Abstract
Introduction There is no methodology to predict aneurysm occlusion using residual volume after flow diverter stent treatment. We retrospectively examined whether residual aneurysm volume at 6 months postoperatively can predict the degree of aneurysm obliteration at 1 year after flow diverter stent treatment. Materials and Methods This single institution study included 101 consecutive patients who underwent flow diverter stent treatment for unruptured cerebral aneurysm. Based on pre-treatment aneurysm volume, the percentage residual volume was calculated 6 months postoperatively. The volume of the aneurysm was determined using the volume calculation function of the cerebral angiography equipment. 1 year postoperatively, patients were classified into two groups: the good obliteration group (GG; O'KellyMarotta [OKM] grading scale: C and D) and the poor obliteration group (PG; OKM: A and B). Statistical analysis was performed to determine if there was a difference in residual aneurysm volume percentage at 6 months postoperatively between the two groups. Results A total of 20 patients were studied: 6 in the GG and 14 in the PG. Mean residual aneurysm volume at 6 months postoperatively in the GG was 33.1% (±34.7), while that in the PG was 80.6% (±24.8) (P=0.018). A residual aneurysm volume of ≥35.2% at 6 months postoperatively was significantly associated with poor aneurysm obliteration at 1 year postoperatively (AUC=0.88, P=0.008). Conclusions Residual aneurysm volume percentage at 6 months after flow diverter stent treatment might be able to predict the likelihood of aneurysm occlusion at 1 year postoperatively
Keywords: Flow diverter, FRED, Pipeline, Unruptured cerebral aneurysm, Cerebral aneurysm, Prediction, Aneurysm volume, O'Kelly-Marotta grading scale, OKM grading scale
Abbreviations
- AUC
Area under the curve
- CI
Confidence Interval
- FRED
The Flow-Redirection Intraluminal Device
- OKM grading scale
The O'Kelly-Marotta grading scale
- ROC curve
Receiver Operatorating Characteristic curve
- SD
Standard Deviation
1. Introduction
The O'Kelly-Marotta (OKM) grading scale1 of residual aneurysm volume is a widely known method for evaluating aneurysm obliteration rate after flow diverter stent treatment. The OKM grading scale classifies a >95% residual aneurysm as Grade A, 5–95% as Grade B, <5% as Grade C, and 0% residual aneurysm as Grade D. However, there is no methodology to predict future aneurysm occlusion using residual volume after flow diverter stent treatment.
A recently published study2 reported limited utility of long-term angiography in patients treated with flow diverter stents who do not have complete aneurysm occlusion and in-stent stenosis on angiography 6 months after treatment. However, there is no rule regarding the appropriate timing for performing cerebral angiography in patients with incomplete aneurysmal occlusion after flow diverter treatment.
Here, we retrospectively examined whether residual aneurysm volume on cerebral angiography at 6 months postoperatively can predict the degree of aneurysm obliteration at 1 year after flow diverter stent treatment, and whether performing cerebral angiography at 1 year postoperatively is worthwhile.
2. Materials and methods
This single-institution study was conducted in accordance with the Declaration of Helsinki and with the approval of our institutional review board. Informed consent was obtained from all patients for participation in the study. A total of 101 consecutive patients who underwent flow diverter stent treatment for unruptured cerebral aneurysm with a maximum diameter of ≥5 mm between June 2020 and February 2022 were included. Dual anti-platelet therapy was performed preoperatively in all patients. Following flow diverter stent treatment, all patients (n = 101, 22.7% male, 59.6 ± 13 years old) underwent cerebral angiography at 6 months and 1 year postoperatively using flat-panel detectors (Artis Q BA Twin; Siemens AG, Forchheim, Germany). Aneurysms that were completely occluded at 6 months postoperatively (OKM: D) were excluded from the study. Aneurysm volume was calculated on angiography images in the cases of incomplete aneurysm occlusion at 6 months postoperatively. The volume of the aneurysm was calculated using the volume calculation function of the cerebral angiography system using three-dimensional rotational angiography (3D-RA) at the time of cerebral angiography (Fig. 1). Using preoperative aneurysm volume as the baseline, aneurysm volume at 6 months postoperatively was calculated as a percentage of the baseline volume (Fig. 2, Fig. 3). Next, patients whose aneurysm obliteration was judged to be ≥ 95% at 1 year postoperatively, i.e. those with Grade C or Grade D obliteration on the OKM grading scale, were classified as the good obliteration group (Fig. 2, Fig. 3), and those whose aneurysm obliteration was judged as ≤5% at 1 year postoperatively, i.e. Grade A or Grade B on the OKM grading scale, were classified as the poor obliteration group (Fig. 2, Fig. 3).
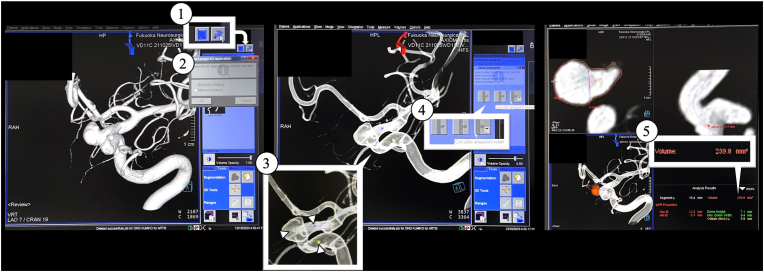
Fig. 1.
Method of using the simplified volume calculation function in the cerebral angiography system and flat-panel detectors (Artis Q BA Twin; Siemens AG, Forchheim, Germany).
(1) After obtaining a three-dimensional rotational angiography (3D-RA) image, the ‘Analyze’ button in the upper right corner of the Analyze screen is pressed.
(2) The ‘Aneurysm Analysis’ button is pressed.
(3) Three points are placed on the aneurysm body and the distal and proximal normal vessels. At this stage, even if the aneurysm is clearly visible, if the device does not recognize the aneurysm, it cannot place a point on the aneurysm body, and therefore, such cases were excluded from this study.
(4) The ‘Analyze’ button is pressed.
(5) The automatic volume analysis system calculates the aneurysm volume.
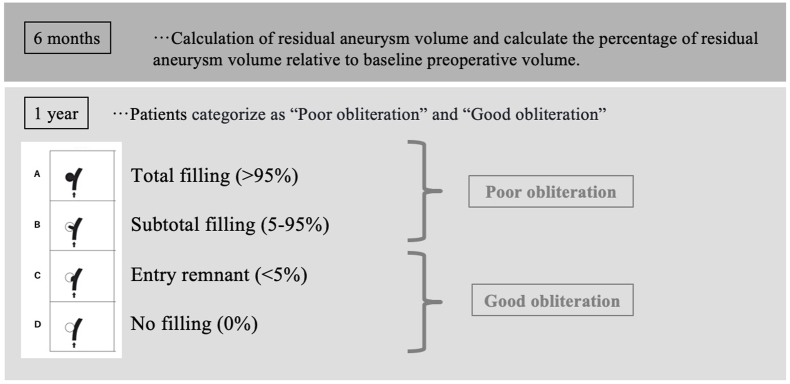
Fig. 2.
Aneurysm volume was calculated using a flat-panel detector (Artis Q BA Twin; Siemens AG, Forchheim, Germany). First, using the preoperative aneurysm volume as the baseline, aneurysm volume 6 months after surgery was calculated as a percentage of that volume. Next, patients whose aneurysm obliteration was judged to be 95% or higher at 1 year after surgery, i.e. Grade C or Grade D obliteration on the OKM grading scale, were classified as the good obliteration group, and those whose aneurysm occlusion was judged to be less than 95% at 1 year after surgery, i.e. Grade A or Grade B obliteration on the OKM grading scale, were classified as the poor obliteration group.
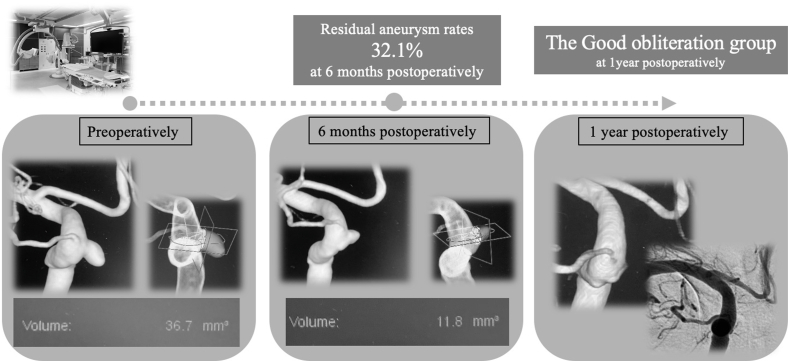
Fig. 3.
Representative case showing details of calculation of aneurysm volume at 6 months and 1 year after surgery.
The preoperative aneurysm volume was 36.7 mm3 and the aneurysm volume 6 months postoperatively was 11.8 mm3, indicating a residual aneurysm volume rate at 6 months postoperatively of 32.1%. This case was classified into the good obliteration group because complete occlusion was achieved 1 year postoperatively.
Residual aneurysm volume rate (%) at 6 months postoperatively and the degree of aneurysm occlusion (good and poor obliteration groups) at 1 year postoperatively were used as parameters in the statistical analysis to investigate whether residual aneurysm volume at 6 months postoperatively can predict the degree of aneurysm obliteration at 1 year postoperatively. Specifically, Statistical analysis was performed to determine whether the good and poor obliteration groups at 1 year postoperatively were associated with various patient parameters, including residual volume fraction at 6 months postoperatively (The following parameters were statistically analyzed: sex, age, maximum diameter of aneurysm, smokers, hypertensives, diabetics, dyslipidemia, aneurysm location, presence of branching vessels from the aneurysm dome, morphology of the aneurysm, and residual aneurysm volume percentage at 6 months postoperatively).
Statistical analysis was performed using SPSS software (IBM SPSS Statistics, Armonk, NY, USA). Variables are expressed as the mean ± standard deviation, the median (interquartile range 25th to 75th percentile), or the number of cases (%), as appropriate. Fisher's exact test was performed for nominal variables. For continuous variables, the normality of the data was evaluated using the Shapiro–Wilk test. Normally and non-normally distributed continuous variables were compared using the Student's t-test and Mann–Whitney U-test, respectively. Differences were considered significant at p values < 0.05. The optimal cutoff value of residual aneurysm volume on the receiver operating characteristic (ROC) curve was determined using the Youden Index.
3. Results
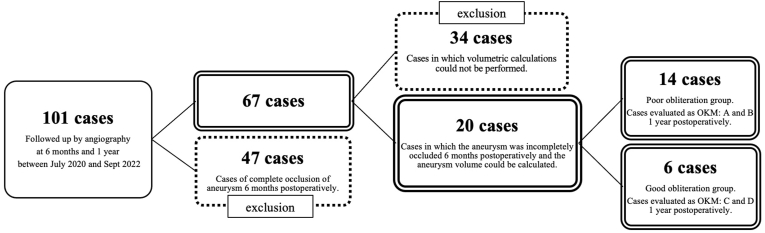
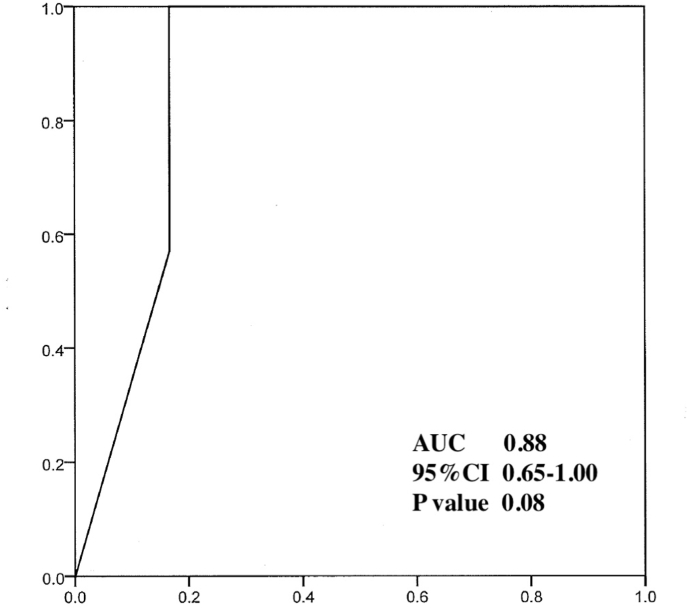
In all cases, the flow diverter stents used in this study were the Flow-Redirection Intraluminal Device (FRED). Cases in which the aneurysm had reached complete occlusion (OKM: D) on cerebral angiography 6 months postoperatively (n = 34) and cases in which the volume could not be calculated by the volume calculation device (n = 47) were excluded from the study. The remaining 20 cases were included in the aneurysm obliteration prediction analysis (Figure 4). At 1 year postoperatively, six patients had achieved good obliteration (Good obliteration group (GG)) and the remaining 14 patients had poor obliteration (Poor obliteration group (PG)). There were no differences in age, aneurysm diameter before treatment, number of smokers, hypertensives, diabetics, dyslipidemics, location of aneurysm, presence of branch vessels from aneurysmal dome, or morphology of aneurysms between the two groups (Table 1). The mean residual aneurysm volume at 6 months postoperatively in the GG was 33.1 (±34.7)%, while that in the PG was 80.6 (±24.8)%, with a significant difference between the two groups (p = 0.018). ROC curve analysis showed that a residual aneurysm volume of ≥35.2% at 6 months postoperatively was significantly associated with poor aneurysm obliteration at 1 year postoperatively (AUC = 0.88, p = 0.008) (Fig. 4) (see Fig. 5).
Table 1.
Background of patients in the good obliteration and poor obliteration groups. There were no differences in age, maximum diameter of aneurysm before treatment, the number of smokers, hypertensives, diabetics, dyslipidemics, or the location of the aneurysm, the presence of branch vessels from the aneurysmal dome, the morphology of aneurysms, or residual aneurysm volume 6 months postoperatively between the two groups.
| Good obliteration group (n = 6) | Poor obliteration group (n = 14) | P value | |
|---|---|---|---|
| Female sex, n (%) | 5 (83.3) | 11 (78.5) | 0.6 |
| Age, yrs (SD) | 55 (±13.9) | 66 (±11.3) | 0.1 |
| Maximum diameter of aneurysm, mm (SD) | 6.4 (±2.3) | 5.8 (±0.8) | 0.4 |
| Smokers, n (%) | 2 (33.3) | 1 (7.1) | 0.2 |
| Hypertensives, n (%) | 2 (33.3) | 8 (57.1) | 0.3 |
| Diabetics, n (%) | 0 (0) | 2 (14.2) | 0.4 |
| Dyslipidemics, n (%) | 0 (0) | 6 (42.8) | 0.07 |
| Location of aneurysm, n (%) | 0.2 | ||
| Cavenous | 1 (16.6) | 2 (14.2) | |
| Cave | 2 (33.3) | 1 (7.1) | |
| Ophthalmic | 1 (16.6) | 2 (14.2) | |
| Dorsal | 1 (16.6) | 0 (0) | |
| Superior Hypophyseal Artery | 0 (0) | 1 (7.1) | |
| Pcom | 1 (16.6) | 5 (35.7) | |
| Acho | 0 (0) | 3 (21.4) | |
| Presence of branch vessels from aneurysmal dome, n (%) | 2 (33.3) | 8 (57.1) | 0.3 |
| Morphology of aneurysms, n (%) | 0.6 | ||
| Sacclar | 4 (66.6) | 10 (71.4) | |
| Fuziform | 2 (33.3) | 4 (28.5) | |
| Residual aneurysm volume 6 months postoperatively, % (SD) |
33.1 (±34.7) | 80.6 (±24.8) | 0.018 |
Fig. 4.
Of the total of 101 cases, 47 cases with complete occlusion 6 months postoperatively and 34 cases in which volume calculations could not be performed by the instrument 6 months postoperatively were excluded. The remaining 20 cases were included in this study.
Fig. 5.
ROC curve analysis showed that a residual aneurysm volume of 35.2% or greater at 6 months after surgery was significantly associated with the likelihood of being included in the poor obliteration group at 1 year after surgery (AUC = 0.88, p = 0.008). The optimal cutoff value of the ROC curve was determined using the Youden Index.
4. Discussion
There was a significant difference in residual aneurysm volume percentage at 6 months postoperatively between GG and PG at 1 year postoperatively, a residual aneurysm volume of ≥35.2% at 6 months postoperatively was significantly associated with poor aneurysm obliteration at 1 year postoperatively.
As predictors of incomplete aneurysm occlusion after flow diverter stent treatment, univariate analysis has shown that the following factors are predictive of incomplete occlusion: elderly age,3,4 sex,5,6 cases involving branching vessels,7, 8, 9 aneurysm size,9, 10, 11 morphology,10,11 and biomarkers extracted from immediate post-procedural digital subtraction angiography by angiographic parametric imaging.12 In a previous multivariate analysis, Ishii et al found that elderly patients over 70 years of age are candidates for incomplete occlusion.13 We reported in a previous multivariate analysis that age under 67.5 years and sidewall-type aneurysms are predictors of complete occlusion.14
However, in all of the previous reports, residual aneurysm volume was only used to describe the course of aneurysm occlusion using the OKM grading scale, and not as a predictor of aneurysm occlusion.
The OKM grading scale is a widely used method for classifying the degree of aneurysm occlusion in response to flow diverter treatment, although the residual volume in Grade B aneurysms varies widely from 5 to 95%. In this study, we found that a residual aneurysm volume of ≥35.2% at 6 months postoperatively was significantly associated with the likelihood of poor obliteration at 1 year postoperatively. Future prospective studies with further stratification of the residual volume in the Grade B group are needed for further confirmation of our results.
There is no rule regarding the appropriate timing for cerebral angiography after flow diverter treatment. Lauzier et al have shown that among patients who undergo treatment of intracranial aneurysms with a Pipeline Embolization Device, the value of long-term angiography in patients demonstrating complete aneurysm occlusion and no in-stent stenosis on angiography at 6 months post-treatment is low.2 However, there is no rule regarding the appropriate timing for performing cerebral angiography in patients with incomplete aneurysmal occlusion after flow diverter treatment. Our study showed that the residual aneurysm volume fraction at 6 months postoperatively might be related to the degree of aneurysm occlusion at 1 year postoperatively. We consider that this result shows the significance of cerebral angiography at 1 year postoperatively in patients with incomplete aneurysmal occlusion at 6 months postoperatively.
All the flow diverter stents used at our hospital for the period of this study were FREDs. There are multiple types of flow diverter stents, of which the FRED is the only stent with a double layer structure. In terms of long-term outcomes, the FRED has been reported to show safety and good occlusion.15 The FRED X, which has a surface coating for thrombus control, has also been reported to have a low complication rate and a good short-term occlusion rate in a multicenter trial.16 Matsukawa et al reported a higher rate of aneurysm occlusion and lesser need for additional treatment of unruptured internal carotid artery aneurysms with FREDs compared to conventional FD stents in a study using propensity score matched analysis.17
A limitation of this study is that the only flow diverter stent used was the FRED. Evaluation of other flow diverter stents would be necessary to make this study more generalizable. Another limitation of this study is the small number of cases included. The volume analysis system provided with the cerebral angiography equipment was used to avoid bias of the person who performed the measurements when calculating the volume. On the other hand, 47 cases that were clearly identifiable as aneurysms, but for which the volume calculation system of the cerebral angiography system did not work, were excluded, resulting in the small number of patients. In addition, this study was conducted at a single institution; a multi-institutional study is needed to produce more generalizable results. Prospective studies are also needed for further generalization.
5. Conclusions
A cut-off value of residual aneurysm volume on cerebral angiography of ≥35.2% at 6 months after flow diverter stent treatment might be able to predict the likelihood of aneurysm occlusion at 1 year postoperatively.
CRediT authorship contribution statement
Shinichiro Yoshida: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Kiyoshi Kazekawa: Conceptualization. Kaisei Kamatani: Conceptualization. Kousei Maruyama: Conceptualization. Kousuke Takigawa: Conceptualization. Noriaki Tashiro: Conceptualization. Yoshiya Hashiguchi: Conceptualization. Masahiro Yasaka: Conceptualization. Hiroshi Aikawa: Conceptualization. Yoshinori Go: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing.
Contributor Information
Shinichiro Yoshida, Email: fkewk902@gmail.com.
Kiyoshi Kazekawa, Email: kazekawa3426@gmail.com.
Kaisei Kamatani, Email: kaisei.kamatani@gmail.com.
Kousei Maruyama, Email: k.maruyama0209@gmail.com.
Kousuke Takigawa, Email: takigawa19841221@gmail.com.
Noriaki Tashiro, Email: noriaki1983@hotmail.co.jp.
Yoshiya Hashiguchi, Email: yoshiyamihoko@yahoo.co.jp.
Masahiro Yasaka, Email: yasakamasahiro@gmail.com.
Hiroshi Aikawa, Email: haikawa35@gmail.com.
Yoshinori Go, Email: go@kouchikukai.or.jp.
References
- 1.O'Kelly C.J., Krings T., Fiorella D., et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16:133–137. doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauzier D.C., Cler S.J., Chatterjee A.R., et al. The value of long-term angiographic follow-up following Pipeline embolization of intracranial aneurysms. J Neurointerventional Surg. 2022;14(6):585–588. doi: 10.1136/neurintsurg-2021-017745. [DOI] [PubMed] [Google Scholar]
- 3.Adeeb N., Moore J.M., Wirtz M., et al. Predictors of incomplete occlusion following pipeline embolization of intracranial aneurysms: is it less effective in older patients? AJNR Am J Neuroradiol. 2017;38:2295–2300. doi: 10.3174/ajnr.A5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maragkos G.A., Ascanio L.C., Salem M.M., et al. Predictive factors of incomplete aneurysm occlusion after endovascular treatment with the Pipeline embolization device. J Neurosurg. 2019;132:1598–1605. doi: 10.3171/2019.1.JNS183226. [DOI] [PubMed] [Google Scholar]
- 5.Bender M.T., Colby G.P., Lin L.M., et al. Predictors of cerebral aneurysm persistence and occlusion after flow diversion: a single-institution series of 445 cases with angiographic follow-up. J Neurosurg. 2018;130:259–267. doi: 10.3171/2017.11.JNS171738. [DOI] [PubMed] [Google Scholar]
- 6.O'Kelly C.J., Spears J., Chow M., et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2013;34:381–387. doi: 10.3174/ajnr.A3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae H.J., Park Y.K., Cho D.Y., et al. Predictors of the Effects of flow diversion in Very Large and Giant aneurysms. AJNR Am J Neuroradiol. 2021;42:1099–1103. doi: 10.3174/ajnr.A7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moshayedi H., Omofoye O.A., Yap E., et al. Factors Affecting the obliteration rate of intracranial aneurysms treated with a single pipeline embolization device. World Neurosurg. 2017;104:205–212. doi: 10.1016/j.wneu.2017.04.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunohara T., Imamura H., Goto M., et al. Neck location on the outer Convexity is a predictor of incomplete occlusion in treatment with the pipeline embolization device: Clinical and angiographic outcomes. AJNR Am J Neuroradiol. 2021;42:119–125. doi: 10.3174/ajnr.A6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro M., Becske T., Nelson P.K. Learning from failure: persistence of aneurysms following pipeline embolization. J Neurosurg. 2017;126:578–585. doi: 10.3171/2015.12.JNS152065. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Kim J., Ahmed A. Effect of aneurysm morphologic parameters on occlusion rates following pipeline embolization. Clin Neurol Neurosurg. 2019;183 doi: 10.1016/j.clineuro.2019.105395. [DOI] [PubMed] [Google Scholar]
- 12.Liang F., Ma C., Zhu H., et al. Using angiographic parametric imaging-derived radiomics features to predict complications and embolization outcomes of intracranial aneurysms treated by pipeline embolization devices. J Neurointerventional Surg. 2022;14(8):826–831. doi: 10.1136/neurintsurg-2021-017832. [DOI] [PubMed] [Google Scholar]
- 13.Ishii A. Advantages and Disadvantages of flow diverter treatment for cerebral aneurysms. Jpn J Neurosurg. 2022;31:98–106. [Google Scholar]
- 14.Yoshida S., Matsukawa H., Maruyama K., et al. Factors predicting effective aneurysm early obliteration after flow re-direction endoluminal device placement for unruptured intracranial cerebral aneurysms. Neuroscience Informatics. 2022;2 [Google Scholar]
- 15.Hohenstatt S., Ulfert C., Herweh C., et al. Long-term follow-up after aneurysm treatment with the flow Redirection endoluminal device (FRED) flow diverter. Clin Neuroradiol. 2023 Oct 13 doi: 10.1007/s00062-023-01346-3. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vollherbst D.F., Lücking H., DuPlessis J., et al. The FRESH study: treatment of intracranial aneurysms with the New FRED X flow diverter with Antithrombotic surface treatment Technology-First multicenter experience in 161 patients. AJNR Am J Neuroradiol. 2023;44(4):474–480. doi: 10.3174/ajnr.A7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsukawa H., Uchida K., Rajbhandari S., et al. Difference in the cumulative incidence of aneurysmal occlusion by Flow Re-direction Endoluminal Device and Pipeline Embolization Device in the treatment of unruptured internal carotid artery aneurysms: a propensity score-matched cohort study. Neurosurg Rev. 2023;46(1):125. doi: 10.1007/s10143-023-02026-z. [DOI] [PubMed] [Google Scholar]