Abstract
Kimchi is a traditional Korean fermented food and harbors diverse bacteria. Therefore, proper temperature management contributes to the fermentation and preservation of kimchi. In this study, we explored fermentation temperature influences the bacterial composition and metabolite variations in kimchi, employing pyrosequencing for bacterial community analysis and mass spectrometry for metabolite profiling. Elevated temperatures within the range of 10–15 °C had a significant impact on the community of lactic acid bacteria (LAB) compared to 4 °C, leading to increased bacterial diversity and richness. We observed a significant increase in Lactiplantibacillus plantarum and Latilactobacillus sakei, alongside a reduction in Lactococcus lactis, during fermentation at 10–15 °C. These changes occurred within a similar pH range across different kimchi fermentation periods.
We performed a liquid extraction via the acetonitrile method, which involved the collection of kimchi samples, and performed LC-MS analysis. Our analysis revealed approximately 5000 metabolites. Notably, we observed a significant increase in metabolite counts, with 3048 metabolites increasing at 10 °C and 2853 metabolites exhibiting a similar trend at 15 °C.
Metabolite analysis showed an increase in lactic and succinic acid with increased glucose and sucrose consumption at 10 and 15 °C. These results indicated that elevated temperatures significantly influenced the glycolysis and tricarboxylic acid cycle, leading to increased acidity during the fermentation process.
These findings show the crucial role played by temperature in controlling the fermentation process, thereby influencing the bacterial succession and the resulting flavor and taste of the product. Therefore, proper temperature management can effectively control kimchi fermentation and yield the desired sensory properties.
Keywords: Temperature, Kimchi, Bacterial community, Metabolite changes, Fermentation process, Lactic acid bacteria
Highlights
-
•
High temperature (10–15 °C) enhanced LAB diversity and altered microbial composition in kimchi compared to low temperature (4 °C).
-
•
High temperature significantly increased total metabolite counts and reduced the glucose and sucrose content.
-
•
Elevated temperatures increase LAB presence for succinic and lactic acids, but reduce malic, citric, and fumaric acids.
1. Introduction
Kimchi is a traditional Korean fermented food comprising salted kimchi cabbage, garlic, leek, ginger, red pepper powder, and varied regional and seasonal ingredients [1]. Food fermentation prevent the growth of undesired bacteria, including foodborne pathogens, and allows for long-term storage of food [2]. Moreover, it confers a noble flavor to food and increases the amount of metabolites, probiotics, and postbiotics, which are beneficial to human health [1,[3], [4], [5], [6], [7]]. Kimchi originated from the tradition of preserving vegetables during the cold winter season [1]. In recent years, specialized refrigerators equipped with precise temperature control functions are widely used for storing kimchi in Korea [8].
Kimchi fermentation conditions are cold, high salinity, hypoxic, and acidic conditions. The lactic acid bacteria (LAB) found in kimchi are psychrophilic or psychrotrophic, facultatively anaerobic, and tolerant to both salt and acid. This feature makes LAB the dominant bacterial group through the kimchi fermentation progress [7,9].
Kimchi is commonly stored at a low temperature (2–6 °C). Alternatively, kimchi can be stored at a relatively high temperature, typically at room temperature (20 °C), for several days before being moved to colder storage. This initial warm temperature facilitates more rapid fermentation, the production and then fermentation of organic acids, and affects bacterial proliferation resulting in increased Lactobacillus [10]. Fermentation at 15 or 25 °C leads to an increased Lactobacillus, along with a rapid reduction in sugars and increase in lactic acid content compared to fermentation at 4 °C [11]. Similar results showed that Leuconostoc was dominant at 4 °C fermentation [12]. These results show that temperature conditions are primary factors that influence bacterial community during the kimchi fermentation. LAB have diverse roles in the kimchi fermentation. The diverse benefits of kimchi are attributed to its probiotic and postbiotic features of LAB [1,8,10]. In addition, LAB produce lactic acid, organic acids, sugar alcohols, amino acids, and volatile metabolites, all of which contribute to the flavor, texture, and nutritional profile of kimchi [1,13,14]. Therefore, the bacterial succession during the fermentation is crucial aspect of kimchi fermentation.
In this study, we explored the relationship among temperature, bacterial community composition, and metabolite profiles during the fermentation of kimchi. We prepared separate batches of kimchi at 4, 10, and 15 °C. We investigated the bacterial community and succession using pyrosequencing technology. Additionally, we quantified the metabolites along with an un-targeted metabolomic approach using mass spectrophotometry technology.
2. Materials and methods
2.1. Kimchi preparation
Kimchi was purchased from a local factory in Gwangju, Korea, and prepared as following raw ingredients: salted kimchi cabbage 70%, red pepper 4%, radish 8%, onions 1.2%, garlic 1.5%, fish sauce 5.3%, glutinous rice 0.6%, and water 9.4%. The kimchi samples were placed in a plastic bag and refrigerated at various temperatures: 4 °C (Kimchi A), 10 °C (Kimchi B), and 15 °C (Kimchi C). The fermentation progress of kimchi was monitored by measuring bacterial count and acidity. Samples were collected from each temperature setting every week for 4 weeks. Briefly, 100 g of each sample was homogenized and subsequently filtered through three layers of sterilized gauze.
2.2. Analysis of viable cell counts and physicochemical properties
The fermentation progress was monitored by measuring bacterial count and acidity. To enumerate total aerobic plate counts and LAB, the filtered homogenized kimchi samples were serially diluted in a 0.85% sodium chloride solution and plated on 3 M Petrifilm Aerobic Count Plates (3 M, St Paul, MN, USA) and MRS agar plate (BD, Franklin Lakes, NJ, USA) for total aerobic and LAB count, respectively. The bacterial cell count is conducted after incubation at 30 °C for 24 h pH and acidity were measured using a pH meter (Mettler Toledo, Columbus, USA) and via titration with 0.1 N NaOH to pH 8.3 to calculate the % lactic acid.
2.3. Bacterial community analysis
Bacterial community analysis was performed using 16S rDNA pyrosequencing of genomic DNA isolated from the samples. A pyrosequencing library was generated using the 16S metagenomics sequencing library preparation protocol (Illumina MiSeq System, Illumina Inc., San Diego, CA, USA) by Theragen Inc. (Suwon, Korea). The V3 and V4 hypervariable regions were amplified using the KAPA HiFi HotStart ReadyMix Kit (KAPA Biosystems, Wilmington, MA, USA). The processing of raw reads began with a quality assessment, and reads with low quality (a score < Q25) were eliminated using Trimmomatic version 0.32. The paired-end sequence data were merged using fastq-mergepairs in VSEARCH version 2.13.4. Subsequently, redundant reads were identified and clustered with the unique reads. Taxonomic assignment was performed using the EzBioCloud 16S rRNA database, followed by a pairwise alignment. Chimeric reads were eliminated using reference-based chimeric detection with the UCHIME algorithm with a threshold set at <97% similarity. After the chimeric filtering step, the reads were compiled and cluster_fast command was utilized to generate additional OTUs. Data analysis was conducted using the EzBioCloud 16S-databased MTP provided by CJ Bioscience (Seoul, Korea) [15].
2.4. Metabolite identification and analysis
Homogenous kimchi samples were centrifuged at 5000 × g for 20 min. The supernatant was extracted using 50% acetonitrile, with 0.5 mmol/L salicin as internal standard [15,16]. Following extraction, samples were filtered using 0.22 μm syringe filter and the diluted with an extraction solvent. Metabolite analysis was performed using a TripleTOF 5600 Plus instrument (SCIEX, Redwood City, CA, USA) coupled to an Acquity ultra UPLC system (Waters, Milford, MA, USA). The metabolites were separated using the Hypersil GOLD C18 column (2.1 × 150 mm, 3 μm; Thermo, Waltham, MA, USA) connected to an Accucore aQ polar endcapped column (2.1 × 100 mm, 2.6 μm; Thermo). The mobile phase comprised solvents A (water) and B (acetonitrile) containing 10 mmol/L ammonium acetate. The injection volume was 10 μl, and the flow rate was set at 0.3 ml/min. Mass spectrometry analysis was executed in negative electrospray ionization mode, utilizing a spray voltage of −4.5 kV and ion transfer temperature was 300 °C; mass spectra generated the following transitions: glucose, m/z 179 → 59; sucrose, m/z 341 → 89; lactic acid, m/z 89 → 73; succinic acid, m/z 117 → 73; citric acid, m/z 191 → 111; malic acid, m/z 133 → 115; and salicin (internal standard), m/z 285 → 123. The fragmentation patterns of the information-dependent acquisition (IDA) mode and multiple reaction monitoring (MRM) mode were controlled using the Analyst software (SCIEX). Peak detection, alignment, and identification were performed using MS-DIAL software with a spectral database (MSP ESI (−)-MS/MS), and MS and MS/MS ion information [17].
2.5. Statistical analysis
The data are presented as the mean ± standard deviation of triplicate experiments. Two-way analysis of variance (ANOVA) with multiple comparison tests, multivariate analysis, metabolite enrichment, and the Spearman rank correlation coefficient were performed using metaboanalyst 5.0 [18] and GraphPad Prism V9 (La Jolla, CA, USA). One-way ANOVA and Duncan's multiple range test were performed using SPSS version 19 (SPSS Inc., Chicago, IL, USA). Statistically significant values are presented as *p < 0.05, **p < 0.005.
3. Results
3.1. Bacteria count analysis of kimchi
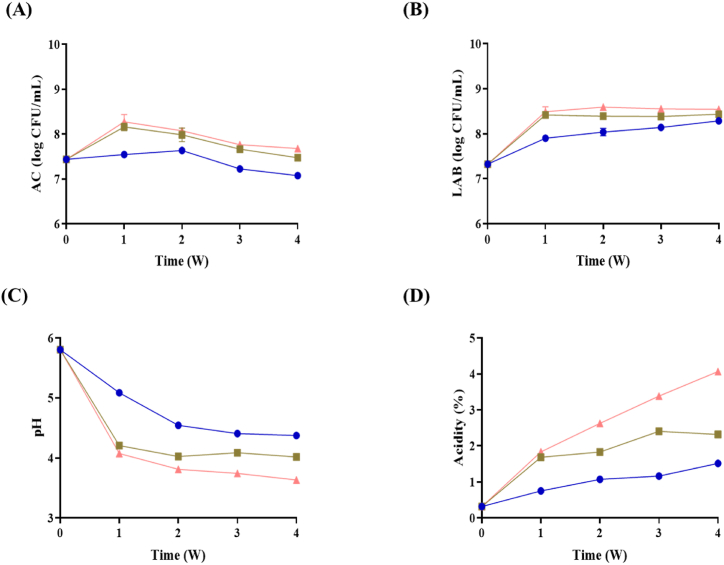
To evaluate the fermentation process, the bacterial population of the kimchi was monitored (Fig. 1A and B). The number of total anaerobic bacteria at the initial time point (week 0) was 7.44 ± 0.01 log CFU/ml and showed no significant increase in Kimchi A. In week 1, the aerobic bacterial count increased slightly in Kimchi B (8.16 ± 0.08 log CFU/ml) and C (8.28 ± 0.16 log CFU/ml), and subsequently decreased throughout the fermentation period (Fig. 1A). Specifically, the number of LAB colonies was 7.33 ± 0.08 log CFU/ml in week 0 and increased to 7.90 ± 0.03, 8.42 ± 0.04, and 8.49 ± 0.11 log CFU/ml in Kimchi A, B, and C, respectively, in week 1 (Fig. 1B). In week 4, LAB count in Kimchi A, B, and C increased to 8.29 ± 0.42–8.55 ± 0.04 log CFU/ml.
Fig. 1.
Bacteria and physicochemical properties of kimchi. The bacteria cell counts and physicochemical properties of kimchi were evaluated at different temperatures. (A) Total viable bacteria, (B) lactic acid bacteria, (C) pH, and (D) acidity. Symbols represent temperature as 4 (●), 10 (■), and 15 °C (▲).
3.2. Physicochemical properties of kimchi
The fermentation of kimchi at different temperatures leads to significant changes in both acidity and pH levels over time (Fig. 1C and D). The acidity profiles of the three kimchi samples showed that the acidity of lower temperature fermented kimchi overlapped the early phase of kimchi fermented at higher temperature. The acidity and pH of Kimchi A, B, and C in week 1 were 0.32% and 5.81, respectively. Kimchi A started with a pH of 5.09 and an acidity of 0.75% in the first week. By the second week, the pH decreased to 4.54, and acidity increased to 1.07%, and by the fourth week, the pH was 4.37 and acidity 1.51%. Kimchi B started with a pH of 4.21 and acidity of 1.69% in the first week. In the second week, the pH dropped to 4.02 and acidity rose to 1.83%. The pH was 4.02 and acidity slightly decreased to 2.32% in the fourth week. Kimchi C began with a pH of 4.07 and an acidity of 1.83% in the first week. The second week marked a drop in pH to 3.81 and an increase in acidity to 2.63%. The fourth week exhibited the lowest pH of 3.63 and highest acidity at 4.07%.
3.3. Change of sugar contents during the kimchi fermentation
The primary free sugars in kimchi at the start of fermentation were glucose, fructose, and sucrose [12,19]. We monitored the reduction of glucose and sucrose to monitor the kimchi fermentation progress (Fig. 2). At week 0, the glucose and sucrose content were 17.16 ± 1.38 and 1.29 ± 0.03 mg/ml, respectively, and then reduced significantly (p < 0.05) as fermentation progressed, with a linear correlation with elevated temperatures. In week 1, the glucose content of Kimchi A, B, and C decreased to 13.54 ± 1.09, 9.41 ± 1.22, and 6.26 ± 0.32 mg/ml, respectively (Fig. 2A). Similarly, sucrose content decreased to 1.20 ± 0.02, 0.93 ± 0.02, and 0.23 ± 0.01 mg/ml, respectively (Fig. 2B). The reduction in sugar content within kimchi samples corresponding to bacterial growth were consistent with LAB growth and increased acidity in kimchi B and C (Fig. 1).
Fig. 2.
Quantification of free sugar contents in kimchi samples using UPLC Q-TOF-MS. (A) Glucose and (B) sucrose contents showed significant difference between kimchi samples. Significant values are presented as *p < 0.05.
3.4. Bacterial community analysis
Bacterial diversity (OTUs) and richness (Shannon index) in Kimchi A, B, and C decreased after one week (Supplementary Table 1). Fig. 3 shows 21 different types of species with abundance exceeding 0.5% through 4 weeks of fermentation. Kimchi B and C showed more diverse LAB species than kimchi A. At the initial time point, 14 different species were identified and Kimchi A showed 12 and 11 species in weeks 1 and 4, respectively. Kimchi B showed the 12 and 20 species in weeks 1 and 4, respectively. Kimchi C showed 15 and 19 species in weeks 1 and 4, respectively. The abundance of LAB also showed the differences in kimchi A, B, and C. Initially (Week 0), kimchi showed Lactococcus lactis, Lactococcus garvieae and Weissella kandleri were present at of 22.96%, 9.30% and 11.11% content, respectively. In kimchi A, L. lactis increased to 40.62% in week 1 and decreased to 15.91% by the fourth week. Conversely, Latilactobacillus sakei content decreased from 13.03% to 12.11% by the fourth week. A significant increase in W. kandleri, from 19.89% in week 1–44.68% was observed in week 4. Kimchi B showed an early abundance of L. sakei, starting at 17.79% in week 1 and rising to 25.77% by the third week, and 20.36% in the fourth week. A remarkable increase was observed in the W. kandleri population, which soared from 27.52% in the first week to 55.84% by the third week, and later reduced to 21.74% in the fourth week. Kimchi C revealed a dominant presence of Lactiplantibacillus plantarum, which initially accounted for 6.17% in week 1 and surged to 35.20% in the second week, followed by a reduction to 19.99% by the fourth week. Similarly, W. kandleri content was 42.88% in week 1 and reduced at 9.00% in week 4. The bacterial population in different temperature conditions showed the close correlation with acidic condition among the three kimchi. For example, L. sakei showed the distinct growth in three kimchi through the fermentation, in kimchi A (pH 5.09 to 4.37) L. sakei content showed the peaked at about pH 4.02–4.07 for week 2 for kimchi B and week 1 for kimchi C, respectively. W. kandleri was also peaked at pH ranged from 5.09 to 4.07 in kimchi A to C.
Fig. 3.
Bacterial community composition in kimchi samples fermented at different temperatures. The composition of the bacterial community in kimchi samples was confirmed by pyrosequencing analysis at the species level. The kimchi samples were stored at various temperatures and collected weekly. Con; initial time point, Kimchi A; 4 °C, Kimchi B; 10 °C, Kimchi C; 15 °C.
3.5. Metabolite analysis
Metabolite information was obtained via an IDA mode using a Q-TOF-MS system (Fig. 4). Further analysis and identification of the metabolites were performed using the MSP spectral library (ESI-MS/MS library). Approximately 5000 peaks as metabolites were detected and aligned across the samples. Changes in metabolites in kimchi samples were analyzed and compared. A heat map showed two distinct patterns of the detected metabolites. Kimchi B and C showed a similar metabolite profile, while Kimchi A had a distinct profile (Fig. 4A). In week 1, 2853 metabolites increased significantly (log2FC > 2, p < 0.05) between Kimchi A and C, while 3048 metabolites between Kimchi A and B (Fig. 4B and C). The metabolites were further analyzed using MS-DIAL using MSP public mass library. Finally, 31 metabolites were identified using an aligned MS and MS/MS fragment ion with higher matching score (>0.9) and included 15 amino acids, 4 nucleic acids, 6 organic acids, 1 sugar, and 5 secondary metabolites (2-hydroxycaproic acid [HICA], phenyllactic acid [PLA], glutathione, citrulline, and ketoglutaric acids [KGA]) (Supplementary Table 2).
Fig. 4.
Comparison of metabolites in kimchi samples stored at different temperatures. The kimchi samples were stored at various temperatures and analyzed weekly. Total metabolites were analyzed using UPLC Q-TOF-MS with an IDA mode and a mass range of 40–600. Metabolite analysis was performed in triplicate. (A) Heat map generated from the average of each metabolite values. Volcano plot comparison of metabolites in (B) Kimchi A and C, and (C) Kimchi A and B, in week 1. Significant values are presented as log2FC > 2 and p < 0.05.
Overall, amino acid content increased throughout the fermentation process, with that of Kimchi B and C exceeding that of Kimchi A after fermentation for a week (Table 1). Glutamine and glutamic acid were the abundant amino acids in kimchi fermentation [20]. Glutamine and glutamic acid content were 5.60 ± 0.04 and 7.03 ± 0.38, respectively, which exceeded other amino acid content. Arginine content was reduced rapidly in kimchi B and C. Initially, the arginine content of Kimchi A, B, and C was 1.05 ± 0.05 and decreased to 0.28 ± 0.02, 0.05 ± 0.01, and 0.07 ± 0.01, respectively. In addition, nucleic acid levels increased throughout the fermentation process and were higher in Kimchi B and C compared to Kimchi A, indicating a different trend of organic acid content through fermentation. Organic acids exhibited a different trend. Lactic and succinic acids increased, while citric, malic, and fumaric acids decreased throughout the fermentation process. Glutathione, KGA, HICA, and PLA increased throughout the fermentation process and exceeded the quantities in Kimchi B and C compared to that in Kimchi A. The citrulline content was lower in Kimchi B and C in weeks 1 and 2 of fermentation and did not exhibit dramatic changes throughout the fermentation process. Mannitol increased during fermentation and was relatively high in Kimchi B and C.
Table 1.
Quantification of metabolites in kimchi samples using UPLC Q-TOF-MS analysis.
| Compounds | Name | Con |
Kimchi A |
Kimchi B |
Kimchi C |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (W) | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Amino acids |
Histidine | 1.13 ± 0.05 gh *,** | 1.10 ± 0.04 gh | 1.51 ± 0.04e | 1.31 ± 0.08f | 0.98 ± 0.03h | 1.71 ± 0.10 cd | 2.08 ± 0.09 ab | 2.05 ± 0.09b | 1.30 ± 0.11f | 2.22 ± 0.13a | 1.61 ± 0.13 de | 1.23 ± 0.06 fg | 1.83 ± 0.09c |
| Aspartate | 1.56 ± 0.10f | 1.51 ± 0.04f | 1.76 ± 0.09 cde | 1.91 ± 0.07c | 1.12 ± 0.05g | 1.84 ± 0.09 cd | 2.24 ± 0.12b | 2.34 ± 0.17b | 1.67 ± 0.12 def | 2.76 ± 0.14a | 1.61 ± 0.10 ef | 1.16 ± 0.12g | 1.01 ± 0.08g | |
| Isoleucine | 1.64 ± 0.09 fg | 1.39 ± 0.02 hi | 1.56 ± 0.04 gh | 1.19 ± 0.03I | 1.82 ± 0.06f | 3.02 ± 0.11 cd | 3.62 ± 0.17a | 3.33 ± 0.18b | 2.87 ± 0.21d | 2.83 ± 0.10d | 3.13 ± 0.18 bc | 2.25 ± 0.21e | 2.84 ± 0.03d | |
| Alanine | 0.22 ± 0.02g | 0.24 ± 0.01g | 0.35 ± 0.03 ef | 0.30 ± 0.03 fg | 0.39 ± 0.02e | 0.43 ± 0.01 de | 0.57 ± 0.06c | 0.55 ± 0.05c | 0.51 ± 0.02 cd | 0.92 ± 0.06a | 0.69 ± 0.11b | 0.71 ± 0.05b | 0.99 ± 0.03a | |
| Arginine | 1.05 ± 0.05a | 0.28 ± 0.02b | 0.11 ± 0.01c | 0.04 ± 0.01 efg | 0.03 ± 0.01 fg | 0.05 ± 0.01 defg | 0.06 ± 0.01 def | 0.07 ± 0.01d | 0.06 ± 0.01 de | 0.07 ± 0.01d | 0.05 ± 0.01 defg | 0.03 ± 0.01g | 0.03 ± 0.01g | |
| Glutamic acid | 5.60 ± 0.04 fg | 5.26 ± 0.23g | 6.15 ± 0.48 ef | 6.75 ± 0.47e | 6.65 ± 0.15e | 10.73 ± 0.34c | 13.03 ± 0.27a | 12.06 ± 0.54b | 8.28 ± 0.89d | 12.61 ± 0.51 ab | 8.83 ± 0.54d | 5.36 ± 0.32g | 5.16 ± 0.12g | |
| Leucine | 2.00 ± 0.42f | 1.08 ± 0.03g | 2.10 ± 0.08f | 0.75 ± 0.05g | 4.45 ± 0.16d | 5.62 ± 0.58c | 7.19 ± 0.13a | 6.21 ± 0.06b | 5.59 ± 0.37c | 3.79 ± 0.21e | 6.30 ± 0.50b | 4.21 ± 0.05 de | 6.65 ± 0.14b | |
| Proline | 0.19 ± 0.01g | 0.20 ± 0.02g | 0.29 ± 0.03f | 0.17 ± 0.02g | 0.34 ± 0.00e | 0.60 ± 0.02 ab | 0.63 ± 0.01a | 0.56 ± 0.01b | 0.58 ± 0.05b | 0.39 ± 0.02 de | 0.49 ± 0.05c | 0.43 ± 0.03d | 0.57 ± 0.05b | |
| Serine | 0.47 ± 0.01f | 0.44 ± 0.04f | 0.66 ± 0.03d | 0.57 ± 0.03e | 0.50 ± 0.03f | 0.74 ± 0.01d | 0.95 ± 0.04b | 0.95 ± 0.01b | 0.71 ± 0.06d | 1.26 ± 0.07a | 0.98 ± 0.06b | 0.81 ± 0.05c | 0.99 ± 0.05b | |
| Lysine | 0.32 ± 0.01g | 0.37 ± 0.02 fg | 0.56 ± 0.09 cd | 0.52 ± 0.01 cde | 0.43 ± 0.04 efg | 0.62 ± 0.07c | 0.76 ± 0.13b | 0.84 ± 0.09 ab | 0.45 ± 0.10 def | 0.92 ± 0.06a | 0.50 ± 0.05 de | 0.14 ± 0.02h | 0.10 ± 0.01h | |
| Phenylalanine | 9.13 ± 0.65 gh | 7.44 ± 0.09I | 9.46 ± 0.50g | 7.99 ± 0.26 hi | 13.56 ± 0.42f | 19.07 ± 0.76 bc | 22.12 ± 0.52a | 19.62 ± 1.15b | 17.78 ± 1.43 cd | 16.52 ± 0.77 de | 21.50 ± 1.23a | 15.87 ± 1.18e | 22.71 ± 0.36a | |
| Threonine | 0.39 ± 0.03h | 0.43 ± 0.03h | 0.57 ± 0.06 fg | 0.48 ± 0.04 gh | 0.61 ± 0.07f | 0.89 ± 0.09e | 0.98 ± 0.02 de | 1.03 ± 0.10 cd | 0.98 ± 0.10 de | 1.16 ± 0.11 bg | 1.11 ± 0.11 bc | 1.09 ± 0.03 bcd | 1.33 ± 0.03a | |
| Tryptophan | 2.50 ± 0.13e | 2.62 ± 0.05e | 4.75 ± 0.21c | 3.27 ± 0.04d | 5.94 ± 0.27a | 5.63 ± 0.37b | 3.28 ± 0.31 de | 0.47 ± 0.07g | 0.08 ± 0.00h | 0.25 ± 0.03 gh | 1.03 ± 0.07f | 0.45 ± 0.05g | 0.89 ± 0.06f | |
| Tyrosine | 2.50 ± 0.04e | 2.02 ± 0.09f | 2.49 ± 0.09e | 1.99 ± 0.08f | 3.44 ± 0.07d | 5.42 ± 0.12b | 5.88 ± 0.35a | 5.49 ± 0.18b | 4.09 ± 0.46c | 3.26 ± 0.14d | 0.86 ± 0.06g | 0.27 ± 0.01h | 0.26 ± 0.01h | |
| Glutamine |
7.03 ± 0.38f |
7.08 ± 0.71f |
9.57 ± 0.74e |
8.25 ± 0.52f |
4.74 ± 0.28g |
10.51 ± 0.55 de |
12.68 ± 0.38c |
11.44 ± 1.24 cd |
7.70 ± 0.94f |
16.44 ± 0.38a |
12.27 ± 1.22c |
9.68 ± 1.01e |
14.34 ± 0.10b |
|
| Nucleic acids |
Adenine | 0.01 ± 0.00f | 0.13 ± 0.01 ef | 0.17 ± 0.02e | 0.22 ± 0.02e | 0.65 ± 0.05d | 1.06 ± 0.04 bc | 1.11 ± 0.08 ab | 1.13 ± 0.12 ab | 1.12 ± 0.17 ab | 0.95 ± 0.11c | 0.95 ± 0.05c | 0.62 ± 0.06d | 1.23 ± 0.04a |
| Guanosine | 0.17 ± 0.01g | 0.02 ± 0.00h | 0.27 ± 0.03f | 0.60 ± 0.03e | 1.62 ± 0.07b | 2.11 ± 0.13a | 1.12 ± 0.02c | 1.05 ± 0.06c | 0.73 ± 0.04d | 1.05 ± 0.07c | 0.76 ± 0.02d | 0.25 ± 0.01 fg | 0.23 ± 0.03 fg | |
| Uracil | 0.79 ± 0.07 de | 0.62 ± 0.02 ef | 0.76 ± 0.02 de | 0.45 ± 0.02f | 0.97 ± 0.09d | 1.74 ± 0.30a | 1.75 ± 0.33a | 1.56 ± 0.07 ab | 1.25 ± 0.10c | 0.98 ± 0.06d | 1.24 ± 0.03c | 0.90 ± 0.07d | 1.34 ± 0.04 bc | |
| Purine |
0.35 ± 0.01e |
0.32 ± 0.01e |
0.36 ± 0.02e |
0.34 ± 0.02e |
0.49 ± 0.02d |
0.79 ± 0.05a |
0.77 ± 0.05a |
0.68 ± 0.07b |
0.55 ± 0.03 cd |
0.57 ± 0.04c |
0.49 ± 0.03d |
0.49 ± 0.07d |
0.53 ± 0.06 cd |
|
| Organic acids |
Citric acid | 24.35 ± 2.18b | 19.27 ± 0.77c | 24.02 ± 1.78b | 17.75 ± 0.91c | 39.01 ± 1.90a | 37.94 ± 1.22a | 8.71 ± 0.47d | 1.70 ± 0.11e | 0.42 ± 0.04e | 0.23 ± 0.02e | 0.22 ± 0.02e | 0.10 ± 0.01e | 0.35 ± 0.01e |
| Malic acid | 53.53 ± 7.29a | 29.05 ± 1.53b | 30.88 ± 1.13b | 22.07 ± 1.16c | 11.44 ± 0.44d | 5.45 ± 0.10e | 2.40 ± 0.12e | 1.98 ± 0.10e | 2.17 ± 0.24e | 2.04 ± 0.23e | 2.24 ± 0.08e | 1.83 ± 0.07e | 3.51 ± 0.09e | |
| Lactic acid | 1.36 ± 0.09h | 5.35 ± 0.04g | 7.33 ± 0.30f | 10.81 ± 0.41e | 7.97 ± 0.34f | 12.91 ± 0.26d | 18.43 ± 1.13 bc | 17.18 ± 0.60c | 13.85 ± 1.46d | 19.15 ± 0.73b | 18.29 ± 1.01 bc | 17.93 ± 1.47 bc | 21.69 ± 1.13a | |
| Fumaric acid | 0.21 ± 0.01a | 0.08 ± 0.01d | 0.15 ± 0.01b | 0.12 ± 0.01c | 0.09 ± 0.00d | 0.01 ± 0.01 ef | 0.01 ± 0.01e | 0.01 ± 0.01e | 0.01 ± 0.00 ef | 0.01 ± 0.00 ef | 0.01 ± 0.00 ef | N.D. *** | N.D. | |
| Pyruvic acid | 0.50 ± 0.05 bc | 0.27 ± 0.02e | 0.20 ± 0.03f | 0.23 ± 0.02 ef | 0.26 ± 0.03e | 0.40 ± 0.02d | 0.47 ± 0.05c | 0.47 ± 0.01c | 0.36 ± 0.02d | 0.56 ± 0.05a | 0.53 ± 0.04 ab | 0.46 ± 0.01c | 0.57 ± 0.02a | |
| Succinic acid |
1.89 ± 0.06 fg |
0.55 ± 0.15g |
3.55 ± 0.74f |
2.12 ± 0.24 fg |
17.02 ± 0.34e |
17.09 ± 0.07e |
21.73 ± 0.55b |
20.49 ± 1.75 bc |
18.97 ± 1.90 cd |
17.75 ± 1.31 de |
25.69 ± 1.06a |
20.51 ± 0.96 bc |
26.72 ± 0.41a |
|
| Sugar |
Mannitol |
0.05 ± 0.01h |
0.30 ± 0.02h |
0.80 ± 0.05 dg |
0.98 ± 0.10g |
1.67 ± 0.08f |
2.79 ± 0.05 de |
3.67 ± 0.21b |
3.18 ± 0.06c |
3.26 ± 0.41c |
3.00 ± 0.11 cd |
2.61 ± 0.23e |
3.30 ± 0.28c |
5.12 ± 0.29a |
| Secondary metabolites | Glutathione | 0.30 ± 0.01e | 0.09 ± 0.01f | 0.70 ± 0.08 cd | 0.27 ± 0.01e | 0.63 ± 0.06d | 0.90 ± 0.06b | 1.32 ± 0.13a | 1.23 ± 0.11a | 1.34 ± 0.19a | 0.59 ± 0.01d | 0.93 ± 0.03b | 0.64 ± 0.04d | 0.82 ± 0.03 bc |
| Citrulline | 0.58 ± 0.03b | 0.79 ± 0.05a | 0.40 ± 0.03c | 0.24 ± 0.02 de | 0.24 ± 0.04 de | 0.27 ± 0.03d | 0.26 ± 0.02 de | 0.28 ± 0.03d | 0.29 ± 0.02d | 0.20 ± 0.05e | 0.35 ± 0.03c | 0.29 ± 0.03d | 0.37 ± 0.01c | |
| KGA | 0.53 ± 0.08a | 0.06 ± 0.01f | 0.08 ± 0.01f | 0.06 ± 0.01f | 0.14 ± 0.02e | 0.20 ± 0.02 de | 0.28 ± 0.01b | 0.26 ± 0.03 bc | 0.20 ± 0.02d | 0.29 ± 0.01b | 0.27 ± 0.05b | 0.18 ± 0.02 de | 0.21 ± 0.01 cd | |
| HICA | 4.37 ± 0.14g | 22.96 ± 0.80f | 32.36 ± 2.51e | 32.71 ± 1.48e | 36.57 ± 1.08e | 82.39 ± 1.87b | 98.58 ± 2.46a | 98.59 ± 1.22a | 60.70 ± 6.51d | 95.27 ± 4.99a | 94.78 ± 5.07a | 73.72 ± 7.66c | 100.0 ± 0.00a | |
| PLA | 1.27 ± 0.04h | 13.78 ± 0.44g | 19.90 ± 0.45f | 23.09 ± 0.96f | 23.41 ± 1.37f | 51.35 ± 2.61d | 61.40 ± 2.76c | 62.44 ± 5.45c | 39.05 ± 3.03e | 62.88 ± 3.63c | 70.22 ± 3.19b | 57.76 ± 3.31c | 83.23 ± 4.31a | |
The numbers indicate peak intensity. *(a–i) values with different superscript letters within a row significantly (p < 0.05). **Values are mean ± standard deviations of three (n = 3) measurements. ***Not detected.
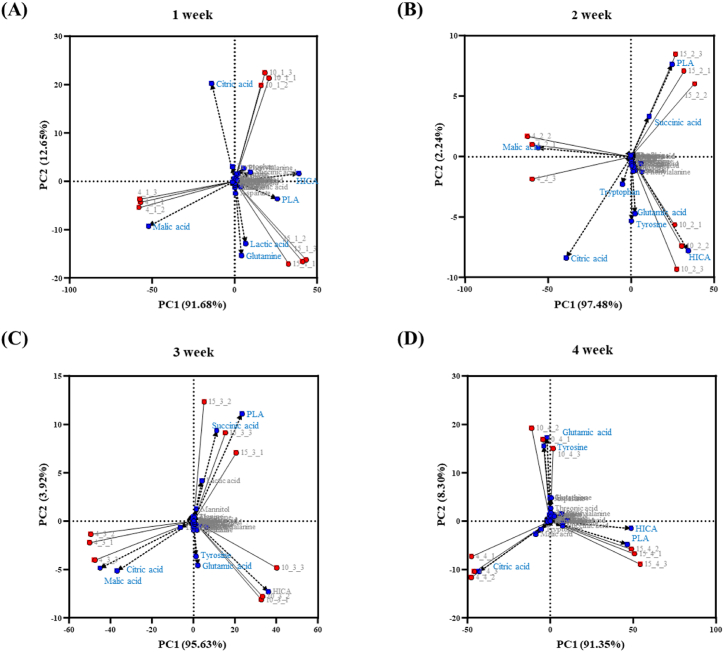
Principal Component Analysis (PCA) was performed to explore the variances in metabolites under three temperature conditions (Fig. 5). PC1 accounted for 91.35–97.48% of the total variance for fermentation. The PCA showed that temperature primarily influenced the metabolites, with kimchi samples clustering along PC1 and showing clear metabolite clustering at varying temperatures. The plot visualized the separation of Kimchi A, whereas Kimchi B and C clustered together and had similar metabolic profiles. The biplot revealed that organic acids played a significant role in driving the differentiation among the samples (Fig. 5A–D). Malic and citric acid showed the same trend in Kimchi A, while lactic acid, succinic acid, HICA, and PLA showed the opposite trend.
Fig. 5.
PCA of metabolites. The PCA score plot revealed changes in major metabolites according to the fermentation period. The PCA and biplot score plot were derived from metabolome analysis in (A) week 1, (B) week 2, (C) week 3, and (D) week 4. Blue: loading, Red: PC scores. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We quantified the contents of organic acids, prominently clustered in the PCA results, in kimchi samples (Fig. 6). In week 1, Kimchi A exhibited relatively high levels of citric and malic acid, measuring 11.92 ± 0.09 and 3.31 ± 0.14 mg/L, respectively, compared to Kimchi B (5.62 ± 1.86 mg/L and N.D) and C (0.50 ± 0.02 mg/L and N.D) (Fig. 6A and B). In contrast, lactic and succinic acid in Kimchi A were generated relatively slowly with 30.44 ± 2.96 and 1.38 ± 0.07 mg/L, respectively, compared to Kimchi B (96.11 ± 11.96 and 2.74 ± 0.07 mg/L) and C (133.90 ± 4.34 and 3.49 ± 0.33 mg/L) (Fig. 6C and D). Overall, the changes in organic acids in Kimchi B and C appeared to occur more rapidly than in Kimchi A and these results are consistent with those of the metabolite comparison with IDA acquisition (Table 1).
Fig. 6.
Organic acid contents in kimchi samples. The analysis of organic acid contents performed using UPLC Q-TOF-MS with a MRM mode. (A) Lactic acid, (B) succinic acid, (C) citric acid, and (D) malic acid. Significant values are presented as *p < 0.05.
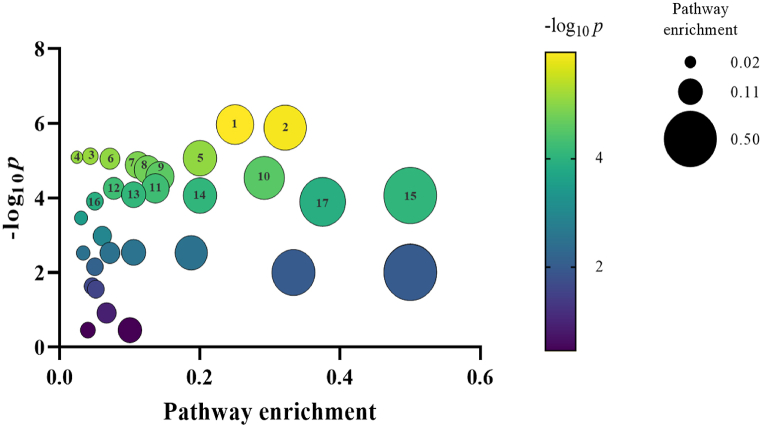
In addition, metabolite enrichment analysis between Kimchi A and C showed that TCA cycle (p = 1.08E-06), Alanine, aspartate and glutamate metabolism (p = 1.31E-06), Tryptophan metabolism (p = 8.13E-06), and tyrosine metabolism (p = 8.89E-06) were significantly different in week 1 (Fig. 7).
Fig. 7.
Metabolite enrichment analysis. The metabolites between Kimchi A and C in week 1 were analyzed using metaboanalyst 5.0. Color intensity (purple to yellow) reflects statistical significance, while the circle diameter covaries with increasing pathway enrichment (hit metabolites/total metabolites).). 1: Citrate cycle (p = TCA cycle) (p = 1.08E-06), 2: Alanine, aspartate and glutamate metabolism (p = 1.31E-06), 3: Propanoate metabolism (p = 7.61E-06), 4: Tryptophan metabolism (p = 8.13E-06), 5: Butanoate metabolism (p = 8.62E-06), 6: Tyrosine metabolism (p = 8.89E-06), 7: Ubiquinone and other terpenoid-quinone biosynthesis (p = 1.30E-05), 8: Glyoxylate and dicarboxylate metabolism (p = 1.74E-05), 9: beta-Alanine metabolism (p = 2.67E-05), 10: Aminoacyl-tRNA biosynthesis (p = 2.91E-05), 11: Pyruvate metabolism (p = 5.54E-05), 12: Glycolysis/Gluconeogenesis (p = 5.55E-05), 13: Pantothenate and CoA biosynthesis (p = 8.19E-05), 14: Phenylalanine metabolism (p = 8.63E-05), 15: Phenylalanine, tyrosine and tryptophan biosynthesis (p = 8.63E-05), 16: Valine, leucine and isoleucine degradation (p = 1.22E-0.4), 17: Valine, leucine and isoleucine biosynthesis (p = 1.27E-04). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 8 shows the correlation between bacteria and metabolites. Correlation coefficient analysis was conducted using the Spearman's rank correlation coefficient with the species (>0.5% presence) and the metabolites in week 1. These results indicated that LAB present in kimchi generally showed a distinct correlation with organic acids. Among the 21 species, 16 were positively correlated with lactic acid and succinic acid, 14 species negatively correlated with citric acid, and 18 species were negatively correlated with fumaric acid and malic acid.
Fig. 8.
Correlation between LAB and metabolites. The distance correlation test using Spearman's rank correlation coefficient was performed to assess the relationship between LAB and metabolites. The significant correlations were visually represented, with metabolites listed along the top and LAB- displayed along the left side. Positive and negative correlations are indicated by red and blue colors, respectively. *p < 0.05 and **p < 0.005. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, kimchi fermentation under different temperature conditions (4, 10, and 15 °C) was evaluated through microbial community variation and metabolites analysis. The findings of the present study showed that kimchi fermented at relatively high temperatures (10 and 15 °C) rapidly increased LAB count, and reduced the sugar content (Fig. 1).
Previous studies have demonstrated that the bacterial composition of initial stages of kimchi fermentation is primarily influenced by raw materials [12,21]. However, fermentation at different temperatures results in distinct bacterial composition [11,12]. For example, fermentation at 10 °C increases the L. plantarum, L. spicheri, and L. citreum, while L. mesenteroides is more prevalent at the initial stage of fermentation at 4 °C. Both W. confusa and L. carnosum emerge at both temperatures [12]. Similarly, another study found that L. mesenteriodes is dominant during the immature stage at 8 °C, whereas L. plantarum is prevalent during the over-ripening stage at 25 °C [11]. These studies also reported a rapid reduction in pH with elevated temperatures, suggesting that reduction in acidity is a key factor driving the diversity of LAB during fermentation.
In our study, we utilized pyrosequencing to analyze the bacterial populations in kimchi, which marks significant advancement over previous studies that predominantly relied on PCR-based methods for their analyses [11,12]. We observed that L. plantarum peaked in kimchi C at the latter part of fermentation. While L. mesenteroides was most abundant in the early stage of kimchi A. These observations are in line with previous studies, demonstrating consistent patterns in bacterial differences and abundance. The bacterial population in different temperature conditions showed close correlation with acidic conditions among the three kimchi. For example, L. sakei showed distinct growth at similar pH ranges among the three kimchi through fermentation, L. sakei content peaked around week 2 for kimchi B and week 1 for kimchi C (Fig. 1, Fig. 3).
LAB are renowned for ability to metabolize sugars into lactic acid through fermentation. As fermentation progresses, the LAB population grows, leading to increased lactic acid production, a process primarily observed in the early stages of fermentation [19,22]. In addition to lactic acid production, LAB also metabolize various organic acids. The metabolite enrichment set showed that TCA cycle and some amino acids metabolism pathways were most significantly different between Kimchi A and C (Fig. 6). Malic and citric acids are converted to lactic acid during the TCA cycle. TCA cycle involves an intermediate metabolite for numerous amino acids and may be related with changes in amino acids during the fermentation [23].
Previous studies exhibited an increase in succinic acid and a reduction in fumaric, malic, and citric acids during kimchi fermentation [19,24,25]. The production of these organic acids exhibits a temperature-sensitive profile; fermentation at temperatures ranging from 10 to 25 °C increases the content of lactic, acetic, and succinic acids, while malic and citric acids either show no significant changes [11] or are reduced [10,24]. Another study showed that the addition of L. sakei in kimchi fermentation led to a rapid decrease of malic, citric, and succinic acid [25]. Moreover, L. sakei and L. plantarum, abundant in kimchi fermented at B and C, possess the malolactic acid enzyme and can convert malic acid into lactic acid [26,27].
This finding underscores the significance of bacterial composition as a key factor influencing the variation in organic acid content. This indicates that increased temperatures encourage bacterial succession, followed by a competition among the bacteria under acidic conditions. This suggests that increased temperatures favor bacterial succession, followed by competition between bacteria under acidic conditions and resulting in distinct metabolite profiles. This highlights the critical role of temperature in determining the quality of kimchi. Effective temperature management can be employed to control fermentation and achieve the desired sensory attributes in kimchi. Overall, this study offers valuable insights into the impact of temperature on the bacterial community and metabolite profiles of kimchi, providing insight into the enhancement of quality and storage. However, this study is limited with respect to the sample numbers and diversity. Additionally, the LAB present in the kimchi originate from the raw materials, which are subject to variations owing to seasonal and regional differences. Therefore, further studies are needed to elucidate the relationship between temperature and bacterial metabolism to refine our comprehension and control of kimchi fermentation.
5. Conclusion
In this study, we thoroughly examined the effect of temperature on both bacterial communities and metabolite changes during kimchi fermentation. In this study, we proved that an increase in temperature to 10 or 15 °C significantly enhanced the growth and diversity of LAB compared to a temperature of 4 °C. Elevated temperatures led to reductions in sugar content and associated with increased the key organic acids and resulted in the rapid acidification of kimchi. The acid sensitivity of the bacterial succession suggests that variations in temperature lead to shifts in bacterial composition as well as metabolite production in kimchi fermentation.
Data availability statement
All data supporting the findings of this study are available from the corresponding author [J.H.Lee] upon reasonable request.
CRediT authorship contribution statement
Sera Jung: Writing – original draft, Validation, Resources, Methodology, Investigation, Formal analysis, Data curation. In Min Hwang: Validation, Methodology, Formal analysis, Data curation. Jong-Hee Lee: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research received support from a grant [KE2301-1] provided by the World Institute of Kimchi, which is funded by the Ministry of Science and ICT. Additionally, it was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and ICT [NRF–2021R1F1A1046425], Republic of Korea.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27174.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Patra J.K., Das G., Paramithiotis S., Shin H.S. Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front. Microbiol. 2016;7:1493. doi: 10.3389/fmicb.2016.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skowron K., Budzyńska A., Grudlewska-Buda K., Wiktorczyk-Kapischke N., Andrzejewska M., Wałecka-Zacharska E., Gospodarek-Komkowska E. Two faces of fermented foods—the benefits and threats of its consumption. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.845166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park K.Y., Jeong J.K., Lee Y.E., Daily J.W., 3rd Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food. 2014;17:6–20. doi: 10.1089/jmf.2013.3083. [DOI] [PubMed] [Google Scholar]
- 4.Park B., Hwang H., Chang J.Y., Hong S.W., Lee S.H., Jung M.Y., Sohn S.O., Park H.W., Lee J.H. Identification of 2-hydroxyisocaproic acid production in lactic acid bacteria and evaluation of microbial dynamics during kimchi ripening. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-10948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang H., Lee J.-H. Characterization of arginine catabolism by lactic acid bacteria isolated from kimchi. Molecules. 2018;23:3049. doi: 10.3390/molecules23113049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung S., Hwang H., Lee J.-H. Effect of lactic acid bacteria on phenyllactic acid production in kimchi. Food Control. 2019;106 doi: 10.1016/j.foodcont.2019.06.027. [DOI] [Google Scholar]
- 7.Chang J.H., Shim Y., Cha S.K., Chee K. Probiotic characteristics of lactic acid bacteria isolated from kimchi. J. Appl. Microbiol. 2010;109:220–230. doi: 10.1111/j.1365-2672.2009.04648.x. [DOI] [PubMed] [Google Scholar]
- 8.Hongu N., Kim A.S., Suzuki A., Wilson H., Tsui K.C., Park S. Korean kimchi: promoting healthy meals through cultural tradition. J. Ethn. Foods. 2017;4:172–180. doi: 10.1016/j.jef.2017.08.005. [DOI] [Google Scholar]
- 9.Jung J.Y., Lee S.H., Jeon C.O. Kimchi microflora: history, current status, and perspectives for industrial kimchi production. Appl. Microbiol. Biotechnol. 2014;98:2385–2393. doi: 10.1007/s00253-014-5513-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim E.-J., Seo S.-H., Park S.-E., Lim Y.-W., Roh S.W., Son H.-S. Initial storage of kimchi at room temperature alters its microbial and metabolite profiles. LWT-Food Sci. Technol. 2020;134 doi: 10.1016/j.lwt.2020.110160. [DOI] [Google Scholar]
- 11.Lee H.Y., Haque M.A., Cho K.M. Changes in physicochemical property and lactic acid bacterial community during kimchi fermentation at different temperatures. J. Appl. Biol. Chem. 2020;63:429–437. doi: 10.3839/jabc.2020.056. [DOI] [Google Scholar]
- 12.Yeun H., Yang H.S., Chang H.C., Kim H.Y. Comparison of bacterial community changes in fermenting kimchi at two different temperatures using a denaturing gradient gel electrophoresis analysis. J. Microbiol. Biotechnol. 2013;23:76–84. doi: 10.4014/jmb.1210.10002. [DOI] [PubMed] [Google Scholar]
- 13.Shim S.-M., Kim J.Y., Lee S.M., Park J.-B., Oh S.-K., Kim Y.-S. Profiling of fermentative metabolites in kimchi: volatile and non-volatile organic acids. J. Korean Soc. Appl. Biol. Chem. 2012;55:463–469. doi: 10.1007/s13765-012-2014-8. [DOI] [Google Scholar]
- 14.Lee M.-E., Jang J.-Y., Lee J.-H., Park H.-W., Choi H.-J., Kim T.-W. Starter cultures for kimchi fermentation. J. Microbiol. Biotechnol. 2015;25:559–568. doi: 10.4014/jmb.1501.01019. [DOI] [PubMed] [Google Scholar]
- 15.Jung S., An H., Lee J.-H. Red pepper powder is an essential factor for ornithine production in kimchi fermentation. LWT-Food Sci. Technol. 2021;137 doi: 10.1016/j.lwt.2020.110434. [DOI] [Google Scholar]
- 16.Rojas-Escudero E., Alarcón-Jiménez A.L., Elizalde-Galván P., Rojo-Callejas F. Optimization of carbohydrate silylation for gas chromatography. J. Chromatogr. A. 2004;1027:117–120. doi: 10.1016/j.chroma.2003.10.131. [DOI] [PubMed] [Google Scholar]
- 17.Tsugawa H., Cajka T., Kind T., Ma Y., Higgins B., Ikeda K., Kanazawa M., VanderGheynst J., Fiehn O., Arita M. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods. 2015;12:523–526. doi: 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Z., Zhou G., Ewald J., Chang L., Hacariz O., Basu N., Xia J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022;17:1735–1761. doi: 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- 19.Jeong S.H., Lee S.H., Jung J.Y., Choi E.J., Jeon C.O. Microbial succession and metabolite changes during long‐term storage of kimchi. J. Food Sci. 2013;78:M763–M769. doi: 10.1111/1750-3841.12095. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y.-J., Lee H.-W., Yang J.-H., Hong S.-W., Park S.-H., Lee M.-A. Changes in quality properties of kimchi based on the nitrogen content of fermented anchovy sauce, Myeolchi Aekjeot, during fermentation. Food Sci. Biotechnol. 2018;27:1145–1155. doi: 10.1007/s10068-018-0349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S., Jung J., Jeon C. Source tracking and succession of kimchi lactic acid bacteria during fermentation. J. Food Sci. 2015;80:M1871–M1877. doi: 10.1111/1750-3841.12948. [DOI] [PubMed] [Google Scholar]
- 22.Park S.-E., Yoo S.-A., Seo S.-H., Lee K.-I., Na C.-S., Son H.-S. GC–MS based metabolomics approach of Kimchi for the understanding of Lactobacillus plantarum fermentation characteristics. LWT-Food Sci. Technol. 2016;68:313–321. doi: 10.1016/j.lwt.2015.12.046. [DOI] [Google Scholar]
- 23.D'Este M., Alvarado-Morales M., Angelidaki I. Amino acids production focusing on fermentation technologies–A review. Biotechnol. Adv. 2018;36:14–25. doi: 10.1016/j.biotechadv.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 24.You S.-Y., Yang J.-S., Kim S.H., Hwang I.M. Changes in the physicochemical quality characteristics of cabbage kimchi with respect to storage conditions. J. Food Qual. 2017:7. doi: 10.1155/2017/9562981. 2017. [DOI] [Google Scholar]
- 25.Lee J.-J., Choi Y.-J., Lee M.J., Park S.J., Oh S.J., Yun Y.-R., Min S.G., Seo H.-Y., Park S.-H., Lee M.-A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.109591. [DOI] [PubMed] [Google Scholar]
- 26.Sumby K.M., Bartle L., Grbin P.R., Jiranek V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019;103:2033–2051. doi: 10.1007/s00253-018-09608-8. [DOI] [PubMed] [Google Scholar]
- 27.Oguro Y., Nishiwaki T., Shinada R., Kobayashi K., Kurahashi A. Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. J. Biosci. Bioeng. 2017;124:178–183. doi: 10.1016/j.jbiosc.2017.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available from the corresponding author [J.H.Lee] upon reasonable request.