Abstract
A teenage girl presented with fever and altered mental status. MRI showed diffuse leptomeningeal enhancement of the brain and spine. She was diagnosed by a positive cerebrospinal fluid (CSF) culture with tuberculous (TB) meningitis and was started on anti-TB medications and corticosteroids. Her mental status improved, but she was noted to have proximal weakness of the lower extremities. In the course of tapering corticosteroids at week 11 of anti-TB therapy, she became acutely confused and febrile. MRI demonstrated interval development of tuberculomas in the brain and a mass lesion in the thoracic spine causing cord compression. Given the clinical picture was suggestive of a paradoxical reaction, the dose of corticosteroids was increased. Infliximab was added when repeat MRI revealed enlargement of the mass lesion in the spine with worsening cord compression. She was successfully tapered off of corticosteroids. Over several months, the patient’s motor function recovered fully, and she returned to ambulating without assistance.
Keywords: Tuberculosis, Meningitis, Paradoxical reaction, Host-directed therapy, Tumor necrosis factor-alpha inhibitor
The following case was presented as part of the Centers for Disease Control & Tuberculosis Centers of Excellence TB Expert Network: Unplugged! series.
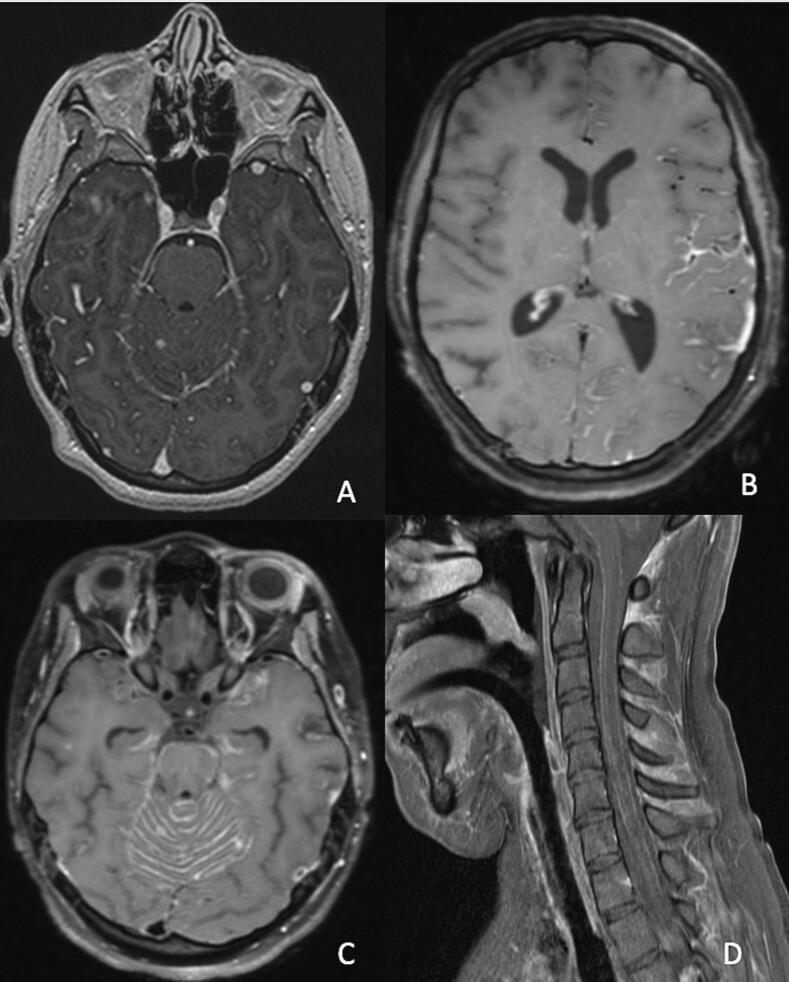
Dr. Ryo Miyakawa, pediatric infectious diseases fellow: A 16-year-old girl who had immigrated to the United States from Latin America as a young child presented to an emergency department (ED) with headache, myalgias, nausea, and vomiting for 1 day. She had no hypoxemia, and her chest radiograph was normal. She was diagnosed with coronavirus disease 2019 (COVID-19) based on polymerase chain reaction (PCR) and was discharged from the ED. She returned four weeks later with fever, shortness of breath, paresthesias in both feet, and inability to ambulate and was admitted to the hospital. Chest computed tomography (CT) revealed bilateral reticulonodular infiltrates. Brain magnetic resonance imaging (MRI) demonstrated supratentorial and posterior fossa leptomeningeal enhancement along with three small contrast-enhancing foci in the parenchyma (Fig. 1). Spine MRI was normal. She was diagnosed with multi-inflammatory syndrome in children (MIS-C) and treated with high-dose corticosteroids and intravenous immunoglobulin (IVIG).
Fig. 1.
Brain and spine MRI obtained several weeks after initial presentation. Axial post-contrast T1-weighted images demonstrated supratentorial and posterior fossa leptomeningeal enhancement along with three small contrast-enhancing foci in the brain parenchyma four weeks after initial presentation (A). Multifocal nodular leptomeningeal enhancement was also seen involving the left Sylvian fissure (B), posterior fossa (C), and cervical spine (D) five weeks after initial presentation.
She presented one week later with fever, altered mental status, and agitation. Physical examination, including neurologic examination, was normal. Initial lumbar puncture (LP) revealed a cerebrospinal fluid (CSF) neutrophilic pleocytosis with 215 white blood cells (WBC)/mm3, low glucose of 28 mg/dL, and elevated protein of 67 mg/dL (Table 1). A CSF meningitis/encephalitis multiplex PCR test was negative. Progressive leptomeningeal enhancement was observed on repeat brain MRI. Bone marrow biopsy revealed hemophagocytosis, and a presumptive diagnosis of hemophagocytic lymphohistiocytosis with central nervous system involvement was made. She received anakinra, etoposide, repeat IVIG and high dose corticosteroids without improvement.
Table 1.
Cerebrospinal fluid results by time of anti-TB therapy initiation.
| Timing in relation to initiation of anti-TB therapy | 2 weeks prior to anti-TB therapy | 1 day prior to anti-TB therapy | Week 1 | Week 2 | Week 7 | Week 11 | Week 13 |
|---|---|---|---|---|---|---|---|
| Source of cerebrospinal fluid | LP | LP | LP | Ventricular shunt | LP | LP | LP |
| WBC (per mm3) | 215 | 108 | 265 | 8 | 290 | 310 | 178 |
| Neutrophils (%) | 83 | 86 | 55 | 48 | 21 | 60 | 37 |
| Lymphocytes (%) | 17 | 14 | 25 | 43 | 65 | 24 | 52 |
| Monocytes (%) | 0 | 0 | 18 | 9 | 13 | 15 | 10 |
| RBC (per mm3) | 3 | 30 | 25 | 4 | 5 | 147 | 3 |
| Glucose (mg/dL) | 28 | 31 | 31 | 46 | 47 | 45 | 53 |
| Protein (mg/dL) | 67 | 166 | 624 | 61 | 1707 | 2203 | 2747 |
| AFB culture | Not performed | Mycobacterial tuberculosis complex | Mycobacterial tuberculosis complex | Negative | Negative | Negative | Negative |
AFB: acid fast bacilli, LP: lumbar puncture, RBC: red blood cell, TB: tuberculosis, WBC: white blood cell.
On further history, her family reported she was healthy prior to presentation. She had been ruled out for tuberculosis (TB) disease and diagnosed with latent tuberculosis infection (LTBI) as part of an immigration health screening. She completed approximately 2 months of the prescribed 6-month course of isoniazid.
Upon transfer to our facility, ophthalmologic examination revealed bilateral chorioretinal lesions. Laboratory tests were notable for pancytopenia and mild transaminitis (alanine aminotransferase 29 IU/L, aspartate transferase 66 IU/L, total bilirubin 0.4 mg/dL). Repeat LP revealed an ongoing CSF neutrophil-predominant pleocytosis, persistently low glucose of 31 mg/dL, and an increase in protein to 166 mg/dL compared with the initial LP (Table 1). Repeat chest CT continued to show diffuse reticulonodular infiltrates.
Dr. Felicia Chow, neuro-infectious diseases specialist: Brain and spine MRI with and without contrast (Fig. 1) revealed multifocal nodular leptomeningeal enhancement including of the posterior fossa, multiple cranial nerves (e.g., II/VII/VIII), the optic chiasm, and left Sylvian fissure. Major arteries of the anterior and posterior circulations were patent, and there was no evidence of infarcts or intracranial hemorrhage. Diffuse leptomeningeal enhancement was present throughout the spine.
Dr. Miyakawa: Due to the patient’s altered mental status, sputum could not be induced for microbiologic evaluation. A bronchoalveolar lavage (BAL) specimen was acid-fast bacilli (AFB) smear negative, but Mycobacterium tuberculosis complex without evidence of rifampin resistance was detected by PCR. AFB cultures of the BAL specimen, gastric aspirates, and CSF were subsequently positive for M. tuberculosis complex.
The sum of these findings was consistent with pulmonary TB and intracranial and intraspinal TB meningitis (TBM) with ocular involvement. The patient was started on daily isoniazid 300 mg, rifampin 600 mg, and pyrazinamide 2 g via nasogastric tube, along with intravenous (IV) amikacin 1 gm and dexamethasone 10 mg (0.13 mg/kg/day; equivalent to prednisone approximately 60 mg/day) in line with standard of care recommendations [1]. We added levofloxacin 750 mg IV daily after considering the risk of drug resistance. Local city and state public health departments were contacted for further recommendations.
Dr. Janice Louie, TB clinic director/infectious diseases specialist: We recommended changing rifampin to IV formulation and increasing the dose to 20 mg/kg/day. We also advised switching amikacin to linezolid 600 mg IV daily for better penetration into the central nervous system (CNS) [2], [3]. However, she did not tolerate linezolid due to worsening pancytopenia. We further recommended increasing dexamethasone to 0.4 mg/kg/day, which has been shown to confer mortality benefit in TBM among those 15 years of age or older [4]. Of note, according to the updated pediatric recommendations available, many experts would include a fluoroquinolone in this initial regimen of TBM, regardless of concern for drug resistance [5].
Dr. Chow: Unlike the rapidly changing landscape in the treatment of pulmonary TB, little progress has been made in recent decades to improve the dismal outcomes associated with TBM. Multiple clinical trials are in progress evaluating both intensified anti-TB regimens that aim to enhance mycobacterial killing through better drug exposures in the CNS and adjunctive host-directed therapy targeting inflammation, which is a major driver of TBM-related morbidity and mortality. For example, several trials are investigating the use of high-dose rifampin in both adults and children with TBM [6], [7], [8], [9], [10], along with the utility of linezolid [7], [9], [10], [11] and aspirin [7], [8], [12]. Even with new information from these trials on the horizon, a more flexible platform to efficiently test novel therapies for TBM has been proposed as a necessary way forward to truly change clinical practice and outcomes [13], [14].
Dr. Chris Keh, state public health officer/infectious diseases specialist: Pyrazinamide was stopped after pyrosequencing identified the organism as Mycobacterium bovis and did not identify genetic mutations associated with resistance to rifampin, isoniazid, or fluoroquinolones. Phenotypic drug susceptibility testing later confirmed susceptibility to isoniazid and rifampin and resistance to pyrazinamide.
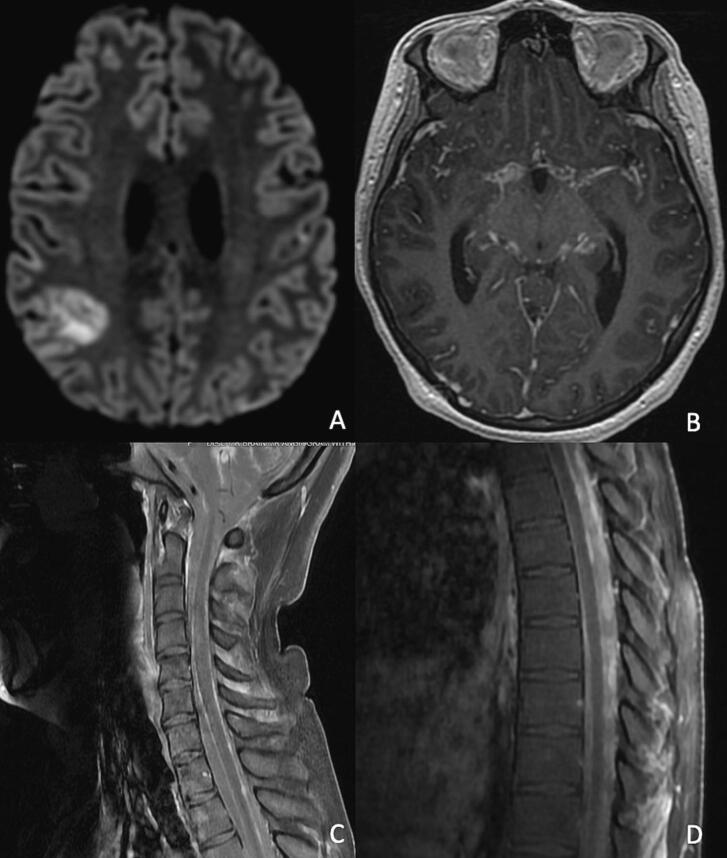
Dr. Miyakawa: The patient was continued on three-drug therapy with isoniazid 300 mg PO daily, rifampin 20 mg/kg IV daily, and levofloxacin 750 mg IV daily. A brain MRI in week 2 of anti-TB therapy revealed a subacute infarct in the right parietal lobe with mild irregularity and stenosis of the bilateral supraclinoid internal carotid, middle cerebral, and anterior cerebral arteries and developing hydrocephalus, along with marked interval worsening of diffuse leptomeningeal enhancement (Fig. 2). A ventriculoperitoneal shunt was placed, and aspirin 81 mg daily was started a few days post-operatively. CSF sampled at the time of shunt placement was markedly different compared with prior CSF samples obtained via LP (Table 1). Post-operatively, after the patient was extubated and her sedation was lifted, she was noted to be awake and interactive but with weakness of the lower extremities.
Fig. 2.
Brain and spine MRI obtained after initiation of anti-TB therapy. A subacute infarct in the right parietal lobe on axial diffusion weighted images (A) and interval worsening of diffuse leptomeningeal enhancement on axial post-contrast T1-weighted images (B) at week 2 of anti-TB therapy. Progression of leptomeningeal enhancement of the spine with new areas of nodular enhancement along the dorsal surface of the cervical and thoracic spinal cord on sagittal post-contrast T1-weighted images (C-D) at week 5 of anti-TB therapy.
Dr. Chow: Discordant profiles of CSF sampled from different compartments is a common phenomenon in CNS infections with or without hydrocephalus, especially with basilar meningitis [15]. In TBM, markers of inflammation are typically more pronounced in lumbar CSF, whereas ventricular CSF tends to have higher concentrations of markers of brain injury [16], [17]. In addition to discrepant CSF parameters, microbiological and molecular testing can detect organisms in CSF from one compartment but not the other. As a result, when interpreting results, it is critical to know which compartment CSF is sampled from and to be aware that CSF from different compartments may not be comparable.
Dr. Miyakawa: Dexamethasone taper was started in week 4 of anti-TB therapy. The patient continued to make slow but steady improvement in her neurologic status. However, repeat MRI in week 5 of anti-TB therapy demonstrated progression of leptomeningeal enhancement of the spine, particularly with new areas of nodular enhancement along the dorsal surface of the thoracic spinal cord, as well as an increase in nodular enhancement throughout the brain (Fig. 2). Given ongoing uncertainty regarding the effectiveness of treatment while awaiting culture conversion, linezolid was added back after her pancytopenia resolved to optimize the anti-TB regimen. Simultaneously, however, as paradoxical worsening was a potential explanation of the radiologic progression, further decrease of the dexamethasone dose was paused.
By week 7, she had regained some movement of her lower extremities with relative preservation of strength distally. However, she was still unable to lift them anti-gravity and complained of neuropathic pain in the lower extremities. Tone was normal to decreased. Patellar reflexes were diminished bilaterally, while ankle jerks were 2 +. No clonus or Babinski reflex was present. A sensory level was absent, though the sensory exam was limited. Bowel and bladder function were intact. Repeat LP showed a persistent CSF pleocytosis, now lymphocyte-predominant, a marked rise in protein, and normalization of the glucose compared with CSF from an LP obtained prior to initiation of anti-TB therapy (Table 1).
The etiology of the patient’s persistent lower extremity weakness was unclear. In light of the progression of disease in the thoracic spine on the most recent week 5 MRI, a myelopathy from paradoxical worsening causing paraplegia remained a top concern, as was a painful polyradiculopathy from meningeal inflammation. She had also lost 12 kg since admission and had diffusely diminished muscle bulk, which, combined with the proximal greater than distal pattern of weakness, raised the alternate possibility that corticosteroid-associated and/or critical illness myopathy could be contributing to her weakness. On balance, with modest but appreciable improvement in the neurologic examination and in some CSF parameters, the evidence for paradoxical worsening was not convincing enough to warrant increasing the dose of corticosteroids or continuing to pause the taper, especially when weighed against the possibility that corticosteroid toxicity was contributing to her proximal weakness. As a result, the decision was made to resume the dexamethasone taper.
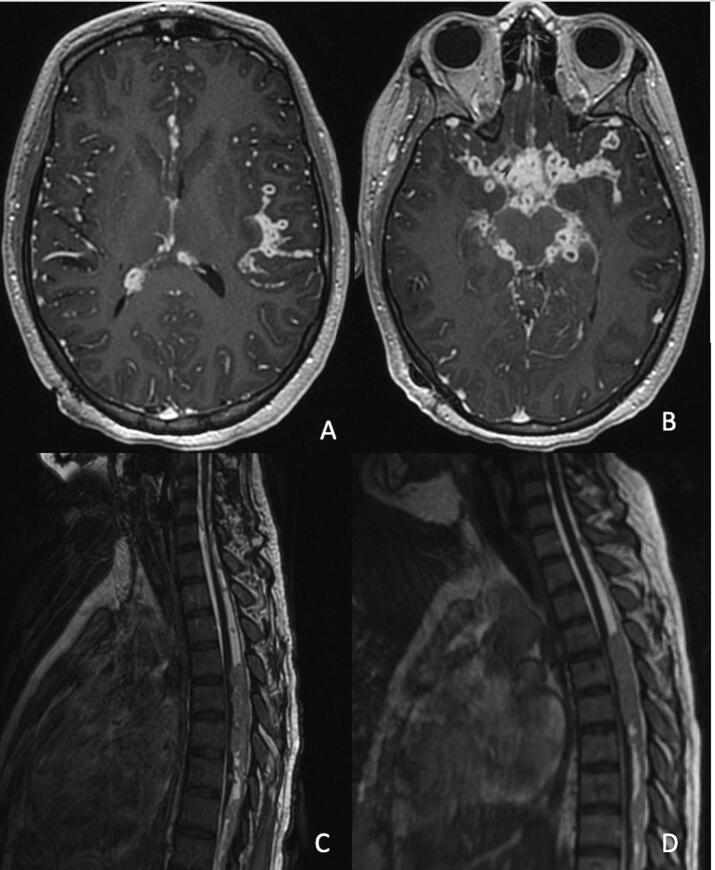
In week 11 of anti-TB therapy, the patient acutely developed confusion and intermittent fevers. As she had been clinically improving in the weeks leading up to this decline and CSF AFB culture from week 7 still showed no growth, therapeutic failure of anti-TB drugs was thought to be unlikely. Concern for a paradoxical reaction was high, in part due to recent re-initiation of a dexamethasone taper. The dose of dexamethasone was increased to 0.2 mg/kg/day, and her clinical status improved within 24 h. Repeat LP showed a stable CSF pleocytosis but now with a predominance of neutrophils along with a markedly elevated protein (Table 1). Repeat brain MRI showed prominent progression of leptomeningeal enhancement and numerous nodular lesions at the skull base (Fig. 3). Since the last spine MRI, there was interval development of bulky, mass-like leptomeningeal disease and intrathecal soft tissue spanning T4 to T12 with ventral displacement of the spinal cord and cord flattening/compression (Fig. 3).
Fig. 3.
Brain and spine MRI obtained at weeks 11 and 13 of anti-TB therapy. Axial post-contrast T1-weighted images demonstrated progression of leptomeningeal enhancement and numerous nodular lesions at the skull base (A-B) at week 11 of anti-TB therapy. Sagittal T2-weighted views of mass-like leptomeningeal disease and intrathecal soft tissue spanning T4 to T12 with ventral displacement, flattening, and compression of the spinal cord (C) at week 11 of anti-TB therapy and enlargement of the mass lesion with worsening cord compression (D) at week 13 of anti-TB therapy.
Dr. Chow: When the patient declined, the top considerations were treatment failure with or without drug resistance versus paradoxical worsening. Focusing on her CSF results, her protein was continuing to rise at week 7. Elevated CSF protein is common in patients with TBM and can frequently worsen over the course of anti-TB treatment. Extremely high CSF protein may be the result of a blockage in the flow of CSF leading to build up of protein in the lumbar space. Therefore, a rising CSF protein, as was observed in this patient’s LP in week 7 and 11, is not surprising and does not necessarily indicate treatment failure or inadequate response to treatment. Of the other CSF parameters, the improvement in CSF glucose by week 7 is reassuring and suggests response to treatment, as does the fact that CSF AFB culture showed no growth. On the other hand, the shift back to a neutrophil-predominant pleocytosis, along with marked progression of inflammatory changes on the brain and spine MRI with interval development of peripherally-enhancing tuberculomas in the brain and a mass lesion in the spine, following a period of initial clinical improvement, was highly suspicious for a paradoxical reaction [18], [19], [20]. In addition, the patient was felt to be at high risk for a paradoxical reaction based on the high percentage of neutrophils in her baseline CSF, which has been shown to be associated with the development of paradoxical inflammatory response to TBM treatment in individuals both with and without HIV [21], [22]. Furthermore, the timing was consistent with a paradoxical reaction, which usually occurs within 3 months of initiation of anti-TB therapy and, often, between 4 and 8 weeks [19], [22].
Dr. Louie: We agreed with this assessment. Arguing against treatment failure was the fact that her chorioretinal lesions and reticulonodular infiltrates of the lungs had improved. Repeat AFB cultures of CSF were negative (Table 1) and gastric aspirate cultures in week 3 and 7 of anti-TB therapy were also negative. Rifampin, levofloxacin, and linezolid had been given IV to reduce concerns for malabsorption, and therapeutic drug monitoring [23] showed levels in appropriate ranges. Lastly, her symptoms resolved promptly with an increase in corticosteroid dose.
Dr. Miyakawa: Given the extramedullary mass lesion in the thoracic spine with associated cord compression seen on the week 11 MRI, dexamethasone was increased further to 0.3 mg/kg/day. Two weeks later, repeat LP in week 13 of anti-TB therapy showed an improvement in the CSF pleocytosis and reduction in the proportion of neutrophils (Table 1). However, repeat MRI demonstrated enlargement of the mass lesion in the thoracic spine with worsening spinal cord compression (Fig. 3). Clinically, she continued to have weakness of her lower extremities, although there was slow improvement.
Dr. Keh: Corticosteroid wean in patients with TBM is often challenging. In this case, we anticipated a slow radiologic response to the increase in corticosteroid dosing and recognized that the enlarging mass lesion with worsening spinal cord compression on MRI could be due to radiologic lag. However, the precarious location of the lesion in the spine was concerning, and the patient’s neurologic examination remained difficult to interpret. An additional challenge of this case was balancing the need for long-term corticosteroids against corticosteroid-associated myopathy, though it was unclear how much this was playing a role in her lower extremity weakness. We discussed the utility of host-directed therapy, hoping to minimize neurologic injury from the compressive lesion, while also allowing for further corticosteroid weaning.
Dr. Chow: Corticosteroids are the cornerstone of treatment for paradoxical reactions and other inflammatory complications of CNS TB, although the optimal approach in terms of dosing and duration of corticosteroids is not well established. My initial response for patients with these inflammatory complications tends to be to re-increase the dose of corticosteroids, often to the same high doses that we use at the start of treatment of TBM [24]. Some patients with paradoxical reactions, however, are refractory even to high-dose corticosteroids, or clinically deteriorate when corticosteroids are tapered. Others may develop adverse effects to long-term corticosteroid use. For such patients, alternative host-directed therapies targeting the immune-mediated inflammatory response are a reasonable option to help manage paradoxical reactions [25], [26], [27], [28], [29], [30], [31], [32], [33]. Thalidomide, which has anti-inflammatory effects in part through its impact on tumor necrosis factor (TNF)-alpha production, has been used to successfully treat inflammatory complications of CNS TB, especially in children [25]. TNF-alpha inhibitors, including infliximab, have also been used to treat paradoxical reactions in CNS TB in both adults [30], [31], [32], [33] and children [27]. Published and unpublished experience suggests that TNF-alpha inhibitors, which increase the risk of LTBI reactivation [34], [35], can be used safely in TB disease, including TBM, provided patients are concurrently treated with appropriate anti-TB therapy [36]. Although shown in case series and cohorts to be effective in patients with inflammatory complications of CNS TB, data from randomized clinical trials of these targeted anti-inflammatory therapies are lacking.
Dr. Miyakawa: Thalidomide is only available through the risk evaluation and mitigation strategy (REMS) program (https://www.thalomidrems.com) in the United States. Considering the challenges of obtaining thalidomide and its risk in this teenage girl, potentially over the course of several months, we elected to treat with infliximab, which has been used successfully in pediatric populations [27]. The patient received three doses of infliximab 500 mg (approximately 7.5 mg/kg/dose), the first at week 12 of anti-TB therapy, followed by a second dose 2 weeks later, and a third dose after another 4 weeks, as dexamethasone was tapered.
Dr. Joel Ernst, TB immunology researcher/infectious diseases specialist: Understanding the immunologic response to TB is critical to understanding the role of host-directed therapy in treating TB. M. tuberculosis has many distinct molecules that are inflammatory. They include a variety of lipoproteins, lipoarabinomannans, capsular alpha glucan, trehalose dimycolate (TDM), muropeptides, cyclic-di-AMP, and phosphatidylinositol mannans [37]. All of these are recognized by immune cells and exert proinflammatory effects, even when the bacteria are dead. Receptors for these molecules include toll-like receptors, C-type lectins, NOD-like receptors, and cytosolic DNA/RNA sensors [37]. These receptors activate signals that induce cytokines, including TNF, interleukin (IL)-1, IL-6, and interferon beta [38]. When macrophages and dendritic cells are infected by M. tuberculosis, bacterial antigens are presented to and recognized by CD4+ and CD8+ T-cells.
TNF is secreted by macrophages and by T-cells and plays a central role in responding to TB infection with antimicrobial and proinflammatory effects. It activates macrophage microbicidal mechanisms and promotes apoptosis of infected macrophages [39], [40]. While apoptosis is a noninflammatory cell death, TNF prevents T-cell apoptosis and induces chemokines and expression of their receptors. Many of these cytokines are proinflammatory (e.g., IL-1, IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF)). TNF also induces secretion of anti-inflammatory chemokines (e.g., IL-10, IL1-Ra, soluble TNFR, IL-11), and they can help TNF downregulate its proinflammatory effect, along with many genes whose transcription is activated by TNF and block TNF signaling. These anti-inflammatory effects of TNF are often not sufficient in those with TBM, and corticosteroids are often used to help decrease inflammation.
There are a few reasons why TNF blockade may work better than dexamethasone. First, patients may exhibit dexamethasone resistance due to decreased expression of glucocorticoid receptors from its long-term use [41]. M. tuberculosis also triggers IL-6, which inhibits responses to glucocorticoids [42]. Lastly, excess TNF drives distinct mechanisms of inflammation via blockade of T-cell apoptosis and promotion of lymphocyte accumulation that cannot be suppressed by other mechanisms [43].
Dr. Miyakawa: After initiation of infliximab, the patient continued to gradually improve. By week 14 of anti-TB therapy and around the time of the second dose of infliximab, she was ambulating with a walker. By month 8 of anti-TB therapy, she was ambulating without assistance. An MRI obtained 1 year after the initiation of anti-TB therapy showed persistent nodular leptomeningeal enhancement in the brain and spinal cord. The lesion in the thoracic spine had decreased in size with improvement in the mass effect on the thoracic spinal cord.
The patient completed 4 months of linezolid and a total of 1 year of oral isoniazid, rifampin, and levofloxacin. At one year post-treatment, she continued to do well with a normal neurological examination and no signs of relapse.
CRediT authorship contribution statement
Ryo Miyakawa: Writing – original draft, Visualization. Janice Louie: Writing – review & editing. Chris Keh: Writing – review & editing. Lisa Chen: Writing – review & editing. Babak Javid: Writing – review & editing. Joel D. Ernst: Writing – review & editing. Neela Goswami: Writing – review & editing, Project administration. Felicia C. Chow: Writing – review & editing, Visualization, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ryo Miyakawa reports financial support was provided by National Institute of General Medical Sciences. Felicia Chow reports financial support was provided by National Institutes of Health Fogarty International Center. Joel Ernst reports a relationship with National Institutes of Health that includes: funding grants. Joel Ernst reports a relationship with Bill & Melinda Gates Foundation that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the many clinicians at the University of California, San Francisco Benioff Children’s Hospital and local public health officials who were involved in the direct care of the patient.
This work was supported by the National Institute of Health’s Fogarty International Center (R21TW011035 to F.C.C.) and the National Institute of General Medical Sciences (T32GM007546 to R.M.). The funding source did not have a role in the collection, analysis, interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Ethical statement
Local laws and institutional guidelines were followed in preparing this manuscript. Institutional ethical approval was not necessary for this manuscript.
References
- 1.American Academy of Pediatrics. Committee on Infectious Diseases. Report of the Committee on Infectious Diseases. Evanston, Ill.
- 2.Sun F., Ruan Q., Wang J., et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob Agents Chemother. Oct 2014;58(10):6297–6301. doi: 10.1128/AAC.02784-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Lu J., Liu J., Zhao Y., Ni X., Zhao S. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr Infect Dis J. Jun 2016;35(6):607–610. doi: 10.1097/INF.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 4.Thwaites G.E., Nguyen D.B., Nguyen H.D., et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Infectious Diseases American Academy of Pediatrics. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (32nd Edition). American Academy of Pediatrics; 2021.
- 6.Marais S., Cresswell F.V., Hamers R.L., et al. High dose oral rifampicin to improve survival from adult tuberculous meningitis: a randomised placebo-controlled double-blinded phase III trial (the HARVEST study) Wellcome Open Res. 2019;4:190. doi: 10.12688/wellcomeopenres.15565.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maitre T, Bonnet M, Calmy A, et al. Intensified tuberculosis treatment to reduce the mortality of HIV-infected and uninfected patients with tuberculosis meningitis (INTENSE-TBM): study protocol for a phase III randomized controlled trial. Trials. Nov 08 2022;23(1):928. doi:10.1186/s13063-022-06772-1. [DOI] [PMC free article] [PubMed]
- 8.Griffiths A. SURE: Short intensive treatment for children with tuberculous meningitis. Accessed 07/19/2023, https://www.isrctn.com/ISRCTN40829906.
- 9.National Institute of Allergy and Infectious Diseases. Trial of a Six-Month Regimen of High-Dose Rifampicin, High-Dose Isoniazid, Linezolid, and Pyrazinamide Versus a Standard Nine-Month Regimen for the Treatment of Adults and Adolescents With Tuberculous Meningitis. Accessed 07/19/2023, https://classic.clinicaltrials.gov/ct2/show/NCT05383742.
- 10.Chow F. Adjunctive Linezolid for the Treatment of Tuberculous Meningitis (ALTER). Accessed 07/19/2023, https://classic.clinicaltrials.gov/ct2/show/NCT04021121.
- 11.Sahib A, Bhatia R, Srivastava MVP, et al. Escalate: Linezolid as an add on treatment in the intensive phase of tubercular meningitis. A randomized controlled pilot trial. Tuberculosis (Edinb). Sep 2023;142:102351. doi:10.1016/j.tube.2023.102351. [DOI] [PubMed]
- 12.Davis A.G., Wasserman S., Stek C., et al. A phase 2A trial of the safety and tolerability of increased dose rifampicin and adjunctive linezolid, with or without aspirin, for human immunodeficiency virus-associated tuberculous meningitis: the LASER-TBM trial. Clin Infect Dis. 2023;76(8):1412–1422. doi: 10.1093/cid/ciac932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thwaites G.E., Watson J., Thuong Thuong N.T., Huynh J., Walker T., Phu N.H. Which trial do we need? a global, adaptive, platform trial to reduce death and disability from tuberculous meningitis. Clin Microbiol Infect. Jul 2023;29(7):826–828. doi: 10.1016/j.cmi.2023.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson R.J., Donovan J., Thwaites G.E., van Crevel R., Wasserman S. Treatment of tuberculous meningitis: overdue for concerted action. Tuberculosis (Edinb) Sep 2023;142 doi: 10.1016/j.tube.2023.102361. [DOI] [PubMed] [Google Scholar]
- 15.Khan SF, Macauley T, Tong SYC, et al. When Ventricular Cerebrospinal Fluid Assessment Misleads: Basal Meningitis and the Importance of Lumbar Puncture Sampling. Open Forum Infect Dis. Jul 01 2019;6(7)doi:10.1093/ofid/ofz324. [DOI] [PMC free article] [PubMed]
- 16.Rohlwink U.K., Mauff K., Wilkinson K.A., et al. Biomarkers of cerebral injury and inflammation in pediatric tuberculous meningitis. Clin Infect Dis. 2017;65(8):1298–1307. doi: 10.1093/cid/cix540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohlwink UK, Figaji A, Wilkinson KA, et al. Tuberculous meningitis in children is characterized by compartmentalized immune responses and neural excitotoxicity. Nat Commun. Aug 21 2019;10(1):3767. doi:10.1038/s41467-019-11783-9. [DOI] [PMC free article] [PubMed]
- 18.Thwaites G.E., Macmullen-Price J., Tran T.H., et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. Mar 2007;6(3):230–236. doi: 10.1016/S1474-4422(07)70034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A.K., Malhotra H.S., Garg R.K., et al. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC Infect Dis. 2016.306.;06(21):16. doi: 10.1186/s12879-016-1625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y., Hu Z., Wang F., et al. Worsening CSF parameters after the start of anti-tuberculosis treatment predicts intracerebral tuberculoma development. Int J Infect Dis. Dec 2020;101:395–402. doi: 10.1016/j.ijid.2020.09.1457. [DOI] [PubMed] [Google Scholar]
- 21.Marais S., Meintjes G., Pepper D.J., et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. Feb 2013;56(3):450–460. doi: 10.1093/cid/cis899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai M.S., Nor H.M., Kadir K.A.A., et al. Paradoxical manifestation is common in HIV-negative tuberculous meningitis. Medicine (Baltimore) Jan 2016;95(1):e1997. doi: 10.1097/MD.0000000000001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahid P., Dorman S.E., Alipanah N., et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan J., Thanh N.T., Thwaites G.E., Phu N.H. Severe paradoxical reaction in tuberculous meningitis. IDCases. 2021;23:e01009. doi: 10.1016/j.idcr.2020.e01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Toorn R, Solomons RS, Seddon JA, Schoeman JF. Thalidomide Use for Complicated Central Nervous System Tuberculosis in Children: Insights From an Observational Cohort. Clin Infect Dis. 03 01 2021;72(5):e136-e145. doi:10.1093/cid/ciaa1826. [DOI] [PubMed]
- 26.Keeley AJ, Parkash V, Tunbridge A, et al. Anakinra in the treatment of protracted paradoxical inflammatory reactions in HIV-associated tuberculosis in the United Kingdom: a report of two cases. Int J STD AIDS. 07 2020;31(8):808-812. doi:10.1177/0956462420915394. [DOI] [PMC free article] [PubMed]
- 27.Abo Y.N., Curtis N., Osowicki J., et al. Infliximab for paradoxical reactions in pediatric central nervous system tuberculosis. J Pediatric Infect Dis Soc. 2021;10(12):1087–1091. doi: 10.1093/jpids/piab094. [DOI] [PubMed] [Google Scholar]
- 28.Caraffa E., Russo G., Vita S., et al. Intracranial tuberculous mass lesions treated with thalidomide in an immunocompetent child from a low tuberculosis endemic country: a case report. Medicine (Baltimore) Jul 2018;97(29):e11186. doi: 10.1097/MD.0000000000011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackmore T.K., Manning L., Taylor W.J., Wallis R.S. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47(10):e83–e85. doi: 10.1086/592695. [DOI] [PubMed] [Google Scholar]
- 30.Santin M, Escrich C, Majòs C, Llaberia M, Grijota MD, Grau I. Tumor necrosis factor antagonists for paradoxical inflammatory reactions in the central nervous system tuberculosis: Case report and review. Medicine (Baltimore). Oct 23 2020;99(43):e22626. doi:10.1097/MD.0000000000022626. [DOI] [PMC free article] [PubMed]
- 31.Marais BJ, Cheong E, Fernando S, et al. Use of Infliximab to Treat Paradoxical Tuberculous Meningitis Reactions. Open Forum Infect Dis. Jan 2021;8(1):ofaa604. doi:10.1093/ofid/ofaa604. [DOI] [PMC free article] [PubMed]
- 32.Molton J.S., Huggan P.J., Archuleta S. Infliximab therapy in two cases of severe neurotuberculosis paradoxical reaction. Med J Aust. 2015;202(3):156–157. doi: 10.5694/mja14.00716. [DOI] [PubMed] [Google Scholar]
- 33.Manesh A, Gautam P, Selwyn Selva Kumar D, et al. Effectiveness of adjunctive high dose infliximab therapy to improve disability free survival among patients with severe CNS tuberculosis: a matched retrospective cohort study. Clin Infect Dis. Jul 05 2023;doi:10.1093/cid/ciad401. [DOI] [PubMed]
- 34.Dixon W.G., Hyrich K.L., Watson K.D., et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology biologics register (BSRBR) Ann Rheum Dis. Mar 2010;69(3):522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Fan W, Yang G, et al. Risk of tuberculosis in patients treated with TNF-α antagonists: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. Mar 22 2017;7(3):e012567. doi:10.1136/bmjopen-2016-012567. [DOI] [PMC free article] [PubMed]
- 36.Wallis R.S., Kyambadde P., Johnson J.L., et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18(2):257–264. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 37.Philips J.A., Ernst J.D. Tuberculosis pathogenesis and immunity. Annu Rev Pathol. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 38.Cooper A.M., Mayer-Barber K.D., Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. May 2011;4(3):252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Garra A., Redford P.S., McNab F.W., Bloom C.I., Wilkinson R.J., Berry M.P. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 40.Dorhoi A., Kaufmann S.H. Tumor necrosis factor alpha in mycobacterial infection. Semin Immunol. Jun 2014;26(3):203–209. doi: 10.1016/j.smim.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Huang H., Wang W. Molecular mechanisms of glucocorticoid resistance. Eur J Clin Invest. Feb 2023;53(2):e13901. doi: 10.1111/eci.13901. [DOI] [PubMed] [Google Scholar]
- 42.Hardin J., MacLeod S., Grigorieva I., et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84(9):3063–3070. [PubMed] [Google Scholar]
- 43.Mehta A.K., Gracias D.T., Croft M. TNF activity and T cells. Cytokine. Jan 2018;101:14–18. doi: 10.1016/j.cyto.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]