Abstract
Fish protein hydrolysates were obtained from cultured rainbow trout (Oncorhynchus mykiss) viscera using commercial and endogenous enzymes. Two methods were employed for hydrolysis: acid autolysis (also known as silage) at room temperature for 10 days in acidic conditions, until total solubilisation, and enzymatic hydrolysis using Alcalase 2.4 LFG, Protana Prime, and the endogenous enzymes in the viscera. The effectiveness of both methods in releasing free amino acids (FAA) was assessed. After evaluating the results, the most effective enzymatic hydrolysis was optimized. The findings indicated that enzymatic hydrolysis with Alcalase, Protana Prime and endogenous enzymes combined for 7 h at a dose of 1% of protein, and a 7-day acid autolysis yielded the highest degree of hydrolysis (83.8% and 75.8%), a yield of FAA from viscera of 5.9% and 3.2%, and a yield of FAA from total protein of 71.3% and 52.5%, respectively. In conclusion, the use of commercial enzymes was more efficient in releasing amino acids, but endogenous enzymes showed a strong proteolytic capacity during acid autolysis, suggesting it also as a promising method to produce FAA-rich hydrolysates.
Keywords: Fish viscera, Silage, Fish protein hydrolysates, Free amino acids, Endogenous enzymes
Graphical abstract
Highlights
-
•
Enzymatic hydrolysis and acid autolysis were carried out using rainbow trout viscera.
-
•
Release of free amino acids was achieved in both, enzymatic hydrolysis and silage.
-
•
Commercial enzymes hydrolysed more effectively than endogenous enzymes.
-
•
Endogenous enzymes seem promising to produce free amino acids rich hydrolysates.
1. Introduction
Aquaculture has grown exponentially during the last decades, even actually surpassing traditional fishing in volume terms since 2012. Global aquaculture production in 2020 reached almost 123 million tonnes and is continuously growing year by year [1]. Spain is the country with the highest aquaculture output in the European Union (EU 27) and mussels, seabass, seabream and rainbow trout are the most produced species [2]. The rise in the production of fish involves a rise in fish by-products, disposing of which could represent an environmental risk [[3], [4], [5]]. These by-products are made up primarily of heads, skin, scales, bones, viscera and muscle, which account for up to 70% of the total fish weight depending on the species, size and type of processing [1,4,6]. In the case of rainbow trout, the skin-on-fillet yield may vary between 43.2 and 51.4% and the viscerosomatic index can range between 8 and 18% depending on the genetic variety and the size of the fish [7]. It is estimated that nowadays 60% of fish is processed by the transformation industry, so the majority of fishery by-products are generated by this sector, which are generally processed to make fishmeal and fish oil for animal feeding [8]. However, these protein-rich by-products could be processed into other value-added products.
Fish viscera are part of the by-products used to produce fishmeal and make up 12–18% of the whole fish weight [9]. However, their processing at fishmeal plants can be complicated as they are prone to rapid degradation due to high microbial counts in the gastrointestinal tract [10] and because their content can reach up to 40%. These by-products can serve as valuable raw materials for other purposes, such as agro-industrial applications or for the production of protein hydrolysates [11]. Viscera that do not meet the required standards for fishmeal production, can be converted into stabilized liquid or solid forms of fertilizers, as they contain a lot of nutrients such as nitrogen or phosphorus. Traditionally, fish waste was used to fertilize crops by Egyptians, Incas, Mayans, and Norwegians [12]. Nowadays, production of bio-based fertilizers is rising due to the need to substitute inorganic fertilizers produced from non-renewable resources [13].
Enzymatic processes can be used to obtain fish protein hydrolysates (FPHs) that contain free amino acids (FAA) and low molecular weight peptides. These FPHs are high-value products with bioactive properties [5,6,14]. Hydrolysis can be acidic, alkaline or enzymatic, being the most widely-used due to its mildness and ease of control [11]. FPHs can be used in many sectors, for example, animal feed, nutraceuticals, aromatic compounds, biofertilizers and biostimulants [5,[11], [12], [13]]. With regard to commercial enzymes, Alcalase has been found to be one of the most effective for the hydrolysis of fish by-products [[15], [16], [17], [18]].
Fish silage is commonly used to obtain FPHs in areas with high densities of fisheries but where fishmeal processing plants are not economically viable [10]. In fact, in 2014 more than 250,000 tonnes of fish by-products were preserved by silage only in Norway, representing 41% of the total fish by-products generated in the country [19]. Silage is the process of liquefaction and stabilization of minced fish at room temperature. Typically, formic acid is added to achieve a pH range of 3.5–4.5 to prevent microbial growth [3]. Hydrolysis of proteins occurs thanks to the acid endoproteases that are located at the fish viscera, in particular aspartic protease (pepsin), which have been reported to be highly stable at pH values between 1.0 and 5.0 [20] and trigger the breakdown of proteins and make it possible to obtain low molecular weight peptides [21,22]. Also, acid-preserved fish silage can completely or partially replace fishmeal in feed for fish, as a 6-day-old silage can contain a similar protein content to good quality fishmeal [23]. However, the level of valorisation achieved can be low to medium comparing with FPHs.
As mentioned, a feasible option to valorise fish by-products is to produce FPHs as intermediary products to produce biostimulants, which are substances applied to plants that enhance nutrition efficiency and abiotic stress tolerance [24]. Among other effects, they increase microbial biomass, soil respiration and soil fertility. There are three major groups based on the source and content: humic substances, products containing hormones, and products containing amino acids. Fish protein hydrolysates can be used to produce amino acid-based biostimulants, which have proved to increase osmoprotection, nutrient availability and metal chelation, apart from protecting the plant from heavy metals [25].
In this work, rainbow trout viscera were treated with two different methods (enzymatic hydrolysis and acid autolysis) to compare the amino acid release in order to use the hydrolysates as intermediary products to produce biostimulants for plants.
2. Materials and methods
2.1. Fish viscera
All the fish viscera used in this study were obtained from cultured rainbow trout (Oncorhynchus mykiss) kindly provided by Caviar Pirinea SL (Yesa, Navarra). In the fish used in these trials, the average weight of the viscera collected was 463.0 ± 98.0 g (n = 15), the average weight of the fish being 3.195 ± 0.057 kg. The percentage of viscera in relation to the fish weight was 15%. Viscera were composed of stomach, gut, swim bladder, pancreas, kidney and gonads, without liver, which is lost during gutting due to its fragility. Viscera were transported in plastic bags on ice in isotherm containers as soon as fish were eviscerated, delivered every other day, and stored at −20 °C at their arrival until use.
Before every process, viscera were minced using a Grindomix GM 300 knife mill (Retsch GmbH, Haan, Germany) and the mince was defatted by decantation, in the case of the production of enzymatic hydrolysates at 40 °C, and in the cases of the optimization of enzymatic hydrolysis and fish silage at room temperature to avoid the inactivation of native enzymes [26,27]. Composition of raw material was determined for each trial (Table 1).
Table 1.
Composition of fresh viscera before being processed into fish silage, enzymatic hydrolysates and optimized enzymatic hydrolysates.
| Dry matter (%) | Protein (%) | Ash (%) | |
|---|---|---|---|
| Fish silage | 76.6 ± 2.3 | 6.6 ± 0.2 | 0.2 ± 0.03 |
| Enzymatic hydrolysates | 59.5 ± 0.3 | 6.4 ± 0.1 | 0.4 ± 0.04 |
| Optimization of enzymatic hydrolysates | 56.2 ± 4.3 | 5.5 ± 0.2 | 0.6 ± 0.03 |
2.2. Commercial enzymes
The commercial enzymes chosen for the enzymatic hydrolysis were Alcalase 2.4 LFG, a broad-spectrum endo-protease, and Protana Prime, an exopeptidase blend, supplied by Novozymes A/S (Bagsværd, Denmark). The preference for Alcalase was based on the findings of several studies in the literature [14,[27], [28], [29]] while the choice of Protana Prime was based on the recommendations of the enzyme supplier and on previous experimental results.
2.3. Production of fish silage
The schematic silage process is outlined in Fig. 1. The pH of the defatted fish viscera mince was adjusted to 4 by adding approximately 0.4% of 98% formic acid (Panreac Química S.L.U., Barcelona, Spain). Then, the minced material was put into 50 mL Falcon tubes and was stored at room temperature for 10 days. Three replicates of each sample were prepared and agitated every day. Following the silage period, the pH was measured, and the tube contents were subjected to a temperature of 90 °C for 15 min to deactivate the enzymes. Subsequently the tubes were centrifuged at 4347×g for 15 min in an oscillating rotor to separate the solubilized protein from the remaining oil, emulsion interphase, and undigested solids.
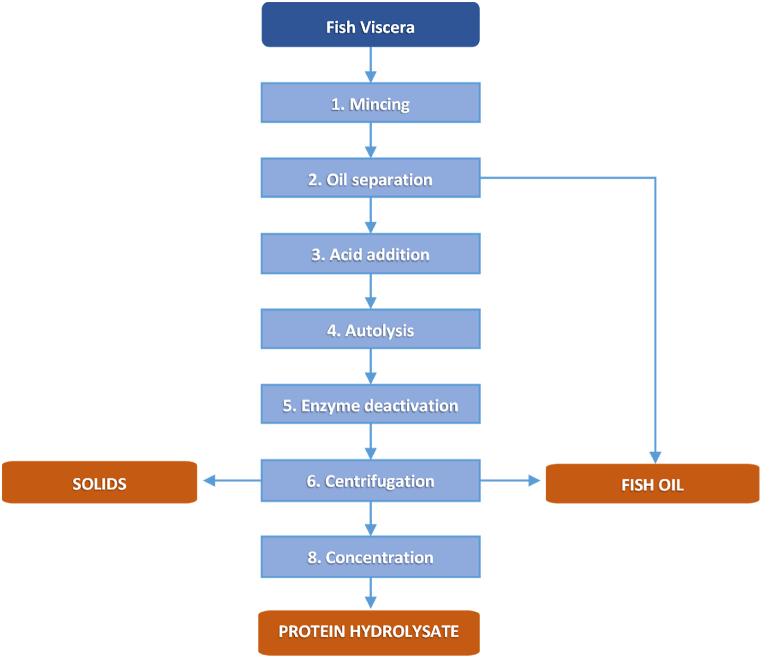
Fig. 1.
Schematic flowchart of rainbow trout viscera processed by acid autolysis (silage), with the previous mincing, oil separation, acid addition, and followed by centrifugation and concentration.
2.4. Production of enzymatic hydrolysates
The schematic hydrolysis process is outlined in Fig. 2.
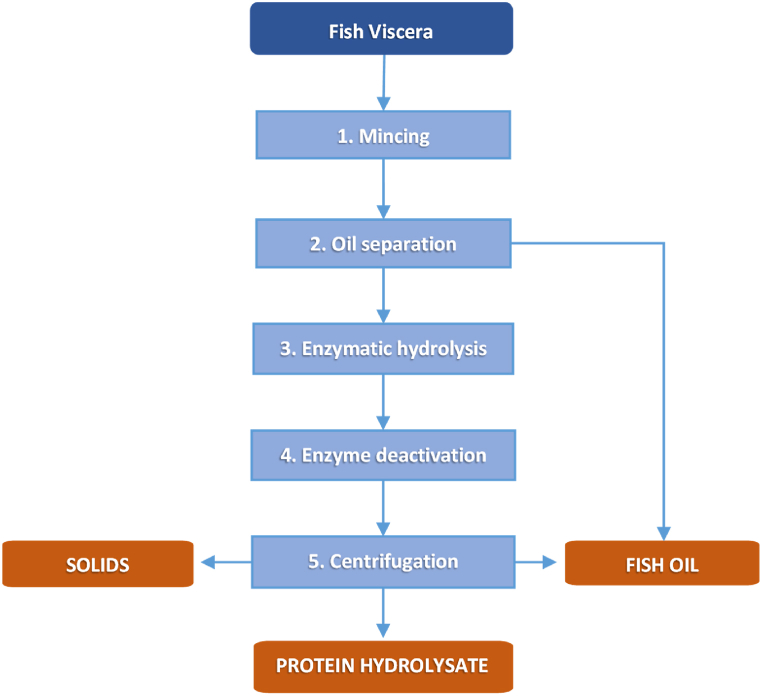
Fig. 2.
Schematic flowchart of rainbow trout viscera processed by enzymatic hydrolysis, with the previous mincing, oil separation and the following enzyme deactivation with heat and centrifugation to separate the protein hydrolysate from remaining oil and solids.
Lab-scale hydrolysis was carried out in batches of 500 mL in a Sell Symphony 7100 Bathless Dissolution system (Distek Inc., North Brunswick, NJ, USA). To adjust the pH, a solution of 10 M sodium hydroxide was prepared, and measurements were carried out using a pH-meter (GLP 21+, Crison Instruments, Barcelona, Spain). All hydrolysis was carried out using a 1:1 dilution with water (250 g of defatted viscera plus 250 g of distilled water).
Two commercial enzymes with different actions, a serine endopeptidase (Alcalase 2.4. FLG) and an exopeptidase (Protana Prime), were tested in combination with and without previous inactivation of the endogenous enzymes of the viscera. The selected hydrolysis combinations are detailed in the experimental flow chart in Fig. 3. The hydrolysis conditions (time, pH and temperature) were determined according to Novozymes’ recommended optimal conditions for each enzyme activity (Table 2). The efficiency of the enzymes was compared when using them sequentially following the recommended protocol (3 h Alcalase and 4 h Protana Prime) and simultaneously. To ensure that Alcalase worked during the same time that when using the enzymes sequentially, and the results could be comparable, the total time when using them simultaneously was set at 7h. Both enzymes were also tested independently to evaluate their effectiveness when used alone, serving as controls for comparison when used in combination. An additional test was done using only the endogenous enzymes of the viscera (ENDO) taking into account the information regarding endogenous enzymes of fish viscera [20] as autolysis has been also proposed as a cost-effective option for obtaining bioactive hydrolysates [30].
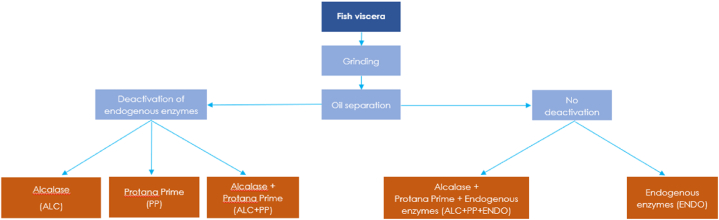
Fig. 3.
Hydrolysis trial design for the rainbow trout viscera. Fish protein hydrolysates were obtained through separated and simultaneous use of commercial (Alcalase and Protana Prime) and endogenous enzymes. Viscera was grinded and defatted prior to enzymatic hydrolysis.
Table 2.
Enzymes, combinations of enzymes and conditions (time, temperature, pH, and dose) used in the experiments of enzymatic hydrolysis.
| Time (h) | Temperature (°C) | pH | Dose (w/w protein) | |
|---|---|---|---|---|
| Alcalase | 3 | 60 | 7 | 1% |
| Protana Prime | 4 | 55 | 7 | 1% |
| Alcalase + Protana Prime | 3 + 4 (7h) | 60–55 | 7 | 1% + 1% |
| Endogenous enzymes | 7 | 55 | 7 | – |
| Alcalase + Protana Prime + Endogenous enzymes | 3 + 4 (7h) | 60–55 | 7 | 1% + 1% |
In the cases where the endogenous enzymes had to be deactivated, the minced material was subjected to heat treatment at 90 °C for 15 min prior to the hydrolysis. The objective of not deactivating the endogenous enzymes was to avoid the thermal deactivation step to reduce energy consumption and at the same time, verify if the presence of endogenous enzymes can have positive synergistic or summation effect with the commercial enzymes.
Following the hydrolysis, the reactor content was heated at 90 °C for 15 min to deactivate the enzymes and subsequently centrifuged in 500 mL containers with an oscillating rotor at 4347×g for 15 min to separate the solubilized protein from the remaining oil, emulsion interphase, and undigested solids.
2.5. Chemical analyses
The proximate composition of the samples was analysed according to the Association of Official Analytical Chemists (AOAC) Official Methods. Dry matter content was determined by drying them at 100 °C until reaching constant weight (method 934.01). Crude protein content was determined by Kjeldahl methodology (method 955.04). Ash content was determined by heating samples at 500 °C for 24 h and then at 700 °C for 2 h.
Free amino acid content was determined by high performance liquid chromatography with diode array detection (RP-HPLC-DAD). The samples were prepared by adding 8 mL of HCl 0.1 N to 1 g of sample, followed by agitation for 30 min and filtration through 0.45 μm filters. Samples and standards were derivatized in the injection needle using borax, OPA and FMOC. The column used in the analysis was Poroshell HPH-C18, 4.6 × 100 mm, 2.7 μm (Agilent Technologies, Madrid, Spain). The chromatography was performed in an isocratic system with acetonitrile (45%), methanol (45%) and water (10%). The detection wavelengths were 262 and 338 nm.
Size exclusion high performance chromatography (SEC-HPLC) was performed in order to determine the peptide molecular weight distribution of the hydrolysates. Analytes were separated using AdvanceBio SEC LC column 300 Å, 7.8 × 300 mm, 2.7 μm (Agilent Technologies, Spain) connected to a diode array detector. Samples were eluted with 50% acetonitrile and 50% water with 0.1% of trifluoroacetic acid as mobile phase at a flow rate of 0.7 mL/min after a 1 μL injection at a temperature of 30 °C. DAD signal was measured at 214 nm. Each sample was filtered through a 0.45 μm PVDF filter and diluted to a protein concentration of 2 g/L before injection.
2.6. Statistical analysis
Experimental factors were analysed via ANOVA (analysis of variance) and were considered significant when their probability (p value) was less than 0.05. Multiple range analysis was carried out using Fisher's LSD method. For the comparison of one variable between two groups Student's test was used. Statgraphics Centurion XVI analysis software (16.2.04 version, Statgraphics Technologies, Inc., Virginia) was used to perform the statistical analysis of the results.
3. Results and discussion
To evaluate the effectiveness of both enzymatic hydrolysis and acid autolysis, the responses analysed were the degree of hydrolysis (DH, calculated by dividing the FAA content by the protein content), the yield of FAA from viscera weight and the yield of FAA from total protein present in viscera.
3.1. Acid autolysis
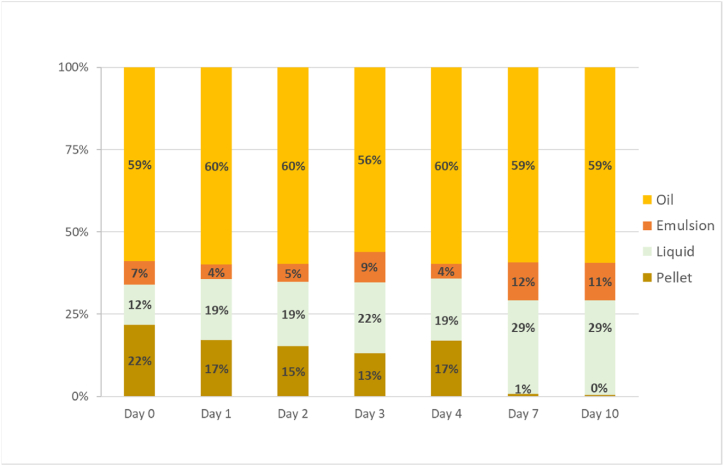
Fish viscera silage was kept for 10 days and samples were taken on days 0, 1, 2, 3, 4, 7 and 10. Evolution of pH during the autolysis varied from 4.02 to 4.12. Fig. 4 shows the evolution of the phase distribution during the 10 days of the silage. The percentage of oil does not change while liquid phase increases and pellet decreases. This means that the undigested proteins from the pellet cleavage along this period due to the endogenous enzymes present in the viscera, and therefore peptides with low molecular weight and FAA pass to the liquid phase as they can dissolve in water, which explains the increase of the relative volume of the liquid phase.
Fig. 4.
Evolution of the relative distribution of the silage fractions along the silage period, n = 3.
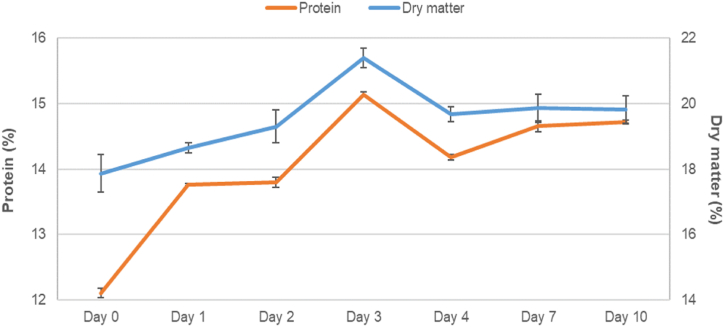
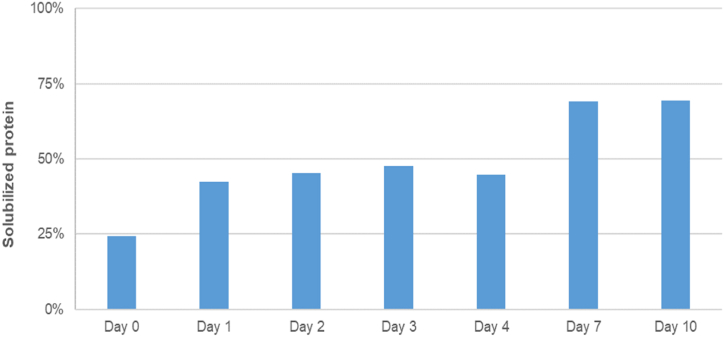
Protein and dry matter increased steadily until day 7 (Fig. 5). The peak observed in day 3 may be due to a problem in the manual separation of phases, when part of the undigested pellet fine particles could have contaminated the liquid phase. Thus, Fig. 6 shows that the solubilized protein (extraction of the total protein into the liquid phase) increased through the days reaching the maximum peak in days 7 and 10.
Fig. 5.
Comparison of protein (%) and dry matter (%) composition throughout 10 days of silage in the liquid phase, n = 3.
Fig. 6.
Evolution of solubilized protein (%, extraction of the total protein into the liquid phase) throughout 10 days of silage, n = 3.
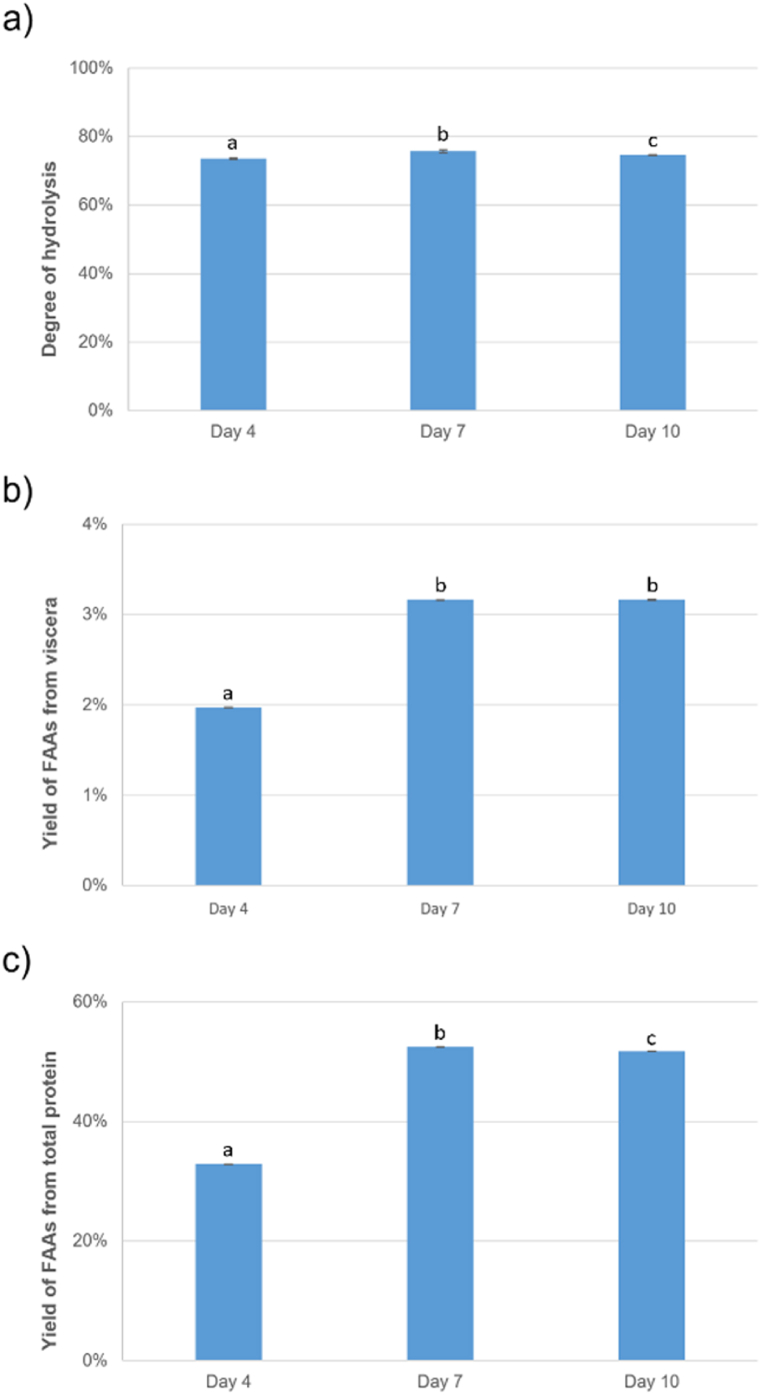
According to Fig. 7, it was found that the degree of hydrolysis was very similar during the last 3 samplings (Fig. 7-a) but on the other hand the yield of FAA from viscera (Fig. 7- b) and from total protein (Fig. 7-c) increased considerably at days 7 and 10. This means that solubilisation of protein (calculated by dividing the protein content in the liquid phase by the total protein content) continued over time keeping the same degree of hydrolysis around 75%.
Fig. 7.
Comparison of a) degree of hydrolysis, b) yield of FAA from viscera, and c) yield of FAA from total protein of the days 4, 7 and 10 of the acid autolysis, n = 3. Different small superscript letters indicate significant differences (p < 0.05).
Regarding the FAA profile (Table 3), the total percentage of Gln and Tyr fell while Glu, Leu and Lys were the most prevalent during the silage, in the case of glutamic acid finishing day 10 at 10.3 ± 0.3 % of the total FAA. The profile was similar to the one reported by Santana et al. (2023) [31], where a 7-day acid silage of tambaqui viscera was carried out. Analysis of variance showed that there was a significant difference between days of silage in terms of the amino acid profile.
Table 3.
Amino acid profile (% of the total FAA) of the result of the acid autolysis of trout viscera in days 4, 7 and 10 of silage, n = 3. Different small superscript letters indicate significant differences (p < 0.05).
| Amino acids | Day 4 | Day 7 | Day 10 |
|---|---|---|---|
| Glu | 9.4 ± 0.1a | 10.0 ± 0.05 b | 10.3 ± 0.3 b |
| Leu | 8.9 ± 0.4a | 9.2 ± 0.2a | 9.4 ± 0.5a |
| Lys | 8.4 ± 0.5a | 8.8 ± 0.4a | 9.1 ± 0.7a |
| Arg | 6.9 ± 0.3a | 7.5 ± 0.2a | 7.7 ± 0.4a |
| Ala | 6.0 ± 0.2a | 6.6 ± 0.04 b | 6.8 ± 0.3 b |
| Val | 5.5 ± 0.03a | 5.8 ± 0.01 b | 5.9 ± 0.1 b |
| Ser | 5.0 ± 0.1a | 5.5 ± 0.1 ab | 5.8 ± 0.3 b |
| Ile | 5.2 ± 0.1a | 5.5 ± 0.04a | 5.5 ± 0.3a |
| Thr | 4.6 ± 0.1a | 4.9 ± 0.1 b | 5.0 ± 0.1 b |
| Phe | 4.5 ± 0.1a | 4.6 ± 0.1a | 4.5 ± 0.4a |
| Gly | 4.0 ± 0.2a | 4.4 ± 0.2 ab | 4.8 ± 0.4 b |
| Asp | 4.2 ± 0.1a | 4.5 ± 0.001b | 4.7 ± 0.001b |
| Pro | 3.7 ± 0.3a | 4.3 ± 0.5a | 4.3 ± 0.02a |
| Gln | 3.9 ± 0.1a | 3.4 ± 0.03 b | 3.1 ± 0.1c |
| Asn | 3.2 ± 0.0a | 3.4 ± 0.005 b | 3.6 ± 0.1 b |
| Tyr | 3.3 ± 0.04a | 3.3 ± 0.1 b | 3.0 ± 0.1 b |
| Met | 3.1 ± 0.1a | 3.1 ± 0.1a | 3.1 ± 0.2a |
| His | 1.9 ± 0.01a | 2.0 ± 0.001b | 2.1 ± 0.04 b |
| Trp | 2.0 ± 0.5a | 1.7 ± 0.2a | 2.1 ± 0.8a |
| OHPro | 0.9 ± 0.3a | 0.9 ± 0.5a | 1.0 ± 0.4a |
| Cys | 0.4 ± 0.002a | 0.4 ± 0.0001 b | 0.4 ± 0.001c |
The molecular weight distribution results from Table 4 indicate that proteins and high molecular weight peptides broke down over time as the concentration of peptides of 1–3 kDa decreased whereas peptides of less than 3 kDa increased to reach a percentage of 72.8 ± 0.1. This indicates the proteolytic capacity at room temperature and pH 4. Molecular weight distribution on days 7 and 10 showed no significant difference, however both were significantly different from day 4, as they increased the percentage of peptides of less than 0.3 kDa (p < 0.05).
Table 4.
Molecular weight distribution of the peptides and average molecular weight of the silage at days 4, 7 and 10, n = 3. Different small superscript letters indicate significant differences (p < 0.05).
| <0.3 kDa (%) | 0.3–1 kDa (%) | 1–3 kDa (%) | Average molecular weight (Da) | |
|---|---|---|---|---|
| Day 4 | 67.9 ± 0.9a | 19.2 ± 0.04a | 12.9 ± 0.9a | 383.7 ± 17.4a |
| Day 7 | 72.1 ± 0.2 b | 18.3 ± 0.1 b | 9.6 ± 0.2 b | 311.3 ± 3.9 b |
| Day 10 | 72.8 ± 0.1 b | 18.3 ± 0.5 b | 9.0 ± 0.6 b | 298.0 ± 9.0 b |
It was concluded that the time necessary for the most efficient extraction of protein from the viscera is between 7 and 10 days.
3.2. Hydrolysis with commercial enzymes
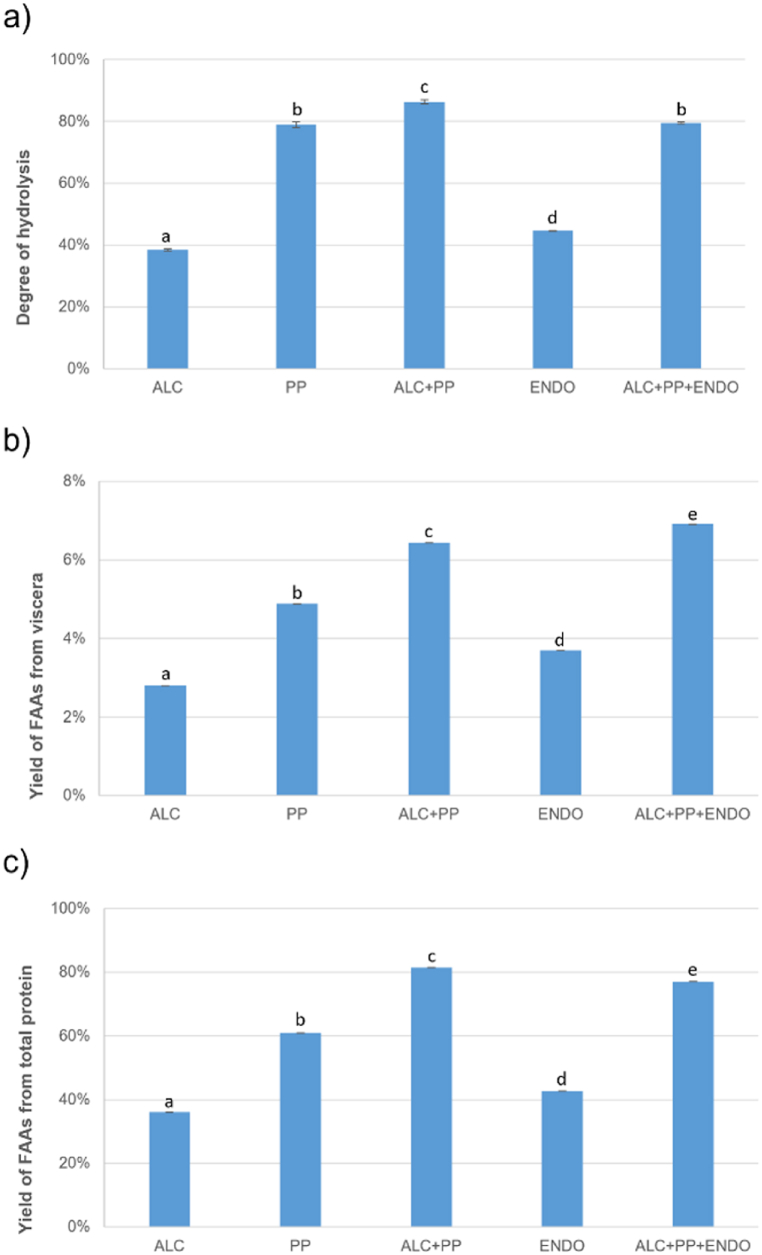
Fig. 8 demonstrates that the most effective processes involved simultaneous use of Alcalase and Protana Prime, with or without endogenous enzymes. The degree of hydrolysis expressed as FAA/total protein in the sample (%) yielded 86.3 ± 0.7 and 79.5 ± 0.3 (Fig. 8-a), the yields of FAA from viscera (%) 6.4 ± 0.01 and 6.9 ± 0.01 (Fig. 8-b) and yields of FAA from total protein present in viscera (%) 81.5 ± 0.01 and 77.0 ± 0.01 (Fig. 8-c) for ALC + PP and ALC + PP + ENDO, respectively. Statistical analysis showed that all results of hydrolysis were significantly different, except from PP and ALC + PP + ENDO in terms of the degree of hydrolysis (p > 0.05). While the yield of FAA by weight of viscera was higher with ALC + PP + ENDO, probably due to a higher protein solubilisation, the protein breakdown was lower compared to ALC + PP, resulting in a slightly lower degree of hydrolysis and less FAA produced per grams of protein present in the viscera. When the purpose of the hydrolysis is to obtain hydrolysates with specific biological or technological functionalities, the inactivation of the endogenous enzymes ensures the standardization and reproducibility of the process, as the activity of the endogenous enzymes is affected by the seasonality or the fish species [29]. In order to avoid that the endogenous enzymes interfere with the commercial ones, a previous deactivation step is usually included when the raw material includes fish viscera [11,28,29,32]. However, when the purpose is to produce the maximum yield in FAA, it can be expected that the endogenous enzymes could contribute to the hydrolysis or, at least, that omitting the inactivation step could help reducing the energy costs. In the present work, the presence of endogenous enzymes did not contribute to the amino acid release and seemed to interfere with the commercial enzymes resulting in a hydrolysis degree even slightly lower, may be due to a competition for specific sites of the substrate. Other authors have reported a similar result depending on the type of enzyme used [26].
Fig. 8.
Comparison of a) degree of hydrolysis, b) yield of FAA from viscera, and c) yield of FAA from total protein of the different enzymatic hydrolysis, n = 3. ALC: Alcalase, PP: Protana Prime, ENDO: endogenous enzymes. Different small superscript letters indicate significant differences (p < 0.05).
It was verified that the combination of Alcalase and Protana Prime is very effective to produce free amino acids due to the synergistic effect of exoprotease and endoprotease actions together. As expected, the exopeptidase activity of PP resulted in a higher production of FAA, therefore in a higher hydrolysis degree, than Alcalase, an endopeptidase. Protana Prime provided a higher hydrolysis degree as the endogenous enzymes, probably because it was working at its optimal temperature and pH conditions, while it is known that the optimal pH of the endogenous alkaline proteases is higher, as reported by other authors [20]. Moreover, when comparing the yield in FAA of ALC and ENDO with the yield of the combination of ALC + PP, it seems evident that the endogenous enzymes did not present a high exopeptidase activity.
3.3. Optimization of enzymatic hydrolysis
Deactivating endogenous enzymes as well as heating during the hydrolysis process involve heating costs and therefore environmental and economic impact. The enzymes also represent an important part of the processing costs. Therefore, the combination of Alcalase and Protana Prime without previous endogenous enzymes deactivation was selected for a further experiment where the enzymes were used sequentially or combined, at their optimal temperature conditions each or simultaneously, and simultaneously at a lower dose [33] as shown in Table 5. Each hydrolysis was carried out in duplicate.
Table 5.
Conditions chosen for the optimization of the hydrolysis using Alcalase, Protana Prime and endogenous enzymes (ALC + PP + ENDO).
| Time (h) | Temperature (°C) | pH | Dose (w/w protein) |
|---|---|---|---|
| 3 + 4 | 60–55 | 7 | 1% |
| 7 | 60 | 7 | 1% |
| 7 | 60 | 7 | 0.5% |
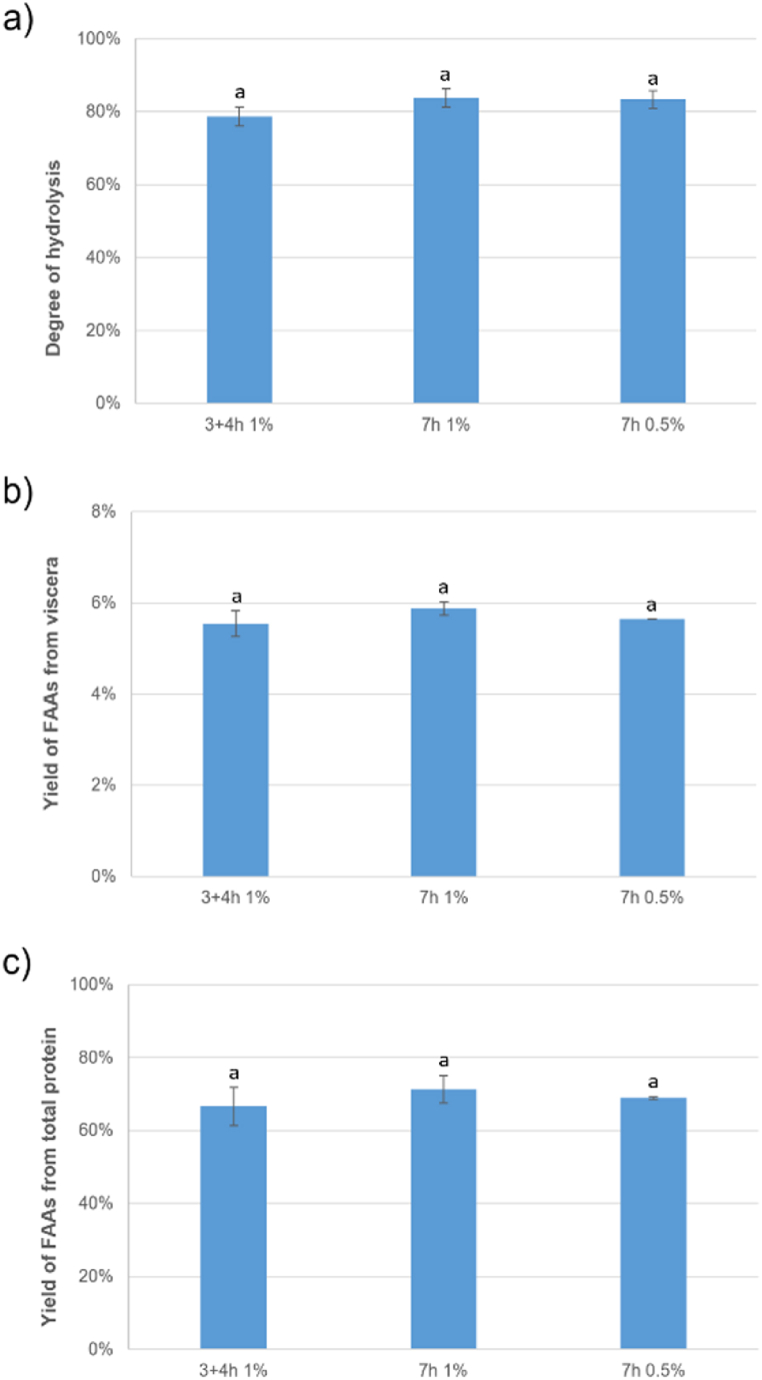
No significant differences were observed in the degree of hydrolysis (Fig. 9-a) or in the yield in FAA both, from viscera weight (Fig. 9-b) and from total protein content in the viscera (Fig. 9-c). This indicates that reducing the enzyme dosage by half did not have a negative impact on the hydrolysis process as reported previously by Valcarcel et al. (2020) [15] and that Alcalase and Protana Prime enzymes can effectively hydrolyse both simultaneously and sequentially without competing. However, there is limited research on the sequential use of Alcalase and other enzymes for producing fish or fish by-product hydrolysates, and the few studies available do not compare this approach with simultaneous use of the enzymes.
Fig. 9.
Comparison of a) degree of hydrolysis, b) yield of FAA from viscera, and c) yield of FAA from total protein of the optimization of the hydrolysis of Alcalase, Protana Prime and endogenous enzymes combined, used sequentially (3 + 4 h) or simultaneously (7 h) and at two different doses (1 and 0.5 %), n = 3. Different small superscript letters indicate significant differences (p < 0.05).
Table 6 shows that the profile of FAA in the simultaneous hydrolysis was very similar to the sequential hydrolysis, except in the case of Glu and Pro, which had higher percentages in the simultaneous hydrolysis for both doses employed (12.9 ± 0.2 and 3.9 ± 0.2, respectively with a 1% enzyme dose). However, reducing of the enzyme dosage by half did not appear to have significant effect.
Table 6.
Profile of amino acids (% from total FAA) of hydrolysates of rainbow trout viscera produced with Alcalase, Protana Prime and endogenous enzymes used sequentially (3 + 4 h) or simultaneously (7 h) and at two different doses (1 and 0.5 %), n = 3. Different small superscript letters indicate significant differences (p < 0.05).
| Amino acids | 3+4h, 1% | 7h, 1% | 7h, 0.5% |
|---|---|---|---|
| Glu | 11.8 ± 0.5a | 12.9 ± 0.2 b | 12.7 ± 0.7 b |
| Leu | 8.9 ± 0.3a | 9.5 ± 0.1 b | 9.3 ± 0.4 ab |
| Ala | 6.9 ± 0.3a | 7.5 ± 0.1 b | 7.3 ± 0.3 b |
| Val | 6.6 ± 0.4a | 7.1 ± 0.1a | 7.0 ± 0.4a |
| Ser | 5.6 ± 0.2a | 6.2 ± 0.05 b | 6.0 ± 0.3 b |
| Ile | 5.4 ± 0.3a | 5.9 ± 0.1 b | 5.7 ± 0.3 ab |
| Thr | 5.0 ± 0.3a | 5.4 ± 0.1a | 5.3 ± 0.3a |
| Gly | 4.7 ± 0.1a | 5.4 ± 0.03 b | 5.1 ± 0.4c |
| Lys | 4.7 ± 0.4a | 4.9 ± 0.2a | 4.7 ± 0.2a |
| Arg | 4.6 ± 0.4a | 4.8 ± 0.2a | 4.6 ± 0.1a |
| Asp | 4.0 ± 0.1a | 4.5 ± 0.1 b | 4.4 ± 0.3 b |
| Asn | 3.9 ± 0.2a | 4.3 ± 0.04 b | 4.1 ± 0.3 ab |
| Phe | 3.9 ± 0.2a | 4.2 ± 0.1 b | 4.0 ± 0.2a |
| Pro | 2.9 ± 0.6a | 3.9 ± 0.2 b | 3.8 ± 0.6 b |
| Met | 3.0 ± 0.2a | 3.2 ± 0.03a | 3.2 ± 0.2a |
| Tyr | 3.1 ± 0.2a | 3.1 ± 0.2a | 3.1 ± 0.2a |
| His | 2.2 ± 0.1a | 2.3 ± 0.04a | 2.2 ± 0.1a |
| Trp | 2.3 ± 0.5a | 1.9 ± 0.4a | 2.1 ± 0.4a |
| Gln | 1.6 ± 0.2a | 1.6 ± 0.1a | 1.4 ± 0.1 b |
| OHPro | 1.2 ± 0.2a | 1.4 ± 0.3a | 1.5 ± 0.3a |
| Cys | 0.3 ± 0.02a | 0.4 ± 0.01 b | 0.3 ± 0.01a |
Regarding the molecular size profile of the protein fragments of the resulting hydrolysates, Table 7 illustrates the production of peptides of different chain length below 3 kDa. All hydrolysates were not significantly different in all the molecular weight ranges (p > 0.05). However, it was observed that the protein from the 7-h hydrolysis with a 1% enzyme dose was more extensively hydrolysed as indicated by the lower percentage of 1–3 kDa peptides and the higher percentage of the smallest peptides and FAA (<0.3 kDa), resulting in a smaller average molecular size. These conditions enhance the cleavage of the peptide bonds, leading to higher yields of FAA and small peptides. This finding is consistent with the high degree of hydrolysis shown in Fig. 9-a.
Table 7.
Molecular weight distribution of peptides and average molecular weight of the hydrolysates obtained with Alcalase + Protana Prime + Endogenous enzymes with the different combinations and doses, n = 3. Different small superscript letters indicate significant differences (p < 0.05).
| Conditions | <0.3 kDa (%) | 0.3–1 kDa (%) | 1–3 kDa (%) | Average molecular weight (Da) |
|---|---|---|---|---|
| 3+4h, 1% | 63.5 ± 1.8a | 28.1 ± 0.4a | 8.4 ± 1.4a | 350.9 ± 31.2a |
| 7h, 1% | 64.7 ± 0.5a | 28.1 ± 0.1a | 7.2 ± 0.4a | 326.7 ± 8.6a |
| 7h, 0.5% | 62.9 ± 1.5a | 28.0 ± 0.4a | 9.1 ± 1.0a | 364.0 ± 23.6a |
For the enzymatic hydrolysis, it was concluded that Alcalase and Protana Prime working simultaneously at a dose of 1% of protein alongside endogenous enzymes of viscera for 7 h is the most effective method to get low molecular weight peptides and FAA.
3.4. Enzymatic hydrolysis vs acid autolysis
Table 8 shows that the yield of FAA from total protein was significantly higher with ALC + PP + ENDO (71.3%) compared to silage (52.5%). This result suggests that ALC + PP + ENDO had a greater ability to break protein into FAA compared to silage and that the commercial enzymes showed stronger proteolytic activity than endogenous enzymes in the silage. However, the degree of hydrolysis was only slightly lower (83.8% vs 75.8%) because more protein was solubilized in the silage. This seems to indicate that the endoprotease activity of the endogenous enzymes can be sufficient in the silage conditions while the exoprotease activity needs probably a longer time to achieve similar results. This is in accordance with the fact that, in acidic conditions, the predominant enzyme activity is derived from the aspartic endopeptidase pepsin.
Table 8.
Comparison of the composition (%) of the optimized enzymatic hydrolysate (ALC + PP + ENDO, 7h, 1%) and the 7-day acid autolysis. Different small superscript letters indicate significant differences (p < 0.05).
| ALC + PP + ENDO, 7h, 1% | 7-day acid autolysis | |
|---|---|---|
| Dry matter | 8.6 ± 0.2a | 19.9 ± 0.4 b |
| Protein d.m. | 63.9 ± 0.2a | 73.8 ± 0.08 b |
| Ash d.m. | 7.1 ± 0.03a | 5.2 ± 0.02 b |
| FAA d.m. | 50.3 ± 3.7a | 49.2 ± 1.2a |
| Glu | 12.9 ± 0.2a | 10.0 ± 0.05 b |
| Leu | 9.5 ± 0.1a | 9.2 ± 0.2 b |
| Ala | 7.5 ± 0.1a | 6.6 ± 0.04 b |
| Val | 7.1 ± 0.1a | 5.8 ± 0.01 b |
| Ser | 6.2 ± 0.05a | 5.5 ± 0.1 b |
| Ile | 5.9 ± ± 0.1a | 5.5 ± 0.04 b |
| Thr | 5.4 ± 0.1a | 4.9 ± 0.1 b |
| Gly | 5.4 ± 0.03a | 4.4 ± 0.2 b |
| Lys | 4.9 ± 0.2a | 8.8 ± 0.4 b |
| Arg | 4.8 ± 0.2a | 7.5 ± 0.2 b |
| Asp | 4.5 ± 0.1a | 4.5 ± 0.09a |
| Asn | 4.3 ± 0.04a | 3.4 ± 0.005 b |
| Phe | 4.2 ± 0.1a | 4.6 ± 0.1 b |
| Pro | 3.9 ± 0.2a | 4.3 ± 0.5a |
| Met | 3.2 ± 0.03a | 3.1 ± 0.1a |
| Tyr | 3.1 ± 0.2a | 3.3 ± 0.1a |
| His | 2.3 ± 0.04a | 2.0 ± 0.001 b |
| Trp | 1.9 ± 0.4a | 1.7 ± 0.2a |
| Gln | 1.6 ± 0.1a | 3.4 ± 0.03 b |
| OHPro | 1.4 ± 0.3a | 0.9 ± 0.5a |
| Cys | 0.4 ± 0.2a | 0.4 ± 0.0001a |
| Degree of hydrolysis (FAA/protein) | 83.8 ± 2.6a | 75.8 ± 0.4 b |
| Yield of FAAs from viscera | 5.9 ± 0.1a | 3.2 ± 0.002 b |
| Yield of FAAs from total protein in viscera | 71.3 ± 3.8a | 52.5 ± 0.01 b |
| <0.3 kDa | 64.7 ± 0.5a | 72.1 ± 0.2 b |
| 0.3–1 kDa | 28.1 ± 0.1a | 18.3 ± 0.1 b |
| 1–3 kDa | 7.2 ± 0.4a | 9.6 ± 0.2 b |
When comparing the profile of the FAA, it was observed that the enzymatic hydrolysis yielded higher concentrations of Glu, Asn, Ser, His, Gly, Thr, Ala, Val, and Ile while silage yielded higher concentrations of Gln, Arg, Phe and Lys. However, Asp, Tyr, Cys, Met, Trp, Leu, OHPro and Pro showed comparable results. In both cases, the percentage of glutamic acid stands out but enzymatic hydrolysis highlights (Table 8).
Also, in Table 8 the distribution of peptide sizes is displayed for both procedures, indicating that they were both efficient in producing more than 90% of peptides below 1 kDa, with most of them being even smaller than 0.3 kDa. While acid autolysis yielded a higher percentage of FAA, dipeptides, and tripeptides (72.1%), enzymatic hydrolysis produced a higher percentage of peptides below 1 kDa (92.8% vs 90.4%), which can be attributed to the difference in the DH (83.8% vs 75.8%) and indicates that enzymatic hydrolysis cleaved peptides more efficiently. This supports the idea that the endoprotease activity of the endogenous enzymes may be enough while the lower yield in free amino acids indicates a lack of exoprotease activity as compared with the use of Protana Prime. The results obtained were similar to the ones obtained by Nikoo et al. (2021) [30], where the predominant peptide fractions were dipeptides and tripeptides (180–500 Da, 51.6–58.4%) followed by free amino acids (<180 Da, 27.3–32.9%).
According to ANOVA, the procedures were significantly different in all parameters (p < 0.05) except from free amino acids in dry matter (FAA d.m.), asparagine (Asp), proline (Pro), methionine (Met), tyrosine (Tyr), tryptophan (Trp), hydroxyproline (OHPro) and cysteine (Cys).
Both enzymatic hydrolysate and 7-day silage could be used as intermediary products to produce amino acid-based biostimulants according to Spanish legislation (RD 506/2013), as a minimum of 6% of free amino acids is required in their composition. The contents of free amino acids in dry matter base for the enzymatic hydrolysate and the silage were 53.6% and 55.9% respectively.
Despite the results, long acid autolysis periods might lead to the degradation of FAA essential amino acids (EAA), while other authors argue that, maintaining the silage at a lower pH can minimize these loses even over very long storage periods [34]. Previous studies reported protein loses of 17.91% after 91 days of storage at pH 4–4.5, and a reduction in EAA mainly during the first month of silage [23]. As described by Nikoo et al. (2021) [30], higher autolysis temperatures could lead to strong oxidizing conditions and amino acid loses and, therefore, autolysis should be carried out over shorter periods in controlled pH and temperature conditions suitable for the endogenous enzymes in viscera. However, the acid autolysis conditions tested in the present study, the silage duration and temperature, were not so strong and, consequently, a significant amino acid degradation was not observed during the studied period.
Another point for optimization should be maximizing the previous oil separation. Oil constitutes a valuable co-product and both the enzymatic hydrolysis, and the silage process can affect its quality through oxidation. Other authors have previously demonstrated that oil obtained by centrifugation after a mild thermal treatment before the hydrolysis significantly reduces the oxidation values and is more stable [35]. Moreover, the presence of oil reduces the efficiency of the enzymatic hydrolysis by preventing the access of the enzymes to the protein and reducing the energy transfer in the heating of the mass in the hydrolysis process. In the present study, 53% of the oil extraction rate was achieved, a result similar to that obtained by Opheim et al. (2015) [26] at 20 °C. While they achieved a higher extraction, near 85% at 40 °C, in previous experiments we did not see a significant difference heating the sample at 40 °C. The difference may be explained by the raw material used (salmon backbones versus trout viscera). At industrial scale, they also showed that oil can be separated by means of a two-phase decanter centrifuge.
4. Conclusions
The combination of the commercial enzymes Alcalase and Protana Prime proved to be highly effective in breaking down protein into small peptides and FAA. Endogenous enzymes were also found to be very effective in both silage and enzymatic hydrolysis, whether used alone or in combination with commercial enzymes. In terms of acid autolysis, a 7-day process was sufficient to produce a valuable hydrolysate. Both products could potentially be used as amino acid-based bio stimulants according to Spanish regulation. Additionally, it would be worth to investigate whether the yield in free amino acids could be increased using only endogenous enzymes by modulating autolysis in controlled temperature and pH conditions, so saving costs arising from the use of commercial enzymes and leading to a more economically viable process.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Haizea Domínguez: Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Bruno Iñarra: Writing – review & editing, Investigation, Formal analysis, Conceptualization. Jalel Labidi: Writing – review & editing, Supervision. Diego Mendiola: Investigation, Data curation. Carlos Bald: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research has been developed within the SEA2LAND project which has received funding from the European Union Research and Innovation H2020 programme, contract No 101000402 (this work reflects the views of the author(s) only, and the European Union cannot be held responsible for any use which may be made of the information contained therein).
The authors wish to thank Nagore Luengo, Arantza Salvarrey, Ainhoa Bikandi and Jorge Ferrer for their excellent technical assistance.
This paper is contribution nº 1207 from AZTI, Food Research, Basque Research and Technology Alliance (BRTA).
References
- 1.FAO . FAO; Rome: 2022. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. [DOI] [Google Scholar]
- 2.European Commission . Publications Office of the European Union; 2023. Directorate-General for Maritime Affairs and Fisheries, the EU Fish Market – 2023 Edition.https://data.europa.eu/doi/10.2771/38507 ISSN 2363-4154. Available at: [DOI] [Google Scholar]
- 3.Olsen R., Toppe J., Karunasagar I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014;36:144–151. doi: 10.1016/j.tifs.2014.01.007. [DOI] [Google Scholar]
- 4.Marti-Quijal F.J., Remize R., Meca G., Ferrer E., Ruiz M.J., Barba F.J. Fermentation in fish and by-products processing: an overview of current research and future prospects. Curr. Opin. Food Sci. 2020;31:9–16. doi: 10.1016/j.cofs.2019.08.001. [DOI] [Google Scholar]
- 5.Chalamaiah M., Kumar B.D., Hemalatha R., Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes Moya Moreira T., Antunes Pessoa L.G., Vicente Seixas F.A., Porto Ineu R., Gonçalves O.H., Leimann F.V., Pereira Ribeiro R. Chemometric evaluation of enzymatic hydrolysis in the production of fish protein hydrolysates with acetylcholinesterase inhibitory activity. Food Chem. 2022;367 doi: 10.1016/j.foodchem.2021.130728. [DOI] [PubMed] [Google Scholar]
- 7.Crouse C., Knight A., May T., Davidson J., Good C. Performance, processing yields, and fillet composition of specific United States diploid and triploid rainbow trout (Oncorhynchus mykiss) lines eared in a semi-commercial scale freshwater recirculating aquaculture system. Aquaculture Reports. 2023;33 doi: 10.1016/j.aqrep.2023. [DOI] [Google Scholar]
- 8.Ioannis S., Arvanitoyannis A.K. Fish industry waste: treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008;43:726–745. doi: 10.1111/j.1365-2621.2006.01513.x. [DOI] [Google Scholar]
- 9.Rustad T., Storrø I., Slizyte R., R Possibilities for the utilisation of marine by-products. Int J Food Sci Tech. 2011;46(10):2001–2014. doi: 10.1111/j.1365-2621.2011.02736.x. [DOI] [Google Scholar]
- 10.Olsen R., Toppe J. Fish silage hydrolysates: not only a feed nutrient, but also a useful feed additive. Trends Food Sci. Technol. 2017;66:93–97. doi: 10.1016/j.tifs.2017.06.003. [DOI] [Google Scholar]
- 11.Villamil O., Váquiro H., Solanilla J.F. Fish viscera protein hydrolysates: production, potential applications and functional and bioactive properties. Food Chem. 2017;224:160–171. doi: 10.1016/j.foodchem.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja I., Dauksas E., Remme J.F., Richardsen R., Løes A.K. Fish and fish waste-based fertilizers in organic farming – with status in Norway: a review. Waste Manage. (Tucson, Ariz.) 2020;115:95–112. doi: 10.1016/j.wasman.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Chojnacka K., Moustakas K., Witek-Krowiak A. Bio-based fertilizers: a practical approach towards circular economy. Bioresour. Technol. 2020;295 doi: 10.1016/j.biortech.2019.122223. [DOI] [PubMed] [Google Scholar]
- 14.Taheri A., Anvar S.A.A., Ahari H., Fogliano V. Comparison the functional properties of protein hydrolysates from poultry byproducts and rainbow trout (Onchorhynchus mykiss) viscera. Iran. J. Fish. Sci. 2013;12(1):154–169. https://aquadocs.org/handle/1834/37351 [Google Scholar]
- 15.Valcarcel J., Sanz N., Vázquez J.A. Optimization of the enzymatic protein hydrolysis of by-products from seabream (sparus aurata) and seabass (Dicentrarchus labrax), chemical and functional characterization. Foods. 2020;9(10):1503. doi: 10.3390/foods9101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vázquez J.A., Sotelo C.G., Sanz N., Pérez-Martín R.I., Rodríguez Amado I., Valcarcel J. Valorization of aquaculture by-products of salmonids to produce enzymatic hydrolysates: process optimization, chemical characterization and evaluation of bioactives. Mar. Drugs. 2019;17(12):676. doi: 10.3390/md17120676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aspmo S.I., Horn S.J., Eijsink V.G.H. Process Biochem.; 2005. Enzymatic Hydrolysis of Atlantic Cod (Gadus morhua L.) Viscera; pp. 1957–1966. [DOI] [Google Scholar]
- 18.Ramakrishnan V.V., Ghaly A.E., Brooks M.S., Budge S.M. Extraction of proteins from mackerel fish processing waste using Alcalase enzyme. J. Bioprocess. Biotech. 2013;3(2):130. doi: 10.4172/2155-9821.1000130. [DOI] [Google Scholar]
- 19.Richardsen R., Nystoyl R., Strandheim G., Viken A. Analyse marint restråstoff. 2015. https://sintef.brage.unit.no/sintef-xmlui/handle/11250/2454964?locale-attribute=en 2014. (In Norwegian). Available at :
- 20.Vannabun A., Ketnawa S., Phongthai S., Benjakul S., Rawdkuen S. Characterization of acid and alkaline proteases from viscera of farmed giant catfish. Food Biosci. 2014;6:9–16. doi: 10.1016/j.fbio.2014.01.001. [DOI] [Google Scholar]
- 21.Prabha J., Narikimelli A., Sajini M.I., Vincent S. Optimization for autolysis assisted production of fish protein hydrolysate from underutilized fish Pellona ditchela. Int. J. Sci. Eng. Res. 2013;4(12):1863–1869. [Google Scholar]
- 22.Toppe J., Olsen R.L., Peñarubia O.R., James D.G. FAO; Rome: 2018. A Manual on How to Turn Fish Waste into Profit and a Valuable Feed Ingredient or Fertilizer; p. 28.https://www.fao.org/documents/card/fr/c/I9606EN [Google Scholar]
- 23.Van’t Land M., Vanderperren E., Raes K. The effect of raw material combination on the nutritional composition and stability of four types of autolyzed fish silage. Anim. Feed Sci. Technol. 2017;234:284–294. doi: 10.1016/j.anifeedsci.2017.10.009. [DOI] [Google Scholar]
- 24.du Jardin P. Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- 25.Shahrajabian M.H., Cheng Q., Sun W. The effects of amino acids, phenols and protein hydrolysates as biostimulants on sustainable crop production and alleviated stress. Recent Pat. Biotechnol. 2022;16:319–328. doi: 10.2174/1872208316666220412133749. [DOI] [PubMed] [Google Scholar]
- 26.Opheim M., Šližytė S., Sterten H., Provan F., Larssen E., Kjos N.P. Hydrolysis of Atlantic salmon (Salmo salar) rest raw materials - effect of raw material and processing on composition, nutritional value, and potential bioactive peptides in the hydrolysates. Process Biochemistry. 2015;50(8):1247–1257. doi: 10.1016/j.procbio.2015.04.017. [DOI] [Google Scholar]
- 27.Hoyle N.T., Merritt J.H. Quality of fish protein hydrolysates from herring (Clupea harengus) J. Food Sci. 1994;59(1):76–79. doi: 10.1111/j.1365-2621.1994.tb06901.x. [DOI] [Google Scholar]
- 28.Guérard F., Dufossé L., De La Broise D., Binet A. Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using Alcalase. J. Mol. Catal. B Enzym. 2001;11(4–6):1051–1059. doi: 10.1016/S1381-1177(00)00031-X. [DOI] [Google Scholar]
- 29.Bhaskar N., Benila T., Radha C., Lalitha R.G. Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour. Technol. 2008;99:335–343. doi: 10.1016/j.biortech.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Nikoo M., Regenstein J.M., Noori F., Gheshlaghi S.P. Autolysis of rainbow trout by-products: enzymatic activities, lipid and protein oxidation, and antioxidant activity of protein hydrolysates. LWT - Food Sci. Technol. 2021;140 doi: 10.1016/j.lwt.2020.110702. [DOI] [Google Scholar]
- 31.Santana T.M., Dantas F.M., Monteiro dos Santos D.K., Kojima J.T., Pastrana Y.M., De Jesus R.S., Gonçalves L.U. Fish viscera silage: production, characterization, and digestibility of nutrients and energy for tambaqui juveniles. Fishes. 2023;8:111. doi: 10.3390/fishes8020111. [DOI] [Google Scholar]
- 32.Vásquez P., Sepúlveda C.T., Zapata J.E. Functional properties of rainbow trout (Oncorhynchus mykiss) viscera protein hydrolysates. Biocatal. Agric. Biotechnol. 2022;39 doi: 10.1016/j.bcab.2021.102268. [DOI] [Google Scholar]
- 33.Jamil N.H., Halim N.R.A., Sarbon N.M. Optimization of enzymatic hydrolysis condition and functional properties of eel (Monopterus sp.) protein using response surface methodology (RSM) Int. Food Res. J. 2016;23(1):1–9. [Google Scholar]
- 34.Haaland H., Njaa L.R. Total volatile nitrogen - a quality criterion for fish silage? Aquaculture. 1989;79(1–4):311–316. doi: 10.1016/0044-8486(89)90472-9. [DOI] [Google Scholar]
- 35.Šližytė Rasa, Mozuraityte R., Remman T., Rustad T. Two-stage processing of salmon backbones to obtain high-quality oil and proteins. Int. J. Food Sci. Technol. 2018;53:2378–2385. doi: 10.1111/ijfs.13830. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.