Abstract
Introduction
Severe Haemophilia A patients with inhibitors are currently being treated with bypassing agents like activated prothrombin complex concentrates (aPCC) and recombinant factor VIIa. Emicizumab is a recombinant humanized monoclonal antibody, introduced to reduce the bleeding events, improve treatment adherence, and quality of life. However, cost-effectiveness and long-term sustainability of the intervention is not studied in a low middle income setting like India.
Aim
The primary objective of this study was to evaluate the cost-utility of Emicizumab compared to traditional bypassing agents in the treatment of severe haemophilia A patients with inhibitors in India. Secondary objective was to analyze the budgetary impact of introducing Emicizumab for this patient population from the perspective of public health system in India.
Methods
Markov model was created to compare the prophylactic emicizumab therapy against bypassing agents for a hypothetical cohort of 10-year-old adolescents in India. The time horizon was 10 years and model built based on health system perspective. Cost utility was expressed as costs per quality-adjusted life-years (QALYs) gained. All costs were expressed as 2021 US dollars. Probabilistic sensitivity analysis was performed to check the robustness of the estimates.
Results
Prophylactic emicizumab was a cost saving intervention with negative Incremental Cost Utility Ratio (ICUR) against recombinant factor VIIa of −853,573 USD (INR -63,109,773), and negative ICUR of −211,675 USD (INR -15,650,403) against APCC. The estimated total budget for treating all the severe Haemophilia A patients with inhibitors in India was USD 59,042,000 (INR 4,365,329,312) for 10 years’ time horizon (per patient cost of USD 295,210 [INR 21,826,646.56]).
Conclusion
Prophylactic emicizumab therapy is a cost saving intervention when compared to both the bypassing agents as it is less costly and more effective for severe Haemophilia A patients with inhibitors in India.
Keywords: Cost utility analysis, Emicizumab, Haemophilia A, India
1. Introduction
Haemophilia A is a X-linked recessive bleeding disorder, characterised by a decreased or absent circulating factor VIII (FVIII) activity, leading to lifelong bleeding tendency. This disorder accounts for nearly 85% of all the haemophilia cases [1]. It has an incidence of about 1 in 5000 male births. Males are symptomatic and present with spontaneous bleeding episodes and bleeding secondary to trauma and surgeries, while females may have bleeding manifestations or remain asymptomatic carriers [2]. Haemophilia is classified into mild (6–40%), moderate (1–5%) and severe haemophilia (<1%) based on FVIII levels [1]. People with severe haemophilia A are at increased risk of developing severe life-threatening bleeding following trauma and surgeries. They develop spontaneous bleeding into joints (hemarthrosis), soft tissues and muscles leading to chronic disability and impaired quality of life [1].
Replacement of factor-VIII with intravenously delivered factor-VIII concentrates either plasma derived or recombinant is the standard of care for patients with severe Haemophilia A. It is administered in response to bleeding (“on-demand therapy”) or to prevent bleeding (“prophylactic therapy”) [1,2]. However, 20–30% of people with severe haemophilia A develop inhibitors to factor-VIII. Presence of inhibitors increases the morbidity and mortality in patients with severe haemophilia A [1].
Severe Haemophilia A patients with inhibitors are currently being treated with bypassing agents such as activated prothrombin complex concentrates (aPCC) and recombinant activated factor VIIa [1,3]. Intravenous bypassing agents are used on an on-demand basis during the bleeding episodes to arrest bleeding [1]. This does not reduce the occurrence of bleeding episodes and resulting disability. Direct cost of bypassing agents alone account for 98% of the healthcare cost associated with the treatment of severe haemophilia A [4]. High cost of bypassing agents also affects treatment adherence and reduces overall treatment effectiveness and quality of life [5,6].
Emicizumab is a recombinant humanized monoclonal antibody, mimics the function of factor VIII instead of replacing it [6]. It has been approved by US FDA in 2017 for the prevention and reduction of bleeding in haemophilia A patients with inhibitors [7]. It has a half-life of approximately 30 days and is unaffected by the presence of inhibitors [8]. It reduces the annualised bleeding rates and reported to have good safety profile [9]. Less frequent and subcutaneous administration of emicizumab increases the treatment adherence and improves the quality of life of severe haemophilia A patients and decreases burden on the healthcare system [8]. A recently concluded phase III trial has demonstrated safety and efficacy with once-weekly subcutaneous dosing of emicizumab over bypassing agents [10]. However, breakthrough bleeding during prophylactic therapy has to be treated with bypassing agents. Though emicizumab has several advantages over bypassing agents, cost-effectiveness of prophylactic emicizumab in severe haemophilia A patients with inhibitors is not studied in a low middle income setting like India. Hence, the study was done to compare the budget impact and cost-utility of emicizumab against bypassing agents in the treatment of severe haemophilia A patients with inhibitors.
2. Methods
2.1. Model characteristics
Markov model (given the recurrent nature of the bleeds in severe Haemophilia patients) was created using TreeAge Pro Healthcare 2022 software. This model was run for a hypothetical cohort of 10-year-old children with severe haemophilia A with factor VIII inhibitors. The time horizon was 10 years and analysis were performed based on health system perspective.
This model was utilized to estimate the cost-utility of prophylactic emicizumab compared to on-demand bypassing agents (recombinant factor VIIa or APCC) in India. Separate model was run for each of the two bypassing agents. Currently, severe haemophilia A patients with inhibitors are being treated with on-demand bypassing agents i.e., as and when the bleeding occurs. The average recommended dosage of recombinant factor VIIa was taken as 0.2 mg/kg/bleed, while the dosage of APCC was 65 IU/kg/bleed [11]. The recommended dosage of emicizumab consists of a loading dose of 3 mg/kg for every week for first four weeks of initial therapy followed by maintenance dose of 3 mg/kg once in two weeks in subsequent months [11].
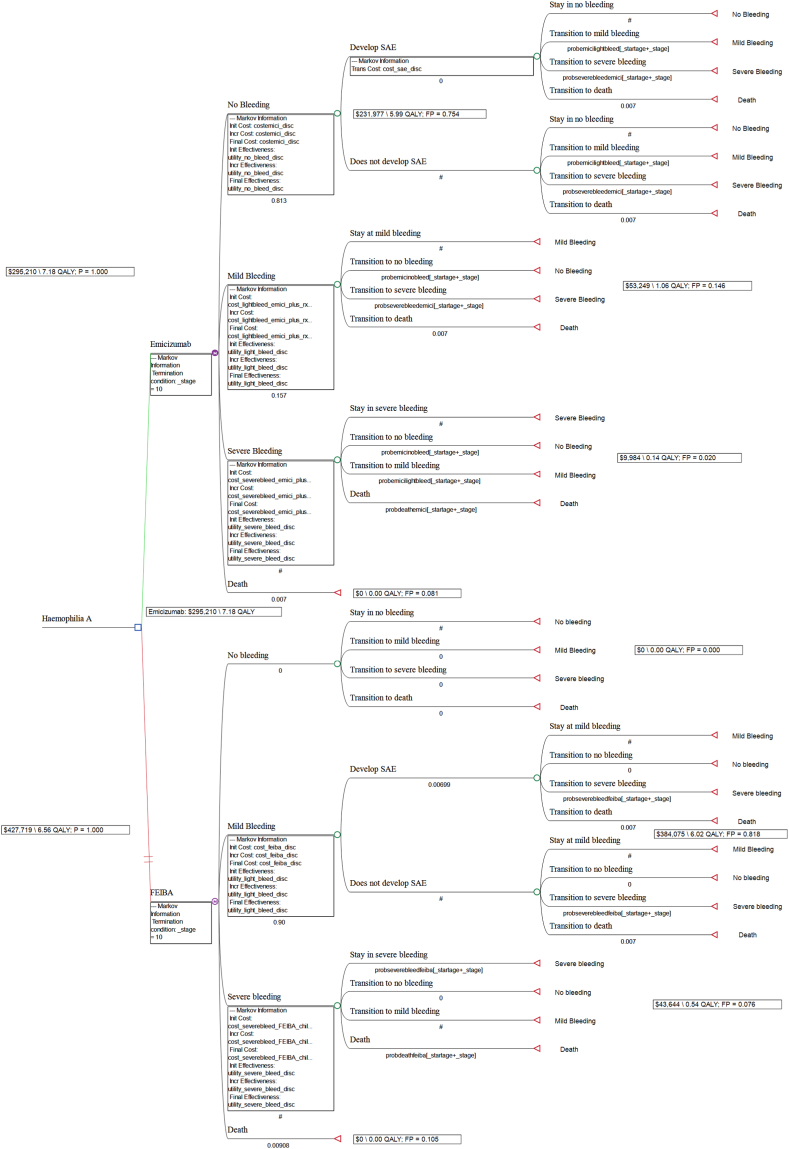
Figure-1 shows the state transition diagram consisting of the health states in model (i.e., no bleeding, mild bleeding, severe bleeding and death). There was also an interim transition state of serious adverse events (SAEs) associated with emicizumab or bypassing agents. Figure-2A and 2B shows the Markov decision tree model for prophylactic emicizumab against on-demand recombinant factor VIIa and APCC separately. The cycle length is one year.
Fig. 1.
State Transition Diagram for all the possible health states in the Markov Model.
Fig. 2.
a: Markov decision tree model for prophylactic emicizumab against recombinant factor VIIa with rollback results
b: Markov decision tree model for prophylactic emicizumab against APCC with rollback results.
2.2. Inputs
Broadly, the following inputs were required in the model: transition probabilities, cost and utility parameters. The values of each individual input with references are detailed in Table-1.
Table 1.
Input parameters for base case and sensitivity analysis.
| Input parameter | Base case values | Parameter range (distribution)┼ | Source |
|---|---|---|---|
| Disease parameters | |||
| Probability of bleeding and death after treatment with emicizumab | |||
| Probability of no bleeding after emicizumab | 0.813 | 0.768–0.95 (beta) | Michael U. Callaghan et al., 2021 (HAVEN 1–4) [10] |
| Probability of mild bleeding after emicizumab | 0.157 | 0.121–0.20 (beta) | Michael U. Callaghan et al., 2021 (HAVEN 1–4) [10] |
| Probability of severe bleeding after emicizumab | Extracted by combining the other states and subtracting by 1 | ±10% (beta) | – |
| Probability of death after emicizumab | 0 | 0–0.04 (beta) | Makris M et al., 2019 [12] |
| Probability of bleeding and death after treatment with recombinant VII A | |||
| Probability of no bleeding after recombinant VII A | 0 | ±10% (beta) | – |
| Probability of mild bleeding after recombinant VII A | 0.90 | ±10% (beta) | Levi M et al., 2005 [16] |
| Probability of severe bleeding after recombinant VII A | Extracted by combining the other states and subtracting by 1 | ±10% (beta) | – |
| Probability of death after recombinant VII A | 0.02277 | ±10% (beta) | Abshire T et al., 2008 [13] |
| Probability of bleeding and death after treatment with APCC | |||
| Probability of no bleeding after APCC | 0 | ±10% (beta) | |
| Probability of mild bleeding after APCC | 0.90 | ±10% (beta) | Zhou ZY et al., 2010 [15] |
| Probability of severe bleeding after APCC | 1-other states | ±10% (beta) | |
| Probability of death after APCC | 0.00908 | ±10% (beta) | Kim CH et al., 2019 [14] |
| Probability of severe adverse events (SAE) | |||
| Probability of SAE after emicizumab | 0 | ±10% (beta) | Pipe SW et al., 2019 [17] |
| Probability of SAE after recombinant VII A | 0.01 | ±10% (beta) | Levi M et al., 2005 [16] |
| Probability of SAE after APCC | 0.00699 | ±10% (beta) | Olasupo OO et al., 2021 [18] |
| Utility values | |||
| Utility value for no bleeding | 0.80 (0.21 SD) | ±10% (beta) | Hoxer CS et al., 2019 [22] |
| Utility value of light bleeding | 0.73 (0.22 SD) | ±10% (beta) | Hoxer CS et al., 2019 [22] |
| Utility value of severe bleeding | 0.67 (0.25 SD) | ±10% (beta) | Hoxer CS et al., 2019 [22] |
| Utility value for death | 0 | – | Hoxer CS et al., 2019 [22] |
| Treatment costs | |||
| Cost of emicizumab per 30 mg vial | 551.2 USD (40,754.00 INR) | ±10% (Gamma) | Price quoted by pharmaceuticals |
| Cost of recombinant VII A per vial | 531.7 USD (39,310.00 INR) | ±10% (Gamma) | Price quoted by pharmaceuticals |
| Cost of APCC per IU | 0.74 USD (55.00 INR) | ±10% (Gamma) | Price quoted by pharmaceuticals |
| Dosage requirement of emicizumab Loading dose (1st month): Maintenance dose (subsequent months): |
3 mg/kg once a week 3 mg/kg once in two weeks |
– | Srivastava A et al., 2020 (WFH Guidelines for the Management of Hemophilia) [11] |
| Dosage requirement of recombinant VII A | 0.2 mg/kg | – | Srivastava A et al., 2020 [11] |
| Dosage requirement of APCC | 65 IU/kg | – | Srivastava A et al., 2020 [11] |
| Unit cost per outpatient visit | 3.978 USD | ±10% (Gamma) | Chauhan AS et al., 2022 [19] |
| Unit cost per inpatient admission | 75.997 USD | ±10% (Gamma) | Chauhan AS et al., 2022 [19] |
| Unit cost per ICU admission | 149.975 USD | ±10% (Gamma) | Chauhan AS et al., 2022 [19] |
| Unit cost per inpatient day | 17.368 USD | ±10% (Gamma) | Chauhan AS et al., 2022 [19] |
| Unit cost per ICU-day | 74.9978 USD | ±10% (Gamma) | Chauhan AS et al., 2022 [19] |
| Exchange rate (USD to INR) | 1 USD = 73.936 INR | – | World Bank official exchange rate for 2021 [21] |
| Discount rate | 3% | – | WHO guide to cost-effectiveness analysis [20] |
APCC - Factor eight inhibitor bypass activity (activated prothrombin complex concentrates); ICU-Intensive Care Unit; INR – Indian Rupees; SAE – Serious Adverse Events; USD – United States Dollars; QALY-Quality adjusted life years.
2.2.1. Transition probabilities
Initial and transition probability of no, mild, severe bleeding and death was obtained from HAVEN 1–4 studies, which reports the long-term effectiveness and safety of prophylactic emicizumab [10,12]. The probabilities for the bypassing agents were obtained from two systematic review and meta-analysis papers, pooling the efficacy and safety estimates for recombinant factor VIIa and APCC [[13], [14], [15]]. The transition probability for SAE due to bypassing agents was also obtained from the same review [[15], [16], [17]].
Probability of no bleeding was kept as zero for patients on either of the on-demand bypassing agents, as the severe haemophilia A patients with inhibitors and not on any prophylactic therapy are bound to have bleeding events. Comprehensive literature review and expert opinion was obtained before setting the transition probabilities for every health states. The compliance to treatment was assumed to be 100% for prophylactic therapy. All-cause mortality was also incorporated into the probability of death in both the intervention cycles.
2.2.2. Costs
The cost estimates included in the model were the cost of prophylactic emicizumab treatment, cost of treating light and severe bleeding events, cost of on-demand treatment with bypassing agents and cost of treating SAEs.
2.2.3. Prophylactic emicizumab treatment
Cost of prophylactic emicizumab treatment consists of the drug costs and the health system costs associated with the provision of drug in the hospitals. The current drug price of emicizumab (trading under the name “Hemlibra”) is USD 551.2 (INR 40,754) per 30 mg vial. Approximately 104 vials will be required for treating a patient weighing 40 kg (assumed for the current study cohort). However, currently, the Roche pharmaceuticals is providing a discount to provide free vials for a period of six months (maintenance phase) treatment in India. Hence, the cost calculation is required for only 56 vials. The scenario analysis without the discount was done to understand the future sustainability of the intervention without the concession from the pharmaceutical company. Health system cost was calculated depending on the number of visits required to the hospital for getting the prophylactic medication. For the loading dose, the patient should visit the facility weekly, while during the maintenance phase, the patient can visit as little as one visit per month or 4 times/month (depending on the preferred dosing schedule). In this study, the assumption is 2 visits/month will be required during maintenance phase, totalling to approximately 26 hospital visits/year. Unit cost per outpatient visit was obtained from the large-scale multicentric costing study conducted in India.
Breakthrough bleeding event during the prophylactic emicizumab therapy should be treated with recombinant factor VIIa [11]. Treatment with APCC for breakthrough bleeding in patients on emicizumab is reported to cause higher risk of serious complications like thrombotic microangiopathy [11]. Hence, the cost of recombinant factor VIIa was included to calculate the cost of health states like mild and severe bleeding event in emicizumab treated patients. For mild bleeding event, the recommended dose of recombinant factor VIIa is 270 μg/kg for one day, while for severe bleeding event, the recommended dose is required (on average) for three days. Hence, for mild bleeding event, cost of recombinant factor VIIa and unit cost of outpatient visits were incorporated, while for severe bleeding event, unit costs for intensive care unit (ICU) stay (for first two days of treating severe bleeding event) and inpatient stay (for one day before discharge) were additionally incorporated.
2.2.4. On-demand bypassing agents
2.2.4.1. Recombinant factor VIIa
As per literature search and expert opinions, severe haemophilia A patients with inhibitors and not on any prophylactic therapy are reported to have two bleeding events per month (averaging to 24 bleeding events per year) and this state is considered as mild bleeding event [18]. For each bleeding event, 8 mg of recombinant factor VIIa (0.2 mg/kg/bleeding event) is required for a patient weighing 40 kgs, totalling to 8 vials (1 vial = 1 mg) [11]. Hence, a total of 192 vials will be required for one-year period. Cost of one vial is approximately USD 531.7 (INR 39,310). Unit costs of health system visit was also added for each bleeding event. For severe bleeding events, additional costs (as mentioned earlier in prophylactic emicizumab costs) were added [19].
2.2.4.2. APCC
Assumption for the number of bleeding event is as same as that for recombinant factor VIIa. For each bleeding event, 65 IU/kg is required, totalling to 2600 IU [11]. Hence, a total of 62,400 IUs will be required for one-year period after adjusting the dose required according to the available dosage formulations of each vial. Cost of APCC per IU is approximately USD 0.74 (INR 55). Unit costs of health system visit was also added for each bleeding event. For severe bleeding events, additional health system costs and additional dosage costs were added [19].
Costs and utility parameters were discounted at a standard recommended rate of 3% per year [20]. All the costs included in the model were updated to the 2021 US dollars at exchange rate of 73.936 INR [21].
2.2.5. Cost of treating SAE
For treating the thrombotic complications (SAEs) associated with the treatment of prophylactic or on-demand drugs, the standard treatment regimen is to give low molecular weight heparin (LMWH) 80 IU/kg as a bolus dose followed by maintenance dose of 18 IU/kg in the hospital setting. This is followed by oral warfarin treatment at home for a period of three months. Cost for LMWH, warfarin and associated health system costs were included in the SAE transition state of the model.
2.2.6. Utility parameters
The utility value for the health states stated in the model is not available for Indian setting. Hence, values from multi-country large-scale previous study was used. The utility value for no bleeding, mild bleeding, severe bleeding and death were 0.80, 0.73, 0.67 and 0 respectively [22].
2.3. Model outputs
2.3.1. Treatment impact
Treatment impact for prophylactic and on-demand therapy were expressed in terms of the number of bleeding events averted and quality adjusted life years (QALYs) gained (which measures the number of healthy years added). All the possible health states have a utility weight (measuring the quality of life) which ranges from 0 (death) to 1 (perfect health).
2.3.2. Determination of cost-utility
Incremental cost-utility ratios (ICURs), a measure of cost-utility, was estimated by dividing the incremental costs (i.e., net costs in treating a severe haemophilia A patient with inhibitor using prophylactic emicizumab therapy or on-demand bypassing agents) and incremental effectiveness (net QALYs gained between the two strategies). Separate ICURs were obtained for emicizumab versus recombinant factor VIIa and APCC.
2.3.3. Probabilistic sensitivity analysis (PSA)
PSA was performed to check the robustness of the base case model results. It was done by changing the key cost and utility parameters in the model results over a set of plausible ranges based on the probability distributions. This helps to quantify the level of confidence in the model outputs with respect to the uncertainty involved in the inputs. Analysis was performed with Monte Carlo simulation for 1000 iterations, with replacement value taken from the probability distribution of parameters. Cost parameters were assigned the gamma distribution while the utility parameters were assigned beta distribution. Costs, QALYs gained and ICURs were obtained for each iteration.
2.3.4. Budget impact analysis
Budget impact analysis was done to assess the financial implications and feasibility of incorporating prophylactic emicizumab as therapy for severe Haemophilia A patients with inhibitors into the public healthcare system in India. The total budget of prophylactic therapy for a 10-year period was estimated by multiplying the approximate number of severe Haemophilia A patients with inhibitors in the country with the prophylactic therapy costs and incremental healthcare system costs. Annual costs for the implementation were also calculated and presented.
3. Results
3.1. Cost utility analysis
The total treatment cost for Prophylactic Emicizumab, On-demand Recombinant factor VIIa and On demand APCC is estimated to be 295,209.7 USD (21,826,624.4 INR), 920,394.6 USD (68,050,295.1 INR) and 427,718.9 (31,623,824.6 INR) respectively. QALYs gained by Prophylactic Emicizumab, On-demand Recombinant factor VIIa and On demand APCC is estimated to be 7.18, 6.45 and 6.55 respectively.
Table-2 shows the results of base case analysis for prophylactic emicizumab against each of the two bypassing agents. Severe Haemophilia A patients with inhibitors and treated with prophylactic emicizumab is reported to gain 7.18 QALYs over the 10 years period after discounting. Prophylactic emicizumab was also found to avert nearly 185 bleeding events against APCC and 179 bleeding events against recombinant factor VIIa.
Table 2.
Base case analysis results from health system perspective of Prophylactic Emicizumab vs bypassing agents for severe haemophilia A patients with inhibitors in India.
| Treatment | Cost of treatment | Incremental cost | Total bleeding events | Bleeding events averted | QALYs gained | Incremental utility | ICUR |
|---|---|---|---|---|---|---|---|
| Prophylactic Emicizumab | 295,209.7 USD (21,826,624.4 INR) | – | 42 | – | 7.18 | – | – |
| On-demand Recombinant Factor VIIa | 920394.6 USD (68,050,295.1 INR) | – | 221 | – | 6.45 | – | – |
| On-demand APCC | 427718.9 (31,623,824.6 INR) | – | 227 | – | 6.55 | – | – |
| Prophylactic Emicizumab vs Recombinant factor VIIa | – | −625,185 USD (−46,223,678.2 INR) | – | 179 | – | 0.732 | −853,573 USD (−63,109,773 INR) |
| Prophylactic Emicizumab vs APCC | – | −132509.2 USD (−9,797,200.2 INR) | – | 185 | – | 0.626 | −211,675 USD (−15,650,403 INR) |
APCC - Factor eight inhibitor bypass activity (activated prothrombin complex concentrates); ICU-Intensive Care Unit; INR – Indian Rupees; SAE – Serious Adverse Events; USD – United States Dollars; QALY-Quality adjusted life years.
Prophylactic emicizumab was found to be a cost saving intervention with negative ICUR against both the bypassing agents. ICUR for prophylactic emicizumab vs recombinant factor VIIa was −853,573 USD (INR -63,109,773), while against APCC, ICUR was −211,675 USD (INR -15,650,403).
3.2. Budget impact analysis
The total budget required for treating all the severe Haemophilia A patients with inhibitors is estimated to be USD 59,042,000 (INR 4,365,329,312) for 10 years’ time horizon (per patient cost of USD 295,210 [INR 21,826,646.56]). The annual cost for health system is estimated to be USD 5,904,200 (INR 436,329,312) (Table 3).
Table 3.
Budget impact analysis from health system perspective of Prophylactic Emicizumab vs bypassing agents for severe haemophilia A patients with inhibitors in India.
| Parameters | Values |
|---|---|
| Approximate number of severe Haemophilia A patients in India | 600 |
| Approximate number of severe Haemophilia A patients with inhibitors in India (30% of the total severe patients) | 200 |
| Cumulative discounted cost of prophylactic emicizumab therapy per patient over 10 years' time horizon | 295,210 USD (21,826,646.56 INR) |
| Total budget required for treating severe haemophilia A patients with inhibitors using prophylactic emicizumab therapy over 10 years in India | 59,042,000 USD (INR 4,365,329,312) |
| Annual budget requirement | 5,904,200 USD (INR 436,329,312) |
3.3. Scenario analysis
Prophylactic emicizumab therapy was still found to be a cost saving intervention without discount in the unit cost of the drug against on-demand recombinant factor VIIa (negative ICUR of −382,502.7 USD [INR -28,280,719.6]). However, on comparison to APCC, the prophylactic emicizumab was not found to be a cost-effective intervention with ICUR of 175,995 USD (INR 13,012,366.3) (Table-4). Hence, application of the existing discount is necessary for adoption of prophylactic emicizumab therapy in health system perspective.
Table 4.
Health system perspective results of Prophylactic Emicizumab (without discount) vs bypassing agents for severe haemophilia A patients with inhibitors in India.
| Treatment | Cost of treatment | Incremental cost | QALYs gained | Incremental utility | ICUR |
|---|---|---|---|---|---|
| Prophylactic Emicizumab | 537,892 USD (39,769,582.9 INR) | – | 7.18 | – | – |
| On-demand Recombinant Factor VIIa | 920394.6 USD (68,050,295.1 INR) | – | 6.45 | – | – |
| On-demand FEIBA | 427718.9 (31,623,824.6 INR) | – | 6.55 | – | – |
| Prophylactic Emicizumab vs Recombinant factor VIIa | – | −382502.7 USD (−28,280,719.6 INR) | – | 0.732 | −522236 USD (38,612,040.9 INR) |
| Prophylactic Emicizumab vs FEIBA | – | 110,173.1 USD (8,145,758.3 INR) | – | 0.626 | 175,995 USD (13,012,366.3 INR) |
APCC - Factor eight inhibitor bypass activity (activated prothrombin complex concentrates); ICU-Intensive Care Unit; INR – Indian Rupees; SAE – Serious Adverse Events; USD – United States Dollars; QALY-Quality adjusted life years.
3.4. PSA results
Figure-3A shows the cost effectiveness scatterplot, where there is clear area of demarcation between prophylactic emicizumab and recombinant factor VIIa, indicating the cost saving nature of prophylactic therapy. Figure-4A further confirms these findings, where the ICUR for the entire 1000 simulation results shows that the prophylactic emicizumab is a cost saving intervention when compared to recombinant factor VIIa. Hence, prophylactic emicizumab therapy occupies the Southeast quadrant of the cost-effectiveness plane, which indicates that the intervention is less costly and more effective.
Fig. 3.
Cost effectiveness scatterplot
3A) Prophylactic Emicizumab vs Recombinant Factor VIIa;
3B) Prophylactic Emicizumab vs APCC.
Fig. 4.
Incremental Cost effectiveness scatterplot
4A) Prophylactic Emicizumab vs Recombinant Factor VIIa;
4B) Prophylactic Emicizumab vs APCC.
Cost-effectiveness scatterplot (Figure-3B) does not have such clear area of demarcation between prophylactic emicizumab and APCC. Figure-4B further reiterates these findings, where the ICUR values are spread across the four quadrants of cost-effectiveness plane. However, major portion of the simulation results still occupied the Southeast quadrant i.e., nearly 63% probability that the prophylactic emicizumab will be a cost saving intervention compared to APCC.
4. Discussion
This study investigated the budget impact and cost utility of the prophylactic emicizumab therapy against on-demand bypassing agents for adolescent severe haemophilia A patients with inhibitors. Two separate Markov models were created, one for each of the bypassing agent against prophylactic emicizumab and the analysis was performed based on a health system perspective. The model findings have showed that emicizumab prophylaxis is a cost saving intervention when compared to each of the two on-demand bypassing agents with negative ICUR. This means that the patients on emicizumab prophylaxis gain additional QALYs and it costs the health system lesser than the existing on-demand bypassing agent treatment (i.e., less costly and more effective). Severe Haemophilia A patients with inhibitors are currently treated with on-demand bypassing agents (recombinant factor VIIa/APCC) across various public health hospitals of India [23]. Prophylactic emicizumab therapy, in addition to being a cost-saving intervention, has several other advantages such as prevention or reduction in the number of bleeding episodes, ease of compliance (given the once or twice monthly prophylactic regimen), reduction in healthcare system costs (subcutaneous administration of agent) and better quality of life for the patients [24].
Though, economic evaluation of prophylactic emicizumab is not conducted in Indian setting, the model findings were in line with previous models developed for other countries like Brazil, France, Italy, Korea, Iran, and Peru [4,6,[25], [26], [27], [28]]. Though, there is difference across these studies in terms of the time horizon, all these studies included Markov models reporting results in terms of health system perspective. This shows that the prophylactic emicizumab therapy will be a cost-saving intervention in Indian setting. Nonetheless, the base case model was run based on the current discounted price of emicizumab provided by the manufacturer in India. The scenario analysis without discount showed it may not be cost-effective against APCC with the same model parameters. Hence, it is important to receive the drug in the current discounted price to sustain the cost-saving nature of intervention.
Since the model parameters were secondary data taken from various sources, there is always a possibility of having variability, inherent uncertainty with the data. Hence, additional sensitivity analysis was performed, which showed that the prophylactic emicizumab has 100% probability of being cost-saving intervention when compared to recombinant factor VIIa, while the probability reduces to 63% against APCC. However, the possible reason for such variation could be the use of undiscounted emicizumab price as the highest range for the cost parameter. As seen with the scenario analysis, the emicizumab therapy may not be a cost-effective intervention against APCC without the current discounted price. Nonetheless, the current model results provide enough evidence that the prophylactic emicizumab therapy is a cost-saving intervention against both the bypassing agents with the current discounted price and requires the same discount to remain a cost-effective intervention.
The total budget impact was estimated to be nearly 59 million dollars (i.e., INR 436 crores) for treating all the existing Haemophilia A patient in India for a period of 10 years (annual cost of 5.9 million dollars i.e., 43.6 crores INR). The per patient cost for 10 years was USD 295,210 [i.e., 2.1 crores INR]. The annual per patient health system cost is estimated to be 0.0001% of GDP of India. Given the fact that India spends up to 2% of GDP on health, this would occupy only 0.0068% of healthcare budget. This information is particularly important for policymakers in the healthcare system to understand the potential impact to the annual national health budget. The per patient costs can be utilized by the respective state health authorities to calculate the budget impact to their healthcare system.
This study has certain strengths. First, this is the first study evaluating cost-utility of emicizumab prophylaxis against on-demand bypassing agents amongst severe Haemophilia A patients with inhibitors in Indian setting. Second, utilization of Markov model helps to account for the recurrent nature of the bleeding events. Third, budget impact results will help the national, regional and state level policymakers to understand the estimated short-term and long-term budget required for the implementation of prophylactic emicizumab therapy. Finally, additional sensitivity analysis helps to account for the uncertainty in model parameters.
This study has certain limitations. First, the study was done in health system perspective, which means that the patient side direct and indirect costs could not be accounted into the model. However, the main objective of the study is to inform the health system about the inclusion of the therapy in their perspective. Nonetheless, the societal perspective model would have further highlighted the cost saving nature associated with emicizumab, given the lower hospital visits, morbidity, mortality rate associated with prophylactic emicizumab therapy. Second, the model included few inputs outside the Indian context as there was no national or regional level data available for some parameters. However, PSA was performed to overcome these limitations and check the robustness of the base case estimates. Finally, the starting age of the model is 10 years and provides estimates for this cohort over the next 10 years timeframe. This is because certain parameters like the drug dosage and certain transition probabilities are age-specific, which could not be accounted in our model. Hence, separate economic evaluation models can be created for children (<10 years) and adults (>19 years).
Despite these limitations, current study provides important guidance to the clinicians and health system actors in India. Prophylactic emicizumab is a cost-saving intervention when compared to the current practice of on-demand bypassing agents for severe haemophilia A patients with inhibitors at the discounted rate. The estimated budget impact is also provided, which can be taken into account before making a switch to this prophylactic therapy. Future studies can also focus on developing separate model for children and adults. Emicizumab is also marketed as a drug that can be used even in Haemophilia A patients without inhibitors. The rationale for such recommendation is that the emicizumab provides better quality of life to the patients by reducing the need for IV injections and frequent visits to hospitals, by reducing the number of bleeding episodes and therefore the disruption of daily activities. Hence, future models can also check the cost-effective nature of this intervention against recombinant factor VIII in patients without inhibitors.
Funding
None.
Ethics approval statement
Ethical approval is not required as the analysis involved data already available from secondary data sources.
Patient consent statement
Not applicable.
Clinical trial registration
Not applicable.
Data availability statement
Data will be made available upon request from the researchers.
CRediT authorship contribution statement
Yuvaraj Krishnamoorthy: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Dhanajayan Govindan: Writing – review & editing, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Narasimhapriyan Kannan: Writing – review & editing, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Data curation. Marie Gilbert Majella: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation. Vishnu Shankar Hariharan: Writing – review & editing, Visualization, Supervision, Software, Resources, Methodology, Investigation, Data curation. Vivek Valliappan: Writing – review & editing, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yuvaraj Krishnamoorthy and Marie Gilbert Majella are Associate Editors in Heliyon Journal. However, both Yuvaraj Krishnamoorthy and Marie Gilbert Majella had no editorial influence or decision-making power regarding the review or acceptance of this particular manuscript within the Heliyon Journal.
Acknowledgements
Nil.
Contributor Information
Yuvaraj Krishnamoorthy, Email: yuvi.1130@gmail.com, yuvaraj@propulevidence.org.
Dhanajayan Govindan, Email: gdhanajayanj@gmail.com.
Narasimhapriyan Kannan, Email: knarasimhapriyan@gmail.com.
Marie Gilbert Majella, Email: gilbert2691@gmail.com.
Vishnu Shankar Hariharan, Email: vishank91@gmail.com.
Vivek Valliappan, Email: vivekvalliappan@gmail.com.
References
- 1.Arruda Valder R., Katherine A. In: Harrison's Principles of Internal Medicine. Jameson J.L., editor. 20thed McGraw Hill; 2018. High. Coagulation disorders; pp. 830–833. [Google Scholar]
- 2.Saini Surbhi, Amy L., Dunn, Haemophilia A., Shaz Beth H., Hillyer Christopher D., Reyes Gil Morayma. Elsevier; 2019. Transfusion Medicine and Haemostasis. 3rded; pp. 677–683. [Google Scholar]
- 3.Barthels M. Clinical efficacy of prothrombin complex concentrates and recombinant factor VIIa in the treatment of bleeding episodes in patients with factor VIII and IX inhibitors. Thromb. Res. 1999;95(4):S31–S38. doi: 10.1016/s0049-3848(99)00082-1. [DOI] [PubMed] [Google Scholar]
- 4.Rasekh H.R., Imani A., Karimi M., Golestani M. Cost-utility analysis of immune tolerance induction therapy versus on-demand treatment with recombinant factor VII for hemophilia A with high titer inhibitors in Iran. Clinicoecon Outcomes Res. 2011;3:207. doi: 10.2147/CEOR.S25909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai-Adisaksopha C., Nevitt S.J., Simpson M.L., Janbain M., Konkle B.A. Bypassing agent prophylaxis in people with hemophilia A or B with inhibitors. Cochrane Database Syst. Rev. 2017;9:Cd011441. doi: 10.1002/14651858.CD011441.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack B., Trossaërt M., Cousin M., Baffert S., Pruvot A., Godard C. Cost-effectiveness of emicizumab vs bypassing agents in the prevention of bleeding episodes in haemophilia A patients with anti-FVIII inhibitors in France. Haemophilia. 2020;00:1–11. doi: 10.1111/hae.14129. [DOI] [PubMed] [Google Scholar]
- 7.Research C for DE and. FDA approves emicizumab-kxwh for hemophilia A with or without factor VIII inhibitors. FDA [Internet]. 2019 [cited 2023 Jan 11]; Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-emicizumab-kxwh-hemophilia-or-without-factor-viii-inhibitors.
- 8.Institute for Clinical and Economic Review . Institute for Clinical and Economic Review; Boston: 2018. Emicizumab for Hemophilia A with Inhibitors: Effectiveness and Value. [Google Scholar]
- 9.McCary I., Guelcher C., Kuhn J., et al. Real-world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. 2020;00:1–6. doi: 10.1111/hae.14005. [DOI] [PubMed] [Google Scholar]
- 10.Callaghan M.U., Negrier C., Paz-Priel I., et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood. 2021;137(16):2231–2242. doi: 10.1182/blood.2020009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A., Santagostino E., Dougall A., et al. WFH Guidelines for the Management of hemophilia. Haemophilia. 2020;26(Suppl 6):1–158. doi: 10.1111/hae.14046. 3rd edition. [DOI] [PubMed] [Google Scholar]
- 12.Makris M., Iorio A., Lenting P.J. Emicizumab and thrombosis: the story so far. J Thromb Haemost. 2019;17:1269–1272. doi: 10.1111/jth.14556. [DOI] [PubMed] [Google Scholar]
- 13.Abshire T., Kenet G. Safety update on the use of recombinant factor VIIa and the treatment of congenital and acquired deficiency of factor VIII or IX with inhibitors. Haemophilia. 2008;14(5):898–902. doi: 10.1111/j.1365-2516.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim C.H., Simmons S.C., Bui C.M., Jiang N., Pham H.P. aPCC vs. rFVIIa for the treatment of bleeding in patients with acquired haemophilia - a cost-effectiveness model. Vox Sang. 2019;114(1):63–72. doi: 10.1111/vox.12726. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z.Y., Hay J.W. Efficacy of bypassing agents in patients with hemophilia and inhibitors: a systematic review and meta-analysis. Clin Ther. 2012;34(2):434–445. doi: 10.1016/j.clinthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Marcel Levi, Peters Marjolein, Buller Harry R. Efficacy and safety of recombinant factor VIIa for treatment of severe bleeding: a systematic review. Crit. Care Med. 2005;33(4):883–890. doi: 10.1097/01.ccm.0000159087.85970.38. [DOI] [PubMed] [Google Scholar]
- 17.Pipe S.W., Shima M., Lehle M., et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–e305. doi: 10.1016/S2352-3026(19)30054-7. [DOI] [PubMed] [Google Scholar]
- 18.Olasupo O.O., Lowe M.S., Krishan A., Collins P., Iorio A., Matino D. Clotting factor concentrates for preventing bleeding and bleeding-related complications in previously treated individuals with haemophilia A or B. Cochrane Database Syst. Rev. 2021 Aug 18;8(8):CD014201. doi: 10.1002/14651858.CD014201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan A.S., Guinness L., Bahuguna P., et al. Cost of hospital services in India: a multi-site study to inform provider payment rates and Health Technology Assessment. BMC Health Serv. Res. 2022;22:1343. doi: 10.1186/s12913-022-08707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edejer T.T.-T., editor. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. World Health Organization; Geneva: 2003. World Health Organization. [Google Scholar]
- 21.World Bank. Official exchange rate (LCU per US$, period average). Available from: https://data.worldbank.org/indicator/PA.NUS.FCRF [accessed December, 2022]..
- 22.Hoxer C.S., Zak M., Benmedjahed K., Lambert J. Utility valuation of health states for haemophilia and related complications in Europe and in the United States. Haemophilia. 2019;25(1):92–100. doi: 10.1111/hae.13634. [DOI] [PubMed] [Google Scholar]
- 23.Peyvandi F., Kavakli K., El-Beshlawy A., Rangarajan S. Management of haemophilia A with inhibitors: a regional cross-talk. Haemophilia. 2022;28(6):950–961. doi: 10.1111/hae.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermans C., Makris M. Disruptive technology and hemophilia care: the multiple impacts of emicizumab. Res Pract Thromb Haemost. 2021;5(4) doi: 10.1002/rth2.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camelo R.M., Barbosa M.M., Araújo M.S., et al. Economic evaluation of immune tolerance induction in children with severe hemophilia A and high-Responding inhibitors: a cost-effectiveness analysis of prophylaxis with emicizumab. Value Health Reg Issues. 2022;34:31–39. doi: 10.1016/j.vhri.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Cortesi P.A., Castaman G., Trifirò G., et al. Cost-effectiveness and budget impact of emicizumab prophylaxis in haemophilia A patients with inhibitors. Thromb Haemost. 2020;120(2):216–228. doi: 10.1055/s-0039-3401822. [DOI] [PubMed] [Google Scholar]
- 27.Lee H., Cho H., Han J.W., et al. Cost-utility analysis of emicizumab prophylaxis in haemophilia A patients with factor VIII inhibitors in Korea. Haemophilia. 2021;27(1):e12–e21. doi: 10.1111/hae.14143. [DOI] [PubMed] [Google Scholar]
- 28.Bitran R., Pena C., Arpon P., et al. Cost-effectiveness study of prophylaxis with emicizumab versus bypassing agents in patients with severe hemophilia A in Peru. Medwave. 2022;22(2) doi: 10.5867/medwave.2022.02.002118. Spanish, English. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request from the researchers.