Abstract
Pachyonychia congenita is an uncommon autosomal dominant skin disorder characterized by hypertrophic nail dystrophy, palmoplantar keratoderma, oral leukokeratosis, and cutaneous cysts. And fissured tongue is rarely reported in patients with pachyonychia congenita. The disease is primarily associated with mutations in five keratin genes, namely KRT6A, KRT6B, KRT6C, KRT16 or KRT17. Herein we report a 9-year-old Chinese girl who has thickened nails, keratinized plaques, and fissured tongue since birth. To investigate the underlying genetic cause, whole-exome sequencing and Sanger sequencing were performed in this patient and her family members. We identified a candidate variant c.1460–2_1460del (p.S487Lfs*21) in the KRT6A gene (NM_005554.4) by whole-exome sequencing. Sanger sequencing revealed the absence of the mutation in both parents, indicating that it is a de novo variant. Thus, the novel heterozygous frameshift mutation c.1460–2_1460del (p.S487Lfs*21) within exon 9 of KRT6A was identified as the genetic cause of the patient. Our study identified a rare de novo heterozygous frameshift mutation in the KRT6A gene in a patient with pachyonychia congenita presenting fissured tongue. Our findings expand the KRT6A gene mutation spectrum of Pachyonychia congenita, and will contribute to the future genetic counseling and gene therapy for this disease.
Keywords: Pachyonychia congenita, Fissured tongue, Keratin, KRT6A, Genodermatosis
Highlights
-
•
We report a patient who has thickened nails, keratinized plaques, and fissured tongue since birth.
-
•
DNA sequencing of the patient revealed the previously unreported c.1460–2_1460delC mutation.
-
•
The mutation, identified as de novo, was absent in the parents' genomic profiles.
-
•
The patient was diagnosed as pachyonychia congenita 6a(PC-k6a).
-
•
The rare fissured tongue in this study may be linked to the mutation locus.
1. Introduction
Pachyonychia congenita (PC) is a rare autosomal dominant disorder that was first reported in 1906 by Jadasshon and Lewandowsky [1]. PC is characterized by symmetrically thickened, dystrophic fingernails and toenails [2]. Most of these features are normally present at or soon after birth. PC-1 (Jadassohn-Lewandowsky syndrome, MIM: 167200) has hypertrophic nail dystrophy, palmoplantar keratoderma, and more prominent oral leukokeratosis. PC-2 (Jackson-Lawler syndrome, MIM: 167210) is characterized by natal teeth, pilosebaceous cysts, hypertrophic nail dystrophy, palmoplantar keratoderma, and with few oral leukoplakias. In addition, Lucker [3] and Mouaci-Midoun [4] reported a late-onset PC, usually between the ages of 40 and 50, called PC tarda. In 1994, Munro et al. showed that the causative gene of PC was located on chromosome 17 at 17q2-q21, the region in which the keratin gene is harbored, suggesting that keratin gene abnormalities are the genetic basis for the nail, epidermis, mucosa, and hair lesions in PC [5]. Since then, people have successively discovered the pathogenic mutations of PC. Up to this point, there are five main pathogenic genes in PC, including KRT6A (NM_005554.4) [6], KRT6B (NM_005555.4) [7], KRT6C (NM_173086.5) [8], KRT16 (NM_005557.4) and KRT17 (NM_000422.3) [9]. It is well known that dyskeratosis nail, painful palmoplantar keratoderma, and oral leukokeratosis of PC-1 are caused by mutations in KRT6A and KRT16 [6]. In contrast, the natal teeth and multiple sebaceous cysts of PC-2 might be caused by KRT6B and KRT17 mutations [7]. Based on an extensive dataset of clinical information from PC patients, as provided by the International Pachyonychia Congenita Research Registry (IPCRR), a more refined molecular-level classification method was proposed during the 2010 International Symposium on Pachyonychia congenita. Based on the classification of keratin mutation genes, PC was classified into PC-k6a, PC-k6b, PC-k6c, PC-k16, and PC-k17. PC-U refers to cases of suspected PC, that either a mutation has not been identified or has not been investigated. This classification facilitates the correlation of PC clinical phenotypes with molecular genetics [[10], [11], [12]].
Keratin constitutes a vital component of the intermediate filament protein family (IFs) and assumes a pivotal role in the cytoskeleton. It significantly contributes to providing mechanical strength and resilience within and between cells and tissues. When the keratin gene is defective and amino acids are substituted or deleted, the abnormal keratin cannot play its role, resulting in the rupture or collapse of the cytoskeleton, resulting in epidermal relaxation and compensatory hyperkeratosis, causing a series of symptoms such as PC [13]. Previous research has elucidated that keratins share a conserved protein structure, featuring the central α-helical rod domain encompassing the 1A, 1B, 2A, and 2B domains. These are intricately linked by the non-helical linkage domains L1, L12, and L2 [11,13,14]. The α-helical rod domain is bordered by the variable V1 and V2 domains. Within the central α-helical rod domain, there exist highly conserved helix boundary motifs, including the helix initiation motif and helix termination motif. These motifs play a crucial role in mediating end-to-end interactions during the heterodimerization of keratins and their assembly into intermediate filaments [15]. Exons 1 and 6 in type I keratin and 1 and 7 in type II keratin encode the helix boundary motifs [16]. Type II keratin also has two sub-domains, H1 and H2, located between the α-helical rod domain and the variable sequences V1 and V2 [16] The highly conserved helical border motif is the site of a high incidence of genetic mutations, some of which cause PC.
Previous studies mainly focused on the typical clinical manifestations and gene mutation hotspots of PC, and there were few reports on PC with fissured tongue. In this study, a new case of PC with fissured tongue was collected. The mutation analysis of the keratin gene was conducted through whole-exome sequencing (WES) and Sanger sequencing, to identify disease-causing mutated genes and further explore genotype-phenotype correlations.
2. Case presentation and findings
2.1. Clinical report
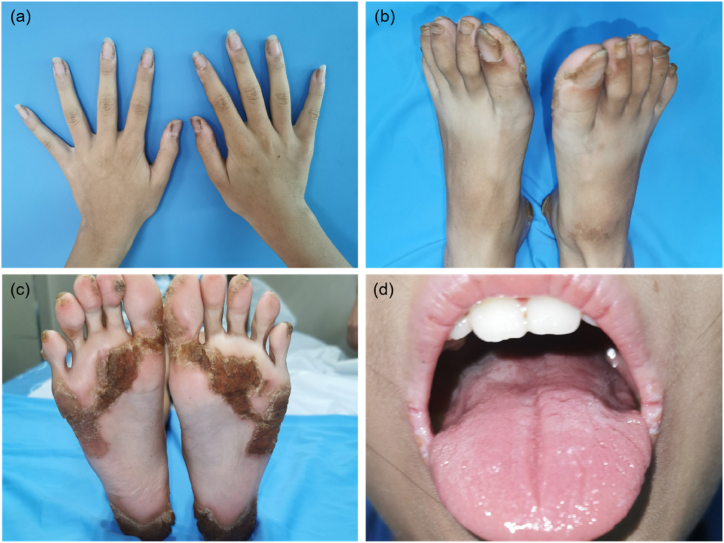
The patient was a 9-year-old Chinese girl who has thickened nails, keratinized plaques on palms, soles, corners of the mouth, and fissured tongue since birth. At the age of 1, the nail grooves of her fingernails and toenails began to turn yellow and thicken. The skin of the palms and soles of the stressed parts was gradually keratinized and thickened, black and chapped. Her parents did not share a consanguineous relationship, and there were no other individuals in the family exhibiting similar symptoms. (Fig. 1). Physical examination revealed normal vision and hearing, normal intelligence and growth, and the nutrition was moderate. Skin examination revealed that the nail grooves of the fingernails and toenails were partly thickened and raised in a wedge shape, and the surface of the nails was rough, opaque, and dirty brown (Fig. 2a and b). The skin of the trunk and extremities was dry, and the palms and soles of the hands and feet had some punctate and flaky keratinized dark brown thickened patches (Fig. 2c). The patient exhibited fissured tongue, oral leukokeratosis, and keratotic plaques in the corners of mouth (Fig. 2d). No natal teeth, follicular sebaceous cysts, hair loss, or other organ abnormalities.
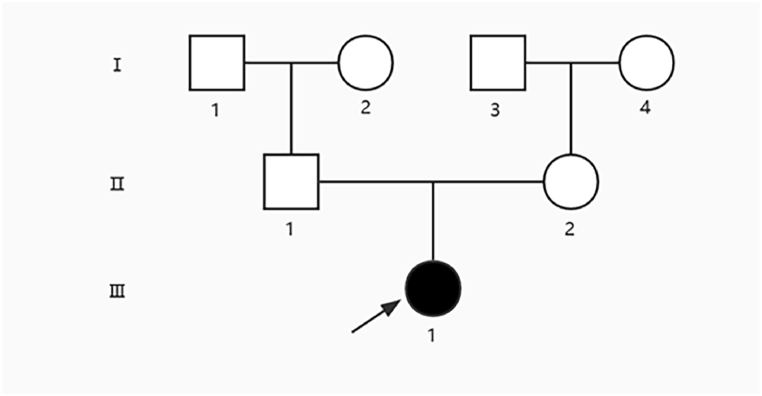
Fig. 1.
Family Pedigree. Pedigree was constructed for the 7-member family with PC. Squares and circles indicate males and females, respectively. Arrow indicates the proband.
Fig. 2.
Clinical Phenotype of the Proband with PC. (a, b) The nail grooves of the fingernails and toenails were partly thickened and raised in a wedge shape, and the surface of the nails was rough, opaque, and dirty brown. (c) The skin on the stressed part of the sole had become keratinized, thickened, darkened, and cracked. (d) The patient exhibited fissured tongue, oral leukokeratosis, and keratotic plaques in the corners of mouth. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Method
After obtaining informed consent, peripheral blood samples were collected from the proband and her parents. Genomic DNA was subsequently extracted from the peripheral blood using a Qiagen kit (Hilden, Germany). The DNA Sample Prep Reagent Set (MyGenostics, Beijing, China) was utilized for preparing standard Illumina libraries. Human samples were obtained according to the principles of the Declaration of Helsinki and approved by the Institutional Review Board from Nanfang Hospital of Southern Medical University. Written informed consent was obtained from all participants.
Biotinylated 100 bp capture probes were specifically designed to cover the coding exons and adjacent 50 bp flanking regions of all targeted genes. The capture experiment was performed following the manufacturer's protocol. In brief, a 500 ng DNA library was combined with Buffer BL and GenCap gene panel probes from MyGenostics Inc. The mixture was heated at 95 °C for 5 minutes, followed by 65 °C for 5 minutes using a PCR machine. Subsequently, 19 μl of Buffer HY (prewarmed to 65 °C, MyGenostics, MD, USA) was added to the mixture. The hybridization process was conducted at 65 °C with PCR lid heat on for 16–24 hours. MyOne beads (Life Technology), 50μl in volume, were washed three times using 50μl of 1X binding buffer each time. Subsequently, the beads were resuspended in 50μl of 1X binding buffer. The hybrid mixture. Following that, the beads were washed with WB1 buffer at room temperature for 15 minutes once, and with WB3 buffer at 65 °C for 10 minutes three times. The bound DNA was eluted using a buffer, and amplified for 13 cycles using the following program: 95 °C for 4 minutes (1 cycle); 98 °C for 30 seconds, 65 °C for 30 seconds, 72 °C for 30 seconds (13 cycles); 72 °C for 5 minutes (1 cycle). The PCR product was purified using SPRI beads (Beckman Coulter) followingthe manufacturer's protocol. The enrichment libraries were sequenced on the Illumina HiSeq X Ten sequencer, generating paired reads of 150 bp. We employed various tools, such as Inheritance State Consistency Analysis (ISCA) for sequence alignment, ANNOVAR for single nucleotide polymorphism (SNP) annotation, and GERP for substitution analysis, to analyze the genome sequencing results. After annotating variants, we focused solely on nonsynonymous variants (NSVs), splice acceptor-site or donor-site mutations (SSMs), and insertions/deletions (indels), which are more likely to be pathogenic than other variants.
Sanger sequencing was conducted on the patient and her parents to validate the candidate mutation in KRT6A gene obtained from WES and to determine its origin. PCR was performed around the c.1460–2_1460del mutation positions in KRT6A (NM_005554.4) according to standard techniques. The QIAquick PCR Purification Kit from Qiagen (Germantown, MD) was used to purify the PCR products, which were then sequenced using ABI Dye Terminator Cycle Sequencing on an ABI 3730XL DNA analyzer (Life Technologies, Carlsbad, CA). Sequence trace files were analyzed using Sequencer 4.3 software (Gene Codes, Ann Arbor, MI).
4. Result and discussion
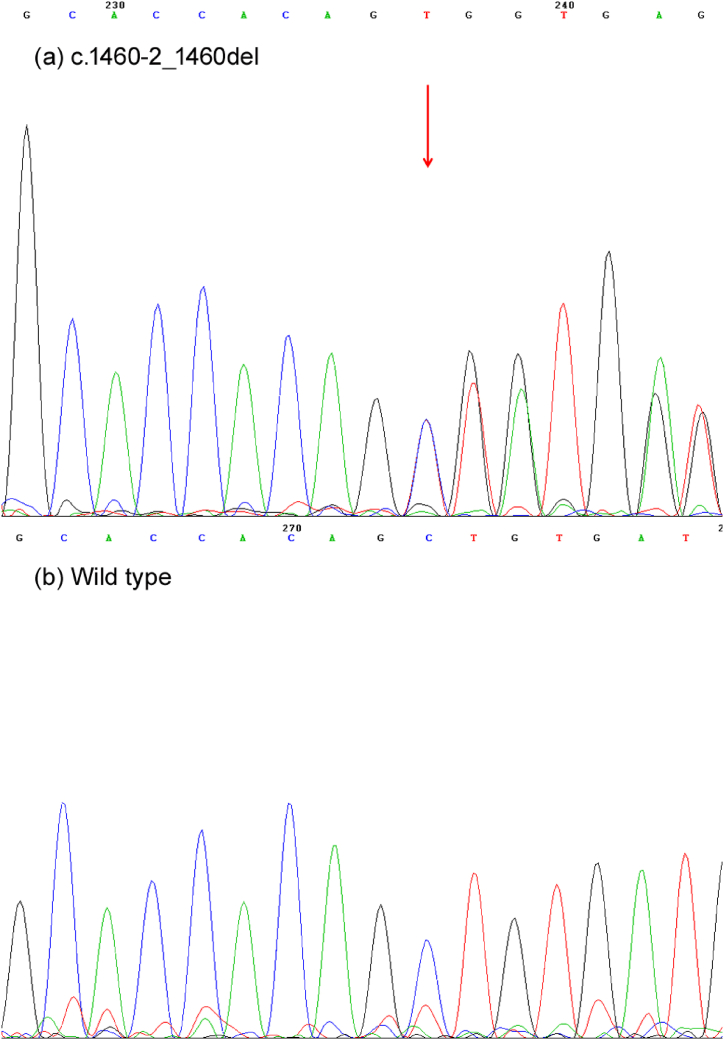
In this study, we identified a rare deletion mutation c.1460–2_1460del in exon 9 of KRT6A gene (NM_005554.4) in the patient through WES. According to ACMG committee guidelines, this variant was likely pathogenic, resulting in a frameshift (p.S487Lfs*21) (Fig. 3a and b). This variant was absent from the 1000 Genomes Project database, ESP6500 (NHLBI Exome Sequencing Project), EXAC (The Exome Aggregation Consortium), EXAC-EAS and gnomAD (Genome Aggregation Database). After Sanger sequencing, the heterozygous mutation was identified in the proband, whereas it was not detected in her parents. This observation suggest that it is a de novo variant. The c.1460–2_1460del has not been reported previously in KRT6A gene. As a rare keratin-related genetic disorder, PC is characterized clinically by hyperkeratosis and yellowing and thickening of the finger (toe) nails, which can involve tissues and organs such as palmoplantar, oral mucosa, teeth, and hair. Since the first identification of the likely pathogenic variant of PC in 1994 [5], researchers around the world have conducted numerous studies for PC variants. The most common likely pathogenic variations are located in KRT6A gene, specifically the codon 172 [17,18]. Most of these mutations are missense mutations and base insertions or deletions, with a smaller number of heterozygous splice site mutations and nonsense mutations leading to frameshift mutations and premature stop codons.
Fig. 3.
Mutant sequence in the KRT6A gene. (a) Sanger sequencing showed the heterozygous c.1460–2_1460del variant in the patient. (b) The patient's parents showed wild-type sequences.
The patient was a sporadic case with typical PC features in this study. She developed dystrophic nails, hyperkeratosis of hands and feet, perioral keratosis, and oral leukoplakia soon after birth. According to the clinical symptoms and physical signs of the patient, combined with DNA sequencing, she was diagnosed as PC-k6a (PC3). The WES identified a potentially pathogenic variant located in exon 9 of KRT6A, suggesting that keratin 6 production might be abnormal in the patient. In normal skin, K6 is restricted to nail bed epithelium, palmoplantar skin, and oral-genital mucosa. This K6 lesion caused compensatory keratosis of the nails, oral mucosa, palms, and soles, resulting in clinical manifestations inconsistent with other types of PC, i.e., younger age of onset, but without skin cysts and tooth birth [18]. The mild manifestation of nail changes might be explained by the variant being located in the tail domain of K6. Generally, mutations in the tail domain have a mild effect on keratin function. The clinical manifestations of the patient further validated the genotype-phenotype correlations.
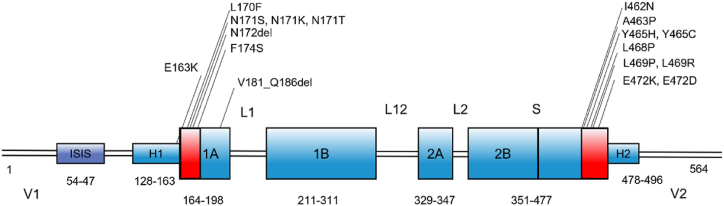
Studies have shown that mutations in KRT6A gene lead to a series of clinical manifestations of PC-k6a, including hyperplastic nail dystrophy, palmoplantar hyperkeratosis, and oral leukoplakia. However, it was uncommon for a fissured tongue to accompany the above symptoms. Fissured tongue is a common dysplasia of the tongue, characterized by splits and grooves in the central or lateral dorsum of the tongue, the etiology of which is currently unknown [19]. Some studies have reported associations between the fissured tongue and systemic diseases, such as psoriasis [20], Melkersson-Rosenthal Syndrome [21], pernicious anemia, macroglossia, etc. Although Melkersson's syndrome may also present with a fissured tongue, it does not have the same clinical manifestations in the skin and nails as PC does. At present, cases of PC patients with fissured tongues are rarely reported. Iraci, S. first reported a case of PC with fissured tongue in 1992 [22]. A 29-year-old white woman with late-onset PC and plantar palmoplantar hyperkeratosis with scrotal tongue (also called fissured tongue) and oral keratosis leukoplakia shortly after birth. Her mother also had a scrotal tongue, but no PC symptoms, suggesting that the scrotal tongue may be genetically related. Vijaikumar reported in 2001 that a 15-year-old boy had PC-2 since birth, with a thickened tongue and groove clefts, but no genetic sequencing was performed [23]. Du et al. also reported a case of a PC-k6a patient with fissured tongue in 2012 [24]. Interestingly, the IVS8-2A > C mutation in KRT6A discovered by Du et al. led to the deletion of exon 9 (c.1460_1470del), which led to the deletion of the same deleted exon in our case. The majority of reported KRT6A mutations are situated within the helix boundary motifs encoded by exons 1 and 7. Specifically, they occur in the 1A transcription initiation region or the 2B helix end [25]. Notably, the most frequently observed mutations include K6a p.Asn172del, K16 p.Asn125Ser, K16 p.Arg127Cys, and K17 p.Asn92Ser (Fig. 4) [18]. Gene mutations are rarely located in the tail region of keratin. In contrast, the likely pathogenic variant in this study is in exon 9, which is not a hotspot region and has been rarely reported. Therefore, there might be a potential correlation between the rare feature of fissured tongue and the variant in exon 9 of the KRT6A gene in this PC case. However, our study has some limitations. We only investigated one sporadic case, and no definite conclusions could be drawn regarding the correlation between this uncommon phenotype of the fissured tongue and the c.1460–2_1460del mutation in KRT6A. The genetic structure of PC with fissured tongue requires cell experiments and animal experiments to further verify the function of the mutant gene.
Fig. 4.
The domain structure and the location of reported mutations of K6a protein. The α-helical rod domain has four domains, the 1A, 1B, 2A, 2B, each domain consists of seven repeat sequences. These domains are connected by linkers, L1, L12, L2. The rod domain is flanked by variable domains V1 and V2. The ‘stutter’ sequence is marked by S. The H1 and H2 sub-domains are present in type II keratins. KRT6A mutation sites source: IPCRR (www.pachyonychia.org).
No PC-related gene mutation was found in other members of the patient's parents, the patient was a de novo mutation. About 40% of PC patients are caused by de novo mutations, which may be due to a mutation triggered by some factor in the germ cells of the father or mother during meiosis, and it is not excluded that their parents are germ cell chimeric carriers [26]. The patient's parents have a very small chance of having another child with the disease, but the patient's offspring have a 50% chance of developing the disease. PC currently lacks effective treatment. Hyperkeratotic skin can be treated with topical keratolytics and glucocorticoid ointment. The nail lesions are often treated by removing the nail, nail bed, and nail matrix. Some studies have demonstrated the effectiveness of retinoids, including isotretinoin, in treating this disease. Chronic plantar pain is a significant symptom that profoundly impacts the quality of life in PC patients. Current studies have confirmed that plantar injection of botulinum toxin (Btx) can reduce or even eliminate pain, blisters, and corpus callosum in PC patients [27]. There is also evidence that oral rosuvastatin can reduce callose thickness in the plantar and relieve pain [28,29]. Some scholars also focus on targeted therapies such as siRNA [30] and mTOR inhibitors [31]. In general, as a genetic disease that significantly reduces the quality of life of patients, it is best to avoid the disease from the source. With the development of molecular biology, genetic counseling, prenatal diagnosis, preimplantation genetic screening (PGS), and other gene-therapy methods can be used for PC patients before giving birth to avoid the occurrence of PC in their offspring.
4.1. Conclusion
In summary, we identified a novel heterozygous frameshift mutation (c.1460–2_1460del) in the KRT6A gene responsible for Pachyonychia congenita with fissured tongue in a Chinese girl. which not only expands our knowledge on KRT6A-related phenotype-genotype correlations, but also provides some preliminary genetic evidence that PC could associate with a rare fissured tongue phenotype. This study may broaden the KRT6A mutation spectrum and give insight into genetic counseling, genetic diagnosis, and gene-therapy for PC-k6a.
Funding
This research was supported by grants from the National Natural Science Foundation of China (NSFC 82173437), the Natural Science Foundation of Guangdong Province (No. 2020A15150875), and the Quanzhou City Science & Technology Program of China (Grant No.2019N026S).
Disclosure of conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
Human samples were obtained according to the principles of the Declaration of Helsinki. This study was approved by the Medical Ethics Committee of Nanfang Hospital of Southern Medical University (NFEC-2022-483). All participants agreed to publish the clinical data and provided written informed consent.
Data availability statement
The data that support the findings of this study are deposited into the NCBI database with the BioProject: PRJNA1039959. The data are also available upon reasonable request from the corresponding author, Changxing Li.
CRediT authorship contribution statement
Jiali Liang: Writing – original draft, Data curation. Ronghua Li: Formal analysis, Data curation. Chenmei Liu: Investigation. Yan Cai: Software. Yifei Liu: Data curation. Pingjiao Chen: Data curation. Kang Zeng: Writing – review & editing. Changxing Li: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank our colleagues and the family members of the patient who contributed to the samples used in this study. We thank the patient's parents for permission to post this information.
Contributor Information
Kang Zeng, Email: npfkzk@163.com.
Changxing Li, Email: lilichangxing@163.com.
List of abbreviations
- PC
Pachyonychia congenita
- KRT6A
Keratin 6a gene
- K6a
Keratin 6a
- MIM
Mendelian Inheritance in Man
- WES
Whole-exome sequencing
- IPCRR
International Pachyonychia Congenita Research Registry
- IFs
Intermediate Filaments
- ISCA
Inheritance State Consistency Analysis
- SNP
single nucleotide polymorphism
- Btx
botulinum toxin
References
- 1.Jadassohn J., Lewandowsky F. Pachyonychia congenita. Ikonographia dermatologica. 1906;1:29. [Google Scholar]
- 2.Feinstein A., Friedman J., Schewach-Millet M. Pachyonychia congenita. J. Am. Acad. Dermatol. 1988;19(4):705–711. doi: 10.1016/s0190-9622(88)70226-1. [DOI] [PubMed] [Google Scholar]
- 3.Lucker G.P., Steijlen P.M. Pachyonychia congenita tarda. Clin. Exp. Dermatol. 1995;20(3):226–229. doi: 10.1111/j.1365-2230.1995.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 4.Mouaci-Midoun N., Cambiaghi S., Abimelec P. Pachyonychia congenita tarda. J. Am. Acad. Dermatol. 1996;35(2 Pt 2):334–335. doi: 10.1016/s0190-9622(96)90663-5. [DOI] [PubMed] [Google Scholar]
- 5.Munro C.S., et al. A gene for pachyonychia congenita is closely linked to the keratin gene cluster on 17q12-q21. J. Med. Genet. 1994;31(9):675–678. doi: 10.1136/jmg.31.9.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden P.E., et al. Mutation of a type II keratin gene (K6a) in pachyonychia congenita. Nat. Genet. 1995;10(3):363–365. doi: 10.1038/ng0795-363. [DOI] [PubMed] [Google Scholar]
- 7.Smith F.J., et al. A mutation in human keratin K6b produces a phenocopy of the K17 disorder pachyonychia congenita type 2. Hum. Mol. Genet. 1998;7(7):1143–1148. doi: 10.1093/hmg/7.7.1143. [DOI] [PubMed] [Google Scholar]
- 8.Wilson N.J., et al. Keratin K6c mutations cause focal palmoplantar keratoderma. J. Invest. Dermatol. 2010;130(2):425–429. doi: 10.1038/jid.2009.215. [DOI] [PubMed] [Google Scholar]
- 9.McLean W.H., et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat. Genet. 1995;9(3):273–278. doi: 10.1038/ng0395-273. [DOI] [PubMed] [Google Scholar]
- 10.McLean W.H., et al. The phenotypic and molecular genetic features of pachyonychia congenita. J. Invest. Dermatol. 2011;131(5):1015–1017. doi: 10.1038/jid.2011.59. [DOI] [PubMed] [Google Scholar]
- 11.Wilson N.J., et al. A large mutational study in pachyonychia congenita. J. Invest. Dermatol. 2011;131(5):1018–1024. doi: 10.1038/jid.2011.20. [DOI] [PubMed] [Google Scholar]
- 12.Shah S., et al. Pachyonychia congenita in pediatric patients: natural history, features, and impact. JAMA Dermatol. 2014;150(2):146–153. doi: 10.1001/jamadermatol.2013.6448. [DOI] [PubMed] [Google Scholar]
- 13.Smith F. The molecular genetics of keratin disorders. Am. J. Clin. Dermatol. 2003;4(5):347–364. doi: 10.2165/00128071-200304050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ahn J., et al. Structural basis for lamin assembly at the molecular level. Nat. Commun. 2019;10(1):3757. doi: 10.1038/s41467-019-11684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob J.T., et al. Types I and II keratin intermediate filaments. Cold Spring Harbor Perspect. Biol. 2018;10(4) doi: 10.1101/cshperspect.a018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamcheu J.C., et al. Keratin gene mutations in disorders of human skin and its appendages. Arch. Biochem. Biophys. 2011;508(2):123–137. doi: 10.1016/j.abb.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu T.T., et al. Genotype‒structurotype‒phenotype correlations in patients with pachyonychia congenita. J. Invest. Dermatol. 2021;141(12):2876–2884.e4. doi: 10.1016/j.jid.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuelov L., et al. Revisiting pachyonychia congenita: a case-cohort study of 815 patients. Br. J. Dermatol. 2020;182(3):738–746. doi: 10.1111/bjd.18794. [DOI] [PubMed] [Google Scholar]
- 19.Pinna R., et al. Genetic and developmental disorders of the oral mucosa: epidemiology; molecular mechanisms; diagnostic criteria; management. Periodontol. 2000. 2019;80(1):12–27. doi: 10.1111/prd.12261. [DOI] [PubMed] [Google Scholar]
- 20.Picciani B.L.S., et al. Fissured tongue in patients with psoriasis. J. Am. Acad. Dermatol. 2018;78(2):413–414. doi: 10.1016/j.jaad.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Hallett J.W., Mitchell B. Melkersson-rosenthal syndrome. Am. J. Ophthalmol. 1968;65(4):542–544. doi: 10.1016/0002-9394(68)93870-1. [DOI] [PubMed] [Google Scholar]
- 22.Iraci S., et al. Pachyonychia congenita with late onset of nail dystrophy--a new clinical entity? Clin. Exp. Dermatol. 1993;18(5):478–480. doi: 10.1111/j.1365-2230.1993.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 23.Vijaikumar M., Thappa D.M., Laxmisha C. Pachyonychia congenita: a case report. Pediatr. Dermatol. 2001;18(6):541–543. doi: 10.1046/j.1525-1470.2001.1862011e.x. [DOI] [PubMed] [Google Scholar]
- 24.Du Z.F., et al. Two novel de novo mutations of KRT6A and KRT16 genes in two Chinese pachyonychia congenita pedigrees with fissured tongue or diffuse plantar keratoderma. Eur. J. Dermatol. 2012;22(4):476–480. doi: 10.1684/ejd.2012.1773. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H.L., et al. A novel missense mutation L468Q of keratin 6a in pachyonychia congenita type 1. J. Eur. Acad. Dermatol. Venereol. 2007;21(3):351–355. doi: 10.1111/j.1468-3083.2006.01930.x. [DOI] [PubMed] [Google Scholar]
- 26.Li Y., et al. A KRT6A mutation p.Ile462Asn in a Chinese family with pachyonychia congenita, and identification of maternal mosaicism: a case report. BMC Med. Genom. 2021;14(1):259. doi: 10.1186/s12920-021-01109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koren A., et al. A treatment protocol for botulinum toxin injections in the treatment of pachyonychia congenita-associated keratoderma. Br. J. Dermatol. 2020;182(3):671–677. doi: 10.1111/bjd.18169. [DOI] [PubMed] [Google Scholar]
- 28.Abdollahimajd F., et al. Pachyonychia congenita: a case report of a successful treatment with rosuvastatin in a patient with a KRT6A mutation. Br. J. Dermatol. 2019;181(3):584–586. doi: 10.1111/bjd.17276. [DOI] [PubMed] [Google Scholar]
- 29.Frommherz L., Has C. Successful treatment of pachyonychia congenita with rosuvastatin. J. Eur. Acad. Dermatol. Venereol. 2020;34(9):e480–e482. doi: 10.1111/jdv.16393. [DOI] [PubMed] [Google Scholar]
- 30.Smith F.J., et al. Development of therapeutic siRNAs for pachyonychia congenita. J. Invest. Dermatol. 2008;128(1):50–58. doi: 10.1038/sj.jid.5701040. [DOI] [PubMed] [Google Scholar]
- 31.Hickerson R.P., et al. Rapamycin selectively inhibits expression of an inducible keratin (K6a) in human keratinocytes and improves symptoms in pachyonychia congenita patients. J. Dermatol. Sci. 2009;56(2):82–88. doi: 10.1016/j.jdermsci.2009.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are deposited into the NCBI database with the BioProject: PRJNA1039959. The data are also available upon reasonable request from the corresponding author, Changxing Li.