Abstract
Background
Data on the durability of booster dose immunity of COVID-19 vaccines are relatively limited.
Methods
Immunogenicity was evaluated for up to 9–12 months after the third dose of vaccination in 94 healthy adults.
Results
Following the third dose, the anti-spike immunoglobulin G (IgG) antibody response against the wild-type was boosted markedly, which decreased gradually over time. However, even 9–12 months after the booster dose, both the median and geometric mean of anti-spike IgG antibody levels were higher than those measured 4 weeks after the second dose. Breakthrough infection during the Omicron-dominant period boosted neutralizing antibody titers against Omicron sublineages (BA.1 and BA.5) and the ancestral strain. T-cell immune response was efficiently induced and maintained during the study period.
Conclusions
mRNA vaccine booster dose elicited durable humoral immunity for up to 1 year after the third dose and T-cell immunity was sustained during the study period, supporting an annual COVID-19 vaccination strategy.
Keywords: SARS-CoV-2, COVID-19 vaccines, Longevity, Immunogenicity, Booster shot
1. Introduction
Over the last few years, the coronavirus disease 2019 (COVID-19) pandemic has caused more than 760 million confirmed cases, including 6.9 million deaths, as of October 1, 2023 [1]. COVID-19 vaccines are effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe disease [[2], [3], [4]]. However, waning humoral immunity has been reported for over 6 months after primary series vaccination [[5], [6], [7]].
Considering the evolution of SARS-CoV-2 variants and the waning of vaccine-induced immunity, periodic revaccination is required to maintain vaccine effectiveness [8]. Several countries, including the United Kingdom and the United States, strongly support a transition to a once-a-year COVID-19 vaccination strategy similar to the influenza vaccine [[8], [9], [10]]. However, data on the durability of booster dose immunity is relatively limited.
We previously reported anti-SARS-CoV-2 humoral immunity up to 6 months after the primary series of mRNA-1273 vaccinations [5]. Unlike the influenza viruses, which exhibit a clear seasonal pattern, the SARS-CoV-2 infection has shown a year-round epidemic pattern [1]. Therefore, evaluating immunity beyond 6 months will be crucial for future annual vaccination schedules. This study aimed to investigate the longevity of humoral and cellular immunities against SARS-CoV-2 after a booster dose. In addition, cross-neutralizing activities were evaluated against variants of concern (VOCs) in a subset of participants.
2. Materials and methods
2.1. Study setting and population
This prospective, multicenter cohort study was conducted as an extension of a previous study [5] and was commenced in June 2021 across four university hospitals in South Korea. We recruited healthy adults (aged ≥19 years) who were scheduled to be administered two-dose mRNA-1273 and had no prior SARS-CoV-2 infection. The study design and population, up to 6 months after the primary vaccination series, have been described previously [5]. Subsequently, most participants received the third booster dose according to national recommendations between November 29, 2021, and February 4, 2022. The post-booster dose long-term immunogenicity was evaluated for up to 9–12 months. Blood samples were collected on the day of the first dose of vaccination (T0); 4 weeks after the first dose (T1); 4 weeks after the second dose (T2); 3 months (T3) and 6 months (T4) after the first dose; and 4 weeks (T5) and 3 months (T6), 5 months (T7), and 9–12 months after the booster dose. After the booster dose vaccination, only two hospitals (Korea University Guro Hospital and International St. Mary's Hospital) participated in this study. At each time point, all participants were tested for SARS-CoV-2 infection using anti-nuclear capsid (N) protein antibody tests. The anti-N antibody was measured using the SARS-CoV-2 immunoglobulin G (IgG) assay (Abbott Laboratories, Chicago, IL, USA). The presence of anti-N antibodies was considered a previous infection. In South Korea, Omicron variants emerged in November 2021 and have rapidly spread, accounting for 71% in January 2022 and >99% in February 2022 [11,12]. Omicron BA.1 exclusively circulated initially, but BA.2 became dominant (>50%) by the end of March 2022. Finally, Omicron BA.5 replaced the dominant strain from the second week of July 2022 to the end of our study. Thus, 4 weeks to 3 months (T5–T6), 3–5 months (T6–T7), and 5–12 months (T7–T8) after the booster dose were considered the BA.1, BA.2, and BA.5-dominant periods, respectively. This study received approval from the Institutional Review Board at each hospital. Written informed consent was obtained from all participants.
2.2. Humoral and cellular immunogenicity assessment
Anti-SARS-CoV-2 spike protein (anti-spike) IgG antibodies were tested using an electrochemiluminescence immunoassay (Elecsys anti-SARS-CoV-2 spike ECLIA; Roche Diagnostics, Pleasanton, CA, USA) according to the manufacturer's protocol. The plaque reduction neutralization test (PRNT) was performed using the wild-type (WT) SARS-CoV-2 virus (bCoV/Korea/KCDC03/2020 NCCP No. 43326) to measure the level of neutralization, as described previously [5]. In brief, serum was serially diluted from 1:20 to 1:20,480, and the mixtures of serum dilution/virus (40 PFU/well) were incubated at 37 °C. Subsequently, Vero E6 cells, 0.5% agarose (Lonza, Basel, Switzerland), 4% paraformaldehyde, and crystal violet were sequentially added according to the protocol. The median neutralizing titer (ND50) indicates the antibody concentration necessary to reduce the virus count by 50%. A positive result was determined when the titer was ≥1:20. We also analyzed cross-neutralizing activity against VOCs, including Omicron BA.1 (GRA: BA.1 NCCP No. 43408) and Omicron BA.5 (GRA: BA.5 NCCP No. 43426), considering the dominant viral strain during the study period. In addition, we performed an interferon (IFN)-γ enzyme-linked immunosorbent spot (ELISpot) assay to evaluate SARS-CoV-2-specific cellular immune responses using peripheral blood mononuclear cells (PBMCs). The detailed method has been previously published [5]. Briefly, IFN-γ antibody-coated ELISpot plates were incubated with RPMI medium 1640 containing 10% fetal bovine serum. PBMCs (3 × 105 cells/well) were then stimulated with SARS-CoV-2 spike peptide pools overnight in a 36 °C 5% CO2 incubator. The results were presented as spot-forming units (SFUs) per million PBMC (SFUs/106 PBMC). The PRNT and ELISpot assays were performed only in a subset of participants using age-stratified sampling. Some samples were not tested using the ELISpot assay because of poor PBMC quality.
2.3. Statistical analyses
We conducted a repeated measures analysis of variance (ANOVA) test to evaluate the changes in antibody titers at T0–T8 within a group of participants. Anti-spike IgG and neutralizing antibody geometric mean titers (GMTs) with 95% confidence intervals (CIs) were calculated after logarithmic transformation. The GMT ratio was calculated as the mean difference of the measurements on a logarithmic scale. As for categorical variables, either the chi-square test or Fisher's exact test was used, whereas either Student's t-test or one-way ANOVA was used for continuous variables, followed by Scheffé’s test for multiple comparisons. The results were considered statistically significant at p < 0.05.
Using an exponential decay model fitted to the data at each time point, antibody decay rates and half-lives were calculated. Individuals who missed even one blood sample collection during the study period were excluded from the analysis of decay rate. The detailed method of the exponential model has been described previously [5]. To contrast the half-life following the second and third doses, the model included the vaccine dose factor while accounting for repeated measurements.
Statistical analyses were performed using SPSS (version 24.0; IBM Corp., Armonk, NY, USA), GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA, USA), and SAS (version 9.4 for Windows; SAS Institute Inc., NC, USA).
3. Results
3.1. Study population
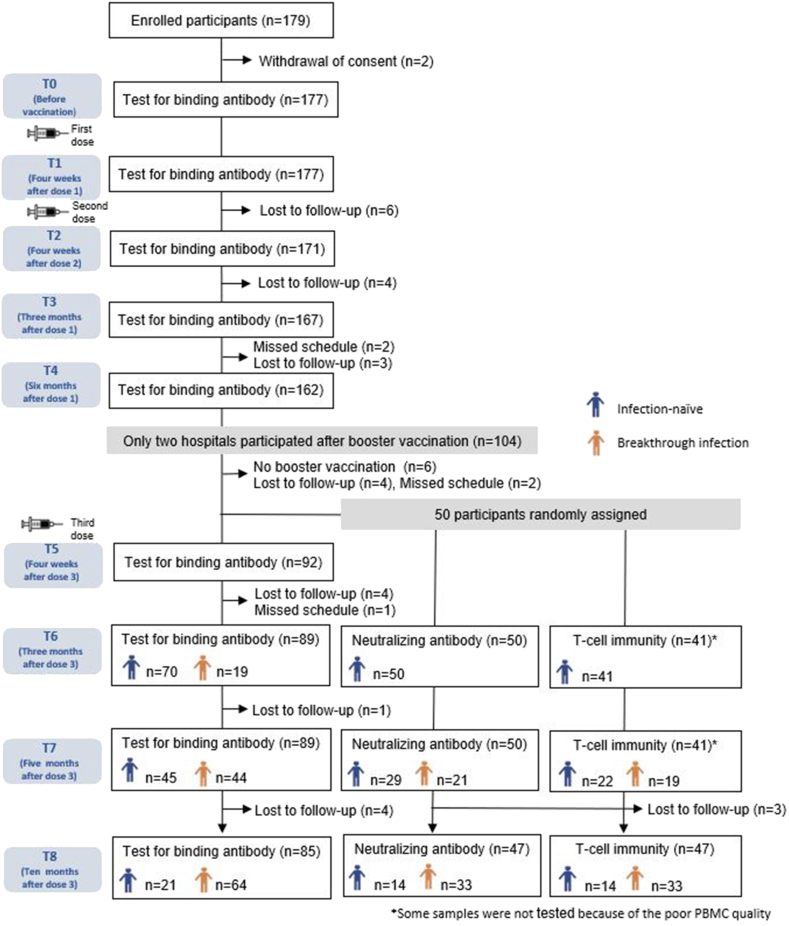
A total of 177 participants from four hospitals who received two doses of mRNA-1273 (primary series) at 28-day intervals were included at the start of this study. The detailed characteristics and schedule of the participants up to 6 months after the start of vaccination have been described previously [5]. In this study, the booster doses (third doses) were administered at 156.0 ± 15.3 days (mean ± standard deviation [SD]) from the second-dose vaccination. After the booster dose vaccination, immunogenicity was evaluated only in the participants from two hospitals (n = 94). All participants received a monovalent mRNA vaccine as a booster dose (mRNA-1273, 88 cases; BNT162b2, 6 cases). Supplementary Table 1 shows the baseline characteristics of the enrolled participants after booster dose administration. The mean age of all participants was 25.3 ± 3.3 years (mean ± SD), and 66% were women. The body mass index (BMI) was 20.9 ± 2.4 kg/m2 (mean ± SD). All the participants were healthy without comorbidities. Owing to missed visits, blood samples were obtained from 82 (87.2%) participants at all time points, 89 (94.7%) at ≥ 3 time points, 90 (95.7%) at ≥ 2 time points, and 94 (100%) at ≥ 1 time point after the booster dose vaccination (Fig. 1). The intervals (mean ± SD) from the booster dose to follow-up time points were as follows: 26.7 ± 5.1 days to T5, 92.4 ± 6.6 days to T6, 155.8 ± 8.5 days to T7, and 300.9 ± 17.9 days to T8. Breakthrough infections were observed in 66 (70.2%) participants during the study period (T5–T6, 19 cases; T6–T7, 25 cases; T7–T8, 22 cases). Among the 66 individuals confirmed with breakthrough infections through anti-N IgG assay, 41 (62%) reported being aware of the infection. All of them experienced mild infections that did not require hospitalization.
Fig. 1.
Study flowchart. Healthy adults (≥19 years) received two doses of the mRNA-1273 vaccine at four hospitals, followed by the third dose administration of mRNA vaccines (mRNA-1273, 88 cases; BNT162b2, 6 cases). After the third dose, participation was limited to two hospitals, and long-term immune responses among them were investigated.
3.2. Kinetics of anti-spike IgG antibody response after the booster dose among infection-naive individuals at each time point
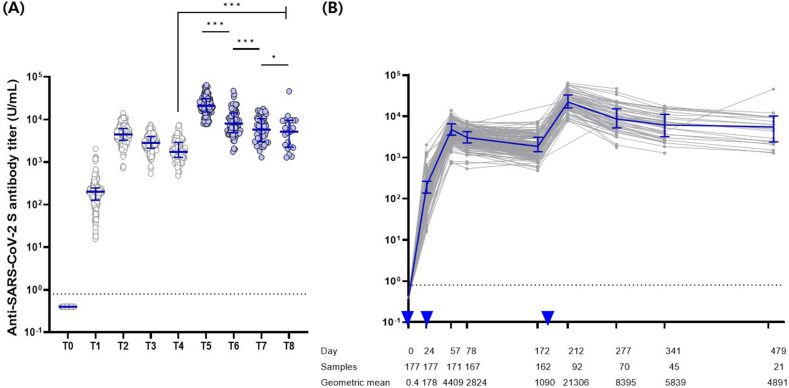
Following the booster dose vaccination, the anti-spike IgG antibody response against WT was boosted; similar to those after primary vaccination, the antibody titers achieved peak responses 4 weeks after the third dose (T5) and declined gradually over the following 9–12 months (Fig. 2), excluding one individual whose titer increased between T7 and T8. The half-life of anti-spike IgG antibodies tended to slow down over time, with 46 days (95% CI, 42.5–50.0), 81.6 days (95% CI, 75.3–89.1), 304.1 days (95% CI, 161.0–2736.6), and 112.7 days (95% CI, 92.3–144.7) at T5–T6, T6–T7, T7–T8, and T5–T8, respectively. Even 9–12 months after the booster dose (T8), both the median and geometric mean of anti-spike IgG antibody levels were higher than those measured 4 weeks after dose 2 (T2). The median (interquartile range) levels of anti-spike antibodies were 5175.0 U/mL (3325.5–6134.0) at T8 and 4476.0 U/mL (2299.0–9543.0) at T2, whereas the GMTs were 4890.8 (95% CI, 3258.8–7340.0) at T8 and 4409.4 (95% CI 4082.6–4762.4) at T2 (p = 0.767).
Fig. 2.
Dynamics of severe acute respiratory syndrome coronavirus 2 spike protein immunoglobulin G responses of infection-naïve individuals at each time point. (A) Median values with interquatile ranges are displayed for each time point (T0–T8), and (B) the trajectory of antibodies are plotted over time elapsed after vaccination. The dashed line represents the antibody positivity threshold, indicating 0.8 U/mL. Two-tailed p-test resulting from paired t-test (*P < 0.05, **P < 0.01, ***P < 0.001). The error bar illustrates the median accompanied by the interquartile range, while the inverted triangle signifies the administration of the vaccine. T0, day of the first dose of vaccine; T1, 4 weeks after the first dose; T2, 4 weeks after the second dose; T3, 3 months after the first dose; T4, 6 months after the first dose; T5, 4 weeks after the third dose; T6, 3 months after the third dose; T7, 5 months after the third dose; T8, 9–12 months after the third dose.
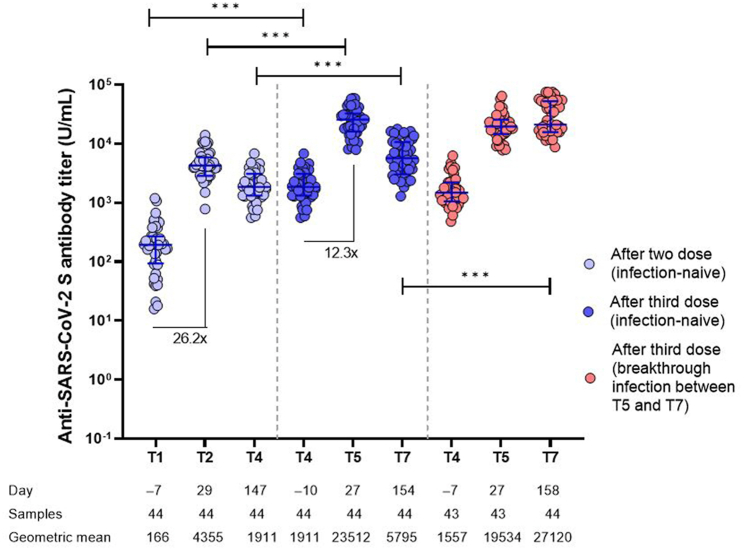
The kinetics of anti-spike IgG antibody among the individuals who were not infected up to 5 months after booster vaccination (T7) and completed all sampling without visit loss (n = 44) showed that the peak (4 weeks) IgG level after the third dose of vaccine was significantly higher than that after the two doses (Fig. 3). IgG waning was faster after the third dose of vaccine than that after the second dose (t1/2 = 63.1 days versus 99.7 days, p < 0.001), although the anti-spike IgG titers 5 months after the third dose were still significantly higher than the peak after the second dose. Comparing uninfected individuals with those who developed breakthrough infection up to 5 months after booster vaccination, the anti-spike IgG titer at pre-booster (third dose) and 4 weeks after the third dose were indistinguishable (Fig. 3).
Fig. 3.
Comparison of humoral immune responses after the second and third doses. Anti-severe acute respiratory syndrome coronavirus 2 spike protein immunoglobulin G antibody titers are presented at baseline (T1), 4 weeks (T2), and 5 months (T4) after the second dose and baseline (T4), 4 weeks (T5), and 5 months (T7) after the third dose. Error bar depicts median with interquartile range. The dashed line represents the antibody positivity threshold, indicating 0.8 U/mL. At the bottom, the elapsed average time since the first dose (days), the number of participants sampled, and the geometric mean antibody titer at each time point are presented. ***P < 0.001.
3.3. Cross-reactive neutralizing immunogenicity against omicron subvariants
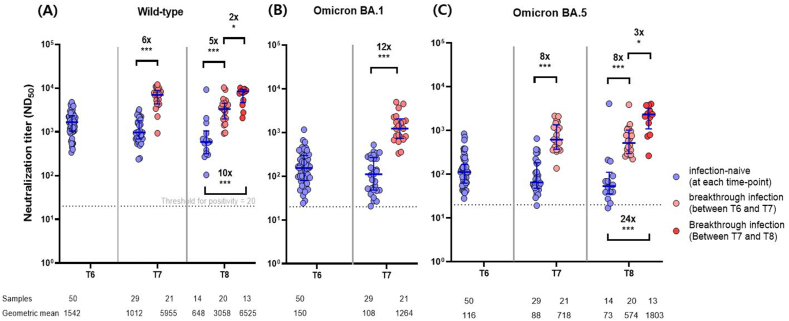
The neutralizing antibody responses against WT SARS-CoV-2 were evaluated at each time point in a subset of randomly selected individuals (n = 50). In addition, cross-neutralization was evaluated on the Omicron variants 3 (T6), 5 (T7), and 9–12 (T8) months after the third dose. Neutralizing activities against Omicron BA.1 and BA.5 were lower than those against the WT at all time points examined. However, all participants showed positive neutralization responses to all variants, except for one individual among the infection-naïve persons who tested negative for Omicron BA.5 at T7 and at T8 (Fig. 4A–C). Compared with the individuals who were SARS-CoV-2 infection-naïve until 5 months after the third dose (T7), those with breakthrough infections at T6–T7 (BA.2-dominant period) showed higher neutralization antibody responses to WT, BA.1, and BA.5 by 6-, 12-, and 8-fold, respectively. A similar pattern was maintained 9–2 months after the third dose; compared with the individuals who were uninfected until 9–12 months after the booster dose (T8), those with breakthrough infection at T6–T7 (BA.2-dominant period) presented higher neutralizing antibody responses to WT and BA.5 by 5- and 8-fold, respectively. However, breakthrough infections at T7–T8 (BA.5-dominant period) appeared to better boost neutralizing activities than those during the BA.2-dominant period. Compared with uninfected individuals until T8, those with breakthrough infection at T7–T8 showed higher neutralizing antibody responses to WT and BA.5 by 10- and 24-fold, respectively.
Fig. 4.
Comparison of neutralizing antibody responses against wild-type (A), Omicron BA.1 (B), and Omicron BA.5 (C) at each time point according to the occurrence of breakthrough infection. At the bottom, the number of participants sampled, and the geometric mean antibody titer at each time point are recorded. T6, 3 months after the third dose; T7, 5 months after the third dose; T8, 9–12 months after the third dose. Error bar depicts median with interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001.
Neutralizing antibody titers against the WT declined faster than those against the Omicron BA.5 variant. In the absence of breakthrough infection, the half-lives of neutralization against WT and Omicron BA.5 were 78.6 days (95% CI, 66.7–95.6) versus 94.7 days (95% CI, 69.5–147.6) at T6–T7 (p < 0.001), 255.1 days (95% CI, 105.6–∞) versus 310.3 days (95% CI, 98.7–∞) at T7–T8 (p < 0.001), and 138.7 days (95% CI, 90.4–301.0) versus 191.7 days (95% CI, 100.7–2006.9) at T6–T8 (p < 0.001).
3.4. Long-term T-cell immunity against severe acute respiratory syndrome coronavirus 2
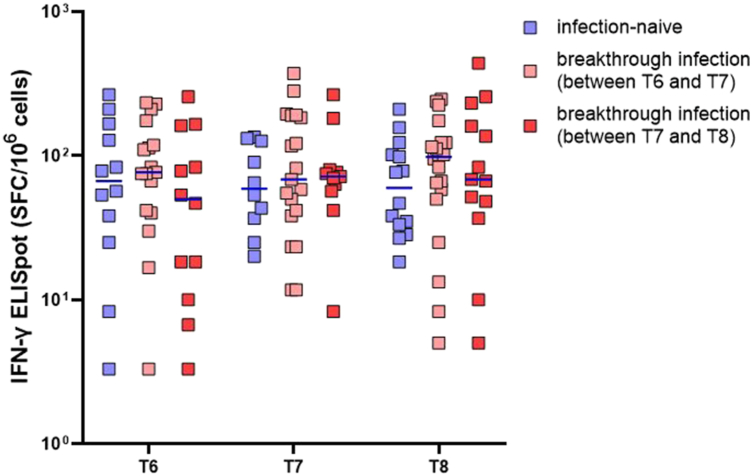
As for the same subset of randomly selected individuals (n = 50), anti-spike T-cell immune responses were also assessed by ELISpot. T-cell immune responses were efficiently induced in all tested individuals (41/41) 3 months after booster vaccination and were sustained up to 12 months post-booster vaccination. To evaluate the T-cell immunity before and after SARS-CoV-2 infection, the participants were classified as SARS-CoV-2 infection-naïve individuals up to T8 (n = 16), those with breakthrough infection between T6 and T7 (n = 21), and those with breakthrough infection between T7 and T8 (n = 13) (Fig. 5). Comparing uninfected individuals with infected individuals, the titer and distribution of T-cell immunity was similar before and after breakthrough infection episodes.
Fig. 5.
Interferon-γ enzyme-linked immunosorbent spot assay results at each time point according to infection status. At the end of the study, there were 16 non-infected individuals, 21 and 13 breakthrough infections at T6–T7 and T7–T8, respectively. The T-cell titers at each time point for these individuals are presented. T6, 3 months after the third dose; T7, 5 months after the third dose; T8, 9–12 months after the third dose. The error bar depicts the median value.
3.5. Characteristics of patients with breakthrough infection
The demographics of individuals who experienced breakthrough infections during the study period versus those who were uninfected are shown in Supplementary Table 2. Although statistically insignificant, those uninfected were rather younger (mean age, 24.2 ± 1.7 years) than were those with breakthrough infection (mean age, 25.7 ± 3.7 years) (p = 0.068). Other parameters, including sex, BMI, the interval between the second and third vaccine dose, and peak anti-spike IgG antibody titers after the booster dose, showed no significant difference between infected and uninfected individuals. However, individuals with breakthrough infection between 3 and 5 months after the booster dose (T6–T7, BA.2-dominant period) showed significantly lower anti-spike IgG antibody titers against WT and neutralizing activities against BA.1 measured at T6 than did those without infection (6200.55 U/mL [95% CI, 4587.63–8380.54] versus 9832.08 U/mL [95% CI, 8082.89–11,959.82], p = 0.009; 110.92 [95% CI, 75.93–162.03] versus 187.26 [95% CI, 136.20–257.44], p = 0.034, respectively). Neutralizing activities against WT were slightly lower in individuals with breakthrough infection at T6–T7 than in those without infection, but statistically insignificant (1274.66 [95% CI, 934.94–1737.82] versus 1770.58 [95% CI, 1404.66–2231.83], p = 0.079). Individuals with a breakthrough infection between 5 and 12 months after the booster dose (T7–T8, BA.5-dominant period) and non-infected individuals did not show significant differences in neutralizing or binding antibody titers at T7. Cellular immunity did not show any relevance with respect to breakthrough infection (Supplementary Table 3 and Supplementary Fig. 1).
4. Discussion
This study showed that the mRNA vaccine booster dose (third dose) triggered a durable anti-spike IgG antibody response for up to 1 year, with titers above the peak after the second dose. Breakthrough infections during the Omicron-dominant period substantially boosted neutralizing antibody titers against Omicron sublineages (BA.1 and BA.5) and the ancestral strain. In particular, breakthrough infections during the BA.5-dominant period conferred substantially higher neutralizing activities against the ancestral strain and Omicron BA.5 than did those during the BA.2-dominant period. Anti-spike IgG antibody titers and neutralizing activity against BA.1 were inversely correlated with breakthrough infection during the BA.2-dominant period.
A crucial question regarding the third dose is whether it induces more durable immune responses than would the two-dose primary series vaccination. Considering the two-dose mRNA vaccination produces substantial memory B and T cells and at least a few long-lived plasma cells [13], it would be reasonable to expect that the booster dose may induce more durable antibodies than would the two-dose primary vaccination. Improved durability after the booster dose has also been observed in previous studies, although the follow-up periods were shorter than that in the present study, mostly within 6 months [[14], [15], [16]]. A few studies have evaluated immunity beyond 6 months post-booster dose, but there are limitations such as the use of inactivated vaccines [17], lack of pre-vaccination serology information [[18], [19], [20]], and the inclusion of individuals with previous or breakthrough COVID-19 infections [[18], [19], [20]]. In one study [18], antibody responses were well-maintained up to 11 months, but the inclusion of some individuals who received a fourth dose at the last sampling point limited the interpretation of the immune durability after the third dose. In another study conducted in South Korea [21], immune responses were evaluated up to 8 months, but those were focused on the breakthrough infection. Unlike influenza, which exhibits a clear seasonal epidemic over 6 months, COVID-19 occurrence continues throughout the year with repeated waxing and waning. Thus, it is crucial to evaluate long-term vaccine immune up to 9–12 months given the annual vaccination strategies. The long-term immunogenicity data in the present study support the clinical data that vaccine effectiveness against SARS-CoV-2 infection lasted for >1 year in the pre-Omicron era [22]. However, in the present study, contrary to previous reports [14], the antibody decay rate (∼5 months) was significantly higher after the third dose than after the second dose. This was likely due to the high antibody titers among the participants before the third-dose vaccination. Pre-vaccination antibody titers may be inversely correlated with memory B-cell reactivation [23]. In a previous study using a mouse model, high levels of immunoglobulins restricted the recall response of memory B cells [24]. The short interval between the second and third doses might have contributed to the high pre-vaccination antibody titers, which would limit memory B-cell reactivation, thereby inducing a faster decay of antibodies after the third-dose vaccination. Nevertheless, notably, the participants in this study maintained high antibody titers for up to 9–12 months after the third-dose vaccination. The absolute quantity of antibodies itself is a surrogate marker preventing breakthrough infection; therefore, it could be justified to administer a short-interval booster during a pandemic, even if the half-life of antibodies decreases.
Consistent with previous findings, this study showed that the WT monovalent vaccine induced lower neutralizing activity against Omicron variants than against the ancestral strain in SARS-CoV-2-naive individuals [15]. In this study, the half-life of neutralizing antibodies against Omicron BA.5 was estimated to be longer than that against WT SARS-CoV-2, which may be attributed to significantly lower neutralizing antibody titers against Omicron BA.5 from the beginning. Therefore, the findings should be interpreted with caution. Considering the previously observed immune evasion of Omicron sublineages, Omicron-containing bivalent vaccines were introduced in late 2022 [25]. However, close surveillance of immune imprinting was required [26]. Nevertheless, enhanced neutralizing activities against the ancestral strain and other omicron variants after Omicron BA.2 or BA.5 breakthrough infection mitigated the concerns of immune imprinting.
This study showed that breakthrough infection at T6–T7 (Omicron BA.2-dominant period) boosted neutralizing antibody titers against the ancestral strain and Omicron subvariants (BA.1 and BA.5). Considering the known limited cross-reactivity after a single exposure to BA.2 in unvaccinated individuals [27], the results in this study are likely due to the prior multiple exposures to SARS-CoV-2 antigen through vaccination. In this study, the improved cross-neutralizing activities were more pronounced in individuals infected during the BA.5-dominant period than in those infected during the BA.2-dominant period. First, the longer interval from booster vaccination to BA.5 breakthrough infection versus BA.2 might contribute to higher cross-neutralizing activities. In addition, there was a chance that the difference in BA.2 and BA.5 antigenicity might affect the degree of cross-neutralizing activities.
At the end of this study, the cumulative infection rate in the study participants was 70%, which was consistent with the seropositivity rate in South Korea at that time [28]. This study found a possible inverse correlation between breakthrough infection during the BA.2-dominant period and humoral immunity, including anti-spike IgG antibody titers and neutralizing activities against the WT and the BA.1 variant. The observed inverse relationship between breakthrough infection and humoral immunity in this study aligns with findings from earlier reports [[29], [30], [31], [32]], although a direct comparison is difficult because of differences in immune assay methods, the timing of blood sampling before infection, and circulating variants. In this study, breakthrough infection at T7–T8 was not significantly related to the humoral immunity measured at T7. However, this should be interpreted with caution because of the small sample size and the wide interval between T7 and T8 (approximately 5 months).
This study demonstrated that cellular immunity was efficiently induced in all tested individuals for up to 9–12 months after the booster dose, which is encouraging evidence that the T-cell memory of mRNA vaccines is long-lived. T-cell immunity might be further boosted after the third dose, as our previous study using the same assay demonstrated detectable cellular immunity in only 90% of participants 3 months after vaccination [5]. The longevity of cellular immunity observed in this study has great implications in the Omicron era, as T-cell epitopes are generally well conserved between variants [33]. As T cells play an important role in the control and clearance of SARS-CoV-2 [13], this suggests that vaccine effectiveness against severe infections may be maintained in the long term, even with the emergence of variants. In this study, T-cell immunity was similar before and after breakthrough infection episodes between infected and uninfected individuals. While the limited sample size necessitates caution in interpretation, this could be explained theoretically, as follows: at the time of infection, low levels of circulating memory T cells may be sufficient for the control of severe infection, given that memory T cells can rapidly proliferate. Thus, T-cell immunity is only weakly boosted in SARS-CoV-2-recovered individuals [13,34].
This study had several limitations. First, this study focused on healthy adults, excluding elderly individuals and patients with comorbidities. Second, owing to the high rate of breakthrough infections (approximately 70%), it was challenging to secure a sufficient number of individuals to evaluate the long-term persistence of vaccine-induced immunity. Third, neutralizing antibody and cellular immunity assays were conducted in a subset of the individuals, while the small sample size may have led to statistically insignificant results. Fourth, CD8 and CD4 T cells were not distinguished in the analysis; the bulk PBMC IFN-γ ELISPOT assay signal originated from a mixture of CD8 and CD4 T cells. However, given that either CD8 or CD4 T cells can contribute to viral clearance [35], the persistence of T-cell immunity for up to a year observed in this study would be significant in the context of preventing severe infections. Finally, there is a chance that some patients could have had undetected prior SARS-CoV-2 infection. A previous study reported that anti-N IgG waned rapidly [13] and anti-N IgG might not be induced among vaccinated individuals [36]. In fact, in the present study, most participants showed immune waning over time, as expected; however, one participant showed increasing antibody titers at T8, although the anti-N IgG antibody test was negative.
In conclusion, booster dose mRNA vaccination elicits a durable humoral antibody response for up to 1 year with titers above the peak after the second dose and induces sustained spike protein-specific T-cell immunity, supporting the annual COVID-19 vaccination strategy. Repeated vaccinations at short intervals may decrease the half-life of the vaccine-induced antibodies.
Ethical approval statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Korea University Guro Hospital (2021GR0099), Ajou University Hospital (AJIRB BMR SMP21 267), Kangnam Sacred Hallym University Hospital (HKS2021 05 023), and International St. Mary's Hospital (S21MIME0045).
Informed consent statement
Informed consent was obtained from all participants involved in the study.
Data availability
The authors declare that all data supporting the results of this study are available in the paper and Supplementary Information. Source data will be provided upon request.
CRediT authorship contribution statement
Min Joo Choi: Writing – original draft, Visualization, Resources, Formal analysis, Data curation, Conceptualization. Hakjun Hyun: Writing – review & editing. Jung Yeon Heo: Writing – review & editing, Resources. Yu Bin Seo: Writing – review & editing, Resources. Ji Yun Noh: Writing – review & editing. Hee Jin Cheong: Writing – review & editing. Woo Joo Kim: Writing – review & editing. Hwa Jung Kim: Formal analysis. Ju-yeon Choi: Investigation. Young Jae Lee: Investigation. Eun Joo Chung: Investigation. Su-Hwan Kim: Investigation. Hyeonji Jeong: Investigation. Byoungguk Kim: Validation, Supervision, Investigation, Conceptualization. Joon Young Song: Writing – original draft, Validation, Supervision, Resources, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Joon Young Song reports financial support was provided by Korea Disease Control and Prevention Agency. Joon Young Song reports a relationship with Korea Disease Control and Prevention Agency that includes: board membership.
Acknowledgments
This research was funded by the Korea Disease Control and Prevention Agency, grant number 2021ER260300. We would like to thank all the individuals who volunteered to participate in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27211.
Contributor Information
Min Joo Choi, Email: cowgow@naver.com.
Joon Young Song, Email: infection@korea.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO. Coronavirus (COVID-19) Dashboard, https://covid19.who.int/, [accessed 5 October 2023].
- 2.Bobrovitz N., Ware H., Ma X., Li Z., Hosseini R., Cao C., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect. Dis. 2023;23:556–567. doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A., Oda G., Holodniy M. Effectiveness of messenger RNA–based vaccines during the emergence of the severe acute respiratory syndrome coronavirus 2 omicron variant. Clin. Infect. Dis. 2022;75:2186–2192. doi: 10.1093/cid/ciac325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Health Security Agency. COVID-19 vaccine monthly surveillance reports (week 39 2021 to week 23 2023), https://www.gov.uk/government/publications/covid-19-vaccine-weekly-surveillance-reports, [accessed 5 October 2023].
- 5.Choi M.J., Heo J.Y., Seo Y.B., Yoon Y.K., Sohn J.W., Noh J.Y., et al. Six-month longitudinal immune kinetics after mRNA-1273 vaccination: correlation of peak antibody response with long-term, cross-reactive immunity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tré-Hardy M., Cupaiolo R., Wilmet A., Antoine-Moussiaux T., Della Vecchia A., Horeanga A., et al. Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. J. Infect. 2021;83:559–564. doi: 10.1016/j.jinf.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. FDA briefing document: Future vaccination regimens addressing COVID-19, https://www.fda.gov/media/164699/download.VaccinesandRelatedBiologicalProductsAdvisoryCommitteeMeeting; [Accessed. 5 October 2023].
- 9.Oliver S. Advisory Committee on Immunization Practices Meeting COVID-19 Vaccines, Advisory Committee on Immunization Practices meeting. Atlanta, Georgia. 2023. COVID-19 vaccine: considerations for future planning. [Google Scholar]
- 10.Joint Committee on Vaccination and Immunisation. JCVI statement on the COVID-19 vaccination programme for 2023, https://www.gov.uk/government/publications/covid-19-vaccination-programme-for-2023-jcvi-interim-advice-8-november-2022/jcvi-statement-on-the-covid-19-vaccination-programme-for-2023-8-november-2022, [accessed 5 October 2023]..
- 11.GISAID. hCoV-19 variants dashboard, https://gisaid.org/hcov-19-variants-dashboard/, [accessed 5 October 2023].
- 12.Korea Disease Control and Prevention Agency [Press release], https://ncov.kdca.go.kr/tcmBoardList.do?pageIndex=5&brdId=3&brdGubun=&board_id=&search_item=1&search_content=, [accessed 5 October 2023].
- 13.Sette A., Crotty S. Immunological memory to SARS‐CoV‐2 infection and COVID‐19 vaccines. Immunol. Rev. 2022;310:27–46. doi: 10.1111/imr.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilboa M., Regev-Yochay G., Mandelboim M., Indenbaum V., Asraf K., Fluss R., et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia H., Zou J., Kurhade C., Cai H., Yang Q., Cutler M., et al. Neutralization of Omicron SARS-CoV-2 by 2 or 3 doses of BNT162b2 vaccine. bioRxiv. 2022 doi: 10.1101/2022.1101.1121.476344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Yin Y., Cui Q., Huang W., Zou Q., Shen T. Long‐term variations and potency of neutralizing antibodies against Omicron subvariants after CoronaVac‐inactivated booster: a 7‐month follow‐up study. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heftdal L.D., Pérez-Alós L., Hasselbalch R.B., Hansen C.B., Hamm S.R., Møller D.L., et al. Humoral and cellular immune responses eleven months after the third dose of BNT162b2 an mRNA-based COVID-19 vaccine in people with HIV–a prospective observational cohort study. EBioMedicine. 2023;93 doi: 10.1016/j.ebiom.2023.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu P., Faraone J.N., Evans J.P., Zheng Y.M., Yu L., Ma Q., et al. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022;387:1329–1331. doi: 10.1056/NEJMc2210546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Munro A.P.S., Wright A., Feng S., Janani L., Aley P.K., et al. Persistence of immune responses after heterologous and homologous third COVID-19 vaccine dose schedules in the UK: eight-month analyses of the COV-BOOST trial. J. Infect. 2023;87:18–26. doi: 10.1016/j.jinf.2023.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y.J., Choi J.Y., Yang J., Baek J.Y., Kim H.J., Kim S.H., et al. Longitudinal kinetics of neutralizing antibodies against circulating SARS-CoV-2 variants and estimated level of group immunity of booster-vaccinated individuals during omicron-dominated COVID-19 outbreaks in the Republic of Korea, 2022. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.01655-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilz S., Theiler-Schwetz V., Trummer C., Krause R., Ioannidis J.P.A. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022;209 doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel R.R., Painter M.M., Lundgreen K.A., Apostolidis S.A., Baxter A.E., Giles J.R., et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185:1875–1887.e8. doi: 10.1016/j.cell.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pape K.A., Taylor J.J., Maul R.W., Gearhart P.J., Jenkins M.K. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA. FDA news release. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use, [accessed 5 October 2023].
- 26.Reynolds C.J., Pade C., Gibbons J.M., Otter A.D., Lin K.M., Muñoz Sandoval D., et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377 doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rössler A., Netzl A., Knabl L., Schäfer H., Wilks S.H., Bante D., et al. BA.2 and BA.5 omicron differ immunologically from both BA.1 omicron and pre-omicron variants. Nat. Commun. 2022;13:7701. doi: 10.1038/s41467-022-35312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korea Centers for Disease Control & Prevention. Agency. Korea National Institute of Heath announced the results of the second national COVID-19 seropositive rate survey, https://www.korea.kr/briefing/pressReleaseView.do?newsId=156547686, [accessed 5 October 2023].
- 29.Perry J., Osman S., Wright J., Richard-Greenblatt M., Buchan S.A., Sadarangani M., et al. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS One. 2022;17 doi: 10.1371/journal.pone.0266852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koutsakos M., Reynaldi A., Lee W.S., Nguyen J., Amarasena T., Taiaroa G., et al. SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity. 2023;56:879–892.e4. doi: 10.1016/j.immuni.2023.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Follmann D., Janes H.E., Buhule O.D., Zhou H., Girard B., Marks K., et al. Antinucleocapsid antibodies after SARS-CoV-2 infection in the blinded phase of the randomized, placebo-controlled mRNA-1273 COVID-19 vaccine efficacy clinical trial. Ann. Intern. Med. 2022;175:1258–1265. doi: 10.7326/M22-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the results of this study are available in the paper and Supplementary Information. Source data will be provided upon request.