Abstract
Background
The HER2DX risk-score has undergone rigorous validation in prior investigations involving patients with early-stage human epidermal growth factor receptor 2 (HER2)-positive (HER2+) breast cancer. In this study, we present the outcomes of the HER2DX risk-score within the most recent release of the Sweden Cancerome Analysis Network—Breast (SCAN-B) HER2+ cohort. This updated examination benefits from a larger patient sample, an extended follow-up duration, and detailed treatment information.
Materials and methods
Clinical and RNAseq data from the SCAN-B dataset were retrieved from Gene Expression Omnibus (GSE81538). Among the 6600 patients, 819 had HER2+ breast cancer, with 757 individuals with research-based HER2DX risk-scores and corresponding survival outcomes. The HER2DX risk-score was evaluated (i) as a continuous variable and (ii) using predefined cut-offs. The primary endpoint for this study was overall survival (OS). The Kaplan–Meier method and Cox models were used to estimate OS and a multistate model with four states was fitted to better characterize patients’ follow-up.
Results
The median follow-up time was 7.5 years (n = 757). The most common systemic therapy was chemotherapy with trastuzumab (82.0%) and most tumors were classified as T1-T2 (97.1%). The HER2DX risk-score as a continuous variable was significantly associated with OS after adjustment for clinical variables and treatment regimen [hazard ratios (HR) per 10-unit increment = 1.31, 95% confidence interval (CI) 1.13-1.51, P < 0.001] as well as within predefined risk groups (high versus low; HR = 2.57, 95% CI 1.36-4.85, P < 0.001). Patients classified as HER2DX high-risk also had higher risk of (i) breast cancer recurrence and (ii) death without previous recurrence. Within the subgroup of HER2+ T1N0 tumors (n = 297), those classified as high-risk demonstrated inferior OS compared to low-risk tumors (7-year OS 77.8% versus 96.8%, P < 0.001). The HER2DX mRNA ERBB2 score was associated with clinical HER2 status (area under the receiver operating characteristic curve = 0.91).
Conclusions
In patients with early-stage HER2+ breast cancer, HER2DX risk-score provides prognostic information beyond clinicopathological variables, including treatment regimen with or without trastuzumab.
Key words: HER2, breast cancer, trastuzumab, chemotherapy, HER2DX
Highlights
-
•
The HER2DX genomic test was validated in the SCAN-B cohort in 757 patients with early-stage HER2+ breast cancer.
-
•
The HER2DX risk-score was associated with efficacy outcomes after adjustment for clinical variables and treatment regimen.
-
•
Within the subgroup of HER2+ T1N0 tumors, those classified as HER2DX high-risk had inferior OS.
-
•
The HER2DX mRNA ERBB2 score was associated with clinical HER2 status.
Introduction
Human epidermal growth factor receptor 2-positive (HER2+) breast cancer constitutes ∼20% of all breast cancer cases and contributes significantly to mortality rates. In the context of early-stage disease, (neo)adjuvant chemotherapy alongside anti-HER2 trastuzumab has consistently shown substantial advancements in survival outcomes.1 Nonetheless, the diversity among patients in terms of clinical and biological aspects introduces variability in prognosis and treatment response.2,3
Various strategies have been developed to improve treatment for early-stage HER2+ breast cancer, with the goal of enhancing survival and quality of life. These include adding more effective therapies and extending duration of HER2-directed therapies4, 5, 6, 7 by using drugs like pertuzumab or neratinib to boost HER2 pathway inhibition,8,9 and switching to trastuzumab emtansine (T-DM1) for patients who do not achieve a pathological complete response (pCR) after initial neoadjuvant therapy.10 However, while chemotherapy with trastuzumab alone effectively treats most patients, concerns about excessive treatment have led to a need for more tailored approaches. As a result, the traditional ‘one-size-fits-all’ treatment approach for early-stage HER2+ breast cancer is becoming outdated.
Beyond tumor size, other factors strongly influence how early-stage HER2+ breast cancer behaves and responds to treatment. These factors include hormone receptor status, breast cancer intrinsic subtypes,11,12 and the presence of stromal tumor-infiltrating lymphocytes.13,14 These aspects relate closely to treatment results and survival. However, current treatment choices mainly rely on traditional measures like tumor size, lymph node involvement, hormone receptor levels, and tumor response to preoperative therapy. Creating a tool that combines clinical and biological factors provides more useful insights than considering them separately.
In this direction, the HER2DX genomic test was created in 2022 based on the expression of 27 genes and clinical attributes.15, 16, 17, 18, 19, 20, 21 The test delivers two scores that predict both long-term prognosis (i.e. risk-score) and the likelihood of pCR in early HER2+ breast cancer following trastuzumab-based neoadjuvant therapy. Merging biological data related to immune response, cellular differentiation, tumor growth, and HER2 gene expression with clinical factors like tumor size and nodal status, the HER2DX assay has demonstrated its potential to enhance treatment decision making.15,16,19,21
The Sweden Cancerome Analysis Network—Breast (SCAN-B) is a collaborative and multidisciplinary initiative aimed at addressing clinical and practical challenges in breast cancer diagnosis and treatment.22, 23, 24, 25 The performance of the HER2DX risk-score was initially investigated in the initial release of the SCAN-B cohort, comprising 378 patients diagnosed with early-stage HER2+ breast cancer.15 However, this analysis solely considered overall survival (OS) data as a survival outcome, with no inclusion of coded treatment regimens. Here, we embark on an extensive evaluation of the HER2DX risk-score’s effectiveness and clinical utility using a broader scope, involving 757 patients from the updated SCAN-B cohort and including treatment information.
Materials and methods
SCAN-B patient dataset
The SCAN-B was launched in 2010 and has enrolled over 6000 patients, collecting tumor and blood samples at a consistent pace. The initiative operates across multiple centers covering ∼85% of breast cancer cases in southern Sweden. For this study, the clinical and RNAseq data from SCAN-B was obtained from Gene Expression Omnibus (GSE81538).22, 23, 24, 25 Among 6600 patients, 819 (12.4%) had HER2+ breast cancer and 757 (92.4%) had research-based HER2DX risk-score and survival outcomes available. The complete dataset used in this study can be found in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.102388.
HER2DX risk-score
The HER2DX assay is based on four different gene signatures comprising 27 genes, including: the 14-gene immunoglobulin (IGG) module, the 4-gene tumor cell proliferation signature, the 5-gene luminal differentiation signature, and the 4-gene HER2 amplicon signature.15 To implement HER2DX, the standardized assay was applied to RNAseq data, following the previously outlined procedure.15 Notably, the HER2DX assay is tailored for the nCounter platform, necessitating appropriate normalization techniques when transferring it to other gene expression platforms. To assess the compatibility between nCounter and RNAseq, our earlier analysis involving 30 paired HER2DX tumor samples revealed a mean correlation coefficient of 0.89 (with a standard deviation of 0.16) across all 27 genes.26 The concordance rate between these two genomic platforms for the HER2DX risk-score reached 96.7%. We assessed the HER2DX risk-score through two approaches: (i) as a continuous variable (from 0 up to 100) and (ii) as a risk group categorized into low and high using an established cut-off (high > 50).15
Endpoints
The primary endpoint for this study was OS defined as the time from breast cancer diagnosis until death from any cause.24,27 Secondary endpoint was (i) recurrence-free interval (RFI) and (ii) death with and without a previous breast cancer recurrence. In this study RFI was defined as the time from breast cancer diagnosis until any recurrence (local, regional, or distant), with death being a censoring event. The comprehensive definition of all collected endpoints in the SCAN-B cohort was reported elsewehere.23
Statistical analysis
A descriptive analysis was carried out to summarize the study population. OS was estimated using the Kaplan–Meier method and multivariable Cox models were used to evaluate the association between baseline variables and OS in terms of hazard ratios (HRs) with 95% confidence interval (CI). Cumulative incidence functions were computed for RFI in a competing risk setting. Fine and Gray competing risk regression models were used to obtain a sub-distribution HR with 95% CI. To better characterize the temporal evolution of breast cancer status in a single model, a Markov multistate model28,29 based on four states (alive without recurrence, recurrence, death without recurrence, and death after recurrence) and three possible transitions was defined (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102388). Stratified Cox proportional hazard models were fitted for each transition to estimate the difference between HER2DX risk groups.30 As the percentage of missing data did not exceed 5% in any variable, multiple imputation of random missing values was carried out via the mice R package (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102388). The median follow-up was calculated using the reverse Kaplan–Meier method. A significance level of 0.05 was set for a two-sided test and all analyses were undertaken using the R statistical software version 4.1.2.
Results
Participant characteristics
The analysis of the HER2+ cohort encompassed 757 patients with survival outcome, with a median follow-up period of 7.5 years. Briefly, the mean age was 65.0 years, the representation of clinical T1 disease was 58.3%, N0 (56.0%), hormone receptor positivity (71.5%), and Nottingham histological grade 3 (70.1%). Most of the patients received chemotherapy with trastuzumab (82.0%). Of note, 42 (5.5%) patients did not receive any adjuvant treatment (Table 1). No clear associations between the type of systemic therapy administered and baseline clinicopathological variables were identified (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.102388).
Table 1.
Baseline characteristics
| Overall cohort (n = 757) | T1-N0 tumors (n = 297) | |
|---|---|---|
| Age, years, median (IQR) | 65 (50-75) | 60 (50-70) |
| Age, years, n (%) | ||
| <65 | 468 (61.8) | 198 (66.7) |
| >65 | 289 (38.2) | 99 (33.3) |
| Clinical tumor stage, n (%) | ||
| T1 | 441 (58.3) | 297 (100) |
| T2 | 294 (38.8) | 0 (0) |
| T3-4 | 22 (2.9) | 0 (0) |
| Clinical nodal stage, n (%) | ||
| N0 | 424 (56.0) | 297 (100) |
| N1 | 233 (30.8) | 0 (0) |
| N2 | 100 (13.2) | 0 (0) |
| Nottingham histological grade, n (%) | ||
| 1-2 | 226 (29.9) | 117 (39.4) |
| 3 | 531 (70.1) | 180 (60.6) |
| Hormone receptor status, n (%) | ||
| Negative | 216 (28.5) | 73 (24.6) |
| Positive | 541 (71.5) | 224 (75.4) |
| Treatment, n (%) | ||
| None | 42 (5.5) | 18 (6.1) |
| CT and/or ET | 94 (12.4) | 26 (8.8) |
| Trastuzumab + CT | 181 (23.9) | 68 (22.9) |
| Trastuzumab + CT + ET | 440 (58.1) | 185 (62.3) |
| HER2DX-risk group, n (%) | ||
| Low-risk | 251 (33.2) | 209 (70.4) |
| High-risk | 506 (66.8) | 88 (29.6) |
CT, chemotherapy; ET, endocrine therapy; IQR, interquartile range.
HER2DX risk-score findings
The HER2DX risk-score classified 33.2% of patients (n = 251) into the HER2DX low-risk category. Analyzing the clinicopathological variables based on the HER2DX risk-score, we observed distinct representations in two cases: T1 versus T2-4 in HER2DX low-risk (47.4% versus 13.3%), and N0 versus N1-2 in HER2DX low-risk (59.2% versus 0%). Nevertheless, the distribution of HER2DX risk groups was more homogeneous regarding age, hormone receptor status, histological grade, and treatment regimen (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102388).
HER2DX risk-score and OS
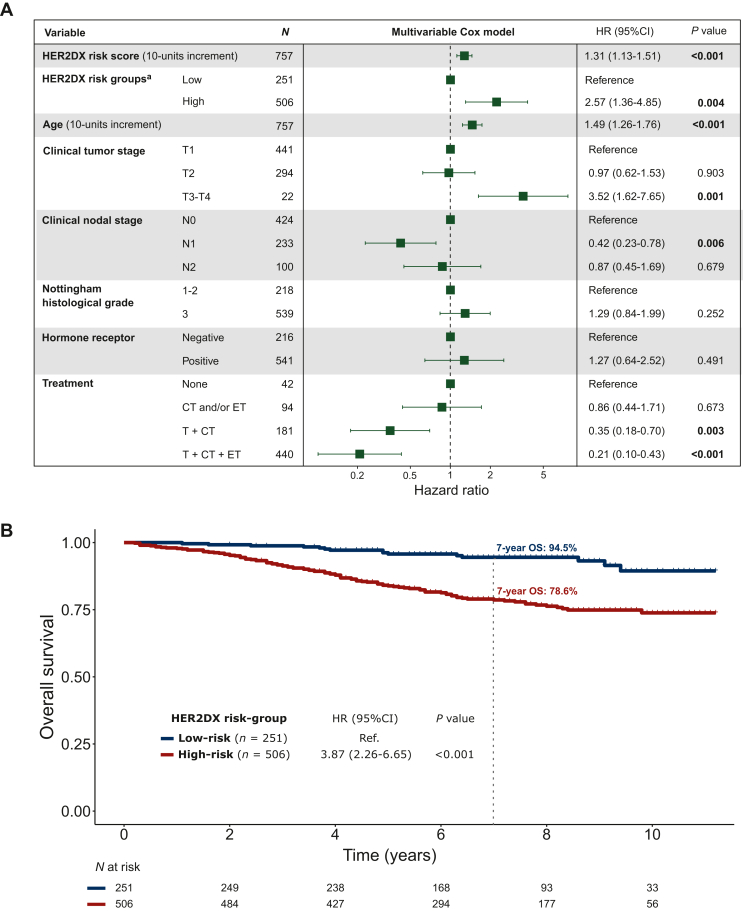
HER2DX risk-score as a continuous variable was statistically associated with OS after adjustment by clinical variables and treatment regimen (HR per 10-unit increment = 1.31, 95% CI 1.13-1.51, P < 0.001). However, no association was observed between hormone receptor status and OS in the multivariable model (Figure 1A). The predefined HER2DX risk groups (high versus low) also identified patients with different OS outcomes. The HER2DX low-risk group displayed a 7-year OS rate of 94.5%, compared to 78.6% for the HER2DX high-risk group (HR = 3.87, 95% CI 2.26-6.65, P < 0.001) (Figure 1B). Consistent results were observed in the RFI analysis (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.102388).
Figure 1.
Association of HER2DX risk-score with overall survival in the HER2-positive cohort of 757 patients. (A) Multivariable Cox model to evaluate OS (n = 757). (B) Kaplan–Meier curves by HER2DX risk group.
CI, confidence interval; CT, chemotherapy; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; T, Trastuzumab.
aA separate multivariable model was estimated using HER2DX risk groups instead of HER2DX risk-score. To avoid multicollinearity, HER2DX risk groups and HER2DX risk-score cannot be included in the same model.
Comprehensive understanding of the breast cancer course
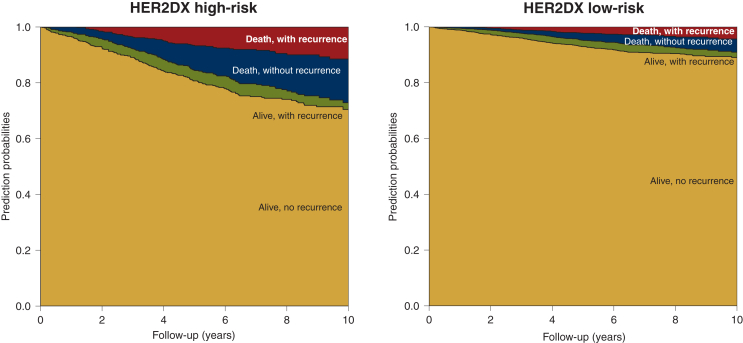
To characterize the temporal evolution of breast cancer status, a multistate model was fitted stratifying by the HER2DX risk group. Patients classified as HER2DX high-risk had higher risk of breast cancer recurrence during follow-up (HR = 2.44, 95% CI 1.37-4.36, P < 0.001), with an elevated posterior probability of experiencing mortality due to breast cancer (the probability at 7 years was 8.1% and 2.8% in the HER2DX high- and low-risk groups, respectively). The risk of death without a previous recurrence was also higher in HER2DX high-risk patients (HR = 3.74, 95% CI 1.86-7.53, P < 0.001) with a 7-year probability of 12.3% compared with 3.6% in the HER2DX low-risk group. Overall, the probability of being alive without recurrence at 7 years was 70.1% and 90.8% in the HER2DX high- and low-risk groups, respectively (Figure 2 and Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.102388).
Figure 2.
Multistate model showing the prediction probabilities of being in each state by the HER2DX risk group during a follow-up of 10 years.
HER2DX risk-score in stage I disease
A total of 297 patients with HER2+ stage I disease (i.e. T1N0) were identified. Among these, the majority received chemotherapy with trastuzumab (85.2%). The majority of tumors were hormone receptor positive (75.4%), and Nottingham histological grade 3 (60.6%). Of note, 18 (6.1%) patients did not receive any adjuvant treatment (Table 1).
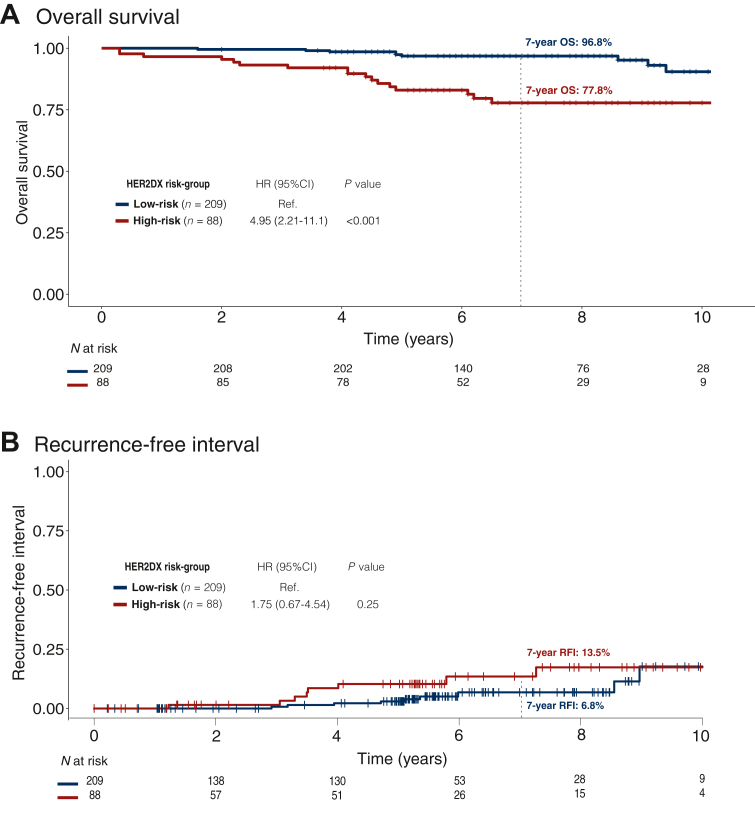
In the subgroup of patients with stage I disease, the HER2DX risk-score was associated with OS and RFI after adjusting for clinical variables and treatment regimen (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.102388). Additionally, the risk groups were also significantly associated with OS (Figure 3). Specifically, the HER2DX low-risk group displayed a 7-year OS rate of 96.8%, compared to 77.8% for the HER2DX high-risk group (HR = 4.95, 95% CI 2.21-11.1, P < 0.001). The findings were observed independently of the hormone receptor status (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.102388).
Figure 3.
Association of HER2DX risk-score with survival outcomes in patients with HER2+ stage 1 disease (n = 297). (A) OS Kaplan–Meier curves by the HER2DX risk group. (B) Cumulative incidence function of RFI by the HER2DX risk group.
CI, confidence interval; HR, hazard ratio; OS, overall survival; RFI, recurrence-free interval.
HER2DX ERBB2 mRNA score and clinical HER2 status
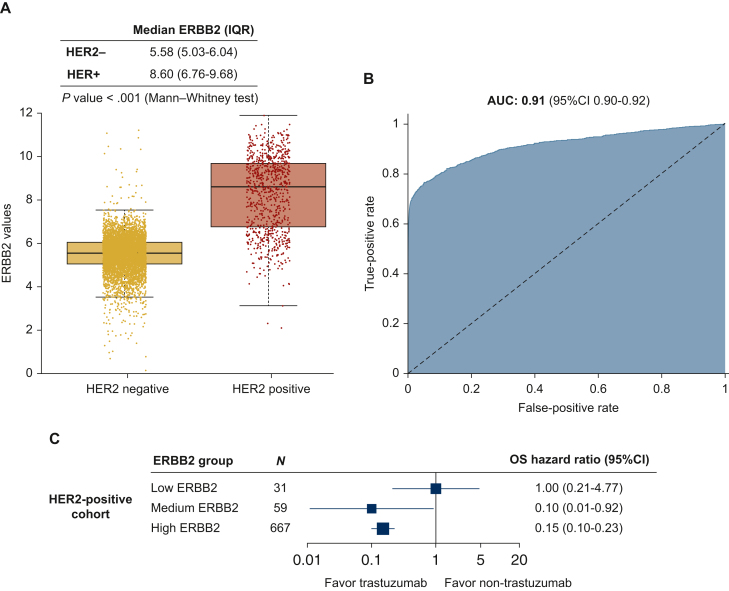
ERBB2 mRNA expression can help identify clinical HER2 status,15 according to the American Society of Clinical Oncology/College of American Pathologists guidelines.31 The HER2DX mRNA ERBB2 score to predict HER2 status was tested in a SCAN-B-independent dataset of 6505 cases with HER2 information (5686 HER2-negative and 819 HER2+). The area under the receiver operating characteristic curve of ERBB2 expression to predict clinical HER2 status was 0.91 (95% CI 0.90-0.92). Few HER2+ cases were identified as ERBB2-low (4.4%, 36/819) (Figure 4A and B).
Figure 4.
HER2DX ERBB2 mRNA score to predict clinical HER2 status and to identify benefit from trastuzumab. (A) Boxplot with HER2DX ERBB2 mRNA score values by HER2 positive and negative patients. (B) Area under the ROC curve with 95% confidence interval of HER2DX ERBB2 mRNA score to predict HER2 status in all patients (n = 6505). (C) Forest plot with the benefit of trastuzumab in terms of OS according HER2DX ERBB2 groups in the HER2-positive cohort with efficacy outcomes (n = 757).
AUC, area under the ROC curve; CI, confidence interval; HER2, human epidermal growth factor receptor 2; OS, overall survival; IQR, interquartile range; ROC, receiver operating characteristic.
HER2DX ERBB2 mRNA score and benefit from trastuzumab
ERBB2 mRNA expression within HER2+ breast cancer can help identify patients with a high response to anti-HER2 therapies.15,20 In SCAN-B, 171 patients with HER2+ disease did not receive trastuzumab. Thus, we explored the relationship between ERBB2 mRNA score and survival outcomes according to trastuzumab use in the SCAN-B cohort after adjusting for tumor size and nodal status. Patients classified as HER2DX ERBB2-low did not show a clear benefit of trastuzumab (HR = 1.00, 95% CI 0.21-4.77), whereas this benefit was clear in HER2DX ERBB2-medium (HR = 0.10, 95% CI 0.01-0.92) and HER2DX ERBB2-high (HR = 0.15, 95% CI 0.10-0.23) (Figure 4C).
Discussion
The present study contributes valuable insights into the prognostic value of the HER2DX risk-score in early-stage HER2+ breast cancer, utilizing an extensive analysis of 757 patients from the SCAN-B dataset. Our findings confirm previous observations of the HER2DX risk-score in other cohorts,15,16,19,21 as well as in SCAN-B with <380 patients and less follow-up (i.e. 1.9 versus 7.5 years).15 In addition, the present study confirmed the association between ERBB2 mRNA scores and clinical HER2 status and opens the door to identify patients with HER2+ breast cancer with low or null benefit from trastuzumab (i.e. ERBB2-low).
In the past, the one-size-fits-all approach in treating HER2+ breast cancer tumors has proven insufficient, given the vast differences among patients in terms of both clinical and biological factors. This opens up the opportunity to tailor treatments for individuals who might not require the most aggressive regimens.2 In stage I disease, 3 months of paclitaxel plus 1 year of trastuzumab is considered the standard of care for most patients based on the results of the APT trial,19 a single-arm study involving 410 patients. While this treatment strategy has gained widespread adoption, there is ongoing debate regarding its applicability to specific patient subsets not comprehensively represented in the APT trial. This includes individuals with hormone receptor-negative tumors or those with tumor sizes between 2 and 3 cm, who were eligible but not well-represented in the study. Furthermore, the effectiveness of adjuvant trastuzumab in patients with tumors <1 cm in size remains uncertain, as this particular group was not part of pivotal randomized phase III clinical trials exploring adjuvant trastuzumab. Therefore, HER2DX stands to aid in better identifying stage I disease patients who could benefit from a less-intensive treatment regimen and those who might derive greater benefit from a more aggressive approach.19
In terms of reducing trastuzumab treatment duration, several non-inferiority studies have shown a slight decrease in recurrence risk and a minor increase in heart-related side-effects when using the treatment for 12 months instead of a shorter period.4, 5, 6, 7,32 While clinical guidelines have not endorsed shorter trastuzumab durations for most patients, HER2DX could help pinpoint patients at low risk of recurrence, making them suitable for this approach. For instance, individuals with comorbidities, those who experience cardiac toxicity or stage I with HER2DX low-risk might be good candidates for shorter trastuzumab treatment. In contrast, some patients with HER2DX high-risk stage I tumors could potentially benefit from more intense treatment, such as a combination of multiagent adjuvant chemotherapy along with trastuzumab administered for a year.
For stage II-III disease, more intense treatments involving drugs like pertuzumab, T-DM1, and neratinib are being considered either concurrently with, as an alternative to, or following a year of trastuzumab therapy.8, 9, 10 Notably, the stage II-III patients assessed in this study in the SCAN-B dataset received treatments solely based on chemotherapy, trastuzumab, and endocrine therapy. Nevertheless, the HER2DX low-risk group exhibited an exceptional 7-year prognosis. On one hand, the added benefit from pertuzumab and neratinib is relatively modest (<3% in terms of reducing recurrence).8,9 On the other hand, T-DM1 has exhibited significant outcomes, elevating the likelihood of survival without invasive disease by 13.7% at the 7-year mark compared to standard trastuzumab,10 particularly for patients not achieving a pCR following standard anti-HER2-based chemotherapy.33 It is noteworthy that in KATHERINE, two out of three patients in the control group experienced no events after 7 years. Given this scenario, there is an urgent necessity to accurately identify which patients with stage II-III HER2+ disease could derive benefits from these intensified therapies, aiming for efficacy while minimizing unnecessary side-effects and financial burdens.
In addition, our findings in this study shed light on the critical role of ERBB2 mRNA expression as a potential predictive biomarker for the effectiveness of trastuzumab treatment in patients with HER2+ breast cancer. Indeed, not all patients with HER2+ breast cancer derive the same level of benefit from trastuzumab, and identifying those who are most likely to respond optimally to the treatment is of paramount importance to personalize therapeutic decisions. In a prior study, HER2DX ERBB2 mRNA score has shown to predict pCR following five cycles of trastuzumab-pertuzumab and endocrine therapy in hormone-sensitive HER2+ breast cancer in tumor samples from the PER-ELISA phase II trial, where the pCR rate in the HER2DX ERBB2 mRNA-low group was 0.0%, versus 7.7% and 53.3% in the HER2DX ERBB2 mRNA-med and -high groups, respectively.20 Similarly, HER2DX ERBB2 mRNA score has been associated with overall response following T-DM1 monotherapy in the advanced HER2+ setting, with an observed overall response rate of 0%, 29%, and 56% in HER2DX ERBB2-low, -med, and -high groups, respectively.34 Collectively, the results from the SCAN-B analysis and the two studies suggest a consistent pattern.
While our study provides promising evidence of the HER2DX risk-score’s clinical value, certain limitations should be acknowledged. Firstly, despite the robustness of our findings, further validation in larger, diverse patient cohorts is essential to confirm the generalizability of the HER2DX risk-score across different populations and clinical contexts. Additionally, the study’s retrospective nature and the potential for unaccounted confounding factors could influence our results. Moreover, as treatment regimens were not consistently coded in the SCAN-B dataset, we could not fully explore the interplay between HER2DX risk-score and specific chemotherapeutic approaches (e.g. anthracycline use), which may influence patient outcomes. Lastly, the study was unable to estimate the risk of breast cancer mortality due to the lack of information regarding the specific cause of death.
In conclusion, this comprehensive analysis of the HER2DX risk-score’s prognostic significance within the SCAN-B dataset provides valuable insights into its potential as a valuable tool for clinical decision making in early-stage HER2+ breast cancer. Further studies and prospective trials will refine the integration of this test into routine clinical practice.
Acknowledgments
Funding
This work was supported by Reveal Genomics (no grant number) and National Cancer Institute of the National Institutes of Health [grant number R01CA229409] to LAC.
Role of the funder
In addition to financial support, the funders actively contributed to various aspects of the research, including study design, data collection, analysis, interpretation, and report writing.
Disclosure
GV has received a speaker’s fee from MSD, Pfizer, GSK, and Pierre Fabre; has held an advisory role with AstraZeneca; and received consultant fees from Reveal Genomics. TP has received honoraria for speaker activities from AstraZeneca, Pfizer, Novartis, Veracyte, and Argenetics, and has held an advisory role with Novartis. FBM has a patent application (EP21383165). LP is listed as an inventor on patent PCT/EP2021/070788. OMS has declared travel expenses and consulting fees from Roche and Reveal, and speaker fees from Eisai, Daiichi-Sankyo, and Novartis. JC has ownership interests in MedSIR, Nektar, and Leuko; has held consulting or advisory roles in Celgene, Cellestia Biotech, AstraZeneca, Roche, Seattle Genetics, Daiichi-Sankyo, ERYTECH Pharma, Polyphor, Athenex, Lilly, Servier, Merck Sharp & Dohme, GlaxoSmithKline, Leuko, Clovis Oncology, Bioasis, Boehringer Ingelheim, Ellipses Pharma, HiberCell, Bioinvent, GEMoaB, Gilead Sciences, Menarini, Zymeworks, and Reveal Genomics; has received research funding from ARIAD, AstraZeneca, Baxalta, Bayer, Eisai, Guardant Health, Merck Sharp & Dohme, Pfizer, Puma Biotechnology, Queen Mary University of London, Roche, and Piqur; has patents, royalties, or other intellectual property from pharmaceutical combinations of a Pi3k Inhibitor and a microtubule destabilizing agent (Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A, Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/ 0338368 A1); and has received travel expenses from Roche, Pfizer, Eisai, Novartis, Daiichi-Sankyo, and Gilead Sciences. EC has received honoraria for advisory roles from Pfizer, AstraZeneca, Daiichi-Sankyo, Roche, Novartis, Lilly, and MSD; and has received a speaker’s fee from Roche and Lilly. MM reports research grants from Puma; consulting/advisory fees from Roche, Novartis, AstraZeneca, Daiichi-Sankyo, Seagen, Lilly, and Sanofi; speakers’ honoraria from Seagen, Lilly, AstraZeneca, Pfizer, Daiichi-Sankyo, and Roche; a leadership or fiduciary role as Chairman, GEICAM and as a member of the Board of Directors for TRIO. PC reports speakers’ bureaus with AstraZeneca, GlaxoSmithKline, Novartis, and Roche; travel, accommodation, and expenses from Celgene, GlaxoSmithKline, and Novartis; and research funding from Merck Serono, Novartis, and Roche. LAC reports participation on a Data Safety Monitoring Board or Advisory Board of Sanofi Aventis, Novartis, Genentech/Roche, GSK, AstraZeneca/Daiichi-Sankyo, and Aptitude Health; and has a spouse serving on the board of Falcon Therapeutics and spouse involvement in a neural stem cell therapy patent. NH received consulting fees from Pierre Fabre and Roche. AV has the DNADX patent filed (EP22382387.3). GC served as consultant or advisor for Roche, Lilly, and Bristol-Myers Squibb; served on the speaker’s bureau for Roche, Pfizer, and Lilly; received travel funding from Pfizer and Roche; and received honoraria from Roche, Pfizer, Lilly, Novartis, and Seagen, all outside the submitted work. PV is one of the stockholders of Reveal Genomics, and also reports personal fees from NanoString. JSP is an equity stockholder and consultant for Reveal Genomics and is listed as an inventor on patent applications for the Breast PAM50 assay. CMP is an equity stockholder and consultant of BioClassifier LLC and Reveal Genomics, and is listed as an inventor on patent applications for the Breast PAM50 assay. AP reports advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, Bristol-Myers Squibb, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc, and Lilly; lecture fees from Roche, Pfizer, Novartis, Amgen, Bristol-Myers Squibb, NanoString Technologies, and Daiichi-Sankyo; institutional financial interests from Boehringer, Novartis, Roche, NanoString, Sysmex Europa GmbH, Medica Scientia Innovation Research, SL, Celgene, Astellas, and Pfizer; is a stockholder and consultant of Reveal Genomics and SL; and is listed as an inventor on patent applications for the HER2DX assay. SMT reports consulting or advisory roles for Novartis, Pfizer, Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol-Myers Squibb, Seattle Genetics, CytomX Therapeutics, Daiichi-Sankyo, Gilead, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Sumitovant Biopharma, Zetagen, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Biopharma, Bayer, Incyte Corp, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR, and Hengrui USA; research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol-Myers Squibb, Eisai, AstraZeneca, Gilead, NanoString Technologies, Seattle Genetics, and OncoPep; and travel support from Eli Lilly, Sanofi, Gilead, and Pfizer. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22:1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schettini F., Prat A. Dissecting the biological heterogeneity of HER2-positive breast cancer. Breast. 2021;59:339–350. doi: 10.1016/j.breast.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Sáez O., Prat A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract. 2021;17:594–604. doi: 10.1200/OP.21.00172. [DOI] [PubMed] [Google Scholar]
- 4.Pondé N., Gelber R.D., Piccart M. PERSEPHONE: are we ready to de-escalate adjuvant trastuzumab for HER2-positive breast cancer? NPJ Breast Cancer. 2019;5:1. doi: 10.1038/s41523-018-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pivot X., Romieu G., Debled M., et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393:2591–2598. doi: 10.1016/S0140-6736(19)30653-1. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H., Fraser J., Wildiers H., et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4:1199–1206. doi: 10.1001/jamaoncol.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte P., Frassoldati A., Bisagni G., et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER study. Ann Oncol. 2018;29:2328–2333. doi: 10.1093/annonc/mdy414. [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G., Procter M., de Azambuja E., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan A., Delaloge S., Holmes F.A., et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 10.von Minckwitz G., Huang C.-S., Mano M.S., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2018;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 11.Prat A., Carey L.A., Adamo B., et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106:dju152. doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schettini F., Pascual T., Conte B., et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2020;84 doi: 10.1016/j.ctrv.2020.101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salgado R., Denkert C., Campbell C., et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1:448–455. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prat A., Guarneri V., Paré L., et al. A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: a retrospective study with an external evaluation. Lancet Oncol. 2020;21:1455–1464. doi: 10.1016/S1470-2045(20)30450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prat A., Guarneri V., Pascual T., et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villacampa G., Tung N.M., Pernas S., et al. Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer. Ann Oncol. 2023;34:783–795. doi: 10.1016/j.annonc.2023.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waks A.G., Ogayo E.R., Paré L., et al. Assessment of the HER2DX assay in patients with ERBB2-positive breast cancer treated with neoadjuvant paclitaxel, trastuzumab, and pertuzumab. JAMA Oncol. 2023;9:835–840. doi: 10.1001/jamaoncol.2023.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bueno-Muiño C., Echavarría I., López-Tarruella S., et al. Assessment of a genomic assay in patients with ERBB2-positive breast cancer following neoadjuvant trastuzumab-based chemotherapy with or without pertuzumab. JAMA Oncol. 2023;9:841–846. doi: 10.1001/jamaoncol.2023.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolaney S.M., Tarantino P., Graham N., et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273–285. doi: 10.1016/S1470-2045(23)00051-7. [DOI] [PubMed] [Google Scholar]
- 20.Guarneri V., Brasó-Maristany F., Dieci M.V., et al. HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: a correlative analysis from the PerELISA trial. eBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarantino P., Villacampa G., Graham N., et al. Combined analysis of the HER2DX genomic tool in adjuvant APT and ATEMPT trials. Ann Oncol. 2023;8(1 suppl 4) [Google Scholar]
- 22.Saal L.H., Vallon-Christersson J., Häkkinen J., et al. The Sweden Cancerome Analysis Network - Breast (SCAN-B) Initiative: a large-scale multicenter infrastructure towards implementation of breast cancer genomic analyses in the clinical routine. Genome Med. 2015;7:20. doi: 10.1186/s13073-015-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staaf J., Häkkinen J., Hegardt C., et al. RNA sequencing-based single sample predictors of molecular subtype and risk of recurrence for clinical assessment of early-stage breast cancer. NPJ Breast Cancer. 2022;8:94. doi: 10.1038/s41523-022-00465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brueffer C., Gladchuk S., Winter C., et al. The mutational landscape of the SCAN-B real-world primary breast cancer transcriptome. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahlgren M., George A.M., Brueffer C., et al. Preexisting somatic mutations of estrogen receptor alpha (ESR1) in early-stage primary breast cancer. JNCI Cancer Spectr. 2021;5:pkab028. doi: 10.1093/jncics/pkab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres E.S., Marín-Aguilera M., Jares P., et al. 13P analytical validation of HER2DX test for early-hER2+ breast cancer. ESMO Open. 2023;8 doi: 10.1016/j.esmoop.2024.102903. [DOI] [PubMed] [Google Scholar]
- 27.Brooke H.L., Talbäck M., Hörnblad J., et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 29.de Boer A.Z., Bastiaannet E., Schetelig J., et al. Breast cancer mortality of older patients with and without recurrence analysed by novel multi-state models. Eur J Cancer. 2022;174:212–220. doi: 10.1016/j.ejca.2022.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Meira-Machado L., de Uña-Álvarez J., Cadarso-Suárez C., et al. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18:195–222. doi: 10.1177/0962280208092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 32.Conte P., Bisagni G., Piacentini F., et al. Nine-week versus one-year trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: 10-year update of the ShortHER phase III randomized trial. J Clin Oncol. 2023;41:4976–4981. doi: 10.1200/JCO.23.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loibl S., Mano M., Untch M., et al. Presented at the 2023 San Antonio Breast Cancer Symposium; San Antonio, TX: December 5-9, 2023. Phase III study of adjuvant ado-trastuzumab emtansine vs trastuzumab for residual invasive HER2-positive early breast cancer after neoadjuvant chemotherapy and HER2-targeted therapy: KATHERINE final IDFS and updated OS analysis. [Google Scholar]
- 34.Brasó-Maristany F., Griguolo G., Chic N., et al. HER2DX ERBB2 mRNA expression in advanced HER2-positive breast cancer treated with T-DM1. J Natl Cancer Inst. 2022;115:332–336. doi: 10.1093/jnci/djac227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.