Abstract
Autoimmune diseases (ADs) are one of the groups of chronic illnesses that impose a significant burden of disease and health costs worldwide. Age is a crucial risk factor for the onset of ADs. Theoretically, it is inferred that with organic and immune system aging, the loss of immune tolerance and specificity of immune activity becomes more intense, the probability of autoimmunity is increasing. However, there is a group of individuals whose prevalence of ADs is very low or non-existent, despite the biological aging. This paradox in autoimmunity raises questions. Centenarians, individuals who are over 100 years old, are possibly the most successful model of biological aging in humans. Most of these individuals exhibit a favorable health phenotype. To date, primary data evidence and potential hypotheses explaining this phenomenon are lacking globally, even though this paradox could provide valuable, original, and relevant information regarding the understanding of risk or protective factors, biological drivers, and biomarkers related to autoimmunity. Herein we discuss some hypothesis that may explain the absence of ADs in centenarians, including inflammaging, immunosenescence and immune resilience, immune system hyperstimulation, proteodynamics, and genetics.
Keywords: Longevity, Centenarians, Autoimmunity, Autoimmune diseases, Genetics, Proteodynamics
Abbreviations
- ADs
Autoimmune diseases
- CMV
Cytomegalovirus
- CRP
C-reactive protein
- DNA
Deoxyribonucleic acid
- EBV
Epstein-Barr virus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- GWAS
Genome-wide association study
1. Introduction
Autoimmune diseases (ADs) constitute one of the most prevalent chronic conditions, with higher healthcare costs and a greater disease burden worldwide [1]. The intensity and persistence of symptoms are among the most important and disabling factors among these patients [2]. For this reason, they represent great interest for public health and global health, with their control, resolution, and personalized approach being a priority [3].
It is well known that the etiology of these diseases is multifactorial, where they all share characteristics and a common origin [4]. Heritable and environmental factors interacting over time are the main causes of ADs. The loss of tolerance, specifically the lack of specificity during the immune response for the recognition of self-antigens, is one of the most relevant pathophysiological mechanisms in understanding these conditions [4]. The more frequent the AD and the later it appears, the more women are affected, indicating that genetics together with female hormones are drivers of autoimmunity [5]. During the aging process and through continuous exposure to various stressors, pathogens, and other environmental factors throughout life (i.e., exposome), accompanied by the aging of the immune system (i.e., immunosenescence) and the onset of age-related chronic diseases, a persistent proinflammatory systemic environment theoretically increases the probability of developing an AD [6]. This is because the immune system's responsiveness would be lower and irregular when exposed to a greater number of stressors and causes of cellular dysregulation. For this reason, age has been considered to be an important risk factor for autoimmunity [6,7].
Aging implies a complex array of changes and remodeling in homeostatic mechanisms that control the immune system, both in terms of numbers and functions of the different cellular subsets. Rather than being a mere process of immunosenescence, age-related transformations redesign the immune architecture and the balance between proinflammatory and anti-inflammatory protective factors [8]. Cellular senescence occurs in response to endogenous and exogenous stresses, including telomere dysfunction, oncogene activation and persistent deoxyribonucleic acid (DNA) damage [9]. Immunosenescence includes three events: a reduction in immune response, an increase in the inflammatory and oxidation background (inflammaging and oxiinflammaging), and a production of autoantibodies [8].
However, there is a group of humans, increasingly observed, that contradicts this paradigmatic view, and whose health phenotype raises numerous questions for which there are currently no answers. Centenarians represent the most successful model of biological aging in humans [10]. These individuals, who have a chronological age equal to or greater than 100 years, have special health characteristics, mostly partially known [11,12], that contradict the previously described theoretical concept of autoimmunity in the elderly. Unfortunately, there is a lack of robust evidence describing or discussing autoimmunity in centenarians. Even studies describing the health phenotypes of centenarians worldwide report that the prevalence of ADs in this population is practically nil, except for some series mentioning imprecise data and methodology [[13], [14], [15]]. Therefore, this field represents a niche of original, novel, and relevant knowledge for the in-depth understanding of new pathophysiological mechanisms, protective and risk factors for autoimmunity, based on the identification of new markers, signaling pathways, and targets related to aging, adaptation, or remodeling of the immune system. Herein we discuss current questions and gaps regarding the understanding of autoimmunity in centenarians, proposing possible hypotheses that would explain this scenario.
In centenarians, natural autoimmunity prevails over latent autoimmunity. The former refers to a network that serves to protect the organism from outer and inner danger but may also contribute to ADs [16]. The later corresponds to the presence of pathogenic autoantibodies or autoimmune biomarkers in the absence of clinical symptoms or fulfillment of classification criteria for AD [17]. Natural autoimmunity is characterized by IgM autoantibodies, protection against infection and a protagonist role in gut microbiota homeostasis. Their ability to bind both self and non-self-molecules allows them to inhibit pathogenic autoimmunity [16]. This explains why centenarians may disclosed natural autoantibodies but not ADs [18]. In fact, a discrepancy between a high prevalence of autoantibodies and the absence of ADs in centenarians has been described [14,[19], [20], [21], [22], [23], [24], [25]] (Table 1).
Table 1.
Autoantibodies reported in healthy Centenarians.
| N | Autoantibody | Comment | Year, (reference) |
|---|---|---|---|
| 34 | Tg (2.9%), TPO (5.8%), PCA (0), AC (0) | The prevalence of serum anti-Tg and anti-TPO antibodies was low in centenarians as compared with older adults and did not differ from that in younger healthy controls. | 1992 [19] |
| 26 | PCA (0), Tg (4%), ACA IgG (58%), ACA IgM (23%), ANA (27%), ds- DNA (0), ENA (0), h-GAL (58%) | 2002 [20] | |
| 140 | Tg (10.7%), TPO (9.3%) | Of an initial cohort of 209 centenarians, 6 (2.87%) disclosed hypothyroidism, and another 6 hyperthyroidism. The 140 centenarians evaluated were free of thyroid disease. | 1999 [14] |
| 20 | RF (25%), ANA (15%) | ANA were positive in three centenarians at titers >1:160, by IFI. Authors hypothesized that a raised sCD30 levels in centenarians, could be responsible, at least in part, for the appearance of circulating autoantibodies without definite clinical consequences at advanced age | 2001 [21] |
| 26 | PCA (0), Tg (4%), ACA IgG (58%), ACA IgM (23%), ANA (27%), ds- DNA (0), ENA (0), h-GAL (58%) | 1997 [22] | |
| 24 | Tg (4.16%), TPO (4.16%) | The occurrence of anti-Tg and anti-TPO positivity was lower in centenarians than in old and in young controls | 2002 [23] |
| 52–140 | Tg (11.4%), TPO (10%), PCA (18.6%), ANA (14.3%), IgM RF (26.6%), IgA RF (18.7%), MPO-ANCA (10.8%), ANCA (18%), PR3-ANCA (7.2%), oxLDL (15%), IgM ACA (14.3%), IgG ACA (2.1%), PR3-ANCA (7.2%), Histone (3.8%), Ro (1.9%) | Anti-La, anti-Sm, anti-U1RNP, and IgG/IgM antibodies to β2GP1 were not detected. | 2004 [24] |
| 77 | IgG β2GP1 (54.3%), IgM β2GP1 (8.6%), IgG ACA (20.7%), IgM ACA (2.59%) | Lupus anticoagulant was not detected. No vascular events were reported. | 2004 [18] |
| 33 | PARP | Class I 8 (24,24%), Class II 13 (39.4%), Class III 12 (36.36%). Classes depended on the magnitude of optical density results. | 2009 [25] |
Abbreviations: AC: adrenal cells; ACA: anti-cardiolipin antibodies; ANA: anti-nuclear antibodies; ANCA: antineutrophil cytoplasmic antibodies; β2GP1: beta 2 glycoprotein 1; h-GAL: natural anti-h-galactosyl antibodies; ds-DNA: anti-double strand DNA; ENA: anti-extractable nuclear antigens; IFI: indirect immunofluorescence; MPO: myeloperoxidase; oxLDL: oxidized low-density lipoprotein; PARP: poly(ADP-ribose) polymerase; PCA: parietal cell antibodies; RF: rheumatoid factor; Tg: thyroglobulin; TPO: thyroid peroxidase.
On the contrary, latent autoimmunity is defined by the presence of biomarkers, including IgG and IgM autoantibodies, that anticipate the development of clinical signs and symptoms of an AD, and therefore they are predictors of disease [17]. The transit from latent autoimmunity to clinically manifest autoimmunity will depend upon several factors including the pathogenicity of autoantibodies (i.e., affinity, isotype switching, glycosylation, rise in the titers, epitope spreading), the milieu (e.g., tertiary lymphoid structures), environmental factors and both epigenetic and genetic characteristics of the individual (discussed in 25). Therefore, the study of autoimmunity and centenarians will examine several and unknown mechanisms of self-antigen response [26].
2. Immunosenescence and inflammaging in the aging process: immune system decline or favorable evolutionary response?
Immunosenescence is a multifactorial phenomenon resulting from cellular aging, affecting both the innate and adaptive immune systems [27]. This condition has been significantly associated with numerous age-related chronic diseases due to changes in the immune response, alteration of cellular regulation, reduced activity in inflammation control, and loss of autophagy (i.e., a highly conserved pathway that degrades cellular components through lysosomes), and cellular waste lysis capabilities [28]. Immunosenescence is intimately related to a persistent state of low-grade inflammation during aging, known as inflammaging [6,29]. Decreased proteasome activity, mitochondrial dysfunction, disruption in control, and increased circulation of pathogen-associated molecular patterns, damage-associated molecular patterns, elevated expression of inflammatory cytokines, and advanced glycation end products are some of the mechanisms that establish the state of inflammaging, linked to immunosenescence [6,[27], [28], [29]].
The causal association between being centenarian and disclosing an AD may not exist [14,30]. What explains this phenomenon? In recent years, some authors have attempted to explain that both immunosenescence and inflammaging could be favorable evolutionary responses rather than just the decline of the immune system [6].
An aged, senescent immune system has a causal role in driving systemic ageing and therefore represents a key therapeutic target to extend healthy ageing [31]. Further, immune resilience, defined as the capacity to preserve and/or rapidly restore immune functions that promote disease resistance (immunocompetence) and control inflammation in infectious diseases as well as other causes of inflammatory stress, is a trait observed across the age spectrum aligned with a specific immunocompetence-inflammation balance linked to favorable immunity-dependent health outcomes [32]. People with optimal immune resilience have health and survival advantages [32]. This, in turn, may reduce the chance of developing cancer, ADs, pulmonary conditions, or cardiometabolic diseases, even when exposed to risk factors throughout life [10,32].
During the aging process, both senescence and persistent inflammation generate systemic damage to target organs, triggering metabolic, vascular, neuroendocrine, and other alterations that facilitate the onset of age-related chronic diseases such as essential hypertension, type 2 diabetes mellitus, dyslipidemia, cancer, dementia, and others [29,33,34]. However, the prevalence of these diseases in centenarians is significantly lower compared to younger age groups [10]. This suggests a unique and specific property of a successful response to cellular dysregulation. This observation has led certain authors to discuss age-related immunological changes because of a mix between resilience and immunological remodeling versus immunological maladaptation [28].
It is known that there are multiple routes to achieve an exceptional longevity. In fact, centenarians are categorized in survivors, delayers, and escapers from the major diseases because of their heterogeneous phenotypes and probably genotypes [35]. Most of centenarians disclose the escaper phenotype [10,35].
To address the absence of ADs in centenarians, we shall discuss five connected hypotheses:
-
1)
Inflammaging, is an evolutionary response that allows controlling a pathogenic and injurious process for some tissue or organ. Thus, it could be argued that centenarians, as a model of more successful biological aging, possess a unique inflammaging, remodeled and adapted to their organic status, with an adequate immune response that prevents the acceleration of systemic aging. C-reactive protein (CRP), IL-12, TNF-α, IFN-γ, IL-6, and IL-10 are increased in plasma of centenarians as compared with young adults and old adults, while IL/23, IL-1β, and Th17/Treg cell ratio are decreased [36]. Decreased Treg cells in centenarians are prone to secrete more anti-inflammatory cytokines. According to the authors, these results, suggest that centenarians alleviated inflammaging by decreasing the ratio of Th17/Treg cells and changing them into anti-inflammatory secretory phenotypes, which provided a novel mechanism for anti-aging research [36].
-
2)
Immunosenescence in centenarians has a specific cellular signature and phenotype, result of a favorable adaptation to the exposure to stressors throughout their lives. In this way, the loss of immunological activity is minimal compared to other younger age groups. However, to demonstrate this, evidence is needed that distinguishes between the expression of biomarkers and molecular regulators associated with biological aging and the health phenotype of centenarians compared to other age groups. If this is true, immunosenescence would evidently be a favorable adaptive evolutionary response and not just the decline of the immune system. Alternatively, escaper centenarians may disclose an immune resilience erosion-resistant phenotype [32]. As mentioned, immunosenescence is intimately related to inflammaging [6,29].
-
3)
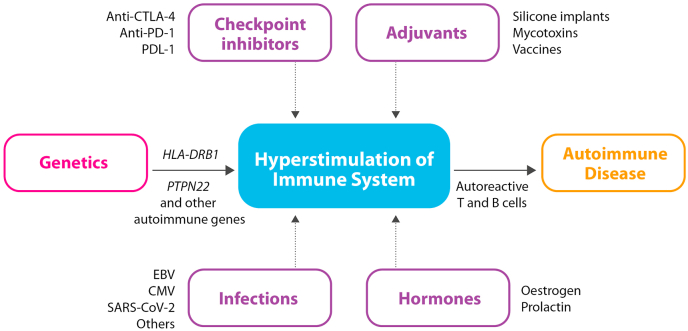
Autoimmune ecology refers to the interactions between individuals and their environment leading to a breakdown in immune tolerance and, therefore, to the development of one or more ADs in such an individual [37]. Hyperstimulation of the individual's immune system by diverse environmental factors, will lead to the production of inflammatory cytokines, autoantibodies, and auto-reactive T- and B-cells, and eventually to the development of ADs [38]. If the hyperstimulation is long lasting it may lead to lymphoma (B large cell lymphoma) or to multiple myeloma [39]. Infections (i.e., Epstein-Barr virus, cytomegalovirus, SARS-CoV-2, Herpes virus) [40,41], chemical substances, which are widely used in pharmacology and medicine, including vaccines [42,43], external adjuvants [44], and immune checkpoint inhibitors [45] are among the main environmental factors that may provoke immune hyperstimulation, ultimately leading to an autoimmune response (Fig. 1).
Fig. 1.
The development of autoimmunity through hyperstimulation from environmental factors. Abbreviations: EBV: Epstein-Barr virus; CMV: cytomegalovirus.
Centenarians may outgrow hyperstimulation or may not have been exposed to strong external stimuli (i.e., low grade exposome). Additionally, as will be discussed below, they may have a genetic and epigenetic background that protects them from hyperstimulation.
-
4)
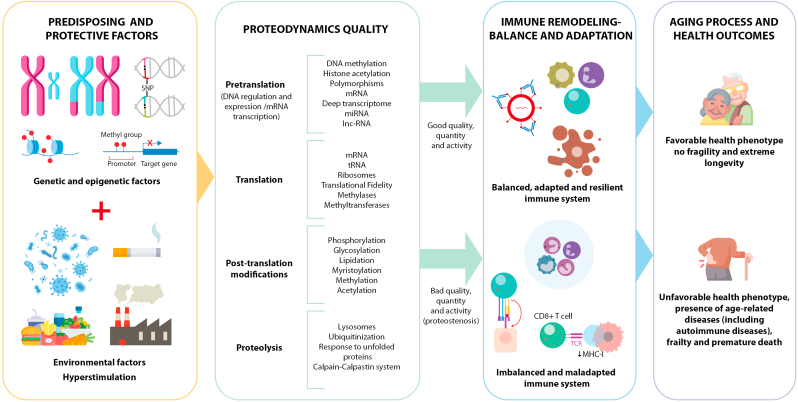
Proteodynamics, defined as the process of regulating the synthesis, modification, and elimination of proteins at the cellular level [46] is based on an efficient and self-regulated proteostasis signature, which delays or avoids proteostenosis and the establishment of a geroproteome based on proteins with deficient functionality, mutated, leading to the accumulation of residues resistant to proteolytic systems. This implies that, compared to the elderly, centenarians have a more effective DNA maintenance capacity, better transcriptional fidelity, translation with lower error frequency, and a better response to post-translational modifications and autophagy [46]. In this way, a better cellular and molecular response to stressors would be maintained, avoiding injurious mechanisms to tissues and organs, and delaying oxidation, dysfunction, and cellular aging (Fig. 2).
-
5)
Genetic and epigenetic factors. Various studies have explored the correlation between specific genetic variants and inflammation, longevity, and extreme longevity phenotypes in humans [47,48]. These variants are frequently situated in genes that play roles in the regulation of metabolism, inflammation, immunity, and stress response. Some of these genes also contribute to the regulation of chromatin state and histone methylation, indicating an epigenetic dimension to longevity and the centenarian phenotype. Recent studies have particularly focused on genes such as FOXO3A, APOE, IL10, and TP53, as summarized in Table 2.
Fig. 2.
Factors associated with onset of ADs during longevity. Potential determinants are disclosed, which determine the quality of immune adaptation, balance, and remodeling (regulated or unregulated inflammaging) during the aging process (immunosenescence). The paradox is presented that, even in the presence of risk factors and immunosenescence, possibly the quality of proteodynamics (representing the quality, quantity, and activity of proteostasis) is essential in immune regulation during aging. This establishes a pattern of immune adaptation, balance, and remodeling, allowing the triggering of a regulated/unregulated inflammaging phenotype, leading to specific health outcomes in older adults (successful aging and protection against ADs or decline and premature death). Abbreviations: lnc-RNA: Long non-coding RNA; miRNA: MicroRNA; mRNA: Messenger RNA; tRNA: Transfer RNA.
Table 2.
Summary of genes associated with inflammation and extreme longevity.
| Gene | Functiona |
|---|---|
| FOXO3A | At 6q21, encodes a master transcription factor that regulates the expression of genes involved in cellular stress response, apoptosis, and inflammation. |
| APOE | At 19q13.32, encodes a protein that transports cholesterol and lipids in the blood and brain. APOE gene has three common alleles (ε2, ε3 and ε4) and six related genotypes (ε3ε3, ε3ε2, ε2ε2, ε3ε4, ε4ε4, and ε2ε4). |
| CHRNA3 | At 15q25.1, encodes the cholinergic receptor nicotinic alpha 3 subunit. Despite being associated with several pathologies, a variant near this locus affecting extreme longevity has also been suggested. |
| IL10 | At 1q32.1, encodes the immunoregulatory cytokine IL-10 who main function is to limit and terminate inflammatory response, mediating the inhibition of effects of T cells, monocytes and macrophages. |
| TP53 | At 17p13.1, encodes a tumor suppressor protein containing transcriptional activation, DNA binding, and oligomerization domains. The encoded protein responds to diverse cellular stresses to regulate expression of target genes, thereby inducing cell cycle arrest, apoptosis, senescence, DNA repair, or changes in metabolism. |
| HLA | At 6p21. The main function of HLA class I gene products (HLA-A, -B, and -C) is to present endogenous peptides to responding CD8+ T cells while the class II coded molecules HLA-DR, -DP, and –DQ have restricted expression and process exogenous peptides for presentation to CD4+ helper T cells. The association of HLA genes with longevity seems to be population and gender specific. There are no definitive conclusions linking its genetic variation to centenarian's phenotype. |
Adapted from GeneCards (https://www.genecards.org).
Notably, two major functional categories emerge for these four genes: cellular mechanisms of inflammatory regulation and cell cycle control. For example, FOXO3A is activated by oxidative stress and inhibits the production of pro-inflammatory cytokines like IL-6 and TNF-α. Genetic variants such as rs2802292, rs13217795, rs2764264, and rs7762395 have been predominantly associated with extreme longevity across various centenarian cohorts [47].
APOE, known for its ε4 variant linked to an increased risk of Alzheimer's disease and cardiovascular disease, exerts pro-inflammatory effects. APOE ε4 heightens the expression of inflammatory mediators such as IL-1β, IL-6, and C-reactive protein. Studies indicate that the ε2 genotype (rs429358) is positively linked to longevity, supported by the low prevalence of the APOE ε4 allele among centenarians [47].
At the immune response level, the rs1800896 variant in IL10 has demonstrated an association with elevated levels of IL-10. Centenarian cohorts from Japan, Italy, Jordan, and Bulgaria exhibit a higher frequency of the GG genotype, suggesting a role of IL-10 gene in extreme longevity phenotypes [47].
Regarding cell cycle regulation, the position identified as rs1042522 in TP53, traditionally known for its role in an individual's cancer risk, has now been associated with aging. Studies across diverse cohorts consistently support a positive association of this SNP with longevity and extreme longevity [47].
HLA genes and longevity has been an ongoing area of interest, and as of the present date, there are still no definitive conclusions linking its genetic variation to longevity phenotype (Table 2). There is an ever-growing interest in longevity and centenarian phenotypes, which highlight the need for new studies that utilize consider cutting-edge molecular technology, which can support and refine the current data. Overall, current state of the art sequencing methods such as whole genome sequencing efforts, as well as homogeneous analysis methods are needed to conclusively demonstrate the role of genetics, including HLA system, in extreme longevity (Table 2).
In the realm of methylations and histone modifications, the mechanisms through which genetic and environmental factors regulate longevity are captivatingly elucidated by their influence on chromatin states. Chromatin states, governed by modifications such as DNA methylation and histone modification, play a pivotal role. Histone modifications, including acetylation and methylation, are often labeled as ‘epigenetic' since they don't alter the genetic sequence itself but rather impact the accessibility and function of DNA [49]. Specific histone marks are linked to distinct chromatin states and gene expression patterns, such as H3K4me3 associated with gene activation and H3K27me3 with gene repression [49,50].
The involvement of methylations and histone modifications in lifespan strategies, favoring a reversible nature of chromatin modifications, suggests that therapeutically targeting chromatin regulators could extend both lifespan and health span, contributing to the phenotype of centenarians.
Currently, research into genes with a positive relationship to the aging process and exceptional longevity, as well as the methylation states and histone modifications of centenarian genomes, is in its early stages. Ongoing and future investigations should prioritize Genome-Wide Association Studies (GWAS) enriched by tools facilitating the study of genome methylation states and histone modifications in individuals with extreme longevity. This focus is crucial for demonstrating the interactions established by different genes and their regulatory processes, thereby clarifying their positive relationship with longevity and the phenotype of extreme longevity.
Despite the existence of studies demonstrating how certain protein signatures are inherited from centenarians to their offspring [51,52], and how these individuals acquire strengths and properties of cellular responses that would allow maintaining adequate proteodynamics [51,52], translational biomarkers, signaling pathways, and specific determinants during the different phases of the immune system's proteodynamics are still unknown. These factors would explain the difference between immunosenescence in rapidly aging humans who develop a phenotype of poor health, and the immune resilience, remodeling, and adaptation in centenarians that confer a certain delay or protection in the development of age-related chronic diseases.
3. Organic and immunological remodeling in centenarians
Unique age-related changes in centenarians have been described, and compared to younger age groups, allowing for an understanding of specific pathways through which centenarians age more slowly, better, and exhibit better cellular plasticity that could delay or modify the cellular senescence signature. In addition to the known decrease of Th17/Treg cell ratio [36], a recent study analyzing a multi-modal profile of peripheral blood cells from seven subjects, identified that individuals with extreme longevity (including centenarians) have alterations in various immune cell populations [53]. The increase in the myeloid/lymphocyte ratio, compared to younger age groups (20–89 years), as well as the proportion of cytotoxic CD4+ T cells (more pronounced in men than in women), and the decrease in the population of naive and memory CD4+ T cells are some of these variations specific to individuals with extreme longevity. The shift in the distribution of these cell populations, which also include B cells, natural killer cells, monocytes with CD14+ and CD16+ subtypes, and dendritic cells with myeloid and plasmacytoid subtypes, has sparked interest in forming three clusters of immune cell types related to aging: 1) Cluster of cells aging-related (which increases or decreases according to the aging process); 2) Cluster of cells specific to extreme longevity (which increase or decrease exclusively in individuals with extreme longevity); 3) Cluster of cells specific to aging but not extreme longevity (which increase or decrease with age, but these changes do not continue into extreme longevity) [53].
The discovery of these changes in the expression and distribution of immune cell populations in different age groups supports the hypothesis of remodeling and adaptive or maladaptive evolutionary response, determining the biological aging and health status of the individual during aging. Particularly, the identification of different clusters reflecting the specific expression of certain cell populations allows distinguishing which cell lines are related to a more significant dynamic, self-regulation, and proteodynamics for healthy aging, compared to others. Other similar studies have also identified this trend of immune signatures [32,54], suggesting it as the basis for the immune resilience characteristic of centenarians who, despite inflammatory stress during exposure to harmful factors and pathogens, maintain their longevity and favorable health outcomes, including resistance to infections [32,54]. All of the above represents the maintenance of favorable immunological tolerance, with proper control of inflammation and cellular regulation and plasticity. This could possibly explain the finding of low or no prevalence of ADs in centenarians.
Nevertheless, the phenotype of healthy aging that allows for extreme longevity is a multifactorial condition, depending on the integrity and proper functionality of other organs. Metabolomic and proteomic signatures, as well as metagenomic findings, have identified changes specific to the metabolic response, regulation, and recolonization of microbiota in centenarians, which have been associated with a better health status [51,[55], [56], [57], [58], [59], [60]]. The accumulation of residues, therefore, the concentration of certain compounds is associated with common causes of disease and death [55,57,61]. Thus, self-regulation, proper proteodynamics, and effective cellular plasticity and adaptation (including immunosenescence and inflammaging) would be determinants in the complex remodeling of cellular functions and populations in extreme longevity, allowing the evasion or delay of age-related chronic diseases, including ADs (Fig. 2).
To better understand this phenomenon, it is necessary to precisely identify the biological drivers that determine the regulation of protein expression or co-expression in different cell lines related to aging and extreme longevity, and establish common pathways that allow the identification of potential therapeutic targets for the development of senolytics (a class of drugs that selectively clear senescent cells) that delay or prevent the establishment of a senescent signature associated with cellular damage. Still, much is unknown about immunosenescence, immune resilience, inflammaging, and evolutionary immune remodeling in centenarians, as evidence remains heterogeneous, and there are gaps in the study of complex variables such as ancestry, exposome, gender, and heritability, which, are also important determining factors in the pathophysiology of ADs.
Lastly, the search for universal senescence biomarkers is constantly challenged by the evidence that senescence phenotypes are highly heterogeneous and may differ depending on the initial trigger and the cell type under study [9]. Transcriptomic and proteomic studies up to the single-cell level in relevant cell and tissue types will be of paramount importance to find unique or common markers of the senescence state and may represent a turning point for senescence-based translational medicine applications [9].
4. Conclusion
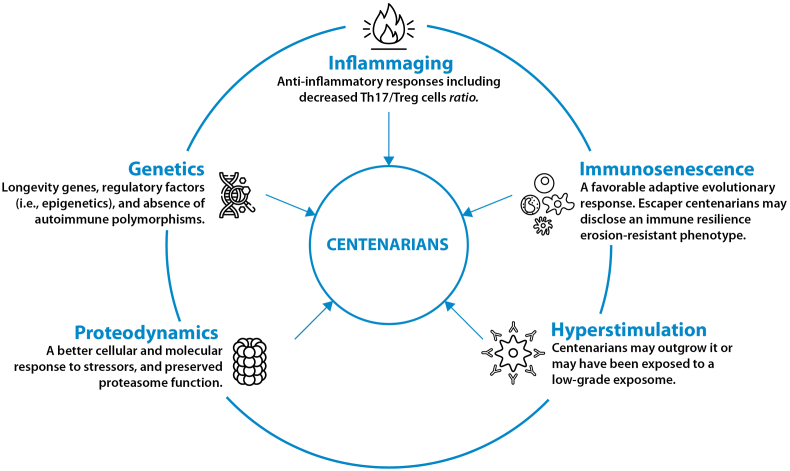
Although it is inferred that centenarians should present ADs and other types of pathologies related to immunosenescence and immune resilience, the truth is that the evidence does not confirm this assertion. One key question is how the central and peripheral tolerance are maintained in centenarians to allow natural autoimmunity but avoid ADs. The reasons to explain this phenomenon is the object of current research. Nevertheless, factors explaining the absence of ADs in centenarians include but are not limited to inflammaging, immunosenescence and immune resilience, immune system hyperstimulation, proteodynamics, and genetics (Fig. 3).
Fig. 3.
Interacting factors that protect centenarians from autoimmune diseases.
CRediT authorship contribution statement
Juan-Manuel Anaya: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization. Ivan David Lozada-Martinez: Writing – review & editing, Writing – original draft, Investigation, Data curation. Isaura Torres: Writing – review & editing, Writing – original draft, Investigation, Data curation. Yehuda Shoenfeld: Writing – review & editing, Writing – original draft, Investigation, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr Y Renaudineau
Data availability
No data was used for the research described in the article.
References
- 1.Conrad N., Misra S., Verbakel J.Y., Verbeke G., Molenberghs G., Taylor P.N., Mason J., Sattar N., V McMurray J.J., McInnes I.B., Khunti K., Cambridge G. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401:1878–1890. doi: 10.1016/S0140-6736(23)00457-9. [DOI] [PubMed] [Google Scholar]
- 2.Spierings J., Sloeserwij A., Vianen M.E., de Boer J.H., Sigurdsson V., van de Wijgert J.H.H.M., van Laar J.M. Health-related quality of life in patients with immune mediated inflammatory diseases: a cross-sectional, multidisciplinary study. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108392. [DOI] [PubMed] [Google Scholar]
- 3.Miller F.W. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 2023;80 doi: 10.1016/j.coi.2022.102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anaya J.-M., Beltrán S. The autoimmune tautology revisited. J. Transl. Autoimmun. 2023;7 doi: 10.1016/j.jtauto.2023.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintero O.L., Amador-Patarroyo M.J., Montoya-Ortiz G., Rojas-Villarraga A., Anaya J.-M. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J. Autoimmun. 2012;38 doi: 10.1016/j.jaut.2011.10.003. J109–J119. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A., Bientinesi E., Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res. Rev. 2021;71 doi: 10.1016/j.arr.2021.101422. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Casals M., Brito-Zerón P., López-Soto A., Font J. Systemic autoimmune diseases in elderly patients. Autoimmun. Rev. 2004;3:376–382. doi: 10.1016/j.autrev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Watad A., Bragazzi N.L., Adawi M., Amital H., Toubi E., Porat B.-S., Shoenfeld Y. Autoimmunity in the elderly: insights from basic science and clinics - a mini-review. Gerontology. 2017;63:515–523. doi: 10.1159/000478012. [DOI] [PubMed] [Google Scholar]
- 9.Di Micco R., Krizhanovsky V., Baker D., d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozada-Martinez I.D., Marín J.S., Castelblanco-Toro S.M., Mazenett-Granados E.A., Suárez J.F., Sarmiento M., Anaya J.-M. Demographics and clinical characteristics of a new population of Centenarians in Colombia. COOLCEN Cohort Arch. Gerontol. Geriatr. Plus. 2024 doi: 10.1016/j.aggp.2024.100006. [DOI] [Google Scholar]
- 11.Willcox D.C., Willcox B.J., Poon L.W. Centenarian studies: important contributors to our understanding of the aging process and longevity. Curr. Gerontol. Geriatr. Res. 2010:1–6. doi: 10.1155/2010/484529. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borras C., Ingles M., Mas-Bargues C., Dromant M., Sanz-Ros J., Román-Domínguez A., Gimeno-Mallench L., Gambini J., Viña J. Centenarians: an excellent example of resilience for successful ageing. Mech. Ageing Dev. 2020;186 doi: 10.1016/j.mad.2019.111199. [DOI] [PubMed] [Google Scholar]
- 13.Mossakowska M., Pawlinska-Chmara R., Broczek K.M. Asthma, allergy, and respiratory symptoms in centenarians living in Poland. J. Physiol. Pharmacol. 2008;59(Suppl 6):483–489. [PubMed] [Google Scholar]
- 14.Andersen‐Ranberg K., Jeune B., Høier‐Madsen M., Hegedüs L. Thyroid function, morphology and prevalence of thyroid disease in a population‐based study of Danish centenarians. J. Am. Geriatr. Soc. 1999;47:1238–1243. doi: 10.1111/j.1532-5415.1999.tb05205.x. [DOI] [PubMed] [Google Scholar]
- 15.Mutasim D.F. Autoimmune bullous dermatoses in the elderly. Drugs Aging. 2003;20:663–681. doi: 10.2165/00002512-200320090-00004. [DOI] [PubMed] [Google Scholar]
- 16.Avrameas S., Alexopoulos H., Moutsopoulos H.M. Natural autoantibodies: an undersugn hero of the immune system and autoimmune disorders—a point of view. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anaya J.-M., Monsalve D.M., Rojas M., Rodríguez Y., Montoya-García N., Mancera-Navarro L.M., Villadiego-Santana A.M., Rodríguez-Leguizamón G., Acosta-Ampudia Y., Ramírez-Santana C. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meroni P.L., Mari D., Monti D., Coppola R., Capri M., Salvioli S., Tincani A., Gerli R., Franceschi C. Anti-beta 2 glycoprotein I antibodies in centenarians. Exp. Gerontol. 2004;39:1459–1465. doi: 10.1016/j.exger.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Mariotti S., Barbesino G., Caturegli P., Giacomelli T., Pinchera A., Monti D., Cossarizza A., Franceschi C., Sansoni P., Passeri G., Fagiolo U. Thyroid and other organ-specific autoantibodies in healthy ceritenarians. Lancet. 1992;339:1506–1508. doi: 10.1016/0140-6736(92)91265-A. [DOI] [PubMed] [Google Scholar]
- 20.Candore G., Grimaldi M.P., Listi F., Ferlazzo V., Colonna-Romano G., Motta M., Malaguarnera M., Fradá G., Lio D., Caruso C. Prevalence of non organ-specific autoantibodies in healthy centenarians. Arch. Gerontol. Geriatr. 2002;35:75–80. doi: 10.1016/S0167-4943(02)00106-1. [DOI] [PubMed] [Google Scholar]
- 21.Gerli R., Monti D., Bistoni O., Mazzone A.M., Peri G., Cossarizza A., Di Gioacchino M., Cesarotti M.E.F., Doni A., Mantovani A., Franceschi C., Paganelli R. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech. Ageing Dev. 2001;121:37–46. doi: 10.1016/S0047-6374(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 22.Candore G., Di Lorenzo G., Mansueto P., Melluso M., Fradà G., Li Vecchi M., Pellitteri M.E., Drago A., Di Salvo A., Caruso C. Prevalence of organ-specific and non organ-specific autoantibodies in healthy centenarians. Mech. Ageing Dev. 1997;94:183–190. doi: 10.1016/S0047-6374(96)01845-3. [DOI] [PubMed] [Google Scholar]
- 23.Magri F., Muzzoni B., Cravello L., Fioravanti M., Busconi L., Camozzi D., Vignati G., Ferrari E. Thyroid function in physiological aging and in centenarians: possible relationships with some nutritional markers. Metabolism. 2002;51:105–109. doi: 10.1053/meta.2002.28968. [DOI] [PubMed] [Google Scholar]
- 24.Andersen-Ranberg K., Høier-Madsen M., Wiik A., Jeune B., Hegedüs L. High prevalence of autoantibodies among Danish centenarians. Clin. Exp. Immunol. 2004;138:158–163. doi: 10.1111/j.1365-2249.2004.02575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisa A., Monti D., Franceschi C., Scovassi A.I. Autoantibodies to poly(ADP-ribose) polymerase in centenarians: a reappraisal of grabar's hypothesis. Gerontology. 2009;55:427–429. doi: 10.1159/000213652. [DOI] [PubMed] [Google Scholar]
- 26.Molano-González N., Rojas M., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Rodríguez Y., Rodríguez-Jimenez M., Ramírez-Santana C., Anaya J.-M. Cluster analysis of autoimmune rheumatic diseases based on autoantibodies. New insights for polyautoimmunity. J. Autoimmun. 2019;98:24–32. doi: 10.1016/j.jaut.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2018;8 doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulop T., Larbi A., Hirokawa K., Cohen A.A., Witkowski J.M. Immunosenescence is both functional/adaptive and dysfunctional/maladaptive. Semin. Immunopathol. 2020;42:521–536. doi: 10.1007/s00281-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., Miller A.H., Mantovani A., Weyand C.M., Barzilai N., Goronzy J.J., Rando T.A., Effros R.B., Lucia A., Kleinstreuer N., Slavich G.M. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariotti S., Chiovato L., Franceschi C., Pinchera A. Thyroid autoimmunity and aging. Exp. Gerontol. 1998;33:535–541. doi: 10.1016/S0531-5565(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 31.Yousefzadeh M.J., Flores R.R., Zhu Y., Schmiechen Z.C., Brooks R.W., Trussoni C.E., Cui Y., Angelini L., Lee K.-A., McGowan S.J., Burrack A.L., Wang D., Dong Q., Lu A., Sano T., O'Kelly R.D., McGuckian C.A., Kato J.I., Bank M.P., Wade E.A., Pillai S.P.S., Klug J., Ladiges W.C., Burd C.E., Lewis S.E., LaRusso N.F., Vo N.V., Wang Y., Kelley E.E., Huard J., Stromnes I.M., Robbins P.D., Niedernhofer L.J. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahuja S.K., Manoharan M.S., Lee G.C., McKinnon L.R., Meunier J.A., Steri M., Harper N., Fiorillo E., Smith A.M., Restrepo M.I., Branum A.P., Bottomley M.J., Orrù V., Jimenez F., Carrillo A., Pandranki L., Winter C.A., Winter L.A., Gaitan A.A., Moreira A.G., Walter E.A., Silvestri G., King C.L., Zheng Y.-T., Zheng H.-Y., Kimani J., Blake Ball T., Plummer F.A., Fowke K.R., Harden P.N., Wood K.J., Ferris M.T., Lund J.M., Heise M.T., Garrett N., Canady K.R., Abdool Karim S.S., Little S.J., Gianella S., Smith D.M., Letendre S., Richman D.D., Cucca F., Trinh H., Sanchez-Reilly S., Hecht J.M., Cadena Zuluaga J.A., Anzueto A., Pugh J.A., Abdalla M.I., Adams S.G., Adebayo Y., Agnew J., Ali S., Anstead G., Balmes M., Barker J., Baruch-Bienen D., Bible V., Birdwell A., Braddy S., Bradford S., Briggs H., Corral J.M., Dacus J.J., Danaher P.J., DePaul S.A., Dickerson J., Doanne J., Ehsan A., Elbel S., Escalante M., Escamilla C., Escamilla V., Farrar R., Feldman D., Flores D., Flynn J., Ford D., Foy J.D., Freeman M., Galley S., Garcia J., Garza M., Gilman S., Goel M., Gomez J., Goyal V.K., Grassmuck S., Grigsby S., Hanson J., Harris B., Haywood A., Hinojosa C., Ho T.T., Hopkins T., Horvath L.L., Hussain A.N., Jabur A., Jewell P., Johnson T.B., Lawler A.C., Lee M., Lester C.S., Levine S.M., Lewis H.V., Louder A., Mainor C., Maldonado R., Martinez C., Martinez Y., Maselli D., Mata C., McElligott N., Medlin L., Mireles M., Moreno J., Morneau K., Muetz J., Munro S.B., Murray C., Nambiar A., Nassery D., Nathanson R., Oakman K., O'Rorke J., Padgett C., Pascual-Guardia S., Patterson M., Perez G.L., Perez R., Perez R., Phillips R.E., Polk P.B., Pomager M.A., Preston K.J., Proud K.C., Rangel M., Ratcliffe T.A., Reichelderfer R.L., Renz E.M., Ross J., Rudd T., Sanchez M.E., Sanders T., Schindler K.C., Schmit D., Sehgal R.T., Solorzano C., Soni N., Tam W.S., Tovar E.J., Trammell Velasquez S.A., Tyler A.R., Vasquez A., Veloso M.C., Venticinque S.G., Villalpando J.A., Villanueva M., Villegas L., Walker M., Wallace A., Wallace M., Wang E., Wickizer S., Williamson A., Yunes A., Zentner K.H., Agan B.K., Root-Bernstein R., Clark R.A., Okulicz J.F., He W. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 2023;14:3286. doi: 10.1038/s41467-023-38238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J., Huang X., Dou L., Yan M., Shen T., Tang W., Li J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Targeted Ther. 2022;7:391. doi: 10.1038/s41392-022-01251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogrodnik M., Gladyshev V.N. The meaning of adaptation in aging: insights from cellular senescence, epigenetic clocks and stem cell alterations. Nat. Aging. 2023;3:766–775. doi: 10.1038/s43587-023-00447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evert J., Lawler E., Bogan H., Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J. Gerontol. A: Biol. Sci. Med. Sci. 2003;58:M232–M237. doi: 10.1093/gerona/58.3.M232. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L., Ge M., Zhang Y., Wu X., Leng M., Gan C., Mou Y., Zhou J., Valencia C.A., Hao Q., Zhu B., Dong B., Dong B. Centenarians alleviate inflammaging by changing the ratio and secretory phenotypes of T Helper 17 and regulatory T cells. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.877709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anaya J.-M., Restrepo-Jiménez P., Ramírez-Santana C. The autoimmune ecology: an update. Curr. Opin. Rheumatol. 2018;30:350–360. doi: 10.1097/BOR.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 38.Ryabkova V.A., Churilov L.P., Shoenfeld Y. Hyperstimulation of the immune system as a cause of autoimmune diseases. Annal. Russ. Acad. Med. Sci. 2020;75:204–213. doi: 10.15690/vramn1276. [DOI] [Google Scholar]
- 39.Watad A., Bragazzi N.L., Amital H., Shoenfeld Y. Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast. Isr. Med. Assoc. J. 2019;21:517–519. [PubMed] [Google Scholar]
- 40.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.-M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arango M.T., Shoenfeld Y., Cervera R., Anaya J.M. In: Autoimmunity: from Bench to Bedside. first ed. Anaya J.M., Shoenfeld Y., Rojas-Villarraga A., Levy R.A., Cervera R., editors. El Rosario University Pres; Bogotá: 2013. Infection and autoimmune diseases; pp. 303–320. [PubMed] [Google Scholar]
- 42.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018;15:586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez Y., Rojas M., Beltrán S., Polo F., Camacho-Domínguez L., Morales S.D., Gershwin M.E., Anaya J.-M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022;132 doi: 10.1016/j.jaut.2022.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen Tervaert J.W., Martinez-Lavin M., Jara L.J., Halpert G., Watad A., Amital H., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun. Rev. 2023;22 doi: 10.1016/j.autrev.2023.103287. [DOI] [PubMed] [Google Scholar]
- 45.Dang Q.M., Watanabe R., Shiomi M., Fukumoto K., Nobashi T.W., Okano T., Yamada S., Hashimoto M. Rheumatic immune-related adverse events due to immune checkpoint inhibitors—a 2023 update. Int. J. Mol. Sci. 2023;24:5643. doi: 10.3390/ijms24065643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frankowska N., Bryl E., Fulop T., Witkowski J.M. Longevity, centenarians and modified cellular proteodynamics. Int. J. Mol. Sci. 2023;24:2888. doi: 10.3390/ijms24032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos-Lozano A., Santamarina A., Pareja-Galeano H., Sanchis-Gomar F., Fiuza-Luces C., Cristi-Montero C., Bernal-Pino A., Lucia A., Garatachea N. The genetics of exceptional longevity: insights from centenarians. Maturitas. 2016;90:49–57. doi: 10.1016/j.maturitas.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Bin-Jumah M.N., Nadeem M.S., Gilani S.J., Al-Abbasi F.A., Ullah I., Alzarea S.I., Ghoneim M.M., Alshehri S., Uddin A., Murtaza B.N., Kazmi I. Genes and longevity of lifespan. Int. J. Mol. Sci. 2022;23:1499. doi: 10.3390/ijms23031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders Y.Y., Liu H., Zhang X., Hecker L., Bernard K., Desai L., Liu G., Thannickal V.J. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol. 2013;1:8–16. doi: 10.1016/j.redox.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sebastiani P., Federico A., Morris M., Gurinovich A., Tanaka T., Chandler K.B., Andersen S.L., Denis G., Costello C.E., Ferrucci L., Jennings L., Glass D.J., Monti S., Perls T.T. Protein signatures of centenarians and their offspring suggest centenarians age slower than other humans. Aging Cell. 2021;20 doi: 10.1111/acel.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tedone E., Arosio B., Gussago C., Casati M., Ferri E., Ogliari G., Ronchetti F., Porta A., Massariello F., Nicolini P., Mari D. Leukocyte telomere length and prevalence of age-related diseases in semisupercentenarians, centenarians and centenarians' offspring. Exp. Gerontol. 2014;58:90–95. doi: 10.1016/j.exger.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Karagiannis T.T., Dowrey T.W., Villacorta-Martin C., Montano M., Reed E., Belkina A.C., Andersen S.L., Perls T.T., Monti S., Murphy G.J., Sebastiani P. Multi-modal profiling of peripheral blood cells across the human lifespan reveals distinct immune cell signatures of aging and longevity. EBioMedicine. 2023;90 doi: 10.1016/j.ebiom.2023.104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ledón N., Añé-Kourí A.L., Ramos M.B., Lorenzo-Luaces P., Silva A., Pereira K., Lage A., Saavedra D. Immunosenescence and inflammatory markers in Cuban centenarians: implications for survival. Aging Clin. Exp. Res. 2023 doi: 10.1007/s40520-023-02567-9. [DOI] [PubMed] [Google Scholar]
- 55.Cheng S., Larson M.G., McCabe E.L., Murabito J.M., Rhee E.P., Ho J.E., Jacques P.F., Ghorbani A., Magnusson M., Souza A.L., Deik A.A., Pierce K.A., Bullock K., O'Donnell C.J., Melander O., Clish C.B., Vasan R.S., Gerszten R.E., Wang T.J. Distinct metabolomic signatures are associated with longevity in humans. Nat. Commun. 2015;6:6791. doi: 10.1038/ncomms7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montoliu I., Scherer M., Beguelin F., DaSilva L., Mari D., Salvioli S., Martin F.-P.J., Capri M., Bucci L., Ostan R., Garagnani P., Monti D., Biagi E., Brigidi P., Kussmann M., Rezzi S., Franceschi C., Collino S. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging. 2014;6:9–25. doi: 10.18632/aging.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collino S., Montoliu I., Martin F.-P.J., Scherer M., Mari D., Salvioli S., Bucci L., Ostan R., Monti D., Biagi E., Brigidi P., Franceschi C., Rezzi S. Metabolic signatures of extreme longevity in Northern Italian centenarians reveal a complex remodeling of Lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J., Abe F., Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luan Z., Sun G., Huang Y., Yang Y., Yang R., Li C., Wang T., Tan D., Qi S., Jun C., Wang C., Wang S., Zhao Y., Jing Y. Metagenomics study reveals changes in gut microbiota in centenarians: a cohort study of Hainan centenarians. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aiello A., Accardi G., Aprile S., Caldarella R., Carru C., Ciaccio M., De Vivo I., Gambino C.M., Ligotti M.E., Vasto S., Zinellu A., Caruso C., Bono F., Candore G. Age and gender-related variations of molecular and phenotypic parameters in A cohort of Sicilian population: from young to centenarians. Aging Dis. 2021;12:1773. doi: 10.14336/AD.2021.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Q., Wu C., Zhu Q., Gao R., Lu J., Valles-Colomer M., Zhu J., Yin F., Huang L., Ding L., Zhang X., Zhang Y., Xiong X., Bi M., Chen X., Zhu Y., Liu L., Liu Y., Chen Y., Fan J., Sun Y., Wang J., Cao Z., Fan C., Ehrlich S.D., Segata N., Qin N., Qin H. Metagenomic and metabolomic remodeling in nonagenarians and centenarians and its association with genetic and socioeconomic factors. Nat Aging. 2022;2:438–452. doi: 10.1038/s43587-022-00193-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.