Abstract
Originally designed as anti-hyperglycemic drugs, Glucagon-Like Peptide-1 receptor agonists (GLP-1Ra) and Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have demonstrated protective cardiovascular effects, with significant impact on cardiovascular morbidity and mortality. Despite several mechanisms have been proposed, the exact pathophysiology behind these effects is not yet fully understood. Cardiovascular imaging is key for the evaluation of diabetic patients, with an established role from the identification of early subclinical changes to long-term follow up and prognostic assessment. Among the different imaging modalities, CMR may have a key-role being the gold standard for volumes and function assessment and having the unique ability to provide tissue characterization. Novel techniques are also implementing the possibility to evaluate cardiac metabolism through CMR and thereby further increasing the potential role of the modality in this context. Aim of this paper is to provide a comprehensive review of changes in CMR parameters and novel CMR techniques applied in both pre-clinical and clinical studies evaluating the effects of SGLT2i and GLP-1Ra, and their potential role in better understanding the underlying CV mechanisms of these drugs.
Keywords: Cardiovascular magnetic resonance, SGLT2i, GLP-1Ra, Diabetic cardiomyopathy
Introduction
Currently, type 2 of diabetes mellitus (DM2) affects more than 500 million people [1–3].
Among the well-known systemic manifestations of type 2 diabetes mellitus (DM2), cardiovascular (CV) diseases represent the most relevant complications, accounting for the prevalent cause of morbidity and mortality [4–9]. Two classes of medications designed as novel therapeutic strategies for DM2, namely Glucagon-Like Peptide-1 receptor agonists (GLP-1Ra) and Sodium-glucose cotransporter-2 inhibitors (SGLT2i), have demonstrated to reduce CV mortality and the occurrence of heart failure (HF) in patients with DM2 [8–10]. Notably, this effect was observed with SGLT2i, regardless of the presence of DM2 [10–15]. Although the precise mechanisms underlying these cardioprotective effects remains not completely understood, several studies have proposed that they may act independently of glycemic control attributing their beneficial effects to direct as well as indirect actions on the CV system [11, 12].
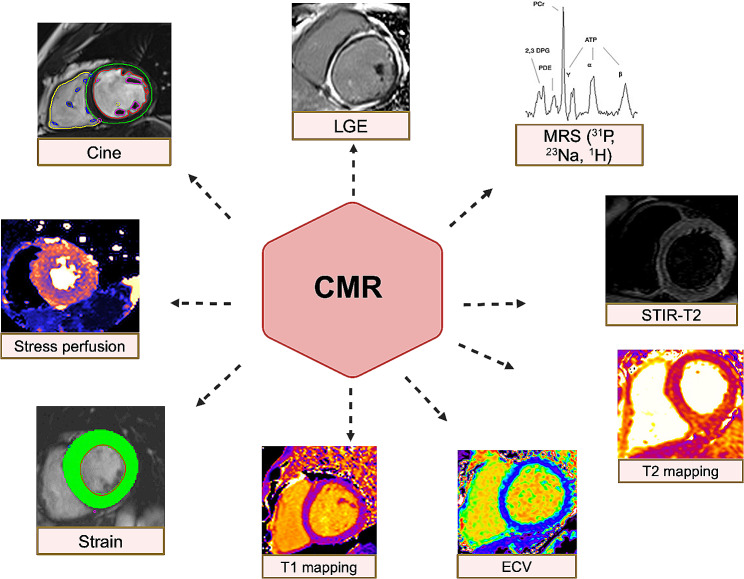
Cardiac remodeling, defined as changes in the cardiac geometry and/or function, often precedes the development and progression of HF and is associated with poor clinical outcomes [16–18]. The evaluation of early, subclinical, changes at CV level induced by GLP-1Ra and SGLT2i will be key to unravel their cardioprotective effects [19, 20]. Among the different imaging modalities, Cardiovascular Magnetic Resonance (CMR) may play a pivotal role in this regard, being not only the gold standard for volumetric and function assessment [21], but also providing tissue characterization with the possibility to image myocardial fibrosis/necrosis, oedema and, when applying a stress protocol, the presence of inducible myocardial ischaemia [22]. Novel CMR sequences have also been recently developed to allow a non-invasive assessment and quantification of microvascular ischaemia [23] and to image cardiac inflammation and energetics [24]. Evaluating changes in CMR parameters can therefore add meaningful piece to the puzzle describing the mechanisms of action underlying the beneficial CV effects of GLP-1Ra and SGLT2i (Fig. 1).
Fig. 1.
Examples of CMR sequences used to evaluate cardiovascular effects of SGLT2i or GLP-1Ra. ECV: extracellular volume; MRS: magnetic resonance spectroscopy; LGE: late gadolinium enhancement; STIR-T2: Short-TI Inversion Recovery
The aim of this article is to provide a narrative review of the existing evidence in the literature regarding the established and potential role of CMR in assessing the cardiovascular effects of GLP-1Ra and SGLT2i.
SGLT2i effects on cardiovascular system
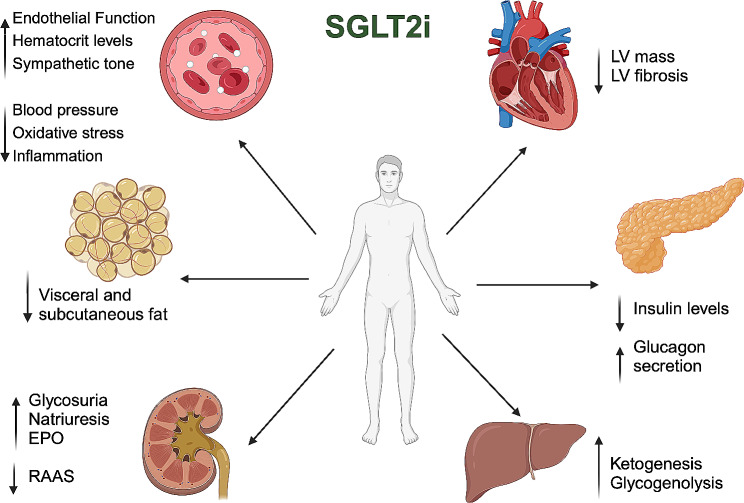
Originally considered solely as hypoglycemic drugs, SGLT2i operate by reducing glucose reabsorption through the blocking of the SGLT2 receptor in the proximal renal tubule, consequently inducing glycosuria [25–27] (Fig. 2). This, in turn, reduces plasma insulin levels and promote glucagon secretion, responsible for lipolysis and lipid oxidation, with the effect of an overall reduction in visceral and subcutaneous fat and a weight loss of ~ 2-3 kg [28–34]. Additionally, the natriuretic effect of SGLT2i inhibits the renin-angiotensin-aldosterone system (RAAS), resulting in a modest reduction in both systolic and diastolic blood pressure [28, 35]. The increased diuresis, coupled with a direct promotion of erythropoiesis, contributes to the observed rise in hematocrit levels in patients receiving these medications [36–39]. However, a similar effect has been observed with other drugs that do not impact mortality [40].
Fig. 2.
Summary of the effects of sodium-glucose cotransporter-2 inhibitors
EPO: Erythropoietin. LV: left ventricular; RAAS: renin-angiotensin-aldosterone system;
The “Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes” (EMPAREG-OUTCOME) [41], “Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes” (CANVAS) [42], “Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes” (DECLARE-TIMI 58) [43] studies have provided evidence that SGLT2i reduces major renal and CV endpoints as hospitalizations and mortality due to HF in patients with DM2.
Subsequently, trials like “Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction” (DAPA-HF) [10] and “Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction” (DELIVER) [13] for dapaglifozin, as well as the “Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure” (EMPEROR-Reduced) [14] the “Empagliflozin in Heart Failure with a Preserved Ejection Fraction” (EMPEROR-Preserved) [15] trials for empaglifozin, have demonstrated a reduction in CV events regardless of LV ejection fraction (EF) and the presence of DM2.
Beneficial effects on reduced HF hospitalization and mortality have been described also in patients with history of prior myocardial infarction (MI), although safety and efficacy of these therapies early after acute MI remain uncertain. The Emmy trial [44] in fact demonstrated significant reduction of NT-proBNP with early initiation of empagliflozin after MI, while treatment with dapaglifozin in the DAPA-MI had only limited impact on CV outcomes including HF hospitalization and CV death, with a benefit observed only in terms of cardiometabolic outcomes [45]. Ongoing trials will provide more insights about the role of SGLT2i in this context [46]. Promising results in terms of MI and stroke risk reduction have been instead demonstrated with the SGLT1/2 inhibitor sotaglifozin [47, 48], with a benefit similar to what observed with GLP1Ras but with the advantage of an additional proved reduction in HF-related hospitalization. Reduction in visceral obesity, increased atherosclerotic plaque stability, and gut microbiome modulation are all potential mechanisms that may contribute to this protective effect [49–51]. Consequently, these drugs are now recommended as a cornerstone of HF treatment by the European Society of Cardiology (ESC) guidelines [10–15]. While the improved glycemic control, lowered blood pressure levels and observed weight reduction after SGLT2i treatment all contribute to improved clinical outcomes, none of these factors can fully explain the overall beneficial effect on the CV system. The improvement of endothelial function and arterial wall stiffness, attributed to increased vasodilation and nitric oxide production, as well as the inhibition of oxidative stress and inflammation [52–55] have been proposed as additional potential mechanisms and described in both animal and clinical models after SGLT2i treatment [56–58]. Modulation of endothelial dysfunction may be also implicated in the amelioration of renal function observed even in the absence of diabetes [59]. Furthermore, SGLT2i reduce circulating catecholamine levels [60] and impact myocardial remodeling and fibrosis, through modulation of several chemokine pathways (IL-6, TNF-α, monocyte chemoattractant protein-1), calcium homeostasis [61, 62], authophagy [60, 63–68] and RAAS inhibition [69] in pre-clinical models. All these proposed mechanisms will be discussed in detail in the following paragraphs.
Cardioprotective effects of SGLT2i in preclinical models
The cardioprotective SGLT2i effects have been investigated in animal models with and without DM2 [69–71]. SGLT2i proved to reduce myocardial hypertrophy, fibrosis and cardiomyocyte apoptosis and, in HF models, to improve systolic function, cardiac dilatation and reduce both atrial and ventricular fibrosis [72, 73].
These results were confirmed in non-diabetic, doxorubicin-treated mice where the treatment with doxorubicin prevented the deterioration of early LV function parameters, such as geometrical deformation indices [74]. The study also showed for the first time expression of SGLT-1 receptors in the heart, opening the way for clinical testing of SGLT-1/2 antagonists, such as sotagliflozin with favorable results both in diabetic [48] and non-diabetic HF patients [47].
To further explore the mechanisms behind the effects of SGLT2i on cardiac remodeling, several studies have utilized the information arising from CMR imaging.
For instance, the effects of a two-month course of empaglifozin on diastolic function were evaluated in a porcine model of nondiabetic HF induced by occlusion of proximal left anterior descending artery [75]. Semiautomatically generated LV filling profiles were used to derive values of peak filling rate and first filling volume to estimate the amount of ventricle filled during either LV active relaxation or suction [76]. Both parameters were found to be higher in SGLT2i-treated animals, reflecting a beneficial effect on diastolic function in this group. Additionally, the reduction in left atrial volume compared to controls suggested a decrease in left atrial pressure after SGLT2i treatment. We know from previous work that two main mechanisms have been recognized in the development of diastolic disfunction: increased interstitial fibrosis and augmented cardiomyocyte stiffness [77]. Interestingly, empaglifozin-treated pigs had reduced intramyocardial fibrosis demonstrated by lower collagen deposition and decreased extracellular volume measured at T1 mapping and ECV analysis [75]. Empaglifozin was also able to improve nitric oxide signaling and impact titin phosphorylation with beneficial effects on cardiomyocyte stiffness [75].
Other potential mechanisms with a proved role in cardiac remodeling are disturbances in ionic homeostasis [78]; elevated myocardial intracellular sodium ([Na+]i) has been found in models of HF and diabetic cardiomyopathy (DC), and linked to detrimental effects on mitochondrial function and myocardial energetics [79, 80]. The ([Na+]i) overload activates in fact the Na+/Ca2+ exchanger, with increased efflux of calcium from the mitochondria to the cytosol and increased calcium influx from the extracellular environment. The result is an overall rise in intracellular calcium, disrupted calcium gradients, and subsequent disturbances in oxidative phosphorylation and ATP levels [81]. Moreover, as most cardiac contractile proteins are calcium-sensitive, calcium plays a pivotal role in maintaining efficient excitation-contraction processes [82]. Disturbances of calcium homeostasis may therefore explain, at least in part, the impairment of contractile function observed in Diabetic Cardiomyopathy (DC) [24, 77–80, 83].
Accordingly, magnetic resonance spectroscopy (MRS) is a new imaging technique providing in vivo metabolic information of the examined tissue [24, 69, 84]. By exploiting the unique signal generated by different nuclei, MRS enables the detection of several metabolites and offers non-invasive assessment of myocardial energetics. For instance, phosphorus-31 Nuclear MRS (31P-MRS) can track myocardial PCr/ATP ratio (a marker of the myocardial energetic state), often compromised in DM2 patients [24, 85, 86]. Using both 31P and 23Na MRS, Croteau et al. [79] demonstrated decreased PCr/ATP ratio and elevated ([Na+]i) in a mice model of DC. A one-month treatment with ertugliflozin corrected the ([Na+]i) increase, improved the PCr/ATP ratio, and reversed myocardial hypertrophy, diastolic and systolic dysfunction [79, 80].
Ongoing research employing a novel imaging technique, manganese-enhanced magnetic resonance imaging, may soon provide insights into the effects of SGLT2i on the homeostasis of another ion, calcium (NCT04591639). The technique exploits the ability of manganese, a calcium analogue, to significantly impact the T1 relaxation time, allowing for the identification of myocardial areas with normal calcium handling.
Chronic glucose overload and ectopic lipid accumulation have both been observed in DM2 and linked to HF development [82]. However, their exact contribution to myocardial dysfunction remains unclear. Joubert et al [87] sought to address this question by using a lipodystrophic mouse model, devoid of lipotoxic features, to demonstrate that glucotoxicity itself can trigger cardiomyopathic changes including LV hypertrophy and diastolic dysfunction. CMR images showed increased wall thickness, mildly reduced EF and impaired longitudinal strain in these mice, alterations that were corrected by subsequent administration of glucose-lowering drugs. Interestingly, in this model, the effects of dapagliflozin on cardiac remodeling were superior to those induced by pioglitazone. Despite both drugs counteract glucotoxicity and reduce the amount of advanced glycation end-products, these results suggest that other metabolic pathways may be implied in the benefits observed with SGLT2i. One postulated hypothesis revolves around a shift in cardiac metabolism from fatty acid and glucose oxidation (the primary sources of fuel under physiological conditions but impaired in DM2 and HF) towards the more efficient utilization of ketone bodies [84]. At this regard, Hyperpolarized [3–13 C]acetoacetate, a novel ketone probe applied to MRS to track the conversion of [3-13C]acetoacetate into its metabolic products, was used to test the effects of empaglifozin in diabetic rats with HF. Despite an increase in the overall amount of circulating ketone bodies, their use at the cardiac level after empagliflozin administration remained surprisingly stable. Nevertheless, the drug once again confirmed a significant impact on afterload (reduced EDV and stroke volume at CMR analysis) [88]. However, another study using 31P-MRS to measure cardiac PCr/ATP levels as a marker of myocardial energetics [89], demonstrated a 45% increase in cardiac PCr/ATP in diabetic mice treated with a single dose of empaglifozin, correlating with the increase of circulating ketones but not with plasma glucose levels [85]. The results underscore the role that changes in myocardial energetics towards more efficient pathways may have in the cardioprotective effects of SGLT2i.
As previously mentioned, CMR is an invaluable imaging modality due to its ability to characterize tissue. In the ischemic setting this unique property allows for the identification of myocardial oedema in T2-weighted (T2w) sequences (area at risk) and myocardial scar (infarcted area) in late gadolinium enhancement (LGE) sequences. One of the potential protective mechanisms implicated in the reduction of cardiovascular events observed after SGLT2i treatment may involve the impact of these drugs on reducing post-ischemic damage. Pre-treatment with empaglifozin for one week in mice with acute myocardial infarction (MI) resulted in a significantly larger myocardial salvage area (identified by the difference between the area at risk -hyperintense in T2w- and the infarcted area measured at LGE), smaller infarct size, and overall improved cardiac function [90].
Cardioprotective effects of SGLT2i in clinical models
One of the key findings in CMR studies involving patients treated with SGLT2i is the beneficial effect on cardiac remodeling [32, 91]. A recently published metanalysis [92] of 9 randomized clinical trials and 1385 patients reported that SGLT2i treatment significantly reduced both LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) as well as LVM and LVM index. Patients treated with SGLT2i also had a significant improvement on LVEF, irrespective of the time to follow-up used or of the HF phenotype. In DM2 patients, the effect on LVMi is also independent from the diabetes duration [93]. SGLT2i had instead no effect on LVM and LVMi in a cohort of non-diabetic patients, with LVH but no HF [94]. If these changes result from primarily alterations in cardiomyocyte size, extracellular volume, or a combination of both is still matter of debate [95–98]. The use of CMR imaging that with T1 mapping analysis has the potential to estimate ECV, may be the appropriate tool to non-invasively provide answers to this question. In a pre-specified analysis of the EMPA-HEART [99] both ECV and indexed ECV were significantly reduced in diabetic patients treated with empaglifozin compared to placebo. Intracellular volume (ICV), calculated as (1-ECV) x (LVMi/1.05), did not differ significantly between the two groups [99]. This 1.4%, reduction in ECV in a relatively short time frame (6 months) is particularly relevant when read in the light of the data published by Wong et al [100], where a 3% increase in ECV in diabetic patients was associated with a 52% increase in the risk of death or HF hospitalization. The reduction of ECV was confirmed by another study in non-diabetic patients, where the authors also demonstrated a reduction in cardiomyocyte volume after empaglifozin treatment [32, 101]. Ongoing trials (NCT03782259, NCT04490681) will provide further evidence about the impact of SGLT2i on ECV.
Preclinical models have highlighted the role that a shift towards more efficient energetic pathway can have in the benefit observed with SGLT2i therapy. However, similar studies using 31P-MRS to measure cardiac PCr/ATP levels at rest and during dobutamine stress failed to prove significant changes in cardiac energetics in both HFrEF and HFpEF [101]. Interestingly, what is significantly reduced after SGLT2i treatment is the amount of epicardial and subcutaneous adipose tissue, associated with a concomitant reduction in circulating inflammatory biomarkers [32]. Epicardial adipose tissue (EAT) serves as a lipid storage and its reduction may represent an indirect proof of the switch of myocardial fuel triggered by SGLT2i [102]. Notably, excess or abnormalities of EAT are linked to increased CV risk [103]. The EMPACEF study [104] however did not confirm the impact of empaglifozin on myocardial or epicardial fat. These conflicting results may be explained by the shorter treatment received in the EMPACEF study (12 weeks) [104], compared to the 6 months used in the EMPA-TROPISM study [32]. The reduction in aortic stiffness demonstrated after SGLT2i, with consequent reduced afterload and improved cardiac efficiency, may represent an additional mechanism involved in the overall beneficial effects in terms of CV risk [32]. Despite the undeniable benefits demonstrated in HF patients, there are mixed data regarding the effect of SGLT2i on LVEF [32, 65, 105–107]. The reasons behind these conflicting results may be the heterogeneity of patients’ selection in published studies, often with small sample size used, no stratification by EF subgroup, NYHA class distribution and degree of LV dilatation. Further studies are certainly needed to better highlight the impact of these features on efficacy of SGLT2i in the clinical setting.
Table 1 summarizes the major findings of the studies discussed in this section.
Table 1.
Studies assessing clinical cardioprotective effects of SGLT2i by Cardiac Magnetic Resonance
| Study | HF | Diabetes | SGLT2i | Duration of Therapy | Imaging Findings | ||
|---|---|---|---|---|---|---|---|
| Santos-Gallego et al. [32] | HFrEF | No | Empaglifozin | 6 months | Improvement of LV volumes, LV mass, LV systolic function, functional capacity | ||
| Brown et al. [91] | No | Yes | Dapaglifozin | 12 months | LVM Reduction | ||
| Connelly et al. [94] | No | No | Empaglifozin | 6 months | No change in LV volumes and function | ||
| Mason et al. [95] | No | Yes | Empaglifozin | 6 months | LVMi and ECV reduction | ||
| Cohen et al. [96] | No | Yes | Empaglifozin | 6 months | Reduced EDV; No changes in ESV, EF, LVM or markers of cardiac fibrosis | ||
| Hsu et al. [97] | No | Yes | Empaglifozin | 6 months | No improvement in LV function, structure, adiposity, and diffuse fibrosis | ||
| Oldgren et al. [98] | No | Yes | Dapaglifozin | 6 weeks | Reduced LA volume. Decreased Peak global radial strain. No changes in peak global longitudinal and circumferential strains. Unchanged cardiac fatty acid uptake | ||
| Verma et al. [99] | No | Yes | Empaglifozin | 6 months | LVMi Reduction | ||
| Hundertmark et al. [101] | HFrEF/HFpEF | No | Empaglifozin | 12 weeks | No improvement in cardiac energetics (PCr/ATP) at rest and during stress | ||
| Gaborit et al. [104] | No | Yes | Empaglifozin | 12 weeks | No change in LVM, LVEF, epicardial fat, diastolic function. | ||
| Lee et al. [106] | HFrEF | Yes | Empaglifozin | 36 weeks | LV volumes reduction | ||
| Singh et al. [107] | HFrEF/HFpEF | Yes | Dapaglifozin | 12 months | No effect on LV remodeling | ||
Legend to Table 1: CMR: cardiac magnetic resonance; EDV: end diastolic volume; ESV: End systolic volume; ECV: extracellular volume; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LA: left atrial; LGE: late gadolinium enhancement; LVEF: left ventricle ejection fraction; LVM left ventricular mass; LVMi: left ventricular mass index; PCr/ATP: phosphocreatine/ATP ratio
GLP-1Ra effects on cardiovascular system
In 2005 GLP-1Ra have been approved to treat DM2 [108]. Although with different structure, duration of action, mode of administration and clinical effectiveness, these drugs overall act similarly by inducing a glucose-dependent insulin release and glucagon suppression [108–113].
In addition, they slow gastric emptying and, by their influence on central nervous system, reduce body weight [114]. GLP-1 receptors have been found in both the glomerulus and renal tubule and use of GLP-1Ra has been associated with increased natriuresis, diuresis, reduced albuminuria and suppression of the RAAS [115–119].
Beyond the metabolic effect, a significant reduction of major adverse CV events (MACE) was observed in patients treated with some of these drugs estimated at 14% when using as outcome a compositum of CV death, nonfatal MI and nonfatal stroke [115].
The underlying mechanisms are still object of current research. Surely multifactorial, they encompass physiological changes of multiple organs involved in central metabolism, systemic regulation of energy expenditure and inflammation and multiple hemodynamic factors, including modulation of blood pressure, heart rate, myocardial geometry and function, endothelial function, vascular tone and regulation of blood volumes [120].
However, not all the GLP-1 Ra are equal when looking at cardioprotection. Lixisenatide, a short acting GLP1Ra failed to demonstrate CV benefits, while liraglutide, semaglutide, dulaglutide and efpeglenatide demonstrated to lower CV events [116]. Reduced mortality was also noted with liraglutide, semaglutide and exenatide [117]. Protective effects in HF patients are controversial, with limited and non-homogenous evidence among the different molecules. The results of the FIGHT and LIVE trial in fact failed to demonstrate a protective effect of liraglutide in patients with both acute and chronic HF, respectively [121, 122]. Moreover, in a post-hoc analysis of the REWIND trial, dulaglutide administration did not reduce HF-related events [123]. Nevertheless, treatment with semaglutide demonstrated to improve HF related symptoms in non-diabetic patients with HFpEF, and a recent meta-analysis encompassing eight trials and 60,080 patients demonstrated an overall reduction of HF-related hospital admission by 11% [115, 124]. The exact reasons of these heterogeneous results are still unknown although may be partially explained by a dose-response effect, with greater CV protection being detectable only when using higher doses of the drug [125].
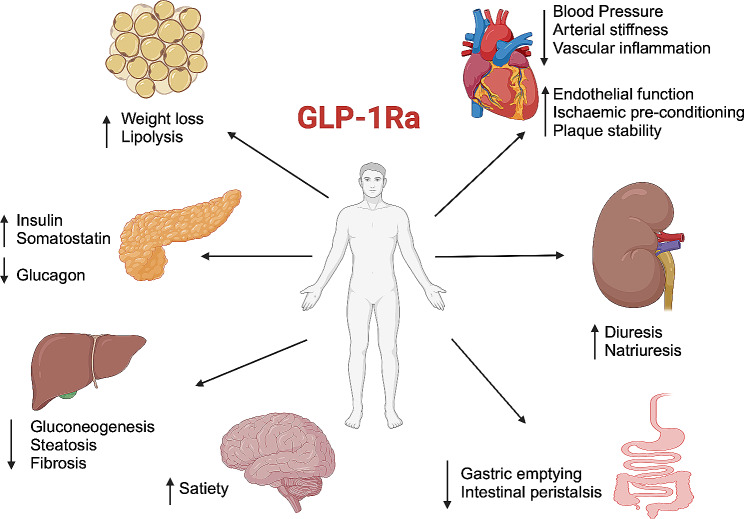
Following this evidence, the 2019 European Society of Cardiology (ESC) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases, advise the use of GLP1-RAs in class I DM2 patients at high CV risk to reduce CV [126]. The indication was later confirmed by the 2021 ESC Guidelines on cardiovascular disease prevention (i.e. class I indication for GLP1-R in patients with DM2 and atherosclerotic cardiovascular disease to reduce CV and cardiorenal outcomes) [127]. Data published so far suggests that the overall benefit observed is mediated by a decrease of atherosclerosis-related event [128–130]. Again, the mechanism behind these effects seems to be various and not yet fully understood (Fig. 3). They have a beneficial effect on systolic blood pressure, although the reduction is only modest (2–6 mmHg) and insufficient to explain by itself the overall effects on CV mortality [119]. GLP-1Ra also lower total and LDL cholesterol and triglycerides [125, 131]. However, it seems the reduction in atherosclerosis development and progression with plaque stabilization and reduced inflammation the most critical factor in terms of CV risk reduction [129, 130].
Fig. 3.
Summary of the effects of Glucagon-Like Peptide-1 receptor agonists
Cardioprotective effects of GLP-1Ra in preclinical models
One of the postulated hypothesis about the protective cardiovascular effects of GLP-1Ra look at the potential detrimental effects of lipotoxicity on cardiac function. It is in fact proved that ectopic fat accumulation and the subsequent imbalance of fatty metabolism is linked with organ damage [119, 132]. In addition, ectopic cardiac fat build-up is strictly related to the development of cardiac dysfunction [131]. The dysregulation of β-oxidation with an excess of availability of its metabolic by-products, is known to cause an increase in reactive oxygen species, contributing to oxidative stress [133]. This pro-inflammatory milieu eventually impacts calcium homeostasis with a direct effect on cardiac function. In addition, cardiac steatosis increases the amount of intramyocardial collagen resulting in reduced relaxation and diastolic dysfunction [134]. However, the exact role of lipotoxicity in the development and progression of DC remain not completely understood, underscoring the need of advanced techniques able to fill this gap in evidence. CMR imaging provides a promising no-invasive approach. Beyond the accurate heart function assessment, proton magnetic resonance spectroscopy (1H-MRS) is effective in detecting triglycerides within the myocardium, with good accuracy when compared to biochemical assays [135, 136]. In addition, microvascular disease is a well-known hallmark of DC that can impact the CV prognosis of patients affected by DM2 [84, 137, 138]. CMR is also helpful to non-invasively assess myocardial blood flow and microvascular ischemia [23]. In detail, arterial spin labeling (ASL) CMR has been used in animal models to assess myocardial blood flow without the use of any contrast agent [139]. Applying this multiparametric CMR protocol (1H-MRS and ASL) Abdesselam et al [140] demonstrated that cardiac abnormalities induced in mice after a 4-weeks course of a high-fat high-sucrose diet (i.e. cardiac hypertrophy, lower cardiac output and decrease myocardial blood flow), were reversed by a 14-day course of the GLP1-Ra Exendin-4. The drug reduced both the myocardial triglyceride content and the myocardial wall thickness [140]. At the same time, GLP1-Ra treatment was able to restore cardiac index and myocardial perfusion [140]. It has been also postulated that GLP1-Ra may exert CV beneficial effects by impacting post-MI cardiac remodeling and ischemia-reperfusion (IR) injury [141, 142]. Ischemic remodeling involves complex interactions at the cellular and molecular levels that lead to oxidative stress, inflammation, and drastic changes in pH and calcium levels, all contributing to cardiomyocyte death and excessive fibrosis [143–146]. Exenatide was found to enhance antioxidant enzyme activity, reduce oxidative stress, and decrease cell death in pigs shortly after IR injury, with similar findings in rats [141–147]. This protection against IR injury seems however to be lost in more severe models, when prolonged provoked ischemia results in irreversible damage [148, 149]. During induced ischemia in experimental models, GLP-1 was in fact able to prompt an increase in anaerobic glycolysis in the ischemic regions, to counteract the lack of oxygen supply as demonstrated using a 1-13C glucose clamp combined with MRS-based isotope analysis [150]. In areas with no ischemia and better oxygenation, a metabolic shift toward carbohydrate oxidation was also observed; the more energy-efficient process may help in sustaining cardiac muscle contraction in these specific circumstances.
Cardioprotective effects of GLP-1Ra in clinical models
There are currently few and conflicting CMR data about cardioprotective effects in vivo of GLP-1Ra.
Exenatide (alone or combined with a remote ischemic conditioning approach) failed to demonstrate a beneficial effect in terms of infarct size measured by LGE, myocardial salvage index, transmurality index, LVEF and MVO volume in patients with ST-segment elevation MI receiving primary percutaneous coronary intervention (pPCI) [151]. However, in another study enrolling 172 STEMI-patients using as endpoint CMR salvage index derived from myocardial area at risk in the acute phase, and infarct size by LGE at follow-up (90 ± 21 days after pPCI) exenatide treatment resulted in a significantly larger salvage index and a smaller infarct size (when related to the myocardial area at risk), despite no differences in LVEF or significant changes in the absolute infarct size [147]. Exenatide treatment did not changed significantly LVEF, myocardial perfusion or oxidative metabolism in T2DM patients with LV systolic dysfunction, having an overall similar effect of glargine insulin [152]. Patients with acute MI treated with liraglutide demonstrated instead smaller LVMi suggesting a role in reverse remodeling [153]. A significant effect on diastolic function was instead noted on a study using liraglutide [154] that demonstrated at a CMR analysis using a 4D flow dataset with retrospective valve tracking, improved early (E) and late (A) trans-mitral peak flow rate, E/A ratio values, along with improved early deceleration peak, early peak mitral annular septal tissue velocity (Ea) and estimated LV filling pressure (E/Ea). The LVEF values were slightly reduced, although remaining within normal range.
Finally, one of the postulated hypotheses was that, given the observed reduction in body weight, GLP1-RA could induce concomitant reduction in epicardial adipose tissue (EAT). This was proven in a cohort of T2DM obese patients, where at CMR analysis EAT thickness was significantly reduced by both exenatide [155] and liraglutide [116, 156] treatment. This result was not confirmed in another study evaluating the effects of liraglutide versus placebo on DM2 patients that showed no significant change in EAT or in myocardial triacylglycerol content (a marker of myocardial steatosis) at proton MR spectroscopy [154]. Further larger studies are therefore needed to assess the impact of GLPR1a on EAT and their impact on LV function.
Table 2 summarizes the major findings of the studies discussed in this section.
Table 2.
Studies assessing clinical cardioprotective effects of GLP1Ra by Cardiac Magnetic Resonance
| Study | Heart Failure | Diabetes | GLP-1Ra | Duration of therapy | Results | ||
|---|---|---|---|---|---|---|---|
| Del Blanco et al. [151] | No | No | Exenatide | Premedication before revascularization | No changes in infarct size measured by LGE, myocardial salvage index, transmurality index, LVEF and MVO | ||
| Lønborg et al. [157] | No | No | Exenatide | 15 min before intervention-6 h after the procedure | Increased myocardial salvage index and reduced infarcted size | ||
| Chen et al. [152] | HFrEF | Yes | Exenatide | 26 weeks | No improvement in LV function, structure, adiposity, and diffuse fibrosis | ||
| Nozue et al. [153] | No | No | Liraglutide | 6 months | Prevention of the progression of LV remodeling | ||
| Bizino et al. [158] | No | Yes | Liraglutide | 26 weeks | Reduction of diastolic and systolic function | ||
| Dutour et al. [155] | No | Yes | Exenatide | 26 weeks | EAT reduction | ||
| Zhao et al. [116] | No | Yes | Liraglutide | 3 months | EAT reduction | ||
| Bizino et al. [154] | No | Yes | Liraglutide | 26 weeks | No changes in EAT and myocardial triacylglycerol content | ||
Legend to Table 2: CMR: cardiac magnetic resonance; EAT: epicardial adipose tissue; LV: left ventricular; MVO: microvascular obstruction
Conclusion
The exact mechanisms underlying the beneficial CV effects of SGLT2i and GLP1-RA are not yet completely understood. Several hypotheses have been formulated and tested in preclinical and clinical studies with the aid of CMR imaging, increasingly used in this setting due to its unique ability to provide accurate volumetric and function assessment complemented with tissue characterization. Beyond visualization and quantification of myocardial fibrosis and oedema, the most recent CMR techniques developed to assess myocardial energetics exploiting the specific relaxation properties of different molecules add promising and radiation-free strings to the bow of the modality. Given the unmatched amount of information that can be obtained from a single scan, with increasingly faster and versatile protocols, CMR imaging will certainly add in the following years meaningful pieces to this complex puzzle.
Author contributions
DT: conceptualization; AC and IL writing—original draft preparation; NS, JS, GB, AS, SDR, DT, CBD, SD—review and editing. All authors approved the final version of the manuscript.
Funding
This work was supported by grants from the Italian Ministry of University and Research: PRIN 2020L45ZWA_005, and PNRR-National Center for Gene Therapy and Drugs based on RNA Technology (CN00000041), and from the Italian Ministry of Health: PSC SALUTE 2014–2020 - POS4 “Cal-Hub-Ria” (T4-AN-09), and PNRR MAD-2022-12376814.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniele Torella, Email: dtorella@unicz.it.
Isabella Leo, Email: isabella.leo@unicz.it.
References
- 1.Ogurtsova K, Da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract Giugno. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. novembre. 2019;157:107843. [DOI] [PubMed]
- 3.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. Erratum in: Lancet. 2016;387(10032):1998. [DOI] [PMC free article] [PubMed]
- 4.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Pencina MJ, Vasan RS et al. Increasing Cardiovascular Disease Burden Due to Diabetes Mellitus: The Framingham Heart Study. Circulation. 27 marzo. 2007;115(12):1544–50. [DOI] [PubMed]
- 5.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Savage PJ et al. Trends in All-Cause and Cardiovascular Disease Mortality Among Women and Men With and Without Diabetes Mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 7 aprile. 2009;119(13):1728–35. [DOI] [PMC free article] [PubMed]
- 6.Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 16 Febbraio. 2018;122(4):624–38. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brønden A, Christensen MB, Glintborg D, Snorgaard O, Kofoed-Enevoldsen A, Madsen GK, et al. Effects of DPP ‐4 inhibitors, GLP ‐1 receptor agonists, SGLT ‐2 inhibitors and sulphonylureas on mortality, cardiovascular and renal outcomes in type 2 diabetes: a network meta‐analyses‐driven approach. Diabet Med Agosto. 2023;40(8):e15157. doi: 10.1111/dme.15157. [DOI] [PubMed] [Google Scholar]
- 8.Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, et al. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes Mellitus: a speckle-tracking imaging study. J Am Soc Echocardiogr Dicembre. 2010;23(12):1266–72. doi: 10.1016/j.echo.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 13 gennaio 2021;m4573. [DOI] [PMC free article] [PubMed]
- 10.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 21 novembre. 2019;381(21):1995–2008. [DOI] [PubMed]
- 11.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 21 Settembre. 2021;42(36):3599–726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 1 Ottobre. 2023;44(37):3627–39. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, McMurray JJV, Claggett B, De Boer RA, DeMets D, Hernandez AF et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 22 settembre. 2022;387(12):1089–98. [DOI] [PubMed]
- 14.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with Empagliflozin in Heart failure. N Engl J Med 8 Ottobre. 2020;383(15):1413–24. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 15.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 14 ottobre. 2021;385(16):1451–61. [DOI] [PubMed]
- 16.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol Marzo. 2000;35(3):569–82. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 17.Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL. Imaging, Biomarker, and clinical predictors of Cardiac Remodeling in Heart failure with reduced ejection fraction. JACC Heart Fail Settembre. 2019;7(9):782–94. doi: 10.1016/j.jchf.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Dhingra NK, Mistry N, Puar P, Verma R, Anker S, Mazer CD, et al. SGLT2 inhibitors and cardiac remodelling: a systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail Dicembre. 2021;8(6):4693–700. doi: 10.1002/ehf2.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Withaar C, Meems LMG, Markousis-Mavrogenis G, Boogerd CJ, Silljé HHW, Schouten EM, et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res 27 Luglio. 2021;117(9):2108–24. doi: 10.1093/cvr/cvaa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, et al. A Glucagon-Like Peptide-1 Analog reverses the Molecular Pathology and Cardiac Dysfunction of a mouse model of obesity. Circulation Gennaio. 2013;127(1):74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 21.Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, Captur G, Francois CJ, Jerosch-Herold M, et al. Reference ranges (normal values) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson Dicembre. 2020;22(1):87. doi: 10.1186/s12968-020-00683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salerno M, Sharif B, Arheden H, Kumar A, Axel L, Li D, et al. Recent advances in Cardiovascular magnetic resonance: techniques and applications. Circ Cardiovasc Imaging Giugno. 2017;10(6):e003951. doi: 10.1161/CIRCIMAGING.116.003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leo I, Nakou E, Artico J, Androulakis E, Wong J, Moon JC, et al. Strengths and weaknesses of alternative noninvasive imaging approaches for microvascular ischemia. J Nucl Cardiol Febbraio. 2023;30(1):227–38. doi: 10.1007/s12350-022-03066-6. [DOI] [PubMed] [Google Scholar]
- 24.Tsampasian V, Swift AJ, Assadi H, Chowdhary A, Swoboda P, Sammut E, et al. Myocardial inflammation and energetics by cardiac MRI: a review of emerging techniques. BMC Med Imaging Dicembre. 2021;21(1):164. doi: 10.1186/s12880-021-00695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2(5):e001007. doi: 10.1136/bmjopen-2012-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol Dicembre. 2022;21(1):83. doi: 10.1186/s12933-022-01512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumiller JJ, White JR, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes Mellitus. Drugs Marzo. 2010;70(4):377–85. doi: 10.2165/11318680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co‐transporter‐2 inhibitors in type 2 diabetes mellitus: systematic review and network meta‐analysis. Diabetes Obes Metab Agosto. 2016;18(8):783–94. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 29.Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs Febbraio. 2019;79(3):219–30. doi: 10.1007/s40265-019-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose tissue distribution in patients with type 2 diabetes Mellitus with inadequate Glycemic Control on Metformin. J Clin Endocrinol Metab 1 Marzo. 2012;97(3):1020–31. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 31.Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez Cordero AJ, Fardman B, Sartori S, Sanz J, et al. Not only how much, but also how to, when measuring epicardial adipose tissue. Magn Reson Imaging Febbraio. 2022;86:149–51. doi: 10.1016/j.mri.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, et al. Randomized Trial of Empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol Gennaio. 2021;77(3):243–55. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr Dicembre. 2017;9(1):78. doi: 10.1186/s13098-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, et al. SGLT2 inhibition by Empagliflozin Promotes Fat Utilization and Browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in Diet-induced obese mice. EBioMedicine Giugno. 2017;20:137–49. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansary TM, Nakano D, Nishiyama A. Diuretic effects of Sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci 1 Febbraio. 2019;20(3):629. doi: 10.3390/ijms20030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, et al. Dapagliflozin suppresses Hepcidin and increases erythropoiesis. J Clin Endocrinol Metab 1 Aprile. 2020;105(4):e1056–63. doi: 10.1210/clinem/dgaa057. [DOI] [PubMed] [Google Scholar]
- 37.Docherty KF, Welsh P, Verma S, De Boer RA, O’Meara E, Bengtsson O et al. Iron Deficiency in Heart Failure and Effect of Dapagliflozin: Findings From DAPA-HF. Circulation. 27 settembre. 2022;146(13):980–94. [DOI] [PMC free article] [PubMed]
- 38.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al. How does Empagliflozin reduce Cardiovascular Mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 1 Febbraio. 2018;41(2):356–63. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka A, Shimabukuro M, Teragawa H, Okada Y, Takamura T, Taguchi I, et al. Reduction of estimated fluid volumes following initiation of empagliflozin in patients with type 2 diabetes and cardiovascular disease: a secondary analysis of the placebo-controlled, randomized EMBLEM trial. Cardiovasc Diabetol Dicembre. 2021;20(1):105. doi: 10.1186/s12933-021-01295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R et al. Treatment of Anemia with Darbepoetin Alfa in Systolic Heart Failure. N Engl J Med. 28 marzo. 2013;368(13):1210–9. [DOI] [PubMed]
- 41.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 26 novembre. 2015;373(22):2117–28. [DOI] [PubMed]
- 42.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 17 agosto. 2017;377(7):644–57. [DOI] [PubMed]
- 43.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 24 gennaio. 2019;380(4):347–57. [DOI] [PubMed]
- 44.Von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN, Benedikt M, Wallner M, et al. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J 1 Novembre. 2022;43(41):4421–32. doi: 10.1093/eurheartj/ehac494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paolisso P, Bergamaschi L, Gragnano F, Gallinoro E, Cesaro A, Sardu C, et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: the SGLT2-I AMI PROTECT Registry. Pharmacol Res Gennaio. 2023;187:106597. doi: 10.1016/j.phrs.2022.106597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrington J, Udell JA, Jones WS, Anker SD, Bhatt DL, Petrie MC, et al. Empagliflozin in patients post myocardial infarction rationale and design of the EMPACT-MI trial. Am Heart J Novembre. 2022;253:86–98. doi: 10.1016/j.ahj.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 14 gennaio. 2021;384(2):129–39. [DOI] [PubMed]
- 48.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 14 gennaio. 2021;384(2):117–28. [DOI] [PubMed]
- 49.Marino F, Salerno N, Scalise M, Salerno L, Torella A, Molinaro C, et al. Streptozotocin-Induced type 1 and 2 diabetes Mellitus Mouse models Show different functional, Cellular and molecular patterns of Diabetic Cardiomyopathy. Int J Mol Sci 6 Gennaio. 2023;24(2):1132. doi: 10.3390/ijms24021132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen X, Li L, Sun Z, Zang G, Zhang L, Shao C, et al. Gut microbiota and atherosclerosis-focusing on the Plaque Stability. Front Cardiovasc Med. 2021;8:668532. doi: 10.3389/fcvm.2021.668532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molinaro C, Salerno L, Marino F, Scalise M, Salerno N, Pagano L et al. Unraveling and Targeting Myocardial Regeneration Deficit in Diabetes. Antioxidants. 22 gennaio. 2022;11(2):208. [DOI] [PMC free article] [PubMed]
- 52.Soares RN, Ramirez-Perez FI, Cabral-Amador FJ, Morales-Quinones M, Foote CA, Ghiarone T, et al. SGLT2 inhibition attenuates arterial dysfunction and decreases vascular F-actin content and expression of proteins associated with oxidative stress in aged mice. GeroScience Giugno. 2022;44(3):1657–75. doi: 10.1007/s11357-022-00563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ugusman A, Kumar J, Aminuddin A. Endothelial function and dysfunction: impact of sodium-glucose cotransporter 2 inhibitors. Pharmacol Ther Agosto. 2021;224:107832. doi: 10.1016/j.pharmthera.2021.107832. [DOI] [PubMed] [Google Scholar]
- 54.Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, et al. SGLT2 inhibition via Empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from Frail Hypertensive and Diabetic patients. Hypertens Agosto. 2022;79(8):1633–43. doi: 10.1161/HYPERTENSIONAHA.122.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durante W, Behnammanesh G, Peyton KJ. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int J Mol Sci. 16 agosto. 2021;22(16):8786. [DOI] [PMC free article] [PubMed]
- 56.Irace C, Cutruzzolà A, Parise M, Fiorentino R, Frazzetto M, Gnasso C, et al. Effect of empagliflozin on brachial artery shear stress and endothelial function in subjects with type 2 diabetes: results from an exploratory study. Diab Vasc Dis Res Gennaio. 2020;17(1):147916411988354. doi: 10.1177/1479164119883540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, et al. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res Luglio. 2020;157:104781. doi: 10.1016/j.phrs.2020.104781. [DOI] [PubMed] [Google Scholar]
- 58.Torella D, Iaconetti C, Tarallo R, Marino F, Giurato G, Veneziano C et al. miRNA Regulation of the Hyperproliferative Phenotype of Vascular Smooth Muscle Cells in Diabetes. Diabetes. 1 dicembre. 2018;67(12):2554–68. [DOI] [PubMed]
- 59.Urbanek K, Cappetta D, Bellocchio G, Coppola MA, Imbrici P, Telesca M, et al. Dapagliflozin protects the kidney in a non-diabetic model of cardiorenal syndrome. Pharmacol Res Febbraio. 2023;188:106659. doi: 10.1016/j.phrs.2023.106659. [DOI] [PubMed] [Google Scholar]
- 60.Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, et al. SGLT2 inhibitor–Induced Sympathoinhibition. JACC Basic Transl Sci Febbraio. 2020;5(2):169–79. doi: 10.1016/j.jacbts.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gager GM, Von Lewinski D, Sourij H, Jilma B, Eyileten C, Filipiak K, et al. Effects of SGLT2 inhibitors on Ion Homeostasis and oxidative stress associated mechanisms in Heart failure. Biomed Pharmacother Novembre. 2021;143:112169. doi: 10.1016/j.biopha.2021.112169. [DOI] [PubMed] [Google Scholar]
- 62.Rau M, Thiele K, Hartmann NUK, Möllmann J, Wied S, Hohl M, et al. Effects of empagliflozin on markers of calcium and phosphate homeostasis in patients with type 2 diabetes – data from a randomized, placebo-controlled study. Bone Rep Giugno. 2022;16:101175. doi: 10.1016/j.bonr.2022.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yurista SR, Silljé HHW, Oberdorf-Maass SU, Schouten E, Pavez Giani MG, Hillebrands J, et al. Sodium–glucose co‐transporter 2 inhibition with empagliflozin improves cardiac function in non‐diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail Luglio. 2019;21(7):862–73. doi: 10.1002/ejhf.1473. [DOI] [PubMed] [Google Scholar]
- 64.Santos-Gallego CG, Mayr M, Badimon J. SGLT2 Inhibitors in Heart Failure: Targeted Metabolomics and Energetic Metabolism. Circulation. 13 settembre. 2022;146(11):819–21. [DOI] [PubMed]
- 65.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol Aprile. 2019;73(15):1931–44. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 66.Thirunavukarasu S, Jex N, Chowdhary A, Hassan IU, Straw S, Craven TP, et al. Empagliflozin Treatment is Associated with improvements in Cardiac energetics and function and reductions in Myocardial Cellular volume in patients with type 2 diabetes. Diabetes 1 Dicembre. 2021;70(12):2810–22. doi: 10.2337/db21-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, et al. Empagliflozin Increases Cardiac Energy Production in diabetes. JACC Basic Transl Sci Ottobre. 2018;3(5):575–87. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med Marzo. 2015;21(3):263–9. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu X, Tesiram YA, Towner RA, Abbott A, Patterson E, Huang S, et al. Early myocardial dysfunction in streptozotocin-induced diabetic mice: a study using in vivo magnetic resonance imaging (MRI) Cardiovasc Diabetol. 2007;6(1):6. doi: 10.1186/1475-2840-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naresh NK, Butcher JT, Lye RJ, Chen X, Isakson BE, Gan LM, et al. Cardiovascular magnetic resonance detects the progression of impaired myocardial perfusion reserve and increased left-ventricular mass in mice fed a high-fat diet. J Cardiovasc Magn Reson Dicembre. 2016;18(1):53. doi: 10.1186/s12968-016-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marciniak C, Marechal X, Montaigne D, Neviere R, Lancel S. Cardiac contractile function and mitochondrial respiration in diabetes-related mouse models. Cardiovasc Diabetol Dicembre. 2014;13(1):118. doi: 10.1186/s12933-014-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi L, Zhu D, Wang S, Jiang A, Li F. Dapagliflozin attenuates Cardiac Remodeling in mice Model of Cardiac pressure overload. Am J Hypertens 22 Aprile. 2019;32(5):452–9. doi: 10.1093/ajh/hpz016. [DOI] [PubMed] [Google Scholar]
- 73.Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, et al. The sodium–glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol Dicembre. 2019;18(1):45. doi: 10.1186/s12933-019-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sabatino J, De Rosa S, Tammè L, Iaconetti C, Sorrentino S, Polimeni A, et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc Diabetol Dicembre. 2020;19(1):66. doi: 10.1186/s12933-020-01040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Garcia-Ropero A, Ishikawa K, Watanabe S, et al. Empagliflozin ameliorates diastolic dysfunction and left ventricular Fibrosis/Stiffness in nondiabetic heart failure. JACC Cardiovasc Imaging Febbraio. 2021;14(2):393–407. doi: 10.1016/j.jcmg.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 76.Kawaji K, Codella NCF, Prince MR, Chu CW, Shakoor A, LaBounty TM, et al. Automated segmentation of Routine Clinical Cardiac magnetic resonance imaging for Assessment of Left Ventricular Diastolic Dysfunction. Circ Cardiovasc Imaging Novembre. 2009;2(6):476–84. doi: 10.1161/CIRCIMAGING.109.879304. [DOI] [PubMed] [Google Scholar]
- 77.Pabel S, Wagner S, Bollenberg H, Bengel P, Kovács Á, Schach C, et al. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail Dicembre. 2018;20(12):1690–700. doi: 10.1002/ejhf.1328. [DOI] [PubMed] [Google Scholar]
- 78.Baartscheer A. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res 15 Marzo. 2003;57(4):1015–24. doi: 10.1016/S0008-6363(02)00809-X. [DOI] [PubMed] [Google Scholar]
- 79.Croteau D, Luptak I, Chambers JM, Hobai I, Panagia M, Pimentel DR et al. Effects of Sodium-Glucose Linked Transporter 2 Inhibition With Ertugliflozin on Mitochondrial Function, Energetics, and Metabolic Gene Expression in the Presence and Absence of Diabetes Mellitus in Mice. J Am Heart Assoc. 6 luglio. 2021;10(13):e019995. [DOI] [PMC free article] [PubMed]
- 80.Croteau D, Baka T, Young S, He H, Chambers JM, Qin F, et al. SGLT2 inhibitor ertugliflozin decreases elevated intracellular sodium, and improves energetics and contractile function in diabetic cardiomyopathy. Biomed Pharmacother Aprile. 2023;160:114310. doi: 10.1016/j.biopha.2023.114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doliba NM, Babsky AM, Osbakken MD. The Role of Sodium in Diabetic Cardiomyopathy. Front Physiol. 24 ottobre. 2018;9:1473. [DOI] [PMC free article] [PubMed]
- 82.Vettor R, Inzucchi SE, Fioretto P. The cardiovascular benefits of empagliflozin: SGLT2-dependent and -independent effects. Diabetologia Marzo. 2017;60(3):395–8. doi: 10.1007/s00125-016-4194-y. [DOI] [PubMed] [Google Scholar]
- 83.Athithan L, Gulsin GS, McCann GP, Levelt E. Diabetic cardiomyopathy: pathophysiology, theories and evidence to date. World J Diabetes 15 Ottobre. 2019;10(10):490–510. doi: 10.4239/wjd.v10.i10.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Towner RA, Smith N, Saunders D, Carrizales J, Lupu F, Silasi-Mansat R, et al. In vivo targeted molecular magnetic resonance imaging of free radicals in diabetic cardiomyopathy within mice. Free Radic Res 2 Settembre. 2015;49(9):1140–6. doi: 10.3109/10715762.2015.1050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM, et al. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J 7 Dicembre. 2016;37(46):3461–9. doi: 10.1093/eurheartj/ehv442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P et al. Abnormal Cardiac and Skeletal Muscle Energy Metabolism in Patients With Type 2 Diabetes. Circulation. 24 giugno. 2003;107(24):3040–6. [DOI] [PubMed]
- 87.Joubert M, Jagu B, Montaigne D, Marechal X, Tesse A, Ayer A, et al. The sodium–glucose cotransporter 2 inhibitor Dapagliflozin prevents cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes 1 Aprile. 2017;66(4):1030–40. doi: 10.2337/db16-0733. [DOI] [PubMed] [Google Scholar]
- 88.Abdurrachim D, Teo XQ, Woo CC, Chan WX, Lalic J, Lam CSP, et al. Empagliflozin reduces myocardial ketone utilization while preserving glucose utilization in diabetic hypertensive heart disease: a hyperpolarized 13 C magnetic resonance spectroscopy study. Diabetes Obes Metab Febbraio. 2019;21(2):357–65. doi: 10.1111/dom.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abdurrachim D, Manders E, Nicolay K, Mayoux E, Prompers JJ. Single dose of empagliflozin increases in vivo cardiac energy status in diabetic db/db mice. Cardiovasc Res [Internet] 5 ottobre 2018 [citato 19 gennaio 2024]; Disponibile su: https://academic.oup.com/cardiovascres/advance-article/doi/10.1093/cvr/cvy246/5115996. [DOI] [PubMed]
- 90.Santos-Gallego CG, Requena-Ibáñez JA, Picatoste B, Fardman B, Ishikawa K, Mazurek R et al. Cardioprotective effect of Empagliflozin and circulating ketone bodies during Acute myocardial infarction. Circ Cardiovasc Imaging [Internet]. aprile 2023 [citato 19 gennaio 2024];16(4). Disponibile su: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.123.015298. [DOI] [PubMed]
- 91.Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J 21 Settembre. 2020;41(36):3421–32. doi: 10.1093/eurheartj/ehaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carluccio E, Biagioli P, Reboldi G, Mengoni A, Lauciello R, Zuchi C, et al. Left ventricular remodeling response to SGLT2 inhibitors in heart failure: an updated meta-analysis of randomized controlled studies. Cardiovasc Diabetol 2 Settembre. 2023;22(1):235. doi: 10.1186/s12933-023-01970-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moroney M, Verma R, Hibino M, Mazer CD, Connelly KA, Yan AT, et al. Impact of diabetes duration on left ventricular mass regression with empagliflozin. ESC Heart Fail Giugno. 2023;10(3):2134–40. doi: 10.1002/ehf2.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Connelly KA, Mazer CD, Puar P, Teoh H, Wang CH, Mason T et al. Empagliflozin and Left Ventricular Remodeling in People Without Diabetes: Primary Results of the EMPA-HEART 2 CardioLink-7 Randomized Clinical Trial. Circulation. 24 gennaio. 2023;147(4):284–95. [DOI] [PubMed]
- 95.Mason T, Coelho-Filho OR, Verma S, Chowdhury B, Zuo F, Quan A, et al. Empagliflozin reduces myocardial extracellular volume in patients with type 2 diabetes and coronary artery disease. JACC Cardiovasc Imaging Giugno. 2021;14(6):1164–73. doi: 10.1016/j.jcmg.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 96.Cohen ND, Gutman SJ, Briganti EM, Taylor AJ. Effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes: a cardiac magnetic resonance study. Intern Med J Agosto. 2019;49(8):1006–10. doi: 10.1111/imj.14260. [DOI] [PubMed] [Google Scholar]
- 97.Hsu JC, Wang CY, Su MYM, Lin LY, Yang WS. Effect of Empagliflozin on Cardiac function, adiposity, and diffuse fibrosis in patients with type 2 diabetes Mellitus. Sci Rep 25 Ottobre. 2019;9(1):15348. doi: 10.1038/s41598-019-51949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oldgren J, Laurila S, Åkerblom A, Latva-Rasku A, Rebelos E, Isackson H, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium‐glucose co‐transporter‐2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes: a randomized, placebo‐controlled, exploratory study. Diabetes Obes Metab Luglio. 2021;23(7):1505–17. doi: 10.1111/dom.14363. [DOI] [PubMed] [Google Scholar]
- 99.Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 19 novembre. 2019;140(21):1693–702. [DOI] [PubMed]
- 100.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2 Marzo. 2014;35(10):657–64. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hundertmark MJ, Adler A, Antoniades C, Coleman R, Griffin JL, Holman RR et al. Assessment of Cardiac Energy Metabolism, Function, and Physiology in Patients With Heart Failure Taking Empagliflozin: The Randomized, Controlled EMPA-VISION Trial. Circulation. 30 maggio. 2023;147(22):1654–69. [DOI] [PMC free article] [PubMed]
- 102.Masson W, Lavalle-Cobo A, Nogueira JP. Effect of SGLT2-Inhibitors on Epicardial Adipose tissue: a Meta-analysis. Cells 20 Agosto. 2021;10(8):2150. doi: 10.3390/cells10082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karampetsou N, Alexopoulos L, Minia A, Pliaka V, Tsolakos N, Kontzoglou K et al. Epicardial Adipose Tissue as an Independent Cardiometabolic Risk Factor for Coronary Artery Disease. Cureus [Internet]. 1 giugno 2022 [citato 19 gennaio 2024]; Disponibile su: https://www.cureus.com/articles/98643-epicardial-adipose-tissue-as-an-independent-cardiometabolic-risk-factor-for-coronary-artery-disease. [DOI] [PMC free article] [PubMed]
- 104.Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol Dicembre. 2021;20(1):57. doi: 10.1186/s12933-021-01237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pérez MS, Rodríguez-Capitán J, Requena-Ibáñez JA, Santos-Gallego CG, Urooj Zafar M, Escolar G et al. Rationale and design of the SOTA-P-CARDIA Trial (ATRU-V): Sotagliflozin in HFpEF patients without Diabetes. Cardiovasc Drugs Ther [Internet] 15 giugno 2023 [citato 19 gennaio 2024]; Disponibile su: https://link.springer.com/10.1007/s10557-023-07469-6. [DOI] [PubMed]
- 106.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 9 febbraio. 2021;143(6):516–25. [DOI] [PMC free article] [PubMed]
- 107.Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, et al. Dapagliflozin Versus Placebo on Left ventricular remodeling in patients with diabetes and heart failure: the REFORM Trial. Diabetes Care 1 Giugno. 2020;43(6):1356–9. doi: 10.2337/dc19-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 15 Gennaio. 2005;62(2):173–81. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 109.Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 17 ottobre. 2017;318(15):1460. [DOI] [PMC free article] [PubMed]
- 110.Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK. Dulaglutide, a long-acting GLP-1 analog fused with an fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther Dicembre. 2010;12(6):790–7. [PubMed] [Google Scholar]
- 111.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: Randomized Clinical Trial of the efficacy and safety of oral Semaglutide Monotherapy in Comparison with Placebo in patients with type 2 diabetes. Diabetes Care 1 Settembre. 2019;42(9):1724–32. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 112.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterol Maggio. 2007;132(6):2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 113.Drucker DJ. Incretin Action in the pancreas: potential Promise, possible perils, and Pathological Pitfalls. Diabetes 1 Ottobre. 2013;62(10):3316–23. doi: 10.2337/db13-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nogueiras R, Pérez-Tilve D, Veyrat-Durebex C, Morgan DA, Varela L, Haynes WG, et al. Direct Control of Peripheral Lipid Deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in Diet-Induced obesity. J Neurosci 6 Maggio. 2009;29(18):5916–25. doi: 10.1523/JNEUROSCI.5977-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol Ottobre. 2021;9(10):653–62. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 116.Zhao N, Wang X, Wang Y, Yao J, Shi C, Du J, et al. The Effect of Liraglutide on Epicardial Adipose tissue in type 2 diabetes. Marfella R, curatore. J Diabetes Res 16 Novembre. 2021;2021:1–6. doi: 10.1155/2021/5578216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Røder ME. Major adverse cardiovascular event reduction with GLP-1 and SGLT2 agents: evidence and clinical potential. Ther Adv Chronic Dis Gennaio. 2018;9(1):33–50. doi: 10.1177/2040622317735283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neves JS, Vasques-Nóvoa F, Borges‐Canha M, Leite AR, Sharma A, Carvalho D, et al. Risk of adverse events with liraglutide in heart failure with reduced ejection fraction: a post hoc analysis of the FIGHT trial. Diabetes Obes Metab Gennaio. 2023;25(1):189–97. doi: 10.1111/dom.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 29 agosto. 2017;136(9):849–70. [DOI] [PubMed]
- 120.Sabouret P, Ecarnot F, De Rosa S, Ray KK. What about glucagon-like peptide-1 receptor agonist for all? Recent data and perspectives. Eur Heart J. 14 novembre. 2023;44(43):4499–502. [DOI] [PubMed]
- 121.Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, et al. Effect of liraglutide, a glucagon-like peptide‐1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—a multicentre, double‐blind, randomised, placebo‐controlled trial. Eur J Heart Fail Gennaio. 2017;19(1):69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 122.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of Liraglutide on Clinical Stability among patients with Advanced Heart failure and reduced ejection fraction: a Randomized Clinical Trial. JAMA 2 Agosto. 2016;316(5):500. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet Luglio. 2019;394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 124.Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 21 settembre. 2023;389(12):1069–84. [DOI] [PubMed]
- 125.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of Glucagon-Like Peptide-1 in patients with Acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2 Marzo. 2004;109(8):962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 126.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 7 gennaio. 2020;41(2):255–323. [DOI] [PubMed]
- 127.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 7 settembre. 2021;42(34):3227–337. [DOI] [PubMed]
- 128.Buse JB, Bain SC, Mann JFE, Nauck MA, Nissen SE, Pocock S, et al. Cardiovascular Risk Reduction with Liraglutide: an exploratory mediation analysis of the LEADER Trial. Diabetes Care 1 Luglio. 2020;43(7):1546–52. doi: 10.2337/dc19-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanada J, Obata A, Obata Y, Fushimi Y, Shimoda M, Kohara K, et al. Dulaglutide exerts beneficial anti atherosclerotic effects in ApoE knockout mice with diabetes: the earlier, the better. Sci Rep 14 Gennaio. 2021;11(1):1425. doi: 10.1038/s41598-020-80894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs Liraglutide and Semaglutide reduce atherosclerosis in ApoE–/– and LDLr–/– mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci Dicembre. 2018;3(6):844–57. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edin C, Ekstedt M, Scheffel T, Karlsson M, Swahn E, Östgren CJ et al. Ectopic fat is associated with cardiac remodeling—A comprehensive assessment of regional fat depots in type 2 diabetes using multi-parametric MRI. Front Cardiovasc Med. 28 luglio. 2022;9:813427. [DOI] [PMC free article] [PubMed]
- 132.Marino F, Scalise M, Salerno N, Salerno L, Molinaro C, Cappetta D, et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory phenotype impair Cardiac regeneration and function independently of age. Diabetes 1 Maggio. 2022;71(5):1081–98. doi: 10.2337/db21-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci 26 Aprile. 2023;24(9):7898. doi: 10.3390/ijms24097898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oneglia AP, Szczepaniak LS, Jaffery MF, Cipher DJ, McDonald JG, Haykowsky MJ, et al. Myocardial steatosis impairs left ventricular diastolic–systolic coupling in healthy humans. J Physiol Aprile. 2023;601(8):1371–82. doi: 10.1113/JP284272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med Marzo. 2003;49(3):417–23. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 136.Hammer S, Snel M, Lamb HJ, Jazet IM, Van Der Meer RW, Pijl H, et al. Prolonged caloric restriction in obese patients with type 2 diabetes Mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol Settembre. 2008;52(12):1006–12. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 137.Kellman P, Hansen MS, Nielles-Vallespin S, Nickander J, Themudo R, Ugander M, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson Dicembre. 2017;19(1):43. doi: 10.1186/s12968-017-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, Aung N et al. The Prognostic significance of quantitative myocardial perfusion: an Artificial Intelligence Based Approach using perfusion mapping. Circulation. 14 febbraio 2020;CIRCULATIONAHA.119.044666. [DOI] [PMC free article] [PubMed]
- 139.Kober F, Jao T, Troalen T, Nayak KS. Myocardial arterial spin labeling. J Cardiovasc Magn Reson Dicembre. 2016;18(1):22. doi: 10.1186/s12968-016-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abdesselam I, Pepino P, Troalen T, Macia M, Ancel P, Masi B, et al. Time course of cardiometabolic alterations in a high fat high sucrose diet mice model and improvement after GLP-1 analog treatment using multimodal cardiovascular magnetic resonance. J Cardiovasc Magn Reson Dicembre. 2015;17(1):95. doi: 10.1186/s12968-015-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist Liraglutide activates Cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 1 Aprile. 2009;58(4):975–83. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saleh MG, Sharp SK, Alhamud A, Spottiswoode BS, Van Der Kouwe AJW, Davies NH, et al. Long-term left ventricular remodelling in Rat Model of Nonreperfused myocardial infarction: sequential MR Imaging using a 3T clinical scanner. J Biomed Biotechnol. 2012;2012:1–10. doi: 10.1155/2012/504037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res 23 Maggio. 2014;114(11):1788–803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 144.Ussher JR, Greenwell AA, Nguyen MA, Mulvihill EE. Cardiovascular effects of Incretin-based therapies: integrating mechanisms with Cardiovascular Outcome trials. Diabetes 1 Febbraio. 2022;71(2):173–83. doi: 10.2337/dbi20-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The role of oxidative stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid Med Cell Longev. 2016;2016:1–14. doi: 10.1155/2016/1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol-Heart Circ Physiol 1 Maggio. 1991;260(5):H1406–14. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- 147.Timmers L, Henriques JPS, De Kleijn DPV, DeVries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a Porcine Model of Ischemia and Reperfusion Injury. J Am Coll Cardiol Febbraio. 2009;53(6):501–10. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 148.Demkes EJ, Wenker S, Silvis MJM, Van Nieuwburg MMJ, Visser MJ, Jansen MS et al. Neutral Effects of Combined Treatment With GLP-1R Agonist Exenatide and MR Antagonist Potassium Canrenoate on Cardiac Function in Porcine and Murine Chronic Heart Failure Models. Front Pharmacol. 26 luglio. 2021;12:702326. [DOI] [PMC free article] [PubMed]
- 149.Kyhl K, Lønborg J, Hartmann B, Kissow H, Poulsen SS, Ali HE, et al. Lack of effect of prolonged treatment with liraglutide on cardiac remodeling in rats after acute myocardial infarction. Peptides Luglio. 2017;93:1–12. doi: 10.1016/j.peptides.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 150.Aravindhan K, Bao W, Harpel MR, Willette RN, Lepore JJ, Jucker BM. Cardioprotection Resulting from Glucagon-Like Peptide-1 Administration Involves Shifting Metabolic Substrate Utilization to Increase Energy Efficiency in the Rat Heart. Ma XL, curatore. PLOS ONE. 22 giugno. 2015;10(6):e0130894. [DOI] [PMC free article] [PubMed]
- 151.Del García B, Otaegui I, Rodríguez-Palomares JF, Bayés-Genis A, Fernández-Nofrerías E, Del Vilalta V, et al. Effect of COMBinAtion therapy with remote ischemic conditioning and exenatide on the myocardial infarct size: a two-by-two factorial randomized trial (COMBAT-MI) Basic Res Cardiol Dicembre. 2021;116(1):4. doi: 10.1007/s00395-021-00842-2. [DOI] [PubMed] [Google Scholar]
- 152.Chen WJY, Diamant M, De Boer K, Harms HJ, Robbers LFHJ, Van Rossum AC, et al. Effects of Exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: a randomized controlled trial against insulin glargine. Cardiovasc Diabetol Dicembre. 2017;16(1):67. doi: 10.1186/s12933-017-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nozue T, Yamada M, Tsunoda T, Katoh H, Ito S, Iwaki T, et al. Effects of liraglutide, a glucagon-like peptide-1 analog, on left ventricular remodeling assessed by cardiac magnetic resonance imaging in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels Agosto. 2016;31(8):1239–46. doi: 10.1007/s00380-015-0734-5. [DOI] [PubMed] [Google Scholar]
- 154.Bizino MB, Jazet IM, De Heer P, Van Eyk HJ, Dekkers IA, Rensen PCN, et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia Gennaio. 2020;63(1):65–74. doi: 10.1007/s00125-019-05021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab Settembre. 2016;18(9):882–91. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 156.Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obes Febbraio. 2017;25(2):311–6. doi: 10.1002/oby.21718. [DOI] [PubMed] [Google Scholar]
- 157.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J Giugno. 2012;33(12):1491–9. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 158.Bizino MB, Jazet IM, Westenberg JJM, Van Eyk HJ, Paiman EHM, Smit JWA, et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol Dicembre. 2019;18(1):55. doi: 10.1186/s12933-019-0857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.