Abstract
Introduction
Sulforaphane can induce the transcription factor, Nrf2, promoting antioxidant and anti-inflammatory responses. In this study, hospitalised patients with community-acquired pneumonia (CAP) were treated with stabilised synthetic sulforaphane (SFX-01) to evaluate impact on clinical status and inflammation.
Methods
Double-blind, randomised, placebo-controlled trial of SFX-01 (300 mg oral capsule, once daily for 14 days) conducted in Dundee, UK, between November 2020 and May 2021. Patients had radiologically confirmed CAP and CURB-65 (confusion, urea >7 mmol·L-1, respiratory rate ≥30 breaths·min-1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years) score ≥1. The primary outcome was the seven-point World Health Organization clinical status scale at day 15. Secondary outcomes included time to clinical improvement, length of stay and mortality. Effects on Nrf2 activity and inflammation were evaluated on days 1, 8 and 15 by measurement of 45 serum cytokines and mRNA sequencing of peripheral blood leukocytes.
Results
The trial was terminated prematurely due to futility with 133 patients enrolled. 65 patients were randomised to SFX-01 treatment and 68 patients to placebo. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was the cause of CAP in 103 (77%) cases. SFX-01 treatment did not improve clinical status at day 15 (adjusted OR 0.87, 95% CI 0.41–1.83; p=0.71), time to clinical improvement (adjusted hazard ratio (aHR) 1.02, 95% CI 0.70–1.49), length of stay (aHR 0.84, 95% CI 0.56–1.26) or 28-day mortality (aHR 1.45, 95% CI 0.67–3.16). The expression of Nrf2 targets and pro-inflammatory genes, including interleukin (IL)-6, IL-1β and tumour necrosis factor-α, was not significantly changed by SFX-01 treatment. At days 8 and 15, respectively, 310 and 42 significant differentially expressed genes were identified between groups (false discovery rate adjusted p<0.05, log2FC >1).
Conclusion
SFX-01 treatment did not improve clinical status or modulate key Nrf2 targets in patients with CAP primarily due to SARS-CoV-2 infection.
Shareable abstract
Treatment with stabilised synthetic sulforaphane (SFX-01, 300 mg oral capsule) once daily for 14 days did not modulate key Nrf2 targets or improve clinical outcomes in patients hospitalised with CAP mainly due to SARS-CoV-2 infection https://bit.ly/4280Tvb
Introduction
Dysregulated inflammatory responses are implicated in the pathogenesis of community-acquired pneumonia (CAP) [1], a leading cause of morbidity and mortality worldwide [2]. In particular, hyperinflammation and cytokine storm are well-established contributors to coronavirus disease 2019 (COVID-19) pneumonia [3], resulting in tissue damage and in the most severe cases, acute respiratory distress syndrome (ARDS) and death [4]. Therapies that reduce mortality in hospitalised COVID-19 patients primarily target overactive inflammatory responses, including corticosteroids, anti-interleukin (IL)-6 receptor monoclonal antibodies and Janus kinase inhibitors [5, 6, 7, 8].
Despite evidence of similar inflammatory involvement in non-COVID-19 pneumonia and ARDS [1], therapeutic advancement in CAP has been relatively neglected since the widespread introduction of antibiotics in the 1950s. Effective therapies and vaccination have dramatically reduced hospitalisation rates and mortality from COVID-19 [9, 10]; however, severe disease is persisting [6], and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become one pathogen among many that can cause CAP [11]. The development of broad-spectrum anti-inflammatory therapies that are effective in CAP remains critical [12].
Oxidant–antioxidant imbalance or oxidative stress can trigger and perpetuate inflammation by activating pro-inflammatory pathways (e.g. NF-κB), inducing metabolic dysfunction and driving tissue damage and cell death [13, 14, 15]. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a master regulator of antioxidant and anti-inflammatory responses. Nrf2 targets and upregulates the expression of antioxidant and cytoprotective genes encoding proteins such as NAD(P)H:quinone oxidoreductase 1 (NQO1), haem oxygenase-1 (HO-1) and glutathione-S-synthetase (GSS), which participate in reactive metabolite and oxidant detoxification [16], in addition to directly inhibiting the transcription of inflammatory cytokines including IL-6, IL-1β and tumour necrosis factor (TNF)-α implicated in cytokine storm [17]. In a murine model of pneumococcal pneumonia, loss of Nrf2 resulted in defective bacterial clearance and increased lung injury [18]. Lung biopsies from COVID-19 patients demonstrated suppression of Nrf2 gene expression, an effect that was abrogated utilising Nrf2 inducers in vitro, resulting in beneficial antiviral and anti-inflammatory responses [19]. Pre-clinical research therefore supports Nrf2 activation as a target with potential benefits in both COVID-19 and non-SARS-CoV-2 pneumonia [20].
Sulforaphane is a naturally occurring potent activator of Nrf2 which acts by inhibiting the usually rapid ubiquitination and degradation of Nrf2 triggered by binding to Kelch-like ECH-associated protein 1 (Keap1) [21, 22], and has demonstrated protective effects in animal models of acute lung inflammation [23] as well as potential benefit in chronic respiratory disease [24, 25]. SFX-01 is an α-cyclodextrin-encapsulated, synthetic, stabilised sulforaphane (1-isothiocyanato-4-methyl-sulfinylbutane) formulation utilised to date in two phase I (clinicaltrials.gov identifiers NCT01948362, NCT02055716) and two phase II clinical trials (clinicaltrials.gov identifiers NCT02614742, NCT02970682), with reportedly good safety profile [26].
We hypothesised that treatment with an Nrf2 activator may improve clinical outcomes in CAP by promoting antioxidant and anti-inflammatory responses. We performed a randomised, double-blind, placebo-controlled trial of SFX-01 compared with placebo in patients hospitalised with CAP.
Methods
Trial design and patients
The STAR-COVID-19 trial (SFX-01 Treatment for Acute Respiratory Infections) was a phase II double-blind, randomised, placebo-controlled, trial conducted at Ninewells Hospital, Dundee, UK. Inclusion criteria were age ≥18 years, hospitalisation with CAP (defined as new radiographic infiltrate on chest radiograph or computed tomography scan <48 h after hospitalisation) and an increased risk of mortality (CURB-65 (confusion, urea >7 mmol·L-1, respiratory rate ≥30 breaths·min-1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years) score ≥1 or bilateral radiographic infiltrates) without requirement for mechanical ventilation. Patients were required to be tested for SARS-CoV-2 infection by reverse transcriptase (RT) quantitative PCR at enrolment.
Key exclusion criteria were inability to provide informed consent, hospital-acquired pneumonia, alanine aminotransferase and/or aspartate aminotransferase more than five times the upper limit of normal and stage 4 chronic kidney disease or requiring dialysis. Complete eligibility criteria are provided in the supplementary material.
Trial oversight
The trial was approved by the Scotland A research ethics committee (20/SS/0092). All patients or legal representatives provided written informed consent. An independent, external data safety monitoring committee reviewed adverse event data. The study was prospectively registered with EudraCT (identifier 2020-003486-19).
Trial procedures
Patients were screened for eligibility up to 24 h prior to randomisation and randomised within 96 h of admission to hospital for CAP. Patients were randomised to treatment with either oral SFX-01 (300-mg capsules) or placebo once daily for 14 days via a central web-based randomisation system (TRuST). Randomisation was stratified by pneumonia severity (CURB-65 score 0–2 versus 3–5). Justification of the dose used is described in detail in the supplementary material.
Patients’ clinical status and safety were evaluated daily while hospitalised and on days 3, 5, 8, 11, 15 and 29 after discharge. Discharged patients continued to receive treatment at home and were invited back to the research unit on day 15 for a follow-up visit including blood sampling.
Primary and secondary outcome measures
The primary study objective was to evaluate the clinical efficacy of SFX-01 compared to placebo, on top of standard care, using the World Health Organization (WHO) seven-point ordinal scale as an outcome measure of clinical status at day 15. Secondary outcome measures included time to an improvement of one category on the WHO scale, clinical status and mean change (WHO scale and National Early Warning Score (NEWS)), time to discharge or NEWS of ≥2 (maintained for ≥24 h) whichever occurred first, oxygen-free days, duration and incidence of new oxygen use or new mechanical ventilation, and ventilator-free days from day 1 to 29, duration of hospitalisation, and 15- and 28-day mortality. Safety of SFX-01 was evaluated by cumulative incidence of adverse events, serious adverse events (SAEs) and discontinuation of treatment. Patients who discontinued study treatment were asked to remain in the study and attend study visits.
Exploratory objectives and substudy
A pre-specified substudy was performed to evaluate effects of SFX-01 on Nrf2 and the systemic immune response. Peripheral blood was collected at days 1, 8 and 15. Serum cytokines were quantified using the Olink Target 48 cytokine panel and RNA-stabilised whole blood utilised for mRNA sequencing (mRNAseq). Serum cytokine analysis was performed on the intention-to-treat (ITT) population, excluding those receiving tocilizumab due to profound effects of the treatment on cytokine levels, while gene expression analysis was performed on the per-protocol population.
Gene expression changes observed in the present study were compared to publicly available data from isolated peripheral blood mononuclear cells incubated for 24 h with or without 15 µM l-sulforaphane (l-SFN) followed by RNAseq (Gene Expression Omnibus accession number GSE160353). Detailed methods for exploratory end-points and additional analyses are included in the supplementary material.
Statistical analyses
The study was originally intended to enrol 300 participants, with details of the power calculation shown in the supplementary material. The pre-specified futility analysis was performed by the data monitoring committee on unblinded data for the first 100 subjects. Adjudication on termination for futility used conditional power of detecting odds ratios of 2 and 1.5 given the emerging treatment effect after 100 participants. Conditional power was calculated under two scenarios: 1) the treatment effect after 100 subjects extended for the duration of the trial and 2) odds ratios of 2 and 1.5 for the remainder of the trial. Termination of the trial would be recommended if all analyses showed conditional power <20%.
Primary efficacy analyses were based on the ITT population. Safety analyses were based on a modified ITT population consisting of all participants who were randomised and received at least one dose of randomised therapy. A per-protocol analysis was performed including all participants who completed randomly assigned therapy. The primary end-point, the WHO seven-point ordinal scale, was evaluated using mixed-effects ordinal logistic regression, assuming proportional odds, adjusted for the stratifying factor of CURB-65 score as random effect. Secondary outcomes of time to event were evaluated using Cox proportional hazards regression adjusted for CURB-65 score. Number of days free from oxygen, new oxygen use, days free from ventilation, new ventilation use and adverse events between the SFX-01 and placebo groups were analysed using negative binomial regression.
Pre-specified subgroup analyses were performed for the primary outcome based on age, sex, SARS-CoV-2 positivity, detection of pathogens, and subgroups based on the WHO scale at baseline.
Serum biomarker data were analysed using a mixed-model repeated measures approach (supplementary material). For mRNAseq data, using the Wald test and Benjamini–Hochberg procedure, a false discovery rate adjusted p-value of <0.05 with a log2 fold-change (log2FC) of >1 or <−1 between treatment groups was considered significant. Full details of exploratory analyses are presented in the supplementary material.
Results
From 20 November 2020 to 5 May 2021, 133 participants were randomised: 65 to the SFX-01 arm and 68 to placebo (figure 1).
FIGURE 1.
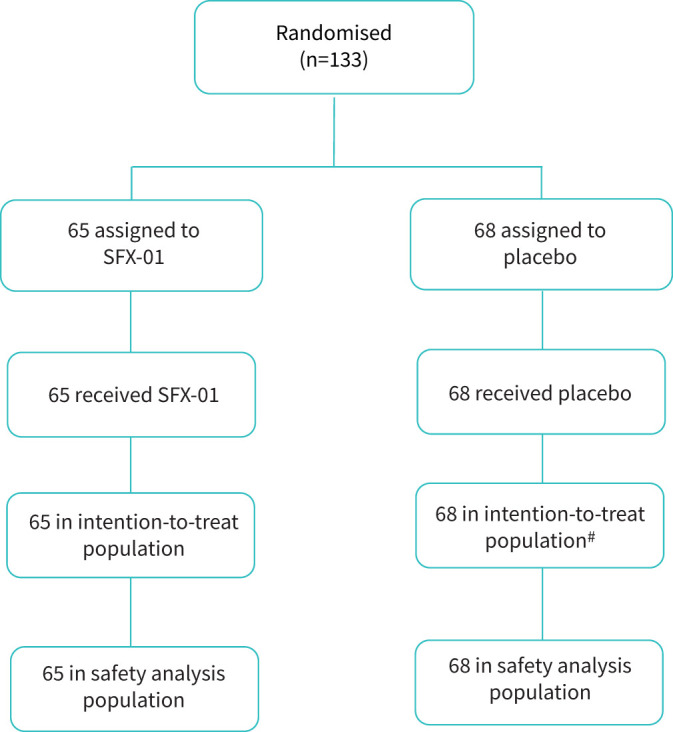
Consolidated Standards of Reporting Trials diagram detailing flow of participants in STAR-COVID-19 (SFX-01 Treatment for Acute Respiratory Infections). #: one participant in the placebo group withdrew from the study and day 15 status was unknown.
Baseline characteristics of the study population were well balanced (table 1). 78.5% of the SFX-01 group and 76.5% of the placebo groups were SARS-CoV-2 positive by RT-PCR. 70.8% of the SFX-01 group and 69.1% of the placebo group also received corticosteroids, predominantly dexamethasone. 18.5% received tocilizumab in the SFX-01 group and 11.8% in the placebo group (table 1).
TABLE 1.
Baseline study participant clinical characteristics
| Placebo | SFX-01 | |
| Participants | 68 | 65 |
| Gender | ||
| Male | 42 (61.8) | 36 (55.4) |
| Female | 26 (38.2) | 29 (44.6) |
| Ethnicity | ||
| English/Welsh/Scottish/Northern Irish/British | 61 (89.7) | 62 (95.4) |
| Indian | 0 | 1 (1.5) |
| Pakistani | 2 (2.9) | 1 (1.5) |
| Chinese | 2 (2.9) | 0 |
| Any other Asian background | 1 (1.5) | 0 |
| African | 2 (2.9) | 1 (1.5) |
| Age years | 63.6±13.8 | 61.6±12.7 |
| SARS-CoV-2 PCR status | ||
| Negative | 16 (23.5) | 14 (21.5) |
| Positive | 52 (76.5) | 51 (78.5) |
| Past medical history | ||
| Chronic cardiac disease | 16 (23.5) | 10 (15.4) |
| Hypertension | 27 (39.7) | 18 (27.7) |
| COPD | 9 (13.2) | 6 (9.2) |
| Chronic pulmonary disease | 2 (2.9) | 5 (7.7) |
| Asthma | 5 (7.4) | 9 (13.8) |
| Chronic kidney disease (eGFR <44 mL·min−1, on dialysis or previous transplant) | 2 (2.9) | 1 (1.5) |
| Moderate or severe liver disease | 1 (1.5) | 1 (1.5) |
| Mild liver disease | 1 (1.5) | 0 (0.0) |
| Chronic neurological disorder | 1 (1.5) | 3 (4.6) |
| Malignant neoplasm | 9 (13.2) | 2 (3.1) |
| Chronic haematological disease | 3 (4.4) | 4 (6.2) |
| Obesity | 21 (30.9) | 16 (24.6) |
| Diabetes with complications | 12 (17.6) | 8 (12.3) |
| Diabetes without complications | 6 (8.8) | 2 (3.1) |
| Rheumatological disorder | 5 (7.4) | 2 (3.1) |
| Smoking status | ||
| Current | 4 (5.9) | 4 (6.2) |
| Former | 34 (50.0) | 40 (61.5) |
| Never | 30 (44.1) | 21 (32.3) |
| CURB-65 score | ||
| <3 | 64 (94.1) | 62 (95.4) |
| 3–5 | 4 (5.9) | 3 (4.6) |
| Seven-point WHO ordinal scale# | ||
| 3 | 28 (41.2) | 24 (36.9) |
| 4 | 33 (48.5) | 30 (46.2) |
| 5 | 7 (10.3) | 11 (16.9) |
| NEWS | 3.78±2.28 | 4.74±2.56 |
| Median (interquartile range) | 4.0 (2.0–6.0) | 5.0 (3.0–7.0) |
| Range | 0.0–8.0 | 0.0–11.0 |
Data are presented as n, n (%) or mean±sd, unless otherwise stated. SFX-01: 1-isothiocyanato-4-methyl-sulfinylbutane; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; eGFR: estimated glomerular filtration rate; CURB-65: confusion, urea >7 mmol·L-1, respiratory rate ≥30 breaths·min-1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years; WHO: World Health Organization; NEWS: National Early Warning Score. #: 3=hospitalised, not requiring supplemental oxygen, 4=hospitalised, requiring supplemental oxygen, 5=hospitalised, on noninvasive ventilation or high-flow oxygen devices.
Primary end-point
At day 15, 70.8% in the SFX-01 group and 69% in the placebo group had been discharged from hospital. One patient withdrew from the study and was excluded from the analysis due to unknown day 15 status. The adjusted odds ratio (aOR) from a proportional odds model in the ITT population was 0.87 (95% CI 0.41–1.83), indicating that SFX-01 treatment did not improve clinical status compared with placebo at day 15 (p=0.71) (table 2).
TABLE 2.
Estimates of treatment effect on the primary outcome World Health Organization Clinical Status Scale at day 15
| Placebo | SFX-01 | Model | OR (95% CI) | p-value | |
| Participants | 68 | 65 | |||
| Clinical status | |||||
| Not hospitalised, no limitations on activities | 3 (4.4) | 0 | Unadjusted | 0.84 (0.41–1.71) | 0.62 |
| Not hospitalised, limitations on activities | 44 (64.7) | 46 (70.8) | Adjusted# | 0.87 (0.41–1.83) | 0.71 |
| Hospitalised, not requiring supplemental oxygen | 5 (7.4) | 4 (6.2) | |||
| Hospitalised, requiring supplemental oxygen | 4 (5.9) | 1 (1.5) | |||
| Hospitalised, on noninvasive ventilation or high-flow oxygen devices | 2 (2.9) | 3 (4.6) | |||
| Hospitalised, on invasive mechanical ventilation or ECMO | 1 (1.5) | 0 | |||
| Death | 8 (11.8) | 11 (16.9) | |||
| Missing | 1 (1.5) | 0 |
Data are presented as n or n (%), unless otherwise stated. SFX-01: 1-isothiocyanato-4-methyl-sulfinylbutane; ECMO: extracorporeal membrane oxygenation. #: primary outcome: adjusted for CURB-65 (confusion, urea >7 mmol·L-1, respiratory rate ≥30 breaths·min-1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years) score and baseline seven-point ordinal scale; adjusted OR >1.0 indicates a benefit of SFX-01 treatment.
In the per-protocol analysis, the aOR was 0.68 (95% CI 0.29–1.62; p=0.38; SFX-01 group n=43, placebo group=62). Pre-specified subgroup analyses at day 29 were consistent with the primary result (supplementary tables S2–7 and supplementary figure S1). In particular, SFX-01 treatment did not improve outcomes in patients positive for SARS-CoV-2 (aOR 0.82, 95% CI 0.31–2.20; p=0.61).
Secondary end-points
There were 26 deaths during the 28-day study period; 11 in the placebo group and 15 in the SFX-01 group (adjusted hazard ratio (aHR) 1.45, 95% CI 0.67–3.16; p=0.35). 19 deaths occurred on or before day 15; eight in the placebo arm and 11 in the SFX-01 arm (aHR 1.46, 95% CI 0.59–3.62; p=0.42) (table 3).
TABLE 3.
Estimates of treatment effects on secondary end-points
| Placebo | SFX-01 | Effect estimate (unadjusted) | p-value | Effect estimate (adjusted # ) | p-value | |
| Participants | 68 | 65 | ||||
| 15-day mortality¶ | 8 (11.8) | 11 (16.9) | 1.45 (0.58–3.61) | 0.91 | 1.46 (0.59–3.62) | 0.92 |
| 28-day mortality ¶ | 11 (16.2) | 15 (23.1) | 1.45 (0.66–3.15) | 0.35 | 1.45 (0.67–3.16) | 0.35 |
| Clinical improvement by day 29¶ | 57 (83.8) | 51 (78.5) | 1.02 (0.70–1.49) | 0.91 | 1.02 (0.70–1.49) | 0.92 |
| Discharged or NEWS ≤2 at day 29¶ | 61 (89.7) | 55 (84.6) | 0.83 (0.56–1.24) | 0.37 | 0.80 (0.54–1.20) | 0.28 |
| Oxygen-free days+ | 20.3±10.1; n=67 | 19.8±10.6; n=65 | 0.98 (0.71–1.34) | 0.89 | 0.98 (0.72–1.34) | 0.91 |
| 25.0 (18.0–28.0) | 25.0 (19.0–28.0) | |||||
| Duration of new oxygen use+ | 1.8±3.5; n=28 | 1.0±1.7; n=24 | 0.55 (0.16–1.91) | 0.35 | 0.49 (0.15–1.64) | 0.25 |
| 0.0 (0.0–2.0) | 0.0 (0.0–1.5) | |||||
| Ventilation-free days+ | 23.5±9.4; n=67 | 21.7±10.6; n=65 | 0.93 (0.72–1.19) | 0.53 | 0.93 (0.72–1.20) | 0.57 |
| 28.0 (27.0–28.0) | 28.0 (22.0–28.0) | |||||
| Duration of new ventilation use+ | 0.8±2.5; n=60 | 1.7±4.7; n=54 | 2.11 (0.52–8.51) | 0.30 | 2.11 (0.53–8.51) | 0.29 |
| 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | |||||
| Mechanical ventilation-free days+ | 24.6±8.0; n=67 | 23.6±8.6; n=65 | 0.96 (0.82–1.13) | 0.63 | 0.96 (0.82–1.13) | 0.63 |
| 28.0 (28.0–28.0) | 28.0 (28.0–28.0) | |||||
| Duration of new mechanical ventilation use+ | 0.5±2.0; n=39 | 0.6±2.0; n=41 | 1.09 (0.09–13.29) | 0.94 | 2.10 (0.17–25.74) | 0.56 |
| 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | |||||
| Duration of hospitalisation + | 7.4±7.7; n=57 | 6.2±7.3; n=51 | 0.84 (0.56–1.27) | 0.41 | 0.84 (0.56–1.26) | 0.40 |
| 5.0 (3.0–9.0) | 3.0 (1.0–8.0) |
Data are presented as n, n (%), mean±sd or median (interquartile range), unless otherwise stated. SFX-01: 1-isothiocyanato-4-methyl-sulfinylbutane; NEWS: National Early Warning Score. #: adjusted for CURB-65 (confusion, urea >7 mmol·L-1, respiratory rate ≥30 breaths·min-1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years) score and baseline seven-point ordinal scale; ¶: effect estimates are presented as hazard ratio (95% CI); +: effect estimates are presented as incidence rate ratio (95% CI).
Improvement in the WHO seven-point scale by at least one category over 29 days was seen in 78.5% of the SFX-01 group and 83.8% of the placebo group. Time to clinical improvement of one category, time to first discharge, duration of hospitalisation, or clinical status or mean change in clinical status, or NEWS from baseline at any of the time points was not different (table 3).
There were no significant differences in secondary outcomes relating to oxygen or ventilator use (table 3).
Safety analysis
Rate of study medication discontinuation was significantly higher in the SFX-01 group (33.8%) than in the placebo group (8.8%) (incidence rate ratio (IRR) 3.79, 95% CI 1.53–9.34; p=0.004) (supplementary table S10). The main reasons for discontinuation were adverse events. Gastrointestinal adverse events were the most common reasons for discontinuation and were documented in 10 participants (nine in the SFX-01 group and one in the placebo group).
42 (64.6%) participants in the SFX-01 arm and 30 (44.1%) in the placebo arm reported at least one adverse event (IRR 1.48, 0.92–2.36; p=0.10) (table 4). The most common adverse event reported in the SFX-01 group was gastrointestinal disorders affecting 33 (60%) patients, compared to 10 (23.8%) patients in the placebo group. There were 34 SAEs in total; 18 in the placebo arm and 16 in the SFX-01 arm.
TABLE 4.
Details of total adverse events during the trial period
| Placebo | SFX- 01 | |
| Participants | 68 | 65 |
| Participants with no adverse events | 38 (55.9) | 23 (35.4) |
| Participants with adverse events | 30 (44.1) | 42 (64.6) |
| Adverse events | 42 | 55 |
| Severity | ||
| Mild | 13 (31.0) | 35 (63.6) |
| Moderate | 11 (26.2) | 4 (7.3) |
| Severe | 18 (42.9) | 16 (29.1) |
| System organ class level | ||
| Cardiac disorders | 2 (4.8) | 1 (1.8) |
| Eye disorders | 1 (2.4) | 0 |
| Gastrointestinal disorders | 10 (23.8) | 33 (60.0) |
| General disorders and administration site conditions | 2 (4.8) | 1 (1.8) |
| Infections and infestations | 15 (35.7) | 15 (27.3) |
| Metabolism and nutrition disorders | 1 (2.4) | 0 |
| Musculoskeletal and connective tissue disorders | 1 (2.4) | 0 |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (2.4) | 1 (1.8) |
| Nervous system disorders | 2 (4.8) | 2 (3.6) |
| Renal and urinary disorders | 2 (4.8) | 0 |
| Respiratory, thoracic and mediastinal disorders | 4 (9.55) | 2 (3.6) |
| Skin and subcutaneous tissue disorders | 1 (2.4) | 0 |
| Adverse event leading to study drug discontinuation | 5 (11.9) | 20 (36.4) |
Data are presented as n or n (%). SFX-01: 1-isothiocyanato-4-methyl-sulfinylbutane.
Serum cytokines and systemic inflammation
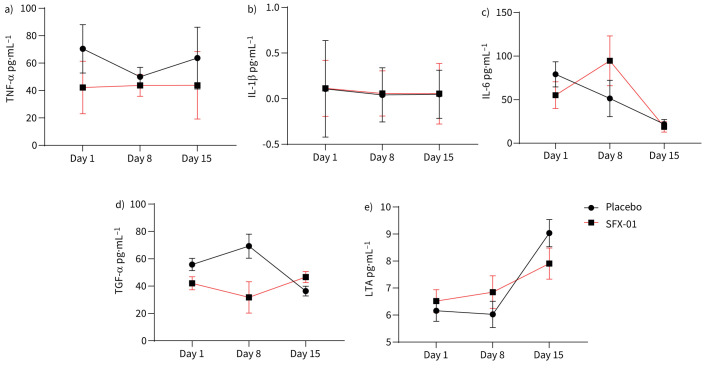
Serum cytokine analysis was performed in all participants for whom serum was available at baseline, day 8 and day 15 (n=230 samples). There were no significant differences between the SFX-01 and placebo group at any study time point in key cytokines including IL-6, TNF-α or IL-1β (figure 2a–c).
FIGURE 2.
Serum cytokine levels and effects of 1-isothiocyanato-4-methyl-sulfinylbutane (SFX-01) treatment. At days 1, 8 and 15, serum was obtained from participants and 45 inflammation-associated cytokines measured. Established Nrf2 targets, a) tumour necrosis factor (TNF)-α, b) interleukin (IL)-1β and c) IL-6 were not significantly affected by SFX-01 treatment compared with levels in the placebo group. Of the 45 cytokines measured, only the epidermal growth factor family member d) transforming growth factor (TGF)-α was significantly higher by day 15 in the SFX-01 group than in the placebo group, and only the apoptosis-inducer e) lymphotoxin-α (LTA) was significantly reduced in the SFX-01 group. Data were analysed by a mixed-model repeated measures approach; data represent model-derived mean±se. Day 1: SFX-01 n=61, placebo n=63; day 8: SFX-01 n=17, placebo n=21; day 15: SFX-01 n=33, placebo n=38.
Of all the serum proteins measured, only the epidermal growth factor family member, transforming growth factor (TGF)-α (p=0.003) (figure 2d) and lymphotoxin-α (LTA) (p=0.049) (figure 2e) were significantly different in the SFX-01- and placebo-treated groups by day 15.
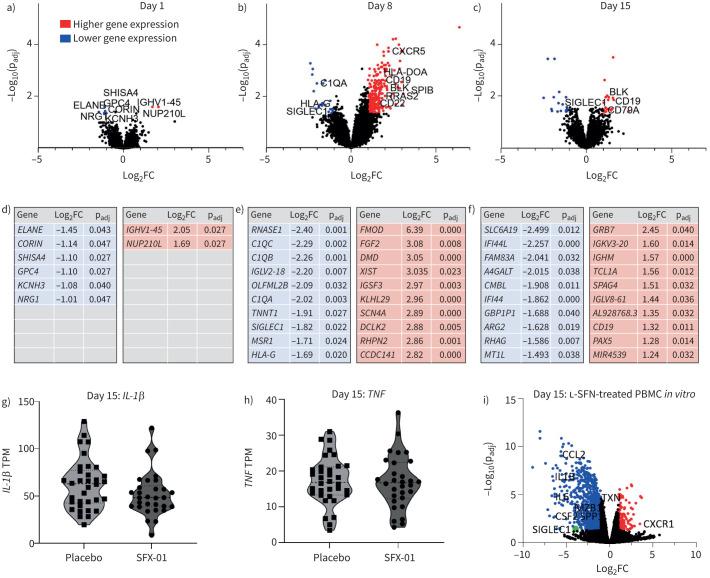
Peripheral blood leukocyte gene expression
SFX-01 effects on peripheral blood immune cell gene expression were determined using mRNAseq. At baseline, eight statistically significant differentially expressed genes (DEGs) were identified between SFX-01-treated and placebo-treated individuals; at day 8 there were 310 DEGs (286 of which were upregulated in the SFX-01 group); and at day 15, 42 DEGs were identified (figure 3a–c). The top 10 upregulated and downregulated genes with largest fold change are shown in figure 3d–f. However, mRNA levels of classical Nrf2 targets such as NQO1, GSS and HO-1 (table 5), and inflammatory cytokines such as IL-6, TNF-α or IL-1β (figure 3g–h and supplementary figure S2), were unchanged at all time points. TGF-α and LTA gene expression was also unchanged (supplementary figure S2).
FIGURE 3.
Peripheral blood leukocyte gene expression and effects of 1-isothiocyanato-4-methyl-sulfinylbutane (SFX-01) treatment and comparison with in vitro sulforaphane treatment. At a) day 1, b) day 8 and c) day 15, peripheral blood leukocyte gene expression analysed by mRNA sequencing was compared between SFX-01- and placebo-treated individuals who completed 14 days of trial treatment and had not discontinued drug at the relevant sampling time point. a) Eight significantly differentially expressed genes (DEGs) were identified between the SFX-01 and placebo groups at day 1; b) 310 DEGs were identified at day 8; and c) 42 at day 15. Higher (red) and lower (blue) gene expression levels in the SFX-01 are shown. d–f) The top 10 significant DEGs (adjusted p-value (padj) <0.05 and log2 fold change (FC) >1 or <−1) between SFX-01- and placebo-treated individuals. Red indicates higher expression in the SFX-01 group compared with the placebo group; blue indicates lower expression. Data were analysed using the Wald test and Benjamini–Hochberg procedure. g, h) Transcript per million (TPM) values representing relative expression levels of genes of interest: IL-1β and TNFα at day 15. Day 1: SFX-01 n=59, placebo n=59; day 8: SFX-01 n=14, placebo n=18; day 15: SFX-01 n=30, placebo n=34. i) Re-analysis of a published dataset (GSE160353); differential gene expression analysis of isolated human peripheral blood mononuclear cells (PBMCs) treated with l-sulforaphane (l-SFN) (15 µM) or vehicle for 24 h and processed for RNA sequencing (n=4 individuals), 1032 significant DEGs identified (false discovery rate-adjusted p-value <0.05 and log2 fold change >1 or <−1).
TABLE 5.
Direct Nrf2 gene targets in l-sulforaphane (SFN)-treated human peripheral blood mononuclear cells (PBMCs) in vitro and in vivo in peripheral blood leukocytes in the STAR-COVID-19 (SFX-01 Treatment for Acute Respiratory Infections) trial of 1-isothiocyanato-4-methyl-sulfinylbutane (SFX-01)
| Pathway | In vitro l-SFN: PBMCs | In vivo SFX-01: day 8 | In vivo SFX-01: day 15 | ||||
| Adjusted p-value | Log2FC | Adjusted p-value | Log2FC | Adjusted p-value | Log2FC | ||
| FECH | Haem production | 0.69 | 0.16 | 0.70 | 0.313 | 0.98 | −0.02 |
| FTL | Haem/iron metabolism | 0.05 | −0.44 | 0.98 | −0.02 | 0.78 | 0.13 |
| G6PD | NADPH regeneration | 0.97 | −0.03 | 0.98 | −0.02 | 0.27 | 0.37 |
| GCLC | GSH production | 0.002 | 0.92 | 0.67 | 0.24 | 0.9 | −0.06 |
| GCLM | GSH production | 0.003 | 0.97 | 0.78 | 0.15 | 0.82 | −0.1 |
| GSR | GSH production | 0.33 | 0.35 | 0.84 | −0.09 | 0.8 | 0.06 |
| GSS | GSH production | 0.61 | 0.18 | 0.84 | 0.08 | 0.72 | 0.08 |
| GSTM2 | ROS detoxification | 0.43 | −0.42 | 0.39 | 0.38 | 0.26 | 0.34 |
| GSTM3 | ROS detoxification | 0.71 | 0.22 | 0.12 | 1.07 | 0.88 | 0.1 |
| HMOX1 | Haem/iron metabolism | 0.62 | −0.24 | 0.33 | −0.44 | 0.67 | 0.14 |
| ME1 | NADPH regeneration | 0.73 | 0.28 | 0.26 | −0.68 | 0.27 | −0.42 |
| NQO1 | ROS detoxification | 0.24 | 0.45 | 0.33 | 0.45 | 0.87 | 0.06 |
| OSGIN1 | Various including autophagosome formation | 0.34 | 0.72 | 0.84 | −0.14 | 0.37 | 0.35 |
| PGD | NADPH regeneration | <0 . 001 | −1 . 03 | 0.88 | −0.11 | 0.52 | 0.28 |
| PRDX1 | TXN-based antioxidant system | 0.6 | −0.16 | 0.70 | 0.15 | 0.66 | 0.1 |
| RXRA | Lipid metabolism | 0.6 | −0.2 | 0.51 | 0.26 | 0.49 | 0.21 |
| SPP1 | Various including inflammatory signalling | 0 . 004 | −2 . 75 | 0.65 | 0.42 | 0.93 | 0.09 |
| SRXN1 | TXN-based antioxidant system | 0.53 | −1.11 | 0.86 | −0.15 | 0.78 | 0.18 |
| TALDO1 | NADPH regeneration | 0.05 | −0.43 | 0.85 | −0.12 | 0.58 | 0.21 |
| TKT | NADPH regeneration | 0.01 | −0.62 | 0.64 | −0.24 | 0.5 | 0.22 |
| TXN | TXN-based antioxidant system | <0 . 0001 | −1 . 55 | 0.98 | 0.02 | 0.88 | 0.09 |
| TXNRD1 | TXN-based antioxidant system | 0.15 | 0.42 | 0.84 | −0.1 | 0.85 | 0.07 |
Bold type represents significantly differentially expressed genes between l-SFN-treated and -untreated PBMCs; false discovery rate adjusted p-value <0.05 and log2 fold change (log2FC) >1 or <−1. GSH: glutathione; ROS: reactive oxygen species; TXN: thioredoxin.
At day 8, gene ontology analysis suggested relevant differential pathways in the SFX-01 group as transcriptional regulation, B-cell receptor signalling (supplementary figure S5) and proliferation. Both subunits of the B-cell antigen receptor CD79 (CD79A and CD79B) and downstream signalling molecules BLK and BLNK, proteins involved in costimulatory regulation CR2, CD19 and CD22, and the lymphocyte cytokine receptor CXCR5, were upregulated. The antiapoptotic factor BCL2, plus proliferation promoters RRAS2 and RASGRP3 were also significantly increased in the SFX-01 group.
Out of the significant DEGs upregulated at day 8 in the SFX-01 group, 13 remained significantly upregulated at day 15, including several B-cell-associated genes (e.g. CD79A, CD19, BLK, PAX5, FcγRII) plus genes regulating B-cell survival (TCL1A). Of the DEGs downregulated at day 8, only SIGLEC1, an interferon-signalling gene and macrophage/monocyte-associated gene, was still significantly altered at day 15. Furthermore, CD200, a macrophage/monocyte suppressor, was significantly upregulated at both time points.
Enrichment analysis identified further pathways associated with SFX-01 treatment at both days 8 and 15 (supplementary figures S3 and S4 and supplementary tables S19 and S20). Although TNF gene expression was not significantly altered, interestingly, pathways relating to both myeloid cell and adaptive responses and also TNF production were downregulated in the SFX-01 group at day 8. At day 15, pathways centred heavily around DNA and RNA processing, but smaller clusters were identified relating to viral responses and interferon signalling, which were suppressed in the SFX-01 group.
Differences in effects of SFX-01 treatment in vivo and L-SFN treatment in vitro
In vivo data in the present study were compared to data from human PBMCs treated for 24 h with or without 15 µM l-SFN in vitro. 1032 genes were significantly differentially expressed in l-SFN-treated PBMCs (false discovery rate (FDR) <0.05, log2FC >1 or <−1) (figure 3i), and 801 when a more stringent FDR of <0.01 was applied. Downregulated pathways include inflammatory cytokine production and also response to oxidative stress, demonstrating significant anti-inflammatory effects. Notably, however, several Nrf2-associated genes unaltered by SFX-01 in our study, including NQO1, GPX, HMOX1, GSS and G6PD, also showed no significant differences with l-SFN treatment in human PBMCs at this time point (table 5) [27].
Of the DEGs found at day 8 in STAR-COVID-19 trial participants, 250 were identified in PBMCs, and 30 were significantly differentially expressed with l-SFN treatment. 25 of these were upregulated at day 8 in vivo; however, all 25 were significantly downregulated in l-SFN-treated PBMCs, including HLA-DOA, -DOB and -DQA1, and CD22. The remaining five were downregulated in both the day 8 in vivo and PBMC datasets: OLFML2B, RNASE1 MAFB, ODF3B and SIGLEC1.
Of the 13 DEGs consistently upregulated in SFX-01 treatment at both day 8 and day 15, 12 were identified in the PBMCs, and four of these genes were significantly differentially expressed with l-SFN, although these were all downregulated in vitro. In particular, CD19 was reduced in l-SFN-treated PBMCs, with Gene Ontology analysis demonstrating significant changes in B-cell receptor signalling, B-cell surface molecules and regulation of B-cell proliferation.
The only gene to be consistently significantly altered in the same direction at both day 8 and 15 with SFX-01 in vivo, and in PBMCs treated with l-SFN in vitro, was SIGLEC1 (supplementary material).
Discussion
In this double-blind randomised trial, SFX-01 did not improve day 15 clinical status in hospitalised patients with CAP. Additionally, we observed an increased rate of treatment discontinuation, mainly due to gastrointestinal adverse effects in the SFX-01 group. The study was terminated early after pre-specified criteria for futility were met. Subgroup analyses including in CAP patients with and without SARS-CoV-2 infection were all consistent with the primary results.
The antioxidant transcription factor Nrf2 is reduced in acute lung infection including in COVID-19 [18, 19]. Animal models as well as human studies have shown significant beneficial effects of Nrf2 activation with sulforaphane or sulforaphane-rich preparations [20, 23, 24, 26, 28, 29, 30, 31]. In view of the negative results we observed, we therefore investigated further whether Nrf2 activation had been achieved in our study. Analyses of serum cytokine levels and peripheral blood leukocyte gene expression evidenced that SFX-01 treatment did not result in Nrf2 activation, shown by a lack of antioxidant gene induction [16] or effects on key inflammatory cytokines such as IL-6 and IL-1β [17, 32]. Our study is unable to answer why SFX-01 failed to activate Nrf2 targets. Interestingly, a study of sulforaphane in people with asthma showed that Nrf2 activation was highly heterogeneous between individuals [25].
A plausible reason that SFX-01 did not achieve the antioxidant or inflammatory modulation shown in other models may be suboptimal dosing. It should also be noted that Nrf2 activation with SFX-01 has not yet been confirmed in human trials, but was deemed highly likely considering the effects of sulforaphane demonstrated in the literature, and SFX-01 administration resulted in anti-inflammatory effects in animal models [28]. SFX-01 has been utilised in two other phase II trials (clnicaltrials.gov identifiers NCT02614742, NCT02970682) with a twice-daily 300 mg dose for 29 days in subarachnoid haemorrhage or up to 6 months in patients with metastatic breast cancer. These studies did not investigate Nrf2-related gene expression to confirm target engagement. Use of a higher dose in the COVID-19 population is unlikely to be feasible given the treatment discontinuation rates and prevalence of gastrointestinal-associated adverse events observed in the present study.
COVID-19 patient lung biopsy samples have shown Nrf2 suppression by SARS-CoV-2 infection [19], although Nrf2 status in immune cells still requires further investigation; one further explanation for lack of SFX-01 efficacy could be that infection-related Nrf2 suppression cannot be overcome by the administered therapy. In the present study, we observed no effects of SFX-01 on classical Nrf2 targets; however, we observed effects of treatment on B-cell activation and proliferation. There are contradictory reports of sulforaphane effects on B-cells. Expansion and activation of B-cells was demonstrated in severe COVID-19, although B-cell responses are required for robust adaptive immunity [33]. In both murine arthritis [34] and lupus [35] models, sulforaphane inhibited B-cell proliferation and was associated with reduction in plasma cell numbers, and Nrf2 knockout in mice with chronic airway inflammation showed enhanced plasma cell infiltration and B-cell activation [36]. In contrast, sulforaphane-treated PBMCs demonstrated dose-dependent reductions in numbers of monocytes, consistent with significant reductions in several monocyte-associated genes in our present study, and also increased numbers of dendritic cells, CD19+ B-cells and T-lymphocytes [37].
To further understand these changes, gene expression data in the present study were compared to published RNAseq data from in vitro sulforaphane-treated human PBMCs [27]. In the additional dataset analysed, several of the B-cell associated genes upregulated in the present dataset showed the opposite trend in PBMCs, in support of results from murine models. Only one gene, SIGLEC1, was significantly downregulated in PBMCs and at both trial time points. Interestingly, interferon-inducible siglec1 (also known as CD169) is capable of direct viral binding at the cell surface [38] and is upregulated in myeloid cells in COVID-19 patients [39]. Nrf2 activation in these cells was indicated by anti-inflammatory pathway induction; however, several Nrf2 targets including NQO1 were not detectably changed at the sampling time. By contrast, the mRNA levels for the classical Nrf2 targets NQO1, HMOX1 and AKR1C1 were significantly upregulated by a shorter incubation time (6 h) and lower concentrations of sulforaphane (2 or 5 μM) in ex vivo-treated human PBMCs [32]. Interestingly, there was no concentration dependence in the induction of these genes, with the higher (5 μM) sulforaphane concentration even showing a slightly diminished effect for the induction of NQO1 and AKR1C1 [32], in agreement with results from a human study with topical administration of sulforaphane-rich extracts showing diminished efficacy by the highest sulforaphane dose used [40]. Taken together, while underdosing in our study is a possible explanation, excessive dosing could also theoretically be associated with a reduced effect on Nrf2 targets.
Ours is not the first study to question the effects of sulforaphane administration on Nrf2 activation in vivo. Several human studies of sulforaphane found no effects on Nrf2 antioxidant targets [41, 42, 43]. While there are limitations to comparison of the present trial data with the PBMC dataset, including lack of inflammatory stimulus, use of a different sulforaphane formulation, concentration and treatment period, the results highlight the critical need for in vivo data on sulforaphane effects in addition to applying caution in extrapolation from in vitro findings.
Sulforaphane is reported to have activity which is not dependent on Nrf2 activation. For example, suboptimal dosing without Nrf2 activation demonstrated significant effects on fibrosis [44], sulforaphane improved macrophage phagocytosis via Nrf2-independent mechanisms [45] and inhibited interferon-γ and TNF-α-mediated pro-inflammatory responses in both an Nrf2-dependent and Nrf2-independent manner [46], in addition to Nrf2-independent antiviral effects [20]. Further investigation to understand the mechanism of action of sulforaphane utilising appropriate dosing and sampling is required. Importantly, as we are unable to demonstrate Nrf2 activation by SFX-01, our study does not exclude possible clinical benefits with alternative Nrf2-activating drugs in CAP. Other Nrf2 activators, such as the cyanoenone triterpenoids, are currently in various stages of drug development, and our study highlights that a number of challenges still remain [47].
During the COVID-19 pandemic a large number of trials were set up to test repurposed and novel therapeutics particularly in hospitalised patients. It was, and remains, important to rapidly establish the lack of efficacy of drugs so that resources can be invested into other targets. Therefore, while this trial is negative, it provides a clear answer to a relevant question about the potential efficacy of SFX-01 in this population. Key strengths of this study include the gold standard double-blind placebo-controlled design and the inclusion of CAP patients including both COVID-19 and non-COVID-19 CAP, recognising that in future SARS-CoV-2 will be just one of several circulating pathogens responsible for CAP in hospitalised patients; excessive systemic inflammation in pneumonia is a driver of high mortality rates in CAP, and further human studies of anti-inflammatory agents are critical.
In conclusion, 300 mg SFX-01 once-daily treatment for 14 days in hospitalised patients with CAP did not result in Nrf2 activation or improved clinical status.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00917-2023.SUPPLEMENT (1.3MB, pdf)
Acknowledgements
We acknowledge the members of the STAR-COVID data monitoring committee: Aran Singanayagam (Imperial College, London, UK), Timothy Hinks (University of Oxford, Oxford, UK), Oriol Sibila (Hospital Clinic, Barcelona, Spain), Alex McConnachie (University of Glasgow, Glasgow, UK) and Petra Rauchhaus (University of Dundee, Dundee, UK). This trial was delivered by Tayside Clinical Trials Unit, a UKCRC registered clinical trials unit. Thanks to Clare Clarke, Jennifer Taylor, Angela Strachan, Heather Loftus and Jodie Strachan (Ninewells Hospital and Medical School, Dundee, UK) and Diane Cassidy (University of Dundee). We thank all study participants and their families.
Provenance: Submitted article, peer reviewed.
This study is registered at https://eudract.ema.europa.eu/ with identifier number 2020-003486-19. Deidentified patient data are available from the corresponding author following publication along with the study protocol and data dictionary.
Ethics statement: The trial was approved by the Scotland A research ethics committee (20/SS/0092). All patients or legal representatives provided written informed consent. An independent, external data safety monitoring committee reviewed adverse event data.
Author contributions: Conception and design of study: M.B. Long, A.T. Dinkova-Kostova and J.D. Chalmers. Acquisition of data: M.B. Long, H. Abo-Leyah, Y.H. Giam, R.C. Hull, H.R. Keir, T. Pembridge, D.A. De Lima, L. Delgado, S.K. Inglis, C. Hughes, A. Gilmore, B.J.M. New and J.D. Chalmers. Analysis and/or interpretation of data: M.B. Long, H. Abo-Leyah, Y.H. Giam, T. Vadiveloo, R.C. Hull, G. MacLennan, A.T. Dinkova-Kostova and J.D. Chalmers. Drafting the manuscript: M.B. Long, H. Abo-Leyah, Y.H. Giam, A.T. Dinkova-Kostova and J.D. Chalmers. Revising the manuscript critically for important intellectual content: all authors. Approval of the version of the manuscript to be published: all authors.
Conflict of interest: H.R. Keir reports receiving personal fees for educational lecture from Insmed Inc., outside the submitted work.
Conflict of interest: A.T. Dinkova-Kostova participates on the Evgen Pharma Scientific Advisory Board, outside the submitted work.
Conflict of interest: J.D. Chalmers reports support for the present manuscript from Lifearc; grants or contracts from AstraZeneca, Genentech, Gilead Sciences, GlaxoSmithKline, Insmed, Grifols, Novartis and Boehringer Ingelheim, outside the submitted work; consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, Insmed, Grifols, Novartis, Boehringer Ingelheim, Pfizer, Janssen, Antabio and Zambon, outside the submitted work; and is an associate editor of this journal.
Conflict of interest: The remaining authors have nothing to disclose.
Support statement: This study was funded by LifeArc. IMP, placebo and staff support were provided by Evgen Pharma. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8: 1233–1244. doi: 10.1016/S2213-2600(20)30404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, Denouel A, Tietjen AK, et al. Global and regional burden of hospital admissions for pneumonia in older adults: a systematic review and meta-analysis. J Infect Dis 2020; 222: Suppl. 7, S570–S576. doi: 10.1093/infdis/jiz053 [DOI] [PubMed] [Google Scholar]
- 3.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584: 463–469. doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RECOVERY Collaborative Group . Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022; 400: 359–368. doi: 10.1016/S0140-6736(22)01109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers JD, Crichton ML, Goeminne PC, et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J 2021; 57: 2100048. doi: 10.1183/13993003.00048-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis 2021; 73: 2257–2264. doi: 10.1093/cid/ciab079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roche N, Crichton ML, Goeminne PC, et al. Update June 2022: management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J 2020; 60: 2200803. doi: 10.1183/13993003.00803-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyams C, Challen R, Begier E, et al. Incidence of community acquired lower respiratory tract disease in Bristol, UK during the COVID-19 pandemic: a prospective cohort study. Lancet Reg Health Eur 2022; 21: 100473. doi: 10.1016/j.lanepe.2022.100473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dela Cruz CS, Evans SE, Restrepo MI, et al. Understanding the host in the management of pneumonia. An official American Thoracic Society workshop report. Ann Am Thorac Soc 2021; 18: 1087–1097. doi: 10.1513/AnnalsATS.202102-209ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 2000; 16: 534–554. doi: 10.1034/j.1399-3003.2000.016003534.x [DOI] [PubMed] [Google Scholar]
- 14.Kratzer E, Tian Y, Sarich N, et al. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol 2012; 47: 688–697. doi: 10.1165/rcmb.2012-0161OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majewska E, Kasielski M, Luczynski R, et al. Elevated exhalation of hydrogen peroxide and thiobarbituric acid reactive substances in patients with community acquired pneumonia. Respir Med 2004; 98: 669–676. doi: 10.1016/j.rmed.2003.08.015 [DOI] [PubMed] [Google Scholar]
- 16.Cho HY, Jedlicka AE, Reddy SP, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 2002; 26: 175–182. doi: 10.1165/ajrcmb.26.2.4501 [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 2016; 7: 11624. doi: 10.1038/ncomms11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez JC, Dang H, Martin JR, et al. Nrf2 modulates host defense during Streptococcus pneumoniae pneumonia in mice. J Immunol 2016; 197: 2864–2879. doi: 10.4049/jimmunol.1600043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olagnier D, Farahani E, Thyrsted J, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun 2020; 11: 4938. doi: 10.1038/s41467-020-18764-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ordonez AA, Bullen CK, Villabona-Rueda AF, et al. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun Biol 2022; 5: 242. doi: 10.1038/s42003-022-03189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C, Eggler AL, Mesecar AD, et al. Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol 2011; 24: 515–521. doi: 10.1021/tx100389r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Talalay P, Cho CG, et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA 1992; 89: 2399–2403. doi: 10.1073/pnas.89.6.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Z, Niu Z, Wu S, et al. Protective mechanism of sulforaphane in Nrf2 and anti-lung injury in ARDS rabbits. Exp Ther Med 2018; 15: 4911–4915. doi: 10.3892/etm.2018.6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey CJ, Thimmulappa RK, Sethi S, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 2011; 3: 78ra32. doi: 10.1126/scitranslmed.3002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown RH, Reynolds C, Brooker A, et al. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res 2015; 16: 106. doi: 10.1186/s12931-015-0253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galea I, Copple IM, Howat DW, et al. SFX-01 reduces residual disability after experimental autoimmune encephalomyelitis. Mult Scler Relat Disord 2019; 30: 257–261. doi: 10.1016/j.msard.2019.02.027 [DOI] [PubMed] [Google Scholar]
- 27.Royce SG, Licciardi PV, Beh RC, et al. Sulforaphane prevents and reverses allergic airways disease in mice via anti-inflammatory, antioxidant, and epigenetic mechanisms. Cell Mol Life Sci 2022; 79: 579. doi: 10.1007/s00018-022-04609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colapietro A, Rossetti A, Mancini A, et al. Multiple antitumor molecular mechanisms are activated by a fully synthetic and stabilized pharmaceutical product delivering the active compound sulforaphane (SFX-01) in preclinical model of human glioblastoma. Pharmaceuticals 2021; 14: 1082. doi: 10.3390/ph14111082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egner PA, Chen JG, Zarth AT, et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res 2014; 7: 813–823. doi: 10.1158/1940-6207.CAPR-14-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axelsson AS, Tubbs E, Mecham B, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 2017; 9: eaah4477. doi: 10.1126/scitranslmed.aah4477 [DOI] [PubMed] [Google Scholar]
- 31.Yagishita Y, Fahey JW, Dinkova-Kostova AT, et al. Broccoli or sulforaphane: is it the source or dose that matters? Molecules 2019; 24: 3593. doi: 10.3390/molecules24193593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Zimmerman AW, Singh K, et al. Biomarker exploration in human peripheral blood mononuclear cells for monitoring sulforaphane treatment responses in autism spectrum disorder. Sci Rep 2020; 10: 5822. doi: 10.1038/s41598-020-62714-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephenson E, Reynolds G, Botting RA, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med 2021; 27: 904–916. doi: 10.1038/s41591-021-01329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon SJ, Jhun J, Ryu J, et al. The anti-arthritis effect of sulforaphane, an activator of Nrf2, is associated with inhibition of both B cell differentiation and the production of inflammatory cytokines. PLoS One 2021; 16: e0245986. doi: 10.1371/journal.pone.0245986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du P, Zhang W, Cui H, et al. Sulforaphane ameliorates the severity of psoriasis and SLE by modulating effector cells and reducing oxidative stress. Front Pharmacol 2022; 13: 805508. doi: 10.3389/fphar.2022.805508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugade AA, Vethanayagam RR, Nasirikenari M, et al. Nrf2 regulates chronic lung inflammation and B-cell responses to nontypeable Haemophilus influenzae. Am J Respir Cell Mol Biol 2011; 45: 557–565. doi: 10.1165/rcmb.2010-0321OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazarakis N, Anderson J, Toh ZQ, et al. Examination of novel immunomodulatory effects of L-sulforaphane. Nutrients 2021; 13: 602. doi: 10.3390/nu13020602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lempp FA, Soriaga LB, Montiel-Ruiz M, et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature 2021; 598: 342–347. doi: 10.1038/s41586-021-03925-1 [DOI] [PubMed] [Google Scholar]
- 39.Bedin AS, Makinson A, Picot MC, et al. Monocyte CD169 expression as a biomarker in the early diagnosis of coronavirus disease 2019. J Infect Dis 2021; 223: 562–567. doi: 10.1093/infdis/jiaa724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinkova-Kostova AT, Fahey JW, Wade KL, et al. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev 2007; 16: 847–851. doi: 10.1158/1055-9965.EPI-06-0934 [DOI] [PubMed] [Google Scholar]
- 41.Duran CG, Burbank AJ, Mills KH, et al. A proof-of-concept clinical study examining the NRF2 activator sulforaphane against neutrophilic airway inflammation. Respir Res 2016; 17: 89. doi: 10.1186/s12931-016-0406-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sudini K, Diette GB, Breysse PN, et al. A randomized controlled trial of the effect of broccoli sprouts on antioxidant gene expression and airway inflammation in asthmatics. J Allergy Clin Immunol Pract 2016; 4: 932–940. doi: 10.1016/j.jaip.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise RA, Holbrook JT, Criner G, et al. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PLoS One 2016; 11: e0163716. doi: 10.1371/journal.pone.0163716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swiderski K, Read SJ, Chan AS, et al. Investigating the potential for sulforaphane to attenuate gastrointestinal dysfunction in mdx dystrophic mice. Nutrients 2021; 13: 4559. doi: 10.3390/nu13124559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suganuma H, Fahey JW, Bryan KE, et al. Stimulation of phagocytosis by sulforaphane. Biochem Biophys Res Commun 2011; 405: 146–151. doi: 10.1016/j.bbrc.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA 2008; 105: 15926–15931. doi: 10.1073/pnas.0808346105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinkova-Kostova AT, Copple IM. Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol Sci 2023; 44: 137–149. doi: 10.1016/j.tips.2022.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00917-2023.SUPPLEMENT (1.3MB, pdf)