Abstract
ABSTRACT: Albuminuria, an established biomarker of the progression of chronic kidney disease, is also recognized as a biomarker for the risk of cardiovascular disease. Elevated urinary albumin excretion indicates kidney damage and systemic vascular disease, including myocardial capillary disease and arterial stiffness. Albuminuria is associated with an increased risk of coronary artery disease, stroke, heart failure, arrhythmias, and microvascular disease. There are now several therapeutic agents that can lead to albuminuria lowering and a reduction in cardiovascular risk. However, screening for albuminuria is still low. Considering the importance of multidisciplinary management of patients with cardiovascular disease, it is crucial that health care professionals managing such patients are aware of the benefits of albuminuria surveillance and management.
Keywords: albuminuria, cardiovascular disease, chronic kidney disease, diabetes
Subject Categories: Biomarkers, Clinical Studies, Heart Failure, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- AER
albumin excretion rate
- MACE
major adverse cardiovascular events
- MRA
mineralocorticoid receptor antagonist
- RAAS
renin–angiotensin–aldosterone system
- SGLT2
sodium–glucose cotransporter 2
- T2D
type 2 diabetes
- UACR
urine albumin:creatinine ratio
Cardiologists caring for people with suspected or clinical cardiovascular disease (CVD) use biomarkers to support the assessment of disease risk. Since the introduction of creatine kinase that revolutionized the diagnosis of acute myocardial ischemia, a range of biomarkers have been identified and used in clinical practice as our understanding of CVD pathophysiology continues to improve. Inflammation has been recognized as an integral component of the atherosclerotic process and can be evaluated by measuring plasma CRP (C‐reactive protein) CRP levels. Patients with high‐sensitivity CRP levels of ≥2 mg/L are at high risk of major adverse cardiovascular events (MACE). 1 In several clinical trials, it was also found that lowering plasma CRP levels was associated with significantly reduced coronary artery disease (CAD) outcomes and cardiovascular mortality. 2 , 3 Myocardial stretch, observed in various pathophysiological conditions, leads to the production of pro‐B‐type natriuretic peptide, which is broken down into B‐type natriuretic peptide and N‐terminal pro‐B‐type natriuretic peptide. Both proteins are routinely used as reliable cardiac biomarkers for the early diagnosis of heart failure (HF). 4 Damage to the myocardium leads to necrosis of cardiomyocytes, resulting in the release of troponin I and T, which are also routinely used in clinical practice and serve as powerful biomarkers for myocardial ischemia. 3 , 4
Albuminuria, the excretion of higher than normal amounts of the protein albumin in the urine (>30 mg/L), is also a risk factor for CVD and incident chronic kidney disease (CKD). 5 Albuminuria is seen in the general population as frequently as elevated CRP levels, and its association with CVD is just as strong. In the Third National Health and Nutrition Examination Survey, elevated CRP levels (≥0.22 mg/dL) and clinically raised CRP levels (>1.00 mg/dL) were present in 27.6% and 6.7% of the population, respectively. 6 In comparison, in adults ≥65 years (the age at which most CVD presents), albuminuria is present in ~15% to 20% of the population and in ~35% to 40% of similarly aged individuals with diabetes. 7 Elevated CRP levels confer ~45% increased risk of CAD, 8 and albuminuria is associated with ~40% increased risk of clinical CAD. 9 Despite these prognostic attributes, albuminuria has been underappreciated and underused as a biomarker of disease risk by the cardiology community. In this review, we aim to make the reader aware of the cardiovascular significance of albuminuria. First, we discuss the physiology and pathophysiology of urinary albumin excretion, followed by the association of albuminuria with CVD (Table 1). 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Last, we review the medications now available for lowering levels of albuminuria, which in turn are associated with a reduction in CVD risk and cardiorenal events.
Table 1.
Summary of the Associations of Albuminuria With Cardiovascular Disease
| Cardiovascular disease | Association with albuminuria |
|---|---|
| CAD |
Increased severity of CAD 10 High coronary artery calcium score 11 Predictor of silent ischemia 12 Underdeveloped collateral vessels in areas of CAD 13 Poor coronary artery bypass graft outcomes 14 , 15 Risk predictor of CAD 16 |
| Stroke | Stroke risk predictor 17 , 18 |
| Arterial stiffness | Predictor of arterial stiffness 19 , 20 , 21 , 22 , 23 , 24 |
| Myocardial capillary disease | Reduced myocardial flow reserve 25 , 26 , 27 |
| Heart failure |
Predictor of heart failure 28 , 29 Predictor of systolic dysfunction 30 |
| Arrhythmia |
Increased prevalence and risk of atrial fibrillation 36 , 37 , 38 Increased percentage of time in atrial fibrillation 36 Increased prevalence of nonsustained ventricular tachycardia 36 |
CAD indicates coronary artery disease.
Albuminuria
Albumin is a small, negatively charged protein that represents 10% of total body protein and 50% of total plasma protein. The physiologic role of albumin is to maintain plasma oncotic pressure and to transport various endogenous and exogenous ligands through the bloodstream to target cells. 39 In healthy individuals, the endothelial layer of the glomerulus serves as a barrier that minimizes albumin movement from the blood into the urine. 39 A urine albumin level of <30 mg in a 24‐hour urine sample is considered normal. Pathologic albuminuria (≥30 mg in a 24‐hour urine sample) involves structural damage in the glomerulus, which is associated with an increased risk for undesirable cardiovascular and kidney outcomes. 5
Cardiovascular and kidney disease share many risk factors and pathological processes that can lead to albuminuria (Figure 1). Albuminuria is often detected in hypertension and diabetes, both of which are also risk factors for CVD. 40 Although it is primarily mentioned in the context of diabetic kidney disease, albuminuria has a strong, independent association with hypertension. Among 9198 patients in a primary care setting, microalbuminuria (based on test strips that detect ≥20 mg/L urine albumin concentrations) was found in 43% of patients with hypertension only and 51% of patients with diabetes only; patients with both conditions had the highest prevalence of microalbuminuria (58%). 41
Figure 1. Reciprocal mechanisms of cardiac and renal disease leading to albuminuria.
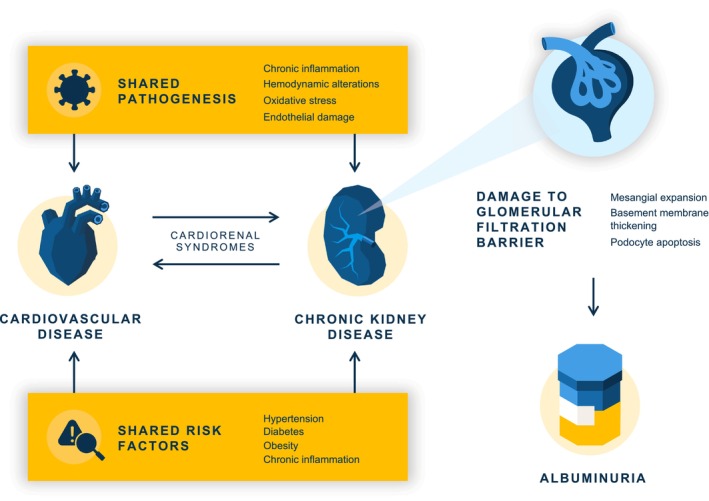
Injury to the glomerulus resulting in albuminuria is seen in cardiovascular disease because of the shared pathological processes with chronic kidney disease and because of compensatory mechanisms in chronic cardiovascular disease.
Hemodynamic alterations induced by hyperglycemia and hypertension lead to mechanical stretch and shear stress on endothelial cells, initiating processes that damage the glomerular filtration barrier. This barrier consists of the endothelial surface layer (the glycocalyx), fenestrated endothelial cells, glomerular basement membrane, and glomerular epithelial cells (podocytes). Albuminuria reflects damage to these structures and is characterized by mesangial expansion (mesangial cell proliferation and excess production of matrix proteins in the central region of the glomerulus provide a supporting framework to the glomerular capillaries), basement membrane thickening, and podocyte apoptosis. Subsequent podocyte injury and detachment further increase albuminuria, decreasing the capillary filtration surface and ultimately leading to glomerulosclerosis. 42
Elevated levels of inflammatory markers are also associated with the presence of albuminuria, providing another link to the association of albuminuria with CVD. 43 Many of these inflammation products are present in processes associated with the development of atherosclerosis. 39 Beyond the shared pathogenetic processes, there is also a strong interplay of the cardiovascular and renal systems where dysfunction of one system can lead to dysfunction of the other, termed cardiorenal syndromes. 44 In chronic cardiac dysfunction, compensatory mechanisms to improve cardiac output and reduce venous congestion can lead to renal hypoperfusion and kidney damage, including albuminuria. 44
Albuminuria is classified into 1 of 3 categories, A1 to A3, depending on the severity (Figure 2). 45 There are several methods to detect albuminuria in daily clinical practice (Table 2). The gold standard is the 24‐hour measurement of albumin excretion rate (AER), as albuminuria has a high short‐term within‐person variability. 46 However, this test is cumbersome and not convenient for routine clinical practice. The spot urine albumin:creatinine ratio (UACR) is another method of measuring albuminuria, and it quantifies the amount of urinary albumin per gram of urinary creatinine, which accounts for differences in urine concentration. The use of this ratio is based on the assumption that creatinine excretion is approximately 1 g per day. Because the amount of creatinine excreted into the urine varies, the ratio of albumin to creatinine is only an approximation of 24‐hour AER.
Figure 2. GFR and albuminuria grid to reflect the risk of CKD progression and requirements for monitoring.
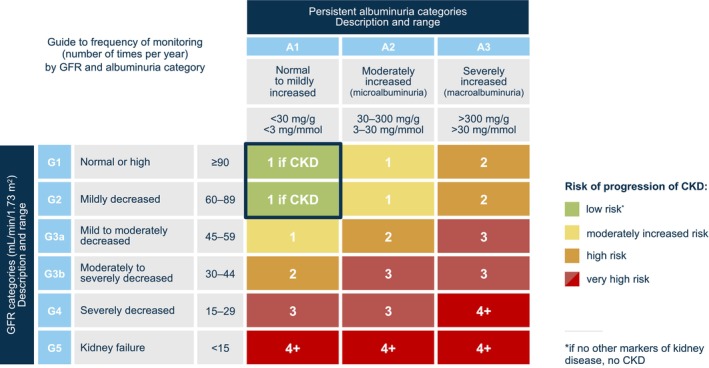
The numbers in the boxes are a guide to the frequency of monitoring (number of times per year). Albuminuria measured as urinary albumin:creatinine ratio. CKD indicates chronic kidney disease; and GFR, glomerular filtration rate. Adapted from Levin A, et al., KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease: Chapter 2: Definition, identification, and prediction of CKD progression; Kidney International Supplements, Vol 3, 63–71, Copyright (2013), with permission from Elsevier. 45
Table 2.
Overview of Urine Albumin Measurement Methods in Clinical Practice
| Test | Benefits | Drawbacks | Best use |
|---|---|---|---|
| 24‐h AER | Accurate measurement of albuminuria excretion | Cumbersome | When accurate albuminuria diagnosis is required |
| Random spot UACR | Convenient, good screening test for microalbuminuria, high sensitivity | Not a good estimate of 24‐h AER, variability owing to exercise, posture, hydration level, extremes of diet or muscle mass, requires retesting | Screening (if first morning spot UACR is not convenient) |
| First morning spot UACR | Stronger correlation with 24‐h AER than random spot UACR | Affected by variability of albuminuria, requires retesting | Screening |
| Urine dipstick | Immediate results, convenient, low cost | Poor specificity and sensitivity to microalbuminuria | Screening (when UACR is unavailable), repeat if negative |
AER indicates albumin excretion rate; and UACR, urinary albumin:creatine ratio.
Several factors can affect spot UACR measurements, such as muscle mass, exercise level, hydration level, and posture. Changes in creatinine generation, as seen with extremes of diet or muscle mass, can also affect UACR measurements. Dietary habits such as red meat consumption or high intake of animal fat have been associated with albuminuria, especially in diseases associated with CKD. 47 , 48 However, the evidence that high protein intake is cross‐sectionally or prospectively associated with albuminuria is not conclusive. 49 Albuminuria is also associated with intake of food with a high acid load in various populations. 50 , 51 The effects of all these factors can be minimized by using a first morning urine sample. A first morning urine sample has a stronger correlation with 24‐hour urine albumin excretion than a random spot sample, as the latter tends to report higher levels of UACR compared with a morning specimen. 52 Owing to the natural variability of albumin excretion, repeating the measurements is advisable. Nonetheless, studies using simultaneously collected samples for 24‐hour AER and first spot morning UACR show that UACR is a good screening test for the presence of microalbuminuria, though not a good diagnostic test for quantifying 24‐hour AER. 53 A conventional dipstick can be used for screening; however, it cannot sufficiently detect microalbuminuria and its sensitivity is poor relative to UACR, hence, its use for albuminuria stratification is discouraged. 54
Association of Albuminuria With Cardiovascular Disease
Coronary Artery Disease
Many studies have documented a strong association of albuminuria with the presence or development of CAD. As compared with normoalbuminuric individuals, people with albuminuria have increased severity of CAD 10 ; an association with high coronary artery calcium score 11 ; the presence of undetected, silent ischemia 12 ; low collateral vessel development in areas of CAD 13 ; and poorer outcomes after coronary artery bypass graft surgery. 14 , 15 One study demonstrated albuminuria to be a risk equivalent to prior myocardial infarctions. 16 In that study, patients with albuminuria and no prior history of myocardial infarction had similar rates of vascular events as patients with prior myocardial infarctions and normoalbuminuria. A meta‐analysis reported the relative risk of developing clinical CAD in people with albuminuria to be 1.41 (95% CI, 1.17–1.69). 9
In a cross‐sectional study from Korea, 45 006 participants without previous CVD events underwent coronary computed tomography and a urine dipstick test as part of a health examination program. A coronary artery calcium score of >100 was clinically significant. Participants were divided into 3 groups based on urine dipstick albumin results as follows: negative (−), trace (±), and positive (+1 to +4). The participants with clinically significant coronary artery calcium score were 2.0%, 2.8%, and 4.9% for the negative, trace, and positive urine dipstick groups, respectively. Compared with the negative group, the odds ratio (OR) for significant coronary artery calcium score was 1.62 (95% CI, 1.08–2.42) in the positive group and 1.34 (95% CI, 1.07–1.66) in the trace group. 11 In a prospective observational study, a random sample of 760 Japanese men from the general population without CVD or other severe diseases were followed up for an average of 5 years. 55 Coronary artery calcification progression was associated with albuminuria (UACR >30 mg/g) after adjustment for diabetes, hypertension, and CRP, independent of change in estimated glomerular filtration rate (eGFR; relative risk, 1.20 [95% CI, 1.01–1.43]; P=0.04). For macroalbuminuria (UACR >300 mg/g) and coronary artery calcification, the Brazilian Longitudinal Study of Adult Health identified an even higher OR of 4.31 (95% CI, 1.27–14.64) among 4189 patients without previous CVD. 56
The mechanisms by which albuminuria could be associated with CAD have been shown to be through impaired vasodilation 57 and increased inflammation factors. 58 In a report from the Cardiovascular Health Study, albuminuria was not associated with the risk of subclinical atherosclerosis in 3312 participants, age ≥ 65 years, without hypertension or diabetes (OR, 1.14 [95% CI, 0.59–2.23]). In contrast, it was associated with subclinical atherosclerosis in participants with hypertension (OR, 1.58 [95% CI, 1.08–2.30]) or diabetes (OR, 2.51 [95% CI, 1.27–4.94]). 59 The association between albuminuria and clinical CAD may involve destabilization of the underlying atherosclerotic vascular disease in those with hypertension and diabetes and endothelial dysfunction in those without hypertension and diabetes. More recent reports identified an association between albuminuria and subclinical atherosclerosis in the absence of diabetes, 60 so the actual pathophysiology requires further investigation.
Stroke
The association between albuminuria and the incidence of stroke has been confirmed in 2 meta‐analyses. A meta‐analysis of 38 studies with 1 735 390 participants concluded that any level of albuminuria was associated with greater stroke risk even after adjustments for other cardiovascular risk factors (relative risk, 1.72 [95% CI, 1.51–1.95]). 18 In a recent meta‐analysis of 7 studies with 159 302 participants, 17 the risk of stroke was also increased (hazard ratio [HR], 1.84 [95% CI, 1.49–2.28]; P < 0.01) with albuminuria compared with its absence. In a subgroup analysis, high levels of UACR were associated with an increased risk of ischemic stroke (HR, 1.60 [95% CI, 1.43–1.80]; P < 0.01) and hemorrhagic stroke (HR, 1.76 [95% CI, 1.22–1.45]; P < 0.01). High levels of UACR were not associated with higher risk of stroke in patients with either type 2 diabetes (T2D) (HR, 2.25 [95% CI, 0.55–9.17]; P = 0.26) or hypertension (HR, 0.95 [95% CI, 0.28–3.22]; P = 0.93). As no information on the degree of control of hyperglycemia or blood pressure was available, the absence of association between albuminuria and risk of stroke should not be considered definite in these populations.
Arterial Stiffness, Vascular Calcification, and Peripheral Arterial Disease
Cross‐sectional and prospective studies suggest a statistically significant bidirectional association of albuminuria with arterial stiffness. In the Jackson Heart Study of African Americans, higher carotid‐femoral pulse wave velocity was associated with prevalent albuminuria (OR, 1.66 [95% CI, 1.32–2.11]; P < 0.001). 19 In the multiethnic Healthy Life in an Urban Setting Study (Amsterdam, Netherlands), aortic stiffness was associated with albuminuria in individuals with T2D (OR, 2.55 [95% CI, 1.30–4.98]) but not in those without diabetes (OR, 0.96 [95% CI, 0.63–1.45]). 20 A study from China reported UACR to be related to carotid‐femoral pulse wave velocity in participants with high‐normal albuminuria and macroalbuminuria. 21 Another study reported that the prevalence of microalbuminuria and macroalbuminuria increased with increasing brachial‐ankle pulse wave velocity. 22 Prospectively, increased carotid‐femoral pulse wave velocity was associated with incident albuminuria in the Framingham Offspring Study. 23 In the Taichung Community Health Study, baseline albuminuria was associated with the development of arterial stiffness in men (OR, 4.47 [95% CI, 1.04–19.31]), and the change in pulse wave velocity was positively associated with the change in UACR. 61 In another prospective diabetes study, arterial stiffness was more strongly associated with albuminuria than was a decrease in renal function as measured by eGFR. 24
Vascular calcification is a common cause of arterial stiffness 62 and has been linked to albuminuria in several studies, in addition to the studies mentioned earlier in relation to coronary artery calcification. Aortic stiffness and femoral artery microcalcifications were significantly increased in patients with T2D with preserved kidney function and macroalbuminuria (UACR >300 mg/g) when compared with those without albuminuria. 63 High urinary protein‐to‐creatinine ratio was independently associated with high aortic arch calcification score (unstandardized coefficient β: 0.315; P=0.002) among 482 predialysis patients with CKD stages 3A to 5 (eGFR <60 mL/min/1.73 m2). 64
Epidemiologic studies have shown an association between peripheral arterial disease and albuminuria. In diabetes, patients with albuminuria (micro‐ and macroalbuminuria combined) have been shown to be 1.90 times more likely to have peripheral arterial disease (95% CI, 1.19–3.04) than those with no albuminuria. 65 The Okinawa Peripheral Arterial Disease Study also demonstrated an association between peripheral arterial disease and albuminuria, which may be a result of arterial stiffness in younger individuals. 66
Myocardial Capillary Disease
The association of albuminuria with myocardial flow reserve (also called microvascular capillary flow) has been studied mostly in people with diabetes. Myocardial flow reserve is the ratio of stress myocardial blood flow to rest myocardial blood flow and correlates with cardiovascular outcomes. In 1 study, myocardial flow reserve decreased progressively in relation to the amount of urinary albumin excretion (normoalbuminuria: 2.9, SD 1.1; microalbuminuria: 2.3, SD 1.0; macroalbuminuria 1.8, SD 0.7; P < 0.0001). 25 In another study, people with T2D free of overt CVD who had albuminuria had a high prevalence of coronary microvascular dysfunction. 26 Finally, in mild‐to‐moderate CKD, patients with albuminuria show reduced coronary vasodilator capacity. 27
Heart Failure
In a cross‐sectional analysis of 1214 adults with HF from the National Health and Nutrition Examination Survey 1999 to 2012, 22.1% had microalbuminuria and 10.4% had macroalbuminuria. In adjusted analyses, the odds of having albuminuria in those with HF (n=1214) were 1.89‐fold higher than in those without HF (n=37 961). 28 Likewise, high normal UACR levels (<30 mg/g) were associated with subsequent HF among 10 975 individuals in the ARIC (Atherosclerosis Risk in Communities) study. 29 Individuals with UACR of 5–9 mg/g and 10–29 mg/g had adjusted HRs for HF of 1.54 (95% CI, 1.12–2.11) and 1.91 (95% CI, 1.38–2.66), respectively, compared with UACR of <5 mg/g. In the same study, micro‐ and macroalbuminuria had adjusted HR of 2.49 (95% CI, 1.77–3.50) and 3.47 (95% CI, 2.10–5.72), respectively. These estimates appeared to be independent of CAD and eGFR.
In people with HF and preserved ejection fraction, increased UACR was associated with increased right ventricular and left ventricular remodeling and with systolic dysfunction. 30 Patients with diabetes and persistent microalbuminuria have markers of diffuse cardiac and diastolic dysfunction. 31 , 32 Even low‐grade albuminuria (<30 mg/g) is associated with left ventricular hypertrophy and left ventricular diastolic dysfunction in hypertensive patients, especially in people <70 years old. 33
Albuminuria affects prognosis in people with HF. In 1 study, the level of UACR on admission for HF was correlated with the risk of subsequent rehospitalization for HF. 34 A meta‐analysis of 11 studies of patients with HF revealed a statistically significant increased risk of all‐cause mortality with microalbuminuria and macroalbuminuria. 35
Arrhythmia
A meta‐analysis of 3 major cohort studies (the Jackson Heart Study, the Multi‐Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study) found a stepwise increase in the adjusted risk of incident atrial fibrillation across microalbuminuria (HR, 1.47 [95% CI, 1.20–1.79]) and macroalbuminuria (HR, 1.76 [95% CI, 1.18–2.62]). 38 More recently, in the ARIC study, albuminuria was consistently associated with higher atrial fibrillation prevalence and percentage of time in atrial fibrillation, and higher prevalence of nonsustained ventricular tachycardia. 36 In a population study from Sweden, 37 the excess risk of atrial fibrillation in individuals with T2D increased with worsening glycemic control and renal complications, including albuminuria.
Other Cardiovascular Associations
Albuminuria is also associated with other conditions of the cardiovascular system that may be encountered by different specialists in addition to cardiologists. Among patients with T2D, a cross‐sectional study of 250 participants showed a definite association between urinary albumin excretion level and severity of diabetic retinopathy, a hallmark of underlying microvascular disease. 67 Even in the absence of diabetes, patients with retinopathy have higher albuminuria levels than those without, as seen in 2271 patients with hypertension. 68 The association between microvascular disease and albuminuria is also seen in relation to diabetic peripheral neuropathy, where a change in UACR ≥30% may indicate a risk for new‐onset diabetic peripheral neuropathy. 69
Several other associations between albuminuria and cardiovascular conditions are of interest. Among 8574 participants followed up for an average 8.6 years, the Prevention of Renal and Vascular End‐Stage Disease study found that microalbuminuria (AER: 30–300 mg/24 h) was independently associated with an increased risk of venous thromboembolism (HR, 2.00 [95% CI, 1.34–2.98]; P < 0.001). 70 The UACR is also a significant neurologic prognostic predictor in patients with aneurysmal subarachnoid hemorrhage; the mechanism of association between the 2 disorders is not known. 71 Microalbuminuria is also a prognostic marker of cardiopulmonary complications after major surgery. However, its independent prognostic significance remains to be established. 72
The Impact of Albuminuria Lowering on Cardiovascular Outcomes
The progression of albuminuria is strongly associated with diabetes and blood pressure control. 40 , 73 If either or both are not well controlled, it is unlikely that albuminuria levels will be meaningfully lowered. Weight loss, 74 lipid control, 75 and smoking cessation 76 also lower albuminuria levels and reduce renal deterioration. There are several classes of therapeutic agents that lower albuminuria and can reduce the risk of CVD. Renin–angiotensin–aldosterone system (RAAS) inhibitors, sodium–glucose cotransporter 2 (SGLT2) inhibitors, and steroidal and nonsteroidal mineralocorticoid receptor antagonists (MRAs) are of particular interest.
An early clinical trial suggested that RAAS blockade could lead to reduced CVD morbidity and mortality in high‐risk patients, many of whom had albuminuria. 77 A more recently published report from the Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration examined the impact of albuminuria change in 28 cohorts with CKD (including 693 816 individuals, 80% with diabetes). 78 Most of the cohorts included in this analysis would have been treated with RAAS inhibitors such as angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers. A 43% increase in UACR over 2 years was associated with an adjusted HR for cardiovascular mortality of 1.14 (95% CI, 1.06–1.22), whereas a 30% decrease in UACR had a statistically nonsignificant adjusted HR of 0.94 (95% CI, 0.87–1.02). In contrast, the adjusted HR for end‐stage kidney disease after a 30% decrease in UACR during a baseline period of 2 years was 0.83 (95% CI, 0.74–0.94). Conversely, a 43% increase in albuminuria was associated with a 1.16 (95% CI, 1.03–1.31) increased risk of end‐stage kidney disease. In sum, albuminuria lowering (mostly with RAAS blockade) was not strongly protective against CVD but was effective for renal protection. Regardless of their effect on albuminuria, a meta‐analysis on patients with CKD showed that angiotensin‐converting enzyme inhibitors (OR, 0.82 [95% CI, 0.71–0.92]) and angiotensin II receptor blockers (OR, 0.76 [95% CI, 0.62–0.89]) reduced the risk of MACE (defined as a composite of cardiovascular death, fatal or nonfatal myocardial infarction, stroke, or HF). 79
SGLT2 inhibitors block mediators of glucose uptake across apical cell membranes. In the kidney, SGLT2 accounts for more than 90% of glucose reabsorption from the glomerular ultrafiltrate. SGLT2 inhibitors were initially developed for the treatment of diabetes but have been found to have cardiovascular‐protective effects, derived from glucose‐lowering, reduced blood volume, lowered blood pressure, and lowered weight. 80
At present, there are 3 double‐blind, randomized, placebo‐controlled studies in renally impaired individuals that have reported results with the use of SGLT2 inhibitors: CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation, investigating canagliflozin) 81 ; DAPA‐CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease, investigating dapagliflozin) 82 ; and EMPA‐KIDNEY (Study of Heart and Kidney Protection With Empagliflozin, investigating empagliflozin). 83 In the CREDENCE study involving 4401 patients with T2D, UACR >300 to 5000 mg/g, and being treated with RAAS inhibitors, canagliflozin lowered UACR by 31% (95% CI, 27–36) and significantly increased the likelihood of achieving a 30% reduction in UACR (OR, 2.69 [95% CI, 2.35–3.07]) compared with placebo. Each 30% decrease in UACR over the first 26 weeks was independently associated with reduced risk of MACE (HR, 0.92 [95% CI, 0.88–0.96; P < 0.001) and hospitalization for HF or cardiovascular death (HR, 0.86 [95% CI, 0.81–0.90]; P < 0.001). In the DAPA‐CKD study involving 4304 patients with UACR 200 to 5000 mg/g, CVD mortality and hospitalization for HF were reduced by 29% (HR, 0.71 [95% CI, 0.55–0.92]; P = 0.009) following treatment with dapagliflozin. Moreover, dapagliflozin resulted in a geometric mean percentage change of albuminuria of −35.1% (95% CI, −39.4 to −30.6; P < 0.0001) in patients with T2D and −14.8% (95% CI, −22.9 to −5.9; P = 0.0016) in patients without T2D over the follow‐up visits. 82 In the EMPA‐KIDNEY study involving 6609 patients with eGFR of ≥20 to <45 mL/min/1.73 m2, regardless of the level of albuminuria, or with an eGFR of ≥45 to <90 mL/min/1.73 m2 and UACR ≥200 mg/g, empagliflozin reduced geometric mean UACR by 19% (95% CI, 15–23). 83 The primary composite outcome of CVD mortality or kidney disease progression was significantly lower with empagliflozin versus placebo (HR, 0.72 [95% CI, 0.64–0.82]; P < 0.001). This benefit was primarily observed in patients with severely increased albuminuria at baseline. There was no statistically significant treatment effect on the secondary composite of hospitalization for HF or CVD mortality (HR, 0.84 [95% CI, 0.67–1.07]; P = 0.15), possibly owing to the low number of events. 83 Nevertheless, the totality of evidence from these 3 studies suggests that lowering albuminuria with the use of SGLT2 inhibitors is associated with CVD protection. It is unknown whether this is a causal relationship and to what degree albuminuria needs to be reduced to confer CVD protection. Dapagliflozin and empagliflozin are now approved by the US Food and Drug Administration to reduce the risk of cardiovascular death and HF hospitalization in patients with HF or T2D. Canagliflozin is currently approved only for patients with T2D.
Two other classes of medications that reduce albuminuria levels and the risk of CVD are the steroidal and nonsteroidal MRAs. Overstimulation of the MR in cardiomyocytes, mesangial cells, podocytes, and endothelial and vascular smooth muscle cells leads to inflammation and fibrosis in the heart, kidneys, and blood vessels. 84 These processes are especially noticeable in HF and CKD. Blocking MRs with spironolactone (first‐generation steroidal MRA) and eplerenone (second‐generation steroidal MRA) has been shown to be effective in reducing cardiovascular mortality and morbidity in patients with chronic HF. Finerenone, a nonsteroidal MRA, shows a higher selectivity for the MR than steroidal MRAs and low affinity for off‐target androgen, glucocorticoid, and progesterone receptors in vitro. 85 Nonsteroidal MR antagonism with finerenone demonstrates better protective effects on cardiorenal function than spironolactone or eplerenone, possibly owing to more balanced tissue distribution in the heart and kidney, as well as a lower incidence of adverse events such as hyperkalemia and gynecomastia. 86
In a meta‐analysis of 4 randomized control studies involving 13 945 patients 87 with T2D and CKD, there was significant UACR lowering from baseline in the finerenone groups compared with the placebo groups (mean difference, 30% [95% CI, −0.33 to −0.27]). The data also showed a lower risk of ≥40% decrease in eGFR from baseline in the finerenone group versus the placebo group (relative risk, 0.85 [95% CI, 0.78–0.93]). The incidence of MACE was reduced by 13% with the lowering of albuminuria (HR, 0.87 [95% CI, 0.76–0.98]; P = 0.03), driven primarily by a lower incidence of hospitalization for HF (HR, 0.71 [95% CI, 0.56–0.90]). 88 , 89 Finerenone is approved by the Food and Drug Administration and the European Medicines Agency to reduce the risk of renal and cardiovascular events in patients with CKD and T2D. Using mediation analysis, a post‐hoc analysis of two finerenone studies that included more than 12 000 individuals with CKD and T2D showed that 84% and 37% of the reduction of renal and CVD with finerenone was due to reduction of albuminuria. 90
There are several other therapeutic agents that lower albuminuria and reduce the risk of CVD. Thiazide diuretics are often used in addition to RAAS blockade for the management of hypertension or HF and can decrease albuminuria by >35%. 91 Statins, one of the most prescribed medications in cardiology with proven cardiovascular benefits, also decrease albuminuria and the risk for cardiovascular events in patients with CKD due to any cause, including diabetes. 92 , 93 Glucagon‐like peptide‐1 receptor agonists used in diabetes have been shown to reduce the risk of progression of albuminuria and to be associated with a lower risk of MACE compared with placebo (OR, 0.89 [95% CI, 0.84–0.94]). 94
Need for More Albuminuria Screening
Elevated levels of urine albumin are one of the earliest signs of CKD and damage of the kidney microvasculature. 95 It follows that screening for elevated urine albumin and managing patients early on have the potential to lower the risk of end‐stage kidney failure (need for dialysis or transplantation) and the risk of concomitant cardiovascular complications including ischemia, arrhythmia, and HF. 96 This is especially so today, given the availability of medications that lower albuminuria levels and provide cardiorenal protection (Figure 3). The benefit to patients seems clear but the case for an economic benefit of more albuminuria screening can also be made. In 1 retrospective analysis, the costs of care for patients with T2D with moderately and severely increased albuminuria were $3580 and $12 830 higher annually, respectively, compared with patients with normal to mildly increased albuminuria levels. 97
Figure 3. Importance of screening for albuminuria: a graphical abstract.
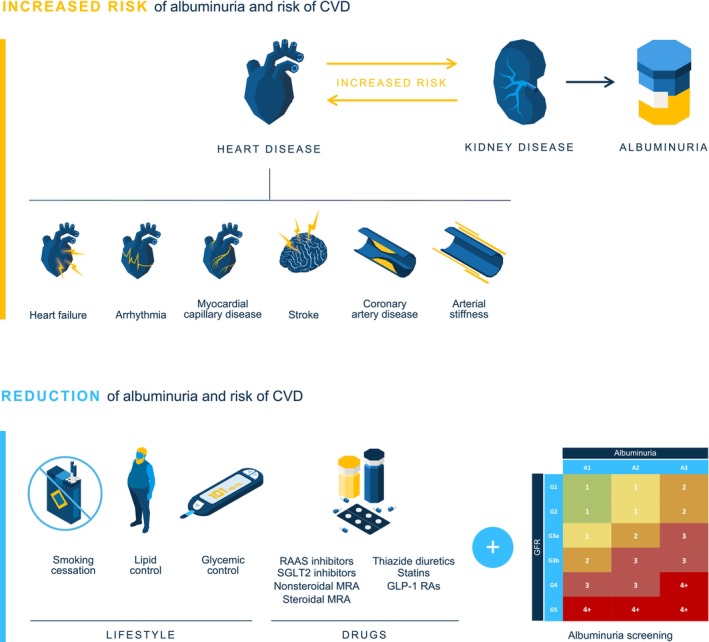
Screening for albuminuria is important to identify patients at risk of CVD and CKD and to intervene early with cardiorenal‐protective therapies that can help slow disease progression and improve patient outcomes. CKD indicates chronic kidney disease; CVD, cardiovascular disease; GLP‐1 RA, Glucagon‐like peptide‐1 receptor agonist; MRA, mineralocorticoid receptor antagonist; RAAS, renin–angiotensin–aldosterone system; and SGLT2, sodium–glucose cotransporter 2.
Several guidelines recommend screening for albuminuria (Table 3). For patients with diagnosed CKD, the Kidney Disease: Improving Global Outcomes guidelines recommend that UACR should be tested at least annually and more often for individuals at higher risk of renal disease progression based on the eGFR categories (Figure 2). 45 For patients with diabetes, the American Diabetes Association recommends screening UACR annually in patients with type 1 diabetes ≥5 years after diagnosis and in all patients with T2D, regardless of treatment. Moreover, in patients with diabetes and urinary albumin ≥300 mg/g creatinine or an eGFR of 30 to 60 mL//min/1.73 m2, the American Diabetes Association recommends monitoring UACR twice a year to guide therapy. 100 Albuminuria screening is also recommended for patients presenting with hypertension. Both the American College of Cardiology/American Heart Association and the International Society of Hypertension recommend routine urine dipstick testing or UACR testing to assess albuminuria. 98 , 99 Beyond CKD, diabetes, and hypertension, there are no guideline recommendations for albuminuria screening.
Table 3.
Guideline Recommendations in Relation to Albuminuria Screening
| Guideline | Population | Preferred testing method | Timing |
|---|---|---|---|
| Kidney Disease: Improving Global Outcomes – diabetes management in CKD 96 | At risk for and with CKD to detect progression | First morning spot UACR | Annually, and 1–4 times per year depending on the stage of CKD (see Figure 2) |
| American College of Cardiology/American Heart Association – high blood pressure in adults 98 | With hypertension to detect hypertension‐mediated organ damage or screening for secondary hypertension | First morning spot UACR | Optional |
| International Society of Hypertension – hypertension 99 | With hypertension to detect hypertension‐mediated organ damage or screening for secondary hypertension | First morning spot UACR or urine dipstick | Routinely |
| American Diabetes Association – CKD in diabetes 100 | Anyone with diabetic kidney disease or at risk of kidney disease (type 1 diabetes with duration of >5 y; type 2 diabetes regardless of treatment) to detect progression of disease | Random spot UACR | Annually, or 1–4 times per year depending on the stage of CKD (see Figure 2) |
CKD indicates chronic kidney disease; and UACR, urine albumin:creatinine ratio.
Despite the rationale and clinical evidence for the value of urine albumin screening, real‐world evidence suggests that adherence to recommended testing schedules is low. 101 , 102 , 103 In a large US‐based cohort of 1 881 446 patients with T2D, Folkerts and colleagues observed that fewer than 50% of patients were screened for albuminuria during the 1‐year follow‐up. 101 A US population‐based study of patients with T2D showed a 1‐year median testing rate of 51.6% for both ACR and eGFR and 52.9% for ACR alone. 103 Another large study spanning multiple international cohorts that included over 3 million participants reported low UACR testing rates in patients with diabetes (35.1%) and extremely low rates in patients with hypertension (4.1%). 102
There are several reasons for this low adherence to the guidelines on albuminuria testing (Table 4). A systematic review of 13 271 records screened identified only 1 study that examined drivers of nonadherence to albuminuria testing. 104 Reasons for nonadherence in patients without diabetes based on clinical scenarios in primary care (hypertension and eGFR ≥60 mL/min/1.73 m2 and hypertension and eGFR <60 mL/min/1.73 m2) included the perception that albuminuria test results would not significantly affect management, limited time, testing not recommended by guidelines, cost, and poor patient adherence. 105 Although specialists may be more aware of relevant guidelines, 106 primary care practitioners, who have the most patient contact, often have modest knowledge of guideline recommendations, such as those related to hypertension. 107 , 108 The lack of awareness of the association of albuminuria with CVD and lack of awareness of the guidelines could relate to inconsistent advice from the guidelines themselves. There is currently no consensus in clinical guidelines regarding how and when to use the 2 key measures of CKD (eGFR and albuminuria) to improve CVD risk prediction, even though 1 large analysis of multiple data sets demonstrated improved cardiovascular risk prediction with the addition of measures of CKD. 109 This is a critical missed opportunity to refine personalized CVD‐preventative therapies according to CKD status.
Table 4.
Factors That May Contribute to Low Albuminuria Testing Rates
| Level | Factors |
|---|---|
| Practitioner | Lack of awareness of the association of albuminuria with cardiovascular disease |
| Lack of awareness of the guidelines | |
| Lack of time / high workload | |
| Inadequate financial incentives / cost | |
| Institutional | Fragmented health care |
| Patients have limited access to health care | |
| Patient | Low disease awareness |
| Poor adherence | |
| Guidelines | Inconsistent advice among the guidelines |
Inadequate financial incentives and fragmented health care with limited coordination/communication between providers may also affect albuminuria testing rates. Patients with limited access to health care or low disease awareness are also less likely to seek review from their clinicians and adhere to screening requests. Overcoming barriers and increasing screening rates of patients at risk are possible, especially because the ready availability and relative low cost of screening tests renders screening of populations at risk, such as those with diabetes or hypertension, very cost effective. 110 Several institutions, including primary care and secondary care, have employed simple measures such as electronic health record‐based reminders and patient and clinician education to improve albuminuria screening rates. 111 , 112
Beyond CVD and CKD
The reader of this review should be aware of the significant pervasive effects of albuminuria on health. Albuminuria is also associated with musculoskeletal, pulmonary, cerebral, endocrine, and cognitive disorders, as well as with injury (eg, falls, fractures). 113 It is also associated with a 40% increased risk of hospitalization and a 40% increased number of hospital days for a broad range of conditions. 113
Conclusions
Albuminuria is a marker of increased physiological stress and an indicator of the need for intensive medical attention. Its recognition and treatment could lead to improved medical outcomes and reduced medical costs. It is well established that there is a strong association of albuminuria with several manifestations of CVD and CVD mortality. Despite this knowledge, rates of albuminuria screening in high‐risk patients, such as those with diabetes or hypertension, are low. Health care professionals are urged to screen for albuminuria per current guidelines. Improving adherence to recommended albuminuria testing guidelines is likely to provide substantial clinical and economic benefits. Albuminuria diagnosis supports the identification of patients at risk of CVD and CKD and presents a crucial early opportunity to intervene with cardiorenal‐protective therapy to slow disease progression and improve patient outcomes. Guidelines should also be more explicit in their recommendations, with clear guidance on who to screen, when to screen, and how to manage albuminuria.
Sources of Funding
Medical writing support was funded by Bayer US, in accordance with Good Publication Practice (GPP 2022) guidelines (DeTora LM, et al. Ann Intern Med. 2022;175:1298–1304).
Disclosures
Youssef M.K. Farag and Jeffrey Durthaler are employees of Bayer US Pharmaceuticals. Joshua I. Barzilay has no disclosures to report.
Acknowledgments
The authors would like to thank Constantinos Bezos, 3 Stories High, United Kingdom, for his medical writing support.
This article was sent to Tiffany M. Powell‐Wiley, MD MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Carrero JJ, Andersson Franko M, Obergfell A, Gabrielsen A, Jernberg T. hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: a healthcare‐based study. J Am Heart Assoc. 2019;8:e012638. doi: 10.1161/jaha.119.012638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridker PM. A test in context: high‐sensitivity C‐reactive protein. J Am Coll Cardiol. 2016;67:712–723. doi: 10.1016/j.jacc.2015.11.037 [DOI] [PubMed] [Google Scholar]
- 3. Lyngbakken MN, Myhre PL, Røsjø H, Omland T. Novel biomarkers of cardiovascular disease: applications in clinical practice. Crit Rev Clin Lab Sci. 2019;56:33–60. doi: 10.1080/10408363.2018.1525335 [DOI] [PubMed] [Google Scholar]
- 4. Dhingra R, Vasan RS. Biomarkers in cardiovascular disease: statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovasc Med. 2017;27:123–133. doi: 10.1016/j.tcm.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, Fassett R, Ansquer JC, Dixon P, Davis TM, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54:32–43. doi: 10.1007/s00125-010-1854-1 [DOI] [PubMed] [Google Scholar]
- 6. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C‐reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- 7. Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x [DOI] [PubMed] [Google Scholar]
- 8. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804 [DOI] [PubMed] [Google Scholar]
- 9. Xia F, Liu G, Shi Y, Zhang Y. Impact of microalbuminuria on incident coronary heart disease, cardiovascular and all‐cause mortality: a meta‐analysis of prospective studies. Int J Clin Exp Med. 2015;8:1–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Ozyol A, Yucel O, Ege MR, Zorlu A, Yilmaz MB. Microalbuminuria is associated with the severity of coronary artery disease independently of other cardiovascular risk factors. Angiology. 2012;63:457–460. doi: 10.1177/0003319711423528 [DOI] [PubMed] [Google Scholar]
- 11. Song JJ, Lee KB, Hyun YY, Kim H. Trace albumin in the urine dipstick test is associated with coronary artery calcification in Korean adults. Nephron. 2018;140:169–174. doi: 10.1159/000490954 [DOI] [PubMed] [Google Scholar]
- 12. Giovacchini G, Cappagli M, Carro S, Borrini S, Montepagani A, Leoncini R, Mazzotta G, Sambuceti G, Mariani G, Volterrani D, et al. Microalbuminuria predicts silent myocardial ischaemia in type 2 diabetes patients. Eur J Nucl Med Mol Imaging. 2013;40:548–557. doi: 10.1007/s00259-012-2323-5 [DOI] [PubMed] [Google Scholar]
- 13. Topsakal R, Kaya MG, Duran M, Gunebakmaz O, Dogan A, Inanc T, Yarlioglues M, Celik A, Ergin A. The relation between microalbuminuria and coronary collateral vessel development in patients with unstable coronary artery disease. Coron Artery Dis. 2009;20:431–434. doi: 10.1097/MCA.0b013e3283277650 [DOI] [PubMed] [Google Scholar]
- 14. George LK, Molnar MZ, Lu JL, Kalantar‐Zadeh K, Koshy SK, Kovesdy CP. Association of pre‐operative albuminuria with post‐operative outcomes after coronary artery bypass grafting. Sci Rep. 2015;5:16458. doi: 10.1038/srep16458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shafranskaya KS, Kashtalap VV, Kutikhin AG, Barbarash OL, Barbarash LS. Microalbuminuria and prediction of cardiovascular complications in patients with coronary artery disease and type 2 diabetes mellitus after CABG surgery. Heart Lung Circ. 2015;24:951–959. doi: 10.1016/j.hlc.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 16. Rein P, Saely CH, Vonbank A, Fraunberger P, Drexel H. Is albuminuria a myocardial infarction risk equivalent for atherothrombotic events? Atherosclerosis. 2015;240:21–25. doi: 10.1016/j.atherosclerosis.2015.02.037 [DOI] [PubMed] [Google Scholar]
- 17. Li M, Cheng A, Sun J, Fan C, Meng R. The role of urinary albumin‐to‐creatinine ratio as a biomarker to predict stroke: a meta‐analysis and systemic review. Brain Circ. 2021;7:139–146. doi: 10.4103/bc.bc_64_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly DM, Rothwell PM. Proteinuria as an independent predictor of stroke: systematic review and meta‐analysis. Int J Stroke. 2020;15:29–38. doi: 10.1177/1747493019895206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagarajarao HS, Musani SK, Cobb KE, Pollard JD, Cooper LL, Anugu A, Yano Y, Moore JA, Tsao CW, Dreisbach AW, et al. Kidney function and aortic stiffness, pulsatility, and endothelial function in African Americans: the Jackson Heart Study. Kidney Med. 2021;3:702–711.e701. doi: 10.1016/j.xkme.2021.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayfron‐Benjamin CF, Amoah AGB, Maitland‐van der Zee AH, Moll van Charante EP, Galenkamp H, van den Born BJ, Agyemang C. Associations between macrovascular and renal microvascular dysfunction in type 2 diabetes and non‐diabetes: the HELIUS study. Microvasc Res. 2021;136:104162. doi: 10.1016/j.mvr.2021.104162 [DOI] [PubMed] [Google Scholar]
- 21. Ye C, Gong J, Wang T, Luo L, Lian G, Wang H, Chen W, Xie L. Relationship between high‐normal albuminuria and arterial stiffness in Chinese population. J Clin Hypertens (Greenwich). 2020;22:1674–1681. doi: 10.1111/jch.13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang Y, Fan F, Jia J, He D, Sun P, Wu Z, Huo Y, Zhang Y. Brachial‐ankle pulse wave velocity is independently associated with urine albumin‐to‐creatinine ratio in a Chinese community‐based cohort. Int Urol Nephrol. 2020;52:713–720. doi: 10.1007/s11255-020-02404-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasan RS, Short MI, Niiranen TJ, Xanthakis V, DeCarli C, Cheng S, Seshadri S, Mitchell GF. Interrelations between arterial stiffness, target organ damage, and cardiovascular disease outcomes. J Am Heart Assoc. 2019;8:e012141. doi: 10.1161/jaha.119.012141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim JH, Kim SS, Kim IJ, Kim BH, Park JY, Lee CW, Suk JH, Shin SH, Son SP, Kim MC, et al. Arterial stiffness is more associated with albuminuria than decreased glomerular filtration rate in patients with type 2 diabetes mellitus: the REBOUND study. J Diabetes Res. 2017;2017:7047909. doi: 10.1155/2017/7047909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potier L, Chequer R, Roussel R, Mohammedi K, Sismail S, Hartemann A, Amouyal C, Marre M, Le Guludec D, Hyafil F. Relationship between cardiac microvascular dysfunction measured with 82Rubidium‐PET and albuminuria in patients with diabetes mellitus. Cardiovasc Diabetol. 2018;17:11. doi: 10.1186/s12933-017-0652-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Scholten BJ, Hasbak P, Christensen TE, Ghotbi AA, Kjaer A, Rossing P, Hansen TW. Cardiac (82)Rb PET/CT for fast and non‐invasive assessment of microvascular function and structure in asymptomatic patients with type 2 diabetes. Diabetologia. 2016;59:371–378. doi: 10.1007/s00125-015-3799-x [DOI] [PubMed] [Google Scholar]
- 27. Imamura S, Hirata K, Orii M, Shimamura K, Shiono Y, Ishibashi K, Tanimoto T, Yamano T, Ino Y, Kitabata H, et al. Relation of albuminuria to coronary microvascular function in patients with chronic kidney disease. Am J Cardiol. 2014;113:779–785. doi: 10.1016/j.amjcard.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 28. Odutayo A, Hsiao AJ, Emdin CA. Prevalence of albuminuria in a general population cohort of patients with established chronic heart failure. J Card Fail. 2016;22:33–37. doi: 10.1016/j.cardfail.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 29. Blecker S, Matsushita K, Köttgen A, Loehr LR, Bertoni AG, Boulware LE, Coresh J. High‐normal albuminuria and risk of heart failure in the community. Am J Kidney Dis. 2011;58:47–55. doi: 10.1053/j.ajkd.2011.02.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2014;2:586–596. doi: 10.1016/j.jchf.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jørgensen PG, Biering‐Sørensen T, Mogelvang R, Fritz‐Hansen T, Vilsbøll T, Rossing P, Jensen JS. Presence of micro‐ and macroalbuminuria and the association with cardiac mechanics in patients with type 2 diabetes. Eur Heart J Cardiovasc Imaging. 2018;19:1034–1041. doi: 10.1093/ehjci/jex231 [DOI] [PubMed] [Google Scholar]
- 32. Swoboda PP, McDiarmid AK, Erhayiem B, Ripley DP, Dobson LE, Garg P, Musa TA, Witte KK, Kearney MT, Barth JH, et al. Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc. 2017;6:e005539. doi: 10.1161/jaha.117.005539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang T, Zhong H, Lian G, Cai X, Gong J, Ye C, Xie L. Low‐grade albuminuria is associated with left ventricular hypertrophy and diastolic dysfunction in patients with hypertension. Kidney Blood Press Res. 2019;44:590–603. doi: 10.1159/000500782 [DOI] [PubMed] [Google Scholar]
- 34. Matsumoto Y, Orihara Y, Asakura M, Min KD, Okuhara Y, Azuma K, Nishimura K, Sunayama I, Kashiwase K, Naito Y, et al. Urine albumin‐to‐creatinine ratio on admission predicts early rehospitalization in patients with acute decompensated heart failure. Heart Vessel. 2022;37:1184–1194. doi: 10.1007/s00380-022-02025-y [DOI] [PubMed] [Google Scholar]
- 35. Mehta R, Ning H, Bansal N, Cohen J, Srivastava A, Dobre M, Michos ED, Rahman M, Townsend R, Seliger S, et al. Ten‐year risk‐prediction equations for incident heart failure hospitalizations in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort Study and the Multi‐Ethnic Study of Atherosclerosis. J Card Fail. 2022;28:540–550. doi: 10.1016/j.cardfail.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim ED, Soliman EZ, Coresh J, Matsushita K, Chen LY. Two‐week burden of arrhythmias across CKD severity in a large community‐based cohort: the ARIC study. J Am Soc Nephrol. 2021;32:629–638. doi: 10.1681/asn.2020030301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seyed Ahmadi S, Svensson AM, Pivodic A, Rosengren A, Lind M. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: a Swedish cohort study. Cardiovasc Diabetol. 2020;19:9. doi: 10.1186/s12933-019-0983-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R, Katz R, Kestenbaum B, Mathew J, Robinson‐Cohen C, et al. eGFR and albuminuria in relation to risk of incident atrial fibrillation: a meta‐analysis of the Jackson Heart Study, the Multi‐Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study. Clin J Am Soc Nephrol. 2017;12:1386–1398. doi: 10.2215/cjn.01860217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raja P, Maxwell AP, Brazil DP. The potential of albuminuria as a biomarker of diabetic complications. Cardiovasc Drugs Ther. 2021;35:455–466. doi: 10.1007/s10557-020-07035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barzilay JI, Peterson D, Cushman M, Heckbert SR, Cao JJ, Blaum C, Tracy RP, Klein R, Herrington DM. The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: the cardiovascular health study. Am J Kidney Dis. 2004;44:25–34. doi: 10.1053/j.ajkd.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 41. Marques da Silva P, Carvalho D, Nazaré J, Martins L, Aguiar C, Manso MC, Carqueja T, Polónia J. Prevalence of microalbuminuria in hypertensive patients with or without type 2 diabetes in a Portuguese primary care setting: the RACE (micRoAlbumin sCreening survEy) study. Rev Port Cardiol. 2015;34:237–246. doi: 10.1016/j.repc.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 42. Benzing T, Salant D. Insights into glomerular filtration and albuminuria. N Engl J Med. 2021;384:1437–1446. doi: 10.1056/NEJMra1808786 [DOI] [PubMed] [Google Scholar]
- 43. Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, O'Donnell CJ, Kathiresan S, Meigs JB, Keaney JF Jr, Rong J, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011;26:920–926. doi: 10.1093/ndt/gfq471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCullough PA, Amin A, Pantalone KM, Ronco C. Cardiorenal nexus: a review with focus on combined chronic heart and kidney failure, and insights from recent clinical trials. J Am Heart Assoc. 2022;11:e024139. doi: 10.1161/jaha.121.024139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kidney Disease: Improving Global Outcomes . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;3:1‐150. doi: 10.1038/kisup.2012.73 [DOI] [PubMed] [Google Scholar]
- 46. Lin CH, Lai YC, Chang TJ, Jiang YD, Chang YC, Chuang LM. Visit‐to‐visit variability in albuminuria predicts renal function deterioration in patients with type 2 diabetes. J Diabetes Investig. 2022;13:1021–1029. doi: 10.1111/jdi.13761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Real Rodrigues CC, Riboldi BP, Rodrigues TDC, Sarmento RA, Antonio JP, de Almeida JC. Association of eating patterns and diabetic kidney disease in type 2 diabetes: a cross‐sectional study. J Ren Nutr. 2023;33:261–268. doi: 10.1053/j.jrn.2022.09.011 [DOI] [PubMed] [Google Scholar]
- 48. Abbate M, Mascaró CM, Montemayor S, Barbería‐Latasa M, Casares M, Gómez C, Ugarriza L, Tejada S, Abete I, Zulet M, et al. Animal fat intake is associated with albuminuria in patients with non‐alcoholic fatty liver disease and metabolic syndrome. Nutrients. 2021;13:1548. doi: 10.3390/nu13051548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Remer T, Kalotai N, Amini AM, Lehmann A, Schmidt A, Bischoff‐Ferrari HA, Egert S, Ellinger S, Kroke A, Kühn T, et al. Protein intake and risk of urolithiasis and kidney diseases: an umbrella review of systematic reviews for the evidence‐based guideline of the German Nutrition Society. Eur J Nutr. 2023;62:1957–1975. doi: 10.1007/s00394-023-03143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banerjee T, Tucker K, Griswold M, Wyatt SB, Harman J, Young B, Taylor H, Powe NR. Dietary potential renal acid load and risk of albuminuria and reduced kidney function in the Jackson Heart Study. J Ren Nutr. 2018;28:251–258. doi: 10.1053/j.jrn.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 51. Kabasawa K, Hosojima M, Takachi R, Nakamura K, Ito Y, Saito A, Sawada N, Tsugane S, Tanaka J, Narita I. Association of estimated dietary acid load with albuminuria in Japanese adults: a cross‐sectional study. BMC Nephrol. 2019;20:194. doi: 10.1186/s12882-019-1352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–443. doi: 10.1681/asn.2008030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Houlihan CA, Tsalamandris C, Akdeniz A, Jerums G. Albumin to creatinine ratio: a screening test with limitations. Am J Kidney Dis. 2002;39:1183–1189. doi: 10.1053/ajkd.2002.33388 [DOI] [PubMed] [Google Scholar]
- 54. Park JI, Baek H, Kim BR, Jung HH. Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: a population‐based study. PLoS One. 2017;12:e0171106. doi: 10.1371/journal.pone.0171106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ganbaatar N, Kadota A, Hisamatsu T, Araki SI, Kume S, Fujiyoshi A, Kadowaki S, Torii S, Kondo K, Segawa H, et al. Relationship between kidney function and subclinical atherosclerosis progression evaluated by coronary artery calcification. J Atheroscler Thromb. 2022;29:1359–1371. doi: 10.5551/jat.63030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suh‐Chiou C, Moysés RM, Bittencourt MS, Bensenor IM, Lotufo PA. Chronic kidney disease and coronary artery calcification in the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Clin Cardiol. 2017;40:1309–1315. doi: 10.1002/clc.22829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koyoshi R, Hitaka‐Yoshimine Y, Shiga Y, Kuwano T, Sugihara M, Ike A, Iwata A, Sako H, Morito N, Kawamura A, et al. Associations between microalbuminuria and parameters of flow‐mediated vasodilatation obtained by continuous measurement approaches. Clin Exp Hypertens. 2018;40:715–720. doi: 10.1080/10641963.2018.1425422 [DOI] [PubMed] [Google Scholar]
- 58. von Scholten BJ, Reinhard H, Hansen TW, Schalkwijk CG, Stehouwer C, Parving HH, Jacobsen PK, Rossing P. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all‐cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J Diabetes Complicat. 2016;30:248–255. doi: 10.1016/j.jdiacomp.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 59. Cao JJ, Barzilay JI, Peterson D, Manolio TA, Psaty BM, Kuller L, Wexler J, Bleyer AJ, Cushman M. The association of microalbuminuria with clinical cardiovascular disease and subclinical atherosclerosis in the elderly: the Cardiovascular Health Study. Atherosclerosis. 2006;187:372–377. doi: 10.1016/j.atherosclerosis.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 60. Kimura T, Ueno T, Doi S, Nakashima A, Doi T, Ashitani A, Kawano R, Yamane K, Masaki T. High‐normal albuminuria is associated with subclinical atherosclerosis in male population with estimated glomerular filtration rate ≥60 mL/min/1.73 m2: a cross‐sectional study. PLoS One. 2019;14:e0218290. doi: 10.1371/journal.pone.0218290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lin CC, Tang KT, Li CI, Liu CS, Lai MM, Lin WY, Lin CC. Gender difference in the relationship of albuminuria and arterial stiffness in Chinese adults ‐ a 6.6‐year follow‐up longitudinal study. Kidney Blood Press Res. 2018;43:1479–1487. doi: 10.1159/000493663 [DOI] [PubMed] [Google Scholar]
- 62. Chen Y, Zhao X, Wu H. Arterial stiffness: a focus on vascular calcification and its link to bone mineralization. Arterioscler Thromb Vasc Biol. 2020;40:1078–1093. doi: 10.1161/atvbaha.120.313131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reijrink M, Sluiter JKE, te Velde‐Keyzer CA, de Borst MH, van Praagh GD, Greuter MJW, Luurtsema G, Boersma HH, Pol RA, Hillebrands JL, et al. Severely increased albuminuria in patients with type 2 diabetes mellitus is associated with increased subclinical atherosclerosis in femoral arteries with Na [18F]F activity as a proxy ‐ the DETERMINE study. Atherosclerosis. 2023;117199. doi: 10.1016/j.atherosclerosis.2023.117199 [DOI] [PubMed] [Google Scholar]
- 64. Su WY, Wu PY, Huang JC, Chen SC, Chang JM. Increased proteinuria is associated with increased aortic arch calcification, cardio‐thoracic ratio, rapid renal progression and increased overall and cardiovascular mortality in chronic kidney disease. Int J Med Sci. 2020;17:1102–1111. doi: 10.7150/ijms.45470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wattanakit K, Folsom AR, Criqui MH, Kramer HJ, Cushman M, Shea S, Hirsch AT. Albuminuria and peripheral arterial disease: results from the multi‐ethnic study of atherosclerosis (MESA). Atherosclerosis. 2008;201:212–216. doi: 10.1016/j.atherosclerosis.2007.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ishida A, Nakachi‐Miyagi M, Kinjo K, Iseki K, Ohya Y. A high normal ankle‐brachial index is associated with proteinuria in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study. J Hypertens. 2014;32:1435–1443; discussion 1443. doi: 10.1097/hjh.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 67. Dash S, Chougule A, Mohanty S. Correlation of albuminuria and diabetic retinopathy in type‐II diabetes mellitus patients. Cureus. 2022;14:e21927. doi: 10.7759/cureus.21927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li J, Zhang W, Zhao L, Zhang J, She H, Meng Y, Zhang Y, Gu X, Zhang Y, Li J, et al. Positive correlation between hypertensive retinopathy and albuminuria in hypertensive adults. BMC Ophthalmol. 2023;23:66. doi: 10.1186/s12886-023-02807-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhong M, Yang YR, Zhang YZ, Yan SJ. Change in urine albumin‐to‐creatinine ratio and risk of diabetic peripheral neuropathy in type 2 diabetes: a retrospective cohort study. Diabetes Metab Syndr Obes. 2021;14:1763–1772. doi: 10.2147/dmso.S303096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mahmoodi BK, Gansevoort RT, Veeger NJ, Matthews AG, Navis G, Hillege HL, van der Meer J. Microalbuminuria and risk of venous thromboembolism. JAMA. 2009;301:1790–1797. doi: 10.1001/jama.2009.565 [DOI] [PubMed] [Google Scholar]
- 71. Terao Y, Oji M, Toyoda T, Inoue H, Fukusaki M, Hara T. An observational study of the association between microalbuminuria and increased N‐terminal pro‐B‐type natriuretic peptide in patients with subarachnoid hemorrhage. J Intensive Care. 2015;3:42. doi: 10.1186/s40560-015-0108-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lucio C, Marco A, Rossella P, Silvia C, Jacopo V, Gianpaolo R, Vito Aldo P, Francesco P. Microalbuminuria as a predictor of cardiopulmonary complications after thoracic surgery. Eur Respir J. 2015;46:PA2514. doi: 10.1183/13993003.congress-2015.PA2514 [DOI] [Google Scholar]
- 73. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/cjn.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miricescu D, Balan DG, Tulin A, Stiru O, Vacaroiu IA, Mihai DA, Popa CC, Enyedi M, Nedelea AS, Nica AE, et al. Impact of adipose tissue in chronic kidney disease development (review). Exp Ther Med. 2021;21:539. doi: 10.3892/etm.2021.9969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lv J, Ren C, Hu Q. Effect of statins on the treatment of early diabetic nephropathy: a systematic review and meta‐analysis of nine randomized controlled trials. Ann Palliat Med. 2021;10:11548–11557. doi: 10.21037/apm-21-2673 [DOI] [PubMed] [Google Scholar]
- 76. Su S, Wang W, Sun T, Ma F, Wang Y, Li J, Xu Z. Smoking as a risk factor for diabetic nephropathy: a meta‐analysis. Int Urol Nephrol. 2017;49:1801–1807. doi: 10.1007/s11255-017-1638-3 [DOI] [PubMed] [Google Scholar]
- 77. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/nejm200001203420301 [DOI] [PubMed] [Google Scholar]
- 78. Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, Black C, Brunskill NJ, Carrero JJ, Feldman HI, et al. Change in albuminuria and subsequent risk of end‐stage kidney disease: an individual participant‐level consortium meta‐analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–127. doi: 10.1016/s2213-8587(18)30313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, Zhao N, Liu L, Lv J, Zhang H, et al. Renin‐angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta‐analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 80. Liu B, Wang Y, Zhang Y, Yan B. Mechanisms of protective effects of SGLT2 inhibitors in cardiovascular disease and renal dysfunction. Curr Top Med Chem. 2019;19:1818–1849. doi: 10.2174/1568026619666190828161409 [DOI] [PubMed] [Google Scholar]
- 81. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 82. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 83. Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes. 2010;17:199–204. doi: 10.1097/med.0b013e3283391989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rico‐Mesa JS, White A, Ahmadian‐Tehrani A, Anderson AS. Mineralocorticoid receptor antagonists: a comprehensive review of finerenone. Curr Cardiol Rep. 2020;22:140. doi: 10.1007/s11886-020-01399-7 [DOI] [PubMed] [Google Scholar]
- 86. Agarwal R, Anker SD, Filippatos G, Pitt B, Rossing P, Ruilope LM, Boletis J, Toto R, Umpierrez GE, Wanner C, et al. Effects of canagliflozin versus finerenone on cardiorenal outcomes: exploratory post hoc analyses from FIDELIO‐DKD compared to reported CREDENCE results. Nephrol Dial Transplant. 2022;37:1261–1269. doi: 10.1093/ndt/gfab336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zheng Y, Ma S, Huang Q, Fang Y, Tan H, Chen Y, Li C. Meta‐analysis of the efficacy and safety of Finerenone in diabetic kidney disease. Kidney Blood Press Res. 2022;47:219–228. doi: 10.1159/000521908 [DOI] [PubMed] [Google Scholar]
- 88. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
- 89. Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Schloemer P, Tornus I, Joseph A, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143:540–552. doi: 10.1161/circulationaha.120.051898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Agarwal R, Tu W, Farjat AE, Farag YMK, Toto R, Kaul S, Lawatschek R, et al. Impact of Finerenone‐Induced Albuminuria Reduction on Chronic Kidney Disease Outcomes in Type 2 Diabetes: A Mediation Analysis. Ann Intern Med. 2023;176:1606–1616. doi: 10.7326/M23-1023 [DOI] [PubMed] [Google Scholar]
- 91. Trujillo H, Caravaca‐Fontán F, Caro J, Morales E, Praga M. The forgotten antiproteinuric properties of diuretics. Am J Nephrol. 2021;52:435–449. doi: 10.1159/000517020 [DOI] [PubMed] [Google Scholar]
- 92. Qin X, Dong H, Fang K, Lu F. The effect of statins on renal outcomes in patients with diabetic kidney disease: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2017;. 33:e2901. doi: 10.1002/dmrr.2901 [DOI] [PubMed] [Google Scholar]
- 93. Su X, Zhang L, Lv J, Wang J, Hou W, Xie X, Zhang H. Effect of statins on kidney disease outcomes: a systematic review and meta‐analysis. Am J Kidney Dis. 2016;67:881–892. doi: 10.1053/j.ajkd.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 94. Mannucci E, Gallo M, Giaccari A, Candido R, Pintaudi B, Targher G, Monami M. Effects of glucose‐lowering agents on cardiovascular and renal outcomes in subjects with type 2 diabetes: an updated meta‐analysis of randomized controlled trials with external adjudication of events. Diabetes Obes Metab. 2023;25:444–453. doi: 10.1111/dom.14888 [DOI] [PubMed] [Google Scholar]
- 95. Zhang J, Deng Y, Wan Y, He S, Cai W, Xu J. Association between serum albumin level and microvascular complications of type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:2173–2182. doi: 10.2147/DMSO.S373160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kidney Disease: Improving Global Outcomes Diabetes Work Group . KDIGO 2022 Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127. doi: 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 97. Zhou Z, Chaudhari P, Yang H, Fang AP, Zhao J, Law EH, Wu EQ, Jiang R, Seifeldin R. Healthcare resource use, costs, and disease progression associated with diabetic nephropathy in adults with type 2 diabetes: a retrospective observational study. Diabetes Ther. 2017;8:555–571. doi: 10.1007/s13300-017-0256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 99. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/hypertensionaha.120.15026 [DOI] [PubMed] [Google Scholar]
- 100. American Diabetes Association Professional Practice Committee . Chronic kidney disease and risk management: standards of medical care in diabetes–2023. Diabetes Care. 2023;46:S191–S202. doi: 10.2337/dc23-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Folkerts K, Petruski‐Ivleva N, Comerford E, Blankenburg M, Evers T, Gay A, Fried L, Kovesdy CP. Adherence to chronic kidney disease screening guidelines among patients with type 2 diabetes in a US administrative claims database. Mayo Clin Proc. 2021;96:975–986. doi: 10.1016/j.mayocp.2020.07.037 [DOI] [PubMed] [Google Scholar]
- 102. Shin JI, Chang AR, Grams ME, Coresh J, Ballew SH, Surapaneni A, Matsushita K, Bilo HJG, Carrero JJ, Chodick G, et al. Albuminuria testing in hypertension and diabetes: an individual‐participant data meta‐analysis in a global consortium. Hypertension. 2021;78:1042–1052. doi: 10.1161/hypertensionaha.121.17323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Stempniewicz N, Vassalotti JA, Cuddeback JK, Ciemins E, Storfer‐Isser A, Sang Y, Matsushita K, Ballew SH, Chang AR, Levey AS, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care. 2021;44:2000–2009. doi: 10.2337/dc20-2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Groehl F, Garreta‐Rufas A, Meredith K, Harris J, Rossing P, Hobbs FDR, Wanner C. The drivers of non‐adherence to albuminuria testing guidelines and the clinical and economic impact of not identifying chronic kidney disease. Clin Nephrol. 2023;100:145–156. doi: 10.5414/cn111106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Abdel‐Kader K, Greer RC, Boulware LE, Unruh ML. Primary care physicians' familiarity, beliefs, and perceived barriers to practice guidelines in non‐diabetic CKD: a survey study. BMC Nephrol. 2014;15:64. doi: 10.1186/1471-2369-15-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Erhardt L, Komajda M, Hobbs FDR, Soler‐Soler J. Cardiologists' awareness and perceptions of guidelines for chronic heart failure. The ADDress your Heart survey. Eur J Heart Fail. 2008;10:1020–1025. doi: 10.1016/j.ejheart.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 107. Al‐Ali KA, Al‐Ghanim FA, Al‐Furaih AM, Al‐Otaibi N, Makboul G, El‐Shazly MK. Awareness of hypertension guidelines among family physicians in primary health care. Alexandria J Med. 2013;49:81–87. doi: 10.1016/j.ajme.2012.07.003 [DOI] [Google Scholar]
- 108. Ale O, Braimoh RW. Awareness of hypertension guidelines and the diagnosis and evaluation of hypertension by primary care physicians in Nigeria. Cardiovasc J Afr. 2017;28:72–76. doi: 10.5830/cvja-2016-048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Matsushita K, Kaptoge S, Hageman SHJ, Sang Y, Ballew SH, Grams ME, Surapaneni A, Sun L, Arnlov J, Bozic M, et al. Including measures of chronic kidney disease to improve cardiovascular risk prediction by SCORE2 and SCORE2‐OP. Eur J Prev Cardiol. 2023;30:8–16. doi: 10.1093/eurjpc/zwac176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yeo SC, Wang H, Ang YG, Lim CK, Ooi XY. Cost‐effectiveness of screening for chronic kidney disease in the general adult population: a systematic review. Clin Kidney J. 2023;sfad137. doi: 10.1093/ckj/sfad137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kam S, Angaramo S, Antoun J, Bhatta MR, Bonds PD, Cadar AG, Chukwuma VU, Donegan PJ, Feldman Z, Grusky AZ, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11:e001591. doi: 10.1136/bmjoq-2021-001591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Willison A, Tully V, Davey P. All patients with diabetes should have annual UACR tests. Why is that so hard? BMJ Qual Improv Rep. 2016;5:u209185.w203747. doi: 10.1136/bmjquality.u209185.w3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barzilay JI, Buzkova P, Shlipak MG, Bansal N, Garimella P, Mukamal KJ. Hospitalization rates in older adults with albuminuria: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2020;75:2426–2433. doi: 10.1093/gerona/glaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]


