Abstract
Optimal transcriptional regulatory circuits are expected to exhibit stringent control, maintaining silence in the absence of inducers while exhibiting a broad induction dynamic range upon the addition of effectors. In the Plac/LacI pair, the promoter of the lac operon in Escherichia coli is characterized by its leakiness, attributed to the moderate affinity of LacI for its operator target. In response to this limitation, the LacI regulatory protein underwent engineering to enhance its regulatory properties. The M7 mutant, carrying I79T and N246S mutations, resulted in the lac promoter displaying approximately 95% less leaky expression and a broader induction dynamic range compared to the wild‐type LacI. An in‐depth analysis of each mutation revealed distinct regulatory profiles. In contrast to the wild‐type LacI, the M7 mutant exhibited a tighter binding to the operator sequence, as evidenced by surface plasmon resonance studies. Leveraging the capabilities of the M7 mutant, a high‐value sugar biosensor was constructed. This biosensor facilitated the selection of mutant galactosidases with approximately a seven‐fold improvement in specific activity for transgalactosylation. Consequently, this advancement enabled enhanced biosynthesis of galacto‐oligosaccharides (GOS).
Evolved mutant LacI displayed less leaky expression. Leveraging the mutant into a biosensor for high‐throughput screening of enzymes for biosynthesis of galacto‐oligosaccharides.

INTRODUCTION
Genetic regulatory proteins whose expression is induced by small molecules have been comprehensively used as biological tools for controlling recombinant expression, fine‐tuning metabolic fluxes, dynamically controlling biosynthetic pathways, monitoring metabolite concentrations and high‐throughput screening for the overproduction of value‐added chemicals in cell factories (Xia et al., 2019). Among bacterial regulatory proteins, the lac repressor LacI, traditionally induced by allolactose, known as 6‐O‐β‐d‐galactopyranosyl‐α‐d‐glucopyranose, which is the transgalactosylation product of β‐galactosidase on lactose (4‐O‐β‐d‐galactopyranosyl‐α‐d‐glucopyranose) (Gilbert & Mullerhi, 1967), paves the way for modern biotechnology. The lac operon, required for the transport and metabolism of lactose, is regulated by LacI. Specifically, in the absence of inducers (such as allolactose), LacI binds to the lac operator sequence within the lac operon (designated as lacO), thus repressing transcription from the lac promoter. In the presence of inducers, LacI, binding to the inducer chemicals, undergoes a conformational change, and subsequently, its affinity for lacO diminishes; consequently, transcription from the promoter increases (Gilbert & Mullerhi, 1967; Matthews & Nichols, 1998). Isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) is a frequently used a gratuitous inducer of LacI, since it is a non‐metabolizable lactose analogue (Lewis, 2005). Despite its widespread application (Ceroni et al., 2010; Kaern et al., 2003; Lapique & Benenson, 2014; Nikel et al., 2022; Studier, 1991; Terpe, 2006), the lac regulatory system is known to exhibit high levels of leaky transcription, indicating that substantial amounts of basal transcription occur even in the absence of inducers (Gatti‐Lafranconi et al., 2013; Kim et al., 2017; Lakshmi & Rao, 2009).
Several studies have tried to explore the relationship between structure and function of LacI repressor (Gordon et al., 1988; Markiewicz et al., 1994; Schmitz et al., 1978; Suckow et al., 1996; Swint‐Kruse et al., 2003; Zhan et al., 2008). The crystal structure of LacI indicated that this repressor protein is a homotetramer. Each subunit contains an N‐terminal DNA‐binding domain, core domain responsible for inducer binding and C‐terminal domain comprising the tetramer interface (Chen & Matthews, 1992; Daber et al., 2007; Friedman et al., 1995; Lewis et al., 1996). The allosteric behaviour of LacI upon binding to the inducer has been extensively studied, which indicates that the O6 hydroxyl of galactoside is essential for establishing a water‐mediated hydrogen‐bonding network that leads to a conformational change that is vital for allosteric transition (Bell & Lewis, 2000; Daber et al., 2007; Matthews et al., 2000; Meyer et al., 2013; Pace et al., 1997; Raman et al., 2014; Swint‐Kruse & Matthews, 2009; Taraban et al., 2008; Xu & Matthews, 2009).
Engineering of regulatory proteins to improve their regulatory properties has broadened their applications in recombinant protein expression, metabolic optimization and biosensing (Bahls et al., 2022; Bertram & Hillen, 2008; Della Corte et al., 2020; Li, Liang, et al., 2017; Li, Maria‐Solano, et al., 2017; Tang & Cirino, 2011; Wang et al., 2017; Xiong et al., 2017; Yao et al., 2020; Zhan et al., 2008; Zhang et al., 2021). Based on semi‐rational approaches, LacI has been engineered to alter its effector specificities, responding to chemicals other than its natural effectors, such as fucose, gentiobiose, lactitol, sucralose and lactulose (Taylor et al., 2016; Wu et al., 2017). Instead, to decrease basal expression, LacIQ1 promoter or its derivatives (Calos, 1978; Calos & Miller, 1981; Glascock & Weickert, 1998), and a hybrid promoter of lacI (Huang et al., 2020), were widely employed in recombinant expression. LacIQ1 promoter leads to a 50‐ to 100‐fold increased expression of lac repressor, greatly repressing the leaky expression because the degree of repression is supposedly dependent on the ratio of intracellular LacI molecules to the lacO operator sites (Calos & Miller, 1981; Glascock & Weickert, 1998; Raibaud & Schwartz, 1984). However, transcriptional leakage is not only related to the number of intracellular repressors, but also relies greatly on the binding affinity of the regulatory protein to its operator DNA sequences, depending on the regulatory properties of the repressor protein itself. Only a few LacI engineering studies have been conducted to address this issue (Gatti‐Lafranconi et al., 2013; Lakshmi & Rao, 2009), and the application potentials of the evolved LacI mutants remain unexplored.
Herein, we demonstrated the feasibility of engineering the best‐ and longest‐studied regulatory protein, lac repressor, for improved regulatory properties. The leaky expression of LacI was significantly reduced, facilitating the development of a high‐quality biosensor for transgalactosylation activity. Galacto‐oligosaccharides (GOS), which are important prebiotic ingredients in functional foods, are mixture of galactose‐containing oligosaccharides produced via the transgalactosylation activity of β‐galactosidase. The evolved LacI mutant in the study exhibited significantly reduced leaky expressions, compared with wild‐type LacI, and has been effectively employed as a regulatory protein in the biosensor used for improved transgalactosylation activity screening. As a proof of concept, this study provides a successful example of high‐quality LacI derivatives with applications in microbial biotechnology.
EXPERIMENTAL PROCEDURES
General
DNA polymerases were purchased from TaKaRa (Dalian, China). Oligonucleotides were synthesized by Synbio Tech (Suzhou, China). Hieff Clone™ Plus Multi One Step Cloning Kit for DNA sequences assembly was purchased from YEASEN Biotechnology Co., Ltd. (Shanghai, China). Epilactose and 6′‐galactosyllactose were purchased from Carbosynth Limited (Berkshire, UK). Other chemicals and reagents were of high quality and obtained from standard commercial sources. DNA sequencing was performed at Life Technologies (Shanghai, China).
All bacteria were routinely cultured in Luria–Bertani (LB) medium (10 g L−1 tryptone, 5 g L−1 yeast extract and 5 g L−1 NaCl). Antibiotics such as ampicillin (100 μg mL−1) and kanamycin (50 μg mL−1) were used when necessary.
Plasmid construction
All strains and plasmids used in this study are shown in Table 1. Sequences for all primers are listed in Table S1.
TABLE 1.
Strains and plasmids used in this study.
| Strains and plasmids | Description | Source |
|---|---|---|
| Strains | ||
| MC1061 | F− araD139 Δ(ara‐leu)7696 galE15 galK16 Δ(lac)X74 rpsL (StrR) hsdR2 (rK − mK +) mcrA mcrB1 | ATCC 53338 |
| BW25113 | F−, DE(araD‐araB)567, lacZ4787(del)::rrnB‐3, LAM−, rph‐1, DE(rhaD‐rhaB)568, hsdR514 | Datsenko and Wanner (2000) |
| BLGR | E. coli BW2113 ΔlacIΔgalKΔrecA | Wu et al. (2017) |
| BL21(DE3) | F′ ompT gal dcm lon hsd SB (rB − mB −) λ(DE3[lacI lacUV5‐T7 gene 1 ind1 sam7 nin5]) | Novagen |
| Plasmids | ||
| pLac7 | LacI expressed under lacI Q1 promoter; Ptac‐gfpuv, pMB1 origin, Ampr | Wu et al. (2017) |
| pLac7‐M7 | LacI‐M7 expressed under lacI Q1 promoter; Ptac‐gfpuv, Ampr | This study |
| pLac7‐I79T | LacI with mutation I79T expressed under lacI Q1 promoter; Ptac‐gfpuv, Ampr | This study |
| pLac7‐N246S | LacI with mutation N246S expressed under lacI Q1 promoter; Ptac‐gfpuv, Ampr | This study |
| pLacO2 | LacI expressed under lacI Q1 promoter; modified Ptac (with two lacO)‐gfpuv, Ampr | This study |
| pLacO3 | LacI expressed under lacI Q1 promoter; modified Ptac (with two lacO)‐gfpuv, Ampr | This study |
| pBAD18‐kan | PBAD; pBR322 origin, Kanr | BioVector NTCC Inc., Beijing, China |
| pBADLacS | PBAD‐6His‐lacS; pBR322 origin, Kanr | Wu et al. (2017) |
| pBADLacS‐R | PBAD‐6His‐lacS; RSF3010 origin, Kanr | This study |
| pBADLacI | PBAD‐6His‐lacI; pBR322 origin, Kanr | Wu et al. (2017) |
| pET28a | T7 promoter, pBR322 origin, Kanr | Novagen |
| pET28a‐LacS | 6His‐LacS or LacS mutant expressed form T7 promoter | Jiang et al. (2015) |
pLac7
Plasmid pLac7 was constructed in previous research (Wu et al., 2017). As shown in Figure S1, Gene lacI was expressed under lacI Q1 promoter (Glascock & Weickert, 1998). Gene gfpuv was expressed under Ptac promoter.
pBADLacS‐R
The lacS gene was amplified using plasmid pBADLacS (Jiang et al., 2015) as template using primes LacS‐GB‐for and LacS‐GB‐rev, then assembled with the PCR product amplified with primers pBAD‐GB‐fwd and pBAD‐GB‐rev using pBAD18‐kan as template, and then, the replication origin of plasmid was replaced with RSF3010 origin, resulting in plasmid pBADLacS‐R (Figure S1).
pLacO2/pLacO3
Additional copies of lacO sequences were inserted into promoter region of Ptac. PCR was performed with plasmid pLac7 as template using primer sets O2‐GB‐fwd/O2‐GB‐rev or O3‐GB‐fwd/O3‐GB‐rev, and the PCR products were individually self‐assembled, resulting in plasmids pLacO2 and pLacO3.
Site‐directed mutagenesis
Single‐mutation I79T or N246S was introduced by PCR amplification with primer sets LacI‐I79T‐fwd/rev or LacI‐N246S‐fwd/rev, respectively, using plasmid pLac7 (Wu et al., 2017) as template. The PCR product was used as MEGA‐primer, the DNA fragment that replaces a homologous region in the template plasmid, to perform megaprimer PCR to amplify the whole plasmid (MEGAWHOP PCR) (Miyazaki, 2011), with plasmid pLac7 (or its derivatives) as template. After digestion with DpnI at 37°C for 12 h, for specific elimination of the template plasmid that can be propagated in E. coli strains and dam‐methylated, the digested product was transformed into E. coli MC1061, resulting in plasmid pLac7‐bearing single‐mutation I79T or N246S.
Library construction
Overlap extension PCR method was used to construct LacI site‐directed saturation library. Gene fragment of lacI was amplified individually with primer sets LacI‐18,22‐for/LacI‐79‐rev or LacI‐79‐for/LacI‐246‐rev, using plasmid pLac7 as template. Equimolar aliquots of the gel‐purified fragments were combined and PCR‐assembled without primers. Finally, outer primers LacI‐18,22‐for and LacI‐246‐rev were added to perform PCR. MEGAWHOP PCR was then performed, with the PCR product containing mutations at all four mutagenesis sites (18, 22, 79 and 246) as a megaprimer. DpnI digestion of the template was done at 37°C for 2 h, and then, DpnI was inactivated at 80°C for 20 min. The PCR product was used to transform strain E. coli MC1061, and ~1 × 106 transformants were collected. Ten randomly picked clones were sequenced, which revealed expected random mutations at targeted positions, with no additional point mutations. Plasmid isolation of all colonies from the agar plates was done to prepare plasmid library.
Error‐prone PCR was applied to construct random mutagenesis libraries of LacS. The lasS gene was amplified using primers LacS‐for and LacS‐rev, with plasmid pBADLacS‐R as template. The PCR mixture was prepared, which contained 5 mM MgCl2, 0.2 mM each of dATP and dGTP, 1 mM each of dCTP and dTTP, 0.1 mM MnCl2 and rTaq DNA polymerase. Then, amplified PCR product containing randomly mutated lacS gene was used as MEGA‐primer with plasmids pBADLacS‐R as template to perform MEGAWHOP PCR. Removement of template and construction of library were performed as described above. Sequencing results revealed that the library contained an average of 2–5 mutations per 1 kbp. All mutant colonies were collected for mutagenesis library.
LacI mutagenesis library screening
The LacI mutagenesis plasmid library (1 × 107 transformants) was used to transform BLGR strain (Table 1). A FACS AriaII sorter (BD, San Jose, USA) was performed for fluorescence‐activated cell sorting (FACS) as described in Wu et al. (2017). In the first round of screening, cells were grown overnight in LB medium and then diluted to OD600 = 0.2 in the same medium. The cells were then cultured for 12 h, and the least fluorescent 8.0 × 106 cells were sorted (representing around 80% of the total cultured cells) for negative screening to remove variants with high leaky expression. The collected cells were subjected to another negative screen in the presence of 10 mM lactose to remove variants responsive to substrate lactose. Afterwards, the sorted cells were induced with 5 mM IPTG for 12 h for positive screening, in which the proportions with the highest fluorescence (around 1 × 105 cells) were sorted (representing around 1% of the total cultured cells). This double negative–positive triple screening strategy was repeated for 4 times, and then, six clones from the library were selected for further studies.
Screening of the LacS random mutagenesis library
BLGR strain harbouring plasmid pLac7‐M7 was transformed with the LacS plasmid library, and 1 × 107 transformants were collected. The cells were grown in LB medium supplemented with 1 μM arabinose and 5 mM lactose for 12 h. The most fluorescent cells (approx. 1 × 105) were collected by FACS from 1 × 107 cells. This sorting strategy was repeated 3 times, and the sorted cells were plated on LB agar plates supplemented with 1 μM arabinose and 5 mM lactose. After incubation of 14 h, colonies exhibiting the highest levels of fluorescence (UV 302 nm, observed by the eye) were selected for rescreening.
Fluorescence assays
A single colony of strain BLGR harbouring plasmid pLac7 was cultured for 12 h in LB medium, then diluted to OD600 = 0.2 with same medium consisting of appropriate concentrations of inducers. The cells were allowed to grow under inducing condition at 37°C for 12 h. Two hundred microliter of culture was centrifuged, and then, the cells were resuspended in 200 μL of 10 mM phosphate‐buffered saline (PBS, pH 7.5). The fluorescence and OD600 were measured with a SynergyMx Multi‐Mode Microplate Reader (BioTek, Vermont, USA) (395‐nm excitation filter and 509/20‐nm emission filter). The background fluorescence due to buffer was subtracted in all measurements. The inducing fold was calculated as the ratio of maximal induced fluorescence to the fluorescence in the absence of inducer (leaky expression) (Li, Liang, et al., 2017; Li, Maria‐Solano, et al., 2017; Tang & Cirino, 2010).
A colony of strain BLGR co‐expressing plasmids pBADLacS‐R and pLac7‐M7 was cultured in LB medium for 12 h, then diluted to OD600 = 0.05 with the same medium consisting of 2 μM arabinose and 5 mM lactose. The cells were grown for 12 h. 200 μL culture was collected to measure the cell density and GFP fluorescence, with a SynergyMx Multi‐Mode Microplate Reader as described above.
All fluorescence data provided in figures were normalized to cell density. All data represent the mean of three independent experiments.
Purification of 6×His‐tagged wild‐type LacI, LacS and their mutants
A single colony of strain E. coli BL21 (DE3)‐expressing wild‐type or mutant LacS from plasmid pET28‐LacS (Jiang et al., 2015) was cultured in LB for 12 h at 37°C. The culture was diluted to OD600 = 0.05 with the same medium. Then, the cells were grown at 37°C until OD600 = 0.8, 0.4 mM IPTG was supplemented and the cells were further grown at 30°C for 10 h. Cells were harvested by centrifugation at 5000 ×g for 10 min at 4°C and then resuspended in 50 mM Tris–HCl buffer (pH 8.0, 300 mM NaCl, 20 mM imidazole) for sonication. The lysate was centrifuged at 4°C for 10 min at 10,000 ×g. The supernatant was applied to Ni‐NTA resin (Sigma‐Aldrich). The column was then washed with 50 mM Tris–HCl buffer (pH 8.0, 300 mM NaCl, 20 mM imidazole), and the bound protein was finally eluted with 50 mM Tris–HCl buffer (pH 8.0, 300 mM NaCl, 250 mM imidazole). Dialysis was performed at 4°C against 50 mM phosphate buffer (pH 6.5) to remove imidazole.
Colonies of strain BW25113‐expressing wild‐type LacI or its mutants from plasmid pBADLacI (Wu et al., 2017) were grown in LB at 37°C for 12 h, respectively. The culture was then diluted to OD600 = 0.05 with the same medium. The cells were grown at 37°C till OD600 = 0.8. A dose of 1 mM l‐arabinose was supplemented, and then, the culture was continuously grown at 30°C for 12 h. The cells were harvested and lysed, and then, the 6×His‐tagged LacI protein was purified as described above. The purified protein was dialysed against 10 mM PBS (pH 7.4).
The purity of proteins was assessed with SDS‐PAGE (Figure S2), and the protein concentration was assayed with the Bradford method (Bradford, 1976).
Surface plasmon resonance assay
A Biacore T100 apparatus (GE Healthcare, Uppsala, Sweden) was used to perform surface plasmon resonance (SPR) assays at 25°C. To compare the binding affinity of purified wild‐type and M7‐mutant LacI for the operator sequence, fragment of tac promoter DNA was amplified using plasmid pLac7 as template, with the 5′ biotin‐conjugated primer Biotin‐Ptac‐for and primer Ptac‐rev. Then, the PCR products were immobilized to a sensor chip SA (GE Healthcare). 200 mM Tris–HCl buffer (pH 7.5, 200 mM KCl, 10 mM EDTA and 0.05% Tween‐20) was used as running buffer. Different concentrations of purified wild‐type LacI (50, 100, 200, 400 and 800 nM) or M7 (0.3125, 0.625, 1.25, 2.5, 5 and 10 nM) were tested. Analytes with different concentrations were injected to the DNA immobilized surface and blank flow cell for 60 s at a flow rate of 30 μL min−1, followed by 120 s dissociation in running buffer. The curves were fitted to 1:1 binding model to obtain affinity data.
Biacore control software was used to collect the data. The refractive index changes as a function of time under constant flow conditions were monitored. The net increase of refractive index over time, compared with that of running buffer alone, was measured, to determine the relative amount of binding. There is an inline subtraction of reference surface during the run. Biacore T100 evaluation software 2.0.1 was used to calculate kinetic association (k a) and dissociation (k d) constants. The equilibrium dissociation constant K D values were calculated with the equation K D = k d/k a.
HPLC identification of the reaction products
The reaction mixture for GOS production (500 μL) consisted of 50 mM phosphate buffer (pH 6.5), 0.438 M lactose and 50 μL crude or purified 6×His‐LacS enzyme. The reaction was incubated at 37 or 70°C for 12 h. The reaction was stopped by cooling at 4°C instantly.
The reaction products of LacS were quantified by Agilent 1200 system (Agilent Technologies, Palo Alto, USA), equipped with a refractive index detector (RID). Separation was achieved with a Cosmosil sugar‐D column (250 × 4.6 mm, 5 μm), (Nacalai tesque, Japan) working at 60°C with the mobile phase of 80% acetonitrile at a flow rate of 1.5 mL min−1. The production of GOS was calculated with a calibration curve prepared with the commercially available authentic 6′‐galactosyllactose. For purified LacS, one unit (U) of enzyme activity was defined as the amount of enzyme required to produce 1 μmol GOS from lactose per hour.
Matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry analysis
The eluted peak assumed to represent GOS was collected and verified using matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF‐MS) on an Applied Biosystems SCIEX TOF/TOF™ 5800 system (Foster, USA).
Structure model of M7 mutant
The LacI protein molecule was selected for the study and its structure was obtained from the website http://www.rcsb.org/ (PDB ID: 2p9h) at a resolution of 2.00 Å. The M7 mutant was homology modelled based on it, using the website SWISS (https://swissmodel.expasy.org). The structural formula of the ligand IPTG was drawn using Chem draw and subsequently turned into a PDB file. Proteins and ligands were dehydrated and hydrogenated using the docking program AutoDock 4.2.6 (Morris et al., 2009). The grid box was located in the centre of the ligand binding site region, the dimensions for the used grid box were 10 × 12 × 12 Angstrom. Finally, the conformation showing the most favourable binding energy was selected for the analysis of the ligand–protein interaction.
Molecular dynamics of LacS and its mutant
The molecular dynamics (MD) simulations were conducted using GROMACS software package (Abraham et al., 2015) with the CHARMM36‐Feb2021 force field for a time scale of 20 ns at 310.15 and 343.15 K. The binding free energy of the protein–ligand complex was calculated from molecular mechanics Poisson–Boltzmann surface area (MM‐PBSA) using the gmx_mmPBSA tool (Genheden & Ryde, 2015).
RESULTS
Engineering LacI for improved regulatory property
Low leaky expression is important for developing a high‐quality regulatory protein‐based biosensor, because a high‐level background of reporter signal greatly interferes with the readout of biosensor response. With high leaky expression, wild‐type LacI failed to behave as a favourable biosensor for effective high‐throughput screening of the β‐galactosidase variants with improved transgalactosylation activity (data not shown). The leaky expression of LacI needs to be greatly repressed to develop an effective biosensor for transgalactosylation activity.
Under allosteric conditions, both the effector and DNA‐binding domains are reported to be potentially related to the regulation of leaky expression without inducer supplementation (Daber et al., 2007; Taylor et al., 2016). In overall 361 amino acids of LacI protein, considering the contributions of both domains, a site‐saturation mutagenesis library containing four amino acid residue positions was designed, with two residues involved in effector binding, I79 and N246, and two residues involved in DNA binding, Q18 and R22 (Lewis et al., 1996). By targeting these four residues, a site‐directed saturation mutagenesis library of approximately 1.0 × 107 LacI mutants was constructed and subjected to ultra‐high‐throughput FACS screening. The E. coli BLGR strain was used for library expression, as wild‐type lacI in this strain was knocked out (Wu et al., 2017) (Table 1). To select LacI mutants with low leaky expression without reducing the induction strength, positive and negative screens were carried out. Negative screenings were carried out without any inducer or with addition of lactose (10 mM), which is an anti‐inducer for wild‐type LacI, to remove mutants exhibiting high leaky expressions or those responsive to both lactose and IPTG, respectively. Then, positive screening was conducted with the addition of 5 mM IPTG. After four rounds of the above‐described triple screen, six mutants displaying lower basal expression and full‐induction expression levels similar to wild‐type LacI in the presence of IPTG were selected, among which M7 mutant displayed approximately 95% less leaky expression than wild‐type LacI (Figure 1A). The lac promoter regulated by M7 exhibited approximately 200‐fold induction of GFPuv expression under conditions of IPTG induction, wherein half‐maximal induction was observed at ~1 mM IPTG. Under the same experimental conditions, wild‐type LacI showed an inducing fold of approximate 10‐fold, and the half‐maximal induction was observed at ~0.05 mM IPTG (Figure 1B), reaching an almost full induction at approximately 0.5 mM IPTG. Thus, M7 displayed a much higher fold induction than wild‐type LacI, largely because of the reduced basal expression. Sequencing showed that the beneficial M7 mutant harboured two mutations, I79T and N246S.
FIGURE 1.

LacI engineering. (A) GFP expression after 12‐h cultivation of Escherichia coli BLGR strain expressing LacI wild‐type (WT) or the selected LacI mutants (M1‐M7) from plasmid pLac7, in the presence (+) and absence (−) of 5 mM IPTG. (B) The IPTG dose–response curves of GFP expression in strain BLGR, which expresses wild‐type LacI (WT), M7 mutant (M7) or LacI with single‐mutation N246S (N246S) or I79T (I79T). (C) Basal expression levels (leaky expression) and the final inducing folds (calculated as the ratio of maximal induced fluorescence to the leaky expression) in strain BLGR expressing the indicated LacI regulators. (D) The modelled IPTG binding pocket of mutant M7, compared with wild‐type LacI (WT) (PDB ID: 2P9H) (Daber et al., 2007). (E) Comparison of GFP expression levels in strain BLGR containing pLac7‐M7 or pLac7 derivative with tandem lacOs, pLacO2 or pLacO3. Error bars indicate SD of three independent replicates. All the cells were allowed to grow under inducing condition at 37°C for 12 h before the measurements.
To evaluate the contribution of single mutations, LacI mutants harbouring each of these two amino acid substitutions were constructed. Figure 1C shows that although both mutations led to stringent regulation of expression in the absence of an inducer, compared with wild‐type LacI, the substitution N246S displayed the dominant contribution. An additive effect of I79T and N246S was observed by further lowering the leaky expressions. Half‐maximal induction of N246S and I79T occurred at ~0.2 and 0.09 mM IPTG, respectively. Comparable to the stringency of M7 in the absence of inducer, N246S mutant reached a similar full expression as M7 at ~1 mM IPTG, whereas I79T displayed a more gradual slope of induction than the other two mutants, with a 60‐fold increase over a broad range of 0.001–10 mM IPTG (Figure 1B). Therefore, although all had low basal expression, the M7, N246S and I79T mutants displayed different regulatory profiles and, thus, have the potential to be applied in various circumstances. The interactions of residues 246 and 79 with IPTG in M7 mutant were modelled and compared with the interactions between these two residues and inducer IPTG in the wild‐type LacI (Figure 1D).
In most studies, promoters modified with tandem operators are used to minimize leaky expression (Zhang et al., 2012). For comparison, another copy of the LacI operator, lacO, was inserted into different regions of the promoter Ptac, resulting in plasmids with modified promoters, pLacO2 and pLacO3 (Figure 1E). Although they exhibited basal expression levels comparable to those of the M7 mutant without inducer supplementation, the maximal expression levels severely decreased in pLacO2 and pLacO3, reaching only ~10% and ~1.7% of the full expression of M7. The overall inducing fold for pLacO2 and pLacO3 remained approximately 19‐ and 2.8‐fold, respectively, which is unfavourable for downstream applications. This result reveals the advantages of regulatory protein engineering over promoter modification strategies.
SPR analysis of DNA binding by wild‐type and mutant LacIs
To explore the mechanism underlying the significantly reduced leaky expression regulated by M7 mutant, it was purified, along with the wild‐type LacI, to compare their binding affinities for the tac promoter sequences, using SPR analysis (Figure 2). The binding K D values of wild‐type LacI and M7 mutant for promoter DNA were 41.8 ± 0.38 and 0.28 ± 0.03 nM, respectively, revealing a tight binding of M7 to promoter DNA, which resulted in significantly reduced leaky expression. Other curve‐fitted constants are shown in Table S2.
FIGURE 2.
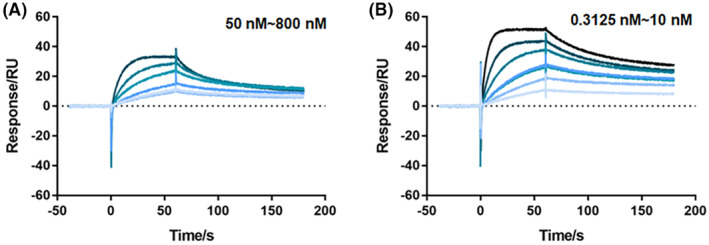
The binding affinities of purified 6×His‐tagged LacI wild‐type (A) and M7 mutant (B) for tac promoter DNA were measured by SPR. Different concentrations of purified wild‐type LacI (50, 100, 200, 400, 800 nM) or M7 (0.3125, 0.625, 1.25, 2.5, 5, 10 nM) were used for determination.
Application of a M7 mutant‐based biosensor in transgalactosylation activity engineering
As an important GOS synthase, β‐galactosidase catalyses the hydrolysis of lactose and formation of transgalactosylation products, including allolactose (transgalactosylated disaccharides) and GOS. Compared with the hydrolysis, transgalactosylation is a minor activity of β‐galactosidases. Thus, how to improve the transgalactosylation activity is critical for efficient GOS production. The M7 mutant was used to develop a high‐quality in vivo high‐throughput screening tool to engineer β‐galactosidase from Sulfolobus solfataricus (LacS) (Nucci et al., 1993) for improved transgalactosylation activity (Figure 3A). LacS is hyperthermostable and has been reported to produce GOS from lactose with high transgalactosylation capability (Hansson & Adlercreutz, 2001; Torres et al., 2010). As reported, at high temperatures, low viscosity and high solubility of saccharides facilitate in vitro synthesis. As our biosensor supports an in vivo screening, we explored the possibility of engineering LacS to produce GOS at a wider range of temperatures (from 37 to 75°C) (Akanuma et al., 2019; Li, Liang, et al., 2017; Li, Maria‐Solano, et al., 2017), facilitating both in vitro and in vivo applications.
FIGURE 3.

Feasibility of the M7 mutant‐based biosensor used for transgalactosylation activity screening. (A) Scheme of screening for improved transgalactosylation activity using biosensor. Inducing effects of IPTG and/or lactose on (B) wild‐type LacI and (C) M7 mutant. (D) GFP fluorescence of strain BLGR harbouring plasmids plac7‐M7 and pBADLacS‐R supplemented with inducer l‐arabinose and substrate lactose at the indicated concentrations. The cells were cultured at 37°C for 12 h.
The feasibility of the biosensor was explored. As shown in Figure 3B,C, similar with wild‐type LacI, since negative screens upon lactose supplementation were performed, M7 mutant was responsive to IPTG, an analogue of allolactose, but not to lactose, indicating that mutations within the effector binding pocket did not significantly alter the recognition properties. In addition, the presence of lactose did not interfere with the induction by IPTG. This recognition specificity is important for the following biosensor development because lactose is a frequently used substrate for GOS biosynthesis. Interestingly, mutations within the binding pocket significantly influenced DNA‐binding behaviour, without disturbing effector recognition.
The biosensor responses were then compared in the presence and absence of LacS expression. As shown in Figure 3D, the fluorescence signals could be detected only in the presence of both l‐arabinose (as inducer of LacS expression) and lactose (the substrate of LacS), indicating that it was reporting the synthesis of transgalactosylation products by LacS using lactose as a substrate. The LacS mutagenesis library was then generated by error‐prone PCR, as mentioned in Experimental Procedures section. The mixed plasmids expressing LacS with random mutations were co‐expressed with the biosensor in the BLGR strain at 37°C, wherein the lacZ gene encoding E. coli β‐galactosidase was deficient. Three sequential rounds of FACS‐positive screening were performed to enrich the most intensely fluorescent cells, which are believed to be GOS hyper‐producers. The cells were plated on LB agar in the presence of 1 μM l‐arabinose (to induce LacS expression) and 5 mM lactose for visual screening.
Four colonies (S4, S5, S19, S21) with the highest GFP fluorescence levels were selected under UV light irradiation (302 nm). Rescreening in test tubes confirmed that the GFP fluorescence emitted by these colonies was induced upon supplementation with lactose, which is not a direct inducer of M7, thereby revealing that fluorescence expression came from allolactose induction, produced by the LacS mutants (Figure 4A). Plasmids expressing LacS mutants from these selected colonies were isolated and used to retransform the BLGR strain harbouring the pLac7‐M7 plasmid, and improved GFP fluorescence under lactose supplementation was confirmed. HPLC analyses demonstrated that the selected variants produced higher concentrations of GOS than wild‐type LacS (Figure 4A). The mutant LacS from the highest producer, mutant S5 (bearing a single‐mutation E112K), was used as a parental enzyme for another round of random mutagenesis. Three variants displaying the strongest GFP fluorescence were selected during the second round, and exhibited the highest GOS production (Figure 4A). The wild‐type LacS and the highest producer, S5–11, were purified. Mutant S5–11 (bearing mutations T23A, Q103R, E112K, M275K, V406I and F441Y) was found to display ~12‐ and 2‐fold increase of GOS production at 37 and 70°C (optimal reaction temperature of wild‐type LacS), respectively, when compared with wild‐type LacS (Figure 4B). The mutant S5–11 enzyme showed ~7‐ and 1.1‐fold increases in specific activity at 37 and 70°C, respectively, when compared with wild‐type LacS (Figure 4C). MALDI‐TOF‐MS revealed that both wild‐type and mutant LacSs mainly produced transgalactosylated trisaccharides from lactose under our experimental conditions (Figure S3).
FIGURE 4.

LacS engineering aided by the M7 mutant‐based biosensor. (A) GFP fluorescence and the GOS production (by crude enzyme) of the selected mutants from two rounds of random mutagenesis. A dose of 1 μM arabinose (inducer) and 5 mM lactose (substrate) was supplemented, and the cells were cultured for 12 h at 37°C. The strain expressing wild‐type LacS (WT) was included as reference. Fluorescence in the presence (+) or absence (−) of substrate lactose was determined. Comparison between GOS production (B) and specific activities (C) of purified wild‐type LacS (WT) and mutant S5‐11 at 37 and 70°C. The reaction was incubated at indicated temperatures for 12 h, supplemented with 0.438 M lactose. One unit (U) of enzyme activity was defined as the amount of enzyme required to produce 1 μmol GOS from lactose per hour in (C). Error bars indicate SD of three independent replicates.
The MM‐PBSA approach (Genheden & Ryde, 2015) is one of the most widely used methods to compute interaction energies among the biomolecular complexes. The average binding free energies of LacS and LacS‐S5–11 with substrate lactose were calculated (Figure S4). The results showed that at 37°C, the average binding free energy of LacS‐S5‐11 was −40 kcal mol–1, lower than that of wild‐type LacS (−27.44 kcal mol–1), indicating that the substrate binding affinity of LacS‐S5‐11 was stronger than that of wild‐type enzyme at this temperature. Additionally, at 70°C, the average binding free energy of LacS‐S5–11 was −39 kcal mol–1, a little bit lower than that of LacS (−33.82 kcal mol–1). The binding free energy difference at 70°C was not as significant as that at 37°C. Accordingly, LacS‐S5–11 mutant exhibited a significant improvement of specific activity at 37°C, the temperature for in vivo screening, compared with 70°C, the optimal reaction temperature of wild‐type LacS (Figure 4C). These results provide an important basis for further investigation of the function and potential applications of the LacS‐S5–11 mutant.
DISCUSSION
Nowadays, regulatory protein and promoter DNA engineering constitute two main strategies for altering the transcriptional behaviour of downstream genes. Promoter engineering has found wide applications in metabolic engineering and cellular therapeutic development (Cazier & Blazeck, 2021). In this study, we explored the strategy of regulatory protein engineering in improving the transcriptional properties and the downstream applications. As a repressor of lac operon, wild‐type LacI has been extensively studied. Attempts have been made to alter the recognition specificity for novel regulatory demands (Taylor et al., 2016; Wu et al., 2017; Zhan et al., 2008); however, as an inherent defect, high transcriptional levels in the absence of inducer retard its application. Thus, an increased stringency of the LacI system is urgently needed. In the present study, leaky expressions were overcome through directed evolution. Conformational changes in LacI were introduced by mutations within the effector binding pocket, resulting in higher affinity of the N‐terminal DNA‐binding domain for the operator DNA, as revealed by SPR analysis. This result indicates the importance of the allosteric effect on the regulatory performance of LacI. However, unlike ligand binding (Della Corte et al., 2020), the prediction of functional alterations introduced by allosteric effects is still in its infancy (Greener & Sternberg, 2018). Therefore, direct evolution becomes a powerful strategy in engineering allosteric regulations.
Tighter binding of DNA requires a higher concentration of IPTG to release the regulatory protein from DNA. Thus, achieving half‐maximal induction of mutants N246 and M7 required higher concentrations of IPTG than the wild‐type LacI (Figure 1B). The extremely low levels of leaky expression and high fold induction have broadened their applications. Stringently controlled tac/lac/trc promoters can be used to design high‐quality genetic circuits, such as fine‐tuning metabolic pathway fluxes and controlling unstable or virulent protein expression. In addition, although the inducing fold of I79T mutant was lower than that for M7 and N246, it exhibited a standard curve‐like response behaviour, showing a gradual increase in response over a wide range of IPTG concentrations. This type of regulation is steady and proportional, rather than a switch‐like induction, and has corresponding application scenarios. Therefore, various regulatory behaviours can be achieved via regulatory protein engineering. Furthermore, as LacI has been employed for protein production not only in E. coli, but also in a range of other organisms, including mammalian cells, we believe that these evolved LacI mutants have extensive applications in biotechnology.
To explore the mechanism leading to significantly lowed leaky expression, we demonstrated with SPR that the M7 mutant displayed a higher binding affinity for the operator than wild‐type LacI. Similarly, in the previous study, LacI mutant W220F, displaying 100‐fold wider dynamic range at 37°C and 10‐fold decrease in leakiness, was obtained. This mutant also required higher concentration of IPTG for inducing and was proved to show increased protein stability in the presence of the operator, compared with wild‐type LacI (Gatti‐Lafranconi et al., 2013). Thus, in the absence of inducer, more inclined binding of operator reduces leaky expression. In the presence of inducer, the operator binding affinity is related to not only the DNA binding pocket itself, but also the inducer binding pocket via allosteric regulation. The conformational change upon inducer binding triggers the release of operator. Although nowadays the computational simulations are widely used (Ding et al., 2010; Jin & Li, 2023), the prediction of allosteric regulation is still difficult. Crystal structure studies have indicated that the allosteric regulation of LacI is induced by a water‐mediated hydrogen‐bonding network in which the O6 hydroxyl of galactoside plays an important role. The binding affinity for operator is not necessarily in a negative correlation with the inducer binding affinity, but closely regulated by the conformational change upon inducer binding. Therefore, the K D calculated from the IPTG dose–response curve (Figure 1B) was an inducing K D value, but not binding K D value, which reflects the IPTG binding affinity of LacI.
Stringency is an important property of high‐quality, regulator‐based biosensors. In our previous study, the use of wild‐type LacI as a high‐throughput screening tool had failed in effectively selecting beneficial LacS variants with improved transgalactosylation activity because of its high level of leaky expression, which led to a rapid increase in fluorescence intensity in cells, even in the absence of the LacS enzyme (data not shown). Herein, equipped with M7 mutant as a high‐quality biosensor, LacS mutants with improved transgalactosylation activities were successfully selected. Glycosidases are carbohydrate‐processing enzymes that primarily catalyse the hydrolysis of glycosidic linkages in glycosides. Many glycosidases also catalyse transglycosylation reactions (Lombard et al., 2014). In addition to lactose hydrolysis, β‐galactosidases catalyse the formation of GOS. Hydrolysis or transgalactosylation occurs within the same catalytic centre, when the incoming acceptor substrate is water or sugar, respectively. To date, limited effective methods exist for improving the minor transglycosylation activity (Rye & Withers, 2000). In this study, we developed the biosensor based on the M7 mutant, allowing in vivo screening of the transgalactosylation activity with an efficient, high‐throughput process, thus providing novel ideas for future transglycosylation activity screening. In addition, as our screening was performed in vivo, the transgalactosylation activity of the LacS mutant exhibited significant improvement at 37°C and relatively minor improvement at 70°C (the optimal reaction temperature of wild‐type LacS), indicating that this mutant is functional over a wider range of reaction temperatures than the wild‐type enzyme, which will broaden its applications, such as producing GOS in gut microbiome (Arnold et al., 2021; Kim et al., 2020).
In conclusion, the well‐known regulatory protein, lac repressor, was engineered for reduced leaky expression, with the aim of developing an effective biosensor for transgalactosylation activity. Aided by this biosensor, mutant β‐galactosidase with significantly improved GOS synthesis capability over a wide range of temperatures has been obtained via directed evolution. This work has revealed the great potential of engineering regulatory proteins for novel biotechnological applications.
AUTHOR CONTRIBUTIONS
Jieyuan Wu: Conceptualization (supporting); data curation (lead); formal analysis (equal); methodology (equal). Chaoning Liang: Conceptualization (equal); methodology (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). Yufei Li: Methodology (equal); validation (equal). Yueting Zeng: Visualization (equal). Xu Sun: Methodology (equal). Peixia Jiang: Methodology (equal). Wei Chen: Methodology (equal). Dandan Xiong: Methodology (equal); validation (equal). Jian‐Ming Jin: Investigation (lead); supervision (equal). Shuang‐Yan Tang: Conceptualization (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interest.
ETHICS STATEMENT
This article does not contain any studies involving human participants or animals performed by any of the authors.
Supporting information
Data S1
ACKNOWLEDGEMENTS
We appreciate Junying Jia and Yuanyuan Chen, Zhenwei Yang and Bingxue Zhou of Institute of Biophysics, Chinese Academy of Sciences, for their technical assistance in flow cytometry analysis and surface plasmon resonance (SPR) assay. We thanked Mr. Ye Mao for helpful discussions. This work was supported by the National Key Research and Development Program of China (Grant 2021YFC2103901), the National Natural Science Foundation of China (Grant Nos. 31971337, 31970080, 31961133016 and 31971382) and Instrument Developing Project of Chinese Academy of Science (YJKYYQ20210032).
Wu, J. , Liang, C. , Li, Y. , Zeng, Y. , Sun, X. , Jiang, P. et al. (2024) Engineering and application of LacI mutants with stringent expressions. Microbial Biotechnology, 17, e14427. Available from: 10.1111/1751-7915.14427
Jieyuan Wu and Chaoning Liang contributed equally to this work.
Contributor Information
Chaoning Liang, Email: laraineliang@163.com.
Jian‐Ming Jin, Email: jinjianming@btbu.edu.cn.
Shuang‐Yan Tang, Email: tangsy@im.ac.cn.
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abraham, M.J. , Murtola, T. , Schulz, R. , Páll, S. , Smith, J.C. , Hess, B. et al. (2015) GROMACS: high performance molecular simulations through multi‐level parallelism from laptops to supercomputers. SoftwareX, 1‐2, 19–25. [Google Scholar]
- Akanuma, S. , Bessho, M. , Kimura, H. , Furukawa, R. , Yokobori, S.‐I. & Yamagishi, A. (2019) Establishment of mesophilic‐like catalytic properties in a thermophilic enzyme without affecting its thermal stability. Scientific Reports, 9, 9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, J.W. , Roach, J. , Fabela, S. , Moorfield, E. , Ding, S. , Blue, E. et al. (2021) The pleiotropic effects of prebiotic galacto‐oligosaccharides on the aging gut. Microbiome, 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahls, M.O. , Platz, L. , Morgado, G. , Schmidt, G.W. & Panke, S. (2022) Directed evolution of biofuel‐responsive biosensors for automated optimization of branched‐chain alcohol biosynthesis. Metabolic Engineering, 69, 98–111. [DOI] [PubMed] [Google Scholar]
- Bell, C.E. & Lewis, M. (2000) A closer view of the conformation of the Lac repressor bound to operator. Nature Structural Biology, 7, 209–214. [DOI] [PubMed] [Google Scholar]
- Bertram, R. & Hillen, W. (2008) The application of Tet repressor in prokaryotic gene regulation and expression. Microbial Biotechnology, 1, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Calos, M.P. (1978) DNA sequence for a low‐level promoter of Lac repressor gene and an up promoter mutation. Nature, 274, 762–765. [DOI] [PubMed] [Google Scholar]
- Calos, M.P. & Miller, J.H. (1981) The DNA sequence change resulting from the IQ1 mutation, which greatly increases promoter strength. Molecular & General Genetics, 183, 559–560. [DOI] [PubMed] [Google Scholar]
- Cazier, A.P. & Blazeck, J. (2021) Advances in promoter engineering: novel applications and predefined transcriptional control. Biotechnology Journal, 16, 2100239. [DOI] [PubMed] [Google Scholar]
- Ceroni, F. , Furini, S. , Giordano, E. & Cavalcanti, S. (2010) Rational design of modular circuits for gene transcription: a test of the bottom‐up approach. Journal of Biological Engineering, 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. & Matthews, K.S. (1992) Deletion of lactose repressor carboxyl‐terminal domain affects tetramer formation. The Journal of Biological Chemistry, 267, 13843–13850. [PubMed] [Google Scholar]
- Daber, R. , Stayrook, S. , Rosenberg, A. & Lewis, M. (2007) Structural analysis of lac repressor bound to allosteric effectors. Journal of Molecular Biology, 370, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko, K.A. & Wanner, B.L. (2000) One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proceedings of the National Academy of Sciences of the United States of America, 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Corte, D. , van Beek, H.L. , Syberg, F. , Schallmey, M. , Tobola, F. , Cormann, K.U. et al. (2020) Engineering and application of a biosensor with focused ligand specificity. Nature Communications, 11, 4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X.M. , Pan, X.Y. , Xu, C. & Shen, H.B. (2010) Computational prediction of DNA‐protein interactions: a review. Current Computer‐Aided Drug Design, 6, 197–206. [DOI] [PubMed] [Google Scholar]
- Friedman, A.M. , Fischmann, T.O. & Steitz, T.A. (1995) Crystal structure of lac repressor core tetramer and its implications for DNA looping. Science, 268, 1721–1727. [DOI] [PubMed] [Google Scholar]
- Gatti‐Lafranconi, P. , Dijkman, W.P. , Devenish, S.R. & Hollfelder, F. (2013) A single mutation in the core domain of the lac repressor reduces leakiness. Microbial Cell Factories, 12, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genheden, S. & Ryde, U. (2015) The MM/PBSA and MM/GBSA methods to estimate ligand‐binding affinities. Expert Opinion on Drug Discovery, 10, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, W. & Mullerhi, B. (1967) The lac operator is DNA. Proceedings of the National Academy of Sciences of the United States of America, 58, 2415–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascock, C.B. & Weickert, M.J. (1998) Using chromosomal lacI(Q1) to control expression of genes on high‐copy‐number plasmids in Escherichia coli . Gene, 223, 221–231. [DOI] [PubMed] [Google Scholar]
- Gordon, A.J.E. , Burns, P.A. , Fix, D.F. , Yatagai, F. , Allen, F.L. , Horsfall, M.J. et al. (1988) Missense mutation in the lac I gene of Escherichia coli. Inferences on the structure of the repressor protein. Journal of Molecular Biology, 200, 239–251. [DOI] [PubMed] [Google Scholar]
- Greener, J.G. & Sternberg, M.J.E. (2018) Structure‐based prediction of protein allostery. Current Opinion in Structural Biology, 50, 1–8. [DOI] [PubMed] [Google Scholar]
- Hansson, T. & Adlercreutz, P. (2001) Optimization of galactooligosaccharide production from lactose using β‐glycosidases from hyperthermophiles. Food Biotechnology, 15, 79–97. [Google Scholar]
- Huang, S.‐Y. , Song, Y.‐H. , Zhuang, X.‐Y. , Gao, Z.‐Y. , Wang, K. , Peng, Y.‐J. et al. (2020) Design and application of an artificial hybrid promoter P luxI‐lacO in genetic circuit to achieve lower basal expression level. Applied Biochemistry and Biotechnology, 191, 893–903. [DOI] [PubMed] [Google Scholar]
- Jiang, P.X. , Mu, S.S. , Li, H. , Li, Y.H. , Feng, C.M. , Jin, J.M. et al. (2015) Design and application of a novel high‐throughput screening technique for 1‐deoxynojirimycin. Scientific Reports, 5, 8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, R. & Li, X. (2023) Backdoor attack and defense in federated generative adversarial network‐based medical image synthesis. Medical Image Analysis, 90, 102965. [DOI] [PubMed] [Google Scholar]
- Kaern, M. , Blake, W.J. & Collins, J.J. (2003) The engineering of gene regulatory networks. Annual Review of Biomedical Engineering, 5, 179–206. [DOI] [PubMed] [Google Scholar]
- Kim, M.G. , Jo, K. , Chang, Y.B. , Suh, H.J. & Hong, K.B. (2020) Changes in the gut microbiome after galacto‐oligosaccharide administration in loperamide‐induced constipation. Journal of Personalized Medicin, 10, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.K. , Lee, D.H. , Kim, O.C. , Kim, J.F. & Yoon, S.H. (2017) Tunable control of an Escherichia coli expression system for the overproduction of membrane proteins by titrated expression of a mutant lac repressor. ACS Synthetic Biology, 6, 1766–1773. [DOI] [PubMed] [Google Scholar]
- Lakshmi, O.S. & Rao, N.M. (2009) Evolving Lac repressor for enhanced inducibility. Protein Engineering, Design & Selection, 22, 53–58. [DOI] [PubMed] [Google Scholar]
- Lapique, N. & Benenson, Y. (2014) Digital switching in a biosensor circuit via programmable timing of gene availability. Nature Chemical Biology, 10, 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M. (2005) The lac repressor. Comptes Rendus Biologies, 328, 521–548. [DOI] [PubMed] [Google Scholar]
- Lewis, M. , Chang, G. , Horton, N.C. , Kercher, M.A. , Pace, H.C. , Schumacher, M.A. et al. (1996) Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science, 271, 1247–1254. [DOI] [PubMed] [Google Scholar]
- Li, G. , Maria‐Solano, M.A. , Romero‐Rivera, A. , Osuna, S. & Reetz, M.T. (2017) Inducing high activity of a thermophilic enzyme at ambient temperatures by directed evolution. Chemical Communications, 53, 9454–9457. [DOI] [PubMed] [Google Scholar]
- Li, H. , Liang, C. , Chen, W. , Jin, J.‐M. , Tang, S.‐Y. & Tao, Y. (2017) Monitoring in vivo metabolic flux with a designed whole‐cell metabolite biosensor of shikimic acid. Biosensors & Bioelectronics, 98, 457–465. [DOI] [PubMed] [Google Scholar]
- Lombard, V. , Ramulu, H.G. , Drula, E. , Coutinho, P.M. & Henrissat, B. (2014) The carbohydrate‐active enzymes database (CAZy) in 2013. Nucleic Acids Research, 42, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz, P. , Kleina, L.G. , Cruz, C. , Ehret, S. & Miller, J.H. (1994) Genetic studies of the lac repressor. XIV. Analysis of 4000 altered Escherichia coli lac repressors reveals essential and non‐essential residues, as well as “spacers” which do not require a specific sequence. Journal of Molecular Biology, 240, 421–433. [DOI] [PubMed] [Google Scholar]
- Matthews, K.S. , Falcon, C.M. & Swint‐Kruse, L. (2000) Relieving repression. Nature Structural Biology, 7, 184–187. [DOI] [PubMed] [Google Scholar]
- Matthews, K.S. & Nichols, J.C. (1998) Lactose repressor protein: functional properties and structure. Progress in Nucleic Acid Research and Molecular Biology, 58, 127–164. [DOI] [PubMed] [Google Scholar]
- Meyer, S. , Ramot, R. , Inampudi, K.K. , Luo, B. , Lin, C. , Amere, S. et al. (2013) Engineering alternate cooperative‐communications in the lactose repressor protein scaffold. Protein Engineering, Design & Selection, 26, 433–443. [DOI] [PubMed] [Google Scholar]
- Miyazaki, K. (2011) MEGAWHOP cloning: a method of creating random mutagenesis libraries via megaprimer PCR of whole plasmids. Methods in Enzymology, 498, 399–406. [DOI] [PubMed] [Google Scholar]
- Morris, G.M. , Huey, R. , Lindstrom, W. , Sanner, M.F. , Belew, R.K. , Goodsell, D.S. et al. (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30, 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikel, P.I. , Benedetti, I. , Wirth, N.T. , de Lorenzo, V. & Calles, B. (2022) Standardization of regulatory nodes for engineering heterologous gene expression: a feasibility study. Microbial Biotechnology, 15, 2250–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci, R. , Moracci, M. , Vaccaro, C. , Vespa, N. & Rossi, M. (1993) Exo‐glucosidase activity and substrate specificity of the beta‐glycosidase isolated from the extreme thermophile Sulfolobus solfataricus . Biotechnology and Applied Biochemistry, 17, 239–250. [PubMed] [Google Scholar]
- Pace, H.C. , Kercher, M.A. , Lu, P. , Markiewicz, P. , Miller, J.H. , Chang, G. et al. (1997) Lac repressor genetic map in real space. Trends in Biochemical Sciences, 22, 334–339. [DOI] [PubMed] [Google Scholar]
- Raibaud, O. & Schwartz, M. (1984) Positive control of transcription initiation in bacteria. Annual Review of Genetics, 18, 173–206. [DOI] [PubMed] [Google Scholar]
- Raman, S. , Taylor, N. , Genuth, N. , Fields, S. & Church, G.M. (2014) Engineering allostery. Trends in Genetics, 30, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye, C.S. & Withers, S.G. (2000) Glycosidase mechanisms. Current Opinion in Chemical Biology, 4, 573–580. [DOI] [PubMed] [Google Scholar]
- Schmitz, A. , Coulondre, C. & Miller, J.H. (1978) Genetic studies of lac repressor. V. Repressors which bind operator more tightly generated by suppression and reversion of nonsense mutations. Journal of Molecular Biology, 123, 431–454. [DOI] [PubMed] [Google Scholar]
- Studier, F.W. (1991) Use of bacteriophage‐T7 lysozyme to improve an inducible T7 expression system. Journal of Molecular Biology, 219, 37–44. [DOI] [PubMed] [Google Scholar]
- Suckow, J. , Markiewicz, P. , Kleina, L.G. , Miller, J. , KistersWoike, B. & MullerHill, B. (1996) Genetic studies of the lac repressor XV: 4000 single amino acid substitutions and analysis of the resulting phenotypes on the basis of the protein structure. Journal of Molecular Biology, 261, 509–523. [DOI] [PubMed] [Google Scholar]
- Swint‐Kruse, L. & Matthews, K.S. (2009) Allostery in the Lacl/GaIR family: variations on a theme. Current Opinion in Microbiology, 12, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swint‐Kruse, L. , Zhan, H.L. , Fairbanks, B.M. , Maheshwari, A. & Matthews, K.S. (2003) Perturbation from a distance: mutations that alter LacI function through long‐range effects. Biochemistry, 42, 14004–14016. [DOI] [PubMed] [Google Scholar]
- Tang, S.Y. & Cirino, P.C. (2010) Elucidating residue roles in engineered variants of AraC regulatory protein. Protein Science, 19, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S.‐Y. & Cirino, P.C. (2011) Design and application of a mevalonate‐responsive regulatory protein. Angewandte Chemie, International Edition, 50, 1084–1086. [DOI] [PubMed] [Google Scholar]
- Taraban, M. , Zhan, H. , Whitten, A.E. , Langley, D.B. , Matthews, K.S. , Swint‐Kruse, L. et al. (2008) Ligand‐induced conformational changes and conformational dynamics in the solution structure of the lactose repressor protein. Journal of Molecular Biology, 376, 466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, N.D. , Garruss, A.S. , Moretti, R. , Chan, S. , Arbing, M.A. , Cascio, D. et al. (2016) Engineering an allosteric transcription factor to respond to new ligands. Nature Methods, 13, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpe, K. (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Applied Microbiology and Biotechnology, 72, 211–222. [DOI] [PubMed] [Google Scholar]
- Torres, D.P.M. , G.M.P.F., Teixeira, J.A. & Rodrigues, L.R. (2010) Galacto‐oligosaccharides: production, properties, applications, and significance as prebiotics. Comprehensive Reviews in Food Science and Food Safety, 9, 438–454. [DOI] [PubMed] [Google Scholar]
- Wang, Q.Z. , Tang, S.Y. & Yang, S. (2017) Genetic biosensors for small‐molecule products: design and applications in high‐throughput screening. Frontiers of Chemical Science and Engineering, 11, 15–26. [Google Scholar]
- Wu, J. , Jiang, P. , Chen, W. , Xiong, D. , Huang, L. , Jia, J. et al. (2017) Design and application of a lactulose biosensor. Scientific Reports, 7, 45994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, P.F. , Ling, H. , Foo, J.L. & Chang, M.W. (2019) Synthetic genetic circuits for programmable biological functionalities. Biotechnology Advances, 37, 107393. [DOI] [PubMed] [Google Scholar]
- Xiong, D. , Lu, S. , Wu, J. , Liang, C. , Wang, W. , Wang, W. et al. (2017) Improving key enzyme activity in phenylpropanoid pathway with a designed biosensor. Metabolic Engineering, 40, 115–123. [DOI] [PubMed] [Google Scholar]
- Xu, J. & Matthews, K.S. (2009) Flexibility in the inducer binding region is crucial for allostery in the Escherichia coli lactose repressor. Biochemistry, 48, 4988–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, J. , He, Y. , Su, N. , Bharath, S.R. , Tao, Y. , Jin, J.‐M. et al. (2020) Developing a highly efficient hydroxytyrosol whole‐cell catalyst by de‐bottlenecking rate‐limiting steps. Nature Communications, 11, 1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, H. , Taraban, M. , Trewhella, J. & Swint‐Kruse, L. (2008) Subdividing repressor function: DNA binding affinity, selectivity, and allostery can be altered by amino acid substitution of nonconserved residues in a LacI/GalR homologue. Biochemistry, 47, 8058–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F. , Carothers, J.M. & Keasling, J.D. (2012) Design of a dynamic sensor‐regulator system for production of chemicals and fuels derived from fatty acids. Nature Biotechnology, 30, 354–359. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , He, Y. , Wu, Z. , Liu, G. , Tao, Y. , Jin, J.‐M. et al. (2021) Whole‐cell biosensors aid exploration of vanillin transmembrane transport. Journal of Agricultural and Food Chemistry, 69, 3114–3123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.


