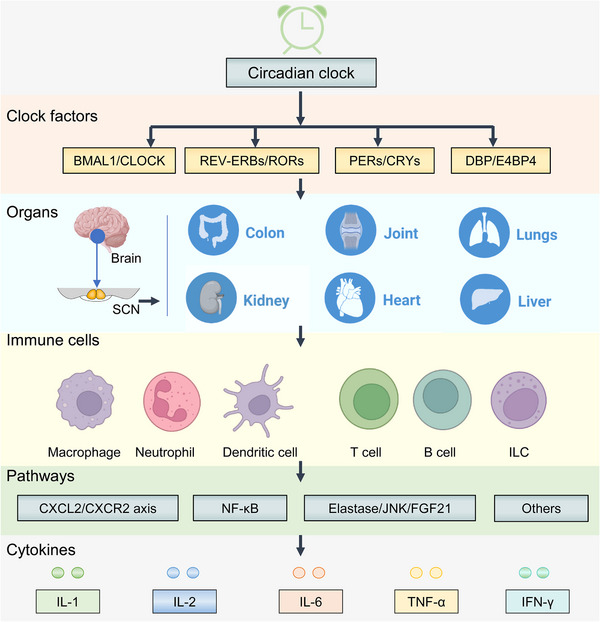
The circadian clock‐controlled immune responses. The circadian clock plays a pivotal role in the regulation of immune responses within immune cells across multiple organs. Through its influence on various signaling pathways and the release of cytokines, the circadian clock orchestrates immune responses in a diverse range of immune cell types. Within these cells, the circadian clock contributes to the precise coordination and modulation of inflammatory signaling pathways and the release of cytokines. CXCL, chemokine ligand; CXCR, CXC‐chemokine receptor; FGF21, fibroblast growth factor 21; IFN, interferon; IL, interleukin; ILC, innate lymphoid cell; JNK, c‐Jun N terminal kinase; NF‐κB, nuclear factor‐κB; TNF, tumor necrosis factor.
2.1. Macrophages
Macrophages possess a robust circadian clock that plays a crucial role in driving their inflammatory and immune functions (Figure 3). 66 About 8% of macrophage transcriptome including clock factors, inflammatory cytokines, and regulators of inflammatory cytokines such as pathogen recognition receptors and inflammatory signaling pathways oscillates in a circadian manner. 67 A previous study identified 1403 genes, which account for 8.1% of the total, to be rhythmically expressed in a circadian manner. 68 Another study involving transcriptome and proteome profiling demonstrates that only 15% of circadian proteome show corresponding oscillating mRNA patterns in murine bone marrow‐derived macrophages, suggesting that post‐transcriptional regulation plays a significant role in affecting the clock regulatory output in macrophages. 69 This observation may be attributed to the abundance of proteins involved in degradation and translation. Macrophages exhibit unique circadian post‐transcriptional regulation due to their need for prompt response to a wide range of potential immunological stimuli. 69
FIGURE 3.
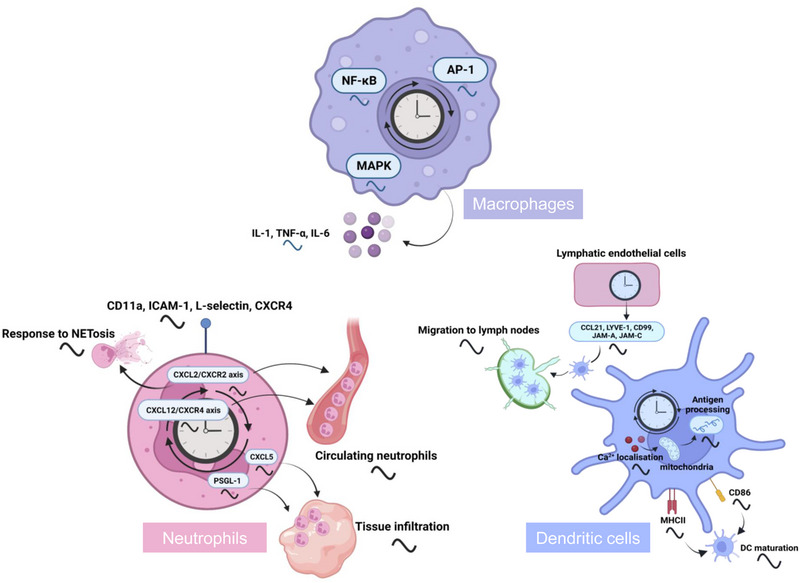
Regulation of myeloid cell behaviors and functions by the circadian clock. The circadian clock orchestrates the rhythmic patterns in various aspects of myeloid cell activities, encompassing their circulation, recruitment to lymph nodes, tissue infiltration, maturation, as well as the synthesis and release of cytokines, antigen processing, and subsequent immune responses. Circadian rhythms are orchestrated via distinct pathways, notably the CXCL2/CXCR2 and CXCL12/CXCR4 axes, and are subject to modulation by regulators such as CXCL5, PSGL‐1, CD86, and MHCII. Furthermore, a variety of regulatory elements, including NF‐κB, AP1, and MAPK, are instrumental in the precise modulation of myeloid cell behaviors and functions.Notably, DCs are categorized into two developmental lineages: a myeloid lineage, shared with phagocytes, and a lymphoid lineage, shared with T cells. AP‐1, activator protein‐1; CCL, CC chemokine ligand; DC, dendritic cell; ICAM‐1, intercellular adhesion molecule‐1; JAM, junctional adhesion molecule; LYVE‐1, lymphatic vessel endothelial hyaluronan receptor 1; MAPK, mitogen‐activated protein kinase; MHC, major histocompatibility complex; PSGL‐1, P‐selectin glycoprotein ligand‐1.
Clock genes, cytokines, and chemokines exhibit robust circadian rhythms in macrophages (Figure 3). 66 , 70 Clock genes such as Rev‐erbα and Per2 display rhythmic expressions with high‐amplitude diurnal oscillations in peritoneal macrophages and central nervous system resident macrophages (microglia). 67 , 71 Tumor necrosis factor (TNF)‐α and interleukin (IL)‐6 secretion are observed in lipopolysaccharides (LPS)‐treated spleen macrophages with a peak phase around the transition time from light to dark, suggestive of a circadian clock‐regulated macrophage‐dependent cytokine secretion. 67 Pro‐inflammatory cytokines show higher levels during the light phase in hippocampal microglia. 72 Disruption or attenuation of core clock genes often disturbs macrophage circadian behaviors or functions. For example, circadian disruption induced by chronic jet lag modifies the rhythmic patterns of M1/M2 macrophages and cytokine levels in the spleen and tumor tissues. 73 Deficiency of Rev‐erbα abolishes the circadian rhythmicity of IL‐6 in LPS‐induced macrophages and endotoxin‐treated mice. 74 Bmal1 deficiency decreases pro‐inflammatory cytokines (e.g., IL‐1β, TNF‐α, and IL‐6) and increases anti‐inflammatory cytokines (e.g., IL‐10) in microglial BV2 cells following LPS stimulation. 75 In glioblastoma, deletion of Clock or its target gene OLFML3 (a microglia‐attracting chemokine) decreases intratumoral microglia infiltration in the tumor microenvironment and increases overall survival. 76 Pathogen recognition receptors, a group of innate immune receptors, serve as upstream regulators of cytokines/chemokines such as IL‐1, TNF‐α, and IL‐6 through shared signaling modules including NF‐κB, activator protein‐1 (AP‐1), and MAPK, 77 and regulation of NF‐κB, AP‐1, and MAPK by circadian clocks may contribute to the diurnal variations of cytokines/chemokines. 78 , 79 , 80
2.2. Neutrophils
Neutrophils are the predominant leukocyte subset in blood. The numbers, behaviors, and functions of neutrophils show apparent diurnal oscillations (Figure 3). The infiltration of neutrophils displays differential circadian rhythmicity in specific subsets and tissues. 81 Under steady‐state conditions, neutrophil counts in the blood show circadian fluctuations. 82 , 83 In both humans and mice, peripheral neutrophil numbers decrease during the rest phase, whereas their numbers increase during the active phase. 84 The levels of CD62− CXC‐chemokine receptor (CXCR) 4+ neutrophils in circulation also follow a circadian rhythm with a nadir in the early active phase in mice. Consistently, blood neutrophil counts are lowest in the early morning in the UK Biobank participants. 83 , 85 This is because the release of young neutrophils and clearance of aged neutrophils from the periphery exhibit a time‐dependent manner. 83 , 85
Neutrophil homing to most naïve tissues such as lung and heart exhibits diurnal variations with a peak at night, whereas infiltration of neutrophils shows no circadian rhythms in the intestine, liver, and white adipose tissue. 86 The ability of neutrophils to form neutrophil extracellular traps (NETs) also follows a circadian oscillation. In humans, the capacity of granule‐loading and NETs formation in neutrophils are higher at around 8:00 a.m. and gradually decrease later in the day. 87 In mice, neutrophils express more enhanced levels of proteins related to granules, NETs generation, and cell migration in the nighttime. 88 Specific deletion of Bmal1 abolishes daily variations in granule contents and NETs formation. 89 Some markers such as CD11a, intercellular adhesion molecule‐1, L‐selectin, and the chemokine receptor CXCR4 in neutrophils exhibit circadian oscillations in humans, while P‐selectin glycoprotein ligand‐1 (PSGL‐1), L‐selectin, CD11a, and CD29 display rhythmic expression in mice (Figure 3). 81 Antagonistic chemokine signaling drives neutrophil release from bone marrow through two main receptors CXCR4 and CXCR2. BMAL1 coordinates diurnal changes in the transcriptional and migratory properties of circulating neutrophils by regulating chemokine CXCL2 and chemokine receptor CXCR2 (Figure 3). 90 Genetic disruption of the circadian clock has an impact on neutrophil trafficking. CXCL12/CXCR4 axis controlled by the central clock underlies the circadian variations of neutrophil numbers in blood (Figure 3). Genetic deletion of Cxcr4 in myeloid cells leads to a blunted oscillation of neutrophil infiltration into the blood. 91 Downregulation of CXCL12 in the daytime stimulates the egress of neutrophils from the bone marrow into the blood. 92 In addition, ablation of Bmal1 in myeloid cells leads to a lack of rhythmic donor neutrophil migration into the spleen, which is attributed to a reduced PSGL‐1 (an adhesion molecule involved in immune cell trafficking) expression. 93
2.3. Dendritic cells (DCs)
DCs play a pivotal role in the adaptive immune system, orchestrating antigen phagocytosis, processing, and presentation to naïve T cells, thereby initiating immune responses in pathological settings. The intricate orchestration of this process involves the expression of core clock genes (i.e., Per1, Per2, Bmal1, and Clock) and CCGs within DCs, which exhibit inherent circadian rhythmicity (Figure 3). 94 Synchronized DCs demonstrate robust oscillations in the expressions of Bmal1, Rev‐erbα, and Per2.
The circadian rhythm regulates the differentiation, behaviors, and functions of DCs. First, loss of Rev‐erbs increases the expressions of surface molecules and genes (i.e., major histocompatibility complex II [MHCII] and CD86) that are important for DC maturation and immune responses. Besides, Rev‐erbα deficiency shows no effects on CD11b+CD11c+ DC populations, whereas Rev‐erbβ deficiency increases the percentage of CD11b+CD11c+ DC cells, suggesting a unique role of Rev‐erbβ in CD11b+CD11c+ DC development. 95 In addition, the circadian rhythm of antigen processing in DCs is noticeably disrupted upon deletion of Bmal1. These findings collectively highlight the substantial contribution of molecular clock in governing the antigen‐processing capabilities of DCs. 96 Furthermore, migration of DCs into lymph nodes also exhibits a circadian pattern, which peaks during the rest phase in mice (Figure 3). 82 Circadian‐regulated functionality of DCs intricately shapes adaptive immune responses. For instance, intravenous administration of ovalbumin‐loaded DCs into mice in the daytime prominently augments the population of CD8+ T cells within the spleen, compared to nighttime administration. 96
Biological clocks have emerged as crucial regulators of the trafficking DCs into lymph nodes. First, circadian clock genes are essential for maintaining the rhythmic migration of DCs into lymph nodes, involving both lymphatic endothelial cells (LECs) and DCs. Circadian expression of factors such as CC chemokine ligand 21 (CCL21), lymphatic vessel endothelial hyaluronan receptor 1, CD99, junctional adhesion molecule (JAM)‐A, and JAM‐C, which are associated with DC migration, is found to be dependent on the presence of functional BMAL1 in LECs. Notably, rhythmic expression of CCR7, a receptor for CCL21, is directly modulated by BMAL1 in DCs (Figure 3). 97 BMAL1 also plays a significant role in DC antigen processing by influencing Ca2+ localization, mitochondrial dynamics, and metabolic processes, thereby impacting adaptive immune responses. 96 Additionally, REV‐ERBα has been identified as a contributor to DC development, as it enhances the expression of MHCII and the co‐stimulatory receptor CD86, both of which serve as markers for DC maturation (Figure 3). 95
2.4. T cells
Lymphocytes, as a prominent subset of immune cells responsible for immune recognition, exhibit significant circadian alterations in their numbers, including granulocytes and lymphocytes (Figure 4). T lymphocytes, a principal subset of lymphocytes, are identifiable through surface markers such as CD3, CD4, and CD8. These cells, upon activation, initiate a process of proliferation and differentiation, culminating in the secretion of cytokines, notably interferon (IFN)‐γ and IL‐2. Diverse subtypes of T cells, notably within the CD4+ and CD8+ subsets, display circadian rhythms. The number of antigen‐stimulated IFN‐γ+CD4+ T cells is higher during the nighttime, while their proportion significantly decreases during the daytime. 98 CD4‐positive T cells exhibit pronounced circadian oscillations in various parameters, including clock gene expressions, cytokine release (IL‐2, IL‐4, IFN‐γ), and CD40L expression post stimulation (Figure 4). 98 Natural regulatory T cells (Treg), crucial in shaping adaptive immune responses, exhibit a clear circadian rhythm, with their highest levels occurring at night and lowest during the day. 99 Additionally, molecular clocks within T cells play a role in regulating their trafficking behaviors, including homing and egress within lymph nodes. 100 Circadian clocks also participate in the regulation of T‐cell differentiation. It has been established that molecular clocks, such as BMAL1, CLOCK, and REV‐ERBα, directly govern the differentiation of T helper 17 (TH17) cells. 101
FIGURE 4.
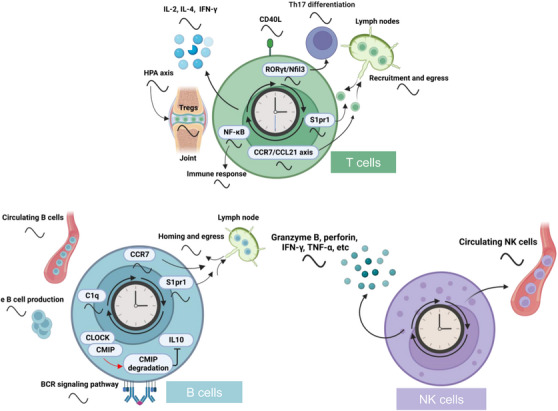
Regulation of lymphocyte behaviors and functions by the circadian clock. Circadian rhythms of lymphocyte behaviors and functions are regulated by the circadian clock at diverse aspects such as circulation of lymphocyte populations, recruitment, and egress to lymph nodes, tissue infiltration, cell differentiation, and maturation, as well as the release of cytokines, perforin, granzyme B and immune responses. The rhythmicity of these behaviors and functions are driven by clocks via different pathways such as the CCR7/CCL21 axis, CMIP, NF‐κB, S1pr1, and C1q. BCR, B‐cell receptor; CCR, CC chemokine receptor; CMIP, c‐Maf inducing protein; Nfil3, nuclear factor interleukin‐3; NK, natural killer; RORγt, retinoid‐related orphan receptor gamma t; S1pr1, sphingosine 1‐phosphate receptor 1; TH17, T helper 17.
The rhythmic oscillations of T‐cell numbers, reactivity, functions, and trafficking behaviors are reliant on biological clocks or circadian cues. To begin with, the circadian rhythm of T‐cell numbers is primarily affected by endogenous cortisol production, characterized by higher concentrations in the early morning and lower concentrations at midnight. This cortisol production is under the control of the circadian clock (Figure 4). 102 Recruitment of T cells to lymph nodes is determined by the rhythmicity of T cells themselves as well as the microenvironment, which are regulated by CCR7–CCL21 axis. Additionally, oscillatory expression of sphingosine 1‐phosphate receptor 1 (S1pr1), controlled by BMAL1 and CLOCK, governs the rhythmic egress of T cells (Figure 4). 101 Moreover, NF‐κB pathway influenced by the cell‐intrinsic clock has been identified as a potential mechanism mediating the rhythmicity of T‐cell immune responses (Figure 4). 100 Furthermore, diurnal variations in T‐cell function are influenced by the circadian oscillation of DC function, which is regulated by the biological clock. 96 Last, ablation of Clock has been found to diminish the capacity for TH17‐cell differentiation and reduce TH17‐cell frequencies in the gut through the regulation of retinoid‐related orphan receptor gamma t (Rorγt) transcription and Nfil3 expression (Figure 4). 102
2.5. B cells
B cells, as a crucial component of the adaptive immune system, display diurnal variations in their numbers. In human blood, B‐cell numbers exhibit a significant peak during nighttime, followed by decreased levels during the daytime. 103 Additionally, B‐cell counts in lymph nodes also display rhythmicity, with the highest levels during the early dark phase, which is closely associated with circadian behaviors such as homing and egress (Figure 4). 101 Moreover, mRNA levels of canonical clock genes, including Bmal1, Clock, and Per2, exhibit oscillations in splenic B cells under the daily light–dark cycle and constant conditions. 104 B‐cell receptor (BCR) signaling pathway plays a critical role in the activation, proliferation, and differentiation of B cells. Notably, loss of CRY, a circadian protein, leads to a significant activation of BCR‐proximal signaling pathways and affects B‐cell functions. 105 Hence, circadian clock plays a vital role in modulating B cell numbers, development, and functions.
Regulation of B‐cell numbers, development, and functions relies on both circadian clocks and external cues. First, the diurnal rhythm in circulating B‐cell numbers in humans is influenced by the rhythmic plasma cortisol levels. This diurnal oscillation of B‐cell numbers coincides inversely with plasma cortisol levels. 106 Second, the biological clock also plays a crucial role in modulating B‐cell numbers. Genetic deletion of clock proteins has been shown to impair B‐cell development and leads to a significant reduction in B‐cell populations in peripheral blood, spleen, and bone marrow. 107 Additionally, loss of Bmal1 specifically in B cells disrupts the overall oscillation of B‐cell numbers in lymph nodes by regulating the diurnal expressions of key regulators such as Ccr7 and S1pr1, which are essential for lymphocyte trafficking (Figure 4). 101 Moreover, CLOCK forms a complex with c‐Maf inducing protein (CMIP) to induce the degradation of CMIP, resulting in restricted transcription of the Il‐10 gene in B cells. This, in turn, suppresses the immune regulatory functions of B cells (Figure 4). 28
2.6. ILCs
The ILC family is stratified into two principal subtypes: cytotoxic and helper‐like ILCs. The cytotoxic subset encompasses natural killer (NK) cells, renowned for their inherent ability to target and eliminate malignant or pathogen‐infected cells. Conversely, the helper‐like ILCs are segregated into three primary groups: ILC1, ILC2, and ILC3. Each group exhibits distinct functional characteristics and plays a pivotal role in orchestrating immune responses.
NK cells, a crucial subset of lymphocytes distinguished by the absence of the CD3‐T cell receptor (TCR) complex, play a pivotal role in safeguarding the host against microbial infections and malignant transformations by means of discharging cytolytic granules or TNF‐α and triggering the Fas pathway. 108 Recent studies have elucidated that within NK cells, the expression patterns of core circadian genes, including Bmal1, Clock, Per1, and Per2, display a distinct circadian rhythm. This rhythmic expression extends to cytolytic granules, notably granzyme B and perforin, as well as NK cell‐associated cytokines such as IFN‐γ and TNF‐α. These findings indicate a time‐dependent modulation of NK cells’ cytolytic activity, underpinned by the circadian oscillation of both gene expressions and effector functions (Figure 4). 109 Additionally, circulating NK cell counts undergo a 24‐h oscillation in humans, displaying peak levels during the early morning and diminishing during the night (Figure 4). 110 Nevertheless, the underlying mechanism responsible for the diurnal variations in NK cell numbers remains elusive.
Disruption of clock genes through genetic manipulation or exposure to environmental factors has provided valuable insights into the distinct roles of biological clocks in regulating the functions of NK cells. For instance, specific knockdown of Per2 using RNA interference techniques causes a significant decrease in the levels of granzyme B and perforin within the NK cell line RNK16. 111 Additionally, loss of Per1 alters the rhythmic levels of perforin, granzyme B, and IFN‐γ released by splenic NK cells (Figure 4). 112 Furthermore, chronic shift‐lag condition accelerates the aging process of NK cells, characterized by a reduced expression of Ly49 and impaired releases of granular CD107a and IFN‐γ. 113 Repeated phase adjustments in the light–dark cycle similarly disrupt the rhythmic expressions of clock genes, cytolytic factors, and cytokines in NK cells, thereby impeding their time‐dependent cytolytic activities. 114
In comparison to ILC1 and ILC2, ILC3 exhibits higher expressions of a range of clock genes, such as Bmal1, Clock, Nfil3, Dbp, Pers, Crys, and Rev‐erbs, which shows more distinct rhythmicity. 23 The interplay between circadian clocks and ILC3 has garnered increasing attention in recent research. External cues like light exposure, dietary patterns, and microbiota composition, along with intrinsic circadian mechanisms, have been identified as modulators of the rhythmic expressions of clock genes (i.e., Clock and Rev‐erbα) in ILC3. 23 Furthermore, circadian clock exerts a regulatory influence over the quantity, behavior, and functional attributes of ILC3. Notably, ablation of Bmal1 leads to a marked decrease in the population of ILC3 within the small intestine of mice. 115 REV‐ERBα, a pivotal transcription factor for ILC3, significantly influences various ILC3 subsets. 116 Perturbations in circadian rhythm have been observed to impact cytokine secretion in ILC3. For instance, Rev‐erbα deficiency substantially diminishes the numbers of NKp46+ ILC3 cells, reduces RORγt expression, and lowers IL‐22 production, while paradoxically elevating IL‐17 secretion. 116 In ILC3, BMAL1 deficiency shows enhanced expressions of RORγt‐dependent target genes, including IL17a, IL17f, and IL22. 115 Investigations into various types of ILCs, beyond ILC3, remain relatively sparse. However, notable advancements have been made in understanding the role of RORα in the differentiation of ILC2. These cells are integral to type II inflammation, allergic responses, and defense mechanisms against parasitic infections. The significance of RORα in the differentiation process of ILC2 underscores the critical functions of these cells in the immune response. 61 Additionally, ILC2, which is under circadian regulation, play a vital role in the maintenance of eosinophil homeostasis. 117
The circadian regulation of helper‐like ILCs is governed by a multitude of mechanisms. BMAL1 deficiency leads to a decrease in ILC3 counts in the small intestine, and an increased presence of ILC3s in the mesenteric lymph nodes attributed to loss of gut homing signals such as CCR9, α4β7 integrin, and CXCR4. 23 Furthermore, gut microbiota and pro‐apoptotic proteins, such as Bim and Bax, are identified as key factors in controlling the hyper‐activation and loss of intestinal ILC3 in BMAL1‐deficient mice. The elimination of microbiota through antibiotic treatment partially mitigates the hyper‐activation of Bmal1‐deficient ILC3 and restores cellular homeostasis in the gut. 115 However, regulatory mechanisms of helper‐like ILCs by circadian clocks have remained largely unexplored.
3. CIRCADIAN REGULATION OF NON‐IMMUNE CELL FUNCTION
Circadian oscillations manifest within a diverse array of non‐immune cellular populations. Our investigative endeavors have been profoundly directed toward three distinct categories of rhythmically orchestrated cell types, namely, hepatocytes, adipocytes, and intestinal epithelial cells, all of which assume pivotal roles in physiological processes. Hepatocytes, which constitute the majority of liver mass, are central to an array of biochemical and metabolic functions. 118 Adipocytes, along with their secretory products, are involved in numerous physiological processes and significantly influence metabolic health. 119 , 120 Intestinal epithelial cells, essential for food digestion, nutrient absorption, and maintaining intestinal epithelial homeostasis, play a vital role in responding to diseases. 121 A substantial body of evidence demonstrates that circadian clock genes are intricately involved in the physiological functions of these cells.
3.1. Hepatocytes
Hepatocytes, as the principal parenchymal cells in the liver, orchestrate a myriad of cellular processes including drug metabolism, drug detoxification, and immune cell activation, all critical for preserving liver homeostasis. 118 The circadian clock mechanism exerts regulatory control over the diurnal rhythmicity of gene expressions and functional processes within hepatocytes. Perturbation of REV‐ERBs disrupts the natural diurnal rhythm and induces alterations in the metabolomic profiles observed across diverse liver cell categories. 122 Furthermore, disruption of CLOCK function results in modified diurnal patterns of cytochrome P450 enzymes within hepatocytes, including but not limited to CYP2A4/5 and CYP2B10. 123
The hepatocyte clock plays a vital role in sustaining rhythmic gene expression patterns in immune cells. Specifically, hepatocyte‐specific knockout of Rev‐erbs leads to marked changes in the rhythmic gene expression profiles of nonparenchymal liver cells, with macrophages being notably affected. 124 Hepatocyte‐targeted Rev‐erbs knockdown disrupts the rhythmicity of genes linked to the vitamin D receptor signaling pathway, a pathway known to mitigate liver inflammation and steatosis. It also enhances the circadian rhythmicity of genes associated with inflammatory responses in macrophages. 124 , 125 , 126 The interplay between hepatocytes and macrophages, facilitated by mediators including hepatocyte‐derived sterol regulatory‐element binding protein (SREBP) cleavage‐activating protein and a range of polypeptides, plays a critical role in the transmission of circadian rhythmic signals. 124 In conclusion, circadian clocks are integral to the regulation of gene expressions and cellular functions in hepatocytes, underscoring their role in maintaining hepatic and systemic physiological balance.
3.2. Adipocytes
Adipose tissue dynamically responds to the daily fluctuations in energy availability and demand, a crucial aspect of maintaining energy homeostasis. This adaptability is facilitated by a circadian modulation of signals, where adipose physiology is governed in a time‐of‐day dependent manner. This rhythmic regulation is primarily achieved through the transcriptional control of CCGs, which are regulated by adipocyte clocks. 127 Adipocyte clocks orchestrate the timing of the local transcriptome, which includes critical genes involved in lipogenesis and lipolysis, such as Atgl, Hsl, caveolin 2, acyl‐CoA synthetase, phosphatidate phosphatase, Lpl, peroxisome proliferator‐activated receptor α/γ, coactivator 1β, and Srepb1α. 127
The BMAL1/CLOCK heterodimer in adipocyte assumes a central role in the regulation of gene expressions, specifically activating the transcription of Atgl and Hsl genes by binding to E‐box promoter elements. 128 Notably, adipocyte‐selective deletion of Rev‐erbα, under normal conditions, exhibits a relatively modest impact on the expressions of lipogenic genes. However, in the context of diet‐induced obesity, this deletion exerts a profound influence on metabolic health. It leads to the development of a metabolically advantageous phenotype characterized by diminished adipose tissue inflammation and distinct alterations in the dynamics of collagen fibrosis. These effects are attributed to the regulatory control of collagen genes. 129 Furthermore, circadian clock exerts temporal control over adipogenic differentiation. BMAL1 transcriptionally regulates genes associated with the actin dynamic‐MRTF/SRF cascade, thereby influencing actin cytoskeleton organization and SRF activity. Additionally, circadian clock exerts precise temporal control over adipogenic differentiation. BMAL1 orchestrates the transcriptional regulation of genes associated with the actin dynamic‐myocardin‐related transcription factor/serum response factor (MRTF/SRF) cascade, thereby influencing the organization of the actin cytoskeleton and modulating SRF activity. This regulatory mechanism holds pivotal importance in the transformation of adipogenic progenitor cells into mature adipocytes, the process of beige adipogenesis, and the enhancement of thermogenic capacity. 130 In summary, circadian clocks play a vital and multifaceted role in both the functions and differentiation of adipocytes, underscoring their significance in the realm of adipose tissue physiology and the broader context of systemic energy homeostasis.
3.3. Intestinal epithelial cells
Intestinal epithelial cells, a single layer of tightly interconnected columnar cells, form the primary defensive barrier of the intestinal mucosa. 131 Circadian clock regulates cellular processes in intestinal epithelial cells including growth, proliferation, differentiation, and cell signaling. 132 Of particular note, BMAL1 plays a central role in the regulation of intestinal epithelial cell regeneration by modulating cytokine activity, cell cycle progression, and cell proliferation dynamics. It is worth highlighting that knockdown of Bmal1 results in the disruption of the rhythmic pattern of cellular proliferation. 133 , 134 Furthermore, TNF cytokine signaling and P21 emerge as key contributors to BMAL1‐mediated cell proliferation control. The former fosters proliferation among epithelial precursors, thereby facilitating the replacement of damaged cells, while the latter assumes a pivotal role in regulating diurnal rhythms in cell production. 134 The epithelial clock also exerts regulatory control over essential molecules such as Wee1, cyclins D1, and E, all of which are indispensable for the generation and maintenance of circadian rhythms in the cell cycle progression of intestinal epithelial cells. 135 Additionally, intestinal stem cells, vital for the continuous replenishment of differentiated epithelial cells, are influenced by clock components including BMAL1 or CRY. These components modulate transcripts linked to regeneration and stem cell signaling, encompassing pathways like Wnt and Hippo signaling within the intestinal epithelium. 136
The circadian clock serves as a key regulator in managing both endogenous substances and xenobiotics within intestinal epithelial cells, highlighting its essential role in cellular processing and homeostasis. Intestinal BMAL1 transcriptionally enhances DGAT2 expression, an enzyme crucial for triacylglycerol synthesis, by binding to an E‐box in its promoter, thereby facilitating dietary fat absorption in the gut. 137 Intestinal exporter MRP2, which plays a key role in the disposition and elimination of various drugs, is also impacted by the intestinal circadian clock. Loss of Bmal1 in intestinal epithelial cells diminishes the expression and transport activity of MRP2, as well as their diurnal rhythms, affecting the pharmacokinetics and toxicity of medications like methotrexate. 138
4. CIRCADIAN REGULATION OF CELLULAR SIGNALING PATHWAYS
The circadian clock is crucial in regulating signaling pathways via transcriptional and translational modulation. 13 NF‐κB, JAKs/STATs, and MAPKs signaling pathways, central to inflammatory responses and diverse diseases, are significantly influenced by circadian rhythms. 42 The following sections critically examine the mechanistic connections between the circadian molecular clock and these signaling pathways: NF‐κB, JAKs/STATs, and MAPKs.
4.1. NF‐κB signaling pathway
NF‐κB signaling pathway, comprising a family of inducible transcription factors, is a pivotal regulator of inflammatory responses. 140 Circadian clock modulates various aspects of the NF‐κB signaling pathway targets (Figure 5). 141 Clock factors form an intricate network with NF‐κB proteins, influencing their function and interaction. REV‐ERBα, a key clock component, regulates the levels of p65, a subunit of NF‐κB, orchestrating the expressions of inflammatory genes and the release of cytokines and chemokines through three distinct mechanisms. First, Rev‐erbα suppresses the Toll‐Like Receptor 4, thereby blocking upstream pro‐inflammatory signals in the NF‐κB pathway. 142 Second, REV‐ERBα disminishes the nuclear level of p65 protein, inhibiting its transcriptional activation of target genes. Third, REV‐ERBα directly binds to the promoters of inflammatory genes (e.g., [NOD‐, LRR‐ and pyrin domain‐containing protein 3]Nlrp3, Il‐1β, and Il‐6), suppressing their transcription and expressions. Multiple mechanisms are proposed for the regulation of NF‐κB signaling by the clock protein BMAL1. BMAL1 regulates NF‐κB/NLRP3 axis via REV‐ERBα, which acts as a repressor of NF‐κB/NLRP3. Supporting this, BMAL1 influences the timing of gene expression in response to inflammatory activation by modulating the epigenetic status of enhancers, partly through the regulation of REV‐ERBs and eRNA transcription in macrophages. 143 Biochemical and biophysical analyses have shown that p65 subunit of NF‐κB binds to the transactivation domain of BMAL1 through protein–protein interaction, 144 with additional mechanisms including an increase in the phosphorylation of IκB. 144 CLOCK directly interacts with p65 protein, and CLOCK overexpression leads to an increase in specific phosphorylated and acetylated transcriptionally active forms of p65. 145 CRY1, another clock component, binds to adenylyl cyclase, limiting cAMP production and inactivating protein kinase A, which results in NF‐κB activation through phosphorylation of p65 at S276. 146 These studies provide a comprehensive mechanistic framework for understanding the intricate relationship between the circadian clock and NF‐κB signaling.
FIGURE 5.
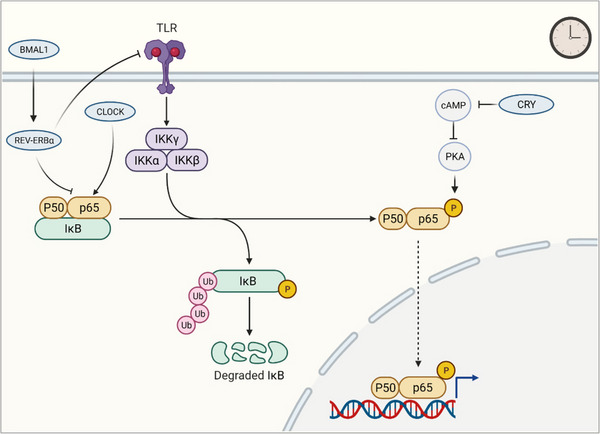
Regulation of NF‐κB signaling pathway by the circadian clock. Circadian clocks regulate NF‐κB signaling pathway through diverse aspects including transcription, post‐translational modification, and protein–protein interactions. REV‐ERBα regulates NF‐κB via modulation of p65 subunit and TLR4. BMAL1 acts as an NF‐κB/NLRP3 repressor through REV‐ERBα. CLOCK interacts with the p65 subunit. CRY1 binds to adenylyl cyclase to limit cAMP production and inactivates PKA, subsequently resulting in NF‐κB activation through phosphorylation of p65. cAMP, cyclic adenosine monophosphate; CLOCK, circadian locomotor output cycles kaput; CRY, cryptochrome; IKK, IκB kinase; NF‐κB, nuclear factor‐κB; PKA, protein kinase A; cAMP, cyclic adenosine monophosphate; REV‐ERBα, nuclear reverse erythroblastosis virus heme receptor α; TLR, Toll‐like receptors.
4.2. MAPK signaling pathway
MAPK signaling pathways are composed of three families including extracellular‐signal‐regulated kinase (ERK), c‐Jun NH2‐terminal kinase (JNK), and p38. Clock factors physically and/or genetically interact with MAPKs to regulate inflammatory responses. BMAL1 decreases ERK phosphorylation in chondrocytes 147 and inactivates p38 phosphorylation after traumatic brain injury. 78 REV‐ERBα suppresses osteoblast differentiation by inhibiting the p38 MAPK pathway, 148 while ROR‐γ‐knockdown downregulates p38 MAPK expression in hepatocytes. 149 These regulatory mechanisms are attributed to circadian rhythms in the expression of MAPK genes and/or proteins such as ERK and MAPKs (upstream of p38 and JNK kinases). 150
4.3. JAK‐STAT signaling pathway
JAK‐STAT signaling pathway is critical for orchestrating the immune system. The circadian clock modulates JAK‐STAT signaling pathway via two separable mechanisms. First, REV‐ERBα abrogates JAK/STAT3 signaling pathway as evidenced by reduced phosphorylated JAK1, JAK2, and STAT3. This effect was attained by up‐regulating the suppressor of cytokine signaling 3, an inhibitor of the JAK/STAT3. 151 In addition to the core clock genes, miR‐279 acting as an effector of clock‐controlled behavioral output, targets Upd (the ortholog of the JAK/STAT ligand) to circadian behavioral output. 152 Consequently, STAT92E and phosphorylated HOP (JAK/STAT components) are expressed in a circadian manner. 153 The regulation of JAK/STAT3 by circadian clock bears significance for the etiology of inflammatory and autoimmune disorders.
5. CIRCADIAN REGULATION OF DISEASES
Dysregulation of circadian rhythms within distinct cellular populations stands out as a pivotal mechanistic factor contributing to a wide spectrum of pathological conditions spanning multiple tissue types. These afflictions encompass a range of conditions intimately linked to typical circadian patterns, including inflammatory diseases, cancers, and systemic diseases (Figure 6 and Table 1). 154
FIGURE 6.
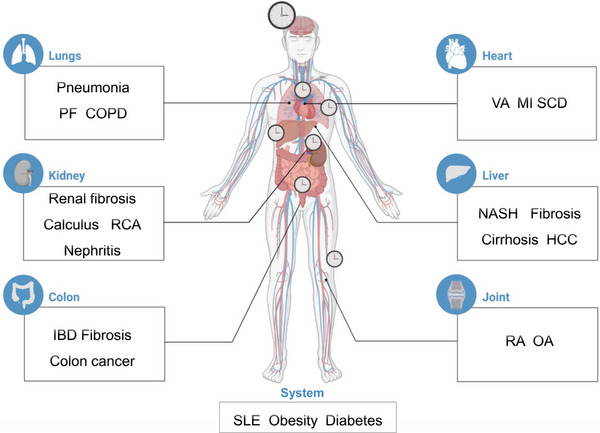
The role of the circadian clock in diseases. Circadian clocks situated in the lung, kidney, colon, heart, liver, and joint tissues exert regulatory effects over diseases. COPD, chronic obstructive pulmonary disease; HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease; MI, myocardial infarction; NASH, nonalcoholic steatohepatitis; OA, osteoarthritis; PF, pulmonary fibrosis; RA, rheumatoid arthritis; RCA, right coronary artery; SCD, sudden cardiac death; SLE, systemic lupus erythematosus; VA, ventricular arrhythmias.
TABLE 1.
The effects of circadian disruption on cell functions in diseases.
| Cell types | Models | Diseases | Findings | Refs |
|---|---|---|---|---|
| Microglia | Bmal1 deficiency | Asthma | Decrease pro‐inflammatory cytokines and increase anti‐inflammatory cytokines in microglial BV2 cells following LPS stimulation | 75 |
| Macrophages | Rev‐erbα deficiency | Rheumatoid arthritis | Abolish the circadian rhythmicity of IL‐6 in LPS‐induced macrophages and endotoxin‐treated mice | 74 |
| Macrophages | Jet lag | Melanoma | Induce the loss or inversion of daily patterns of M1 and M2 macrophages and cytokine levels | 73 |
| Microglia | Clock deficiency | Glioblastoma | Decrease intratumoral microglia infiltration in the tumor microenvironment and increase overall survival | 76 |
| Neutrophils | Rev‐erbα deficiency | Hepatic I/R | Aggravate hepatic I/R injury and significantly increases the number of neutrophils infiltrated to I/R liver | 155 |
| Neutrophils | Bmal1 deficiency | IBD | Abolish daily variations in granule contents and NETs formation | 89 |
| NK cells | Per1 deficiency | Lung cancer | Disrupt and alter the rhythmic levels of perforin, granzyme B, and IFN‐γ released by splenic NK cells | 112 |
| NK cells | Chronic shift‐lag | Autoimmune disease | Accelerate the aging process of NK cells, characterized by reduced expression of Ly49 and impaired release of granular CD107a and IFN‐γ | 113 |
| NK cells | Per2 deficiency | Non‐small cell lung cancer | Confer greater resistance to LPS‐induced endotoxic shock, which could be attributed to decreased production of IFN‐γ/IL‐1β and suppression of NK cell function | 156 |
| NK cells | Bmal1 deficiency | IBD | Reduce ILC3s counts in the small intestine and increase the frequency of ILC3s in the mesenteric lymph nodes | 23 |
| ILCs | Rev‐erbα deficiency | IBD | Reduce the cell numbers of NKp46+ ILC3 subset, RORγt expression, and IL‐22 production, whereas increase IL‐17 secretion paradoxically | 116 |
| ILCs | Bmal1 deficiency | IBD | Decrease in the population of ILC3 within the small intestine of mice | 115 |
| DCs | Clock deficiency | Autoimmune inflammation | Diminish the capacity for TH17‐cell differentiation and reduce TH17‐cell frequencies in the gut | 102 |
| T cells | Clock deficiency | B‐cell lymphoma | Impair B‐cell development and lead to a significant reduction in B‐cell populations in peripheral blood, spleen, and bone marrow | 107 |
| B cells | Bmal1 deficiency | Lymphomas | Disrupt the oscillation of B‐cell numbers in lymph nodes | 101 |
Abbreviations: Bmal1, Brain and Muscle ARNT‐Like 1; Clock, circadian locomotor output cycles kaput; DCs, dendritic cells; I/R, ischemia/reperfusion; IBD, inflammatory bowel disease; IFN‐γ, interferon‐γ; IL, interleukin; ILC3s, Group 3 innate lymphoid cells; LPS, lipopolysaccharides; NETs, neutrophil extracellular traps; NK, natural killer; Per1, period 1; Per2, period 2; Rev‐erbα, nuclear reverse erythroblastosis virus heme receptor α; RORγt, retinoic acid receptor‐related orphan receptor γt; TH17, T helper 17.
5.1. Inflammatory and immune diseases
5.1.1. Inflammatory bowel disease (IBD)
IBD, a chronic affliction of the colon that significantly impacts individuals' lives, primarily comprises two types: ulcerative colitis and Crohn's disease. 157 IBD is characterized as a chronic and recurrent inflammatory disorder of the intestinal tract. Research involving chronic colitis in mice has demonstrated a diurnal pattern in disease severity, marked by elevated levels of inflammatory factors (IL‐1β and IL‐6) during the daytime and reduced levels at night. 158
In contrast, disruptions in circadian rhythm have been observed to intensify a pro‐inflammatory state and foster the development of IBD. Shift workers, who often endure non‐traditional work schedules leading to abnormal sleep–wake cycles, are at an increased risk for developing IBD. 159 In mammals, deficiencies in clock genes, including Bmal1, Per1/2, and Rev‐erbα, are associated with heightened susceptibility to dextran sulfate sodium/trinitrobenzene sulfonicacid (DSS/TNBS)‐induced IBD. Conversely, overexpression of these genes can mitigate the severity of colon inflammation. 25
The progression of IBD is intricately regulated by the circadian clock through various molecular mechanisms. These encompass key factors such as NLRP3 inflammasome, NF‐κB signaling pathway, and immune cell populations, notably PDL1+ B cells and CD4+ T cells. 102 , 160 In addition to inflammation‐related factors, non‐inflammatory factors, including genes associated with the intestinal barrier such as tight junction protein 1 and mucin 2, along with intestinal epithelial cells, play significant roles in the circadian clock‐mediated regulation of IBD. Disruption of circadian clock specifically within epithelial cells elicits a profound exacerbation in colitis severity and mortality. This adverse outcome can be attributed to an upsurge in the production of inflammatory mediators, including signal transducer and activator of transcription, as well as gut microbiota‐derived short‐chain fatty acids. 161 This evidence underscores a significant role of circadian rhythms in the pathophysiology of IBD, highlighting the need for further research to fully understand these complex interactions.
5.1.2. Rheumatoid arthritis (RA)
RA, an autoimmune disorder, is marked by chronic inflammation in the articular cartilage, joints, and surrounding tissues. The symptoms of RA exhibit significant circadian oscillations, with increased severity in joint swelling, stiffness, and pain observed during the early morning. 162 , 163 , 164 This escalation correlates with a higher nocturnal release of proinflammatory cytokines such as TNF‐α and IL‐6 in the synovium and pannus. 165 Additionally, anti‐inflammatory hormone cortisol varies in a time‐dependent manner, typically showing insufficiency in the early morning, which contributes to the circadian variation in RA symptoms. 166
Disruptions in circadian clocks have been observed to exert a deleterious influence on the pathogenesis of RA. Extensive investigations have demonstrated that perturbations of the molecular clock, whether induced in vivo through light misalignment and genetic interventions or in vitro through pharmacological manipulation, culminate in the alteration of clock gene expression and exacerbation of joint inflammation.. 139 For instance, deficiency of Cry1 and Cry2, which are pivotal core clock genes, results in a heightened inflammatory state in collagen‐induced RA murine models, thereby underscoring the significance of clock gene integrity in the regulation of RA‐associated inflammation. 167 Conversely,RA itself can engender disturbances in sleep patterns, characterized by difficulties in falling asleep, non‐restorative sleep, and discernible alterations in the expression profiles of clock genes such as BMAL1, CLOCK, CRY, and PER. 168 These bidirectional interactions between circadian rhythms and RA pathophysiology underscore the intricate relationship between the circadian system and immune regulation in the context of this autoimmune disorder.
The development of RA is regulated by circadian clocks through various mediators. These include immune cell infiltration, pro‐inflammatory cytokines (IL‐1β, IL‐6, and TNF‐α), neuroendocrine hormones (cortisol in humans and corticosterone in rodents, and melatonin), mediators of oxidative stress (e.g., p53 and Nrf2), and neurotransmitters. 169 For instance, increased suppression of inflammation is noted during nighttime, attributed to the elevated presence of Treg cells within the joints. 57 This complex interplay highlights a significant effect of circadian rhythms on the progression and symptomatology of RA.
5.2. Cancers
Cancer remains a leading cause of mortality and a significant barrier to extending life expectancy. Emerging research increasingly implicates disruptions in circadian rhythms as significant factors in the onset and progression of various cancers. For instance, shift workers exhibit an approximately 10% heightened risk of developing breast cancer. 170 Furthermore, mice with chronic circadian disruption are more likely to develop HCC (hepatocellular carcinoma), likely due to metabolic and immunologic changes. 171 Additionally, chronic jet lag exacerbates tumor‐induced inflammation in the mouse liver and hypothalamus, which may contribute to cancer‐related cognitive impairments and an unfavorable prognosis. 172 In a contrasting perspective, the levels of specific clock genes, namely, PER1, PER2, PER3, and CRY2, are notably diminished in cancerous tissues, compared to their noncancerous counterparts. This reduction is primarily ascribed to promoter methylation and overexpression of EZH2, a gene integral to epigenetic regulation. 173
Alterations in the components of the circadian clock, including BMAL1, PER1/2, and CRY1/2, have been correlated with an increased risk of cancer development. Specifically, BMAL1 is critical in hematologic malignancies, where its inactivation has been linked to the advancement of cancers. 174 Bmal1 knockout mice show a heightened susceptibility to colitis‐associated colorectal cancer. 160 Mice with hepatocyte Bmal1 deficiency have a protective effect in high‐fat diet‐ and diethylnitrosamine‐induced hepatocellular carcinoma in mice. 175 The repressive arms of the core circadian clock, including PER1 and PER2, are also implicated in cancer. Reduced expression of these genes has been associated with shorter survival in patients with glioma and gastric cancer. 176 These findings highlight the intricate connection between circadian rhythms and cancer, emphasizing the need for further research to understand how circadian disruptions contribute to cancer development and progression.
Inflammation plays a pivotal role in the regulatory effects of the circadian clock on cancer pathogenesis. 177 , 178 , 179 The activation, trafficking, and infiltration of immune cells into tumors are largely contingent on the release and presentation of cancer cell antigens, with inflammation being a central mediator in this mechanism. For instance, rhythmic expression of Bmal1 regulates the numbers of antigen‐specific DCs and the volume of tumors following tumor engraftment at different time points (ZT9 or ZT21). 180 In a melanoma mouse model, chronic jet lag leads to significant immunosuppression within the tumor microenvironment, accelerating tumor growth. This effect is attributed to the elimination of daily variations in macrophage counts and a reduced ratio of M1 to M2 macrophages in the tumor. 73 Additionally, Bmal1 deficiency in mice results in a decrease in Breg+ PDL1+ cell numbers among intestinal intraepithelial lymphocytes and induces the death of activated CD4+ T cells, thereby promoting the progression of colitis‐associated colorectal cancer. 160 Chronic shift‐lag conditions disrupt both the circadian expressions of clock genes and the cytolytic activities of NK cells, potentially fostering tumor growth in the lung. 156 Furthermore, various regulators, such as cyclin‐dependent kinases, suppressor of mother against decapentaplegic (SMAD) family member 4, chemokines, vascular endothelial growth factor A, and telomerase reverse transcriptase, have been implicated in the influence of clock genes on cancer development. 176 Given these insights, it is crucial to explore novel therapeutic approaches targeting cancer cells, leveraging the circadian rhythmic regulation of cancer biology. Such strategies could offer more effective and tailored treatments, potentially improving patient outcomes by aligning therapy with the body's natural circadian rhythms.
5.3. Systemic diseases
5.3.1. Systemic lupus erythematosus (SLE)
The circadian clock is fundamentally implicated in the progression of SLE, characterized by a bidirectional relationship between circadian rhythms and the disease. This association is underscored by a large‐scale population‐based cohort study involving 144,396 patients, which identifies that sleep deprivation markedly increase the susceptibility to SLE. 181 Complementing this finding, additional research suggests that relatives of SLE patients who consistently sleep less than 7 h per night face an elevated risk of developing the disease. 182 Moreover, genetic factors contribute to this correlation as evidenced by the association of single nucleotide polymorphisms in the PER2 gene with both the susceptibility to and clinical manifestations of SLE. 183 Conversely, patients with SLE, or those at risk of developing it, often experience sleep disturbances, which significantly impair their quality of life. 184
Studies have highlighted the role of circadian clock proteins in the pathogenesis of SLE. These studies point to various factors, including immune cells, melatonin, Nrf2, anti‐nuclear antibodies, and glomerular complement precipitation, as contributors to the disease process. 105 , 185 , 186 In experimental models, conditional knockout of E4bp4 in T cells has been shown to enhance the differentiation of T follicular helper cells, thus exacerbating pristane‐induced lupus‐like diseases in mice. 187 Additionally, loss of Cry results in increased mature B‐cell production, overactivation of the BCR‐signaling pathway, and downregulation of C1q expression, thereby accelerating the pathogenesis of SLE. 106 This growing body of evidence underscores a complex interplay between circadian rhythms and immune function in the context of SLE, highlighting the need for further research to fully understand these relationships and their implications for SLE management.
5.3.2. Obesity and diabetes
Immune cells, particularly macrophages, are central to the mechanisms linking circadian rhythms with inflammation‐driven metabolic disorders including obesity and diabetes. Myeloid‐specific Bmal1 knockout increases Ly6Chi macrophage content in adipose tissue and levels of monocyte‐attracting chemokines, as well as inflammatory responses in mice subjected to a high‐fat diet. 188 This causes increased total body adiposity and tissue weight, highlighting the role of BMAL1 in regulating obesity‐related inflammation. 188 Similarly, bone marrow‐derived macrophages from Per1/2 knockout mice show a higher proportion of pro‐inflammatory M1 macrophages, exacerbating systemic insulin resistance and accelerating the progression of diabetes. 74
In addition to the role of immune cells in diabetes, intestinal epithelial cell‐specific Bmal1 knockout can remodel diurnal hepatic metabolism by shifting to gluconeogenesis from lipogenesis during the dark phase, and elevate the production of glucose and insulin insensitivity, leading to an enhanced susceptibility of diabetes in mice. 10 Adipocyte‐specific deletion of Bmal1 results in obesity accompanied with reduced energy expenditure, attenuated food intake rhythm, and increased food intake via decreasing circulating polyunsaturated fatty acid concentration and affecting the levels of neurotransmitters responsible for appetite regulation in hypothalamic feeding centers. 189 Recent advancements in chronobiology have shed light on a significant role of the hepatocyte clock in the regulation of metabolic disorders associated with inflammation. A key finding in this domain is the discovery of Platr4, an anti‐inflammatory long non‐coding RNA, which operates under the control of REV‐ERBα. The primary function of Platr4 is the inhibition of the NLRP3 inflammasome, an essential factor in the body's inflammatory response. This inhibition by Platr4 is particularly noteworthy in the context of nonalcoholic steatohepatitis, where it helps in reducing the disease's severity. 190
Inflammation‐associated metabolic diseases disrupt circadian clocks. For example, in obese patients, activation of NF‐κB hinders the binding of BMAL1 protein to PER2 promoter, reducing PER2 expression and thus impairing circadian clock function. 191 By understanding and manipulating the complex interplay between the circadian clock and inflammation, novel strategies could be developed to treat obesity and diabetes.
6. CONCLUSION
This review provides insights into the regulatory influence of circadian clocks on cellular functions, signaling pathways, and disease processes. The modulation of cell functions and signaling pathways by the circadian clock emerges as a pivotal determinant of the rhythmic intensity observed in various diseases. A deep comprehension of the intricate interplay between cell‐intrinsic clocks and cell functions is of paramount significance for advancing our mechanistic understanding of diseases. Furthermore, this knowledge is essential for the development of innovative chronotherapeutics and targeted drugs. These interventions aim to harness the circadian regulation of cellular functions to enhance disease management strategies.
In normal life, sleep deprivation is closely associated with circadian disruption, resulting in dysregulated immune responses and increased susceptibility to diseases. 192 , 193 , 194 Epidemiological studies have robustly established a significant correlation between sleep deprivation and various cancers, including breast, colorectal, and prostate cancers. 195 , 196 , 197 , 198 Sleep‐deprived models exhibit a reduction in immune cell numbers (e.g., NK cells, T cells, DCs), creating an immunosuppressive environment conducive to accelerated cancer growth. 199 Sleep deprivation disrupts the circadian rhythm to influence inflammatory pathologies. Sleep deprivation, by disrupting circadian rhythms, also exerts an influence on inflammatory pathologies. Interventions targeting the circadian clock can modulate inflammation induced by sleep deprivation through signaling pathways. 200 Consequently, an exploration of novel ligands targeting circadian clocks holds substantial promise for the treatment of inflammation‐related diseases triggered by sleep deprivation.
Our review sheds light on the bidirectional relationship between circadian clocks and inflammation. Disruptions in circadian rhythms amplify inflammatory responses to pathogens and exacerbate disease progression. Conversely, inflammation can influence the expression and functional dynamics of circadian clocks. A key player in this interplay is NF‐κB, which acts as a modulator of circadian rhythm through its interaction with clock genes. NF‐κB modifies circadian rhythms by binding to the regulatory sequences of clock genes, recruiting histone deacetylases for chromatin modification, and interacting with the BMAL1/CLOCK complex at E‐box sites, thereby influencing circadian transcription. 201 The precise mechanism by which disturbances in circadian rhythms initiate inflammation (as an “egg”) and the extent to which existing inflammation (as a “chicken”) impacts these rhythms remain unclear. However, our review acknowledges the intricate interconnection between circadian rhythms and inflammation‐associated diseases. This understanding paves the way for developing targeted therapeutic strategies to effectively manage these complex interactions.
In summary, this review underscores a pivotal role of circadian rhythm disruptions in diverse diseases. This influence is primarily mediated through cell functions and signaling pathways. There exists an urgent necessity for conducting extensive research to discover innovative approaches for intervening in circadian clock‐controlled diseases. This encompasses endeavors to address disturbed circadian rhythms and optimize disease management, thereby potentially enhancing patient outcomes and quality of life.
AUTHOR CONTRIBUTIONS
Y.L., L.H., Y.C., X.W., S.W., and F.L. conceived, edited, and wrote this manuscript. Y.L., L.H., S.W., and F.L. revised this manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The author Yanke Lin is an employee of Guangdong TCRCure Biopharma Technology Co., Ltd. but has no potential relevant financial or non‐financial interests to disclose. The other authors have no conflicts of interest to declare.
ETHICS STATEMENT
The author declares that ethics approval was not needed for this study.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 82104238, 82003914 & 82373940) and the Fundamental Research Funds for the Central Universities. Biorender software (https://app.biorender.com) was used to create the figure under an academic license.
Lin Y, He L, Cai Y, Wang X, Wang S, Li F. The role of circadian clock in regulating cell functions: implications for diseases. MedComm. 2024;5:e504. 10.1002/mco2.504
Yanke Lin and Liangliang He contributed equally to this study.
Contributor Information
Shuai Wang, Email: wangs91@163.com.
Feng Li, Email: li_fengfeng@163.com.
DATA AVAILABILITY STATEMENT
All data are available upon request.
REFERENCES
- 1. Zhao K, Ma B, Xu Y, Stirling E, Xu J. Light exposure mediates circadian rhythms of rhizosphere microbial communities. ISME J. 2021;15(9):2655‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steele TA, St Louis EK, Videnovic A, Auger RR. Circadian rhythm sleep‐wake disorders: a contemporary review of neurobiology, treatment, and dysregulation in neurodegenerative disease. Neurotherapeutics. 2021;18(1):53‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segers A, Depoortere I. Circadian clocks in the digestive system. Nat Rev Gastroenterol Hepatol. 2021;18(4):239‐251. [DOI] [PubMed] [Google Scholar]
- 4. Koronowski KB, Sassone‐Corsi P. Communicating clocks shape circadian homeostasis. Science. 2021;371(6530):eabd0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teichman EM, O'Riordan KJ, Gahan CGM, Dinan TG, Cryan JF. When rhythms meet the blues: circadian interactions with the microbiota‐gut‐brain axis. Cell Metab. 2020;31(3):448‐471. [DOI] [PubMed] [Google Scholar]
- 6. Ozturk N, Ozturk D, Kavakli IH, Okyar A. Molecular aspects of circadian pharmacology and relevance for cancer chronotherapy. Int J Mol Sci. 2017;18(10):2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Jiang W, Chen H, et al. Sympathetic nervous system mediates cardiac remodeling after myocardial infarction in a circadian disruption model. Front Cardiovasc Med. 2021;8:668387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenfield MD, Honing H, Kotz SA, Ravignani A. Synchrony and rhythm interaction: from the brain to behavioural ecology. Philos Trans R Soc Lond B Biol Sci. 2021;376(1835):20200324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bekala A, Plotek W, Siwicka‐Gieroba D, et al. Melatonin and the brain‐heart crosstalk in neurocritically ill patients‐from molecular action to clinical practice. Int J Mol Sci. 2022;23(13):7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen M, Lin Y, Dang Y, et al. Reprogramming of rhythmic liver metabolism by intestinal clock. J Hepatol. 2023;79(3):741‐757. [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Zhang C, Cao Y, Li J, Bi F. Major roles of the circadian clock in cancer. Cancer Biol Med. 2023;20(1):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boone DR, Sell SL, Micci MA, et al. Traumatic brain injury‐induced dysregulation of the circadian clock. PLoS One. 2012;7(10):e46204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tischkau SA. Mechanisms of circadian clock interactions with aryl hydrocarbon receptor signalling. Eur J Neurosci. 2020;51(1):379‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kavakli IH, Ozturk N, Baris I. Protein interaction networks of the mammalian core clock proteins. Adv Protein Chem Struct Biol. 2022;131:207‐233. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Lin Y, Gao L, et al. PPAR‐gamma integrates obesity and adipocyte clock through epigenetic regulation of Bmal1 . Theranostics. 2022;12(4):1589‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yi JS, Diaz NM, D'Souza S, Buhr ED. The molecular clockwork of mammalian cells. Semin Cell Dev Biol. 2022;126:87‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang S, Li F, Lin Y, Wu B. Targeting REV‐ERBalpha for therapeutic purposes: promises and challenges. Theranostics. 2020;10(9):4168‐4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong D, Yang D, Lin L, Wang S, Wu B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol. 2020;178:114045. [DOI] [PubMed] [Google Scholar]
- 19. Bolsius YG, Zurbriggen MD, Kim JK, et al. The role of clock genes in sleep, stress and memory. Biochem Pharmacol. 2021;191:114493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farshadi E, van der Horst GTJ, Chaves I. Molecular links between the circadian clock and the cell cycle. J Mol Biol. 2020;432(12):3515‐3524. [DOI] [PubMed] [Google Scholar]
- 21. Shirato K, Sato S. Macrophage meets the circadian clock: implication of the circadian clock in the role of macrophages in acute lower respiratory tract infection. Front Cell Infect Microbiol. 2022;12:826738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dierickx P, Zhu K, Carpenter BJ, et al. Circadian REV‐ERBs repress E4bp4 to activate NAMPT‐dependent NAD(+) biosynthesis and sustain cardiac function. Nat Cardiovasc Res. 2022;1(1):45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Q, Colonna M. Keeping time in group 3 innate lymphoid cells. Nat Rev Immunol. 2020;20(12):720‐726. [DOI] [PubMed] [Google Scholar]
- 24. Nakao A. Circadian regulation of the biology of allergic disease: clock disruption can promote allergy. Front Immunol. 2020;11:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giebfried J, Lorentz A. Relationship between the biological clock and inflammatory bowel disease. Clocks Sleep. 2023;5(2):260‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nathan P, Gibbs JE, Rainger GE, Chimen M. Changes in circadian rhythms dysregulate inflammation in ageing: focus on leukocyte trafficking. Front Immunol. 2021;12:673405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Timmons GA, O'Siorain JR, Kennedy OD, Curtis AM, Early JO. Innate rhythms: clocks at the center of monocyte and macrophage function. Front Immunol. 2020;11:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Q, Li L, Li C, et al. Circadian protein CLOCK modulates regulatory B cell functions of nurses engaging day‐night shift rotation. Cell Signal. 2022;96:110362. [DOI] [PubMed] [Google Scholar]
- 29. Cermakian N, Labrecque N. Regulation of cytotoxic CD8+ T cells by the circadian clock. J Immunol. 2023;210(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 30. Habbel J, Arnold L, Chen Y, et al. Inflammation‐driven activation of JAK/STAT signaling reversibly accelerates acute myeloid leukemia in vitro. Blood Adv. 2020;4(13):3000‐3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salas A, Hernandez‐Rocha C, Duijvestein M, et al. JAK‐STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(6):323‐337. [DOI] [PubMed] [Google Scholar]
- 32. Sabaawy HE, Ryan BM, Khiabanian H, Pine SR. JAK/STAT of all trades: linking inflammation with cancer development, tumor progression and therapy resistance. Carcinogenesis. 2021;42(12):1411‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong Q, Jie Y, Ma J, Li C, Xin T, Yang D. Renal tubular cell death and inflammation response are regulated by the MAPK‐ERK‐CREB signaling pathway under hypoxia‐reoxygenation injury. J Recept Signal Transduct Res. 2019;39(5‐6):383‐391. [DOI] [PubMed] [Google Scholar]
- 34. Behl T, Upadhyay T, Singh S, et al. Polyphenols targeting MAPK mediated oxidative stress and inflammation in rheumatoid arthritis. Molecules. 2021;26(21):6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnabei L, Laplantine E, Mbongo W, Rieux‐Laucat F, Weil R. NF‐kappaB: at the borders of autoimmunity and inflammation. Front Immunol. 2021;12:716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF‐kappaB signaling in inflammation and cancer. MedComm. 2021;2(4):618‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kunnumakkara AB, Shabnam B, Girisa S, et al. Inflammation, NF‐kappaB, and chronic diseases: how are they linked? Crit Rev Immunol. 2020;40(1):1‐39. [DOI] [PubMed] [Google Scholar]
- 38. Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The nuclear factor kappa B (NF‐kB) signaling in cancer development and immune diseases. Genes Dis. 2021;8(3):287‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma H, Zhou M, Duan W, Chen L, Wang L, Liu P. Anemoside B4 prevents acute ulcerative colitis through inhibiting of TLR4/NF‐kappaB/MAPK signaling pathway. Int Immunopharmacol. 2020;87:106794. [DOI] [PubMed] [Google Scholar]
- 40. De Maeyer RPH, van de Merwe RC, Louie R, et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol. 2020;21(6):615‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, Hu Y, Song B, Xiong Y, Wang J, Chen D. Targeting JAK/STAT signaling pathways in treatment of inflammatory bowel disease. Inflamm Res. 2021;70(7):753‐764. [DOI] [PubMed] [Google Scholar]
- 42. Haftcheshmeh SM, Abedi M, Mashayekhi K, et al. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: focus on NF‐kappaB, JAK/STAT, and MAPK signaling pathways. Phytother Res. 2022;36(3):1216‐1230. [DOI] [PubMed] [Google Scholar]
- 43. Megha KB, Joseph X, Akhil V, Mohanan PV. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine. 2021;91:153712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yadav S, Dwivedi A, Tripathi A. Biology of macrophage fate decision: implication in inflammatory disorders. Cell Biol Int. 2022;46(10):1539‐1556. [DOI] [PubMed] [Google Scholar]
- 45. Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662‐680. [DOI] [PubMed] [Google Scholar]
- 46. Hiam‐Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994‐3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yue T, Shi Y, Luo S, Weng J, Wu Y, Zheng X. The role of inflammation in immune system of diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Front Immunol. 2022;13:1055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Golden TN, Simmons RA. Immune dysfunction in developmental programming of type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(4):235‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang C, Wang Y, Chen D, et al. Natural polysaccharides protect against diet‐induced obesity by improving lipid metabolism and regulating the immune system. Food Res Int. 2023;172:113192. [DOI] [PubMed] [Google Scholar]
- 51. Riedel S, Pheiffer C, Johnson R, Louw J, Muller CJF. Intestinal barrier function and immune homeostasis are missing links in obesity and type 2 diabetes development. Front Endocrinol (Lausanne). 2021;12:833544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Larabee CM, Neely OC, Domingos AI. Obesity: a neuroimmunometabolic perspective. Nat Rev Endocrinol. 2020;16(1):30‐43. [DOI] [PubMed] [Google Scholar]
- 53. Li M, Chen Z, Jiang T, et al. Circadian rhythm‐associated clinical relevance and Tumor Microenvironment of Non‐small Cell Lung Cancer. J Cancer. 2021;12(9):2582‐2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramos CA, Ouyang C, Qi Y, et al. A Non‐canonical function of BMAL1 metabolically limits obesity‐promoted triple‐negative breast cancer. iScience. 2020;23(2):100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou L, Luo Z, Li Z, Huang Q. Circadian clock is associated with tumor microenvironment in kidney renal clear cell carcinoma. Aging. 2020;12(14):14620‐14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shivshankar P, Fekry B, Eckel‐Mahan K, Wetsel RA. Circadian clock and complement immune system‐complementary control of physiology and pathology? Front Cell Infect Microbiol. 2020;10:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hand LE, Gray KJ, Dickson SH, et al. Regulatory T cells confer a circadian signature on inflammatory arthritis. Nat Commun. 2020;11(1):1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Waddell H, Stevenson TJ, Mole DJ. The role of the circadian rhythms in critical illness with a focus on acute pancreatitis. Heliyon. 2023;9(4):e15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carvalho Cabral P, Tekade K, Stegeman SK, Olivier M, Cermakian N. The involvement of host circadian clocks in the regulation of the immune response to parasitic infections in mammals. Parasite Immunol. 2022;44(3):e12903. [DOI] [PubMed] [Google Scholar]
- 60. Felten M, Ferencik S, Teixeira Alves LG, et al. Ventilator‐induced lung injury is modulated by the circadian clock. Am J Respir Crit Care Med. 2023;207(11):1464‐1474. [DOI] [PubMed] [Google Scholar]
- 61. Haspel JA, Anafi R, Brown MK, et al. Perfect timing: circadian rhythms, sleep, and immunity—an NIH workshop summary. JCI Insight. 2020;5(1):e131487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barik S. Molecular interactions between pathogens and the circadian clock. Int J Mol Sci. 2019;20(23):5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mazzoccoli G, Vinciguerra M, Carbone A, Relogio A. The circadian clock, the immune system, and viral infections: the intricate relationship between biological time and host‐virus interaction. Pathogens. 2020;9(2):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boivin DB, Boudreau P, Kosmadopoulos A. Disturbance of the circadian system in shift work and its health impact. J Biol Rhythms. 2022;37(1):3‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colombini B, Dinu M, Murgo E, et al. Ageing and low‐level chronic inflammation: the role of the biological clock. Antioxidants. 2022;11(11):2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kitchen GB, Cunningham PS, Poolman TM, et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc Natl Acad Sci U S A. 2020;117(3):1543‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Keller M, Mazuch J, Abraham U, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106(50):21407‐21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Annamneedi VP, Park JW, Lee GS, Kang TJ. Cell autonomous circadian systems and their relation to inflammation. Biomol Ther (Seoul). 2021;29(1):31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Collins EJ, Cervantes‐Silva MP, Timmons GA, O'Siorain JR, Curtis AM, Hurley JM. Post‐transcriptional circadian regulation in macrophages organizes temporally distinct immunometabolic states. Genome Res. 2021;31(2):171‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Honzlova P, Sumova A. Metabolic regulation of the circadian clock in classically and alternatively activated macrophages. Immunol Cell Biol. 2023;101(5):428‐443. [DOI] [PubMed] [Google Scholar]
- 71. Guzman‐Ruiz MA, Guerrero‐Vargas NN, Lagunes‐Cruz A, et al. Circadian modulation of microglial physiological processes and immune responses. Glia. 2023;71(2):155‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aiello I, Fedele MLM, Roman F, et al. Circadian disruption promotes tumor‐immune microenvironment remodeling favoring tumor cell proliferation. Sci Adv. 2020;6(42):eaaz4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vieira E, Mirizio GG, Barin GR, de Andrade RV, Nimer NFS, La Sala L. Clock genes, inflammation and the immune system‐implications for diabetes, obesity and neurodegenerative diseases. Int J Mol Sci. 2020;21(24):9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang XL, Wolff SEC, Korpel N, et al. Deficiency of the circadian clock gene bmal1 reduces microglial immunometabolism. Front Immunol. 2020;11:586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen P, Hsu WH, Chang A, et al. Circadian regulator CLOCK recruits immune‐suppressive microglia into the GBM tumor microenvironment. Cancer Discov. 2020;10(3):371‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jang JH, Shin HW, Lee JM, Lee HW, Kim EC, Park SH. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediators Inflamm. 2015;2015:794143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li B, Li D, Ni H, et al. The circadian clock regulator Bmal1 affects traumatic brain injury in rats through the p38 MAPK signalling pathway. Brain Res Bull. 2022;178:17‐28. [DOI] [PubMed] [Google Scholar]
- 79. Lee JH, Sancar A. Regulation of apoptosis by the circadian clock through NF‐kappaB signaling. Proc Natl Acad Sci U S A. 2011;108(29):12036‐12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee J, Sul HJ, Choi H, Oh DH, Shong M. Loss of thyroid gland circadian PER2 rhythmicity in aged mice and its potential association with thyroid cancer development. Cell Death Dis. 2022;13(10):898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pick R, He W, Chen CS, Scheiermann C. Time‐of‐Day‐Dependent trafficking and function of leukocyte subsets. Trends Immunol. 2019;40(6):524‐537. [DOI] [PubMed] [Google Scholar]
- 82. Ella K, Csepanyi‐Komi R, Kaldi K. Circadian regulation of human peripheral neutrophils. Brain Behav Immun. 2016;57:209‐221. [DOI] [PubMed] [Google Scholar]
- 83. Casanova‐Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malengier‐Devlies B, Metzemaekers M, Wouters C, Proost P, Matthys P. Neutrophil homeostasis and emergency granulopoiesis: the example of systemic juvenile idiopathic arthritis. Front Immunol. 2021;12:766620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wyse C, O'Malley G, Coogan AN, McConkey S, Smith DJ. Seasonal and daytime variation in multiple immune parameters in humans: evidence from 329,261 participants of the UK Biobank cohort. iScience. 2021;24(4):102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aroca‐Crevillen A, Adrover JM, Hidalgo A. Circadian features of neutrophil biology. Front Immunol. 2020;11:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nuri Pamuk O, Hasni S, et al. Morning stiffness and neutrophil circadian disarming: comment on the article by Orange et al. Arthritis Rheumatol. 2021;73(2):356‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ovadia S, Ozcan A, Hidalgo A. The circadian neutrophil, inside‐out. J Leukoc Biol. 2023;113(6):555‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jerigova V, Zeman M, Okuliarova M. Circadian disruption and consequences on innate immunity and inflammatory response. Int J Mol Sci. 2022;23(22):13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Adrover JM, Del Fresno C, Crainiciuc G, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;51(5):966‐967. [DOI] [PubMed] [Google Scholar]
- 91. Martin C, Burdon PC, Bridger G, Gutierrez‐Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583‐593. [DOI] [PubMed] [Google Scholar]
- 92. De Filippo K, Rankin SM. CXCR4, the master regulator of neutrophil trafficking in homeostasis and disease. Eur J Clin Invest. 2018;48(2):e12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. He W, Holtkamp S, Hergenhan SM, et al. Circadian expression of migratory factors establishes lineage‐specific signatures that guide the homing of leukocyte subsets to tissues. Immunity. 2018;49(6):1175‐1190. e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll‐like receptor 9‐mediated innate and adaptive immunity. Immunity. 2012;36(2):251‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Amir M, Campbell S, Kamenecka TM, Solt LA. Pharmacological modulation and genetic deletion of REV‐ERBalpha and REV‐ERBbeta regulates dendritic cell development. Biochem Biophys Res Commun. 2020;527(4):1000‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cervantes‐Silva MP, Carroll RG, Wilk MM, et al. The circadian clock influences T cell responses to vaccination by regulating dendritic cell antigen processing. Nat Commun. 2022;13(1):7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Holtkamp SJ, Ince LM, Barnoud C, et al. Circadian clocks guide dendritic cells into skin lymphatics. Nat Immunol. 2021;22(11):1375‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bollinger T, Leutz A, Leliavski A, et al. Circadian clocks in mouse and human CD4+ T cells. PLoS One. 2011;6(12):e29801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bollinger T, Bollinger A, Skrum L, Dimitrov S, Lange T, Solbach W. Sleep‐dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155(2):231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Druzd D, Matveeva O, Ince L, et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity. 2017;46(1):120‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yu X, Rollins D, Ruhn KA, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158(9):4454‐4464. [PubMed] [Google Scholar]
- 103. Abo T, Kawate T, Itoh K, Kumagai K. Studies on the bioperiodicity of the immune response. I. Circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J Immunol. 1981;126(4):1360‐1363. [PubMed] [Google Scholar]
- 104. Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav Immun. 2012;26(3):407‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cao Q, Zhao X, Bai J, et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A. 2017;114(47):12548‐12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Aziz IS, McMahon AM, Friedman D, Rabinovich‐Nikitin I, Kirshenbaum LA, Martino TA. Circadian influence on inflammatory response during cardiovascular disease. Curr Opin Pharmacol. 2021;57:60‐70. [DOI] [PubMed] [Google Scholar]
- 107. Sun Y, Yang Z, Niu Z, et al. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology. 2006;119(4):451‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Austin Taylor M, Bennett M, Kumar V, Schatzle JD. Functional defects of NK cells treated with chloroquine mimic the lytic defects observed in perforin‐deficient mice. J Immunol. 2000;165(9):5048‐5053. [DOI] [PubMed] [Google Scholar]
- 109. Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN‐gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol. 2004;172(5):2811‐2817. [DOI] [PubMed] [Google Scholar]
- 110. Bourin P, Mansour I, Doinel C, Roue R, Rouger P, Levi F. Circadian rhythms of circulating NK cells in healthy and human immunodeficiency virus‐infected men. Chronobiol Int. 1993;10(4):298‐305. [DOI] [PubMed] [Google Scholar]
- 111. Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20(5):469‐476. [DOI] [PubMed] [Google Scholar]
- 112. Logan RW, Wynne O, Levitt D, Price D, Sarkar DK. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1(‐/‐) mutant mice. J Interferon Cytokine Res. 2013;33(3):108‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zeng X, Liang C, Yao J. Chronic shift‐lag promotes NK cell ageing and impairs immunosurveillance in mice by decreasing the expression of CD122. J Cell Mol Med. 2020;24(24):14583‐14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Logan RW, Zhang C, Murugan S, et al. Chronic shift‐lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012;188(6):2583‐2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Teng F, Goc J, Zhou L, et al. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci Immunol. 2019;4(40):eaax1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang Q, Robinette ML, Billon C, et al. Circadian rhythm‐dependent and circadian rhythm‐independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci Immunol. 2019;4(40):eaay7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gong J, Tu W, Liu J, Tian D. Hepatocytes: a key role in liver inflammation. Front Immunol. 2022;13:1083780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Desruisseaux MS, Nagajyothi TrujilloME, Tanowitz HB, Scherer PE. Adipocyte, adipose tissue, and infectious disease. Infect Immun. 2007;75(3):1066‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Harvey I, Boudreau A, Stephens JM. Adipose tissue in health and disease. Open Biol. 2020;10(12):200291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Andrews C, McLean MH, Durum SK. Cytokine tuning of intestinal epithelial function. Front Immunol. 2018;9:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Guan D, Xiong Y, Trinh TM, et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science. 2020;369(6509):1388‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhao M, Zhao H, Deng J, Guo L, Wu B. Role of the CLOCK protein in liver detoxification. Br J Pharmacol. 2019;176(24):4639‐4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Guan D, Bae H, Zhou D, et al. Hepatocyte SREBP signaling mediates clock communication within the liver. J Clin Invest. 2023;133(8):e163018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhou Y, Dong B, Kim KH, et al. Vitamin D receptor activation in liver macrophages protects against hepatic endoplasmic reticulum stress in mice. Hepatology. 2020;71(4):1453‐1466. [DOI] [PubMed] [Google Scholar]
- 126. Dong B, Zhou Y, Wang W, et al. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice. Hepatology. 2020;71(5):1559‐1574. [DOI] [PubMed] [Google Scholar]
- 127. Heyde I, Begemann K, Oster H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology. 2021;162(3):bqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shostak A, Meyer‐Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62(7):2195‐2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hunter AL, Pelekanou CE, Barron NJ, et al. Adipocyte NR1D1 dictates adipose tissue expansion during obesity. Elife. 2021;10:e63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Xiong X, Li W, Liu R, Saha P, Yechoor V, Ma K. Circadian clock control of MRTF/SRF pathway suppresses beige adipocyte thermogenic recruitment. J Mol Cell Biol. 2023;14(12):mjac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Subramanian S, Geng H, Tan XD. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao. 2020;72(3):308‐324. [PMC free article] [PubMed] [Google Scholar]
- 132. Parasram K, Karpowicz P. Time after time: circadian clock regulation of intestinal stem cells. Cell Mol Life Sci. 2020;77(7):1267‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tian Y, Zhang D. Biological clock and inflammatory bowel disease review: from the standpoint of the intestinal barrier. Gastroenterol Res Pract. 2022;2022:2939921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stokes K, Cooke A, Chang H, Weaver DR, Breault DT, Karpowicz P. The circadian clock gene BMAL1 coordinates intestinal regeneration. Cell Mol Gastroenterol Hepatol. 2017;4(1):95‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pacha J, Sumova A. Circadian regulation of epithelial functions in the intestine. Acta Physiol. 2013;208(1):11‐24. [DOI] [PubMed] [Google Scholar]
- 136. Parasram K, Bernardon N, Hammoud M, et al. Intestinal stem cells exhibit conditional circadian clock function. Stem Cell Reports. 2018;11(5):1287‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yu F, Wang Z, Zhang T, et al. Deficiency of intestinal Bmal1 prevents obesity induced by high‐fat feeding. Nat Commun. 2021;12(1):5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yu F, Zhang T, Zhou C, et al. The circadian clock gene Bmal1 controls intestinal exporter MRP2 and drug disposition. Theranostics. 2019;9(10):2754‐2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fagiani F, Di Marino D, Romagnoli A, et al. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct Target Ther. 2022;7(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rius‐Perez S, Perez S, Marti‐Andres P, Monsalve M, Sastre J. Nuclear factor kappa B signaling complexes in acute inflammation. Antioxid Redox Signal. 2020;33(3):145‐165. [DOI] [PubMed] [Google Scholar]
- 141. Wang S, Lin Y, Li F, et al. An NF‐kappaB‐driven lncRNA orchestrates colitis and circadian clock. Sci Adv. 2020;6(42):eabb5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fontaine C, Rigamonti E, Pourcet B, et al. The nuclear receptor Rev‐erbalpha is a liver X receptor (LXR) target gene driving a negative feedback loop on select LXR‐induced pathways in human macrophages. Mol Endocrinol. 2008;22(8):1797‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Oishi Y, Hayashi S, Isagawa T, et al. Bmal1 regulates inflammatory responses in macrophages by modulating enhancer RNA transcription. Sci Rep. 2017;7(1):7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang J, Li S, Li X, et al. Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up‐regulating matrix metalloproteinase9 expression. Cancer Cell Int. 2019;19:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Spengler ML, Kuropatwinski KK, Comas M, et al. Core circadian protein CLOCK is a positive regulator of NF‐kappaB‐mediated transcription. Proc Natl Acad Sci U S A. 2012;109(37):E2457‐E2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(31):12662‐12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen G, Zhao H, Ma S, et al. Circadian rhythm protein bmal1 modulates cartilage gene expression in temporomandibular joint osteoarthritis via the MAPK/ERK pathway. Front Pharmacol. 2020;11:527744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kim K, Kim JH, Kim I, Seong S, Kim N. Rev‐erbalpha negatively regulates osteoclast and osteoblast differentiation through p38 MAPK signaling pathway. Mol Cells. 2020;43(1):34‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim SM, Choi JE, Hur W, et al. RAR‐related orphan receptor gamma (ROR‐gamma) mediates epithelial‐mesenchymal transition of hepatocytes during hepatic fibrosis. J Cell Biochem. 2017;118(8):2026‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Goldsmith CS, Bell‐Pedersen D. Diverse roles for MAPK signaling in circadian clocks. Adv Genet. 2013;84:1‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang H, Fu YJBC. NR1D1 suppressed the growth of ovarian cancer by abrogating the JAK/STAT3 signaling pathway. BMC Cancer. 2021;21(1):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Luo W, Sehgal AJC. Regulation of circadian behavioral output via a microRNA‐JAK/STAT circuit. Cell. 2012;148(4):765‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mehta N, Cheng HYM. Micro‐managing the circadian clock: the role of microRNAs in biological timekeeping. J Mol Biol. 2013;425(19):3609‐3624. [DOI] [PubMed] [Google Scholar]
- 154. Xie Y, Tang Q, Chen G, et al. New insights into the circadian rhythm and its related diseases. Front Physiol. 2019;10:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lin Y, Lin L, Gao L, Wang S, Wu B. Rev‐erbalpha regulates hepatic ischemia‐reperfusion injury in mice. Biochem Biophys Res Commun. 2020;529(4):916‐921. [DOI] [PubMed] [Google Scholar]
- 156. Liu J, Malkani G, Shi X, et al. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide‐induced endotoxic shock. Infect Immun. 2006;74(8):4750‐4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Colquhoun C, Duncan M, Grant GJD. Inflammatory bowel diseases: host‐microbial‐environmental interactions in dysbiosis. Diseases. 2020;8(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhou Z, Lin Y, Gao L, Yang Z, Wang S, Wu B. Circadian pharmacological effects of berberine on chronic colitis in mice: role of the clock component Rev‐erbalpha. Biochem Pharmacol. 2020;172:113773. [DOI] [PubMed] [Google Scholar]
- 159. Sobolewska‐Wlodarczyk A, Wlodarczyk M, Szemraj J, Stec‐Michalska K, Fichna J, Wisniewska‐Jarosinska M. Circadian rhythm abnormalities—association with the course of inflammatory bowel disease. Pharmacol Rep. 2016;68(4):847‐851. [DOI] [PubMed] [Google Scholar]
- 160. Liu JL, Wang CY, Cheng TY, et al. Circadian clock disruption suppresses PDL1(+) intraepithelial B cells in experimental colitis and colitis‐associated colorectal cancer. Cell Mol Gastroenterol Hepatol. 2021;12(1):251‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jochum SB, Engen PA, Shaikh M, et al. Colonic epithelial circadian disruption worsens dextran sulfate sodium‐induced colitis. Inflamm Bowel Dis. 2023;29(3):444‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Krijbolder DI, Wouters F, van Mulligen E, van der Helm‐van Mil AHM. Morning stiffness precedes the development of rheumatoid arthritis and associates with systemic and subclinical joint inflammation in arthralgia patients. Rheumatology. 2022;61(5):2113‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Boeth H, Biesen R, Hollnagel J, et al. Quantification of morning stiffness to assess disease activity and treatment effects in rheumatoid arthritis. Rheumatology. 2021;60(11):5282‐5291. [DOI] [PubMed] [Google Scholar]
- 164. Orange DE, Blachere NE, DiCarlo EF, et al. Rheumatoid arthritis morning stiffness is associated with synovial fibrin and neutrophils. Arthritis Rheumatol. 2020;72(4):557‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wu X, Liu X, Jing Z, Chen Y, Liu H, Ma W. Moxibustion benignantly regulates circadian rhythm of REV‐ERBalpha in RA rats. Am J Transl Res. 2020;12(5):1459‐1468. [PMC free article] [PubMed] [Google Scholar]
- 166. Kikyo N. Circadian regulation of macrophages and osteoclasts in rheumatoid arthritis. Int J Mol Sci. 2023;24(15):12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hashiramoto A, Yamane T, Tsumiyama K, et al. Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF‐alpha. J Immunol. 2010;184(3):1560‐1565. [DOI] [PubMed] [Google Scholar]
- 168. Kulkarni A, Demory‐Beckler M, Kesselman MM. The role of clock genes in maintaining circadian rhythm and rheumatoid arthritis pathophysiology. Cureus. 2023;15(5):e39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rao RT, Pierre KK, Schlesinger N, Androulakis IP. The potential of circadian realignment in rheumatoid arthritis. Crit Rev Biomed Eng. 2016;44(3):177‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557‐1562. [DOI] [PubMed] [Google Scholar]
- 171. Mazzoccoli G, Miele L, Marrone G, Mazza T, Vinciguerra M, Grieco A. A role for the biological clock in liver cancer. Cancers. 2019;11(11):1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lawther AJ, Phillips AJK, Chung NC, et al. Disrupting circadian rhythms promotes cancer‐induced inflammation in mice. Brain Behav Immun Health. 2022;21:100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lin YM, Chang JH, Yeh KT, et al. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog. 2008;47(12):925‐933. [DOI] [PubMed] [Google Scholar]
- 174. Taniguchi H, Fernandez AF, Setien F, et al. Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69(21):8447‐8454. [DOI] [PubMed] [Google Scholar]
- 175. Fekry B, Ribas‐Latre A, Drunen RV, et al. Hepatic circadian and differentiation factors control liver susceptibility for fatty liver disease and tumorigenesis. FASEB J. 2022;36(9):e22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Battaglin F, Chan P, Pan Y, et al. Clocking cancer: the circadian clock as a target in cancer therapy. Oncogene. 2021;40(18):3187‐3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21(9):974‐982. [DOI] [PubMed] [Google Scholar]
- 178. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang C, Barnoud C, Cenerenti M, et al. Dendritic cells direct circadian anti‐tumour immune responses. Nature. 2023;614(7946):136‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sang N, Gao RC, Zhang MY, Wu ZZ, Wu ZG, Wu GC. Causal relationship between sleep traits and risk of systemic lupus erythematosus: a two‐sample mendelian randomization study. Front Immunol. 2022;13:918749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Awuah WA, Huang H, Kalmanovich J, et al. Circadian rhythm in systemic autoimmune conditions: potential of chrono‐immunology in clinical practice: a narrative review. Medicine. 2023;102(32):e34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Dan YL, Zhao CN, Mao YM, et al. Association of PER2 gene single nucleotide polymorphisms with genetic susceptibility to systemic lupus erythematosus. Lupus. 2021;30(5):734‐740. [DOI] [PubMed] [Google Scholar]
- 184. Young KA, Munroe ME, Harley JB, et al. Less than 7 hours of sleep per night is associated with transitioning to systemic lupus erythematosus. Lupus. 2018;27(9):1524‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Alexander RK, Liou YH, Knudsen NH, et al. Bmal1 integrates mitochondrial metabolism and macrophage activation. Elife. 2020;9:e54090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Early JO, Menon D, Wyse CA, et al. Circadian clock protein BMAL1 regulates IL‐1beta in macrophages via NRF2. Proc Natl Acad Sci U S A. 2018;115(36):E8460‐E8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wang Z, Zhao M, Yin J, et al. E4BP4‐mediated inhibition of T follicular helper cell differentiation is compromised in autoimmune diseases. J Clin Invest. 2020;130(7):3717‐3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341(6153):1483‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Paschos GK, Ibrahim S, Song WL, et al. Obesity in mice with adipocyte‐specific deletion of clock component Arntl . Nat Med. 2012;18(12):1768‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lin Y, Wang S, Gao L, et al. Oscillating lncRNA Platr4 regulates NLRP3 inflammasome to ameliorate nonalcoholic steatohepatitis in mice. Theranostics. 2021;11(1):426‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Maury E, Navez B, Brichard SM. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat Commun. 2021;12(1):2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Role of sleep deprivation in immune‐related disease risk and outcomes. Commun Biol. 2021;4(1):1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702‐715. [DOI] [PubMed] [Google Scholar]
- 194. Ragnoli B, Pochetti P, Pignatti P, et al. Sleep deprivation, immune suppression and SARS‐CoV‐2 infection. Int J Environ Res Public Health. 2022;19(2):904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Jiao L, Duan Z, Sangi‐Haghpeykar H, Hale L, White DL, El‐Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013;108(1):213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kakizaki M, Inoue K, Kuriyama S, et al. Sleep duration and the risk of prostate cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99(1):176‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hurley S, Goldberg D, Bernstein L, Reynolds P. Sleep duration and cancer risk in women. Cancer Causes Control. 2015;26(7):1037‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99(9):1502‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. De Lorenzo BHP, Novaes EBRR, Paslar Leal T, et al. Chronic sleep restriction impairs the antitumor immune response in mice. Neuroimmunomodulation. 2018;25(2):59‐67. [DOI] [PubMed] [Google Scholar]
- 200. Qin B, Deng Y. Overexpression of circadian clock protein cryptochrome (CRY) 1 alleviates sleep deprivation‐induced vascular inflammation in a mouse model. Immunol Lett. 2015;163(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 201. Shen Y, Endale M, Wang W, et al. NF‐kappaB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genet. 2021;17(11):e1009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request.


