Abstract
CYP-dependent metabolites play a critical role in regulating the cell cycle, as well as the proliferative, invasive, and migratory activity of cancer cells. We conducted a study to analyze the relative gene expression of various CYPs (CYP7B1, CYP27A1, CYP39A1, CYP51, CYP1B1, CYP3A5, CYP4F8, CYP5A1, CYP4F2, CYP2J2, CYP2E1, CYP2R1, CYP27B1, CYP24A1) in 41 pairs of prostate samples (tumor and conventional normal tissues) using qPCR. Our analysis determined significant individual variability in the expression levels of all studied CYPs, both in the tumor and in conventionally normal groups. However, when we performed a paired test between the tumor and normal groups, we found no significant difference in the expression of the studied genes. We did observe a tendency to increase the level of CYP1B1 expression in the tumor group. We also did not find any significant difference between the levels of the studied CYPs in the tumor and conventional normal groups at different stages of prostate cancer and pathomorphological indicators. Correlation analysis revealed the presence of a positive relationship between the expressions of some cholesterol-metabolizing CYP genes, as well as between genes responsible for vitamin D biosynthesis and cholesterol biosynthesis. We observed significant correlative relationships between the expression of CYPs and some prostate cancer-related genes (CDH2, MMP9, SCHLAP1, GCR, CYP17A1, ACTA2, CXCL14, FAP, CCL17, MSMB, IRF1, VDR). Therefore, the expression of CYPs is not directly associated with prostate cancer but is largely determined by genetic, epigenetic factors, as well as endogenous substrates and xenobiotics. The significant correlative relationship between CYPs and genes associated with cancer may indicate common regulatory pathways that may have a synergistic effect on the tumor, ensuring the survival of cancer cells.
Keywords: Cytochrome P450, CYPs, Prostate cancer
Highlights
-
•
CYPs participate in signaling pathways and environmental carcinogenesis.
-
•
CYP-dependent metabolites regulate the cell cycle and migratory of cancer cells.
-
•
Individual variability in CYP genes expression may determine prostate cancer risk.
-
•
The expression of some CYP genes correlates with each other.
-
•
There is a significant relationship between CYPs and cancer-related genes.
1. Introduction
Cytochrome P450 (CYP) family is a group of around 60 enzyme genes that use molecular oxygen along with NADPH to catalyze the hydroxylation of compounds. These enzymes metabolize a wide range of endogenous and exogenous compounds and are involved in signaling pathways as well as environmental carcinogenesis [1,2]. Specifically, cytochrome P450 plays a crucial role in lipid metabolism, including the metabolism of cholesterol and fatty acids, which serve as precursors to hormones and vitamins. Additionally, CYP-dependent oxidation is a key step in the biosynthesis and metabolism of important signaling molecules like androgens, vitamin D, and more [3,4]. These CYP-dependent metabolites have been shown to regulate cell cycle, proliferation, and pro-apoptotic processes. The dysregulation of such processes is a critical factor in carcinogenesis [5].
Prostate cancer is a prevalent type of cancer worldwide. It is known that impaired regulation of cellular differentiation, proliferation, and apoptosis can initiate and significantly contribute to prostate carcinogenesis. These processes are directly linked to changes in lipid metabolism in cells. Additionally, changes in lipid metabolism are crucial in providing energy and macromolecules for biomembrane synthesis. These changes also affect membrane functioning, such as membrane permeability and receptor functioning, which consequently aids the survival of cancer cells [6].
Several studies have shown that CYPs are highly expressed in the prostate gland, implying that these enzymes play a significant role in the metabolism of intracellular signaling molecules. Furthermore, CYPs are involved in the local metabolism of xenobiotics, which can affect the intracellular response to carcinogens and modulate the therapeutic effect in the prostate gland. In our previous work, we reviewed the accumulated data on changes in the expression of certain CYPs and their possible involvement in prostate carcinogenesis [7,8].
It is well established that exposure to xenobiotics can cause cancer, primarily by altering the expression of oncogenes and antioncogenes and activating carcinogens. Xenobiotics can also cause changes in the expression of enzymes that are involved in the metabolism of anticancer drugs and endogenous compounds, which are critical to the development of cancer. A study found that enzymes belonging to the cytochrome P450 family may have a significant role in the initiation, progression, and development of prostate cancer [9].
Therefore, a comprehensive analysis of potential alterations in the expression of particular CYPs in prostate tumors is necessary to enhance the comprehension of the molecular mechanisms of carcinogenesis and discover novel molecular targets for therapy.
Our research aimed to examine how certain CYP genes that are responsible for metabolizing lipids, polyunsaturated fatty acids, androgens, vitamin D, and xenobiotics are expressed in pairs of adenocarcinoma and surrounding conditionally normal prostate tissue samples from patients who have been diagnosed with prostate cancer. Additionally, we wanted to determine if there is a correlation between the expression levels of these genes and certain clinical and pathological data of the patients.
2. Materials and methods
2.1. Obtaining samples of prostate tissues
Surgically extracted material from both adenocarcinoma (tumor group) and conventionally normal tissues (conventional normal group) of the human prostate gland was used for this study. The study analyzed 41 pairs of tumor and conventionally normal tissue samples (T/CNT), as well as two unpaired prostate tumor samples (Table A.1). All samples were obtained from the National Cancer Institute (Kyiv, Ukraine). The prostate tissue samples were collected from 43 patients aged between 48 and 72 years, following The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
2.2. Total RNA isolation and cDNA synthesis
The total RNA was isolated from prostate tissues using the GeneJET RNA purification kit (ThermoSci, USA) following the manufacturer's protocol. The RNA samples were treated with DNAaseI (ThermoSci, Lithuania) according to the manufacturer's instructions. For cDNA synthesis, the Maxima H Minus cDNA synthesis master mix (ThermoSci, Lithuania) reagents were used as per the manufacturer's protocol. The cDNA samples were stored at −70 °C until used.
2.3. Quantitative RT-PCR for determining the levels of relative gene expression
The iCycler iQ5 Multicolor Detection System (Bio-Rad, USA) amplifier was used to perform real-time quantitative PCR (qPCR). A set of Hot FirePol EvaGreeen qPCR Supermix (Solis Biodynes, Estonia) reagents was used according to the manufacturer's protocol. The qPCR conditions were 12 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. The primers used for qPCR can be found in Table A.2. Relative gene expression levels were normalized by the TBP reference gene. The 2-ΔСt method was used to calculate the levels of relative gene expression, as described earlier [[10], [11], [12]].
2.4. Statistical data processing
The results of relative expression studies were analyzed using STATISTICA 10 software. To determine the normality of the distribution of relative gene expression levels, we used the Kolmogorov-Smirnov and Lilliefors tests. The Wilcoxon paired test was used to evaluate the differences between adenocarcinomas and their matched conditionally normal tissues according to the 2-ΔCt model. The Benjamini-Hochberg procedure with FDR = 0.1–0.25 was used to correct for multiple comparisons for these tests.
Kruskal-Wallis and Dunn-Bonferroni tests for multiple comparisons were performed to determine differences in relative expression between all sample groups [[10], [11], [12]].
Statistical significance was determined for all types of analysis using a p-value of <0.05. To identify correlations between gene expression and clinical and pathological characteristics of the samples, as well as correlations between gene expression levels, Spearman's rank correlation test was employed.
3. Results
3.1. Relative levels of CYP genes expression in pairs of tumor and conventionally normal tissue samples (T/CNT) of prostate
We examined the levels of 14 CYP genes' relative expression in 41 pairs of prostate cancer (T) and conventionally normal tissue samples (CNT), as well as two unpaired prostate tumor samples (numbers 40 and 43). Table A.1 displays the clinical and pathological characteristics of the tissue samples and some patient anamnesis data.
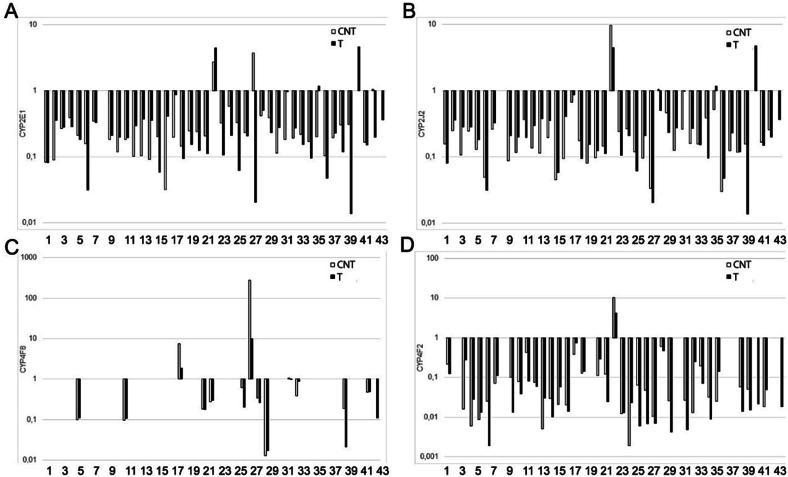
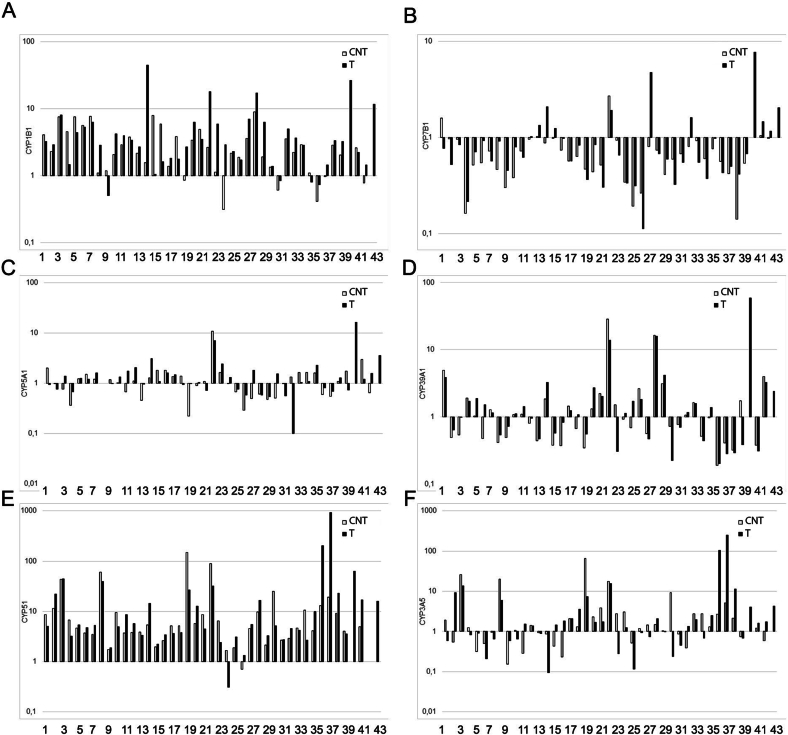
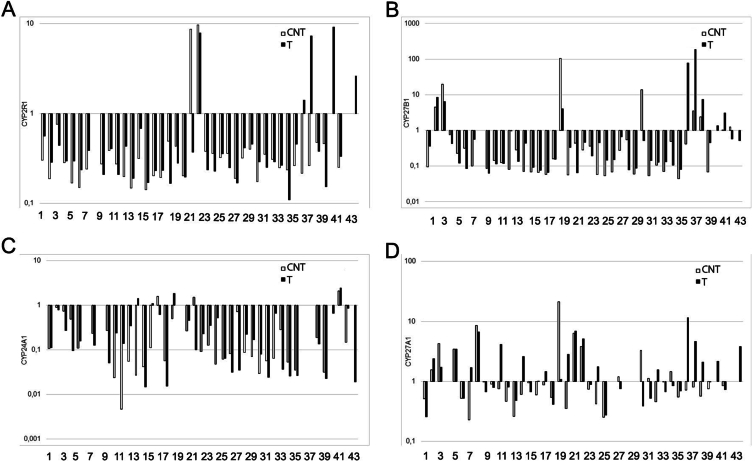
The study found that all of the CYP genes investigated were expressed in the prostate gland in both cancer and CNT groups (Fig. 1, Fig. 2, Fig. 3). However, there was a significant variation in the relative gene expression data among individual samples of all the studied genes in both the prostate tumor and CNT sample groups (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
The relative expression levels of CYP genes involved in the metabolism of polyunsaturated fatty acids in pairs of tissue samples of the human prostate gland. (A) CYP2E1, (B) CYP2J2, (C) CYP4F8, (D) CYP4F2. The horizontal axis represents the number of tissue sample pairs (T/CNT). T – tumor, CNT – conventionally normal tissue samples.
Fig. 2.
The relative expression levels of CYP genes involved in the metabolism of cholesterol, testosterone and xenobiotics in pairs of tissue samples of the human prostate gland. (A) CYP1B1, (B) CYP7B1, (C) CYP5A1, (D) CYP39A1, (E) CYP51, (F) CYP3A5. The horizontal axis represents the number of tissue sample pairs (T/CNT). T – tumor, CNT – conventionally normal tissue samples.
Fig. 3.
The relative expression levels of CYP genes involved in the metabolism of vitamin D in pairs of tissue samples of the human prostate gland. (A) CYP2R1, (B) CYP27B1, (C) CYP24A1, (D) CYP27A1. The horizontal axis represents the number of tissue sample pairs (T/CNT). T – tumor, CNT – conventionally normal tissue samples.
We observed high levels of CYP1B1, CYP4F2, and CYP3A5 gene expression in some patients with a history of long-term smoking (Fig. 1, Fig. 2, Table A.1). However, there were no differences in the expression levels of CYP genes responsible for xenobiotic metabolism in these samples.
3.2. Statistical analysis of the levels of relative gene expression of CYP genes in the prostate cancer and CNT groups
We conducted a comparative analysis of the expression levels of CYP genes between pairs of tumor samples and CNT, as well as between groups of tumor samples and CNT. The study revealed that the available sample of expression levels for most of the studied CYP genes did not follow a normal distribution. Therefore, we utilized descriptive statistics and non-parametric statistical data analysis (Table 1).
Table 1.
Descriptive statistics data of relative expression levels of CYPs in prostate cancer and CNT groups.
| Genes | Tumor group |
CNT group |
||||
|---|---|---|---|---|---|---|
| Median | 25.000 | 75.000 | Median | 25.000 | 75.000 | |
| CYP7B1 | 0.68 | 0.45 | 1.01 | 0.63 | 0.46 | 0.93 |
| CYP27A1 | 1.05 | 0.67 | 2.62 | 0.79 | 0.53 | 1.33 |
| CYP39A1 | 1.14 | 0.54 | 1.89 | 0.93 | 0.49 | 1.65 |
| CYP51 | 5.10 | 3.34 | 16.56 | 5.05 | 3.58 | 9.61 |
| CYP1B1 | 3.25 | 1.73 | 5.94 | 2.30 | 1.35 | 3.84 |
| CYP3A5 | 1.46 | 0.68 | 3.56 | 1.30 | 0.74 | 2.77 |
| CYP4F8 | 0.23 | 0.11 | 0.88 | 0.34 | 0.18 | 0.62 |
| CYP5A1 | 1.14 | 0.77 | 1.59 | 1.08 | 0.63 | 1.55 |
| CYP4F2 | 0.03 | 0.01 | 0.12 | 0.04 | 0.02 | 0.11 |
| CYP2J2 | 0.21 | 0.11 | 0.35 | 0.16 | 0.11 | 0.26 |
| CYP2E1 | 0.20 | 0.16 | 0.36 | 0.20 | 0.15 | 0.32 |
| CYP2R1 | 0.29 | 0.23 | 0.43 | 0.28 | 0.20 | 0.38 |
| CYP27B1 | 0.26 | 0.11 | 0.58 | 0.15 | 0.07 | 0.51 |
| CYP24A1 | 0.15 | 0.04 | 0.53 | 0.10 | 0.05 | 0.28 |
Notes: 25.000 and 75.000 – Percentiles.
We used the Wilcoxon Matched Pairs Test to analyze paired T/CNT samples and found that there were no significant differences in the expression of almost all the studied genes between the conditional normal and tumor groups. However, we did observe a slight increase in the expression level of the CYP1B1 gene in the tumor group compared to the conditional norm, although the p-value (0.059) was not statistically significant.
Our study aimed to investigate whether there are any changes in the expression of CYP genes in tumor samples compared to conditionally normal tissue, based on the stage of the disease and/or the Gleason score (GS). We used the Dunn-Bonferroni post-hoc test for multiple comparisons, and our findings revealed that there was no significant difference in the expression levels of the studied genes between the cancer groups and CNT at different stages and GS indices. However, we did observe a tendency to increase the expression of CYP2E1 in prostate tumor samples at late stages compared to the norm (p = 0.054) in the group of samples with SG 6. Additionally, we noticed a tendency to increase the level of CYP2E1 expression in the cancer group samples relative to the CNT (p = 0.059) at the 2nd and 3rd stages of the disease.
We did not observe a significant difference in the levels of relative expression of CYP genes between the cancer and CNT groups. Therefore, we combined all expression values into a single group and examined potential alterations in gene expression based on the stage of the disease and/or GS.
According to the Kruskal-Wallis Test with Dunn-Bonferroni for multiple comparisons, some significant differences were found for certain CYP genes. The expression level of CYP4F8 was found to be decreased in the group of samples from GS 8 when compared to the group from GS 7 (p = 0.011). Additionally, a tendency to decrease the level of CYP2J2 was observed in the group from GS 9 when compared to SG 7 (p = 0.065). On the other hand, an increase in the level of CYP7B1 expression was detected in the group of samples with stage 4 disease compared to the groups with stage 2 (p = 0.015) and stage 3 (p = 0.022). In the case of CYP27B1, lower expression was detected in stages 2 (p = 0.034) and 4 (p = 0.034) compared to stage 1. Furthermore, there was also a tendency to decrease the expression of CYP51 at stage 2 when compared to stage 1 (p = 0.057).
3.3. Study of the correlations between the expression levels of individual CYP genes and the clinical and pathological characteristics of patients
We analyzed a group of tumor samples to determine the correlation between the expression levels of 14 individual CYP genes and the clinical and pathological characteristics of patients. We used Spearman Rank Order Correlation tests and found that there was no significant correlation.
A combined sample of tumor and conditionally normal tissues was subjected to correlation analysis. The results showed weak but significant correlations (p < 0,050). Firstly, there was a negative correlation between the level of CYP24A1 expression and the index of GS (rs = −0.268). Secondly, there was a negative correlation between the expression levels of certain genes (CYP7B1, CYP5A1, CYP4F2) and age (rs = −0.227, rs = −0.237, rs = −0.266, respectively). Finally, a weak positive correlation was found between the level of CYP2E1 expression and the stage of the disease (rs = 0.218).
3.4. Study of the correlations between the expression levels of individual CYP genes in prostate cancer samples
A study was conducted on a group of prostate cancer samples to analyze the correlation between the expression levels of CYP genes. The study revealed the presence of significant positive and negative correlations between the expression levels of 13 CYP genes. The significant rank correlation coefficients between gene expressions are presented in Table 2. The highest correlation coefficient was found between the genes CYP51 and CYP3A5.
Table 2.
Spearman Rank Order Correlations coefficients (rs) for CYP genes expression in human prostate cancer samples according to the Dunn-Bonferroni test (p < 0.05).
| Genes | CYP51 | CYP1B1 | CYP3A5 | CYP5A1 | CYP4F2 | CYP2E1 | CYP2R1 | CYP27B1 | CYP24A1 |
|---|---|---|---|---|---|---|---|---|---|
| CYP7B1 | − | 0.420 | − | 0.507 | − | − | − | − | − |
| CYP27A1 | 0.583 | − | 0.580 | − | − | − | − | − | − |
| CYP39A1 | − | 0.478 | − | − | − | 0.539 | − | − | − |
| CYP51 | − | − | 0.643 | − | 0.437 | − | 0.622 | 0.601 | − |
| CYP3A5 | 0.643 | − | − | − | 0.594 | − | − | − | − |
| CYP2J2 | − | − | − | − | − | − | 0.406 | − | − |
| CYP2E1 | − | − | − | − | − | − | 0.430 | − | −0.441 |
– The value of the coefficient (rs) is either very low or statistically insignificant.
3.5. Study of correlations between relative expression of certain CYP genes and genes associated with prostate carcinogenesis
In our previous research, we analyzed the expression levels of various genes linked with prostate cancer and prostate tumor microenvironment, in prostate tissue samples from the 43 patients (Table A.1). Our investigation focused on 12 genes, namely CDH2, MMP9, SCHLAP1, GCR, CYP17A1, ACTA2, CXCL14, FAP, CCL17, MSMB, IRF1, and VDR [[10], [11], [12]]. We conducted a correlation analysis of the expression levels of these genes and CYP genes in prostate cancer samples, which revealed some significant positive and negative correlations between the expression levels of certain genes. The most potent positive correlation was found between CYP24A1 and MSMB genes. On the other hand, the strongest negative correlation was detected between CYP24A1 and CCL17 genes (Table 3).
Table 3.
Spearman Rank Order Correlations coefficients (rs) for the expression of CYP genes and some genes associated with carcinogenesis in human prostate cancer samples according to the Dunn-Bonferroni test (p < 0.05).
| Genes | CYP7B1 | CYP27A1 | CYP4F2 | CYP2E1 | CYP27B1 | CYP24A1 |
|---|---|---|---|---|---|---|
| CDH2 | − | 0.800 | 0.700 | − | − | −0.317 |
| MMP9 | − | −0.600 | −0.700 | − | −0.283 | −0.267 |
| VDR | −0.517 | − | − | −0.717 | −0.817 | − |
| SCHLAP1 | − | −0.683 | −0.633 | − | − | − |
| GCR (AG) | − | 0.367 | − | − | − | −0.817 |
| CYP17A1 | 0.733 | − | −0.283 | − | 0.500 | − |
| ACTA2 | − | 0.633 | 0.317 | − | − | −0.667 |
| CXCL14 | − | − | −0.317 | 0.400 | − | −0.833 |
| FAP | −0.483 | −0.267 | −0.333 | −0.533 | −0.717 | − |
| MSMB | 0.267 | − | 0.433 | 0.483 | 0.350 | 0.833 |
| IRF1 (T1) | −0.350 | −0.300 | −0.250 | −0.467 | −0.667 | − |
| CCL17 | − | − | −0.617 | − | − | −0.850 |
– The value of the coefficient (rs) is either very low or statistically insignificant.
4. Discussion
It is known that certain metabolic changes in prostate cells can trigger the development of prostate cancer and contribute to its progression. Studies have shown that changes in lipid metabolism, as well as in the metabolism of hormones and vitamins, are significantly associated with prostate carcinogenesis [[6], [7], [8]]. Moreover, alterations in the metabolism of xenobiotics and anticancer drugs in prostate cells may also play a role in the initiation and progression of prostate cancer. These compounds are predominantly metabolized by the cytochrome P450 enzymes [7,8].
In this study, we have observed the expression of all the cytochrome P450 genes that are responsible for the metabolism of cholesterol, polyunsaturated fatty acids, androgens, vitamin D, and xenobiotics, including drugs, in samples of both prostate cancer and CNT. This suggests that cytochrome P450-dependent metabolic processes of polyunsaturated fatty acids, lipids, vitamin D, and androgens take place in prostate tissues. These processes play a vital role in regulating cell cycle, proliferation, and apoptosis [7,8]. Some of the studied CYPs are also involved in the metabolism of xenobiotics, which can result in the formation and activation of procarcinogens or a decrease in the effectiveness of anticancer drugs in the human prostate gland [7].
It is important to note that the levels of CYP genes expression varied significantly among the samples studied. In some cases, very high or very low expression levels were observed in paired samples from the same patient, which can be attributed to individual differences in CYP genes expression [13]. This may be due to individual metabolic processes or external factors such as smoking and certain medications. [13]. Cigarette smoke compounds are known to affect the expression of several CYP genes, including CYP1B1, CYP3A5, CYP4F2, CYP7B1, and CYP2E1, which have been linked to increased risk of carcinogenesis in respiratory tract tissues [[14], [15], [16], [17], [18]]. Long-term smoking has also been associated with prostate cancer [19]. We found that some smoking patients had relatively high levels of expression of CYP1B1 (numbers 6, 7, 15, 28, 29, 32), CYP3A5 (numbers 30, 33, 34, 35, 38), and CYP4F2 (numbers 28, 32). However, due to a lack of data, we cannot assess the relationship between the expression level of these genes and prostate cancer.
It has been observed that some patients exhibit either extremely high or low levels of expression of CYP genes, which play a key role in controlling the metabolism of cholesterol, fatty acids, and vitamin D. However, due to the unavailability of individual patient data on the metabolism of these compounds, it is difficult to assess the correlation between the variability in gene expression and these processes.
In our previous review of the literature, we discovered significant changes in the expression levels of certain CYP genes in malignant tumors, as compared to normal prostate cells.
Specifically, we found evidence of increased expression of CYP genes that are involved in lipid metabolism, especially CYP1B1 [7]. It is known that CYP1B1 catalyzes the conversion of estradiol to 4-Hydroxy-17β-estradiol and promote the development and progression of hormone-related prostate cancer [20]. In our research, we also observed an increase in the level of expression of this gene in the tumor when compared to conditionally normal tissue. CYP1B1 plays an important role in energy homeostasis, regulation proliferative activity and procarcinogen activation, so an increase in its expression may contribute to carcinogenesis [21].
In a previous study, we discovered that changes in the expression of cytochrome P450 enzymes (which are crucial in the metabolism of vitamin D, a potent anticancer agent) in a tumor compared to normal tissue, depending on their function [8]. Specifically, it was observed significant suppression of the genes CYP27A1 and CYP27B1, which play a critical role in the synthesis of vitamin D, and a significant increase in the expression of CYP24A1, which degrades vitamin D, in prostate cancer samples [8]. However, in our study, we did not find significant changes in these CYPs in the tumor compared to conditionally normal tissue. Other researchers have reported significant suppression of CYP3A5 expression, which regulates testosterone metabolism and the functioning of the androgen receptor, as well as metabolizes most drugs [8]. Nevertheless, we did not observe such changes in the tumor compared to the conditional norm in our work.
We found that there was no significant variation in the expression of the other CYP genes in prostate cancer tissue in comparison to the CNT samples. However, it is worth noting that different authors have employed various approaches in studying changes in relative gene expression. For instance, some studies have analyzed the expression of certain CYP genes in cell lines [[22], [23], [24]], while others have compared the expression levels of other CYPs in prostate cancer patients with those of the corresponding genes in normal and adenoma prostate tissues of other patients [[25], [26], [27]]. In our research, we compared the gene expression levels in a pair of samples taken from cancer and surrounding conventionally normal tissue without signs of carcinogenesis in each individual patient. This approach considers the individual variability of CYPs expression levels, which is essential in characterizing this family of enzymes.
Research has shown that the expression levels of certain CYP genes, particularly CYP27A1 and CYP24A1, are associated with the degree of cell differentiation and the stage of prostate cancer. These genes may also affect the rate and prognosis of the disease [26]. In our study, we found a weak but significant negative correlation between CYP24A1 and the index of SG. Additionally, we observed weak but significant correlations between other CYP genes and the stage of the disease. It is possible that a larger study could reveal new correlations between gene expression levels and clinical or pathological characteristics of patients.
Upon conducting a correlation analysis on the expression levels of CYP genes studied within the prostate tumor group, it was discovered that there is a positive correlation between the expression levels of individual CYP genes that may be functionally related. Specifically, we observed that genes responsible for metabolizing cholesterol are positively correlated with each other in the tumor group. Additionally, we found a positive correlation between the expression of genes responsible for vitamin D biosynthesis and genes that biosynthesize cholesterol, which is the precursor of all fat-soluble vitamins [7,8].
In a study of 43 prostate cancer patients (as presented in Table A.1), we investigated the correlation between the expression of certain genes associated with prostate cancer and CYP genes. The genes in question are CYP17A1, MMP-9, GCR, ACTA2, SChLAP1, CXCL14, FAP, CCL17, VDR, IRF1, and MSMB, which play a role in processes such as proliferation, migration, invasion, angiogenesis, apoptosis, and cell cycle control [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. It was found that the expression of a number of genes of cytochrome P450 had significant correlations with the expression of these genes (Table 3).
It is known that cholesterol is a crucial structural lipid in cells, and an increase in its biosynthesis is linked to an increase in the rate of proliferative processes. Moreover, cholesterol is a precursor to hormones, particularly androgens, which are crucial for prostate carcinogenesis. CYP-dependent derivatives of omega-6 polyunsaturated fatty acids, especially arachidonic acid, regulate the migration and invasion processes of cancer cells [8,9]. We have observed a strong correlation between CYP genes that regulate lipid metabolism and oncogenes, as well as prostate cancer suppressor genes. We found that the expression of the CYP7B1 gene is positively correlated with the CYP17A1 oncogene, which is responsible for testosterone synthesis [32]. The CDH2 oncogene, which controls the proliferative and migratory potential of prostate cancer cells [35], is positively correlated with the CYP27A1 and CYP4F2. Furthermore, we observed a strong negative correlation between the expression level of CYP2E1 and the vitamin D receptor gene, VDR, which is one of the tumor suppressor genes that stimulates proapoptotic processes and inhibits cell proliferation [37]. We have discovered some correlations between the CYP genes, which regulate vitamin D metabolism, and genes that are linked to prostate cancer. The tumor suppressor gene CYP27B1 is negatively correlated with the oncogene FAP, and also negatively correlated with the tumor suppressor genes VDR and IRF1. The potential oncogene CYP24A1 is negatively correlated with almost all the studied oncogenes and positively correlated with the tumor suppressor gene MSMB.
The role of these genes in causing cancer suggests that their functional relationship has a combined effect on the tumor. This promotes the survival of cancer cells and increases their carcinogenic potential.
5. Conclusion
Based on the given data, it can be assumed that changes in the expression level of CYPs in prostate tissues do not have a direct correlation with carcinogenesis in the prostate gland. Instead, it is largely due to the individual characteristics of endogenous metabolism in the tissues of this organ, along with the impact of certain exogenous factors such as smoking, drugs, etc. Further research is required to understand the effect of these factors in detail. The presence of a significant correlation between certain CYP genes and genes associated with prostate carcinogenesis suggests that there may be common regulatory pathways that can have a synergistic effect on the tumor, promoting the survival of cancer cells, and increasing their proliferative, invasive, and migratory potential.
The following are the supplementary data related to this article.
Clinical and pathologic characteristics of tissue samples and patient anamnesis data.
Primer sequences for real-time quantitative PCR.
Author contributions
Conceptualization, O.M. and G.G.; methodology, V.K.; validation, O.M. and G.G.; formal analysis, O.M. and G.G.; investigation, O.M., G.G., O.K. and I.R.; resources, E.S, O.K. and A.T.; data curation, V.K.; writing original draft preparation, O.M.; review and editing, G.G. and V.K.; supervision, V.K.; project administration, V.K. All authors have read and agreed to the published version of the manuscript.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Institute of Molecular Biology and Genetics of NAS of Ukraine (protocol code 32 and date of approval 04/10/2022).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used [Grammarly's generative AI/ https://www.grammarly.com] in order to improve readability and language. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Funding
This work was supported by a grant from the Simons Foundation (Award #1030279, [O.M., G.G., I.R., V.K.]). Financing was carried out from the funds of the State Budget of Ukraine.
CRediT authorship contribution statement
Oksana Maksymchuk: Conceptualization, Formal analysis, Funding acquisition, Investigation, Validation, Writing – original draft. Ganna Gerashchenko: Conceptualization, Formal analysis, Funding acquisition, Investigation, Validation, Writing – review & editing. Inna Rosohatska: Investigation, Funding acquisition. Oleksiy Kononenko: Investigation, Resources. Andriy Tymoshenko: Resources. Eduard Stakhovsky: Resources. Volodymyr Kashuba: Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability
The data used to support the findings of this study are included in the article. Other data that might be useful for the findings of this study will be supplied as supplementary information by the corresponding author upon request.
References
- 1.Nebert D., Dalton T. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 2.Hrycay E.G., Bandiera S.M. Involvement of cytochrome P450 in reactive oxygen species formation and cancer. Adv. Pharmacol. 2015;74:35–84. doi: 10.1016/bs.apha.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Cederbaum A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73. doi: 10.1016/j.redox.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swami S., Krishnan A.V., Feldman D. Vitamin D metabolism and action in the prostate: implications for health and disease. Mol. Cell. Endocrinol. 2011;347:61–69. doi: 10.1016/j.mce.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T.C., Sakaki T., Yamamoto K., Kittaka A. The roles of cytochrome P450 enzymes in prostate cancer development and treatment. Anticancer Res. 2012;32:291–298. [PubMed] [Google Scholar]
- 6.Liu Q., Luo Q., Halim A., Song G. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer. Cancer Lett. 2017;401:39–45. doi: 10.1016/j.canlet.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Maksymchuk O., Kashuba V. Dietary lipids and environmental xenobiotics as risk factors for prostate cancer: the role of cytochrome P450. Pharmacol. Rep. 2019;71:826–832. doi: 10.1016/j.pharep.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Maksymchuk O.V., Kashuba V.I. Altered expression of cytochrome P450 enzymes involved in metabolism of androgens and vitamin D in the prostate as a risk factor for prostate cancer. Pharmacol. Rep. 2020;72:1161–1172. doi: 10.1007/s43440-020-00133-y. [DOI] [PubMed] [Google Scholar]
- 9.Bruno R.D., Njar V.C. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg. Med. Chem. 2007;15:5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerashchenko G.V., Mankovska O.S., Dmitriev A.A., Mevs L.V., Rosenberg E.E., Pikul M.V., Marynychenko M.V., Gryzodub O.P., Stakhovsky E.O., Kashuba V.I. Expression of epithelial-mesenchymal transition-related genes in prostate tumours. Вiopolym. Cell. 2017;33:335–355. doi: 10.7124/bc.00095E. [DOI] [Google Scholar]
- 11.Gerashchenko G.V., Mevs L.V., Chashchina L.I., Pikul M.V., Gryzodub O.P., Stakhovsky E.O., Kashuba V.I. Expression of steroid and peptide hormone receptors, metabolic enzymes and EMT-related genes in prostate tumors in relation to the presence of the TMPRSS2/ERG fusion. Exp. Oncol. 2018;40:101–108. [PubMed] [Google Scholar]
- 12.Gerashchenko G.V., Grygoruk O.V., Kononenko O.A., Gryzodub O.P., Stakhovsky E.O., Kashuba V.I. Expression pattern of genes associated with tumor microenvironment in prostate cancer. Exp. Oncol. 2018;40:315–322. [PubMed] [Google Scholar]
- 13.McDonnell A.M., Dang C.H. Basic review of the cytochrome p450 system. J. Adv. Pract. Oncol. 2013;4:263–268. doi: 10.6004/jadpro.2013.4.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang M., Heerdt P.M., Kopelovich L., Marcus C.B., Altorki N.K., Subbaramaiah K., Dannenberg A.J. Tobacco smoke induces CYP1B1 in the aerodigestive tract. Carcinogenesis. 2004;25:2275–2281. doi: 10.1093/carcin/bgh243. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L.P., Zhu Z.T., He C.Y. Effects of CYP3A5 genetic polymorphism and smoking on the prognosis of non-small-cell lung cancer. Onco Targets Ther. 2016;9:1461–1469. doi: 10.2147/OTT.S94144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia J., Conlon T.M., Sarker R.S., Taşdemir D., Smirnova N.F., Srivastava B., Verleden S.E., Güneş G., Wu X., Prehn C., Gao J., Heinzelmann K., Lintelmann J., Irmler M., Pfeiffer S., Schloter M., Zimmermann R., Hrabé de Angelis M., Beckers J., Adamski J., Bayram H., Eickelberg O., Yildirim A.Ö. Cholesterol metabolism promotes B-cell positioning during immune pathogenesis of chronic obstructive pulmonary disease. EMBO Mol. Med. 2018;10 doi: 10.15252/emmm.201708349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maideen N.M.P. Tobacco smoking and its drug interactions with comedications involving CYP and UGT enzymes and nicotine. World J. Pharmacol. 2019;8:14–25. doi: 10.5497/wjp.v8.i2.14. [DOI] [Google Scholar]
- 18.Ding Y., Yang Y., Li Q., Feng Q., Xu D., Wu C., Zhao J., Zhou X., Niu H., He P., Liu J., Yao H. The correlation between CYP4F2 variants and chronic obstructive pulmonary disease risk in Hainan Han population. Respir. Res. 2020;21 doi: 10.1186/s12931-020-01348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huncharek M., Haddock K.S., Reid R., Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am. J. Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Q., Cao J., Du X., Yang K., Yang X., Liang Z., Shi J., Zhang J. CYP1B1-catalyzed 4-OHE2 promotes the castration resistance of prostate cancer stem cells by estrogen receptor α-mediated IL6 activation. Cell Commun. Signal. 2022;20:31. doi: 10.1186/s12964-021-00807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah B.R., Xu W., Mraz J. Cytochrome P450 1B1: role in health and disease and effect of nutrition on its expression. RSC Adv. 2019;9:21050–21062. doi: 10.1039/c9ra03674a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T.C. 25-Hydroxyvitamin D-1 alpha-hydroxylase (CYP27B1) is a new class of tumor suppressor in the prostate. Anticancer Res. 2008;28:2015–2017. [PubMed] [Google Scholar]
- 23.Nithipatikom K., Brody D.M., Tang A.T., Manthati V.L., Falck J.R., Williams C.L., Campbell W.B. Inhibition of carcinoma cell motility by epoxyeicosatrienoic acid (EET) antagonists. Cancer Sci. 2010;101:2629–2636. doi: 10.1111/j.1349-7006.2010.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfaqih M.A., Nelson E.R., Liu W., Safi R., Jasper J.S., Macias E., Geradts J., Thompson J.W., Dubois L.G., Freeman M.R., Chang C.Y., Chi J.T., McDonnell D.P., Freedland S.J. CYP27A1 loss dysregulates cholesterol homeostasis in prostate cancer. Cancer Res. 2017;77:1662–1673. doi: 10.1158/0008-5472.CAN-16-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leskelä S., Honrado E., Montero-Conde C., Landa I., Cascón A., Letón R., Talavera P., Cózar J.M., Concha A., Robledo M., Rodríguez-Antona C. Cytochrome P450 3A5 is highly expressed in normal prostate cells but absent in prostate cancer. Endocr. Relat. Cancer. 2007;14:645–654. doi: 10.1677/ERC-07-0078. [DOI] [PubMed] [Google Scholar]
- 26.Tannour-Louet M., Lewis S.K., Louet J.F., Stewart J., Addai J.B., Sahin A., Vangapandu H.V., Lewis A.L., Dittmar K., Pautler R.G., Zhang L., Smith R.G., Lamb D.J. Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB J. 2014;28:364–372. doi: 10.1096/fj.13-236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vainio P., Gupta S., Ketola K., Mirtti T., Mpindi J.P., Kohonen P., Fey V., Perälä M., Smit F., Verhaegh G., Schalken J., Alanen K.A., Kallioniemi O., Iljin K. Arachidonic acid pathway members PLA2G7, HPGD, EPHX2, and CYP4F8 identified as putative novel therapeutic targets in prostate cancer. Am. J. Pathol. 2011;178:525–536. doi: 10.1016/j.ajpath.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augsten M., Hägglöf C., Olsson E., Stolz C., Tsagozis P., Levchenko T., Frederick M.J., Borg A., Micke P., Egevad L., Ostman A. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prensner J.R., Iyer M.K., Sahu A., Asangani I.A., Cao Q., Patel L., Vergara I.A., Davicioni E., Erho N., Ghadessi M., Jenkins R.B., Triche T.J., Malik R., Bedenis R., McGregor N., Ma T., Chen W., Han S., Jing X., Cao X., Wang X., Chandler B., Yan W., Siddiqui J., Kunju L.P., Dhanasekaran S.M., Pienta K.J., Feng F.Y., Chinnaiyan A.M. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013;45:1392–1398. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maolake A., Izumi K., Shigehara K., Natsagdorj A., Iwamoto H., Kadomoto S., Takezawa Y., Machioka K., Narimoto K., Namiki M., Lin W.J., Wufuer G., Mizokami A. Tumor-associated macrophages promote prostate cancer migration through activation of the CCL22-CCR4 axis. Oncotarget. 2017;8:9739–9751. doi: 10.18632/oncotarget.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma F., Wang Z., Abdularab A., Dai L., Niu Y., Jiang N. Matrix metalloproteinase 9 and prostate cancer risk: a meta-analysis of 1059 participants. Minerva Urol. Nefrol. 2017;69:324–329. doi: 10.23736/S0393-2249.16.02623-0. [DOI] [PubMed] [Google Scholar]
- 32.Giatromanolaki A., Fasoulaki V., Kalamida D., Mitrakas A., Kakouratos C., Lialiaris T., Koukourakis M.I. CYP17A1 and androgen-receptor expression in prostate carcinoma tissues and cancer cell lines. Curr. Urol. 2019;13:157–165. doi: 10.1159/000499276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh R., Diaz R.J. The remodelling of actin composition as a hallmark of cancer. Transl. Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesch C., Yirga L., Dendl K., Handke A., Darr C., Krafft U., Radtke J.P., Tschirdewahn S., Szarvas T., Fazli L., Gleave M., Giesel F.L., Haberkorn U., Hadaschik B. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur. J. Nucl. Med. Mol. Imaging. 2021;49:385–389. doi: 10.1007/s00259-021-05423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu C.H., Wu C.H., Hsieh P.F., Wu C.Y., Kuo W.W., Ou C.H., Lin V.C.H. Small interfering RNA targeting N-cadherin regulates cell proliferation and migration in enzalutamide-resistant prostate cancer. Oncol. Lett. 2022;23:90. doi: 10.3892/ol.2022.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakellakis M., Flores L.J. Is the glucocorticoid receptor a key player in prostate cancer? A literature review. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000029716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendrickson W.K., Flavin R., Kasperzyk J.L., Fiorentino M., Fang F., Lis R., Fiore C., Penney K.L., Ma J., Kantoff P.W., Stampfer M.J., Loda M., Mucci L.A., Giovannucci E. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J. Clin. Oncol. 2011;29:2378–2385. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergström S.H., Järemo H., Nilsson M., Adamo H.H., Bergh A. Prostate tumors downregulate microseminoprotein-beta (MSMB) in the surrounding benign prostate epithelium and this response is associated with tumor aggressiveness. Prostate. 2018;78:257–265. doi: 10.1002/pros.23466. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y., Wang D., Jiang J., Huang W., Li D., Luo J., Gu W., Mo W., Wang C., Li Y., Gu S., Xu Y. Integrative analysis of AR-mediated transcriptional regulatory network reveals IRF1 as an inhibitor of prostate cancer progression. Prostate. 2020;80:640–652. doi: 10.1002/pros.23976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical and pathologic characteristics of tissue samples and patient anamnesis data.
Primer sequences for real-time quantitative PCR.
Data Availability Statement
The data used to support the findings of this study are included in the article. Other data that might be useful for the findings of this study will be supplied as supplementary information by the corresponding author upon request.