ABSTRACT
Background
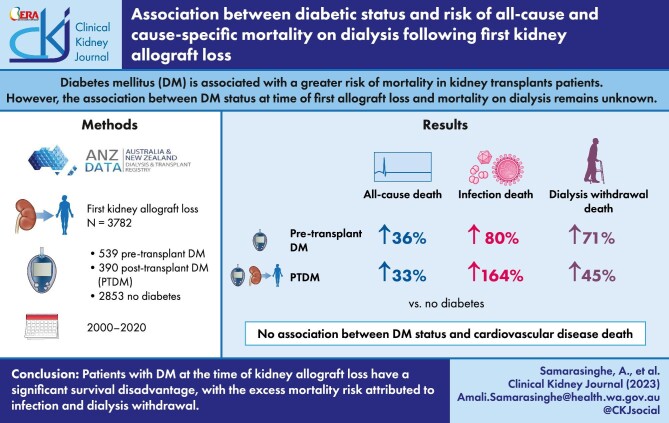
Diabetes mellitus (DM) is associated with a greater risk of mortality in kidney transplant patients, primarily driven by a greater risk of cardiovascular disease (CVD)-related mortality. However, the associations between diabetes status at time of first allograft loss and mortality on dialysis remain unknown.
Methods
All patients with failed first kidney allografts transplanted in Australia and New Zealand between 2000 and 2020 were included. The associations between diabetes status at first allograft loss, all-cause and cause-specific mortality were examined using competing risk analyses, separating patients with diabetes into those with pre-transplant DM or post-transplant diabetes mellitus (PTDM).
Results
Of 3782 patients with a median (IQR) follow-up duration of 2.7 (1.1–5.4) years, 539 (14%) and 390 (10%) patients had pre-transplant DM or developed PTDM, respectively. In the follow-up period, 1336 (35%) patients died, with 424 (32%), 264 (20%) and 199 (15%) deaths attributed to CVD, dialysis withdrawal and infection, respectively. Compared to patients without DM, the adjusted subdistribution HRs (95% CI) for pre-transplant DM and PTDM for all-cause mortality on dialysis were 1.47 (1.17–1.84) and 1.47 (1.23–1.76), respectively; for CVD-related mortality were 0.81 (0.51–1.29) and 1.02 (0.70–1.47), respectively; for infection-related mortality were 1.84 (1.02–3.35) and 2.70 (1.73–4.20), respectively; and for dialysis withdrawal-related mortality were 1.71 (1.05–2.77) and 1.51 (1.02–2.22), respectively.
Conclusions
Patients with diabetes at the time of kidney allograft loss have a significant survival disadvantage, with the excess mortality risk attributed to infection and dialysis withdrawal.
Keywords: allograft loss, diabetes, dialysis, kidney failure, mortality
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
In kidney transplant recipients, those with pre-transplant diabetes mellitus or have developed post-transplant diabetes mellitus (PTDM) have a significant survival disadvantage compared to those without diabetes.
Survival after kidney allograft loss is extremely poor, with less than 1 in 2 patients surviving 10 years post-allograft loss as compared to over 75% of patients surviving beyond 10 years with functioning kidney transplants.
Little is known about the impact of diabetes status on mortality following the loss of kidney allografts.
This study adds:
Compared to patients without diabetes, patients with pre-transplant diabetes mellitus and PTDM were more likely to die on dialysis.
There was no association between diabetes status at time of first allograft loss and CVD-related mortality on dialysis, with the excess risks of mortality in patients with pre-transplant diabetes mellitus or PTDM attributed to infection-related and/or dialysis-withdrawal-related mortality.
The magnitude of the excess risk of mortality in patients with pre-transplant diabetes mellitus or PTDM was greater in younger patients.
Potential impact:
Understanding patient factors associated with a higher risk of mortality on dialysis post-kidney allograft loss is critical for clinicians to tailor how we counsel and monitor failed allograft patients to improve health outcomes.
A more detailed evaluation of the excess risk of infection and withdrawal-related deaths experienced by patients with diabetes post-allograft loss is required.
INTRODUCTION
Diabetes mellitus (DM) is a leading cause of kidney failure. In Australia, the proportion of patients with diabetes on kidney replacement therapy has increased from 46% to 52% between 2009 and 2019, respectively, with diabetic kidney disease the primary cause of kidney failure in 39% of patients in 2019 [1, 2]. Similar proportions of diabetic kidney disease as the primary cause of treated kidney failure have been reported in the United States (48% in 2017 compared to 44% in 2012) and New Zealand (59% in 2019 compared to 52% in 2009) [1–4].
The association between diabetes status and increased mortality risk in patients with treated kidney failure is well established. In patients commencing dialysis, patients with diabetes have up to a 40% greater risk of mortality and up to a 63% greater risk of cardiovascular disease (CVD) mortality, compared to those without diabetes [5]. In kidney transplant recipients, those with pre-transplant diabetes or who have developed post-transplant diabetes (PTDM) have a significant survival disadvantage, with the dominant cause of death attributed to CVD [6]. However, age has been shown to modify the relationship between diabetes status and mortality such that the survival disadvantage in patients with type 2 diabetes is more apparent for younger patients with kidney failure [5, 6].
Survival after allograft loss is poor, with less than one in two patients surviving 10 years post-allograft loss as compared to over 75% of patients surviving beyond 10 years with functioning kidney transplants [7]. In the general population, increasing duration of diabetes is associated with a greater risk of CVD and all-cause death independent of glycaemic control, such that patients with diabetes duration of over 10 years have over a 50% higher risk of fatal and non-fatal CVD compared to patients with diabetes duration of less than 5 years [8, 9]. Prior work, however, has not examined whether disease duration may influence the risk of death on dialysis following kidney allograft loss or whether age modified the association between diabetes status at time of allograft loss and death. Therefore, it is also important to evaluate whether there is a survival difference between those with pre-transplant diabetes and PTDM and how age modified the association.
The aims of this study were 2-fold. First, we examined the association between diabetes status (diabetes diagnosed prior to first transplant and PTDM), all-cause and cause-specific mortality on dialysis after allograft loss. Second, we examined whether patient age at time of kidney allograft loss modified the association between diabetes status and all-cause mortality on dialysis.
MATERIALS AND METHODS
Study population
Using data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry, adult patients (aged at least 18 years at time of first allograft loss) who commenced haemodialysis or peritoneal dialysis following first kidney allograft loss in Australia and New Zealand between 2000 and 2020 were included. Patients where their first kidney allograft was a multi-organ allograft were excluded. Patients without documented diabetes status prior to the time of allograft loss were also excluded. Patients were followed from the commencement of dialysis post-allograft loss until repeat kidney transplantation, death on dialysis or 31 December 2020, whichever occurred first. The conduct of this study was approved by the University of Western Australia Human Research Ethics Committee, Perth, Australia (reference number 2021/ET000573).
Study covariates
Baseline patient characteristics at the time of first kidney allograft loss included age, sex, ethnicity (indigenous and non-indigenous patients), primary cause of kidney failure, body mass index (BMI), comorbid conditions (cardiovascular disease, cerebrovascular accident, peripheral vascular disease, prior cancers) and smoking history (collected at time of kidney replacement therapy). Duration of functioning first kidney allograft and waitlist data [i.e. whether patients were relisted (on the deceased donor transplant wait-list) for repeat transplantation] were also extracted. Data relating to the presence and absence of diabetes were reported by all centres to ANZDATA registry annually.
Exposure factor
The primary exposure factor was diabetes status at the time of first allograft loss, defined as having ‘no diabetes’ (no reported diabetes at any time), ‘pre-transplant DM’ (patients reported to have diabetes prior to first kidney transplantation) or ‘PTDM’ (patients without diabetes prior to first kidney transplantation but have developed diabetes post-transplant).
Clinical outcomes
The study outcomes were all-cause and cause-specific (CVD, dialysis withdrawal and infection-related causes) mortality on dialysis post-allograft loss. CVD mortality was defined as death attributed to a cardiac cause (ischaemic heart disease, heart failure or arrhythmia), using pre-defined definitions according to the ANZDATA registry (available at: https://www.anzdata.org.au/anzdata/services/data-management/data-forms/). Dialysis withdrawal-related mortality was defined as death from dialysis withdrawal because of psychosocial or medical reasons; the latter include withdrawal from dialysis access difficulties, withdrawal from CVD comorbid condition, withdrawal from cerebrovascular comorbid condition, withdrawal from peripheral vascular comorbid conditions, and withdrawal from malignancy. Infection mortality was defined as death from any site-specific infections (bacterial, viral, fungal, or protozoan). Death occurring after repeat (second) kidney transplantation was not considered as an event in this study.
Statistical analysis
Data were expressed as number (percentages) for categorical variables, mean [standard deviation (SD)] for normally distributed continuous variables, and median (interquartile range) for non-normally distributed continuous variables, with comparisons between groups undertaken using chi-square test, analysis of variance (ANOVA) and Kruskal–Wallis test, respectively. The unadjusted incidence rates for all-cause and cause-specific mortality on dialysis were expressed as events [95% confidence interval (95% CI)] per 1000-person years and compared between exposure groups using incidence rate ratios. Cumulative survival at 3 and 5 years post-allograft loss were determined using the Kaplan–Meier method, with the log-rank test used to compare survival between groups. The associations between diabetes status, all-cause and cause-specific mortality on dialysis were examined using a Cox proportional hazards model. For all-cause mortality, repeat kidney transplantation was considered the censored event. For CVD-related mortality, repeat transplantation and non-CVD mortality the censored events; for dialysis-withdrawal mortality, repeat transplantation and non-dialysis withdrawal-related mortality the censored events; and for infection-related mortality, repeat transplantation and non-infection-related mortality the censored events. The estimates of these models were reported as adjusted hazard ratio (HR) and 95% confidence intervals (95% CI). The proportional hazard assumptions of the Cox regression models were checked graphically by plotting the Schoenfeld residuals and there were no departures from the proportional hazards assumption. We constructed four Cox proportional hazards models with incremental levels of adjustment for patient characteristics: (i) Model 1 with diabetes status; (ii) Model 2 with the addition of age, sex, and ethnicity; (iii) Model 3 with the addition of comorbidities of prevalent coronary artery disease, cerebrovascular disease, peripheral vascular disease, prior cancer history, and smoking history at time of dialysis initiation; and (iv) Model 4 with the addition of primary cause of kidney failure, duration of first kidney allograft, BMI, era and residential state/country. These covariates were chosen for inclusion in the multivariable models given the reported biological relationships with mortality in prior studies [5, 6]. Two-way interaction between age (median age thresholds of ≤ or >50 years) and diabetes status was examined in the multivariable-adjusted models for all-cause mortality, with a P-value of <.05 indicating a significant interaction between the covariates. Interactions between diabetes status and prevalent vascular disease for all-cause and CVD death were also examined, with P-value of <.05 indicating significant interaction.
We applied the Fine–Gray competing risk modelling to estimate the adjusted subdistribution HR with 95% CI for all-cause and cause-specific mortality [10]. For all-cause mortality on dialysis, the competing event was repeat transplantation. For cause specific mortality, the competing events were repeat transplantation and other (i.e. non-cardiovascular, non-withdrawal, or non-infectious) causes of mortality. The estimates were provided for the same four models, with increasing level of adjustment of covariates.
In a sensitivity analysis examining the association between waitlist status and mortality, patients were re-categorized according to diabetes and waitlist status. Statistical analyses were performed using the STATA statistical software version 9.4, with P-values of less than .05 considered statistically significant.
RESULTS
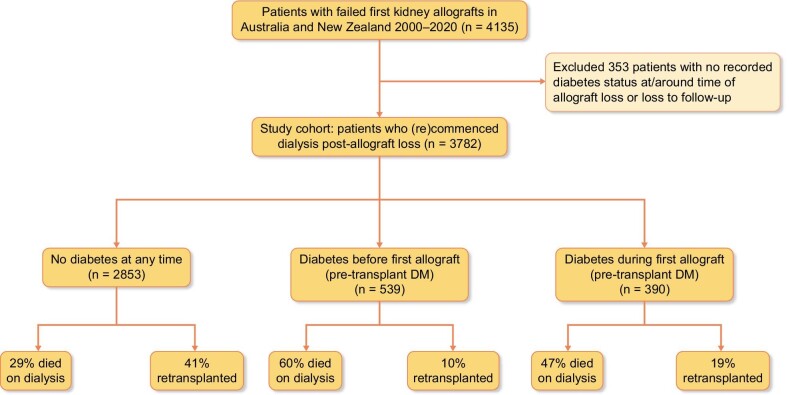
Between January 2000 and December 2020, there were a total of 3782 patients in Australia and New Zealand who commenced dialysis following their first kidney allograft loss (Fig. 1), with a median (IQR) patient follow-up duration of 2.7 (1.1–5.4) years. Of these, 539 (14%) patients had pre-transplant DM [prior to first allograft, 126 (23%) with type 1 DM and 413 (77%) with type 2 DM] and 390 (10%) had developed PTDM during the first allograft. The median (IQR) follow-up periods for patients with no DM, pre-transplant diabetes or PTDM were 2.8 (1.2, 5.7) years, 2.3 (0.9, 4.6) years and 2.5 (0.9, 4.9) years.
Figure 1:
Flow diagram of the study cohort of 3782 patients who commenced on dialysis following failed first kidney allografts in Australia and New Zealand, comprising of 2853 (75%) patients without diabetes, 539 (14%) patients with pre-transplant diabetes mellitus (DM), and 390 (10%) patients who developed post-transplant diabetes mellitus (PTDM).
Table 1 shows the baseline characteristics of the study cohort, stratified by diabetes status. Patients with pre-transplant DM and PTDM were older [mean (SD) 56.5 (10.9), 55.7 (12.6) vs. 46.8 (14.7)], with a greater proportion of patients with prevalent CVD (49%, 36% vs. 15%) and PVD (40%, 16% vs. 7%) at the time of first allograft loss compared to patients without DM. There was an increasing proportion of patients with pre-transplant DM and PTDM post-allograft loss in the more recent decade (2000–2010: 13% and 8%; 2011–2020: 16% and 13%, respectively).
Table 1:
Baseline: characteristics of the study cohort
| No diabetes(n = 2853) | Pre-transplant DM(n = 539) | PTDM(n = 390) | P-value | |
|---|---|---|---|---|
| Patient demographics | ||||
| Age [mean (SD)] | 46.8 (14.7) | 56.5 (10.9) | 55.7 (12.6) | <.001 |
| Female (n, %) | 1107 (38.8) | 185 (34.3) | 151 (38.7) | .141 |
| Race (n, %) | ||||
| Non-indigenous Indigenous | 2768 (97.0)85 (3.0) | 472 (87.6)67 (12.4) | 373 (95.6)17 (4.4) | <.001 |
| Body mass index [mean (SD)] | 28.1 (10.5) | 28.6 (5.8) | 29.9 (7.7) | .002 |
| Patient comorbidities | ||||
| Smoking history (n, %) | ||||
| Non-smoker Former smoker Current smoker Missing | 1638 (57.4)669 (23.4)376 (13.2)170 (6.0) | 246 (45.6)219 (40.6)57 (10.6)17 (3.2) | 203 (52.1)108 (27.7)52 (13.3)27 (6.9) | <.001 |
| Coronary artery disease (n, %) | ||||
| No Yes Missing | 2396 (84.0)439 (15.4)18 (0.6) | 276 (51.2)263 (48.8)0 (0.0) | 249 (63.8)141 (36.2)0 (0.0) | <.001 |
| Cerebrovascular disease (n, %) | ||||
| No Yes Missing | 2673 (93.7)162 (5.7)18 (0.6) | 453 (84.0)86 (16.0)0 (0.0) | 354 (90.8)36 (9.2)0 (0.0) | <.001 |
| Peripheral vascular disease (n, %) | ||||
| No Yes Missing | 2639 (92.5)196 (6.9)18 (0.6) | 325 (60.3)214 (39.7)0 (0.0) | 328 (84.1)62 (15.9)0 (0.0) | <.001 |
| Prior cancer (n, %) | ||||
| No Yes Missing | 2290 (80.3)560 (8.6)3 (1.1) | 453 (84.1)85 (15.8)1 (0.1) | 315 (80.8)75 (19.2)0 (0.0) | .113 |
| Others | ||||
| Primary cause of kidney failure (n, %) Glomerulonephritis Diabetic kidney disease Hypertension/vascular Cystic disease Others | 1597 (56.0)0 (0.0)95 (3.3)225 (7.9)936 (32.8) | 109 (20.2)346 (64.2)11 (2.0)12 (2.3)61 (11.3) | 205 (52.6)0 (0.0)21 (5.4)48 (12.3)116 (29.7) | <.001 |
| Duration of first allograft [years; mean (SD)] | 11.3 (7.6) | 7.4 (5.4) | 13.4 (7.3) | <.001 |
| Era (n, %) 2000–2010 2011–2020 | 1330 (46.6)1523 (53.4) | 209 (38.8)330 (61.2) | 125 (32.1)265 (67.9) | <.001 |
| State/country (n, %) | ||||
| New South Wales/ACT Victoria/Tasmania Queensland South Australia/Northern Territory Western Australia New Zealand | 702 (24.6)736 (25.8)505 (17.7)238 (8.4)234 (8.2)438 (15.3) | 110 (20.4)138 (25.6)84 (15.6)74 (13.7)55 (10.2)78 (14.5) | 100 (25.6)103 (26.4)59 (15.1)48 (12.3)40 (10.3)40 (10.3) | <.001 |
Data expressed as mean (standard deviation [SD]) or number (percentages). ACT: Australian Capital Territory.
Clinical outcomes of study cohort
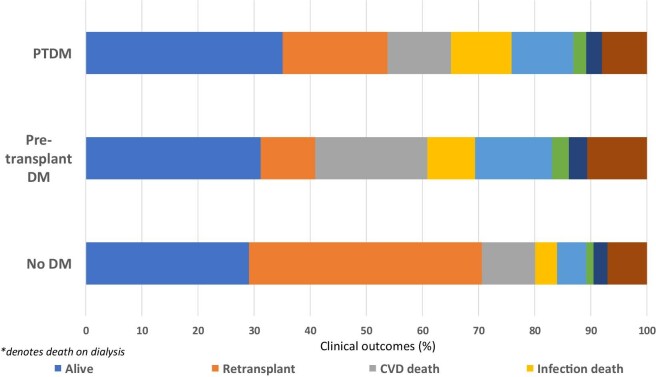
During the follow-up period, 1310 (34.6%) received a second kidney transplant and 1336 (35%) died on dialysis. CVD deaths were the most common cause of death (n = 424, 32%), followed by dialysis withdrawal (n = 264, 20%) and infections (n = 199, 15%). Figure 2 shows the outcomes of the study cohort by diabetes status. A greater proportion of patients without DM received a second kidney transplant compared to those with pre-transplant DM and PTDM (42%, 10% vs. 19%); whereas patients with pre-transplant DM (59%) and PTDM (46%) were more likely to die on dialysis compared to patients without DM (29%). Of patients without DM who died on dialysis, CVD (33%), dialysis withdrawals (18%) and infection (13%) were the most frequent causes of death. These compared with the respective proportions of 34%, 23%, and 14% for patients with pre-transplant DM and 24%, 24%, and 23% for patients who had developed PTDM.
Figure 2:
Bar graph showing the clinical outcomes of patients who commenced dialysis following failed first kidney allografts, stratified by diabetes status. For the outcome of ‘deaths’, the proportion of deaths from cardiovascular disease, infection, dialysis withdrawal, and other causes are shown. CVA: cerebrovascular accident.
Incidence rates for cause-specific and all-cause mortality
Table 2 shows the unadjusted incidence rates for all-cause and cause-specific mortality for each diabetes exposure group. The incidence rate for all-cause mortality on dialysis was significantly higher for patients with pre-transplant DM [185 (95% CI 166, 206) events per 1000-person-years], compared to patients without DM [74 (95% CI 69, 79) events per 1000-person-years] or those with PTDM [137 (95% CI 118, 158) events per 1000 person-years]. The excess mortality for patients with pre-transplant DM was attributed to CVD mortality, with an incidence rate of 61 (95% CI 51, 74) events per 1000 person-years, which was significantly higher compared with 24 (95% CI 21, 27) and 33 (95% CI 24, 44) events per 1000 person-years for patients without DM or with PTDM, respectively. Patients with pre-transplant DM and PTDM had similar incidence rates of infection and dialysis withdrawal-related mortality, and significantly higher compared to patients without DM.
Table 2:
Unadjusted: incidence rates of all-cause and cause-specific mortality by diabetes status at time of allograft loss.
| No diabetesa | Pre-transplant DMb | PTDMc | P-values | |
|---|---|---|---|---|
| All-cause mortality | 73.6 (68.8, 78.7) | 184.9 (165.9, 206.1) | 136.7 (118.3, 158.1) | a vs. b: <0.001a vs. c: <0.001b vs. c: <.001 |
| CVD-related mortality | 23.8 (21.2, 26.8) | 61.2 (50.7, 74.0) | 32.9 (24.5, 44.2) | a vs. b: <0.001a vs. c: 0.058b vs. c: <.001 |
| Infection-related mortality | 9.7 (8.1, 11.7) | 26.1 (19.5, 34.8) | 30.6 (22.6, 41.6) | a vs. b: <0.001a vs. c: <0.001b vs. c: .482 |
| Dialysis withdrawal-related mortality | 12.9 (11.0, 15.1) | 40.8 (32.2, 51.5) | 34.0 (25.5, 45.2) | a vs. b: <0.001a vs. c: <0.001b vs. c: .335 |
Data expressed as events per 1000 person-years. CVD: cardiovascular disease; DM: diabetes mellitus; PTDM: post-transplant diabetes mellitus.
Association between diabetes status and all-cause mortality on dialysis
The 3 and 5-year patient survivals for patients without DM were 79.2% (95% CI 77.5 to 80.8) and 69.4% (67.2, 71.5), respectively. These compared with respective survivals of 57.5% (52.8, 61.9) and 39.0% (34.2, 43.9) for patients with pre-transplant DM; and 66.2% (60.8, 71.1) and 51.1% (44.8, 57.0) for patients with PTDM (log-rank across groups, P < .01).
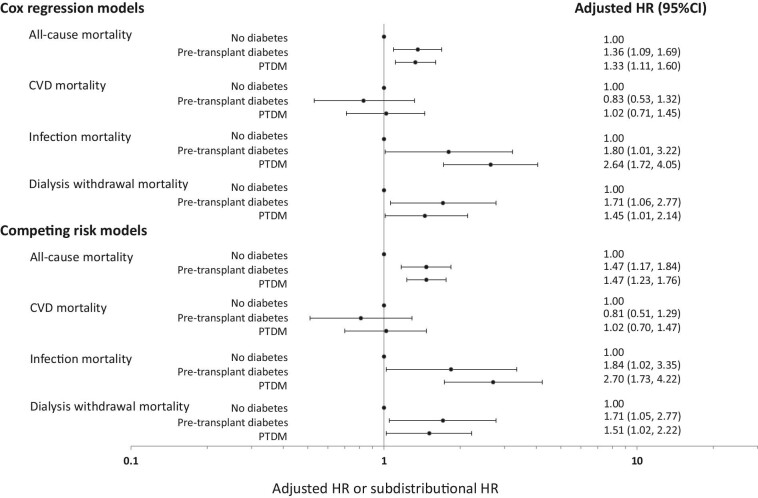
Compared to patients without DM, patients with pre-transplant DM and PTDM were more likely to die on dialysis, with multivariable-adjusted HRs of 1.36 (1.09, 1.69) and 1.33 (1.11, 1.60), respectively (Model 4, Table 3, and Fig. 3). In the analysis restricted to patients with DM, patients with pre-transplant DM were significantly more likely to die compared to patients with PTDM in the unadjusted and partially adjusted models, but this association was no longer significant in the fully adjusted Model 4 [adjusted HR 1.02 (0.79, 1.31)].
Table 3:
Association between diabetes status at time of first kidney allograft loss, all-cause and cause-specific mortality.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Cox proportional hazard models | ||||
| All-cause mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.002.48 (2.18, 2.82)a1.86 (1.58, 2.18)a | 1.001.85 (1.62, 2.12)a1.39 (1.18, 1.63)a | 1.001.39 (1.19, 1.62)1.27 (1.06, 1.51) | 1.001.36 (1.09, 1.69)1.33 (1.11, 1.60) |
| CVD-related mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.002.55 (2.04, 3.19)a1.38 (1.00, 1.90)a | 1.001.96 (1.55, 2.49)a1.10 (0.80, 1.53)a | 1.001.33 (1.02, 1.75)a0.94 (0.66, 1.33)a | 1.000.83 (0.53, 1.32)1.02 (0.71, 1.45) |
| Infection-related mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.002.56 (1.82, 3.61)3.04 (2.13, 4.36) | 1.001.84 (1.28, 2.65)2.30 (1.60, 3.31) | 1.001.62 (1.08, 2.44)2.25 (1.52, 3.32) | 1.001.80 (1.01, 3.22)2.64 (1.72, 4.05) |
| Dialysis withdrawal-related mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.003.25 (2.45, 4.29)2.49 (1.77, 3.50) | 1.002.30 (1.72, 3.08)1.65 (1.17, 2.33) | 1.001.53 (1.10, 2.14)1.49 (1.03, 2.16) | 1.001.71 (1.06, 2.77)1.45 (1.01, 2.14) |
| All-cause mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.003.05 (2.69, 3.45)a2.08 (1.78, 2.44)a | 1.002.11 (1.84, 2.43)a1.49 (1.28, 1.75)a | 1.001.57 (1.34, 1.85)1.33 (1.12, 1.58) | 1.001.47 (1.17, 1.84)1.47 (1.23, 1.76) |
| CVD-related mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.002.40 (1.92, 2.99)a1.30 (0.94, 1.78)a | 1.001.83 (1.43, 2.35)a1.06 (0.77, 1.47)a | 1.001.32 (0.97, 1.79)0.89 (0.62, 1.28) | 1.000.81 (0.51, 1.29)1.02 (0.70, 1.47) |
| Infection-related mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.002.37 (1.68, 3.33)2.98 (2.09, 4.27) | 1.001.72 (1.18, 2.50)2.30 (1.59, 3.33) | 1.001.67 (1.11, 2.51)2.29 (1.53, 3.41) | 1.001.84 (1.02, 3.35)2.70 (1.73, 4.22) |
| Dialysis withdrawal-related mortality | ||||
| No diabetesPre-transplant DMPTDM | 1.002.99 (2.26, 3.95)2.38 (1.70, 3.35) | 1.002.10 (1.56, 2.82)1.62 (1.15, 2.29) | 1.001.57 (1.12, 2.21)1.52 (1.06, 2.19) | 1.001.71 (1.05, 2.77)1.51 (1.02, 2.22) |
Data expressed as adjusted hazard ratios and 95% confidence intervals. Model 1: unadjusted model (diabetes status only); Model 2: Model 1 + adjustments for age, race and sex; Model 3: Model 2 + adjustments for comorbidities (smoking history, coronary artery disease, cerebrovascular disease, peripheral vascular disease, prior cancer); Model 4: Model 3 + adjustments for primary cause of kidney failure, era, body mass index, duration of first allograft and state/country. CVD: cardiovascular disease; DM: diabetes mellitus; PTDM: post-transplant diabetes mellitus.
Comparison between pre-transplant DM and PTDM groups with P-values of <.05.
Figure 3:
Forest plots showing the adjusted hazard ratios (HR) and subdistribution HR and 95% confidence intervals (95% CI) of the association between diabetes status at time of first allograft loss, all-cause, and cause-specific [cardiovascular disease (CVD), dialysis withdrawal, and infection] mortality.
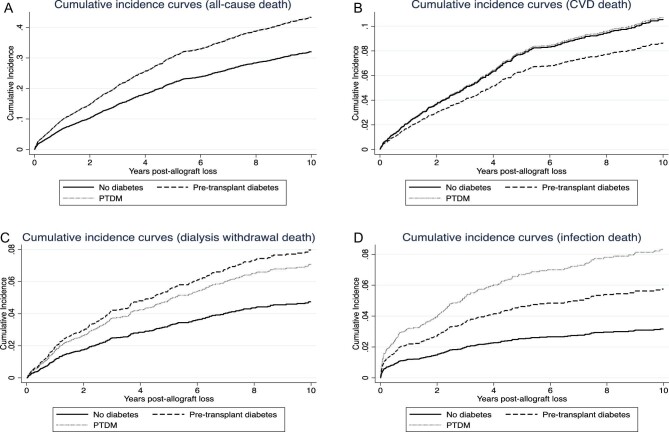
In the competing risk model, the estimates were similar with respective adjusted subdistribution HR for all-cause mortality of 1.47 (1.17, 1.84) and 1.47 (1.23, 1.76) (Table 3 and Fig. 3). If the referent group was changed to PTDM, the adjusted subdistribution HR for pre-transplant DM for all-cause mortality was 1.00 (0.77, 1.30). Adjusted cumulative incidence curves for all-cause mortality by diabetes status are shown in Fig. 4A.
Figure 4:
Adjusted cumulative incidence curves for all-cause mortality on dialysis (A), cardiovascular disease (CVD)-related mortality (B), dialysis withdrawal-related mortality (C) and infection-related mortality (D) post-dialysis initiation following first kidney allograft loss. The solid black line represents patients without diabetes, discontinuous black line represents patients with pre-transplant diabetes mellitus and the solid grey line represents patients with post-transplant diabetes mellitus (PTDM).
Association between diabetes status and cause-specific mortality on dialysis
The 5-year CVD mortality free patient survivals for patients without DM, with pre-transplant DM and PTDM were 87.9% (86.1, 89.4), 71.9% (65.9, 77.0), and 84.4% (78.5, 88.8), respectively (log-rank across groups, P < .01). The respective 5-year infection-mortality free patient survivals were 95.4% (94.3, 96.3), 87.4% (82.9, 90.8) and 85.7% (80.0, 89.9; log-rank across groups, P < .01); and the respective 5-year dialysis withdrawal mortality free patient survivals were 94.1% (92.8, 95.1), 83.8% (79.0, 87.5) and 84.1% (77.8, 88.7) (log-rank across groups, P < .01).
Table 3 and Fig. 3 show the association between diabetes status and cause-specific mortality for both Cox proportional hazards and competing risk models. Patients with pre-transplant DM were significantly more likely to experience CVD mortality compared to patients without DM in the unadjusted and partially adjusted models, but this association was not statistically significant in the fully adjusted Model 4. There was no association between PTDM and CVD mortality in the unadjusted and adjusted models. In the analysis restricted to patients with DM, patients with pre-transplant DM were significantly more likely to experience CVD mortality compared to patients with PTDM in the unadjusted and partially adjusted models, but this association was no longer significant in the fully adjusted Model 4.
Compared to patients without DM, patients with pre-transplant DM and PTDM experienced a higher risk of infection-related mortality, with the magnitude greatest in those with PTDM. Although patients with pre-transplant diabetes and PTDM experienced a higher risk of dialysis withdrawal mortality, this was statistically significant only in the latter group. When restricted only to patients with DM, there were no associations between pre-transplant DM and infection [adjusted HR 0.68 (0.36, 1.29)] and dialysis-withdrawal mortality [adjusted HR 1.18 (0.68, 2.06)], compared to patients with PTDM. Adjusted cumulative incidence curves for CVD, dialysis withdrawal-related, and infection-related mortality by diabetes status are shown in Fig. 4B–D.
Interaction between diabetes status and age for all-cause mortality
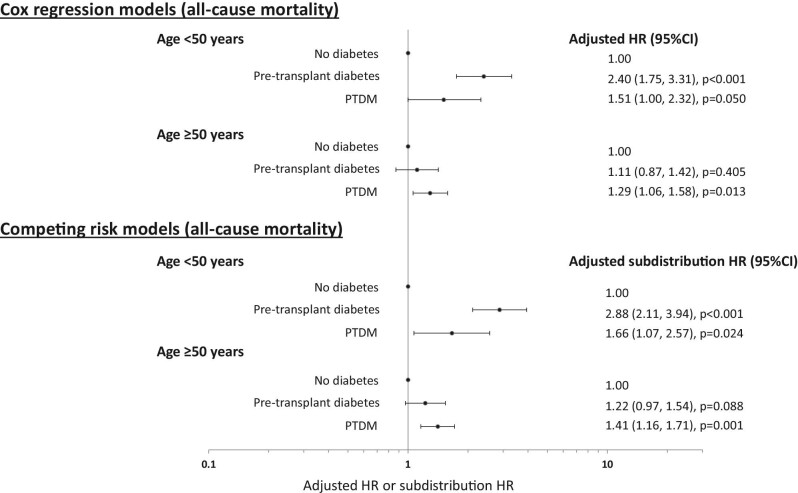
There was a significant interaction between diabetes status and age (P-value for interaction <.01) for all-cause mortality. In younger patients aged ≤50 years, the adjusted HRs for patients with pre-transplant diabetes and PTDM were 2.44 (1.78, 3.34) and 1.53 (1.01, 2.33), respectively, compared to patients without diabetes. This association was only present for patients with PTDM in older patients aged >50 years [1.28 (1.05, 1.56)]. Similar estimates were observed in the competing risk models (Fig. 5).
Figure 5:
Forest plots showing the adjusted hazard ratios (HR) and subdistribution HR and 95% confidence intervals (95% CI) of the association between diabetes status at time of first allograft loss and all-cause mortality, stratified by patient age (< and ≥50 years at time of allograft loss).
There were no statistically significant interactions between diabetes status and prevalent vascular disease for all-cause or CVD death (P-values for interactions of >.1).
Sensitivity analysis: association between diabetes and waitlist status and mortality
Of patients without diabetes, 1120 (39%) were waitlisted for repeat transplantation. This compared with 64 (12%) and 85 (22%) patients with pre-transplant diabetes and PTDM who were waitlisted, respectively. Table S1 (see online supplementary material) shows the respective adjusted HR (95% CI) and SHR (95% CI) for all-cause and cause-specific mortality. Compared to patients without diabetes and who were not waitlisted, patients with either pre-transplant diabetes or those with PTDM and not waitlisted had a higher risk of all-cause mortality. Patients who were waitlisted, irrespective of the presence of pre-transplant diabetes or PTDM, experienced a lower risk of mortality and CVD mortality.
DISCUSSION
In this contemporary study of patients with failed kidney allografts spanning two decades, patients with pre-transplant DM or PTDM experienced a significant survival disadvantage on dialysis, primarily explained by an excess risk of dialysis withdrawal-related and infection-related mortality. The unadjusted rate of CVD mortality was significantly greater in patients with pre-transplant DM compared to those without diabetes or with PTDM, suggesting that the duration of diabetes may have a significant effect on CVD survival on dialysis following kidney allograft loss. There was a significant interaction between diabetes status and age for all-cause mortality on dialysis, with the increased risk of mortality more apparent in younger patients.
Cohort studies have consistently shown that patients with kidney failure and diabetes have poorer outcomes. Two studies showed that patients with kidney failure and diabetes experienced a significant survival disadvantage compared to patients without diabetes [5, 6]. Similar survival disadvantage has also been shown in patients who have developed PTDM after kidney transplantation [11–13]. However, there is little data about the association between diabetes status and outcome on dialysis following kidney allograft loss. In this study, we have shown that patients with pre-transplant DM or PTDM have a greater than 30% risk of mortality on dialysis following first allograft loss and were less likely to be retransplanted compared to patients without diabetes. Despite having a shorter duration of diabetes, patients with PTDM experienced comparable survival disadvantage to those with pre-transplant DM, suggesting that hyperglycaemia in the post-transplant period may have a greater deleterious effect on survival following allograft loss. These findings highlight the continuing prognostic significance of diabetes status following kidney allograft loss and suggest that a greater understanding contributing to this survival disparity is required.
In patients who are receiving dialysis or have received kidney transplants, diabetes is associated with an excess risk of CVD-related mortality compared to those without diabetes [5, 6, 14]. A similar association has been observed for patients who have developed PTDM [12, 15, 16]. Contrary to these studies, our study has shown that there was no association between diabetes status post-allograft loss and CVD-related mortality on dialysis. Kidney transplant recipients with vascular disease experienced an excess risk of vascular related mortality by at least 1.6 times compared to those without vascular disease [17]. The observation in this study is somewhat surprising, although may reflect improvements in the management of CVD risk factors over time, the presence of both confounding and survival bias [a type of selection bias, with this analysis only included patients that have survived after allograft loss without considering those patients that have died (most likely from vascular disease) and did not survive after allograft loss] and a short follow-up period (median follow-up of less than 3 years) such that the patients with pre-transplant diabetes or PTDM may not have had sufficient time to develop severe CVD. It is noteworthy that although patients with pre-transplant diabetes had a higher risk of CVD mortality compared to patients without diabetes or with PTDM in the partially adjusted models, this was no longer statistically significant in the final model suggesting that the relationship between pre-transplant diabetes status and CVD mortality is due to confounding by other patient and transplant-related characteristics.
The higher risks of infection and dialysis withdrawal-related mortality in diabetic patients is not unexpected. The chronic exposure to immunosuppression, combined with exposure to vascular catheters may have further predisposed patients with failed allografts to infection-related complications [18, 19]. The management of patients with failing allografts remains inadequate and often the transition from functioning transplant to dialysis treatment is delayed and fragmented [20–22]. It is important to point out that our study definition of dialysis withdrawal-related death included withdrawals for medical reasons including CVD, which may have affected the estimates of CVD-related mortality. However, given the reasons for dialysis withdrawal are often multifactorial (often an overlap between psychosocial and multiple medical reasons rather than attributed to a dominant cause), study analysis focusing on dialysis withdrawal-related deaths, regardless of the cause is most appropriate as the approach and management of patients undergoing dialysis withdrawals are dissimilar to death from other causes.
In our study, the finding that age modified the association between diabetes status and mortality is consistent with other studies [5, 6, 23–25]. Although the precise pathophysiology remains unclear, it is likely that younger patients with diabetes may represent a distinct clinical phenotype or that the treatment of diabetes or CVD risk factors is inadequate in younger patients. One can also speculate that younger patients may be less disciplined with diabetes management compared with older and more experienced patients, which may have contributed to our study findings, but these data are not collected by the registry.
A prior cohort study of patients with failed first allografts showed that patients reinitiated on dialysis and on the waitlist for repeat transplantation post-allograft loss had superior survival compared to patients reinitiated on dialysis but not waitlisted, likely reflecting differences in patient characteristics and comorbid burden [26]. Similarly, our study has shown that patients who were waitlisted for repeat transplantation following first allograft loss had significantly lower risk of all-cause mortality compared to patients who were not waitlisted, with this survival advantage observed for those with or without diabetes. The survival disadvantage associated with the presence of diabetes (pre-transplant diabetes or PTDM) was no longer apparent if the patients were waitlisted for repeat transplantation, suggesting that the clinical phenotypes of the waitlisted patients were dissimilar to those who were not waitlisted for repeat transplantation. However, given the relatively small cohort sizes of waitlisted patients with pre-transplant diabetes or with PTDM, the risk estimates for mortality in these groups may be imprecise, especially for cause-specific mortality.
Managing patients with failing kidney allografts should be carefully considered, with a focus on the understanding of the barriers and preparation of these patients for dialysis following allograft loss. Identifying patient characteristics, such as diabetes status, that are associated with poorer survival post-allograft loss is essential, as this allows clinicians and patients to allow for early preparation for dialysis or repeat transplantation, and undertake adequate CKD management. Personalised strategies that allow for an efficient transition to dialysis require early recognition, adequate patient education, dedicated clinics focusing on the transition to dialysis, integration of other healthcare services (such as CKD management, dialysis access planning, symptom management, reassessment of re-transplant suitability) and the management of the patient's quality of life and emotional burden that often accompany a failing allograft [21]. There continues to be major gaps in the management of patients with failing allografts, including the titration of immunosuppression, adequate CKD management, clinician and patient perceptions, and expectations of transitional strategies and re-transplant potential. These issues remain poorly defined and implemented within the constraints of the existing healthcare model and resources. A greater understanding of these barriers and deficiencies is critical in determining potential modifiable factors that may contribute to the greater risk of mortality post-allograft loss, especially for infection and dialysis withdrawal mortality [27–30]. In addition, qualitative studies exploring patients’ and clinicians’ perceptions towards the management of failing allograft are essential to optimizing the transition from transplantation to dialysis.
Our study has several strengths and limitations. The large number of patients, extended follow-up duration and completeness of the dataset suggested that selection biases were minimized. However, there was likely to be selection bias because there may have been systematic differences in the management of diabetes and the consideration of patients with diabetes for repeat transplantation. Survival bias was also likely as patients with diabetes who had survived the transplant period and restarted dialysis may have represented a dissimilar clinical phenotype compared to those who had died with a functioning allograft, and therefore the estimates of mortality may not have been generalizable to all patients with diabetes. Even though multiple confounding factors were adjusted for in the analysis, there is likely to be unmeasured residual confounders such as the severity of comorbidities, duration of diabetes, and adequacy of glycaemic control. The ANZDATA registry does not provide a definition for PTDM and therefore misclassification bias of this exposure group was possible. The data we had on infection-related death and dialysis withdrawal did not include the types of infections or specific reasons for dialysis withdrawal which prevents more in-depth analyses of these mortality outcomes. In addition, the continuation (or discontinuation) of immunosuppression following allograft loss or the cumulative exposure to overall immunosuppression during allograft life (e.g. therapeutic drug levels) were not captured by the ANZDATA registry, the differences of which between groups may have contributed to the excess infection deaths in patients with and without diabetes.
Our binational study demonstrated that patients with any form of diabetes at the time of kidney allograft loss who commenced or had reinitiated on dialysis had poorer survival, predominantly driven by an excess of infection and dialysis withdrawal-related deaths. Future studies focusing on the understanding of the barriers and attitudes of clinicians and patients towards the management of patients with allograft loss, transition process from failed allograft to dialysis treatment and the mechanistic pathway driving the excess mortality risk experienced by patients with pre-transplant DM and PTDM are urgently needed to improve health outcomes in this vulnerable population.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to gratefully acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators and patients) that provide information to, and maintain, the ANZDATA database. The data reported here have been supplied by the ANZDATA registry. The interpretation and reporting of these data are the authors’ responsibility and in no way should be seen as official policy or interpretation of the ANZDATA registry.
Contributor Information
Amali Samarasinghe, Department of Renal Medicine, Sir Charles Gairdner Hospital, Perth, Australia.
Germaine Wong, School of Public Health, Faculty of Medicine and Health, Sydney University, Sydney, Australia; Centre for Kidney Research, The Children's Hospital at Westmead, Sydney, Australia; Department of Renal Medicine and National Pancreas Transplant Unit, Westmead Hospital, Sydney, Australia.
Armando Teixeira-Pinto, School of Public Health, Faculty of Medicine and Health, Sydney University, Sydney, Australia; Centre for Kidney Research, The Children's Hospital at Westmead, Sydney, Australia.
David W Johnson, Department of Kidney and Transplant Services, Princess Alexandra Hospital, Queensland, Australia; Australasian Kidney Trials Network, University of Queensland, Queensland, Australia; Translational Research Institute, Queensland, Australia.
Carmel Hawley, Department of Kidney and Transplant Services, Princess Alexandra Hospital, Queensland, Australia; Australasian Kidney Trials Network, University of Queensland, Queensland, Australia; Translational Research Institute, Queensland, Australia.
Helen Pilmore, Department of Renal Medicine, Auckland City Hospital, Auckland, New Zealand; Department of Medicine, Auckland University, Auckland, New Zealand.
William R Mulley, Department of Nephrology, Monash Medical Centre, Melbourne, Australia; Department of Medicine, Monash University, Melbourne, Australia.
Matthew A Roberts, Eastern Health Clinical School, Monash University, Victoria, Australia.
Kevan R Polkinghorne, Department of Nephrology and Medicine, Monash Medical Centre, Melbourne, Australia; Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Neil Boudville, Department of Renal Medicine, Sir Charles Gairdner Hospital, Perth, Australia; Internal Medicine, University of Western Australia Medical School, Perth, Australia.
Christopher E Davies, Faculty of Health and Medical Science, Adelaide University Medical School, South Australia, Australia; Australia and New Zealand Dialysis and Transplant Registry, South Australian Health and Medical Research Institute, Adelaide, Australia.
Andrea K Viecelli, Department of Kidney and Transplant Services, Princess Alexandra Hospital, Queensland, Australia; Australasian Kidney Trials Network, University of Queensland, Queensland, Australia.
Esther Ooi, School of Biomedical Sciences, University of Western Australia, Western Australia, Australia.
Nicholas G Larkins, Department of Nephrology, Perth Children's Hospital, Perth, Western Australia, Australia; School of Paediatrics and Child Health, University of Western Australia, Perth, Western Australia, Australia.
Charmaine Lok, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Division of Nephrology, Department of Medicine, University Health Network-Toronto General Hospital, Toronto, Ontario, Canada.
Wai H Lim, Department of Renal Medicine, Sir Charles Gairdner Hospital, Perth, Australia; Internal Medicine, University of Western Australia Medical School, Perth, Australia.
DATA AVAILABILITY STATEMENT
The data used in this study can be requested from the ANZDATA registry (email: requests@anzdata.org.au).
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
W.H.L. and E.O. extracted and analysed data. W.H.L., G.W., and A.S. wrote, reviewed, and edited the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
FUNDING
D.W.J. is supported by a National Health and Medical Research Council (NHMRC) Leadership Investigator Grant. A.K.V. is supported by a Queensland Advancing Clinical Research Fellowship and an NHMRC Emerging Leader Grant (1196033). G.W. is supported by an NHMRC Career Development Fellowship. E.O. is supported by a Heart Foundation Future Leader Fellowship (Award ID: 102538).
REFERENCES
- 1. Australia and New Zealand Dialysis and Transplant Registry . ANZDATA Registry 43rd Report, Chapter 1: Incidence of Renal Replacement Therapy for End Stage Kidney Disease. Adelaide, Australia, 2020. [Google Scholar]
- 2. Grace B, Excell L, Dent H et al. Australia and New Zealand Dialysis and Transplant Registry. ANZDATA Registry 43rd Report, Chapter 2: New Patients. Adelaide, Australia, 2010.
- 3. United States Renal Data System . 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2019. [Google Scholar]
- 4. United States Renal Data System . 2014 USRDS Annual Data Report Volume 2: End-Stage Renal Disease. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2014. [Google Scholar]
- 5. Lim WH, Johnson DW, Hawley C et al. Type 2 diabetes in patients with end-stage kidney disease: influence on cardiovascular disease-related mortality risk. Med J Aust 2018;209:440–6. 10.5694/mja18.00195 [DOI] [PubMed] [Google Scholar]
- 6. Lim WH, Wong G, Pilmore HL et al. Long-term outcomes of kidney transplantation in people with type 2 diabetes: a population cohort study. Lancet Diabetes Endocrinol 2017;5:26–33. 10.1016/S2213-8587(16)30317-5 [DOI] [PubMed] [Google Scholar]
- 7. Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002;2:970–4. 10.1034/j.1600-6143.2002.21015.x [DOI] [PubMed] [Google Scholar]
- 8. Barkoudah E, Skali H, Uno H et al. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc 2012;1:8–15. 10.1161/xJAHA.111.000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li FR, Yang HL, Zhou R et al. Diabetes duration and glycaemic control as predictors of cardiovascular disease and mortality. Diabetes Obes Metab 2021;23:1361–70. 10.1111/dom.14348 [DOI] [PubMed] [Google Scholar]
- 10. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 11. Lim WH, Lok C, Kim SJ et al. Incidence of major adverse cardiovascular events and cardiac mortality in high-risk kidney-only and simultaneous pancreas-kidney transplant recipients. Kidney Int Rep 2021;6:1423–8. 10.1016/j.ekir.2021.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim WH, Lok CE, Kim SJ et al. Impact of pretransplant and new-onset diabetes after transplantation on the risk of major adverse cardiovascular events in kidney transplant recipients: a population-based cohort study. Transplantation 2021;105:2470–81. 10.1097/TP.0000000000003639 [DOI] [PubMed] [Google Scholar]
- 13. Lin H, Yan J, Yuan L et al. Impact of diabetes mellitus developing after kidney transplantation on patient mortality and graft survival: a meta-analysis of adjusted data. Diabetol Metab Syndr 2021;13:126. 10.1186/s13098-021-00742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez-Perez A, Saez M, Vizcaya D et al. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: a population-based cohort study in the UK. BMJ Open Diab Res Care 2021;9:e002146. 10.1136/bmjdrc-2021-002146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cosio FG, Kudva Y, van der Velde M et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 2005;67:2415–21. 10.1111/j.1523-1755.2005.00349.x [DOI] [PubMed] [Google Scholar]
- 16. Hjelmesaeth J, Hartmann A, Leivestad T et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 2006;69:588–95. 10.1038/sj.ki.5000116 [DOI] [PubMed] [Google Scholar]
- 17. Lim WH, Johnson DW, Hawley CM et al. Impact of diabetes mellitus on the association of vascular disease before transplantation with long-term transplant and patient outcomes after kidney transplantation: a population cohort study. Am J Kidney Dis 2018;71:102–11. 10.1053/j.ajkd.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 18. Chan MR, Oza-Gajera B, Chapla K et al. Initial vascular access type in patients with a failed renal transplant. Clin J Am Soc Nephrol 2014;9:1225–31. 10.2215/CJN.12461213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woodside KJ, Schirm ZW, Noon KA et al. Fever, infection, and rejection after kidney transplant failure. Transplantation 2014;97:648–53. 10.1097/01.TP.0000437558.75574.9c [DOI] [PubMed] [Google Scholar]
- 20. Ansell D, Udayaraj UP, Steenkamp R et al. Chronic renal failure in kidney transplant recipients. Do they receive optimum care?: data from the UK renal registry. Am J Transplant 2007;7:1167–76. 10.1111/j.1600-6143.2007.01745.x [DOI] [PubMed] [Google Scholar]
- 21. Davis S, Mohan S. Managing patients with failing kidney allograft: many questions remain. Clin J Am Soc Nephrol 2022;17:444–51. 10.2215/CJN.14620920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molnar MZ, Ichii H, Lineen J et al. Timing of return to dialysis in patients with failing kidney transplants. Semin Dial 2013;26:667–74. 10.1111/sdi.12129 [DOI] [PubMed] [Google Scholar]
- 23. Nanayakkara N, Curtis AJ, Heritier S et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia 2021;64:275–87. 10.1007/s00125-020-05319-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barker MM, Zaccardi F, Brady EM et al. Age at diagnosis of type 2 diabetes and cardiovascular risk factor profile: a pooled analysis. World J Diabetes 2022;13:260–71. 10.4239/wjd.v13.i3.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sattar N, Rawshani A, Franzen S et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 2019;139:2228–37. 10.1161/CIRCULATIONAHA.118.037885 [DOI] [PubMed] [Google Scholar]
- 26. Couceiro C, Rama I, Comas J et al. Effect of kidney replacement therapy modality after first kidney graft failure on second kidney transplantation outcomes. Clin Kidney J 2022;15:2046–55. 10.1093/ckj/sfac155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnston O, Zalunardo N, Rose C et al. Prevention of sepsis during the transition to dialysis may improve the survival of transplant failure patients. J Am Soc Nephrol 2007;18:1331–7. 10.1681/ASN.2006091017 [DOI] [PubMed] [Google Scholar]
- 28. Kassakian CT, Ajmal S, Gohh RY et al. Immunosuppression in the failing and failed transplant kidney: optimizing outcomes. Nephrol Dial Transplant 2016;31:1261–9. 10.1093/ndt/gfv256 [DOI] [PubMed] [Google Scholar]
- 29. Evans RDR, Bekele S, Campbell SM et al. Assessment of a dedicated transplant low clearance clinic and patient outcomes on dialysis after renal allograft loss at 2 UK transplant centers. Transplant Direct 2018;4:e352. 10.1097/TXD.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wentlandt K, Weiss A, O'Connor E et al. Palliative and end of life care in solid organ transplantation. Am J Transplant 2017;17:3008–19. 10.1111/ajt.14522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study can be requested from the ANZDATA registry (email: requests@anzdata.org.au).