Abstract
Non-typhoidal Salmonella (NTS) is a food-borne zoonotic pathogen with important implications for human health. Despite its importance, the burden of NTS infections in Vietnam is unknown. We conducted a systematic review and a meta-analysis to describe the prevalence and phenotypic antimicrobial resistance (AMR) for NTS over time in Vietnam. Following PRISMA guidelines, we identified 72 studies from PubMed and Google Scholar containing data relevant to ‘Salmonella’, ‘Salmonellosis’, and ‘Vietnam’, or ‘Viet Nam’. Of those, forty-two papers reporting prevalence of NTS, and twenty-six including data on phenotypic resistance were selected for meta-analysis. The prevalence of NTS ranged from 2% to 5% in humans and from 30% to 41% in samples from animals and the farm/slaughterhouse environment. Between 2000 and 2020 we observed a 27.3% (p = 0.044) increase in absolute terms in prevalence among individuals with enteric diseases and a 12.0% (p = 0.047) increase in aquaculture/seafood samples. The most commonly serovars identified across studies included S. Weltevreden (12.0%), followed by S. Typhimurium (10.1%), S. Derby (6.4%), S. London (5.8%), S. Anatum (4.4%), S. Rissen (3.9%), S. Enteritidis (3.7%), S. Albany (3.3%) and S. 4,[5],12:i:- (3.0%). Over the same period, there was an increasing trend in the pooled AMR prevalence for quinolones (15.6% relative increase), 3rd-, 4th-, and 5th-generation cephalosporins (23.7%), penicillins (16.1%), tetracyclines (12.9%), sulfonamides (8.8%), amphenicol (17.8%), and multidrug resistance (11.4%) (all p ≤ 0.014). A broad range of AMR genes (ARGs) were detected in both human and animal populations. The observed prevalence and AMR trends in Vietnam underscore the need of adopting a One Health strategy encompassing surveillance systems, research initiatives, and awareness campaigns to effectively address the challenges posed by NTS.
Keywords: Non-typhoidal Salmonella, Systematic review, Meta-analysis, Antimicrobial resitance, One Health, Vietnam
1. Introduction
Salmonella enterica is a global pathogen responsible for enteric fever, diarrhea and invasive disease. >2500 Salmonella serovars have been identified worldwide [1]. Non-typhoidal Salmonella (NTS) are serovars other than Typhi. In developed countries the economic burden of NTS results from the large number of human gastroenteritis cases and serious sequelae in some cases [2]. Particularly in Africa, invasive NTS infections (iNTS) account for ∼4100 annual deaths, mostly in children with malnutrition, malaria, and HIV [3].
NTS, being zoonotic, can be hosted by many animal species, and may further contaminate the environment, water and food. Given its ubiquity, its impact on human health including implications for food safety in the farm-to-fork continuum and its capacity for acquisition of antimicrobial resistance (AMR) traits [4] control of NTS should follow a One Health approach. These recognize that the health of humans, animals, plants, and the wider environment (including ecosystems) are closely interdependent [5]. In many developed countries with well-established surveillance systems NTS is a notifiable disease and its epidemiological features are well characterized.
Until the early 1990's, Vietnam has been endemic for typhoid fever. However, improvements in sanitation and water quality alongside vaccination efforts have led to massive reductions over the past 25 years [6]. In parallel to this, more attention has been paid to NTS. In 2017 Vietnam established active surveillance of AMR in NTS from pigs and chickens at slaughter points and markets [7].
Over the last two decades Vietnam has been experiencing rapid intensification in food animal production, and has now reached a leading global position especially in aquaculture [8], with some incipient exports of pig and poultry products. Intensification in animal production is associated with increased connectivity between different components in the value chain and may potentially contribute to increased dissemination of NTS strains.
The burden of human disease due to NTS in Vietnam is not well known. This is because of other pressing priorities in the health system as well as the technical challenges of diagnosis of diarrheal pathogens. Paradoxically, the Vietnamese authorities are aware of the importance of food safety in the country. Outbreaks of disease (often due to NTS) are detected and covered by the media. For example, in November 2022, an outbreak in Nha Trang province affected over 600 schoolchildren, resulting in one fatality [9]. There is also awareness of a potential higher risk of bacterial infections associated with seasonal flooding and may increase due to global climate change [10]. Still, Vietnam does not have a nationwide programme to monitor NTS and other foodborne infections in human populations.
A recent review and meta-analysis summarized AMR for NTS and other Enterobacteriales, but did not investigate differences between host animal species or changes over time [11]. The aim of this study is to describe the changes in prevalence and AMR of NTS in Vietnam over time. The goal is to provide a benchmark for future One Health interventions to control NTS in human and animal populations.
2. Methods
2.1. Study protocol and search strategy
We reviewed literature on NTS in Vietnam published in English from 1980 until 2023 following the PRISMA guideline, which includes a total of 27 checklist items [12].
2.2. Inclusion and exclusion criteria for article selection
Initially, the title and abstract of selected articles were screened for information on: (1) Prevalence of detection (per sample/individual); (2) Serovar; (3) Antimicrobial susceptibility; and (4) Antimicrobial resistance gene (ARGs). Articles were excluded if they did not contain any information on either the NTS prevalence or antimicrobial susceptibility or detection of ARGs. Moreover, review articles, book chapter, conference abstracts, letter, and articles written in other languages than English, and duplicated among records were also excluded. Article selection adhered to the Joanna Briggs Institute (JBI) Critical Appraisal Checklist, specifically designed for studies reporting prevalence data [13]. The two authors (N.T.N and D.H·P) independently conducted the selection process. In cases where discrepancies arose in study selection between the two reviewers, a third reviewer (J.C.M) was involved to resolve any disagreements regarding article inclusion.
2.3. Data collection
The date of sample collection was extracted from all studies. In the absence of an explicit date of sample collection, a date of two years prior to publication was assigned; for studies conducted over a time period, a mid-point was defined. From each publication, the following information was extracted: (1) Year of sample collection; (2) Species (for samples collected from humans and animals or their environment) (3) Sample type (faeces, floor swabs, farm waste, carcass, lymph node, caeca, meat, other samples from the abattoir environment); (4) Number of samples; (5) Prevalence of detection (per sample); (6) Number of NTS isolates; (7) Serovar; (8) Prevalence of resistance; (9) Methods used. Human isolates were further categorized based on whether they were recovered from healthy subjects (i.e. enteric carriage), from cases of enteric disease or invasive infection.
2.4. Statistical analyses
Antimicrobial agents were grouped by class [14] and the average prevalence of resistance was calculated for each class. Only studies with prevalence and/or phenotypic resistance data were included in the meta-analyses. Logit-transformed proportions were analysed using a generalized linear mixed-effect model, including a random-effect model to identify the within- and between-study variances [15]. Heterogeneity across selected studies was assessed using the inverse variance index [16]. Studies were further categorized by the source of the isolates: (1) Human-enteric carriage; (2) Human-enteric disease; (3) Humans-invasive infection; (4) Poultry/poultry farm/abattoir environment/poultry meat; (5) Pig/pig farm/abattoir environment/pork; (6) Cattle/cattle farm/abattoir environment/beef; and (7) Aquaculture/seafood. Analyses were conducted on NTS prevalence and phenotypic resistance meta-analyses to identify significant differences among host species. Univariable meta-regression models were performed to investigate trends in NTS prevalence and phenotypic resistance over time. Results of the meta-analysis were presented using forest plots [17]. All statistical analyses and figure were performed using R programming language [18] with the ‘meta’, ‘meta for’, ‘tidyverse’ and ‘ggplot2’ packages.
3. Results
3.1. Article selection
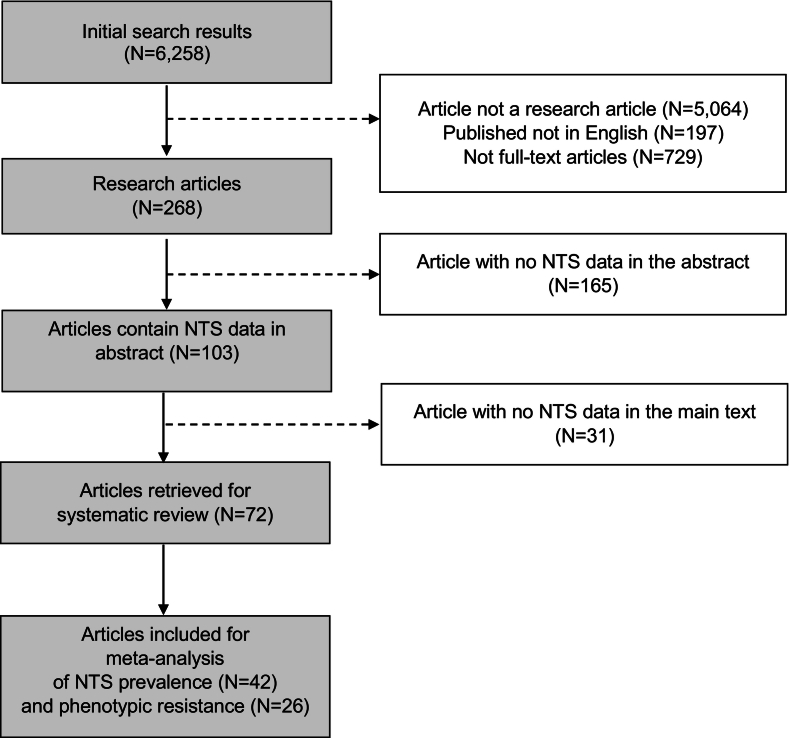
A total of 6258 articles were initially identified. Of those, 95.7% were excluded since they were not research articles (e.g. books, reviews, letters to the editor, datasets, guidelines, etc.), were published in different language other than English, or their full text was unavailable. A further 2.6% were excluded since their abstracts did not contain any data. Of the remaining 103 articles, 31 lacked NTS data in the full-text. Therefore 72 articles were finally selected (Supplementary Table 1). For the meta-analyses, only articles that reported NTS prevalence (n = 42) and phenotypic resistance (n = 26) were selected (Fig. 1).
Fig. 1.
The PRISMA flow diagram of the study selection process.
Of the 72 selected articles, 23 (31.9%) included NTS human data (including invasive disease, enteric disease and carriage). A total of 51 (70.8%) investigated NTS from animals; of those, 35 (68.6%) included data on NTS from pigs, 24 (47.1%) from poultry, 13 (25.5%) from aquaculture and 12 (23.5%) from cattle. A total of seven (9.7%) studies included NTS from environmental samples (i.e. outside animal farms) and wildlife/synantropic fauna (i.e., geckos). Most (40.3%) studies were conducted in southern Vietnam, 30.5% in northern Vietnam and 4.2% in the central region; 8 (11.1%) covered more than one region, and 10 (13.9%) did not specify geographical area. The serovar identity was investigated in 39 (54.2%) studies, 29 by the agglutination test, 7 by Multi-Locus Sequence Typing (MLST) and 1 by Whole Genome Sequencing (WGS). In two studies the method was not specified. In 43 studies (59.7%) isolates were investigated for their antimicrobial susceptibility. The disc diffusion test was used in 32 studies and in 11 the minimum inhibitory concentration (MIC) was analysed using broth dilution (Sensititre), E-test, Vitek, and agar dilution. Full details are given in Supplementary Table 2.
3.2. Asymptomatic carriage in humans
A study in the Mekong Delta found a higher (albeit non-statistically significant) NTS prevalence among healthy adult chicken farmers (4.4%) compared with rural non-farming adult subjects (2.9%) and their urban matched controls (2.0%) [19]. Potentially limitations in sample size (102 individuals in each group) limits the statistical power of detection of a statistical difference.
3.3. Enteric infection
In a 2001-2002 study, NTS was detected in 45/922 acute diarrheic children in the Mekong Delta region [20]. In contrast, NTS was not detected in 587 children with diarrhea in Hanoi (northern Vietnam) during the same period [21]. A subsequent case-control study (2009-2010) in Ho Chi Minh City (HCMC) on 2028 children (<60 months) failed to detect significant difference in prevalence of NTS between acute diarrhea cases and controls (4.0% and 5.6%, respectively) [22]. A prospective study of newborn children in the rural district of Dong Thap province and HCMC (2009-2013) revealed that in 57% (748/1309) diarrhea cases a pathogen was detected. Salmonella was detected by PCR in 18% positive samples after rotavirus (50%), norovirus (24%), and Campylobacter spp. (20%). Interestingly, the percent of children colonized with NTS in the Mekong Delta was three times higher than in a large urban center (HCMC) [23]. Among 16 patients with gastroenteritis symptoms in Ben Tre province (Mekong Delta), 7 (43.8%) were Salmonella-positive [24]. A 2014-2016 study of 3166 children (<16 years) with dysentery admitted in southern (HCMC) hospitals identified NTS by stool culture in 15%. The peak of cases was between May and September (mid-rainy season) [25].
3.4. Invasive infection
A hospital in Hue (central Vietnam) reported four cases of Salmonella meningitis from 2003 to 2008 in <7 month-old children (two S. Claibornei, S. enterica subsp. arizonae, one to S. Paratyphi B and one non-specified) [26]. A 2008-2013 study of 147 individuals with an invasive NTS (iNTS) in HCMC identified 43% as S. Enteritidis and 30% as S. Typhimurium. A total of 71% patients were HIV-positive adults [27]. NTS accounted for 10.9% of 1070 invasive bacterial isolates recorded in HCMC hospitals (2011−2013) [28].
3.5. NTS in animals and food
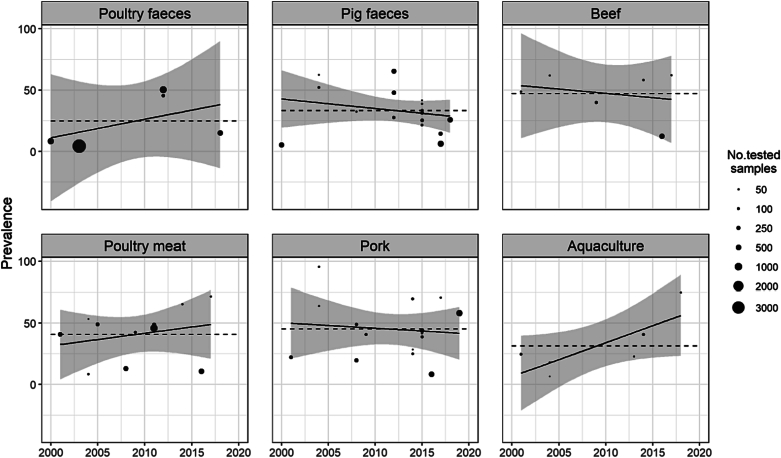
A total of 32 studies reported 23 prevalence estimates for pigs, 18 for poultry, 7 for cattle and 6 for aquaculture (Table 1). The sample prevalence by year is displayed in Fig. 2.
Table 1.
Meta-regression analyses of NST prevalence by different host species and time of study. P-values reflects the significance level of the coefficient being different from 0 obtained generalized linear mixed-effect models with the logit-transformed prevalence.
| Host categories | Meta-analyses |
Univariable meta-regression analyses (Year of sampling) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. prevalence estimates* | I2 | Pooled prevalence (%) | 95% CI | p-value | β | 95% CI | p-value | |
| Individual analyses by separate host species | ||||||||
| Human (enteric carriage) (a) | 3 | 38% | 2.0 | 0.0- 59.0 | 0.200 | 0.334 | -2.31-2.98 | 0.355 |
| Human (diarrhea) (b) | 7 | 97% | 5.0 | 1.0-24.0 | <0.01 | 0.273 | 0.01-0.54 | 0.044 |
| Human (invasive) (c) | 3 | 99% | 4.0 | 1.0-25.0 | <0.01 | 0.227 | -1.90-2.36 | 0.404 |
| Poultry and poultry meat (d) | 18 | 99% | 30.0 | 19.0-43.0 | <0.01 | 0.061 | -0.04-0.16 | 0.220 |
| Pig and pork products (e) | 23 | 98% | 35.0 | 25.0–46.0 | <0.01 | -0.006 | -0.09–0.08 | 0.884 |
| Aquaculture/seafood (f) | 6 | 88% | 30.0 | 12.0–56.0 | <0.01 | 0.120 | 0.00–0.24 | 0.047 |
| Cattle and beef products (g) | 7 | 97% | 41.0 | 22.0-63.0 | <0.01 | -0.032 | -0.19-0.12 | 0.620 |
| Sub-group analyses | ||||||||
| Human studies (a,b,c) | 13 | NC | 4.0 | 1.0-25.0 | 0.610 | 0.273 | 0.09-0.45 | 0.006 |
| Animal studies (d,e,f,g) | 54 | NC | 33.0 | 27.0-40.0 | 0.720 | 0.030 | -0.02-0.08 | 0.244 |
| Human and animal studies (a,b,c,d,e,f,g) | 67 | NC | 24.0 | 18.0-31.0 | <0.01 | 0.071 | 0.01-0.14 | 0.035 |
I2: inverse variance index; NC: not calculated. *The number of prevalence estimates (n = 67) was from 42 studies selected for meta-analyses of prevalence of NTS. Of 67 estimates, seven for human with diarrhea [[20], [21], [22], [23], [24], [25],29], 3 for invasive infection [26,28,30], 3 for enteric carriage [19,21,22], 23 for pig [7,[31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]], 18 for poultry [7,19,31,[34], [35], [36], [37], [38], [39], [40],42,48,50,[53], [54], [55], [56], [57]], 7 for cattle [31,34,35,40,42,50,58], and 6 for aquaculture [31,35,40,54,59,60].
Fig. 2.
Prevalence of NTS detection in animal sources over time. The dashed lines indicate the average prevalence across studies.
The meta-analysis of prevalence is presented in Supplementary Figs. 1 and 2 and summarized in Table 1. Detection in samples ranged from 2% to 5% in humans, and from 30% to 41% for animal samples. High levels of heterogeneity were found among the selected studies within each type (all I2 > 88%, p < 0.01), except for humans with enteric carriage (I2 = 38%, p < 0.20). Humans with enteric disease (+27.3%; p = 0.044), and aquaculture/seafood (+12.0%; p = 0.047) had significant increases over time.
3.6. NTS in animal feeds and other foods at retail
Five of 123 pig commercial feeds (4.1%) were contaminated with NTS (all S. Weltevreden) [52]. The investigation of 9 vegetable samples in canteens in Hanoi (2003-2004), failed to detect NTS [54]. A 2014-2015 study on raw pepper detected NTS in 5/84 samples [61]. A survey of 420 cooked and fermented, ready to eat foods from 21 hospital and university canteens in Hanoi detected Salmonella in 5% of samples [62]. A survey in the Mekong Delta identified NTS in 12.8% fresh vegetable samples [63]. Of 160 vegetable samples collected from markets in HCMC, 17.5% were contaminated with NTS [64]. No studies have been conducted on table or embryonated eggs.
3.7. NTS in wildlife and the environment
A study on wastewater used for aquaculture and crops in Hanoi found Salmonella at a concentration of 108 MPN/100 mL and coliform contamination over 110 times the legal limits, indicating considerable faecal contamination resulting from inadequate waste treatment. A city drainage canal was most contaminated (>455 MPN/100 mL) [65]. A study of 72 household waste and agriculture field samples reported a NTS prevalence of 46-79% (concentration 164-1441 MPN/100 mL) [46]. A study on river and irrigation water detected NTS in 43/141 (30.5%) samples, where river water had highest contamination (75%) [64]. A study of faecal samples from 101 geckos in the Mekong Delta identified NTS in 23.8%. In positive faecal samples, NTS were present at a concentration of 4.5 log CFU/g [66]. In another study, 156 of 959 (16.3%) of geckos collected from Hue and Mekong Delta provinces were NTS positive [67].
3.8. Differences by type of farm and retail type
No significant differences in prevalence were detected between small-scale and household chicken flocks in the Mekong Delta [19]. However, medium-scale pig herds had higher prevalence than small-scale ones [39]. Similarly, a study in northern pig farms reported significant higher prevalence of NTS in medium-scale compared with small-scale farms [43]. A study on chicken farms using European Union (EU) sampling methods (pooled faeces and dust) [68] showed that the prevalence of infection was highest in layers, followed by broilers and breeder flocks. In pig farms, fattening pigs displayed the highest prevalence (both per sample and at farm-level) [38]. A study in HCMC found NTS in 90.0% chicken meat samples from wet markets (52.6% from supermarkets) [42]. The prevalence of NTS in shrimp from wet markets (80%) was also higher than those from supermarkets (60%) [60]. A study on shrimp farms in the Mekong Delta (data from 2015 or earlier) identified a 22.3% prevalence of NTS, but did not detect any differences between farm sizes [59].
3.9. Serovar distribution
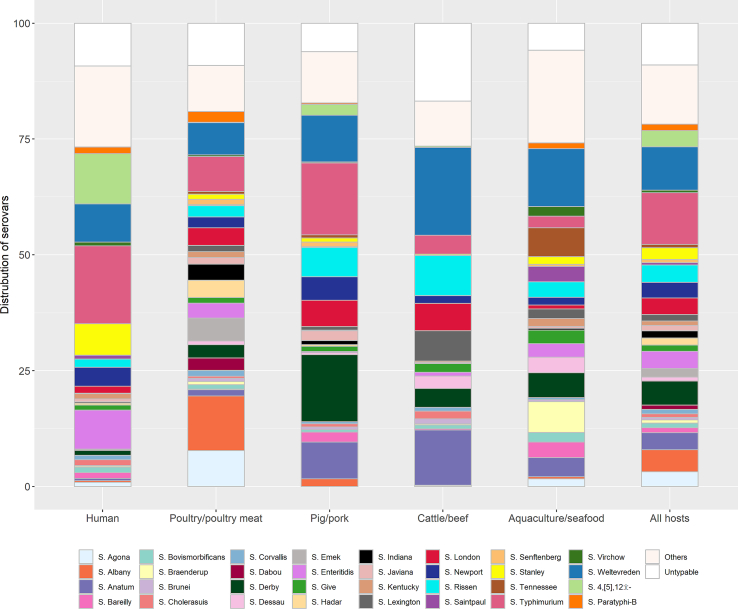
In total, 6235 isolates were tested for their serovar identify. A total of 8.4% were untypable. A total of 109 different serovars were identified (Supplementary Table 3). The most common serovars identified were, in descending order, S. Weltevreden (12.0%), followed by S. typhimurium (10.1%), S. Derby (6.4%), S. London (5.8%), S. Anatum (4.4%), S. Rissen (3.9%), S. enteritidis (3.7%), S. Albany (3.3%) and S. 4,[5],12:i:- (3.0%). Each of the remaining serovars accounted for <3% of the total. The serovar distribution of the five host categories are displayed in Fig. 3.
Fig. 3.
Serovar distribution in five host categories.
By animal host type, the highest correlation with the human serovar distribution corresponded to pork (r = 0.501; p = 0.002), followed by cattle (r = 0.321; p = 0.060), poultry (r = 0.308; p = 0.072), and aquaculture (r = 0.239; p = 0.167). The fraction of NTS isolates identified as S. Weltevreden decreased from 2006 to 2010 to 2016-2020 in aquaculture (from 21.6% to 1.6% of all serovars in individual studies), cattle (28.6% to 4.2%), pigs (18.8% to 6.3%). Over the same time period, S. Derby in pigs decreased from 32.3% to 5%, and S. Anatum in cattle from 28.6% to 3.1%.
In a 2014-2016 study monophasic S. Typhimurium (mSTM) (S. 4,[5],12:i:- ST34) accounted for 120/450 (26.7%) hospitalized children with dysentery [25]. In a study conducted in the Mekong Delta (2012−2013), mSTM was found in ducks (15% of farms) and pigs (6%) [39]. High prevalence of mSTM (11%) were also reported in poultry and pig farms in central Vietnam [38] and in 13.9% pig farms in northern Vietnam [47]. This serovar was also detected in raw meat samples of chicken, pork and beef (1.3-1.7% of isolates) in HCMC [40].
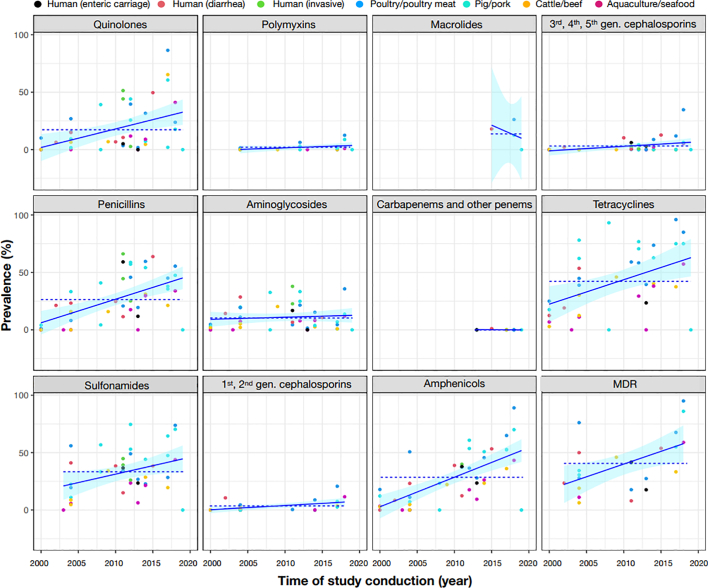
3.10. Phenotypic resistance
Twenty-six (36.1%) studies had data on phenotypic resistance suitable for meta-analyses (Fig. 4, Table 2). Among highest priority critically important antimicrobials (CIAs), macrolides and quinolones had the highest pooled resistance prevalence (12.0% and 10.0% respectively). The pooled prevalence was for 3rd-, 4th-, and 5th-generation cephalosporins and polymyxins was 1.0%. From 2000 to 2020 there was an increase in absolute terms in resistance to quinolones (15.6%, p = 0.001) and to 3rd-, 4th-, and 5th-generation cephalosporins (23.7%; p = 0.005). Regarding high priority CIAs, the highest resistance was seen for penicillins (adjusted pooled prevalence 21.0%), followed by aminoglycosides (8%). No resistance against carbapenems was detected. An increase in AMR levels over time was only found for penicillin antimicrobials (16.1% increase from 2000 to 2020, p < 0.001). Regarding highly important antimicrobials, the highest resistance corresponded to tetracyclines (39.0%), followed by sulfonamides (32.0%) and amphenicols (21.0%).
Fig. 4.
(Crude) prevalence of AMR in NTS over time. The plotted lines correspond to linear regression lines. The dashed lines indicate the mean prevalence among studies. MDR = multi-drug resistance.
Table 2.
Sub-group analyses of resistance prevalence and trends over time. P-values reflects the significance level of the coefficient being different from 0 obtained generalized linear mixed-effect models with the logit-transformed prevalence.
| Antimicrobials (no. of studies) | Host species |
Meta-regression analyses (year of study conduction) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human (Enteric carriage) | Human (Diarrhea) | Human (Invasive) | Poultry/ poultry meat | Pig/pork | Aquaculture/seafood | Cattle/beef | Total⁎ | p-value | β | p-value | |
| Quinolones1 (n) | 2 | 6 | 3 | 9 | 12 | 6 | 6 | 44 | |||
| Pooled prevalence (%) | 4.0 | 12.0 | 23.0 | 19.0 | 8.0 | 5.0 | 6.0 | 10.0 | 0.230 | 0.156 | 0.001 |
| 95% CI | (0.0-94.0) | (4.0-33.0) | (0.0-95.0) | (6.0-45.0) | (2.0-25.0) | (0.0-40.0) | (1.0-34.0) | (6.0-16.0) | (0.06-0.25) | ||
| Polymyxins1 (n) | _ | _ | _ | 4 | 7 | 2 | 3 | 16 | |||
| Pooled prevalence (%) | _ | _ | _ | 4.0 | 2.0 | 1.0 | 0.0 | 1.0 | 0.630 | 0.164 | 0.063 |
| 95% CI | _ | _ | _ | (0.0-25.0) | (0.0-8.0) | (0.0-100.0) | (0.0-100) | (0.0-4.0) | (-0.01-0.34) | ||
| Macrolides1 (n) | _ | _ | _ | _ | 2 | _ | _ | 2 | |||
| Pooled prevalence (%) | _ | _ | _ | _ | 12.0 | _ | _ | 12.0 | 1.0 | nc | nc |
| 95% CI | _ | _ | _ | _ | (1.0-79.0) | _ | _ | (1.0-79.0) | |||
| 3,4,5 gens of Cephalosporins1 (n) | 2 | 6 | 3 | 8 | 9 | 4 | 5 | 37 | |||
| Pooled prevalence (%) | 6.0 | 2.0 | 2.0 | 1.0 | 0.0 | 3.0 | 0.0 | 1.0 | 0.280 | 0.237 | 0.005 |
| 95% CI | (0.0-91.0) | (0.0-17.0) | (0.0-9.0) | (0.0-14.0) | (0.0-13.0) | (1.0-10.0) | (0.0-100) | (0.0-2.0) | (0.07-0.40) | ||
| Penicillins2 (n) | 2 | 6 | 3 | 9 | 12 | 7 | 6 | 45 | |||
| Pooled prevalence (%) | 33.0 | 23.0 | 45.0 | 24.0 | 29.0 | 4.0 | 10.0 | 21.0 | 0.10 | 0.161 | <0.001 |
| 95% CI | (0.0-100.0) | (9.0-48.0) | (12.0-83.0) | (10.0-47.0) | (16.0-47.0) | (0.0-47.0) | (2.0-37.0) | (14.0-29.0) | (0.10-0.22) | ||
| Aminoglycosides2 (n) | 2 | 5 | 2 | 9 | 12 | 7 | 6 | 43 | |||
| Pooled prevalence (%) | 9.0 | 12.0 | 30.0 | 9.0 | 8.0 | 7.0 | 4.0 | 8.0 | <0.01 | 0.024 | 0.061 |
| 95% CI | (0.0-100.0) | (6.0-25.0) | (2.0-92.0) | (4.0-20.0) | (3.0-19.0) | (2.0-19.0) | (1.0-14.0) | (6.0-12.0) | (-0.00-0.05) | ||
| Carbapenems2 (n) | _ | _ | _ | 3 | 3 | _ | _ | 6 | |||
| Pooled prevalence (%) | _ | _ | _ | 0.0 | 0.0 | _ | _ | 0.0 | 1.0 | nc | nc |
| 95% CI | _ | _ | _ | (0.0-100) | (0.0-100.0) | _ | _ | (0.0-100.0) | |||
| Tetracyclines3 (n) | _ | 3 | _ | 9 | 11 | 7 | 6 | 36 | |||
| Pooled prevalence (%) | _ | 29.0 | _ | 61.0 | 51.0 | 14.0 | 25.0 | 39.0 | 0.010 | 0.129 | 0.003 |
| 95% CI | _ | (4.0-81.0) | _ | (39.0-79.0) | (20.0-82.0) | (3.0-47.0) | (11.0-49.0) | (26.0-53.0) | (0.05-0.21) | ||
| Sulfonamides3 (n) | 2 | 4 | 3 | 8 | 11 | 6 | 5 | 39 | |||
| Pooled prevalence (%) | 35.0 | 32.0 | 37.0 | 39.0 | 45.0 | 16.0 | 17.0 | 32.0 | 0.050 | 0.088 | 0.007 |
| 95% CI | (4.0-87.0) | (16.0-53.0) | (20.0-57.0) | (25.0-55.0) | (29.0-61.0) | (4.0-42.0) | (7.0-37.0) | (26.0-39.0) | (0.03-0.15) | ||
| 1,2 gens of Cephalosporins3 (n) | _ | 3 | _ | 6 | 6 | 4 | 5 | 24 | |||
| Pooled prevalence (%) | _ | 6.0 | _ | 2.0 | 0.0 | 0.0 | 1.0 | 1.0 | 0.060 | 0.140 | 0.050 |
| 95% CI | _ | (1.0-30.0) | _ | (0.0-16.0) | (0.0-16.0) | (0.0-97.0) | (0.0-6.0) |
|
(0.00-0.28) | ||
| Amphenicols3 (n) | 2 | 6 | _ | 9 | 12 | 7 | 6 | 42 | |||
| Pooled prevalence (%) | 36.0 | 21.0 | _ | 42.0 | 21.0 | 11.0 | 10.0 | 21.0 | 0.010 | 0.178 | <0.001 |
| 95% CI | (4.0-87.0) | (8.0-46.0) | _ | (24.0-63.0) | (6.0-50.0) | (3.0-36.0) | (2.0-34.0) | (14.0-31.0) | (0.12-0.24) | ||
| Multi-drug resistance (n) | 2 | 4 | _ | 6 | 4 | 2 | 4 | 22 | |||
| Pooled prevalence (%) | 38.0 | 30.0 | _ | 55.0 | 54.0 | 36.0 | 24.0 | 39.0 | 0.380 | 0.114 | 0.014 |
| 95% CI | (5.0-88.0) | (7.0-70.0) | _ | (19.0-87.0) | (17.0-86.0) | (0.0-100) | (7.0-56.0) | (27.0-54.0) | (0.03-0.20) | ||
1Highest priority CIAs, 2high priority CIAs, 3highly important antimicrobials. *The total number of prevalence estimates was from 26 studies selected for meta-analyses of phenotypic AMR prevalence of NTS. Six studies conducted on humans with NTS diarrhea [20,22,25,[69], [70], [71]], 3 on invasive NTS infections [27,28,72], 2 studies on human with NTS enteric carriage [19,70], 12 studies in pigs [7,33,35,36,38,40,42,47,51,52,69,71], 9 studies in poultry [7,19,35,38,40,42,53,69,71], 6 studies on aquaculture [35,40,59,60,69,73,74] and 6 studies on cattle [35,40,42,58,69,71].
NTS from poultry and pigs displayed the highest MDR prevalence (54-55%) compared to other host species (≤39%). Between 2000 and 2020 there was an overall 11.4% increase in MDR (p = 0.014).
Resistance was highly variable across serovars. Particularly high levels of MDR have been reported in S. Typhimurium (80%), mSTM (78.9%), S. Kentucky ST198 (70%) and S. Newport (59.1%) isolates from invasive infections in HCMC [25]. A study in Mekong Delta revealed >37% MDR among S. Typhimurium and mSTM from pigs and poultry [39]. In other studies, 100% of mSTM isolates from pig and poultry were MDR [38,47]. In study on NTS from retail meat, MDR was most common in S. Kentucky (91.7% isolates), S. Derby (87.5%), and S. London (71.4%); in contrast MDR was absent in S. Weltevreden and S. Lexington isolates [42].
A 1995-2001 study on S. Weltevreden from several countries (19 from Vietnam) reported <6% of resistance against all tested antimicrobials [75]. The analysis of 12 Vietnamese S. Weltevreden isolates (2001-2005) from fish and snails imported to the USA revealed full susceptibility to a range of antimicrobials [73]. Similarly, studies in the Mekong Delta reported that all 26 S. Weltevreden isolates from vegetables (2017-2019) and 58 isolates from wild geckos (2012-2015) were fully susceptible against all examined antimicrobials [63,67].
3.11. Genotypic antimicrobial resistance
A total of 17 publications reported ARGs in NTS isolates from humans (N = 5, [25,28,71,76,77]), poultry (N = 7, [37,40,71,[76], [77], [78], [79]]), pigs (N = 9, [37,71,[76], [77], [78], [79], [80], [81], [82]]), cattle (N = 2, [58,79]), aquaculture (N = 5, [40,74,78,83,84]), vegetables (N = 2, [64,74]) and river water (N = 1, [64]). A total of 79 ARGs conferring resistance against 12 antimicrobial classes were identified (Table 3).
Table 3.
Number of studies where ARGs were detected in NTS isolates from different sources in Vietnam.
*Quinolone resistance determinant region mutations consist of gyrA-83, gyrA-87 and parC-80.
Of the ten different β-lactamase encoding genes described, blaCTX-M and blaTEM were most frequently detected in both human and animal isolates. A study investigating organisms associated with bacteremia in HCMC (2011-2013) revealed that blaCTX-M and blaTEM were the only genes detected in ESBL producing S. Cholerasuis strain [28]. Recent study of children in HCMC (2014-2016) reported that 58.7% of NTS trains harbored blaTEM gene [25]. In studies conducted in retail markets (2008-2009) in northern Vietnam, blaTEM was found in 90-92.3% of NTS strains resistant against ampicillin [37,58]. Co-occurrence of blaampC and blaCMY-2 was reported in four S. Braenderup and one S. Typhimurium isolates (all MDR and ESBL producers) from poultry samples collected in markets (2012-2015) in HCMC [40]. Mutations of the gyrA gene was the most detected mechanism conferred to quinolone resistance. Other plasmid-mediated resistant genes such as acc(6’)-Ib-cr, oqxAB and qnrS were also detected. Mutation at codon Ser83 of gyrA gene was detected in 18/23 quilonone resistant NTS from meat samples (2008-2009) while no other mechanisms observed [37]. In a study of NTS from human, qnrS1 was present in around haft of collection (47.8%) whereas mutations of gyrA and parC genes were only detected in 8.4% of isolates [25]. Similarly, the presence of qnrS1 was detected in 32/69 (46.4%) NTS isolates from pigs in slaughterhouse and markets in the North (2014-2019) [81].
A study of 450 NTS isolates from children with diarrhea in HCMC hospitals reported high concordance between AMR phenotype and genotype [25]. This was confirmed by a further study on isolates from human and animals [77]. The study revealed that 84% of ARGs were located on plasmids and 95% of ARGs were within 10 kb of insert sequence IS6/IS26, which allows exchange of ARGs between plasmids and other parts of the genome.
Three studies reported Class 1 integrons in between 13%-28% isolates [71,74,78]. Gene cassettes found in class 1 integrons included dhfrXII, dfrA1, dfrA12, dfrA17 (conferring resistance against trimethoprim), aadA1, aadA2, aadA5, sat (aminoglycosides), and blaPSE, blaOXA-30 (β-lactams). The transfer of integrons and AMR determinants from Salmonella to E. coli through conjugation experiment was possible for 17/83 (20.5%) integron-positive isolates [71].
3.12. Colistin resistance
Colistin resistance was detected in 1/67 (S. Albany) isolate from poultry in 2004 while none of 63 NTS from beef at retail in Hanoi (2009) [58,71]. A 2011-2012 study of NTS (N = 138) from pigs in northern Vietnam identified phenotypic colistin resistance in 2.9% isolates (S. Typhimurium and mSTM) [47]. A 2012-2013 study reported colistin resistance in 3/48 (6.2%) isolates (all S. enteritidis) from chickens, but none from 51 pig isolates [38]. A further study (2013) described high (MIC≥16 mg/L) colistin resistance in a S. Rissen isolate from a Vietnamese pig, mediated by a mcr-1 gene identical (100%) to one identified in a pig in Hong Kong on the same year and harbored by an almost identical (96.86%) plasmid [80]. A study on 69 NTS isolates from pork (2014-2019) revealed that 21.7% were fully resistant (4.3% intermediate); both the mcr-1 and mcr-3 genes were identified [81]. In a further study (2016-2017) of 40 NTS isolates from pork purchased in HCMC markets, colistin resistance was detected in 1 isolate (S. London; MIC = 8 μg/mL), but not in chicken meat (n = 37) and beef (n = 36) [42]. Surveillance data from pigs and chickens sampled at markets from 2017 to 2019 revealed an overall prevalence of colistin resistance of 9% in pigs and 13% in chickens [7]. In a study (2018) of 90 NTS from shrimps purchased at retail in HCMC, colistin resistance was identified in 1 (1.1%) isolate (S. enteritidis; MIC>2 μg/mL) [60].
4. Discussion
Our study demonstrates an increase of NTS in Vietnam over the 2000-2020 period, mostly in human diarrhea cases and aquaculture. Over this period, the aquaculture output in Vietnam has increased ten-fold (from 0.51 to 4.68 million of metric tons) [85]. The Vietnamese per capita meat consumption has increased 154% (from 24 kg in 2000 to 61 kg in 2020) [86]. The observed rises in human NTS diarrhea may also reflect increased exposure due to changes in consumption.
Furthermore, the increase in urbanization experienced over recent decades (i.e. a total of 20.3% of Vietnamese lived in urban settings in 1990 versus 38.8% in 2022) [87] may have contributed to reduction of levels of herd immunity over time.
The data suggests that human challenge with NTS is of higher magnitude in rural environments potentially due to higher challenge associated with higher exposure to farm animals and their excreta. Farmers in the Mekong Delta exhibited a higher prevalence of NTS carriage compared with rural non-farming and urban adults [19] and detection of NTS in children with diarrhea living rural areas was 3.5 higher than those in urban areas [23]. Furthermore, there is evidence of strong protective serological immunity which is transferred from mother to offspring [88].
The data confirms widespread distribution of NTS in farms, slaughterhouse environment and food products. However, contamination in many relevant food sources (i.e. table eggs) remains unknown in Vietnam. Eggs have been incriminated as a primary source of NTS worldwide, particularly due to S. Enteritidis [89,90].
Three studies reported data on NTS carriage in healthy humans in Vietnam, with an overall 2.0% pooled prevalence. Studies from North Africa, Mexico and the Middle East have shown variable prevalence of NTS in healthy individuals globally (range from 3.4 to 11.8%) [[91], [92], [93]].
The data conclusively confirms overall increases in AMR and MDR over time. An early study (1996-1999) of (human) community acquired NTS infection Vietnamese isolates had a comparatively low AMR (<1-13% by antimicrobial). No resistance to nalidixic acid was detected [94]. This suggests that the development of AMR has accelerated over the 2000-2020 period, and this is likely to be a result of the high levels of antimicrobial consumption reported in humans and animals in the country [95].
The prevalence AMR in Vietnamese NTS isolates was generally higher than in the EU. For example, in 2021 the EU average resistance of NTS against tetracycline, ampicillin, gentamicin, and colistin for broilers was, respectively 34.0%, 21.7%, 1.6% and 2.5% [96], compared with 61.0% (tetracyclines), 24.0% (penicillins), 19.0% (aminoglycosides) and 4.0% (polymyxins) in Vietnam. However, the Vietnamese data displayed an upward trend and therefore it is likely that the differences would be even more marked if only recent data were used. It is not known whether these differences reflect a different distribution of serovars or a genuine higher prevalence of AMR given the higher levels of AMU in Vietnam compared with the EU [95].
The relative contribution of exposure via contaminated food or direct contact with animals to human NTS infection in Vietnam is not clear. A study characterizing sequence type and AMR patterns of NTS isolates from humans and animals indicated overlapping between sources. Accordingly, NTS in human blood were likely to have greater similarity (54%) to chicken isolates whereas NTS in human stools could be largely (40%) attributed to pig isolates [72]. In contrast, another study showed that S. typhimurium isolates from humans and animals (duck, pig and chicken) clustered into distinct clades in the phylogeny indicating limited evidence for the transmission of this pathogen [76]. Conducting attribution studies of different animal sources using serovar characterization would be desirable under a One Health approach, but are technically challenging given the vast diversity of NTS in animals and the environment (including geographical diversity) and the difficulties in obtaining representative data. A quantitative microbial risk assessment indicated that the annual probability of acquiring salmonellosis from consumption of boiled pork was 17.7% [41].
S. Enteritidis and S. Typhimurium accounted for 73% of invasive infections in hospital settings in southern Vietnam [27]. This probably reflects a higher pathogenic potential of these serovars which represent 31.5% of isolates recovered from humans across the country (14.9% for all species combined). mSTM is an emerging serovar associated with numerous outbreaks in humans worldwide in association with livestock, particularly pigs [97]. Over the past decade MDR mSTM has become more prevalent in Vietnam, and has been associated with iNTS disease in HIV-patients [76]. In contrast, serovar S. Weltevreden is widespread but has considerably reduced its prevalence over time, and is associated with low levels AMR and low virulence in humans [77,98].
In Vietnam, meat products are often purchased unrefrigerated in informal street markets, which allow for any bacteria present to quickly multiply after slaughter. Chicken meat from wet markets had higher level of contamination with NTS bacteria (median 3.2 log10 MPN/g) than beef or pork [42]. It has been proposed that restricting sales of meat to the cooler morning hours may reduce bacterial contamination [43]. Given the high prevalence of NTS in vegetables [63,64], and the practice of consuming raw leaf vegetables, we suggest that this could be an additional source of NTS to humans.
5. Conclusions
This review demonstrates the magnitude of circulation of NTS in humans, animals and the environment. Levels of human infection appear to be higher in rural settings, probably reflecting environmental exposure and closer animal contact. The observed increases in NTS in aquaculture and poultry over the 2000-2020 period probably reflects intensification of these sectors. The increase in AMR and MDR of NTS isolates over the same period are likely to have repercussions for the treatment of invasive NTS infections. Since most laboratories do not conduct routine investigation of NTS in outpatients the burden of infection in the community is now well known. The prevalence and increased AMR trends of NTS infections underscore the need of adopting a One Health strategy for surveillance, research, and awareness campaigns to address the challenges posed by this pathogen. Unchecked, the rise in NTS infections and AMR could strain healthcare resources, hinder economic progress in the agriculture sector, and jeopardize food safety in Vietnam.
Ethics statement
No ethical approval was required for this review work.
Funding
This study has been funded by the United States Agency for International Development (USAID) through Project OSRO.VIE.001.USA.
CRediT authorship contribution statement
Nguyen Thi Nhung: Conceptualization, Formal analysis, Methodology, Writing – original draft. Doan Hoang Phu: Formal analysis, Investigation, Methodology, Writing – review & editing. Juan J. Carrique-Mas: Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. Pawin Padungtod: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100698.
Appendix A. Supplementary data
Supplementary material 1: Supplementary Tables
Supplementary material 2: Supplementary figures
Data availability
Data will be made available on request.
References
- 1.Eng S.K., Pusparajah P., Ab Mutalib N.S., Ser H.L., Chan K.G., Lee L.H. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8 doi: 10.1080/21553769.2015.1051243. [DOI] [Google Scholar]
- 2.Esan O.B., Perera R., McCarthy N., Violato M., Fanshawe T.R. Incidence, risk factors, and health service burden of sequelae of Campylobacter and non-typhoidal Salmonella infections in England, 2000–2015: a retrospective cohort study using linked electronic health records. J. Infect. 2020;81 doi: 10.1016/j.jinf.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Teklemariam A.D., Al-Hindi R.R., Albiheyri R.S., Alharbi M.G., Alghamdi M.A., Filimban A.A.R., Al Mutiri A.S., Al-Alyani A.M., Alseghayer M.S., Almaneea A.M., Albar A.H., Khormi M.A., Bhunia A.K. Human salmonellosis: a continuous global threat in the farm-to-fork food safety continuum. Foods. 2023;12 doi: 10.3390/foods12091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Salmonella (non-typhoidal) 2018. https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed August 2, 2023)
- 5.WHO One Health. 2024. https://www.who.int/health-topics/one-health#tab=tab_1 (accessed November 7, 2023)
- 6.Nga T.V.T., Duy P.T., Lan N.P.H., Chau N.V.V., Baker S. The control of typhoid fever in Vietnam. Am. J. Trop. Med. Hyg. 2018;99 doi: 10.4269/ajtmh.18-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuat C.V., Hue P.T., Loan N.T.P., Thuy N.T., Hue L.T., Giang V.N., Erickson V.I., Padungtod P. Antimicrobial resistance pilot surveillance of pigs and chickens in Vietnam, 2017–2019. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.618497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FAO The state of world fisheries and aquaculture. 2022. https://www.fao.org/documents/card/en?details=cc0461en (accessed September 18, 2023)
- 9.VNExpress . 2022. Mass food poisoning at Nha Trang school caused by Salmonella bacteria.https://e.vnexpress.net/news/news/mass-food-poisoning-at-nha-trang-school-caused-by-salmonella-bacteria-4538907.htmlhttps://e.vnexpress.net/news/news/mass-food-poisoning-at-nha-trang-school-caused-by-salmonella-bacteria-4538907.html (accessed August 2, 2023) [Google Scholar]
- 10.MoH . 2018. Decision: Action Plan to Respond to the Climate Change of the Health Sector for the Period of 2019-2030 and a Vision to 2050 (in Vietnamese) [Google Scholar]
- 11.Phu D.H., Wongtawan T., Truong D.B., Van Cuong N., Carrique-Mas J., Thomrongsuwannakij T. A systematic review and meta-analysis of integrated studies on antimicrobial resistance in Vietnam, with a focus on Enterobacteriaceae, from a One Health perspective. One Health. 2022;15 doi: 10.1016/j.onehlt.2022.100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn Z., MClinSc S.M., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015;13 doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 14.WHO . 2019. Critically important antimicrobials for human medicine - 6th revision 2018. [Google Scholar]
- 15.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3 doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huedo-Medina T., Sanchez-Meca J., Marin-Martinez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11 doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Harrer M., Cuijpers P., Furukawa T.A., Ebert D.D. Doing Meta-Analysis with R. 2021 doi: 10.1201/9781003107347. [DOI] [Google Scholar]
- 18.R Core Team . 2022. R: A Language and Environment for Statistical Computing, Vienna, Austria. [Google Scholar]
- 19.Trung N.V., Carrique-Mas J.J., Nghia N.H., Tu L.T., Mai H.H., Tuyen H.T., Campbell J., Nhung N.T., Nhung H.N., Minh P.V., Chieu T.T., Hieu T.Q., Mai N.T., Baker S., Wagenaar J.A., Hoa N.T., Schultsz C. Non-Typhoidal Salmonella colonization in chickens and humans in the Mekong Delta of Vietnam. Zoonoses Public Health. 2016 doi: 10.1111/zph.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ly T.L.-K., Duong T.T.T., Nguyen T.T., Tran T.P., Tran T.T.D., Nakadai A., Iwata T., Taniguchi T., Ha T.T., Hayashidani H. Prevalence of Salmonella and Escherichia coli O157 from acute diarrheic children in the Mekong Delta, Vietnam. Journal of Veterinary Epidemiology. 2010;14 doi: 10.2743/jve.14.55. [DOI] [Google Scholar]
- 21.Vu Nguyen T., Le Van P., Le Huy C., Nguyen Gia K., Weintraub A. Etiology and epidemiology of diarrhea in children in Hanoi, Vietnam. Int. J. Infect. Dis. 2006;10 doi: 10.1016/j.ijid.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Thompson C.N., Phan M.V.T., Van Minh Hoang N., Van Minh P., Vinh N.T., Thuy C.T., Nga T.T.T., Rabaa M.A., Duy P.T., Dung T.T.N., Phat V.V., Nga T.V.T., Tu L.T.P., Tuyen H.T., Yoshihara K., Jenkins C., Duong V.T., Le Phuc H., Tuyet P.T.N., Ngoc N.M., Vinh H., Chinh N.T., Thuong T.C., Tuan H.M., Hien T.T., Campbell J.I., Van Vinh Chau N., Thwaites G., Baker S. A prospective multi-center observational study of children hospitalized with diarrhea in Ho Chi Minh City, Vietnam. American Journal of Tropical Medicine and Hygiene. 2015;92 doi: 10.4269/ajtmh.14-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders K., Thompson C., Thuy N., Nguyet N., Tu L., Dung T., Phat V., Van N., Hieu N., Tham N., Ha P., Lien L., Chau N., Baker S. The epidemiology and aetiology of diarrhoeal disease in infancy in southern Vietnam: a birth cohort study. Int. J. Infect. Dis. 2015;35 doi: 10.1016/j.ijid.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vo T.H., Le N.H., Cao T.T.D., Nuorti J.P., Minh N.N.T. An outbreak of food-borne salmonellosis linked to a bread takeaway shop in ben Tre City, Vietnam. Int. J. Infect. Dis. 2014;26 doi: 10.1016/j.ijid.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Duong V.T., The H.C., Nhu T.D.H., Tuyen H.T., Campbell J.I., Van Minh P., Le Phuc H., Chau T.T.H., Ngoc N.M., Vi L.L., Mather A.E., Baker S. Genomic serotyping, clinical manifestations, and antimicrobial resistance of nontyphoidal Salmonella gastroenteritis in hospitalized children in Ho Chi Minh City, Vietnam. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01465-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuan Đ.Q., Hung P.H., Mai P.X., Hao T.K., Van Ha C., Luong N.D., Son N.H., Lien N.T.N., Yamanaka J., Sato N., Matsushita T. Salmonella meningitis: a report from national hue central hospital, Vietnam. Jpn. J. Infect. Dis. 2015;68 doi: 10.7883/yoken.JJID.2014.072. [DOI] [PubMed] [Google Scholar]
- 27.Lan N.P.H., Tu L.T.P., Hien N.H., Thuy L., Mather A.E., Park S.E., Marks F., Thwaites G.E., Chau N.V.V., Thompson C.N., Baker S. Invasive non-typhoidal Salmonella infections in Asia: clinical observations, disease outcome and dominant serovars from an infectious disease hospital in Vietnam. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan N.P.H., Hien N.H., Le Thi Phuong T., Thanh D.P., Thieu N.T.V., Ngoc D.T.T., Tuyen H.T., Vinh P.V., Ellington M.J., Thwaites G.E., Van Vinh Chau N., Baker S., Boinett C.J. Phenotypic and genotypic characteristics of ESBL and AmpC producing organisms associated with bacteraemia in Ho Chi Minh City, Vietnam, Antimicrob resist. Infect. Control. 2017;6 doi: 10.1186/s13756-017-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson C.N., Phan V.T.M., Le T.P.T., Pham T.N.T., Hoang L.P., Ha V., Nguyen V.M.H., Pham V.M., Nguyen T.V., Cao T.T., Tran T.T.N., Nguyen T.T.H., Dao M.T., Campbell J.I., Nguyen T.C., Tang C.T., Ha M.T., Farrar J., Baker S. Epidemiological features and risk factors of Salmonella gastroenteritis in children resident in Ho Chi Minh City, Vietnam. Epidemiol. Infect. 2013;141 doi: 10.1017/S0950268812002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nga T.V.T., Parry C.M., Le T., Lan N.P.H., Diep T.S., Campbell J.I., Hoang N.V.M., Dung L.T., Wain J., Dolecek C., Farrar J.J., Chau N.V.V., Hien T.T., Day J.N., Baker S. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 2012;106 doi: 10.1016/j.trstmh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Tran T.P., Khai L.T.L., Ogasawara N., Nguyen T.T., Okatani A.T., Akiba M., Hayashidani H. Contamination of Salmonella in retail meats and shrimps in the Mekong Delta, Vietnam. J. Food Prot. 2005;68 doi: 10.4315/0362-028x-68.5.1077. [DOI] [PubMed] [Google Scholar]
- 32.Le Bas C., Hanh T.T., Thanh N.T., Thuong D.D., Thuy N.C. Prevalence and epidemiology of Salmonella spp. in small pig abattoirs of Hanoi, Vietnam. Ann N Y Acad Sci. 2006;1081 doi: 10.1196/annals.1373.035. [DOI] [PubMed] [Google Scholar]
- 33.Takeshi K., Itoh S., Hosono H., Kono H., Tin V.T., Vinh N.Q., Thuy N.T.B., Kawamoto K., Makino S. Ichi. Detection of Salmonella spp. isolates from specimens due to pork production chains in Hue City, Vietnam. J. Vet. Med. Sci. 2009;71 doi: 10.1292/jvms.71.485. [DOI] [PubMed] [Google Scholar]
- 34.Vo A.T., van Duijkeren E., Fluit A.C., Heck M.E., Verbruggen A., Maas H.M., Gaastra W. Distribution of Salmonella enterica serovars from humans, livestock and meat in Vietnam and the dominance of Salmonella typhimurium phage type 90. Vet. Microbiol. 2006;113:153–158. doi: 10.1016/j.vetmic.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Van T.T.H., Moutafis G., Istivan T., Tran L.T., Coloe P.J. Detection of Salmonella spp. in retail raw food samples from Vietnam and characterization of their antibiotic resistance. Appl. Environ. Microbiol. 2007;73 doi: 10.1128/AEM.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellerbroek L., Narapati D., Phu Tai N., Poosaran N., Pinthong R., Sirimalaisuwan A., Tshering P., Fries R., Zessin K.H., Baumann M., Schroeter A. Antibiotic resistance in Salmonella isolates from imported chicken carcasses in Bhutan and from pig carcasses in Vietnam. J. Food Prot. 2010;73 doi: 10.4315/0362-028X-73.2.376. [DOI] [PubMed] [Google Scholar]
- 37.Thai T.H., Yamaguchi R. Molecular characterization of antibiotic-resistant Salmonella isolates from retail meat from markets in northern Vietnam. J. Food Prot. 2012;75:1709–1714. doi: 10.4315/0362-028X.12-101. [DOI] [PubMed] [Google Scholar]
- 38.Lettini A.A., Vo Than T., Marafin E., Longo A., Antonello K., Zavagnin P., Barco L., Mancin M., Cibin V., Morini M., Sao M. Dang Thi, Thi T. Nguyen, Trung H. Pham, Le L., Duc T. Nguyen, Ricci A. Distribution of Salmonella serovars and antimicrobial susceptibility from poultry and swine farms in Central Vietnam. Zoonoses Public Health. 2016;63 doi: 10.1111/zph.12265. [DOI] [PubMed] [Google Scholar]
- 39.Tu L.T.P., Hoang N.V.M., Cuong N.V., Campbell J., Bryant J.E., Hoa N.T., Kiet B.T., Thompson C., Duy D.T., Phat V.V., Hien V.B., Thwaites G., Baker S., Carrique-Mas J.J. High levels of contamination and antimicrobial-resistant non-typhoidal Salmonella serovars on pig and poultry farms in the Mekong Delta of Vietnam. Epidemiol. Infect. 2015;143 doi: 10.1017/S0950268815000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen D.T.A., Kanki M., Do Nguyen P., Le H.T., Ngo P.T., Tran D.N.M., Le N.H., Van Dang C., Kawai T., Kawahara R., Yonogi S., Hirai Y., Jinnai M., Yamasaki S., Kumeda Y., Yamamoto Y. Prevalence, antibiotic resistance, and extended-spectrum and AmpC β-lactamase productivity of Salmonella isolates from raw meat and seafood samples in Ho Chi Minh City, Vietnam. Int. J. Food Microbiol. 2016;236 doi: 10.1016/j.ijfoodmicro.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Dang-Xuan S., Nguyen-Viet H., Unger F., Pham-Duc P., Grace D., Tran-Thi N., Barot M., Pham-Thi N., Makita K. Quantitative risk assessment of human salmonellosis in the smallholder pig value chains in urban of Vietnam. Int. J. Public Health. 2017;62 doi: 10.1007/s00038-016-0921-x. [DOI] [PubMed] [Google Scholar]
- 42.Nhung N.T., Van N.T.B., Van Cuong N., Duong T.T.Q., Nhat T.T., Hang T.T.T., Nhi N.T.H., Kiet B.T., Hien V.B., Ngoc P.T., Campbell J., Thwaites G., Carrique-Mas J. Antimicrobial residues and resistance against critically important antimicrobials in non-typhoidal Salmonella from meat sold at wet markets and supermarkets in Vietnam. Int. J. Food Microbiol. 2018;266:301–309. doi: 10.1016/j.ijfoodmicro.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang-Xuan S., Nguyen-Viet H., Pham-Duc P., Unger F., Tran-Thi N., Grace D., Makita K. Risk factors associated with Salmonella spp. prevalence along smallholder pig value chains in Vietnam. Int. J. Food Microbiol. 2019;290 doi: 10.1016/j.ijfoodmicro.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 44.Ngo H.H.T., Nguyen-Thanh L., Pham-Duc P., Dang-Xuan S., Le-Thi H., Denis-Robichaud J., Nguyen-Viet H., Le T.T.H., Grace D., Unger F. Microbial contamination and associated risk factors in retailed pork from key value chains in northern Vietnam. Int. J. Food Microbiol. 2021;346 doi: 10.1016/j.ijfoodmicro.2021.109163. [DOI] [PubMed] [Google Scholar]
- 45.Lan T.T.Q., Lou Gaucher M., Nhan N.T.M., Letellier A., Quessy S. Distribution of virulence genes among Salmonella serotypes isolated from pigs in southern Vietnam. J. Food Prot. 2018;81 doi: 10.4315/0362-028X.JFP-17-408. [DOI] [PubMed] [Google Scholar]
- 46.Pham-Duc P., Nguyen-Viet H., Luu-Quoc T., Cook M.A., Trinh-Thi-Minh P., Payne D., Dao-Thu T., Grace D., Dang-Xuan S. Understanding antibiotic residues and pathogens flow in wastewater from smallholder pig farms to agriculture field in Ha Nam Province, Vietnam. Environ Health Insights. 2020;14 doi: 10.1177/1178630220943206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huong L.Q., Forslund A., Madsen H., Dalsgaard A. Survival of Salmonella spp. and fecal indicator bacteria in Vietnamese biogas digesters receiving pig slurry. Int. J. Hyg. Environ. Health. 2014;217 doi: 10.1016/j.ijheh.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanh T.T., Thanh N.T., Thoa H.Q., Thi L.T., Thuan L.M., Ly N.T.H. Prevalence of Salmonella spp. in poultry in Vietnam. Ann N Y Acad Sci. 2006;1081 doi: 10.1196/annals.1373.034. [DOI] [PubMed] [Google Scholar]
- 49.Yokozawa T., Dang-Xuan S., Hung N.V., Lapar L., Makita K. Transition of Salmonella prevalence in pork value chain from pig slaughterhouses to markets in Hung Yen, Vietnam. Journal of Veterinary Epidemiology. 2016;20 doi: 10.2743/jve.20.51. [DOI] [Google Scholar]
- 50.Binh D. Xuan, Minh N. Ngoc, Nguyet D. Thi. Prevalence of Listeria monocytogenes, E. coli, Salmonella Spp. and Staphylococcus aureus bacteria contamination on meat at public market in the North of Vietnam. SOJ Microbiol Infect Dis. 2017;5 doi: 10.15226/sojmid/5/5/00184. [DOI] [Google Scholar]
- 51.An H., Khai L. The prevalence and antibiotic resistance of Salmonella spp. isolated from pigs and farm environments in Vinh Long province, Can Tho University. Journal of Science. 2018;54 doi: 10.22144/ctu.jsi.2018.091. [DOI] [Google Scholar]
- 52.Minh D.K., Hounmanou Y.M.G., Mai H.B.T., Olsen J.E., Dalsgaard A. Prevalence and genomic characterization of Salmonella Weltevreden in commercial pig feed. Vet. Microbiol. 2020;246 doi: 10.1016/j.vetmic.2020.108725. [DOI] [PubMed] [Google Scholar]
- 53.Ta Y.T., Nguyen T.T., P.B. To, Da X. Pham, Le H.T., Thi G.N., Alali W.Q., Walls I., Doyle M.P. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam. J. Food Prot. 2014;77:57–66. doi: 10.4315/0362-028X.JFP-13-221. [DOI] [PubMed] [Google Scholar]
- 54.Dao H.T.A., Yen P.T. Study of Salmonella, Campylobacter, and Escherichia coli contamination in raw food available in factories, schools, and hospital canteens in Hanoi, Vietnam. Ann. N. Y. Acad. Sci. 2006;1081 doi: 10.1196/annals.1373.033. [DOI] [PubMed] [Google Scholar]
- 55.Ta Y.T., Nguyen T.T., Le H.T., Alali W.Q., Walls I., Wong D.M. Lo Fo, Doyle M.P. Prevalence of Salmonella on chicken carcasses from retail markets in Vietnam. J. Food Prot. 2012;75:1851–1854. doi: 10.4315/0362-028X.JFP-12-130. [DOI] [PubMed] [Google Scholar]
- 56.Tran T.P., Ly T.L.K., Nguyen T.T., Akiba M., Ogasawara N., Shinoda D., Okatani A.T., Hayashidani H. Prevalence of Salmonella spp. in pigs, chickens and ducks in the Mekong Delta, Vietnam. Journal of Veterinary Medical Science. 2004;66 doi: 10.1292/jvms.66.1011. [DOI] [PubMed] [Google Scholar]
- 57.Huong L.Q., Fries R., Padungtod P., Hanh T.T., Kyule M.N., Baumann M.P.O., Zessin K.H. Prevalence of Salmonella in retail chicken meat in Hanoi, Vietnam. Ann. N. Y. Acad. Sci. 2006 doi: 10.1196/annals.1373.032. [DOI] [PubMed] [Google Scholar]
- 58.Thai T.H., Hirai T., Lan N.T., Shimada A., Ngoc P.T., Yamaguchi R. Antimicrobial resistance of Salmonella serovars isolated from beef at retail markets in the north Vietnam. J. Vet. Med. Sci. 2012;74:1163–1169. doi: 10.1292/jvms.12-0053. https://www.ncbi.nlm.nih.gov/pubmed/22673721. [DOI] [PubMed] [Google Scholar]
- 59.Uddin G.M.N., Larsen M.H., Barco L., Phu T.M., Dalsgaard A. Clonal occurrence of Salmonella Weltevreden in cultured shrimp in the Mekong delta, Vietnam. PloS One. 2015;10 doi: 10.1371/journal.pone.0134252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yen N.T.P., Nhung N.T., Van N.T.B., Cuong N.V., Tien Chau L.T., Trinh H.N., Van Tuat C., Tu N.D., Lan N. Phu Huong, Campbell J., Thwaites G., Baker S., Carrique-Mas J. Antimicrobial residues, non-typhoidal Salmonella, Vibrio spp. and associated microbiological hazards in retail shrimps purchased in Ho Chi Minh city (Vietnam) Food Control. 2020;107:106756. doi: 10.1016/j.foodcont.2019.106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harada T., Yamane R., Dang V.C., Nguyen D.P., Nguyen T.A.D., Jinnai M., Shinyayonogi R., Kawahara M., Kanki T., Kawai K., Kawatsu Y., Kumeda Y., Isegawa Y. Yamamoto. Prevalence and antimicrobial susceptibility of Enterobacteriaceae isolated from retail pepper in Vietnam. J. Food Prot. 2017;80 doi: 10.4315/0362-028X.JFP-16-501. [DOI] [PubMed] [Google Scholar]
- 62.Luu-Thi H., Michiels C.W. Microbiological safety of ready-to-eat foods in hospital and university canteens in Hanoi, Vietnam. J. Food Prot. 2021;84 doi: 10.4315/JFP-20-324. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen T.K., Bui H.T., Truong T.A., Lam D.N., Ikeuchi S., Ly L.K.T., Hara-Kudo Y., Taniguchi T., Hayashidani H. Retail fresh vegetables as a potential source of Salmonella infection in the Mekong Delta, Vietnam. Int. J. Food Microbiol. 2021;341 doi: 10.1016/j.ijfoodmicro.2021.109049. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen D.T.A., Awasthi S.P., Hoang P.H., Do Nguyen P., Jayedul H., Hatanaka N., Hinenoya A., Van Dang C., Faruque S.M., Yamasaki S. Prevalence, serovar, and antimicrobial resistance of Nontyphoidal Salmonella in vegetable, fruit, and water samples in Ho Chi Minh City, Vietnam. Foodborne Pathog Dis. 2021;18 doi: 10.1089/fpd.2020.2891. [DOI] [PubMed] [Google Scholar]
- 65.Fuhrimann S., Pham-Duc P., Cissé G., Tram N.T., Thu Ha H., Dung D.T., Ngoc P., Nguyen-Viet H., Anh Vuong T., Utzinger J., Schindler C., Winkler M.S. Microbial contamination along the main open wastewater and storm water channel of Hanoi, Vietnam, and potential health risks for urban farmers. Sci. Total Environ. 2016;566–567 doi: 10.1016/j.scitotenv.2016.05.080. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen K.T., Hasegawa M., Nguyen T.T., Vo T.M.T., Tran T.H.T., Ly T.L.K., Taniguchi T., Hayashidani H. The importance of wild gecko as a source of human Salmonella infection. Journal of Veterinary Medical Science. 2018;80 doi: 10.1292/jvms.18-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen K.T., Hasegawa M., Vo T.M.T., Huynh T.L., Nagata E., Ly T.L.K., Taniguchi T., Hayashidani H. Wild geckos considered as the natural reservoir of Salmonella Weltevreden in southeast Asian countries. Zoonoses Public Health. 2021;68 doi: 10.1111/zph.12873. [DOI] [PubMed] [Google Scholar]
- 68.Snow L.C., Davies R.H., Christiansen K.H., Carrique-Mas J.J., Cook A.J.C., Teale C.J., Evans S.J. Survey of the prevalence of Salmonella on commercial broiler farms in the United Kingdom, 2005/06. Vet. Rec. 2008;163 doi: 10.1136/vr.163.22.649. [DOI] [PubMed] [Google Scholar]
- 69.Ogasawara N., Tran T.P., Ly T.L., Nguyen T.T., Iwata T., Okatani A.T., Watanabe M., Taniguchi T., Hirota Y., Hayashidani H. Antimicrobial susceptibilities of Salmonella from domestic animals, food and human in the Mekong Delta, Vietnam. J. Vet. Med. Sci. 2008;70:1159–1164. doi: 10.1292/jvms.70.1159. http://www.ncbi.nlm.nih.gov/pubmed/19057132 [DOI] [PubMed] [Google Scholar]
- 70.Parisi A., Le Thi Phuong T., Mather A.E., Jombart T., Tuyen H.T., Lan N. Phu Huong, Trang N. Hoang Thu, Carrique-Mas J., Campbell J.I., Glass K., Kirk M.D., Baker S. Differential antimicrobial susceptibility profiles between symptomatic and asymptomatic non-typhoidal Salmonella infections in Vietnamese children. Epidemiol Infect. 2020 doi: 10.1017/S0950268820001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vo A.T.T., van Duijkeren E., Gaastra W., Fluit A.C. Antimicrobial resistance, class 1 Integrons, and genomic Island 1 in Salmonella isolates from Vietnam. PloS One. 2010;5 doi: 10.1371/journal.pone.0009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parisi A., Phuong T.L.T., Mather A.E., Jombart T., Tuyen H.T., Lan N.P.H., Trang N.H.T., Carrique-Mas J., Campbell J.I., Trung N.V., Glass K., Kirk M.D., Baker S. The role of animals as a source of antimicrobial resistant nontyphoidal Salmonella causing invasive and non-invasive human disease in Vietnam, infection. Genetics and Evolution. 2020;85 doi: 10.1016/j.meegid.2020.104534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponce E., Khan A.A., Cheng C.M., Summage-West C., Cerniglia C.E. Prevalence and characterization of Salmonella enterica serovar Weltevreden from imported seafood. Food Microbiol. 2008;25 doi: 10.1016/j.fm.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Bae D., Kweon O., Khan A.A. Isolation and characterization of antimicrobial-resistant nontyphoidal Salmonella enterica serovars from imported food products. J. Food Prot. 2016;79 doi: 10.4315/0362-028X.JFP-15-564. [DOI] [PubMed] [Google Scholar]
- 75.Aarestrup F.M., Lertworapreecha M., Evans M.C., Bangtrakulnonth A., Chalermchaikit T., Hendriksen R.S., Wegener H.C. Antimicrobial susceptibility and occurrence of resistance genes among Salmonella enterica serovar Weltevreden from different countries. J. Antimicrob. Chemother. 2003;52:715–718. doi: 10.1093/jac/dkg426. [DOI] [PubMed] [Google Scholar]
- 76.Mather A.E., Phuong T.L.T., Gao Y., Clare S., Mukhopadhyay S., Goulding D.A., Do Hoang N.T., Tuyen H.T., Lan N.P.H., Thompson C.N., Trang N.H.T., Carrique-Mas J., Tue N.T., Campbell J.I., Rabaa M.A., Thanh D.P., Harcourt K., Hoa N.T., Trung N.V., Schultsz C., Perron G.G., Coia J.E., Brown D.J., Okoro C., Parkhill J., Thomson N.R., Chau N.V.V., Thwaites G.E., Maskell D.J., Dougan G., Kenney L.J., Baker S. New variant of multidrug-resistant Salmonella enterica serovar Typhimurium associated with invasive disease in immunocompromised patients in Vietnam. MBio. 2018;9 doi: 10.1128/mBio.01056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bloomfield S., Duong V.T., Tuyen H.T., Campbell J.I., Thomson N.R., Parkhill J., Le Phuc H., Chau T.T.H., Maskell D.J., Perron G.G., Ngoc N.M., Vi L.L., Adriaenssens E.M., Baker S., Mather A.E. Mobility of antimicrobial resistance across serovars and disease presentations in non-typhoidal Salmonella from animals and humans in Vietnam. Microb Genom. 2022;8 doi: 10.1099/mgen.0.000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van T.T.H., Moutafis G., Tran L.T., Coloe P.J. Antibiotic resistance in food-borne bacterial contaminants in Vietnam. Appl. Environ. Microbiol. 2007;73 doi: 10.1128/AEM.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nghiem M.N., Nguyen V.T., Nguyen T.T.H., Nguyen T.D., Vo T.T.B. Antimicrobial resistance gene expression associated with multidrug resistant Salmonella spp. isolated from retail meat in Hanoi, Vietnam. Int. Microbiol. 2017;20 doi: 10.2436/20.1501.01.288. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Santamarina B., Busch A., Garcia-Soto S., Abdel-Glil M.Y., Linde J., Fries R., Meemken D., Hotzel H., Tomaso H. Draft genome sequence of multi-resistant Salmonella enterica subsp. enterica serovar Rissen strain 19CS0416 isolated from Vietnam reveals mcr-1 plasmid mediated resistance to colistin already in 2013. J Genomics. 2020;8 doi: 10.7150/jgen.42790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holohan N., Wallat M., Luu T. Hai Yen, Clark E., Truong D.T.Q., Xuan S.D., Vu H.T.K., Van Truong D., Huy H. Tran, Nguyen-Viet H., Unger F., Dang S. Thi Thanh, Stabler R.A. Analysis of antimicrobial resistance in non-typhoidal Salmonella collected from pork retail outlets and slaughterhouses in Vietnam using whole genome sequencing. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.816279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.González-Santamarina B., García-Soto S., Dang-Xuan S., Abdel-Glil M.Y., Meemken D., Fries R., Tomaso H. Genomic characterization of multidrug-resistant Salmonella serovars Derby and Rissen from the pig value chain in Vietnam. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.705044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akiyama T., Khan A.A. Isolation and characterization of small qnrS1-carrying plasmids from imported seafood isolates of Salmonella enterica that are highly similar to plasmids of clinical isolates. FEMS Immunol. Med. Microbiol. 2012;64 doi: 10.1111/j.1574-695X.2011.00921.x. [DOI] [PubMed] [Google Scholar]
- 84.Hounmanou Y.M.G., Dalsgaard A., Sopacua T.F., Uddin G.M.N., Leekitcharoenphon P., Hendriksen R.S., Olsen J.E., Larsen M.H. Molecular characteristics and zoonotic potential of Salmonella Weltevreden from cultured shrimp and tilapia in Vietnam and China. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The World Bank Aquaculture Production (Metric Tons) - Vietnam. 2024. https://data.worldbank.org/indicator/ER.FSH.AQUA.MT?locations=VN
- 86.Hannah R., Pablo R., Max R. Meat and Dairy Production, our World in Data. 2024. https://ourworldindata.org/meat-production
- 87.Macrotrends Vietnam Urban Population 1960-2023. 2023. https://www.macrotrends.net/countries/VNM/vietnam/urban-population (accessed November 7, 2023)
- 88.De Alwis R., Tu L.T.P., Quynh N.L.T., Thompson C.N., Anders K.L., Van Thuy N.T., Hieu N.T., Vi L.L., Chau N.V.V., Duong V.T., Chau T.T.H., Tuyen H.T., Nga T.V.T., Van Minh P., Van Tan T., Thu T.N.H., Nhu T.D.H., Thwaites G.E., Simmons C., Baker S. The role of maternally acquired antibody in providing protective immunity against nontyphoidal Salmonella in urban Vietnamese infants: a birth cohort study. J. Infect. Dis. 2019;219 doi: 10.1093/infdis/jiy501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.EFSA Quantitative risk assessment of Salmonella Enteritidis in shell eggs in Europe. EFSA J. 2010;8 doi: 10.2903/j.efsa.2010.1588. [DOI] [Google Scholar]
- 90.Howard Z.R., O’Bryan C.A., Crandall P.G., Ricke S.C. Salmonella Enteritidis in shell eggs: current issues and prospects for control. Food Res. Int. 2012;45 doi: 10.1016/j.foodres.2011.04.030. [DOI] [Google Scholar]
- 91.Zaidi M.B., McDermott P.F., Fedorka-Cray P., Leon V., Canche C., Hubert S.K., Abbott J., León M., Zhao S., Headrick M., Tollefson L. Nontyphoidal Salmonella from human clinical cases, asymptomatic children, and raw retail meats in Yucatan, Mexico. Clin. Infect. Dis. 2006;42 doi: 10.1086/498508. [DOI] [PubMed] [Google Scholar]
- 92.Mbuyi-Kalonji L., Barbé B., Nkoji G., Madinga J., Roucher C., Linsuke S., Hermy M., Heroes A.S., Mattheus W., Polman K., Lutumba P., Phoba M.F., Lunguya O., Jacobs J. Non-typhoidal Salmonella intestinal carriage in a Schistosoma mansoni endemic community in a rural area of the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Rifai R.H., Chaabna K., Denagamage T., Alali W.Q. Prevalence of enteric non-typhoidal Salmonella in humans in the Middle East and North Africa: a systematic review and meta-analysis. Zoonoses Public Health. 2019;66 doi: 10.1111/zph.12631. [DOI] [PubMed] [Google Scholar]
- 94.Isenbarger D.W., Hoge C.W., Srijan A., Pitarangsi C., Vithayasai N., Bodhidatta L., Hickey K.W., Cam P.D. Comparative antibiotic resistance of diarrheal pathogens from Vietnam and Thailand, 1996-1999. Emerg. Infect. Dis. 2002;8 doi: 10.3201/eid0802.010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carrique-Mas J.J., Choisy M., Cuong N.V., Thwaites G., Baker S. An estimation of total antimicrobial usage in humans and animals in Vietnam. Antimicrob. Resist. Infect. Control. 2020;9 doi: 10.1186/s13756-019-0671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.EFSA The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023;21 doi: 10.2903/j.efsa.2023.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun H., Wan Y., Du P., Bai L. The epidemiology of monophasic Salmonella typhimurium. Foodborne Pathog. Dis. 2020;17 doi: 10.1089/fpd.2019.2676. [DOI] [PubMed] [Google Scholar]
- 98.Makendi C., Page A.J., Wren B.W., Le Thi Phuong T., Clare S., Hale C., Goulding D., Klemm E.J., Pickard D., Okoro C., Hunt M., Thompson C.N., Lan N. Phu Huong, Do Hoang N. Tran, Thwaites G.E., Le Hello S., Brisabois A., Weill F.X., Baker S., Dougan G. A phylogenetic and phenotypic analysis of Salmonella enterica serovar Weltevreden, an emerging agent of diarrheal disease in tropical regions. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: Supplementary Tables
Supplementary material 2: Supplementary figures
Data Availability Statement
Data will be made available on request.