Abstract
Glymphatic system is an emerging pathway of removing metabolic waste products and toxic solutes from the brain tissue. It is made of a network of perivascular spaces, filled in cerebrospinal and interstitial fluid, encompassing penetrating and pial vessels and communicating with the subarachnoid space. It is separated from vessels by the blood brain barrier and from brain tissue by the endfeet of the astrocytes rich in aquaporin 4, a membrane protein which controls the water flow along the perivascular space. Animal models and magnetic resonance (MR) studies allowed to characterize the glymphatic system function and determine how its impairment could lead to numerous neurological disorders (e.g. Alzheimer’s disease, stroke, sleep disturbances, migraine, idiopathic normal pressure hydrocephalus). This review aims to summarize the role of the glymphatic system in the pathophysiology of migraine in order to provide new ways of approaching to this disease and to its therapy.
Keywords: Glymphatic system, Perivascular space, Cerebrospinal fluid, Neurological disorders, Migraine, Headache, Cortical spreading depression, CGRP, DTI-ALPS
Introduction
Migraine is a neurovascular disorder involving the trigeminovascular system [1]. It is one of the most frequent and disabling neurological diseases. It affects approximately 14% of the general population, mainly women, with a mean age of 15–40 years [2]. It represents a worldwide social and health concern [3]. Although the efforts in understanding migraine pathophysiology, the exact mechanisms underlying this disease still remain unclear. It has been hypothesized that the glymphatic system (GS) may play a role in migraine pathophysiology and that its disfunction may impact on the clinical spectrum of migraine. In this paper we reviewed current literature to summarize the available data concerning migraine, the GS and its involvement in migraine pathogenesis in order to provide a new insight into future diagnostic and therapeutic perspectives in the field of headache disorders.
Migraine disease
Migraine is a complex neurovascular disorder involving the trigeminovascular system [1]. The current best estimate of global migraine prevalence is 14–15%, and, in terms of burden, migraine accounts for 4.9% of global ill health, quantified as years lived with disability [4]. Migraine causes negative consequences not only in patients immediately affected but also on their families, colleagues, employers, and society [5]. According to the International Classification of Headache disorders (ICHD) 3rd edition, migraine could be divided into episodic migraine, with or without aura, and chronic migraine [6]. Migraine without aura is defined as a strictly unilateral, recurrent and pulsating pain, of moderate to severe intensity, lasting 4–72 h and accompanied by symptoms like nausea and/or vomiting, phono- and/or photophobia, aggravating by the body activity and alleviated by rest. Migraine with aura is characterized by the recurrence of reversible symptoms, preceding headache, which could be visual, sensory, speech/language, brainstem, motor or retinal disturbances. Which should show at least two of the following characteristics: spreading in 5 min and/or in succession, lasting 60 min, being unilateral and followed by headache associated with migraine symptoms. Chronic migraine is defined as a headache occurring on 15 days/month for more than 3 months, which has the features of migraine headache on at least 8 days per month [6].
Neuroinflammation, excess of calcitonin gene-related peptide (CGRP) and cortical spreading depression are the three most studied mechanisms underlying migraine pathogenesis and aura development. A common feature of these mechanisms is the impairment of the glymphatic system [2, 5, 7–23].
The glymphatic system
The lymphatic system, a vast network of vessels and lymphoid organs, assures intrabody fluid homeostasis and immunity by collecting and detoxifying fluid and metabolic waste products in the interstitial space [24, 25]. Although the brain tissue is among the most metabolically active organs of the body, there is no classical lymphatic circulation clearing its metabolites and waste products [26]. Nonetheless, recent studies have demonstrated the existence of the so called glymphatic system (GS). It is a complex network of perivascular space (PVS) surrounding brain vessels and acting as a possible lymphatic circulation. The outer perimeter of the PVS is lined with foot-like protrusions of astrocyte cells [27] and is separated from the vascular wall by a basement membrane called glia limitans [28]. PVS is filled with the cerebrospinal fluid (CSF) [1]. CSF is produced by the choroid plexi located in the brain ventricles, through / ATP-ase and Aquaporin 1 [29]. Despite CSF shares many similarities with blood plasma, it has higher levels of sodium, chloride, and magnesium, and lower levels of potassium, calcium, proteins, and cells [30]. After its synthesis CSF spreads into the ventricles, the subarachnoid space and the PVSs. It enters the brain tissue through para-arterial space and mixes with the interstitial fluid (ISF). CSF-ISF complex and its solutes enter the paravenous space thanks to the water transporter Aquaporin 4 (AQP4) sited into the astrocytes’ endfeet [31]. Once CSF-ISF has reached the subarachnoid space, it passes through the arachnoid granulations into the dural sinuses, the meningeal lymphatics and into the cervical lymphatics [32, 33] (Fig. 1).
Fig. 1.
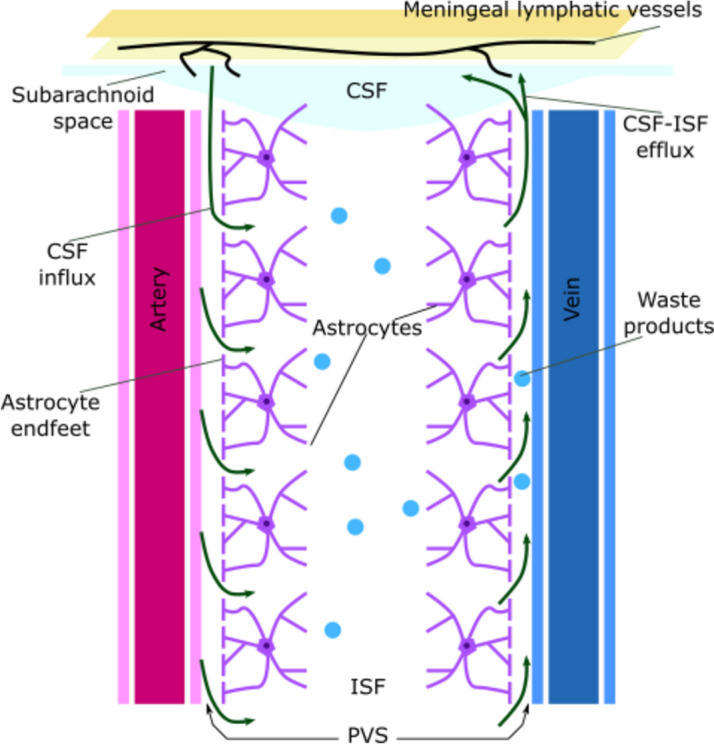
Representation of the glymphatic system and the glymphatic flow. The glymphatic system is made of a network of PVS around arteries and veins throwing metabolic waste products away from the central nervous system. PVS is limited by the endfeets of astrocytes and is filled with the CSF. CSF is produced in the choroid plexi in the lateral ventricles and then is vehicled into the subarachoid space. From the subarachoid space the CSF streams into the PVS around pial arteries. Here CSF enters the brain tissue and mixes with the ISF. CSF-ISF flows into the perivenous space and reach the dura mater sinuses, the meningeal lymphatics and the cervical lymphnodes. PVS: perivascular space; CSF: cerebrospinal fluid; ISF: interstitial fluid
The GS is involved in the drainage of metabolic waste products such as lactic acid, tau protein, Amyloid-β or α-Synuclein. It plays a pivotal role in the exchange of nutrients (like glucose and lipids), neurotransmitters, and neuroactive substances (such as transretin and apoprotein E) [34, 35]. The flow within the GS could be influenced by factors such as changes in the arteriovenous hydrostatic gradient, vascular vasodilation or vasoconstriction, body position, circadian rhythm, respiration, heart rate and intracranial pressure [26, 36, 37].
Glymphatic dysfunction in migraine
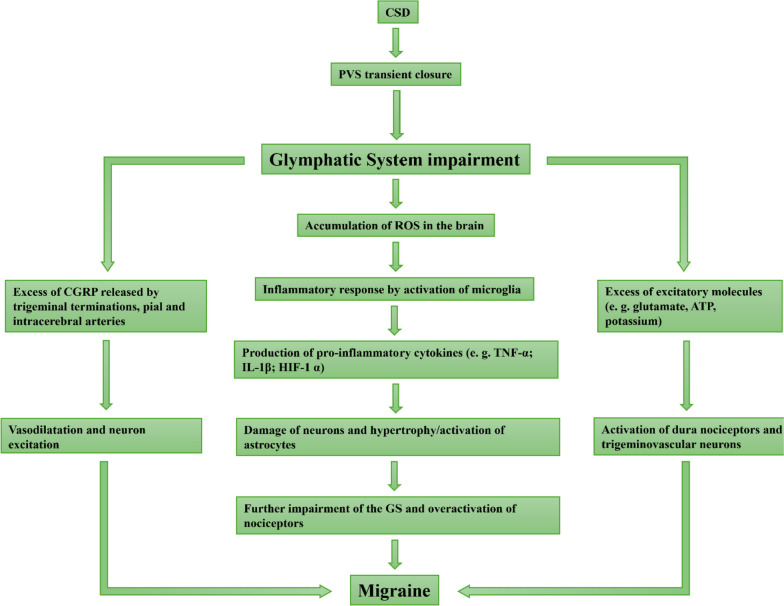
It has been postulated that the GS could contribute to the pathogenesis of migraine. Even though the exact mechanism underlying this relationship remains to be fully elucidated, three main potential mechanisms have been hypotesized: neuroinflammation, calcitonin gene-related peptide (CGRP) dysregulation and cortical spreading depression (CSD) (Fig. 2).
Fig. 2.
Possible pathogenetic mechanisms of migraine involving the glymphatic system dysfunction. CSD can be responsible for a transient PVS closure causing a GS impairment. The GS dysfunction can lead to the accumulation of excitatory, pro-inflammatory and vasodilator molecules involved in the development and the exacerbation of migraine. CSD: cortical spreading depression; PVS: perivascular space; ROS: reactive oxygen species; CGRP: calcitonin gene-related peptide; ATP: adenosine triphosphate; TNF-α: tumor necrosis factor alpha; IL-1β: interleukin 1-beta; HIF-1α: hypoxia-inducible factor 1-alpha
Regarding neuroinflammation, it is known that GS is crucial for removing reactive oxygen species (ROS) [5]. Brain tissue is extremely susceptible to ROS damage due to its high oxygen consumption, lipidic metabolism and poor antioxidants [7]. An excess of ROS in the brain activates the immune response by the microglia production of proinflammatory cytokines such as tumor necrosis factor alfa (TNF α), interleukin 1beta (IL-1β) and HIF-1α [8, 9]. These molecules are released into the extracellular space (ECS) and flow in the glymphatic network. Thus, an impairment of the GS results in the accumulation of proinflammatory cytokines and ROS leading to the degeneration of neurons and to the hypertrophy and activation of astrocytes [10, 11]. The disruption of the astrocytes further aggravates the GS dysfunction and starts a vicious circle of events [12]. Neuroinflammation has been enquired as a possible mechanism linking the GS dysfunction with migraine development. It is known that pro-inflammatory cytokines can exacerbate nociceptive stimuli overactivating neurons and nociceptors [13–16].
CGRP is a key player in the pathogenesis of migraine. It is a neuropeptide produced both in the central and in the peripheral nervous system [17]. CGRP exerts a vasodilator action on blood vessels and acts as a neuron excitability modulator [18]. After its release from trigeminal terminations of meninges, pia mater and intracerebral arteries, CGRP doesn’t cross the blood–brain barrier but could rapidly penetrate the PVS encompassing pial artery [19]. Within the CSF-ISF, it reaches the perivenous space and hence the bigger dura mater sinus. Then, the final step are the lymph nodes of the general lymphatic system [2]. It can been postulated that an impairment of the GS may increase CGRP concentration and thus worsen migraine.
Finally, studies investigated the role of CSD as an additional hypothetic mechanism explaining how the GS dysfunction could lead to the development of migraine [2, 19–23]. CSD is a chemo-electrical excitatory wave propagating across the brain surface. It is supposed to be the major pathophysiological mechanism of migraine aura. When a CSD-wavefront arises, the physiological ions concentration in the CSF changes: and enter into the cells, while flows out of neurons. In physiological conditions, neurons maintain a potential of -70 mV thanks to ions channels and membrane pumps. Depolarization of cells membrane occurs when the relative excess of , adenosine triphosphate and hydrogen ions in the ECS makes their potential achieve the value of approximately -10 mV [19, 21]. CSD can cause a succession of vasoconstriction and vasodilatation of the pial and penetrating arteries, thus modifying the PVS radium and interfering with the normal function of the GS. Moreover, this change in ionic distribution leads to a swelling of neurons whose activity remains temporarily suppressed [20, 21, 23]. At the end of the CSD the excess of is removed both through the CSF and via buffers [20]. Since those evidence is based on studies in the experimental animal, further research is needed to extend these findings to the human [2, 20, 22].
Techniques for investigating the glymphatic system
In animal models, optical imaging techniques, particularly two-photon microscopy, have traditionally held a prominent role in the study of the GS. This is primarily due to their exceptional spatial resolution, which is crucial for capturing tiny PVS. In in vivo studies, after the intracisternal injection of small fluorescent tracers, in anesthetized mice, two-photon microscopy has been used to determine the dynamics and the anatomic structure of the glymphatic flow [31, 37–40]. However, two-photon microscopy doesn’t allow to visualize the deeper regions of brain and for this reason ex vivo studies have been employed to analyze the GS distribution and function in the whole brain or in specific regions [31, 39]. In ex vivo experiments, coronal or sagittal slices of death mice brains injected with fluorescent tracers are visualized under a microscope [41, 42]. To quantitatively determine the fluorescence distribution in the slices an imaging processing is needed: a whole brain slice or a region of interest is chosen and the mean pixel intensity or the coverage area of the fluorescent tracer is manually analyzed in imaging software [31, 41, 43, 44] However, this technique is time consuming and, more important, the fluorescence of the slices doesn’t always faithfully depict the distribution of tracers in the live brains. For these reasons, the use of spectrophotofluorometry on microplate assays is preferred to better quantify the distribution of fluorescent tracers in animal brain slices as a marker of the GS function [39].
MR imaging has been used in animal models to visualize the distribution and to characterize the function of the GS [45, 46]. Iliff et al. used a gadolinium-based contrast agent to describe the GS flow into the brains of living rats [47].
While direct measurement techniques utilizing fluorescence and contrast agent tracers can be used in animal studies, their application in humans is invasive and comes with inherent challenges. Consequently, there is a pressing need to explore alternative noninvasive methods that facilitate the study of the glymphatic system in human subjects [48, 49]. So far, no ideal technique is available to study the GS in humans but several techniques have been employed as tools to provide different type of information on the GS function in humans. Research studies employed MR, positron emission tomography and ultrasound [50–53].
MR offers distinct advantages, including the ability to overcome the limited penetration depth of two-photon microscopy and the capacity to perform whole-brain imaging, in contrast to two-photon microscopy [38, 50]. Several noninvasive MR methodologies offer the opportunity to investigate the dynamics of ISF and CSF flow within the cerebral tissue in human subject: T1 and T2 weighted sequences, T2 fluid-attenuated inversion recovery (FLAIR); PVS imaging; dynamic contrast-enhanced MR imaging (DCE-MRI); diffusion tensor image analysis along the PVS (DTI- ALPS), arterial spin labeling, chemical exchange saturation transfer, and intravoxel incoherent motion [49].
PVS exhibits hyperintensity on T2-weighted imaging, isointensity on proton density weighted imaging and hypointensity on T1 and T2 weighted imaging and FLAIR. The abnormalities of PVS can be detected as ectatic and less regular spaces at MRI. The combination of T1 and T2 weighted imaging, as well as T1 and FLAIR can enhance the sensitivity of PVS identification [34, 54–56].
A common tool to analyze the distribution of PVS in human brains is a visual rating scale based on the Potter scoring which grades PVSs from 0 to 4 according to their numbers in the brain plane calculated in the basal ganglia and in the centrum semiovale on structural brain imaging. Potter scoring also grades midbrain PVSs 0–1 according to their presence or absence [49, 57, 58]. However, this technique is influenced by the experience of observers and by the ceiling effect which affect the inter- and intrareproducibility [49]. For these reasons, Dubost et al. developed an automatic system of grading PVS at MRI in humans [59].
DCE-MRI measures the movement of contrast agents in the ISF within the PVS and brain tissue, eliminating the necessity for intricate modeling or postprocessing techniques. Additionally, intrathecal DCE-MRI has the potential to achieve precise and detailed spatial resolution. The extensive use of DCE-MRI for investigating ISF properties in humans is hampered by several factors. The procedure is invasive, requiring sterile conditions and the expertise of healthcare professionals, it can be uncomfortable for patients and can be biased by limited spatial and temporal resolution of tracers and by movement artifacts [38, 60]. Furthermore, intrathecal injections of gadolinium-based contrast agents may lead to adverse reactions, such as anaphylactic responses, including headache and severe nausea [61], and carry the risk of nephrotoxicity, potentially causing renal failure [62], as well as neurotoxic effects, which may manifest as speech issues, psychotic symptoms, lethargy, and visual impairment [63]. There is also the concern that gadolinium can enter brain tissue through the glymphatic system and deposit in parenchymal tissues, including the dentate nucleus and globus pallidus [64, 65].
DTI-ALPS is a method that utilizes diffusion MR to assess the activity of the glymphatic system by examining the dynamics of ISF within the human brain. This technique entails the analysis of DTI along the PVS, and the outcomes are represented as ALPS scores. When the ALPS index approaches a value of one, it indicates that water diffusion within the PVS has a minimal impact. In contrast, a higher ratio suggests an elevated level of water diffusivity within the PVS. The method proposed for computing the ALPS index using DTI-ALPS is influenced by head rotation, potentially leading to reduced reproducibility and reliability. To address this issue, an additional approach involving DTI reorientation was introduced for ALPS index calculation based on DTI-ALPS. Taoka et al. also proposed a method involving the utilization of diffusion-weighted imaging with a three-axis diffusion gradient direction for the computation of the ALPS index within the framework of diffusion-weighted imaging-ALPS [66].
Arterial spin labeling, chemical exchange saturation transfer and intravoxel incoherent motion are new promising MRI technique that indirect assess the GS function by the analysis of blood–brain barrier permeability, by the estimation of solutes at two order of magnitude lower concentration than traditional MRI and by the evaluation of the diffusion/perfusion effect of blood motion in tiny vessels [49].
Ultra-high field MR imaging, acquired at ≥ 7 T, is a valuable approach to better detect PVS abnormalities in human subjects [67–69]. However, there are some limitations of this technique: ultra-high MRI is more sensitive to movement artifacts, there is a magnetic field dishomogeneity causing a higher difficulty to identify subcortical PVS, there are issues about the radiofrequency absorption rate and a more limited compatibility of medical devices with magnetic coils [34].
Positron emission tomography and ultrasound imaging are two further noninvasive imaging techniques which could be used to investigate the GS [38, 52, 53].
Further details are shown in Table 1.
Table 1.
Investigation of the glymphatic system: different imaging techniques with their advantages and disadvantages
| Investigation tool | Technique | Pros | Cons | |
|---|---|---|---|---|
| Imaging | Optical imaging | Two-photon microscopy | Tracking tracers with high spatial and temporal resolution |
Limited field of view Invasive technique |
| MRI | FLAIR | Facilitates the visualization of perivascular spaces and the assessment of glymphatic function | Low specificity | |
| CE-MRI and DCE-MRI |
Noninvasive technique Whole brain images 3D visualization |
Low spatial and temporal resolution Movement artifacts |
||
| DTI-ALPS | Quantify the diffusion of water molecules within the brain's interstitial space, providing valuable insights into glymphatic function | Low specificity | ||
| Arterial spin labeling, chemical exchange saturation transfer, intravoxel incoherent motion | Blood brain barrier permeability, assess of solutes concentration at two order of magnitude lower than traditional MRI, diffusion/perfusion effect evaluation of blood motion |
Indirect measurements of the glymphatic system function Need of integration with other techniques |
||
| ULTRA-HIGH MRI | Better detection of PVS abnormalities | Movement artifacts, dishomogeneity in magnetic field, difficulty to identify subcortical PVS, radiofrequency absorption rate and lower compatibility of medical devices | ||
| Positron emission tomography | Use of radiolabeled tracers |
Whole brain images Quantify the glymphatic system clearence |
Low spatial resolution Movement artifacts |
|
| Ultrasound Imaging | Transcranial Doppler ultrasound | Study glymphatic pulsations and CSF dynamics in humans | Low specificity | |
Abbreviations: MRI Magnetic Resonance Imaging, FLAIR Fluid-Attenuated Inversion Recovery, CE/DCE MRI Contrast Enhanced/Dynamic Contrast Enhanced Magnetic Resonance Imaging, DTI Diffusion Tensor Imaging, PVS perivascular space, CSF Cerebrospinal fluid
Animal models
There are some studies aimed to elucidate the way in which the GS could be involved in the pathophysiology of migraine in the animal model of this disease [37, 70].
Huang et al. used a nitroglycerin (NTG)-induced migraine mouse model to clarify whether the GS dysfunction is a trigger or an aggravating factor of migraine [70]. The NTG administration in adult mice determined a decrease in the mechanical pain threshold and the stimulation of meningeal nociceptors by the release of nitric oxide, when compared with healthy controls. Using immunofluorescence, Huang et al. demonstrated that there is a reduction in the AQP4 expression in the PVS in mice with NTG induced migraine, especially in those simultaneously treated with an AQP4 inhibitor (TGN-020). The changes in AQP4 distribution led to a massive release of CGRP [70]. Immunofluorescence in NTG-induced migraine models further revealed that TGN-020 administration determined an increased expression of c-Fos as a marker of increased neuronal activity as well as an increase in astrocytes and microglia activation as a marker of neuroinflammation in the medullary dorsal horn. NTG-induced migraine models simultaneously treated with TGN-020 showed a reduction in the distribution of a tracer (TR-d3) when it is injected in the cisterna magna. Taken together these results suggest that the GS dysfunction is an aggravator factor rather than a trigger mechanism of migraine [70].
Schain et al. used an in vivo two-photon imaging technique to determine whether CSD alters the function of the GS in mice models [37]. Using dying tracers they demonstrated that PVS system is a wide, cleansing network encompassing both superficial and penetrating vessels (arteries and veins) and that it is bordered by endothelium, pia mater and brain tissue. PVS diameter is influenced by anatomy: it is larger in case of multiple vessels and of vessels bifurcations. Orthogonal reconstruction of superficial vessels was used to quantify the tridimensional rate between PVS width, vessel lumen and subarachnoid space. This technique was less efficient when used to determine PVS diameter in penetrating vessels. Inducing CSD by pinprick in the brain cortex of non-injected mice, Schain et al. observed that CSD causes an initial constriction of superficial vessels (arteries and veins), followed by a dilatation at 3 min from the beginning of the stimulation and by a final constriction that lasts for about 22 min. CSD causes the complete closure of PVS at 6 min after its induction. Thereafter, PVS slowly reopens but remains partially closed for about 30 min. It still remains unknown the exact mechanism underlying the closure of PVS during CSD but some studies hypothesize that it depends on the swelling of neurons and astrocytic endfeet during CSD [71, 72]. After the injection of tracers, Schain et al. demonstrated that they accumulate into the PVS after the arrival of CSD wavefront and that the glymphatic flow is delayed and slowed [37]. This confirms the hypothesis that CSD causes the storage of excitatory and neuroinflammatory chemicals such as glutamate [73], ATP [74] and potassium [75, 76] in the PVS. At the end of CSD, when PVS reopens, all the excitatory molecules reach and activate the dura nociceptors and central trigeminovascular neurons [77, 78]. This sequence of events sems also to explain the delayed onset of pain in patients suffering from migraine with aura [37].
In summary, experimental studies investigating the glymphatic system in migraine are so far limited. Available data indicate that the GS dysfunction acts more consistently as an aggravator rather than as a causal factor of migraine. Furthermore, it has been demonstrated that a transient closure of the PVS is involved in the development of CSD, the most investigated pathogenetic mechanism of migraine aura, by the accumulation of pro-inflammatory and irritative molecules. These molecules than contribute to the activation of the trigeminovascular nociceptors determining headache pain.
Human findings
Few studies have investigated the role of the GS dysfunction in migraine in humans [2, 79–94].
A recent pilot study using the DTI-ALPS index compared healthy controls with people with migraine, both with and without aura. It demonstrated that there is not a significant difference in the DTI-ALPS index, as a marker of the GS dysfunction, in the two examined groups and also between individuals with and without aura. These findings suggest a weak engagement of the GS impairment in the pathophysiology of migraine, but further research is needed to confirm these observations [80].
Another possible marker of the GS dysfunction are the enlarged perivascular spaces at MRI. Using a 3 T MRI technique, Yuan et al. investigated the correlation between enlarged perivascular spaces, as a marker of GS dysfunction, and migraine in three groups: healthy controls, episodic migraine and chronic migraine. They showed that an increase in the PVS width, especially in the centrum semiovale and in the midbrain, is an independent predictor factor of migraine. In this same study GS dysfunction was not associated with the clinical manifestation and the chronification of migraine [2].
To evaluate whether the GS activity changes during chronification of migraine, Zhang et al. used the DTI-ALPS index on a cohort of people with CM. They compared the results obtained in the CM group with those emerged from healthy controls and from episodic migraine group. During migraine chronification, the DTI-ALPS score is improved rather than diminished [81]. The raise of the DTI-ALPS index in CM seems to be related to the alteration of vascular reactivity induced by the prolonged release of CGRP during each migraine attack. CGRP, in fact, causes a central sensitization thought to be the mechanism underlying migraine chronification. Furthermore, Zhang et al. demonstrated that the improvement of the DTI-ALPS index lateralized on the right hemisphere in CM [81], confirming the results of previous studies investigating the lateralized manifestation of headaches [82–86]. Functional MR imaging studies hypothesized that the predominance of the right hemisphere dysfunction in headaches disorders could depend on abnormal connections between the right thalamus and some ipsilateral cortical regions involved in the regulation of pain (primary somatosensory cortex and premotor cortex) [85]. MR spectroscopy further confirmed that the right thalamus of migraineurs has increased levels of glutamate and glutamine [86]. These findings suggest that in CM there is an improvement of the GS activity. Zhang et al. hypothesized that the GS overactivation during migraine chronification could be a concomitant phenomenon of the vascular reactivity induced by an accumulation of CGRP in the interictal period starting the mechanism of central sensitization. However, this study has some limitations: first, the number of participants was too small to extend the results to the whole population of individuals with CM; second, the DTI-ALPS score, as a marker of the GS activity, is commonly calculated on slices of the lateral ventricle body and so it represents partial function of the entire GS; third, data on the CGRP levels in the brain of the participants were not collected. For these reasons, further researches are needed to validate these findings [81]. More recently, Wu et al. study used MRI techniques to establish whether the GS and the meningeal lymphatic vessels function are altered in people with chronic migraine (both with and without analgesic medical overuse) and episodic migraine, compared with healthy controls. They demonstrated a negative correlation between the DTI-ALPS index in chronic migraine, especially in people with a medical overuse, rather the in episodic migraine or in healthy control, which suggest a dysfunction of the GS in chronic migraine. Furthermore, they observed a negative correlation also between the DTI-ALPS and migraine disability, especially when migraine attacks frequence was > 4 per month. Additionally, they observed that a negative correlation exists also between the DCE-MRI values of time to peak, mean time to enhance, enhancement integral and chronic migraine, suggesting an impairment of the meningeal lymphatic system in chronic migraine [87].
Some studies have provided evidence that cerebral small vessel disease may be associated with a dysfunction of the GS [88]. Following these findings, Ornello et al. hypothesized that GS dysfunction may contribute to the development of white matter hyperintensities (WMHs) in people with migraine [89, 90]. WMHs are a common finding at MR in individuals with migraine but their nature is still unclear [88, 91]. It is supposed that WMHs represent an expression of subtle ischemic suffering of brain tissue caused by an impairment of normal perivascular outflow of CSF-ISF which, in turn, leads to an accumulation of waste products into the ECS [92]. It has been suggested that CSD, the surrogate of migraine aura could determine, through the vasoconstriction of pial and penetrating arteries, a spreading ischemia and the appearance of WMHs at MR [93]. For these reasons, it could be postulated that the WMHs in patients suffering from migraine represent a consequence of the dysfunction of the GS [94]. To evaluate whether WMHs in migraineurs are associated with the GS dysfunction, Ornello et al. used the DTI-ALPS index. Using this technique, the authors did not find that GS dysfunction was associated with WMHs in patients with migraine [94].
To summarize, human studies came with conflictual data regarding the involvement of the GS dysfunction in migraine pathogenesis, especially in people suffering from chronic migraine. Some limitations occurred in the studies design making results not generalizable to the whole population of individuals with migraine. For these reasons, further research is needed to fully elucidate the role of the GS in migraine development as well as to define new techniques of investigating the GS changes in humans suffering from migraine. To move the field forward it would be important to investigate well defined populations, harmonizing methods and possibly using multicenter study design.
Other headache disorders and the glymphatic system
Several studies have reported that there is an association between sleep disturbances and impairment of the GS. Since sleep disturbances are common in migraine and may be present also in other headache disorders it can be postulated that there is an interconnection among all those conditions. In recent years, it has been pointed out the possible bidirectional involvement of sleep abnormalities and GS [95–99]. Animal models demonstrate that the flow into the GS is facilitated during the sleep, especially during the deep sleep [96, 97]. Thus, sleep disturbances can diminish the efficacy of the GS in recycling metabolic waste products, resulting in a greater likelihood of developing migraine and dementia [95, 98] (Fig. 3). Furthermore, awakening causes a greater production of norephinepfrine which reduces the interstitial space and determines an accumulation of molecules involved in the pathogenesis of vary neurological diseases [99]. GS disfunction can also lead to the accumulation of orexin (both A and B) in the brain tissue, especially in the dorsal raphe and in the locus coeruleus causing sleep fragmentation and inefficiency [95].
Fig. 3.
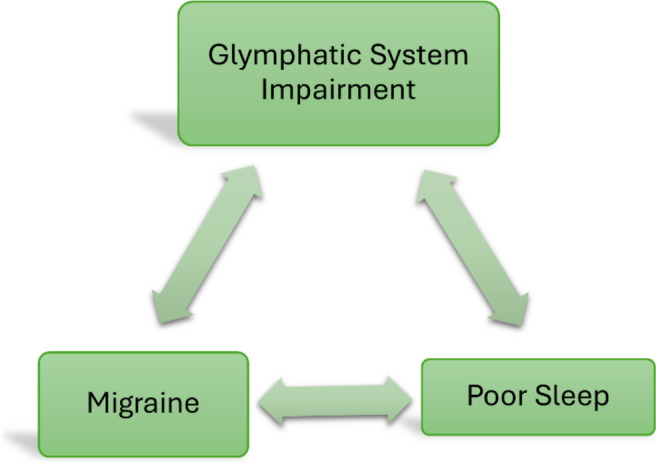
Relationship between glymphatic system impairment, sleep disturbance and headache. Poor sleep can determine an impairment in the glymphatic flow which, in turn, leads to the accumulation of neuroexcitatory and pro-inflammatory chemicals involved in the development of headache. Headache itself can, directly or indirectly (via a dysfunction of the glymphatic system) exacerbate sleep disturbances
Cluster headache (CH) is considered the most painful type of headache [35] and according to the ICHD-3rd edition it is defined as a strictly unilateral orbital, supraorbital or temporal pain that lasts for about 15–80 min and occurs for 1–8 times per day. It is associated with autonomic ipsilateral manifestations such as tearing, conjunctival injection, eyelid edema, excessive sweating, rhinorrhea, miosis, ptosis and agitation [6]. Kim et al. investigated the possible association between GS impairment and CH, focusing on the role of sleep disturbances and ageing. Using the DTI-ALPS index they demonstrated that patients with CH show a decreased activity of the GS and they identified a negative correlation between the DTI-ALPS index reduction in CH and age. These findings confirm the existence of a bidirectional link between CH and sleep disturbances in aged brains: ageing reduces the total time spent in deep sleep causing a decreased removal of waste products from the brain, included those involved in the development of CH; on the other hand, CH causes sleep fragmentation and the accumulation of molecules involved in different neurological diseases (e.g. β-amyloid, tau protein, pro-inflammatory cytokines) [24, 25, 100–103].
The GS inefficiency has been correlated also with the development of idiopathic intracranial hypertension (IIH) [104]. It represents an augmentation of the normal intracranial pressure (ICP) that, in normal conditions, is determined by the balance of three compounds: CSF, brain tissue and blood vasculature. Considering that the skull is a fixed volume, the relative excess of one of these three compounds is sufficient to cause an increment in the intracranial pressure, determining symptoms like nausea, vomiting, tinnitus, headache, and visual impairment. When the glymphatic flow is diminished, an accumulation of CSF into brain tissue and nerves sheaths (particularly in the optic nerve) happens and leads to the typical radiological signs of IIH: congestion of the GS and overflow of CSF into the lymphatic pathway [104–106]. The third radiological sign of IIH, the venous stenosis, both intrinsic and extrinsic [107], is typical in symptomatic patients with papilledema and increased ICP. Particularly, transverse sinus stenosis determines an impairment of the CSF outflow via the venous system. Thus, CSF drainage, as well as ICP, become largely dependent on the efficiency of the glymphatic pathway. For these reasons, ICP in IIH could be extremely variable and this variability could explain why some individuals with chronic migraine, also vestibular migraine, and isolated tinnitus could show radiological signs of IIH without meeting the diagnostic criteria for IIH (Dandy’s criteria), based on the presence of high ICP [108, 109]. Vice versa, increased ICP and papilledema could represent a severe stage of IIH [104]. These observations suggest the need to upgrade Dandy’s criteria for the diagnosis of IIH, introducing the radiological signs as diagnostic criteria and reducing the importance of increased ICP [104].
Traumatic brain injury is frequently associated with the development of post-traumatic headache and chronic migraine via an impairment of the GS [95]. Studies reported that traumatic brain injury causes a regional brain damage that leads to a disruption of the GS with the consequently accumulation of solutes and neurotoxic molecules, as far as excitatory products that feedforward the headache [110].
Conclusions
The study of the glymphatic system is giving more insights on the mechanisms underlying several neurological disorders and particularly sleep, neuroinflammatory and neurodegenerative diseases. Despite a rationale and experimental studies supporting a possible involvement of the glymphatic system in migraine and in other headache disorders so far no conclusions in this regard can be reached. The human studies investigating the glymphatic system in migraine and in other headache disorders are limited and mostly lead to negative results. To move the filed forwards, further improvements in techniques to investigate the glymphatic system in vivo in humans are needed as well as proper designed studies that consider possible confounders and contributing factors.
Acknowledgements
This work was supported by the School of Advanced Studies of the European Headache Federation (EHF-SAS).
Abbreviations
- GS
Glymphatic system
- PVS
Perivascular space
- CSF
Cerebrospinal fluid
- ISF
Interstitial fluid
- AQP4
Aquaporin 4
- MRI
Magnetic resonance imaging
- FLAIR
Fluid attenuated inversion recovery
- DCE-MRI
Dynamic contrast enhancement magnetic resonance imaging
- DTI-ALPS
Diffusion Tensor Imaging Along the Perivascular Space
- ROS
Reactive oxygen species
- TNF-α
Tumor Necrosis Factor alpha
- IL-1β
Interleukin 1-beta
- HIF-1α
Hypoxia-inducible factor 1-alpha
- CGRP
Calcitonin Gene-related Peptide
- CSD
Cortical spreading depression
- NTG
Nitroglycerin
- ATP
Adenosine triphosphate
- WMH
White matter hyperintensity
- CH
Cluster headache
- IIH
Idiopatic intracranial hypertension
- ICP
Intracranial pressure
Authors’ contributions
All Authors equally contributed to the review.
Funding
Not applicable.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Christian Lampl is a consultant or scientific advisor for Novartis and Teva. Simona Sacco reports personal fees as speaker or advisor from Abbott, Allergan-Abbvie, AstraZeneca, Boheringer, Eli Lilly, Lundbeck, Novartis, NovoNordisk, Pfizer, Teva and research grants from Novartis and Uriach. Other authors have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edvinsson L, Villalón CM, MaassenVanDenBrink A. Basic mechanisms of migraine and its acute treatment. Pharmacol Ther. 2012;136(3):319–333. doi: 10.1016/j.pharmthera.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Yuan Z, Li W, Tang H, Mei Y, Qiu D, Zhang M, et al. Enlarged perivascular spaces in patients with migraine: a case–control study based on 3T MRI. Ann Clin Transl Neurol. 2023;10(7):1160–1169. doi: 10.1002/acn3.51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, et al. Migraine: epidemiology and systems of care. Lancet. 2021;397(10283):1485–1495. doi: 10.1016/S0140-6736(20)32160-7. [DOI] [PubMed] [Google Scholar]
- 4.Steiner TJ, Stovner LJ. Global epidemiology of migraine and its implications for public health and health policy. Nat Rev Neurol. 2023;19(2):109–117. doi: 10.1038/s41582-022-00763-1. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Wu G, Tang N, Li L, Liu C, Wang F, Ke S. Glymphatic drainage blocking aggravates brain edema, neuroinflammation via modulating TNF-α, IL-10, and AQP4 after intracerebral hemorrhage in rats. Front Cell Neurosci. 2021;15:784154. doi: 10.3389/fncel.2021.784154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(2018) Headache Classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 38(1):1–211 [DOI] [PubMed]
- 7.Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Kepp O, Galluzzi L, Zitvogel L, Kroemer G. Pyroptosis-A cell death modality of its kind? Eur J Immunol. 2010;40:627–630. doi: 10.1002/eji.200940160. [DOI] [PubMed] [Google Scholar]
- 9.Rainville JR, Hode GE. Inflaming sex differences in mood disorders. Neuropsychopharmacology. 2019;44:184–199. doi: 10.1038/s41386-018-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue JH, Yanamoto H, Nakajo Y, Tohnai N, Nakano Y, Hori T, Iihara K, Miyamoto S. Induced spreading depression evokes cell division of astrocytes in the subpial zone, generating neural precursor-like cells and new immature neurons in the adult cerebral cortex. Stroke. 2009;40:e606–613. doi: 10.1161/STROKEAHA.109.560334. [DOI] [PubMed] [Google Scholar]
- 11.Sukhotinsky I, Dilekoz E, Wang Y, Qin T, Eikermann-Haerter K, Waeber C, Ayata C. Chronic daily cortical spreading depressions suppress spreading depression susceptibility. Cephalalgia. 2011;31:1601–1608. doi: 10.1177/0333102411425865. [DOI] [PubMed] [Google Scholar]
- 12.Gu S, Li Y, Jiang Y, Huang JH, Wang F. Glymphatic dysfunction induced oxidative stress and neuro-inflammation in major depression disorders. Antioxidants (Basel) 2022;11(11):2296. doi: 10.3390/antiox11112296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factoralpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011;152:140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W, Long T, Pan Q, Zhang S, Zhang Y, Zhang D, Qin G, Chen L, et al. Microglial NLRP3 inflammasome activation mediates IL-1beta release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J Neuroinflammation. 2019;16:78. doi: 10.1186/s12974-019-1459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuraiaiyah J, Erritzoe-Jervild M, Al-Khazali HM, Schytz HW, Younis S. The role of cytokines in migraine: a systematic review. Cephalalgia. 2022;42(14):1565–1588. doi: 10.1177/03331024221118924. [DOI] [PubMed] [Google Scholar]
- 16.Spekker E, Tanaka M, Szabo A, Vecsei L. Neurogenic inflammation: the participant in migraine and recent advancements in translational research. Biomedicines. 2021;10(1):76. doi: 10.3390/biomedicines10010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59:659–681. doi: 10.1111/head.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 19.Yi T, Gao P, Zhu T, Yin H, Jin S. Glymphatic system dysfunction: a novel mediator of sleep disorders and headaches. Front Neurol. 2022;13:885020. doi: 10.3389/fneur.2022.885020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee S, Mirzaee M, Tithof J. Quantifying the relationship between spreading depolarization and perivascular cerebrospinal fluid flow. Sci Rep. 2023;13:12405. doi: 10.1038/s41598-023-38938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Lee DA, Lee HJ, Park BS, Ko J, Park SH. Glymphatic system dysfunction in patients with cluster headache. Brain Behav. 2022;12:e2631. doi: 10.1002/brb3.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9:637–644. doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- 23.Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci. 2017;37:2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciurea AV, Mohan AG, Covache-Busuioc RA, Costin HP, Saceleanu VM. The Brain’s glymphatic system: drawing new perspectives in neuroscience. Brain Sci. 2023;13:1005. doi: 10.3390/brainsci13071005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis S. Structure and function of the lymphatic system: an overview. Br J Community Nurs. 2006;11:S4–S6. doi: 10.12968/bjcn.2006.11.Sup2.20841. [DOI] [PubMed] [Google Scholar]
- 26.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci USA. 2002;99(16):10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 28.Rennels M, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 29.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat Rev Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldred AR, Brack CM, Schreiber G. The cerebral expression of plasma protein genes in different species. Comp Biochem Physiol B Biochem Mol Biol. 1995;111:1–15. doi: 10.1016/0305-0491(94)00229-N. [DOI] [PubMed] [Google Scholar]
- 31.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amy- loid b. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adigun OO, Al-Dhahir MA. StatPearls. Treasure Island: StatPearls Publishing; 2023. Anatomy, head and neck: cerebrospinal fluid. [PubMed] [Google Scholar]
- 34.Gouveia-Freitas K, Bastos-Leite AJ. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021;63:1581–1597. doi: 10.1007/s00234-021-02718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kassem NA, Deane R, Segal MB, Preston JE. Role of transthyretin in thyroxine transfer from cerebrospinal fluid to brain and choroid plexus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1310–R1315. doi: 10.1152/ajpregu.00789.2005. [DOI] [PubMed] [Google Scholar]
- 36.Asgari M, de Zélicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Chen D, Liu N, Luan Y, Zhu S, Wang H. Modulation of lymphatic transport in the central nervous system. Theranostics. 2022;12(3):1117–1131. doi: 10.7150/thno.66026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohr T, Hjorth PG, Holst SC, Hrabětová S, Kiviniemi V, Lilius T, et al. The glymphatic system: current understanding and modeling. iScience. 2022;29:104987. doi: 10.1016/j.isci.2022.104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Song J, He XZ, Xiong J, Xue R, Ge GH, et al. Quantitative determination of glymphatic flow using spectrophotofluorometry. Neurosci Bull. 2020;36(12):1524–1537. doi: 10.1007/s12264-020-00548-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai JK, Wang SX, Shan D, Niu HC, Lei H. Super-resolution track-density imaging reveals fine anatomical features in tree shrew primary visual cortex and hippocampus. Neurosci Bull. 2018;34:438–448. doi: 10.1007/s12264-017-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kress BT, Iliff JJ, Xia MS, Wang MH, Wei HLS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, et al. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol. 2018;596:445–475. doi: 10.1113/JP275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei F, Song J, Zhang C, Lin J, Xue R, Shan LD, et al. Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology. 2019;236(4):1367–1384. doi: 10.1007/s00213-018-5147-6. [DOI] [PubMed] [Google Scholar]
- 44.Wei F, Zhang C, Xue R, Shan L, Gong S, Wang G, et al. The pathway of subarachnoid CSF moving into the spinal parenchyma and the role of astrocytic aquaporin-4 in this process. Life Sci. 2017;182:29–40. doi: 10.1016/j.lfs.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Jiang Q. MRI and glymphatic system. Stroke Vasc Neurol. 2019;4:75–77. doi: 10.1136/svn-2018-000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taoka T, Naganawa S. Glymphatic imaging using MRI. J Magn Reson Imaging. 2020;51:11–24. doi: 10.1002/jmri.26892. [DOI] [PubMed] [Google Scholar]
- 47.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brainwide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Li Li X, B, Glymphatic system dysfunction in central nervous system diseases and mood disorders. Front Aging Neurosci. 2022;14:873697. doi: 10.3389/fnagi.2022.873697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamagata K, Saito Y, Andica C, Uchida W, Takabayashi K, Yoshida S et al (2023) Noninvasive magnetic resonance imaging measures of glymphatic system activity. J Magn Reson Imaging. 10.1002/jmri.28977. Online ahead of print [DOI] [PubMed]
- 50.Kaur J, Davoodi-Bojd E, Fahmy LM, Zhang L, Ding G, Hu J, et al. Magnetic resonance imaging and modeling of the glymphatic system. Diagnostics (Basel) 2020;10(6):344. doi: 10.3390/diagnostics10060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Leon MJ, Li Y, Okamura N, Tsui WH, Saint-Louis LA, Glodzik L, et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J Nucl Med. 2017;58:1471–1476. doi: 10.2967/jnumed.116.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DS, Suh M, Sarker A, Choi Y. Brain glymphatic/lymphatic imaging by MRI and PET. Nucl Med Mol Imaging. 2020;54(5):207–223. doi: 10.1007/s13139-020-00665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plog BA, Mestre H, Olveda GE, Sweeney AM, Kenney HM, Cove A, et al. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight. 2018;3(23):e126138. doi: 10.1172/jci.insight.126138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Lin Z, Liu C, Bai R, Wu D, Yang J (2023) Glymphatic imaging in pediatrics. J Magn Reson Imaging [DOI] [PubMed]
- 55.Sepehrband F, Barisano G, Sheikh-Bahaei N, Cabeen RP, Choupan J, Law M, Toga AW. Image processing approaches to enhance perivascular space visibility and quantification using MRI. Sci Rep. 2019;9(1):12351. doi: 10.1038/s41598-019-48910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boespflug EL, Schwartz DL, Lahna D, Pollock J, Iliff JJ, Kaye JA, Rooney W, Silbert LC. MR imaging-based multimodal autoidentification of perivascular spaces (mMAPS): automated morphologic segmentation of enlarged perivascular spaces at clinical field strength. Radiology. 2018;286(2):632–642. doi: 10.1148/radiol.2017170205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39(3–4):224–231. doi: 10.1159/000375153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Wang M, Luan M, Song X, Wang Y, Xu LI. Enlarged perivascular spaces and age-related clinical diseases. Clin Interv Aging. 2023;18:855–867. doi: 10.2147/CIA.S404908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubost F, Adams H, Bortsova G, et al. 3D regression neural network for the quantification of enlarged perivascular spaces in brain MRI. Med Image Anal. 2019;51:89–100. doi: 10.1016/j.media.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Drenthen GS, Elschot EP, van der Knaap N, Uher D, Voorter PHM, Backes WH et al (2023) Imaging interstitial fluid with MRI: a narrative review on the associations of altered interstitial fluid with vascular and neurodegenerative abnormalities. J Magn Reson Imaging [DOI] [PubMed]
- 61.Steward CE, Venkatraman VK, Lui E, Malpas CB, Ellis KA, Cyarto EV, et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J Neuroimaging. 2021;31:569–578. doi: 10.1111/jon.12837. [DOI] [PubMed] [Google Scholar]
- 62.Othersen JB, Maize JC, Woolson RF, Budisavljevic MN. Nephrogenic systemic fibrosis after exposure to gadolinium in patients with renal failure. Nephrol Dial Transplant. 2007;22:3179–3185. doi: 10.1093/ndt/gfm584. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Gao FQ, Zhang B, Luo BN, Yang ZY, Zhao J. Overdosage of intrathecal gadolinium and neurological response. Clin Radiol. 2008;63:1063–1068. doi: 10.1016/j.crad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16:137–153. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 65.Ohashi T, Naganawa S, Iwata S, Kuno K. Age-related changes in the distribution of intravenously administered gadolinium-based contrast agents leaked into the cerebrospinal fluid in patients with suspected endolymphatic hydrops. Jpn J Radiol. 2021;39:433–441. doi: 10.1007/s11604-020-01079-0. [DOI] [PubMed] [Google Scholar]
- 66.Taoka T, Ito R, Nakamichi R, Nakane T, Sakai M, Ichikawa K, et al. Diffusion-weighted image analysis along the perivascular space (DWI–ALPS) for evaluating interstitial fluid status: age dependence in normal subjects. Jpn J Radiol. 2022;40:894–902. doi: 10.1007/s11604-022-01275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab. 2016;36(3):576–580. doi: 10.1177/0271678X15620434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai K, Tain R, Das S, Damen FC, Sui Y, Valyi-Nagy T, Elliott MA, Zhou XJ. The feasibility of quantitative MRI of perivascular spaces at 7T. J Neurosci Methods. 2015;256:151–156. doi: 10.1016/j.jneumeth.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barisano G, Law M, Custer RM, Toga AW, Sepehrband F. Perivascular space imaging at ultrahigh field MR imaging. Magn Reson Imaging Clin N Am. 2021;29(1):67–75. doi: 10.1016/j.mric.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W, Zhang Y, Zhou Y, Zong J, Qiu T, Hu L, et al. Glymphatic dysfunction in migraine mice model. Neuroscience. 2023;15(528):64–74. doi: 10.1016/j.neuroscience.2023.07.027. [DOI] [PubMed] [Google Scholar]
- 71.Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 72.Tomita M, Tomita Y, Unekawa M, Toriumi H, Suzuki N. Oscillating neuro-capillary coupling during cortical spreading depression as observed by tracking of FITC-labeled RBCs in single capillaries. Neuroimage. 2011;56:1001–1010. doi: 10.1016/j.neuroimage.2011.02.078. [DOI] [PubMed] [Google Scholar]
- 73.Molchanova S, Kӧӧbi P, Oja SS, Saransaari P. Interstitial concentrations of amino acids in the rat striatum during global forebrain ischemia and potassium-evoked spreading depression. Neurochem Res. 2004;29:1519–1527. doi: 10.1023/B:NERE.0000029564.98905.5c. [DOI] [PubMed] [Google Scholar]
- 74.Schock SC, Munyao N, Yakubchyk Y, Sabourin LA, Hakim AM, Ventureyra EC, Thompson CS. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res. 2007;1168:129–138. doi: 10.1016/j.brainres.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 75.Vyskocil F, Kritz N, Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 1972;39:255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]
- 76.Ayata C, LauritzenM, Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev. 2015;95:953–993. doi: 10.1152/physrev.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toriello M, Gonzalez-Quintanilla V, Perez-Pereda S, Fontanillas N, Pascual J. The potential role of the glymphatic system in headache disorders. Pain Med. 2021;22(12):3098–3100. doi: 10.1093/pm/pnab137. [DOI] [PubMed] [Google Scholar]
- 80.Lee DA, Lee HJ, Park KM. Normal glymphatic system function in patients with migraine: a pilot study. Headache. 2022;62:718–725. doi: 10.1111/head.14320. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X, Wang W, Bai X, Zhang X, Yuan Z, Jiao B. Increased glymphatic system activity in migraine chronification by diffusion tensor image analysis along the perivascular space. J Headache Pain. 2023;24(1):147. doi: 10.1186/s10194-023-01673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang D, Huang X, Mao C, Chen Y, Miao Z, Liu C, et al. Assessment of normalized cerebral blood flow and its connectivity with migraines without aura during interictal periods by arterial spin labeling. J Headache Pain. 2021;22(1):72–43. doi: 10.1186/s10194-021-01282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michels L, Villanueva J, O’Gorman R, Muthuraman M, Koirala N, Büchler R, et al. Interictal hyperperfusion in the higher visual cortex in patients with episodic migraine. Headache. 2019;59(10):1808–1820. doi: 10.1111/head.13646. [DOI] [PubMed] [Google Scholar]
- 84.De Benedittis G. Headache lateralization and functional cerebral asymmetry: a task-related EEG power spectrum analysis. J Neurosurg Sci. 1987;31(3):109–119. [PubMed] [Google Scholar]
- 85.Amin FM, Hougaard A, Magon S, Sprenger T, Wolfram F, Rostrup E, et al. Altered thalamic connectivity during spontaneous attacks of migraine without aura: a resting-state fMRI study. Cephalalgia. 2018;38(7):1237–1244. doi: 10.1177/0333102417729113. [DOI] [PubMed] [Google Scholar]
- 86.Bathel A, Schweizer L, Stude P, Glaubitz B, Wulms N, Delice S, et al. Increased thalamic glutamate/glutamine levels in migraineurs. J Headache Pain. 2018;19(1):55. doi: 10.1186/s10194-018-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu CH, Chang FC, Wang YF, Lirng JF, Wu HM et al (2023) Impaired glymphatic and meningeal lymphatic functions in patients with chronic migraine. Ann Neurol [DOI] [PubMed]
- 88.Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257. doi: 10.1016/j.neuroimage.2021.118257. [DOI] [PubMed] [Google Scholar]
- 89.Kruit M, van Buchem M, Launer L, Terwindt G, Ferrari M. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia. 2010;30(2):129–136. doi: 10.1111/j.1468-2982.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamedani AG, Rose KM, Peterlin BL, Mosley TH, Coker LH, Jack CR, et al. Migraine and white matter hyperintensities: the ARIC MRI study. Neurology. 2013;81(15):1308–1313. doi: 10.1212/WNL.0b013e3182a8235b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013;81(14):1260–1268. doi: 10.1212/WNL.0b013e3182a6cb32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blair GW, Thrippleton MJ, Shi Y, Hamilton I, Stringer M, Chappell F, et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology. 2020;94(21):e2258–e2269. doi: 10.1212/WNL.0000000000009483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86(4):902–922. doi: 10.1016/j.neuron.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Ornello R, Bruno F, Frattale I, Curcio G, Pistoia F, Splendiani A, Sacco S. White matter hyperintensities in migraine are not mediated by a dysfunction of the glymphatic system—a diffusion tensor imaging magnetic resonance imaging study. Headache. 2023;63:1128–1134. doi: 10.1111/head.14607. [DOI] [PubMed] [Google Scholar]
- 95.Christensen J, Yamakawa GR, Shultz SR, Mychasiuk R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog Neurobiol. 2021;198:101917. doi: 10.1016/j.pneurobio.2020.101917. [DOI] [PubMed] [Google Scholar]
- 96.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vgontzas A, Pavlović JM. Sleep disorders and migraine: review of literature and potential pathophysiology mechanisms. Headache. 2018;58(7):1030–1039. doi: 10.1111/head.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komaroff AL. Does sleep flush wastes from the brain? JAMA. 2021;325(21):2153–2155. doi: 10.1001/jama.2021.5631. [DOI] [PubMed] [Google Scholar]
- 99.Goldman N, Hablitz LM, Mori Y, Nedergaard M. The glymphatic system and pain. Med Acupunct. 2020;32(6):373–376. doi: 10.1089/acu.2020.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–178. doi: 10.1007/s11604-017-0617-z. [DOI] [PubMed] [Google Scholar]
- 102.Shen T, Yue Y, Ba F, He T, Tang X, Hu X, et al. Diffusion along perivascular spaces as marker for impairment of glymphatic system in Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):174. doi: 10.1038/s41531-022-00437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlatedwith high EEG delta power and low heart rate inmice under anesthesia. Sci Adv. 2019;5(2):eaav5447. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicholson P, Kedra A, Shotar E, Bonnin S, Boch AL, Shor N. Idiopathic intracranial hypertension: glymphedema of the brain. J Neuro-Ophthalmol. 2021;41:93–97. doi: 10.1097/WNO.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 105.Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 2015;35:400–411. doi: 10.1097/WNO.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 106.Maralani PJ, Hassanlou M, Torres C, Chakraborty S, Kingstone M, Patel V, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012;67:656–663. doi: 10.1016/j.crad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Lenck S, Vallee F, Labeyrie MA, Touitou V, Saint-Maurice JP, Guillonnet A, et al. Stenting of the lateral sinus in idiopathic intracranial hypertension according to the type of stenosis. Neurosurgery. 2017;80:393–400. doi: 10.1227/NEU.0000000000001261. [DOI] [PubMed] [Google Scholar]
- 108.De Simone R, Ranieri A, Montella S, Erro R, Fiorillo C, Bonavita V. Sinus venous stenosis-associated IIHWOP is a powerful risk factor for progression and refractoriness of pain in primary headache patients: a review of supporting evidences. Neurol Sci. 2011;32(suppl 1):S169–S171. doi: 10.1007/s10072-011-0536-1. [DOI] [PubMed] [Google Scholar]
- 109.Mamikoglu B, Algın O, Mengü G, Erdogan-Küçükdaglı F, Kessler A (2023) Transverse sinus pathologies, vestibular migraine and intracranial hypertension without papilledema. Am J Otolaryngol 44;103931 [DOI] [PubMed]
- 110.Piantino J, Lim MM, Newgard CD, Iliff J. Linking traumatic brain injury, sleep disruption and post-traumatic headache: a potential role for glymphatic pathway dysfunction. Curr Pain Headache Rep. 2019;23:62. doi: 10.1007/s11916-019-0799-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.