Abstract
Background
Galactosemia was introduced into Taiwan's routine newborn screening (NBS) program in 1985. This study presents a 12-year experience, emphasizing disease diagnosis and screening performance.
Method
NBS for galactosemia utilized dried blood spot samples taken 48–72 h post-delivery, with total galactose (TGal) level as the primary marker. Newborns with critical TGal levels were referred immediately, while those with borderline TGal underwent a recall test. GALT activity measurement was applied simultaneously as the second-tier marker. Further confirmatory tests, such as whole exome sequencing (WES), were conducted upon referral.
Results
From January 1st, 2011, to December 31st, 2022, 51 cases were identified from 817,906 newborns. Of these, nine individuals had persistently elevated TGal. Diagnoses included one case of GALT deficiency, one of GALM deficiency, and seven of GALE deficiencies. Notably, the classic galactosemia patient (GALT deficiency) presented with extreme high TGal and was referred to the hospital for diet management immediately. All affected patients were instructed to adopt a galactose-restricted diet. By the median age of 2.5 years, all exhibited normal development and liver function.
Conclusion
The incidence of classical galactosemia and its variants is extremely low in Taiwan. Incorporating WES into NBS has improved our ability to detect various galactosemia forms, enriching our understanding of the genetic underpinnings. While these newly discovered forms often present with milder initial elevations in TGal, specific biochemical investigations and regular monitoring are essential to understanding the long-term implications and outcomes.
Keywords: Galactosemia, GALE deficiency, Epimerase deficiency galactosemia, Newborn screening, Whole exome sequencing
1. Introduction
Galactosemia is an inherited disorder affecting the carbohydrate metabolism of galactose. It is categorized into four distinct types based on the enzymatic deficiencies found in the Leloir pathway: classic galactosemia (galactose-1-phosphate uridylyltransferase deficiency, GALT deficiency, OMIM #230400), galactokinase deficiency (GALK deficiency, also known as type II galactosemia, OMIM #230200), UDP-galactose-4′-epimerase deficiency (GALE deficiency, type III galactosemia, OMIM #230350), and galactose mutarotase deficiency (GALM deficiency, type IV galactosemia, OMIM #618881) [1,2].
While newborns with classic galactosemia often present as asymptomatic at birth, they rapidly develop symptoms upon consuming breast milk or formula milk [3]. The primary therapeutic strategy for classic galactosemia is a restricted diet that excludes lactose and galactose [4]. Such dietary interventions can potentially avert or reverse specific complications, such as hepatic failure or sepsis. This highlights the importance of newborn screening (NBS) for galactosemia [5,6].
Various screening methodologies exist today, each adapted based on primary screening objectives and their efficacy. Utilizing total galactose concentration (TGal) as the sole primary marker has resulted in a significant number of false positives. This led Dutch NBS programs to adopt GALT activity as the first tier of testing instead and to use TGal as the second-tier marker [7]. In the United States, most programs utilize GALT activity in dried blood spots (DBS) as the primary marker [5], either alone or in conjunction with other supplementary markers. These additional markers either serve as secondary tier indicators or as a combined panel to screen for all types of galactosemia [8].
Taiwan introduced routine screening for galactosemia in its NBS program in 1985 [9]. Although many countries utilize GALT assays, Taiwan focuses on TGal as its primary marker. The preference is influenced by the challenges associated with GALT measurement, which can be impacted by external factors such as temperature, humidity, and the prevalent incidence of G6PD deficiency in Taiwan [10]. Furthermore, elevated TGal levels can also be observed in newborns with citrin deficiency, an important screening target in this region [11]. Nonetheless, since 2013, the GALT assay has been incorporated as a second-tier test, and whole exome sequencing (WES) was introduced as a molecular diagnostic test in 2018. These additions have enhanced our diagnostic accuracy and accelerated clinical decision-making. This study demonstrates the development process for a more effective galactosemia NBS strategy and evaluates the efficacy of early therapeutic interventions.
2. Method
2.1. Newborn screening
Screening for galactosemia was conducted using DBS samples taken 48–72 h post-delivery. The National Taiwan University Hospital Newborn Screening Center (NTUH-NBSC) handled the screening for approximately 35–37% of newborns in Taiwan [12]. The DBS TGal concentration was the primary marker, which was evaluated using the R&D's GALMMR2000 Screening Kit (R&D Diagnostics Ltd., Greece) before 2013, the Neonatal Total Galactose kit (Perkin Elmer, Turku, Finland) from 2013 to 2020, and the GSP® Neonatal Total Galactose assay (Perkin Elmer, Turku, Finland) from 2020 onwards. Although the government mandates the inclusion of galactosemia in screening, it has not specifically delineated primary from secondary markers. However, the screening program conforms to the quality assurance standards of the U.S. Centers for Disease Control and Prevention (CDC) and the Japanese newborn screening system.
For the cutoff values, we employed a dual-tier approach. Based on our experience and prior studies [13] and given the exceedingly low incidence of GALT deficiency, the critical cutoff was set at the 99.99th percentile (equivalent to 30 μmol/L) of the normal population to identify cases for immediate referral. Alternatively, newborns hitting the borderline cutoff (99.95th percentile, equal to 15 μmol/L with the Neonatal Total Galactose kit or 18 μmol/L with the GSP® Neonatal Total Galactose assay) needed a second abnormal sample result before being referred. These screened positive cases were directed to designated hospitals for confirmatory testing. Simultaneously, a second-tier GALT enzyme measurement was performed on the DBS of patients with elevated TGal using the Neonatal GALT kit by PerkinElmer (Turku, Finland) after 2013.
2.2. Study population
This study included newborns screened at NTUH-NBSC between January 1st, 2011, and December 31st, 2022, who subsequently required confirmatory testing due to abnormal TGal results. The newborns who had passed away or whose parents declined confirmatory testing were excluded from the study. The retrospective analysis was performed on a diverse set of data, including medical records that included birth history, clinical presentation, physical observations, and biochemical assessments such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T. bil), direct bilirubin (D. bil), TGal, and ammonia levels, molecular genetic investigations, final diagnoses, dietary recommendation, and follow-up conditions. The need for informed consent was waived, and the study received approval from the institutional review board of the National Taiwan University Hospital (NTUH-IRB; No. 202302105RIN).
2.3. Confirmatory testing
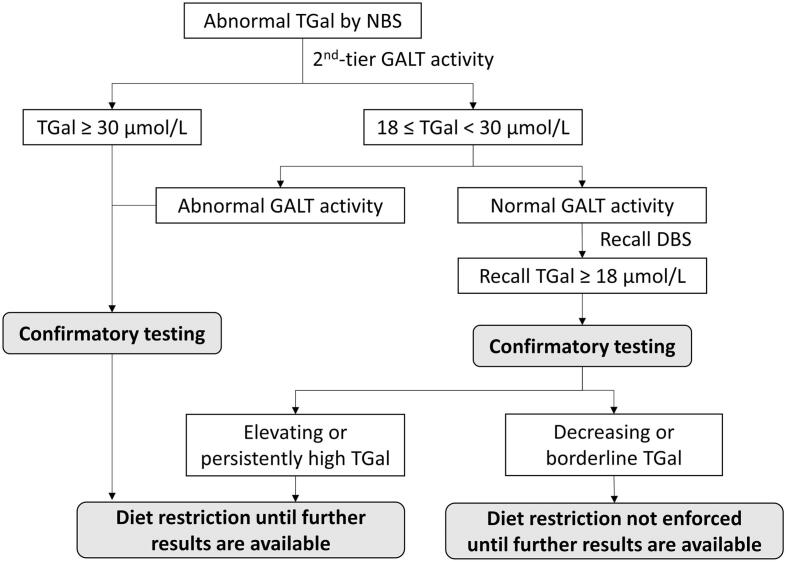
Once referred, newborns underwent a comprehensive physical examination and subsequent biochemical assessments focusing on liver function, complemented by a repeat TGal analysis. Red blood cell GALT activity was measured for those born before 2013, or in cases where the second-tier GALT test could not be obtained. Newborns with rising galactose levels or levels surpassing the critical cutoff were recommended to undergo additional assessments, including GALT, GALE, and GALK gene sequencing. This could involve separate tests in the past or WES more recently. The molecular genetic diagnosis of galactosemia was made by identifying variants categorized as likely pathogenic or pathogenic, according to the ACMG classification [14]. It's important to note that analyzing biochemical markers, such as erythrocyte galactose-1-phosphate (Gal-1-P), plasma-free galactose, and urine galactitol, necessitated specialized sample handling and analysis outsourcing to overseas laboratories; therefore, these markers were not analyzed in our participants. Post-referral, our protocol mandates immediate dietary intervention for newborns with initial TGal levels above critical cutoffs, persistent or increasing TGal upon re-screening, and abnormal GALT enzyme activity. This ensures swift action for those at high risk, such as infants with classic galactosemia. For newborns with borderline or decreasing TGal levels, we proceed more cautiously to avoid unnecessary dietary restrictions and the associated stress (Fig. 1).
Fig. 1.
Diagnostic Flowchart for Galactosemia Screening and Management.
Caption: The flowchart represents the decision-making process for the screening and subsequent management of galactosemia based on total galactose (TGal) levels measured by newborn screening (NBS) and galactose-1-phosphate uridyltransferase (GALT) enzyme activity assays. Extremely elevated TGal levels (over 99.99th percentile, approximately equal to 30 μmol/L) or abnormal GALT activity prompts immediate confirmatory testing, while TGal levels between 99.95th percentile (i.e. 18 when using the GSP® Neonatal Total Galactose assay) and 99.99th percentile (approximately 30 μmol/L) alone require further evaluation of GALT activity. Based on GALT activity and TGal levels, dietary restrictions may be implemented and adjustments made as additional test results become available.
2.4. Statistical analysis
Data were collected and tabulated using Microsoft Excel. We recorded the incidence rates of the different galactosemia subtypes. The Clopper-Pearson method, known as the exact interval, was used to calculate the 95% confidence intervals. This method is particularly appropriate for our analysis because it can handle situations with small sample sizes and rare events. Analyses were conducted using SPSS (version 25.0; IBM Corp, Armonk, NY). A two-tailed p-value below 0.05 was deemed statistically significant. Continuous variable group differences were tested using the Mann–Whitney U test, while Fisher's exact test was applied for categorical variables.
3. Result
From January 1st, 2011, to December 31st, 2022, we identified 51 cases from 817,906 screened newborns. During the study period, no individual with elevated TGal refused confirmatory testing or passed away before their referral visits. Nine displayed persistently elevated galactose levels, with one diagnosed with GALT, one with GALM, and seven with GALE deficiencies (Table 1). The remaining 42 were eventually categorized as transient hypergalactosemia, as they demonstrated normal GALT activity and TGal at the following measurements. No further molecular tests were arranged. Confirmatory testing was performed at a mean age of 16.62 days (range: 6–30 days). It is important to note that while milder forms of galactosemia (e.g., GALE, GALK, or GALM deficiencies) may present with lower TGal levels, there has been no reported cases of false negatives back to NTUH-NBSC. No cases of missed citrin deficiency were reported in subjects categorized with transient hypergalactosemia at NTUH-NBSC during the study period. Additionally, hypergalactosemia was included as an indication for testing citrin deficiency [15] using a second-tier algorithm. Since initiating this testing in 2018, no cases of citrin deficiency associated with hypergalactosemia have been identified.
Table 1.
Genotypic and Phenotypic Variability in Patients with Different Types of Galactosemia:
| No | Gene | Variant 1 | Variant 2 | GALT (U/g Hb) | Sex | BBW (gm) | Initial DBS |
2nd DBS |
Baseline biochemical results |
Age of Tx (Days) | TGal post Tx (μmol/L) | Current age (y) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (day) | Gal (μmol/L) | Age (day) | Gal (μmol/L) | Age (day) | AST (U/L) | ALT (U/L) | T.bil (mg/dL) | D.bil (mg/dL) | ||||||||||
| 1 | GALT | c.1034C > T (p.Ala345Asp) | c.1034C > T (p.Ala345Asp) | 2.6 | F | 3400 | 2 | 86.2 | 7 | 111.7 | 7 | 253 | 162 | 14.5 | 1.7 | 7 | 2.0 | 3.2 |
| 2 | GALE | c.505C > T (p.Arg169Trp) | c.923 A > G (p.Try308Cys) | 9.3 | M | 3410 | 2 | 26.3 | 6 | 31.6 | 10 | 31 | 15 | 10.76 | 0.94 | 8 | 4.1 | 6.8 |
| 3 | GALE | c.925G > A (p.Ala309Thr) | c.528 + 1G > A | 8.3 | M | 2740 | 2 | 20.5 | 9 | 49.5 | 18 | 29 | 20 | 1.83 | 0.76 | 5 | 2.7 | 6.5 |
| 4 | GALE | c.505C > T (p.Arg169Trp) | c.59 T > G (p.Leu20Arg) | 6.5 | F | 3200 | 2 | 37.8 | 8 | 29.52 | 8 | 38 | 11 | 8.1 | 0.7 | 13 | 6.5 | 3.4 |
| 5 | GALE | c.923 A > G (p.Tyr308Cys) | c.520G > C (p.Ala174Pro) | 8.7 | F | 2700 | 3 | 30.6 | 5 | 32.7 | 9 | 27 | 21 | 8.5 | 0.7 | 9 | 4.4 | 2.5 |
| 6 | GALE | c.923 A > G (p.Try308Cys) | c.923 A > G (p.Try308Cys) | 7.2 | F | 3520 | 2 | 23.6 | 10 | 27.4 | 13 | 32 | 17 | 5.4 | 0.7 | 7 | 2.7 | 2.3 |
| 7 | GALE | c.923 A > G (p.Try308Cys) | c.925G > A (p.Ala309Thr) | 8.6 | M | 3180 | 3 | 33.3 | 9 | 26.7 | 9 | 29 | 15 | 3.4 | 0.5 | 12 | 3.1 | 0.9 |
| 8 | GALE | c.505C > T (p.Arg169Trp) | c.528 + 1G > A | 7.9 | F | 3420 | 3 | 44.1 | 6 | 36.4 | 6 | 25 | 6 | 11.6 | 1.1 | 16 | 7.1 | 0.8 |
| 9 | GALM | c.325G > A (p.Gly109Arg) | c.587 T > C (p.Ile196Thr) | 6.0 | M | 3220 | 3 | 17.57 | 9 | 34.1 | 15 | 27 | 15 | 6.8 | 0.7 | 15 | 1.4 | 3.1 |
BBW: birth body weight, NBS: newborn screening, TGal: total galactose concentration, Tx: treatment, AST: Aspartate aminotransferase, ALT: Alanine transaminase, T.bil: total bilirubin, D.bil: direct bilirubin, F: female, M: male, y: year, m: month.
Variants with ClinVar reported as pathogenic/likely pathogenic were shown in bold fonts.
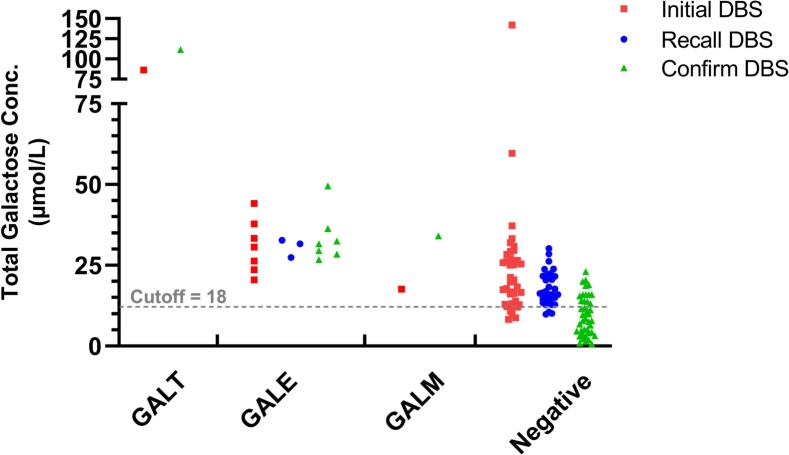
Compared to those with transient hypergalactosemia, patients diagnosed with galactosemia underwent confirmatory testing at earlier ages (16.62 vs. 11.11 days, p = 0.012) and hence started diet restriction sooner. They also received their second DBS earlier (p = 0.049) (Table 2). Notably, persistently and significantly higher TGal concentrations in the initial (35.55 vs. 24.09 μmol/L, p = 0.012) and second DBS (42.17 vs. 15.46 μmol/L, p = 0.0001) were observed in galactosemia patients. Conversely, most transient cases displayed a decreased TGal concentration in consecutive DBS results (Fig. 2). There was no significant difference in sex, gestational age (GA), birth body weight (BBW), or the age at which the first DBS was obtained between the two groups.
Table 2.
Comparative Analysis of Galactosemia Subtypes and Transient Hypergalactosemia.
| Galactosemia |
Transient (n = 42) |
p-value | ||||
|---|---|---|---|---|---|---|
| GALT def (n = 1) |
GALE def (n = 7) |
GALM def (n = 1) |
Total (n = 9) |
|||
| Sex (M) | 0 (0%) | 5 (71.4%) | 1 (100%) | 6 (66.6%) | 21 (50%) | 0.473 |
| GA (weeks) | 39.6 | 38.7 ± 1.2 | 37.7 | 38.7 ± 1.2 | 38.8 ± 1.0 | 0.584 |
| BBW (gm) | 3400 | 3167.14 ± 328.97 | 3220 | 3198.89 ± 295.23 | 2951.07 ± 391.40 | 0.059 |
| Age of confirmation (days) | 7 | 11.14 ± 4.1 | 15 | 11.11 ± 4.08 | 16.62 ± 6.28 | 0.012 |
| Initial DBS | ||||||
| Age (days) | 2 | 2.43 ± 0.53 | 3 | 2.44 ± 0.53 | 2.98 ± 1.81 | 0.145 |
| TGal (μmol/L) | 86.2 | 30.88 ± 8.28 | 17.6 | 35.55 ± 20.77 | 24.09 ± 20.95 | 0.012 |
| 2nd DBS | ||||||
| Age (days) | 7 | 7.57 ± 1.9 | 9 | 7.67 ± 1.73 | 10.36 ± 4.25 | 0.049 |
| TGal (μmol/L) | 111.7 | 33.39 ± 7.82 | 34.1 | 42.17 ± 26.94 | 15.46 ± 7.28 | <0.001 |
M: male, GA: gestational age, BBW: birth body weight, DBS: dried blood spot, TGal: Total galactose concentration, def: deficiency.
Fig. 2.
Variations in Total Galactose Concentration Among Galactosemia Subtypes and Transient Hypergalactosemia.
Caption: The scatter plot illustrates the total galactose concentration (y-axis) for patients across four distinct groups: GALT, GALE, GALM, and transient hypergalactosemia (Negative). Red squares represent the initial dried blood spot (DBS) galactose levels, the retest DBS by blue circles, and the confirmatory DBS by green triangles, where applicable.
Overall, GALE deficiency had the highest incidence among all galactosemia types. The incidences of GALT deficiency, GALE deficiency, and GALM deficiency detected through NBS were 1 in 817,906 (95% confidence interval (CI) 32,305,446 to 146,798), 1 in 116,844 (95%CI: 290,618 to 56,710), and 1 in 817,906 (95%CI: 32,305,446 to 146,798), respectively. The nine diagnosed galactosemia patients were all identified between 2016 and 2022, with the longest follow-up spanning 7 years. Over the past 12 years, no false-negative cases were reported. The overall sensitivity of our screening was 100% (66.4%–100%), specificity was 99.99%, and the positive predictive value (PPV) was 16.3%.
The newborn with GALT deficiency displayed the highest initial TGal at 111.7 μmol/L on day 7. Partial impairment of GALT activity was found in this individual (2.6 U/g Hb, with the normal threshold being >3.5 U/g Hb). Although potentially categorized as Duarte galactosemia (DG) [16], jaundice was observed, and initial liver function tests were abnormal. Consequently, dietary restrictions were initiated on day 7. Jaundice was later resolved, and the liver function test normalized. At 3 years and 3 months old, the patient demonstrated normal development without clinical manifestations such as vomiting, hypoglycemia, bleeding tendency, sepsis, or hepatomegaly. This patient possesses a homozygous GALT c.1034C > T (p.Ala345Asp) variant, classified as uncertain significance (VUS) in ClinVar [17].
Among the seven patients with GALE deficiency, initial TGal ranged between 23.6 and 44.1 μmol/L, and second TGal between 26.7 and 49.5 μmol/L. Based on these findings, a dietary intervention was recommended, involving a galactose-free formula, which commenced at a median age of 9 days (range: 5–16 days). There were no reported icterus, hypoglycemia, or abnormal liver function tests. However, no ophthalmologic consultation was conducted due to the unavailability of ophthalmologists for pre-dietary change cataract assessments. Currently, with a median age of 2 years and 6 months old, all patients have reported normal development, no evidence of liver cirrhosis on abdominal sonography, or growth retardation. The most frequently observed GALE variant was c.923 A > G (p.Tyr308Cys), seen in 5 of the 14 alleles (35.7%), followed by c.505C > T (p.Arg169Trp) (21.4%). ClinVar lists the GALE c.923 A > G variant as VUS, while c.505C > T is reported as likely pathogenic [[17], [18], [19]]. Two variants, GALE c.925G > A (p.Ala309Thr) and c.528 + 1G > A, account for 14.3% each. ClinVar identifies the c.925G > A variant as VUS [17,20]. The c.528 + 1G > A variant was predicted by both MaxEntScan and SpliceAI to result in a donor splicing gain.
Additionally, the first known case of GALM deficiency in Taiwan featured two novel variants: GALM c.325G > A (p.Gly109Arg) and c.587 T > C (p.Ile196Thr). Adhering to dietary recommendations, this patient consumes a 75% galactose-free formula, maintaining normal galactose levels. No other associated symptoms or signs have been reported.
4. Discussion
This 12-year study presents the results of newborn galactosemia screening in Taiwan. Consistent with previous observations [21], we reaffirmed the infrequency of GALT deficiency in our population. We also identified seven patients with GALE deficiency and, notably, the first reported case of GALM deficiency in Taiwan. However, the precise severity of these conditions still needs to be discovered. Despite concerted efforts to enhance the screening program's efficacy, we continue to face the challenge of low PPV.
Our research highlights the varied incidences of different galactosemia forms [22,23]. While the global incidence of classic galactosemia is between 1:40,000 and 1:60,000, it's significantly rarer in the Asian population at approximately 1 in 1,000,000 live births [3,24,25]. The less severe form, DG, has a prevalence of roughly 1 in 4000 in the United States [26,27]. Our data only documented a singular case of GALT deficiency, with enzyme activity levels suggestive of DG, emphasizing its rarity in our cohort. GALK deficiency is most prevalent among the Roma populations of Bulgaria and Bosnia [28]. Among our cohort of approximately 800,000 newborns, we did not identify any cases of GALK deficiency. While GALE deficiency is more common among African Americans, with an incidence of around 1:7000 [26], it remains uncommon in our cohort. However, our observed incidence (1 in 116,844) is higher than that reported in China's Zhejiang Province (1 in 350,000) [19]. GALM deficiency, recently recognized as a variant of galactosemia with an estimated prevalence of 1 in 80,747 in the Japanese population [29], also showed a low incidence in our cohort. Given the overall low prevalence of all galactosemia types in Taiwan, molecular test covering all genetic etiologies that lead to abnormal galactose levels, is necessary for the confirmatory tests for babies with hypergalactosemia, alongside GALT activity or Gal-1-P analysis.
The patient with GALT deficiency, likely presenting with a clinical variant galactosemia with homozygous hypomorphic GALT variants, displayed abnormal liver function tests before diet control. Notably, this patient did not show the hypoglycemia or bleeding tendencies sometimes observed in classic galactosemia patients. The patient's GALT variant c.1034C > T has been identified in a patient presenting with classic galactosemia [30], and a Duarte variant galactosemia patient in conjunction with a Duarte-2 allele [31]. To the best of our understanding, the patient was from a non-consanguineous family. The allele frequency of GALT c.1034C > T (p.Ala345Asp) recorded in Taiwan biobank is 0.0007, which is higher than in other populations (allele frequency 0.00009693 by gnomAD East Asia, and 0 in all other genetic ancestry groups on gnomAD) [32,33]. However, further observation or larger scale database is still warranted. Due to abnormal lab data and uncertain genotype-phenotype correlations, coupled with the absence of targeted biochemical evaluations such as erythrocyte Gal-1-P measurements, we initiated dietary restrictions after discussing with the parents. Remarkably, the patient's liver function abnormalities resolved swiftly after treatment, and the potential future discontinuation of dietary restrictions will be deliberated.
Our program's identification of several GALE deficiency cases can be attributed to the common variants GALE c.923 A > G (p.Tyr308Cys) and c.505C > T (p.Arg169Trp). According to the Taiwan Biobank, both c.923 A > G and c.505C > T are rare variants with allele frequencies of 0.002, and 0.001, respectively [32]. Both have been previously linked with GALE deficiency. The c.505C > T variant has been detected in a compound heterozygous state in patients with peripheral/mild GALE deficiency [18,19]. While our assessment suggests that our patients are likely to have the peripheral form of GALE deficiency [34,35], there is insufficient data to justify recommending a normal diet for affected newborns. These patients initially showed no symptoms and had no systemic or developmental abnormalities. A deeper and more comprehensive understanding of the genotype-phenotype relationship in GALE deficiency is necessary and requires further investigation.
As for GALM deficiency, our understanding is still in its infancy. Given the challenge of measuring mutarotase activity, we recommend a diet that maintains normal galactose levels in the patient. Whether dietary restrictions are necessary and the potential for future systemic issues in GALM deficiency patients remain topics of inquiry. We plan to conduct annual multi-system evaluations to monitor potential long-term effects. Nonetheless, given the small patient number and varied screening protocols, broader and more extended collaborative research is essential.
The suboptimal PPV of the TGal screening method is well-recognized, given that galactose levels may be influenced by a range of etiologies beyond disorders of galactose metabolism [5]. False-positive results can be seen in patients with liver diseases, extrahepatic portosystemic shunts, citrin deficiency, Fanconi–Bickel disease, neonatal diabetes mellitus, among others [11,[36], [37], [38], [39]]. Multiple strategies have been suggested to address this problem. One such strategy, as implemented in our program, is monitoring TGal changes over time. Typically, patients with classic galactosemia or GALK deficiency exhibit extreme elevation of TGal levels, signaling the urgency for prompt specialist referral to evaluate the clinical status of the newborn. On the other hand, most individuals with transient hypergalactosemia exhibit a decline in TGal levels in subsequent tests. Re-evaluating borderline cases might differentiate between transient hypergalactosemia and genuine galactosemia cases, minimizing unnecessary interventions. Increasing the TGal cutoff values could reduce false positives without the risk of increasing false negatives for newborns who are under regular diet. However, For babies who are not drinking milk, such as those using soybean-based formula or those under total parenteral nutrition, using TGal as the primary screening marker may underdiagnose galactosemia patients [5]. Therefore, we routinely re-screen cases after fully oral feeding to decrease the possibility of false negative. Alternatively, assessing markers such as Gal-1-P [40,41] instead of TGal could be advantageous in accurately detecting elevated Gal-1-P levels in newborns. However, a caveat exists: patients with GALK deficiency may present with normal Gal-1-P levels or TGal and might be overlooked in such screenings. A combined evaluation of GALT enzyme activity and TGal may be the optimal two-tier screening for GALT deficiency. Considering the diverse array of screening methods, NBS programs across countries should tailor these strategies according to their national screening policies and the prevalence of galactosemia in their region.
The value of newborn galactosemia screening is debated due to the uncertain long-term benefits of dietary modifications for both classic galactosemia and certain variants of galactosemia. Although NBS for galactosemia has been widely embraced, there are still NBS programs that do not include galactosemia as their routine conditions [6]. Our study emphasizes additional concerns regarding galactosemia NBS, including the extremely rare incidence of the disorder and uncertainties surrounding treatment. While WES/WGS can detect all types of galactosemia, the risk-benefit balance and the cost-effectiveness still need to be determined. Currently, we craft personalized treatment plans and monitoring to prevent potential issues arising from elevated blood galactose levels while avoiding unnecessary stringent galactose restrictions.
There are several limitations in this retrospective study. While our data spans the past 12 years, all identified patients were found after 2016, limiting the maximum follow-up to 7 years. This limited observation timeframe constrains our ability to provide comprehensive insights into long-term outcomes and potential complications. Additionally, the absence of specialized biochemical testing and gene panels might have influenced the incidence rate calculations. Still, all transient hypergalactosemia newborns had normal follow-up blood galactose levels, and no false negatives were noted during the 12 years. Lastly, being a single-center study, our data could have regional biases. Nevertheless, our sample represents over a third of Taiwan's newborns from various regions over 12 years. Furthermore, as parents can freely move and select places for delivery, our cohort should reasonably represent Taiwan's population.
In conclusion, both classical and variant forms of galactosemia are exceedingly rare in our population. Incorporating WES into NBS has enhanced our capability to identify the various forms of galactosemia, improving our understanding of the disease's genetic complexities. Although these recently discovered forms often manifest with milder initial elevations of TGal levels, more specific biochemical investigations and long-term monitoring are paramount to comprehending these variants' long-term implications and outcomes. Screening through a straightforward total galactose assay offers a chance for newborns to avoid severe liver failure. Expanding our collective knowledge of these individuals with rare genetic variants, including the research for tailored treatments where required, will be foundational in the emerging genomic screening era.
CRediT authorship contribution statement
Hui-An Chen: Formal analysis, Writing – original draft. Rai-Hseng Hsu: Validation. Li-Chu Chen: Methodology. Ni-Chung Lee: Data curation. Pao-Chin Chiu: Data curation. Wuh-Liang Hwu: Conceptualization, Resources. Yin-Hsiu Chien: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
None.
Acknowledgments
The authors would like to thank all the members and collaborators of NTUH Newborn Screening Center for their assistance, and the Health Promotion Administration, Ministry of Health and Welfare, Taiwan, for supervising, funding, and coordinating the newborn screening program.
Data availability
Data will be made available on request.
References
- 1.Timson D.J. The molecular basis of galactosemia - past, present and future. Gene. Sep 10 2016;589(2):133–141. doi: 10.1016/j.gene.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 2.Wada Y., et al. Biallelic GALM pathogenic variants cause a novel type of galactosemia. Genet. Med. Jun 2019;21(6):1286–1294. doi: 10.1038/s41436-018-0340-x. [DOI] [PubMed] [Google Scholar]
- 3.Cerone J., Rios A. Galactosemia. Pediatr. Rev. Oct 2019;40(Suppl. 1):24–27. doi: 10.1542/pir.2018-0150. [DOI] [PubMed] [Google Scholar]
- 4.Welling L., et al. International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. J. Inherit. Metab. Dis. Mar 2017;40(2):171–176. doi: 10.1007/s10545-016-9990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquali M., Yu C., Coffee B. Laboratory diagnosis of galactosemia: a technical standard and guideline of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. Jan 2018;20(1):3–11. doi: 10.1038/gim.2017.172. [DOI] [PubMed] [Google Scholar]
- 6.Loeber J.G., et al. Neonatal screening in Europe revisited: an ISNS perspective on the current state and developments since 2010. Int. J. Neonatal Screen. 2021;7(1):15. doi: 10.3390/ijns7010015. https://www.mdpi.com/2409-515X/7/1/15 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welling L., et al. Nine years of newborn screening for classical galactosemia in the Netherlands: effectiveness of screening methods, and identification of patients with previously unreported phenotypes. Mol. Genet. Metab. Mar 2017;120(3):223–228. doi: 10.1016/j.ymgme.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Hong X., He M. Newborn screening for classic galactosemia: biochemical testings, challenges, and future. OBM Genet. 2022;06(03):161. 10.21926/obm.genet.2203161 2022/08/02. [Google Scholar]
- 9.Chien Y.H., Hwu W.L., Lee N.C. Newborn screening: Taiwanese experience. Ann. Transl. Med. Jul 2019;7(13):281. doi: 10.21037/atm.2019.05.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuhrman G., Perez Juanazo S.J., Crivelly K., Smith J., Andersson H., Morava E. False-positive newborn screen using the Beutler spot assay for galactosemia in glucose-6-phosphate dehydrogenase deficiency. JIMD Rep. 2017;36:1–5. doi: 10.1007/8904_2016_34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito E., et al. Type II citrullinaemia (citrin deficiency) in a neonate with hypergalactosaemia detected by mass screening. J. Inherit. Metab. Dis. Feb 2002;25(1):71–76. doi: 10.1023/a:1015198103395. [DOI] [PubMed] [Google Scholar]
- 12.Huang H.P., et al. Tandem mass neonatal screening in Taiwan--report from one center. J. Formos. Med. Assoc. Nov 2006;105(11):882–886. doi: 10.1016/S0929-6646(09)60173-X. [DOI] [PubMed] [Google Scholar]
- 13.Cheung K.L., et al. Classical galactosaemia in Chinese: a case report and review of disease incidence. J. Paediatr. Child Health. Aug 1999;35(4):399–400. doi: 10.1046/j.1440-1754.1999.00373.x. https://www.ncbi.nlm.nih.gov/pubmed/10457302 [Online]. Available: [DOI] [PubMed] [Google Scholar]
- 14.Richards S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. May 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H.A., et al. Improved diagnosis of citrin deficiency by newborn screening using a molecular second-tier test. Mol. Genet. Metab. Aug 2022;136(4):330–336. doi: 10.1016/j.ymgme.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Berry G.T. 2021. Classic Galactosemia and Clinical Variant Galactosemia. [PubMed] [Google Scholar]
- 17.Landrum M.J., et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. Jan 4 2018;46(D1):D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H.D., et al. The molecular basis of UDP-galactose-4-epimerase (GALE) deficiency galactosemia in Korean patients. Genet. Med. Nov-Dec 2005;7(9):646–649. doi: 10.1097/01.gim.0000194023.27802.2d. [DOI] [PubMed] [Google Scholar]
- 19.Tong F., Yang R., Hong F., Qian G., Jiang P., Gao R. A first case report of UDP-galactose-4′-epimerase deficiency in China: genotype and phenotype. J. Pediatr. Endocrinol. Metab. Mar 2016;29(3):379–383. doi: 10.1515/jpem-2014-0462. [DOI] [PubMed] [Google Scholar]
- 20.Nykamp K., et al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet. Med. Oct 2017;19(10):1105–1117. doi: 10.1038/gim.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung K.L., et al. Classical galactosaemia in Chinese: a case report and review of disease incidence. J. Paediatr. Child Health. Aug 1999;35(4):399–400. doi: 10.1046/j.1440-1754.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 22.Badiu Tisa I., Achim A.C., Cozma-Petrut A. The importance of neonatal screening for galactosemia. Nutrients. Dec 20 2022;15, no. 1 doi: 10.3390/nu15010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotb M.A., Mansour L., Shamma R.A. Screening for galactosemia: is there a place for it? Int. J. Gen. Med. 2019;12:193–205. doi: 10.2147/IJGM.S180706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M., West C., Beutler E. Large-scale molecular screening for galactosemia alleles in a pan-ethnic population. Hum. Genet. Aug 2001;109(2):210–215. doi: 10.1007/s004390100552. [DOI] [PubMed] [Google Scholar]
- 25.Porta F., Pagliardini S., Pagliardini V., Ponzone A., Spada M. Newborn screening for galactosemia: a 30-year single center experience. World J. Pediatr. May 2015;11(2):160–164. doi: 10.1007/s12519-015-0017-3. [DOI] [PubMed] [Google Scholar]
- 26.Pyhtila B.M., Shaw K.A., Neumann S.E., Fridovich-Keil J.L. Newborn screening for galactosemia in the United States: looking back, looking around, and looking ahead. JIMD Rep. 2015;15:79–93. doi: 10.1007/8904_2014_302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridovich-Keil J.L., Gambello M.J., Singh R.H., Sharer J.D. In: GeneReviews((R)) Adam M.P., et al., editors. 1993. Duarte variant galactosemia. Seattle (WA) [Google Scholar]
- 28.Bosch A.M., Bakker H.D., van Gennip A.H., van Kempen J.V., Wanders R.J., Wijburg F.A. Clinical features of galactokinase deficiency: a review of the literature. J. Inherit. Metab. Dis. Dec 2002;25(8):629–634. doi: 10.1023/a:1022875629436. [DOI] [PubMed] [Google Scholar]
- 29.Iwasawa S., et al. The prevalence of GALM mutations that cause galactosemia: a database of functionally evaluated variants. Mol. Genet. Metab. Apr 2019;126(4):362–367. doi: 10.1016/j.ymgme.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Estrada S.C., Canson D.M., Silao C.L. Mutational analysis of the GALT gene in Filipino patients. Kobe J. Med. Sci. Aug 9 2013;59(3):E106–E111. https://www.ncbi.nlm.nih.gov/pubmed/24045215 [Online]. Available: [PubMed] [Google Scholar]
- 31.Yang Y.P., Corley N., Garcia-Heras J. Molecular analysis in newborns from Texas affected with galactosemia. Hum. Mutat. Jan 2002;19(1):82–83. doi: 10.1002/humu.9005. [DOI] [PubMed] [Google Scholar]
- 32.Wei C.Y., et al. Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom. Med. Feb 11 2021;6(1):10. doi: 10.1038/s41525-021-00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karczewski K.J., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. May 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgartner M.R., Saudubray J., Walter J. Springer; 2016. Inborn Metabolic Diseases: Diagnosis and Treatment. [Google Scholar]
- 35.Fridovich-Keil J., Bean L., He M., Schroer R. 2016. Epimerase Deficiency Galactosemia. [Google Scholar]
- 36.Tygstrup N. Assessment of liver function: principles and practice. J. Gastroenterol. Hepatol. Jul-Aug 1990;5(4):468–482. doi: 10.1111/j.1440-1746.1990.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 37.Ponziani F.R., et al. Congenital extrahepatic portosystemic shunt: description of four cases and review of the literature. J. Ultrasound. Sep 2019;22(3):349–358. doi: 10.1007/s40477-018-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kentrup H., Altmuller J., Pfaffle R., Heimann G. Neonatal diabetes mellitus with hypergalactosemia. Eur. J. Endocrinol. Oct 1999;141(4):379–381. doi: 10.1530/eje.0.1410379. [DOI] [PubMed] [Google Scholar]
- 39.Peduto A., Spada M., Alluto A., La Dolcetta M., Ponzone A., Santer R. A novel mutation in the GLUT2 gene in a patient with Fanconi-Bickel syndrome detected by neonatal screening for galactosaemia. J. Inherit. Metab. Dis. 2004;27(2):279–280. doi: 10.1023/b:boli.0000028841.00833.f4. [DOI] [PubMed] [Google Scholar]
- 40.Daas S., et al. Addition of galactose-1-phosphate measurement enhances newborn screening for classical galactosemia. J. Inherit. Metab. Dis. Mar 2023;46(2):232–242. doi: 10.1002/jimd.12580. [DOI] [PubMed] [Google Scholar]
- 41.Cohen A.S., Baurek M., Lund A.M., Duno M., Hougaard D.M. Including classical galactosaemia in the expanded newborn screening panel using tandem mass spectrometry for galactose-1-phosphate. Int. J. Neonatal Screen. Jun 2019;5(2):19. doi: 10.3390/ijns5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.