Cardiac rehabilitation (CR) is a well‐established program for secondary prevention of cardiovascular disease. CR is a multimodal intervention that includes education, medication adherence promotion, risk factor management, nutritional counseling, psychosocial support, and structured exercise for patients with myocardial infarction, heart failure, stable angina, or following cardiac surgeries or catheter‐based valve replacement procedures (eg, transcatheter aortic valve replacement). Patients who complete CR have lower mortality and hospitalizations as well as improved functional status and quality of life. 1 Additionally, CR is generally considered cost‐effective based on current studies. 2 Given these benefits, CR is given a class 1A recommendation by the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. However, a significant gap remains in CR participation rates; among Medicare beneficiaries in 2017, only 8% of patients were able to complete the recommended number of CR sessions. 3 Furthermore, there are significant disparities in referral and participation that occur based on race, sex, and socioeconomic status. 4 Barriers to patient participation include transportation issues, work responsibilities, and financial and time constraints. To address these issues, the American Association of Cardiovascular and Pulmonary Rehabilitation, American Heart Association, and American College of Cardiology and national quality improvement initiatives, notably the Million Hearts initiative and the Agency for Healthcare Research and Quality TAKEheart initiative, have endorsed home‐based CR and hybrid delivery models to improve access to CR. 5 , 6 Herein, we offer our perspective on current and future models of CR, and how technology may enable innovative models of CR to enhance health equity.
CURRENT AND INNOVATIVE MODELS OF CR DELIVERY
CR is divided into 3 phases, based on location and intensity. 7 Phase 1 (inpatient) focuses on early mobilization and range‐of‐motion exercises during hospitalization; Phase 2 (early outpatient) includes supervised exercise progression and educational sessions, traditionally based at an outpatient CR center, with 2 to 3 sessions per week, typically lasting 12 to 18 weeks; and Phase 3 (late outpatient) refers to long‐term maintenance of healthy lifestyle habits. In this article, we focus on Phase 2 and provide supporting evidence and roadmap for a novel hybrid CR model to scale the delivery of CR at home during this phase.
A major limitation of center‐based CR is that few accredited facilities offer CR services in the United States. In the United States, 74% of adults live in counties with less than 1 CR center per 100 000 adults, and 14% of adults live in counties without a CR center. 8 This supply shortage is more pronounced in rural and low‐income communities, creating significant disparities in access. The median wait time for patients to start CR is 42 days. 9 With the current limited capacity of CR programs, it is estimated that if all spots were filled, only half of eligible patients could be served. 10 Data show that for each day a patient waits to start CR, they are 1% less likely to enroll. 11 Furthermore, patients may face practical barriers, such as needing transportation, taking time during business hours, and having to miss work. 12 Such practical barriers are also more challenging for those with fewer financial resources, creating further disparities in participation in center‐based CR.
Home‐based CR programs can overcome the barriers to access center‐based CR by safely delivering components of CR at home. Multiple studies have demonstrated the feasibility and potential for success of home‐based CR using technology. 13 , 14 While specific program details vary, most combine human and technology components to deliver components of CR virtually. For example, a smartphone‐based home‐based CR program showed higher uptake, adherence, completion rate, and similar or better functional status compared with traditional center‐based CR in patients with acute coronary syndrome. 15 In 2019, the Kaiser Permanente group created a home‐based CR program in which patients exercised independently for 7 weeks while receiving weekly coaching via a smartphone application and telephone calls with case managers. Compared with a historical, center‐based CR control group, CR completion rate increased by 75%, hospital readmissions decreased by 30%, and cardiovascular mortality decreased by 27%. 16 It should be noted that there is a wide spectrum of technology that is utilized in home‐based CR programs. A recent review described multicomponent interventions that integrate web‐based communication, wearable devices, and mobile applications as more common and more successful in terms of patient adherence and improvement in functional status compared with home‐based CR programs that utilize just 1 component, enabled through individually tailored education and exercise prescriptions based on measured data from connected devices. 17 Future studies are needed to identify which technologies may be most effective for delivering home‐based CR. Taken as a whole, home‐based CR and center‐based CR appear to yield similar benefits for quality of life, all‐cause readmissions, mortality, and cost, based on multiple randomized trials and real‐world studies. 18
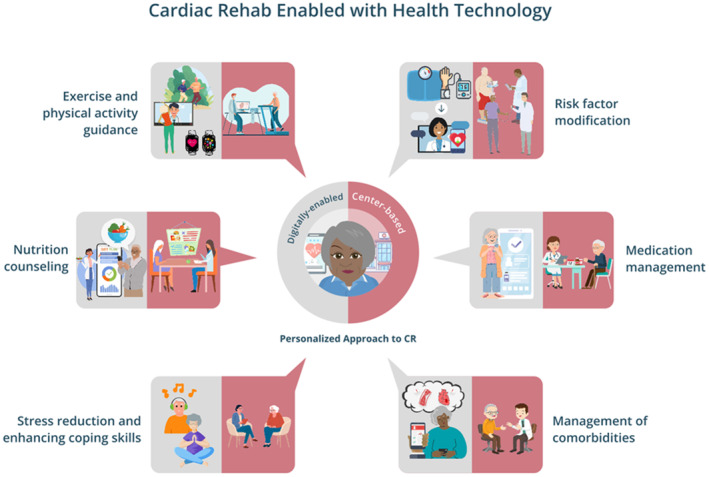
Hybrid CR models, which combine both center‐based CR and home‐based CR, may be able to provide the best practical solution, especially when enhanced by appropriate health technology (Figure). While maintaining in‐person safety evaluation and human‐based components of center‐based CR, remote delivery of various other components of CR can occur through home‐based CR. With appropriate use of health technology, this could scale to rural and low‐income communities and vulnerable populations, allowing for increase in CR supply without exacerbating health disparities. For example, at the Johns Hopkins mTECH Center, the mTECH REHAB trial is testing a hybrid CR program that combines initial in‐center CR sessions with home‐based CR delivered through the Corrie Health technology platform, in order to assess change in functional status plus various safety and clinical outcomes in a broad group of patients with an indication for CR. 19 It is anticipated that in addition to improving clinical outcomes, a hybrid CR model could also demonstrate promotion of health equity through appropriate use of health technology.
Figure 1. Cardiac Rehabilitation (CR) Enabled With Health Technology.
RISK STRATIFICATION
A priority for home‐based or hybrid CR is patient safety. Current clinical practice patterns may be overly conservative, hindering patient participation in home‐based or hybrid CR programs. In 2012, the American Association of Cardiovascular and Pulmonary Rehabilitation published an algorithm to stratify patients based on risk of adverse event, where low‐risk criteria include an ejection fraction >50%, no resting or exercise‐induced dysrhythmias, absence of angina during exercise, a functional capacity of at least 7.0 metabolic equivalents, and lack of cognitive limitations. 20 However, the reported rate of adverse events in CR is very low, with ≈1 event in 400 000 to 800 000 patient hours of exercise. 21 The reported incidence rate of severe adverse events is also low among those participating in home‐based CR, estimated at 1 event in 23 823 patient‐hours of home‐based exercise (with no deaths or hospitalizations), and among which more than half of the patients were technically classified as high‐risk. 22 Moreover, in the HF‐ACTION (Heart‐Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial, where patients with heart failure with reduced ejection fraction (left ventricular ejection fraction <35%) were randomized to undergo a fully center‐based CR versus hybrid CR program, there was no significant difference in safety outcomes between the 2 groups, although these patients would have been considered high risk based on the American Association of Cardiovascular and Pulmonary Rehabilitation criterion of low left ventricular ejection fraction. 23 With more technology available to aid home‐based monitoring of vital signs and other cardiovascular risk factors, the risk of adverse events may be even lower with appropriate clinical oversight. Hence, there is a need to develop a modernized risk stratification tool to avoid inappropriate exclusion from home exercise and to allow more patients to benefit from hybrid CR programs.
A risk calculator is needed that can determine which patients require a center‐based CR visit to optimize transitions between the hospital and home setting. In the same way that patients in the hospital have physical therapy assessments in order to determine placement postdischarge, this risk calculator could be used by inpatient clinicians to help determine which low‐risk and low‐to‐moderate–risk patients can safely participate in home‐based or hybrid CR programs, respectively, to ultimately improve CR uptake. Further studies are needed to develop and validate such risk stratification tools.
KEY FACTORS FOR WIDESPREAD ADOPTION
A major barrier to widespread adoption of home‐based or hybrid CR is the lack of long‐term codified reimbursement. Currently, Medicare reimbursement is mainly available for center‐based CR for up to 36 sessions (or up to 72 sessions for intensive CR). During the COVID‐19 pandemic, the Centers for Medicare and Medicaid Services issued waivers to allow for reimbursement of virtual CR; this reimbursement was specific to synchronous CR programs, which requires direct supervision of patient participation using real‐time 2‐way audiovisual telecommunications technology. To continue reimbursement beyond the public health emergency period, the Sustainable Cardiopulmonary Rehabilitation Services in the Home Act (HR 1406) has been introduced, which allows delivery of virtual CR in the Medicare beneficiary's home through direct supervision by an MD, PA, NP, or a clinical nurse specialist through 2‐way audiovisual telecommunications technology. The introduction of this bill is an important step forward in allowing continued adoption of virtual CR, which can help relieve barriers to CR participation such as travel. Remote therapeutic monitoring and remote physiologic monitoring are currently being reimbursed through their own CPT codes, and can be used to reimburse the health technology aspect of virtual CR. Remote CR can also be utilized as an adjunct to supervised exercise sessions and help enhance the value of CR by providing a way to increase communication between clinicians and patients between CR sessions and enables seamless transitions into long‐term maintenance programs.
NEW INSIGHTS FROM THE INDUSTRY PERSPECTIVE
Industry players in the health care ecosystem include payers, plan‐sponsors, government, employers, vendors, and other stakeholders whose considerations are larger than financial aspects alone. While reimbursement is a major focus of discussion surrounding access to home‐based CR, it is important to recognize that widespread implementation and adoption of new programs, products, and services also remains a barrier.
Scalability afforded by these aforementioned industry stakeholders advance population health by operationalizing initiatives that promote preventive care, manage chronic conditions, and enhance overall health and health care. These players also have a critical responsibility in advancing health equity by ensuring that individuals from all backgrounds have fair and equitable access to health care services and resources.
To support the best interests of individuals while promoting a health care system that is safe and equitable, it is a requisite to carefully evaluate the effectiveness of new technologies and their associated coverage decisions. Using data‐informed decision‐making, assessing the benefit‐to‐risk ratio and considering the diverse needs of the population can enable innovation at scale. Large‐scale implementation and adoption of new solutions involves recognizing and addressing a host of logistical, technological, clinical, and strategic (including cross‐sector partnerships) factors that can facilitate or hinder widespread utilization.
Scalability Is Dependent on Interoperability and Connectivity
The widespread adoption of digital health interventions (DHIs), including home‐based CR, is dependent on interoperability and connectivity to deliver patient‐centric care. This is impacted by informatics frameworks, which is the application of data, information, and knowledge to support and engage patients. Despite the large opportunities new DHIs present, there are barriers in their connectivity to existing data assets and other technologies. There is an overreliance on multiple, isolated technology platforms or applications to address individual populations and/or health conditions rather than a connected, integrated suite of solutions that rely on data‐informed insights, which can lead to point solution fatigue. Many of the core digital health components of home‐based CR (ie, mobile or web‐based platform for curriculum delivery, education, tracking and communication; wearables; and digital health care services) are comparable to other chronic condition and/or care management programs. Thus, integration within existing programs and frameworks can help streamline patients' services, support, and education and allow for solutions that reinforce patient engagement to deliver personalized and timely care.
Digital tools can act as a provider enablement tool to deliver a multitude of support (eg, clinical decision support, communication, data‐informed insights, monitoring) to improve providers' clinical efficiency while concurrently bolstering the provider and patient relationship. Examples of DHIs such as home‐based CR, when applied at scale, have the potential to meet the needs of lower‐risk populations and alleviate issues regarding clinical capacity and resources. However, adoption of DHIs could potentially yield unintended downstream consequences, such as fragmented care, patient safety concerns, technology barriers, alert fatigue, and increased health care utilization, which should also be considered.
Factors Impacting Clinical Product Offerings
Implementation, adoption, and utilization of DHIs requires collaboration within a matrixed organization. DHI's scope of capabilities, associated data flows, and required level of clinical monitoring has a large impact on its utilization. This requires collaboration among various teams and stakeholders, including, but not limited to, medical affairs, clinical quality, legal, privacy, compliance, regulatory, procurement, finance, digital, data governance, product managers, data scientists, and informaticians. Considerations regarding vendor acquisitions and strategy need be considered, such as access to 24/7 technology and clinical support, device fulfillment and training, alignment with business and strategic imperatives, and proper handling of adverse events.
Competency‐based management and ongoing evaluation plans should be established to ensure the effectiveness of home‐based CR programs. Best practice and rigorous evaluation studies to examine DHIs effectiveness utilize randomized controlled trial study designs; however, limitations in conducting randomized controlled trials occur due to practical and ethical constraints. This necessitates alternative approaches, including partnerships with health systems, for evaluating the effectiveness and outcomes of home‐based CR programs. Promoting digital equity becomes paramount in ensuring that home‐based CR programs are accessible and inclusive for all, bridging potential gaps and addressing disparities in access to DHIs (ie, program eligibility), technological capacity for their use (ie, smartphones, broadband internet), and both digital and health literacy to bolster positive health care outcomes. 24 , 25 , 26 , 27
Big Data Begets Big Governance
Digitally connected partnerships are uniquely positioned to reveal novel insights derived from multiple rich data sources, including medical and pharmacy claims, electronic medical records, prior authorization data, electronic health records, and connected devices. Operationalizing these data sources into a singular data warehouse requires establishing interoperability standards and identifying a priori use cases to guide effective data analysis and interpretation. In addition, lack of standardized outcomes can make it challenging to quantify the return on investment. Developing a favorable business case is reliant on eligibility and engagement of patients. Finally, adherence to clinical practice guidelines and the validation of clinical end points are crucial to ensure that the home‐based CR programs align with professional standards and deliver meaningful quality and performance outcomes. 28
CONCLUSIONS
CR is a key component for secondary prevention of cardiovascular disease. Various barriers to center‐based CR participation may be addressed through home‐based and hybrid CR models. Leveraging appropriate health technology could be key to improving access to CR in an equitable manner. In conjunction with new technology, the formulation of effective risk stratification tools for home‐based CR models and reimbursement policies for novel CR models will represent key efforts to grow CR utilization. With evolving CR models and technology, there is an opportunity to scale CR through these steps to benefit patients with cardiovascular disease. Finally, establishing cross‐sector partnerships is essential to design, implement, and evaluate home‐based CR programs. By working together, industry stakeholders and clinicians can navigate the complexities of DHIs operationalization, while advancing equitable population health at large.
Sources of Funding
None.
Disclosures
Under a license agreement between Corrie Health and the Johns Hopkins University, the University owns equity in Corrie Health, and the University and Dr. Marvel and Dr. Martin are entitled to royalty distributions related to the Corrie technology. Additionally, Dr. Marvel and Dr. Martin are founders of and hold equity in Corrie Health. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Marvel has received material support from Apple and iHealth and has received funding from the Maryland Innovation Initiative, Wallace H. Coulter Translational Research Partnership, Louis B. Thalheimer Fund, the PJ Schafer Cardiovascular Research Fund, and the American Heart Association Empowered to Serve Business Accelerator. Dr. Martin has received material support from Apple and iHealth; has received funding from the Maryland Innovation Initiative, Wallace H. Coulter Translational Research Partnership, Louis B. Thalheimer Fund, the Johns Hopkins Individualized Health Initiative, the American Heart Association (20SFRN35380046, 20SFRN35490003, COVID19‐811000, #878924, and #882415), the Patient‐Centered Outcomes Research Institute (ME‐2019C1‐15 328, IHS‐2021C3‐24 147), the National Institutes of Health (P01 HL108800 and R01AG071032), the David and June Trone Family Foundation, the Pollin Digital Innovation Fund, the PJ Schafer Cardiovascular Research Fund, Sandra and Larry Small, CASCADE FH, Google, and Amgen; has received personal fees for serving on scientific advisory boards for Amgen, AstraZeneca, Kaneka, New Amsterdam, Novartis, Novo Nordisk, Sanofi, and 89bio. The remaining authors have no disclosures to report.
This manuscript was sent to Tazeen H. Jafar, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 5.
References
- 1. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 2. Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost‐effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–1410. doi: 10.1136/heartjnl-2017-312809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keteyian SJ, Jackson SL, Chang A, Brawner CA, Wall HK, Forman DE, Sukul D, Ritchey MD, Sperling LS. Tracking cardiac rehabilitation utilization in Medicare beneficiaries: 2017 update. J Cardiopulm Rehabil Prev. 2022;42:235–245. doi: 10.1097/HCR.0000000000000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castellanos LR, Viramontes O, Bains NK, Zepeda IA. Disparities in cardiac rehabilitation among individuals from racial and ethnic groups and rural communities‐a systematic review. J Racial Ethn Health Disparities. 2019;6:1–11. doi: 10.1007/s40615-018-0478-x [DOI] [PubMed] [Google Scholar]
- 5. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home‐based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:69–89. doi: 10.1161/CIR.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 6. Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, Shepard DS, Thomas RJ. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baman JR, Sekhon S, Maganti K. Cardiac rehabilitation. JAMA. 2021;326:366. doi: 10.1001/jama.2021.5952 [DOI] [PubMed] [Google Scholar]
- 8. Wall HK, Stolp H, Wright JS, Ritchey MD, Thomas RJ, Ades PA, Sperling LS. The Million Hearts Initiative: catalyzing utilization of cardiac rehabilitation and accelerating implementation of new care models. J Cardiopulm Rehabil Prev. 2020;40:290–293. doi: 10.1097/HCR.0000000000000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grace SL, Tan Y, Marcus L, Dafoe W, Simpson C, Suskin N, Chessex C. Perceptions of cardiac rehabilitation patients, specialists and rehabilitation programs regarding cardiac rehabilitation wait times. BMC Health Serv Res. 2012;12:259. doi: 10.1186/1472-6963-12-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pack QR, Squires RW, Lopez‐Jimenez F, Lichtman SW, Rodriguez‐Escudero JP, Zysek VN, Thomas RJ. The current and potential capacity for cardiac rehabilitation utilization in the United States. J Cardiopulm Rehabil Prev. 2014;34:318–326. doi: 10.1097/HCR.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 11. Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev. 2011;31:373–377. doi: 10.1097/HCR.0b013e318228a32f [DOI] [PubMed] [Google Scholar]
- 12. Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. A comparison of barriers to use of home‐ versus site‐based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2013;33:297–302. doi: 10.1097/HCR.0b013e31829b6e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:1–151. doi: 10.1002/14651858.CD007130.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golbus JR, Lopez‐Jimenez F, Barac A, Cornwell WK, Dunn P, Forman DE, Martin SS, Schoor EN, Supervia M; Exercise, Cardiac Rehabilitation and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Quality of Care and Outcomes Research; and Council on Cardiovascular and Stroke Nursing . Digital technologies in cardiac rehabilitation: a science advisory from the American Heart Association. Circulation. 2023;148:95–107. doi: 10.1161/CIR.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 15. Yudi MB, Clark DJ, Tsang D, Jelinek M, Kalten K, Joshi SB, Phan K, Ramchand J, Nasis A, Amerena J, et al. SMARTphone‐based, early cardiac REHABilitation in patients with acute coronary syndromes: a randomized controlled trial. Coron Artery Dis. 2021;32:432–440. doi: 10.1097/MCA.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 16. Funahashi T, Borgo L, Joshi N. Saving lives with virtual cardiac rehabilitation. NEJM Catalyst. 2019;5. doi: 10.1056/CAT.19.0624 [DOI] [Google Scholar]
- 17. Lee KCS, Breznen B, Ukhova A, Koehler F, Martin SS. Virtual healthcare solutions for cardiac rehabilitation: a literature review. Eur Heart J Digit Health. 2023;4:99–111. doi: 10.1093/ehjdh/ztad005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnamurthi N, Schopfer DW, Shen H, Rohrbach G, Elnaggar A, Whooley MA. Association of home‐based cardiac rehabilitation with lower mortality in patients with cardiovascular disease: results from the Veterans Health Administration Healthy Heart Program. J Am Heart Assoc. 2023;12:12. doi: 10.1161/JAHA.122.025856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Impact of a Corrie Cardiac Rehabilitation Program (mTECH REHAB) . ClinicalTrials.Gov. https://clinicaltrials.gov/ct2/show/NCT05238103 Accessed July 1, 2023.
- 20. AACVPR stratification algorithm for risk of event . American Association of Cardiovascular and Pulmonary Rehabilitation. 2012. https://registry.dev.aacvpr.org/Documents/AACVPR%20Risk%20Stratification%20Algorithm_June2012.pdf Accessed July 1, 2023.
- 21. Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S. Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med. 2006;166:2329–2334. doi: 10.1001/archinte.166.21.2329 [DOI] [PubMed] [Google Scholar]
- 22. Stefanakis M, Batalik L, Antoniou V, Pepera G. Safety of home‐based cardiac rehabilitation: a systematic review. Heart Lung. 2022;55:117–126. doi: 10.1016/j.hrtlng.2022.04.016 [DOI] [PubMed] [Google Scholar]
- 23. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garvey KV, Craig KJT, Russell RG, Novak L, Moore D, Preininger AM, Jackson GP, Miller BM. The potential and the imperative: the gap in AI‐related clinical competencies and the need to close it. Med Sci Educ. 2021;31:2055–2060. doi: 10.1007/s40670-021-01377-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Novak LL, Russell RG, Garvey K, Patel M, Craig KJT, Snowdon J, Miller B. Clinical use of artificial intelligence requires AI‐capable organizations. JAMIA Open. 2023;6:6. doi: 10.1093/jamiaopen/ooad028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russell RG, Lovett Novak L, Patel M, Garvey GV, Craig KJT, Jackson GP, Moore D, Miller BM. Competencies for the use of artificial intelligence‐based tools by health care professionals. Acad Med. 2023;98:348–356. doi: 10.1097/ACM.0000000000004963 [DOI] [PubMed] [Google Scholar]
- 27. Garvey KV, Thomas Craig KJ, Russell R, Novak LL, Moore D, Miller BM. Considering clinician competencies for the implementation of artificial intelligence‐based tools in health care: findings from a scoping review. JMIR Med Inform. 2022;10:e37478. doi: 10.2196/37478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho MP, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2018;71:1814–1837. doi: 10.1016/j.jacc.2018.01.004 [DOI] [PubMed] [Google Scholar]


