Abstract
Background
Heart failure disproportionately affects individuals residing in rural areas, leading to worse health outcomes. Digital health interventions have been proposed as a promising approach for improving heart failure management. This systematic review aims to identify randomized trials of digital health interventions for individuals living in underserved rural areas with heart failure.
Methods and Results
We conducted a systematic review by searching 6 databases (CINAHL, EMBASE, MEDLINE, Web of Science, Scopus, and PubMed; 2000–2023). A total of 30 426 articles were identified and screened. Inclusion criteria consisted of digital health randomized trials that were conducted in underserved rural areas of the United States based on the US Census Bureau's classification. Two independent reviewers screened the studies using the National Heart, Lung, and Blood Institute tool to evaluate the risk of bias. The review included 5 trials from 6 US states, involving 870 participants (42.9% female). Each of the 5 studies employed telemedicine, 2 studies used remote monitoring, and 1 study used mobile health technology. The studies reported improvement in self‐care behaviors in 4 trials, increased knowledge in 2, and decreased cardiovascular mortality in 1 study. However, 3 trials revealed no change or an increase in health care resource use, 2 showed no change in cardiac biomarkers, and 2 demonstrated an increase in anxiety.
Conclusions
The results suggest that digital health interventions have the potential to enhance self‐care and knowledge of patients with heart failure living in underserved rural areas. However, further research is necessary to evaluate their impact on clinical outcomes, biomarkers, and health care resource use.
Registration
URL: https://www.crd.york.ac.uk/prospero/; Unique identifier: CRD42022366923.
Keywords: digital health intervention, heart failure, randomized controlled trials, underserved rural areas, United States
Subject Categories: Digital Health, Health Equity
Nonstandard Abbreviations and Acronyms
- GDMT
guideline‐directed medical therapy
Clinical Perspective.
What Is New?
This is the first systematic review assessing digital health interventions in underserved rural residents with heart failure in the United States and their impact on health‐related outcomes.
The findings of this study demonstrated that digital health interventions could enhance self‐care abilities and knowledge of heart failure patients living in underserved rural regions.
Nonetheless, despite the promising potential, current research has yet to show a favorable impact on clinical outcomes or the use of health care resources.
What Are the Clinical Implications?
By synthesizing the available evidence, this review will provide valuable insights into the potential of digital health interventions to improve outcomes for rural populations living with heart failure.
Furthermore, the findings of this review may inform the development of tailored digital health interventions that are specifically designed to address the unique challenges faced by rural populations with heart failure.
Ultimately, this research has the potential to inform policy and practice, with the aim of reducing the burden of heart failure on rural populations and improving health outcomes for these underserved communities.
Heart failure (HF) is a highly prevalent condition characterized by considerable morbidity and mortality, impairment of quality of life, and substantial economic burden. Given that over 64 million individuals worldwide, including 6.5 million adults in the United States, are affected by HF, addressing the social and economic impact of this condition is a crucial public health concern. 1 , 2 HF management is longitudinal and requires a multidisciplinary approach, requiring regular visits. Traditional care provisions may not be enough to meet the needs of patients with HF, particularly those residing in underserved rural areas who face additional barriers to accessing cardiovascular care. Commonly cited barriers include distance to health care facilities, limited transportation, parking costs, and infrastructure (eg, quality of roads). These barriers result in underserved rural populations having fewer visits with cardiology, fewer follow‐up visits after HF hospitalization, and less likely to be enrolled in cardiac rehabilitation. 3 Underserved rural populations are at a disproportionate risk for developing HF compared with their urban counterparts, with a 19% higher risk overall and a 34% higher risk for Black men living in rural areas. 4 Digital health interventions including remote cardiovascular monitoring are a promising solution to address the burden of cardiovascular diseases in underserved rural populations, including patients with HF. Multiple randomized clinical trials have demonstrated that various digital health interventions and technologies, including teleconsultations, smartphone applications, wearables, remote monitoring, and predictive analytics, can influence patient behaviors in both the prevention and management of HF. 5 , 6 These tools have the potential to connect underserved rural populations to their care team, regardless of their physical location, allowing for regular monitoring and timely intervention. Although digital health interventions have shown potential benefits for patients with HF in underrepresented groups such as women, 7 older age, 8 and racial and ethnic minority groups, 9 there is insufficient evidence to support their effectiveness for underserved rural areas. Compared with their urban counterparts, patients residing in underserved rural areas of the United States experience a range of socioeconomic challenges such as lower income, lower educational attainment, reduced health literacy, varying health insurance coverage, and limited availability of broadband Internet access. 10 Given the distinct challenges associated with digital health accessibility that underserved rural residents with HF encounter, it is critical to understand the effectiveness of these interventions in this population to identify culturally and linguistically appropriate digital health tools for HF management and improving health care services.
This systematic review aims to identify randomized trials of digital health interventions in underserved rural residents with HF in the United States. We describe the types of interventions and their impact on health‐related outcomes.
Methods
Transparency and Openness Promotion Statement
The authors declare that all supporting data are available within the article (and its supplemental material).
Registration
The systematic review was registered with the International Prospective Register of Systematic Reviews (registration number: CRD42022366923). The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines were followed to ensure the review was conducted systematically and transparently (see Supplemental Material).
Search Strategy
A systematic review of relevant studies on digital health interventions for HF management in underserved rural areas of the United States was conducted using the CINAHL, EMBASE, MEDLINE, Web of Science, Scopus, and PubMed databases. The research question was formulated using the population, intervention, control, and outcomes framework: “In patients with heart failure living in underserved rural areas of the United States, does the use of digital health interventions compared with usual care reduce health care resource use, improve clinical outcomes, and promote self‐care behaviors?” The search terms included keywords such as heart failure, cardiomyopathy, ventricular dysfunction, telemedicine, wearable electronic, mobile applications, mHealth, and digital health, either alone or in combination using Boolean operators in each of the databases searched. The complete electronic search strategy is included in Table S1. The search strategy was developed through an iterative process, with the research team reviewing the results of each search term until a final search strategy was determined. To identify additional relevant articles, the reference lists of relevant articles and systematic reviews were examined, and manual searches were conducted. Duplicate articles were removed, and only studies published in peer‐reviewed journals between January 2000 and April 2023 were considered for inclusion.
Inclusion and Exclusion Criteria
The study aimed to identify randomized controlled trials that evaluated digital health interventions for managing HF in underserved rural areas of the United States. Inclusion criteria were limited to studies that used mobile health, wearables, text messaging, telehealth, or web‐based platforms for remote monitoring of patients and studies that were primarily conducted in underserved rural areas of the United States, or those that reported intervention outcomes through subgroup analysis including underserved rural areas based on the US Census Bureau's classification.
The exclusion criteria comprised studies that assessed internal biosensors (pacemakers, defibrillators, pulmonary artery pressure sensors, and implanted cardiac device diagnostics), artificial intelligence algorithms, retrospective studies, prospective studies without intervention, reviews, case reports, case series, books, risk prediction models, and studies that included all cardiovascular diseases, including those other than HF. Studies that lacked primary data, such as protocols or reviews, as well as studies that lacked full‐text access (eg, conference abstracts only) and nonrandomized trials were also excluded. Two reviewers independently screened eligible studies by title and abstract, and in cases of disagreement, a third reviewer was consulted until a consensus was reached. The study selection process is presented in a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram (Figure 1).
Figure 1. Study flow chart.
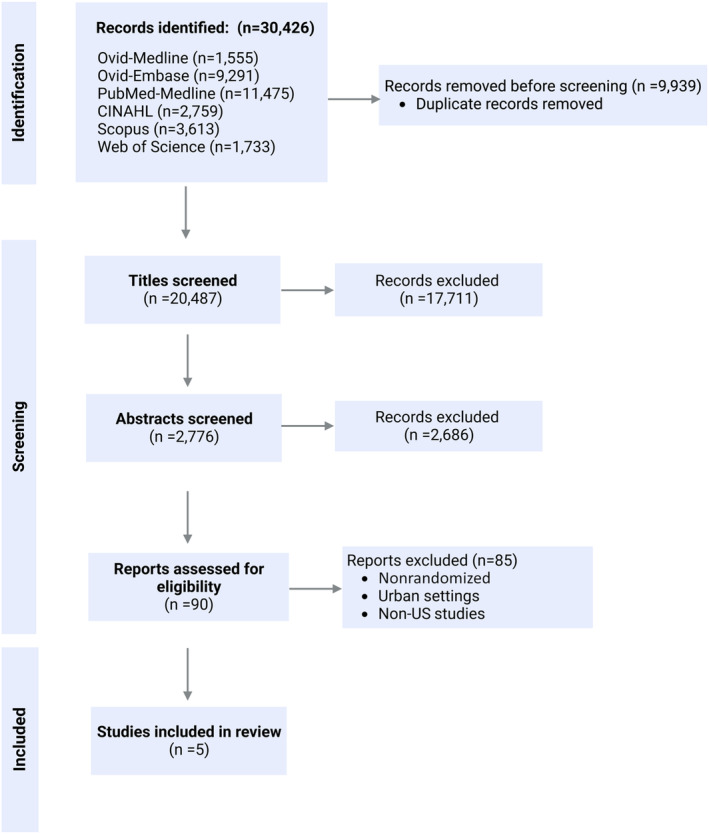
Data Extraction and Assessment of Risk Bias
The relevant data extracted from individual trials included the study design, sample characteristics such as sample size, mean age, percentage of women, and percentage of participants who completed the study. Characteristics of the intervention and control groups, including the intervention modality, duration, and frequency of interaction, were also extracted. We identified the primary outcome and secondary outcomes and extracted the mean differences with the corresponding 95% CIs between the intervention and control groups. We divided outcomes into 4 groups: clinical outcomes (cardiac or HF‐related mortality) and biomarkers, health care resource use (HF readmission, hospitalization, emergency department visits, and clinic visits), self‐care behaviors (such as measuring symptoms and vitals, adhering to a low‐sodium diet, taking prescribed medication, and daily exercise) and others. Data extraction was completed by 2 reviewers using a prespecified format. Any discrepancies were resolved through consensus or consultation with a third reviewer.
We used the National Heart, Lung and Blood Institute study quality assessment tool 11 to evaluate the risk of bias of each study. This tool is widely used to assess the methodological rigor of studies and to identify potential biases that may have affected the cumulative evidence. 12 The tool consists of 14 questions that assess the internal validity of studies. The quality of the studies was then classified as good, fair, or poor based on these assessments.
This study is based on data from published studies and does not require approval from an ethical standards committee.
Results
Search Result
We searched 6 electronic databases for articles published between January 2000 and April 2023 and identified a total of 30 426 articles. After removing duplicates, we screened 20 487 article titles and further narrowed down our selection by reviewing 2776 abstracts. Finally, we screened 90 full‐text publications based on inclusion and exclusion criteria, excluding nonrandomized studies and those conducted in nonrural or socially advantaged settings. We ultimately included 5 studies conducted in underserved rural areas that met our criteria. (Figure 1).
Characteristics and Participants Traits in Studies
Table 1 presents an overview of the key features of the 5 studies included in this analysis, which included a total of 870 participants (42.9% female). These studies were conducted in underserved rural regions of 6 US states: New York, 13 Nebraska, 14 California, 15 , 16 Kentucky, 15 Nevada, 15 and Arizona 17 between 2005 and 2019 (Figure 2, 18 ). All studies included in this analysis were randomized clinical trials, 2 of which were pilot studies. 16 , 17 In the study by Pekmezaris et al, 13 the participants were limited to Black and Hispanic individuals residing in underserved rural areas, and study by Lefler et al 17 exclusively included patients aged 55 years and above.
Table 1.
Participant Characteristics, Study Design, Quality, and Results of Included Studies
| Study | Country | Participants (mean age, y, % female, % completed) | Intervention | Control | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (mean age, y, % female, % completed) | Participants (mean age, y, % female, % completed) | Program duration | Type of intervention | Device | Follow up duration (post) | Follow up periods (mid, post) | Analysis (PP, ITT, or both) | Comparison condition | Result | |||
| Pekmezaris et al, 2019 13 | United States: New York |
104 Black and Hispanic participants (59.9±15.1 y, 41% female, 81.7% completed) |
46 (58.4±15.2 y, 43% female, 76.1% completed) |
58 (61.1±15.0 y, 40% female, 86.2% completed) |
90 d |
TSM: (1) Daily vital self‐monitoring using (American TeleCare LifeView device) (2) Weekly telehealth visits |
American TeleCare LifeView, Telephone |
0 | 0, 90 d | ITT | Outpatient setting, based upon 2013 Heart Failure Clinical Practice Guidelines |
(1) Emergency department visits (RR, 1.37 [95% CI, 0.83–2.27]) (2) Hospitalization: (RR, 0.92 [95% CI, 0.57–1.48]) Length of stay: (TSM=0.54 d vs COM=0.91d) (3) Number of all‐cause hospitalizations: (TSM=0.78 vs COM=0.55; P=0.03) (4) Depression (Patient Health Questionnaire‐4): no significant change (5) Anxiety (Patient Health Questionnaire‐4): (TSM=50–28%; COM=57–13%; P=0.05) (6) Minnesota Living with Heart Failure Questionnaire: no significant change |
| Young et al, 2016 14 | United States: Nebraska | 100 (70.2±12.2 y, 64% female, 95.2% completed) |
51 (68.7±11.8 y, 52.9% female, 94.4% completed) |
49 (71.8±12.6 y, 75.5% female, 96.1% completed) | 12 wk |
PATCH intervention. Two phases: (1) One‐on‐one in‐hospital self‐management training session delivered by telephone (2) Post‐discharge reinforcement sessions (a) Twice a wk for the first 2 wk (b) Once a wk for wk 3–6 (c) Every other wk for wk 7–12 |
Telephone | 3 mo | 0, 3, 6 mo | PP | Usual care: standard discharge teaching for HF (written and verbal information about HF self‐care and scheduled follow‐up doctor appointments) |
(1) Significant improvement in patient reported self‐management adherence at 3 and 6 mo after discharge in intervention vs control: ‐Weighing (mean difference: 1.1, P<0.005) ‐Following a low‐sodium diet (mean difference: 0.9, P<0.005) ‐Taking prescribed medication (mean difference: 0.6, P<0.005) ‐Exercising daily (mean difference: 0.6, P<0.005) (2) No significant difference in physical activity (mean difference: 0.03 to 0.05, P>0.05) ,or clinical biomarkers (3) Significantly greater 30‐d readmission rates in intervention vs control (19.6% vs 6.1%) (4) Significant improvement in self‐efficacy for heart failure self‐management (mean difference: 0.4, P=0.03), self‐management strategies (mean difference: 1.0, P<0.005) and patient activation score (mean difference: 0.3, P=0.06) |
| Caldwell et al, 2005 16 | United States: Northern California | 36 (71±14.7 y, 31% female) | 20 (69±15.9 y, 25% female) | 16 (73±13 y, 37% female) | 1 mo | Usual care+ a simple individualized education and counseling session focused on symptom recognition and fluid weight management, with a phone call at 1 mo for reinforcement | Telephone | 2 mo | 0, 3 mo | PP | Usual care and written material |
(1) Significant improvement in knowledge after 3 mo (18.1 vs 14.9, P=0.01) (2) Significant improvement in self‐care behavior related to daily weights at 3 mo (2.9±1.0 vs 1.9±1.3, P=0.03) (3) No significant change in B‐type natriuretic peptide levels (195±170 vs 302±311 pg/mL, P=0.21) |
| Dracup et al, 2014 15 , REMOTE HF | United States: California, Kentucky, Nevada | 602 (66.1±12.9 y, 40.5% female, 82.24% completed) |
Fluid Watchers LITE: 200 (65.9±12.8 y, 42% female, 78.8% completed) Fluid Watchers LITE‐PLUS: 193 (66.1±12.9, y, 42.5% female, 82.8% completed) |
209 (66.4±12.9 y, 37.3% female, 84.5% completed) |
Fluid Watchers LITE: 4 wk Fluid Watchers LITE‐PLUS: Until content competency was demonstrated: 5.3±3.6 wk (1–19) phone calls |
Fluid Watchers LITE: 2 phone calls at 2‐wk intervals to reinforce the information in the educational session Fluid Watchers LITE‐PLUS: (1) An audiotape of the education session for future review. (2) Biweekly follow‐up phone calls by the research nurse until content competency was demonstrated |
Telephone | 24 mo | 0, 3, 12, 24 mo | ITT | Usual care |
(1) Significant improvement in self‐care scores in both LITE and PLUS groups vs control at 3 and 12 mo (2) No significant difference in the prevalence of combined clinical outcomes (cardiac death and HF hospitalization) (control: 37.8% vs LITE: 28.5% vs PLUS: 38.9%, P=0.058) over 2 y (3) No significant difference in time to HF hospitalization or cardiac death P=0.1 (4) Lower proportion of cardiac death in LITE group compared with control (control: 17.7% vs LITE: 7.5% vs PLUS: 11.9%, P=0.003) (5) Fewer scheduled or nonscheduled office visits for HF in LITE group compared with PLUS and control (control: 12.9% vs LITE: 11.5% vs PLUS: 23.8%, P=0.001) (6) No significant difference in family presence during the visits between interventions (LITE: 35% vs PLUS: 37%, P=0.6). |
| Lefler et al, 2018 17 | United States: Arkansas |
28 (82% <60 y, 43% female, 89.28% completed) |
(1) mHealth connected to a 24‐hour call center: 7 (88.7% completed) (2) Digital home equipment: 11 (81.81% completed) |
Standard care: 10 (100% completed) | 12 wk |
(1) mHealth connected to a 24‐h call center (2) Digital home equipment |
A Cloud DX‐connected Health Kit containing Android Health Tablet with Bluetooth‐paired body weight scale and pulse wave Universal serial bus blood pressure wrist monitor | 0 | 0,12 wk | PP | Standard care: regular instruction with no home equipment |
(1) 100% of mHealth and home equipment groups monitored vitals daily post intervention. (2) 36% had technology anxiety and 32% were afraid of technology. (3) Qualitative interview revealed 4 important themes regarding communication with providers, usefulness of home monitoring, confidence in self‐monitoring, and uncertainty with persistent health problems. |
COM indicates comprehensive outpatient management; HF, heart failure; ITT, intention to treat; mHealth, mobile health; PP, per‐protocol; RR, relative risk; and TSM, telehealth self‐monitoring.
Figure 2. US bubble map based on the location, type of intervention, and number of participants investigated from selected studies.
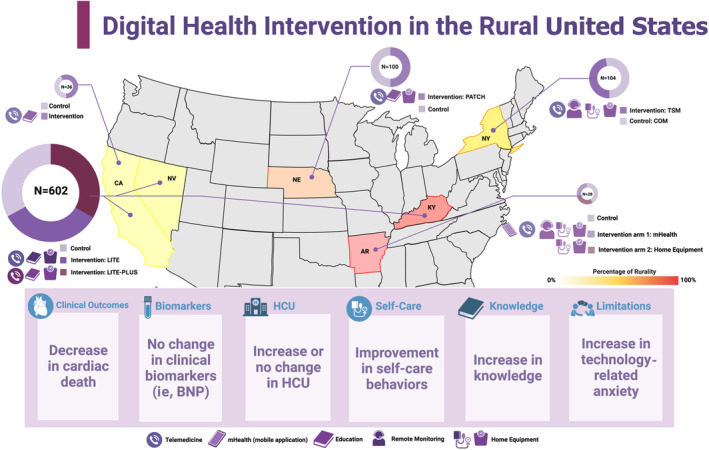
The figure displays the results of 5 studies between 2005 and 2019 13 , 14 , 15 , 16 , 17 included in this review, which were conducted in underserved rural regions of 6 US states: New York, Nebraska, California, Kentucky, Nevada, and Arizona. All of the studies used digital health technologies, including telemonitoring (via phone or video), remote monitoring (via a call center), or mHealth (via mobile devices), and 4 of the studies provided home equipment such as blood pressure cuffs and scales. The ring pie chart indicates the size of each study, including the number of participants and the proportion of intervention and control groups (represented by different colors). The number of participants in each study is also displayed within the chart. The rurality of each state is illustrated by a heat map, which shows a color gradient ranging from light yellow to indicate 0% rurality, progressively darkening to red to represent areas of 100% rurality (Arkansas (44.5%): pure red, Kentucky (41.3%): red, Nebraska (27%): medium red‐orange, New York (12.6%): light red‐orange, California (5.8%): yellow, Nevada (5.9%): yellow). The heat map categorically delineates the rural nature of each study area based on most recent census data (percentage of the population living in rural areas, rural population density, and rural land area). 18 The boxes provide a summary of the key findings from all of the studies. Created with BioRender.com. BNP indicates B‐type natriuretic peptide; COM, comprehensive outpatient management; HCU, health care use; mHealth, mobile health; and TSM, telehealth self‐monitoring.
Quality of Studies
Table 2 displays the findings of the quality assessment conducted by the National Heart, Lung, and Blood Institute on the 5 studies. Of these, 1 study was classified as having good quality, 15 2 studies as fair, 13 , 14 and 2 as poor. 16 , 17 The studies that received a poor‐quality rating were evaluated as such due to various biases including selection, randomization, reporting, attrition, multiple testing, imprecision, and lack of generalizability, possibly attributable to small sample sizes.
Table 2.
Quality Assessment
| Study | Pekmezaris et al, 2019 13 | Young et al, 2016 14 | Caldwell et al, 2005 16 | Dracup et al, 2014 15 | Lefler et al, 2018 17 |
|---|---|---|---|---|---|
| Was the study described as randomized, a randomized trial, a randomized clinical trial, or an randomized controlled trial? | Yes | Yes | Yes | Yes | Yes |
| Was the method of randomization adequate (ie, use of randomly generated assignment)? | Yes | Yes | NR | Yes | NR |
| Was the treatment allocation concealed (so that assignments could not be predicted)? | Yes | Yes | NR | Yes | NR |
| Were study participants and providers blinded to treatment group assignment? | NR | No | NR | Yes | NR |
| Were the people assessing the outcomes blinded to the participants' group assignments? | No | Yes | NR | Yes | NR |
| Were the groups similar at baseline on important characteristics that could affect outcomes (eg, demographics, risk factors, comorbid conditions)? | Yes | No | Yes | Yes | NR |
| Was the overall drop‐out rate from the study at end point 20% or lower of the number allocated to treatment? | Yes | Yes | NR | Yes | Yes |
| Was the differential dropout rate (between treatment groups) at the end point 15 percentage points or lower? | Yes | Yes | NR | Yes | Yes |
| Was there high adherence to the intervention protocols for each treatment group? | Yes | Yes | NR | Yes | Yes |
| Were other interventions avoided or similar in the groups (eg, similar background treatments)? | Yes | Yes | NR | Yes | Yes |
| Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants? | Yes | Yes | No | Yes | Yes |
| Did the authors report that the sample size was sufficiently large to be able to detect a difference in the main outcome between groups with at least 80% power? | No | Yes | No | Yes | No |
| Were outcomes reported or subgroups analyzed prespecified (ie, identified before analyses were conducted)? | Yes | Yes | Yes | Yes | Yes |
| Were all randomized participants analyzed in the group to which they were originally assigned (ie, did they use an intention‐to‐treat analysis?)? | Yes | No | No | Yes | No |
| Quality | Fair | Fair | Poor | Good | Poor |
| Limitations |
|
|
|
|
|
NR indicates not reported.
Intervention Features
The details of interventions are summarized in Table 1. Each of the 5 studies employed telemedicine interventions such as scheduled phone or video visits, 13 , 14 , 15 , 16 , 17 and 4 studies provided home equipment including weight scales and blood pressure cuffs. 13 , 14 , 15 , 17 Two studies used remote monitoring 13 , 17 and 1 study used mobile health technology. 17 Additionally, 3 studies provided education and counseling sessions and used telemedicine to reinforce the content presented to participants. 14 , 15 , 17 In the study by Lefler et al, 17 participants in the home equipment group were encouraged to record their blood pressure and weight using pen and paper. The study by Dracup et al, 15 provided HF symptom diaries to patients for symptom logging. The remaining studies relied on patients reporting HF symptoms during their visits. The PATCH (Self‐Management Adherence in Heart Failure Patients) trial 14 was the only study that emphasized daily salt intake tracking and reinforced medication adherence by providing a pill organizer and reminder alarm.
In the study by Caldwell et al, 16 patients were only given education on weight management and symptoms. The PATCH trial 14 and REMOTE‐HF 15 primarily aimed at educating patients on fluid management and weight, whereas studies by Pekmezaris 13 and Lefler et al, 17 required patients to monitor all vital signs. 13 , 17 REMOTE‐HF trial 15 consisted of 2 intervention groups (LITE and PLUS). Both intervention groups received a face‐to‐face education session delivered by a nurse focused on self‐care. However, the LITE group received only 2 follow‐up phone calls, whereas the PLUS group received biweekly calls until they achieved content competency. The PATCH intervention consisted of an in‐hospital self‐management training session delivered one on one by telephone, as well as postdischarge reinforcement sessions. These reinforcement sessions occurred twice a week during the first 2 weeks, once a week for weeks 3–6, and every other week for weeks 7–12. 14
In the study by Young et al, 14 the educational content for the intervention group was developed based on the components of Lorig's chronic disease self‐management model, 19 Hibbard's patient activation theory, 20 and Bandura's conceptualization of self‐efficacy. 21 On the other hand, a study by Dracup et al, 15 employed the teach‐back strategy, where patients were asked to repeat what they had been taught. In contrast, the educational content for the control group in all studies 13 , 14 , 15 , 16 , 17 consisted of standard discharge teaching for HF, including the American Heart Association guidelines for HF management. 13 None of the studies had a primary focus on optimizing guideline‐directed medical therapies.
Patients in the usual arm in all studies were encouraged to adhere to standards such as monitoring medications, blood pressure, weight, diet, and lipid profile, as well as receiving patient education within their respective study. 13 , 14 , 15 , 16 , 17
Clinical Outcomes, Health Care Resource Use, and Biomarkers
Only the REMOTE‐HF study measured clinical outcomes, 15 including cardiac death and a composite of HF hospitalization and overall cardiac mortality. The prevalence of combined clinical outcomes (cardiac death and HF hospitalization) over 2 years did not differ significantly between the intervention (LITE and PLUS) and control groups (control: 37.8% versus LITE: 28.5% versus PLUS: 38.9%, P=0.058). There was also no significant difference in the time of HF hospitalization or cardiac death (P=0.1). The rate of cardiac death was lower in the LITE group compared with the control group (control: 17.7% versus LITE: 7.5% versus PLUS: 11.9%, P=0.003). However, there was no significant difference between the 2 intervention groups.
Three studies measured changes in health care resource use following intervention. However, there was no significant change in emergency department visits, 13 hospitalization, 13 length of stay, 13 or time to hospitalization 15 in the intervention groups compared with controls. Furthermore, in 2 trials, the number of all‐cause hospitalizations 13 and 30‐day readmissions 14 was greater in the intervention groups than in the control groups. The REMOTE‐HF study found that the LITE intervention group reported a significantly lower number of scheduled or nonscheduled office visits compared with the PLUS and control groups (P=0.001) 15 (Table 1).
Two studies assessed the clinical biomarker BNP (B‐type natriuretic peptide) levels following the interventions. 14 , 16 In both studies, there were no significant changes observed in BNP levels (Table 1).
Self‐Care Behaviors and Knowledge
Four studies evaluated self‐care behavior following the intervention. 13 , 14 , 15 , 16 , 17 These studies reported a significant improvement in patient‐reported self‐management adherence to daily weights, a low‐sodium diet, prescribed medications, and daily exercise in the intervention arms compared with the control arms. 13 , 14 , 15 , 16 , 17 REMOTE‐HF trial led to a significant improvement in self‐care score (measured by the 9‐item European HF Self‐Care Behavior Scale) after 3 and 12 months compared with the control group, with no significant difference between the 2 intervention groups. 15 Only 1 study showed an increase in knowledge in the intervention group compared with the control 16 (Table 1).
Anxiety
The anxiety levels of participants were evaluated in 2 studies. 13 , 17 The study by Pekmezaris et al 13 showed that the intervention group had a higher follow‐up level of general anxiety compared with the control group (Patient Health Questionnaire‐4: intervention=28%; control=13%; P=0.05). Conversely, a study by Lefler et al 17 reported that 36% of participants experienced technology anxiety, and 32% of older adults were afraid of technology at baseline. There were no significant changes observed after the intervention across arms (Table 1).
Discussion
The findings of this systematic review suggest that digital health interventions have the potential to improve the self‐care and knowledge of patients with HF residing in underserved rural areas. However, existing studies have not demonstrated a positive effect on clinical outcomes or health care resource use. Future digital health research should evaluate how such tools can improve HF clinical outcomes or reduce health care use in these high‐risk underserved rural populations.
Digital health technologies hold the potential for reducing disparities in care across rurality and socioeconomic status. In underserved rural areas where access to health care facilities and chronic care management services are limited, digital health technologies can be used to improve access to care via telemedicine consultations and to enable remote monitoring. These interventions can theoretically improve quality of care, improve patient knowledge and behaviors around self‐management, and ultimately reduce hospital admissions, improve quality of life, and increase survival. 22
Role of Digital Health in Improving Knowledge and Self‐Care
Adherence to self‐management guidelines tends to be lower in underserved rural populations with HF. 14 Studies have indicated that nonadherence to self‐management guidelines is responsible for 21% to 55% of hospital readmissions in patients with HF. 23 , 24 , 25 , 26 Self‐management is critical for HF, given the need to identify signs of decompensation and adhere to a complex regimen of medications and lifestyle recommendations around diet and exercise. Effective interventions should be designed to include strategies 27 that promote both self‐efficacy and activation and leverage the potential of digital health interventions. Previous studies have demonstrated 17 that digital health interventions are feasible and have the potential to improve self‐management in older adults with HF, with users reporting feeling more secure knowing that they are under the care and observation of a health care provider. We found digital interventions for patients residing in underserved rural areas led to a significant improvement in patient‐reported self‐management adherence following the interventions. 13 , 14 , 15 , 16 , 17 A 2014 study 14 , 28 examined home‐based, postacute care services to enhance patient activation and improve self‐management adherence in patients with HF discharged from underserved rural hospitals. The study highlighted the importance of leveraging the expertise of advanced practice nurses and tailoring the intervention to the needs of underserved rural patients by developing a conceptual framework to guide the design and implementation of activation‐enhancing interventions.
Further investigation of interpersonal factors, such as cultural beliefs and access to care, would improve our understanding of self‐management behavior in rural populations with HF. The quality of the patient‐provider interaction 29 is an independent predictor of patient activation and self‐management behaviors in populations with various chronic illnesses. By addressing these underlying mechanisms via digital health interventions, it may be possible to improve outcomes and reduce the economic burden for patients with HF in underserved rural areas.
Clinical Outcomes and Health Care Use
HF is characterized by recurrent periods of clinical exacerbation, resulting in high rates of emergency department and inpatient hospital use, leading to poor health outcomes, decreased quality of life, and exorbitant health care costs. Standard outpatient management programs are often resource‐intensive and limited to major urban medical centers. Although some evidence exists suggesting that adequate self‐care is associated with improved health outcomes, the link between HF self‐care and health outcomes remains inconclusive. 30 , 31 Our review revealed that the digital interventions in underserved rural areas, although contributing to improvements in self‐care and knowledge, did not demonstrate significant improvements in either clinical outcomes or health care resource use. There could be multiple reasons for this. First, self‐management and education may require longer time periods to demonstrate benefits. Second, prior trials may have been too small and underpowered to show meaningful effects. Third, effective medical therapies are likely the most established mechanisms of improving clinical outcomes for HF, but interventions around knowledge and self‐care have not focused around optimization of medical therapy. Interventions around education may have greater impact on outcomes if tied to optimization of medical therapy. An illustrative instance of guideline‐directed medical therapy (GDMT) optimization in urban settings is the EPIC HF (Electronically Delivered, Patient‐Activation Tool for Intensification of Medications for Chronic Heart Failure with Reduced Ejection Fraction) study. 6 In this trial, 306 outpatients with HF with reduced ejection fraction were randomized to standard care or an intervention group receiving patient activation tools: a 3‐minute video and a 1‐page checklist emphasizing GDMT importance. The intervention led to a remarkable 20% absolute increase in GDMT initiation or intensification within 30 days. This highlights the significant potential of improving patient engagement for enhancing GDMT rates. Future digital health designs need to consider how to translate self‐management improvements into reduced morbidity and costs, and future digital health trials should be designed with these outcomes in mind.
Although digital health interventions have the potential to improve outcomes for patients with HF, it is essential to consider social determinants of health in the design and implementation of these interventions to ensure equitable access and improved outcomes for all patients with HF. This highlights the need for continued research and development of digital health interventions that are culturally and linguistically appropriate, as well as tailored to meet the specific needs of patients with HF in various environments.
Anxiety
Technology‐related anxiety, especially among older adults, is a significant barrier to the adoption of digital health services in underserved rural areas. Pekmezaris et al 13 found that the intervention group had higher levels of general anxiety during follow‐up compared with the control group. According to the study by Lefler et al, 17 36% of older adults in the intervention group reported having technology anxiety, and 32% were afraid of technology at baseline, with no significant change post intervention. 17 These anxieties may stem from feelings of powerlessness during the process of regaining independence. 17 Educational levels are also highly correlated with technology use; older adults who are more affluent and have higher educational levels have similar rates of technology use as adults 65 and younger. Despite the increase in adoption by older adults, a Pew report found that 73% of older adults still require assistance in setting up or using new electronic devices. 32
Several actions can be taken to address this issue such as providing education and training on technology use, developing user‐friendly technology and digital health services, involving older adults in the design and development of technology (patient partners), addressing privacy and security concerns, and encouraging partnerships between health care providers and community organizations. These steps can improve access to technology and digital health services among older adults living in underserved rural areas and help ensure that everyone has the opportunity to benefit from these innovations.
Limitations
Our systematic review has limitations. First, although limiting the review to randomized trials reduces biases, valuable nonrandomized studies may have been overlooked. Additionally, publication bias may have resulted in the exclusion of relevant studies. Furthermore, due to significant heterogeneity among the studies, we refrained from pooling the study results. Several interventions reviewed in this study were found to be human resource‐intensive, emphasizing the need for future research to include rigorous costing studies that document the costs and cost‐effectiveness of these interventions. Finally, in this review, we focused on digital health interventions in underserved rural areas. However, it is important to examine parallels between successful interventions in rural and urban populations, particularly in underserved communities. 33 Both rural and urban underserved populations encounter barriers to health care access, including shortages of clinicians and challenges of transportation to care. Socioeconomic factors, such as lower income and educational background, contribute to disparities access across both settings. To ensure effective digital health interventions, it is also essential to consider the unique challenges faced by each population. Further research should further explore specific similarities and differences between digital health interventions in underserved rural and urban populations to promote equitable health care solutions for everyone, regardless of their location. 33 It is important to note that findings might not uniformly apply to all contexts, emphasizing the significance of tailored health care solutions that ensure equity across geographical locations.
Future Directions
Future research that demonstrates the potential impact of digital health for improving HF in rural communities should be prioritized. This will require developing interventions that are developed with community input and are culturally appropriate. One illustrative example is the Fostering African‐American Improvement in Total Health (FAITH!) app, which was developed through a community‐based participatory research partnership with Black churches. This app was a successful example of culturally tailored intervention that led to overall improvement of cardiovascular health of the participants. 34 To be effective, interventions will also need to address limited broadband access. To tackle this, mobile health apps should be designed for low‐bandwidth use or interventions should explore satellite Internet options. Interventions must also account for fewer health care resources available in rural environments. Digital health interventions may leverage remote resources and minimize the need for in‐person encounters. In addition to increasing access, interventions will need to address psychosocial barriers to digital literacy among rural communities. This can be addressed through educational initiatives in collaboration with local organizations and educators. The intersection of public policy and public access is critical to bridging the digital divide and advancing digital inclusion. Finally, interventions need to be tested in randomized trials that demonstrate the impact on both clinical outcomes and resource use to identify strategies that are worth the investment needed for broader implementation.
Conclusions
Digital health interventions have the potential to increase access to care, improve patient education and self‐management, and ultimately improve clinical outcomes for patients with HF in underserved rural areas. However, patients in underserved rural areas face unique challenges related to broadband access and digital literacy, which may affect the feasibility and effectiveness of digital health interventions. It is important to address these challenges to design digital health interventions that are accessible and appropriate across a broad range of patients. 17 We found evidence that digital health interventions can be designed to successfully promote self‐efficacy and activation in underserved rural populations with HF. However, continued research is needed to better understand how digital health interventions in HF can also translate to improved clinical outcomes, as well as to investigate potential ripple effects of digital interventions. Increasing the emphasis on the use of GDMT in digital health interventions is one promising approach. By both improving patient self‐efficacy and self‐management and improving quality of care, digital health interventions may be able to help reduce the impact of existing disparities in access for patients with HF in underserved rural areas.
Sources of Funding
Zahra Azizi, Mario Funes Hernandez, Paul Wang, and Alex T. Sandhu were funded by American Heart Association Health Technology and Innovation Strategically Focused Research Network.
Disclosures
None.
Supporting information
Table S1
Z. Azizi and C. Broadwin are co‐first authors.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030956
For Sources of Funding and Disclosures, see page 13.
References
- 1. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272–3287. doi: 10.1093/cvr/cvac013 [DOI] [PubMed] [Google Scholar]
- 2. Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. doi: 10.1161/CIRCHEARTFAILURE.117.004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leung YW, Brual J, Macpherson A, Grace SL. Geographic issues in cardiac rehabilitation utilization: a narrative review. Health Place. 2010;16:1196–1205. doi: 10.1016/j.healthplace.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turecamo SE, Xu M, Dixon D, Powell‐Wiley TM, Mumma MT, Joo J, Gupta DK, Lipworth L, Roger VL. Association of rurality with risk of heart failure. JAMA Cardiol. 2023;8:231–239. doi: 10.1001/jamacardio.2022.5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray R, Indraratna P, Lovell N, Ooi SY. Digital health technology in the prevention of heart failure and coronary artery disease. Cardiovasc Digit Health J. 2022;3:S9–S16. doi: 10.1016/j.cvdhj.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen LA, Venechuk G, McIlvennan CK, Page RL, Knoepke CE, Helmkamp LJ, Khazanie P, Peterson PN, Pierce K, Harger G, et al. An electronically delivered patient‐activation tool for intensification of medications for chronic heart failure with reduced ejection fraction. Circulation. 2021;143:427–437. doi: 10.1161/CIRCULATIONAHA.120.051863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adedinsewo DA, Pollak AW, Phillips SD, Smith TL, Svatikova A, Hayes SN, Mulvagh SL, Norris C, Roger VL, Noseworthy PA, et al. Cardiovascular disease screening in women: leveraging artificial intelligence and digital tools. Circ Res. 2022;130:673–690. doi: 10.1161/CIRCRESAHA.121.319876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guasti L, Dilaveris P, Mamas MA, Richter D, Christodorescu R, Lumens J, Schuuring MJ, Carugo S, Afilalo J, Ferrini M, et al. Digital health in older adults for the prevention and management of cardiovascular diseases and frailty. A clinical consensus statement from the ESC Council for Cardiology Practice/Taskforce on Geriatric Cardiology, the ESC Digital Health Committee and the ESC Working Group on e‐Cardiology. ESC Heart Fail. 2022;9:2808–2822. doi: 10.1002/ehf2.14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hernandez MF, Rodriguez F. Health techequity: opportunities for digital health innovations to improve equity and diversity in cardiovascular care. Curr Cardiovasc Risk Rep. 2023;17:1–20. doi: 10.1007/s12170-022-00711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dracup K, Moser DK, Pelter MM, Nesbitt T, Southard J, Paul SM, Robinson S, Hemsey JZ, Cooper L. Rural patients' knowledge about heart failure. J Cardiovasc Nurs. 2014;29:423–428. doi: 10.1097/JCN.0b013e31829cbcf3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Study quality assessment tools. The National Heart, Lung and Blood Institute. 2013. Accessed September 1, 2023. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools
- 12. Sperotto F, Daverio M, Amigoni A, Gregori D, Dorste A, Allan C, Thiagarajan RR. Trends in in‐hospital cardiac arrest and mortality among children with cardiac disease in the intensive care unit: a systematic review and meta‐analysis. JAMA Netw Open. 2023;6:e2256178. doi: 10.1001/jamanetworkopen.2022.56178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pekmezaris R, Nouryan CN, Schwartz R, Castillo S, Makaryus AN, Ahern D, Akerman MB, Lesser ML, Bauer L, Murray L, et al. A randomized controlled trial comparing telehealth self‐management to standard outpatient management in underserved Black and Hispanic patients living with heart failure. Telemed J E Health. 2019;25:917–925. doi: 10.1089/tmj.2018.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young L, Hertzog M, Barnason S. Effects of a home‐based activation intervention on self‐management adherence and readmission in rural heart failure patients: the PATCH randomized controlled trial. BMC Cardiovasc Disord. 2016;16:176. doi: 10.1186/s12872-016-0339-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dracup K, Moser DK, Pelter MM, Nesbitt TS, Southard J, Paul SM, Robinson S, Cooper LS. Randomized, controlled trial to improve self‐care in patients with heart failure living in rural areas. Circulation. 2014;130:256–264. doi: 10.1161/circulationaha.113.003542 [DOI] [PubMed] [Google Scholar]
- 16. Caldwell MA, Peters KJ, Dracup KA. A simplified education program improves knowledge, self‐care behavior, and disease severity in heart failure patients in rural settings. Am Heart J. 2005;150:983–983.e12. doi: 10.1016/j.ahj.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 17. Lefler LL, Rhoads SJ, Harris M, Funderburg AE, Lubin SA, Martel ID, Faulkner JL, Rooker JL, Bell DK, Marshall H, et al. Evaluating the use of mobile health technology in older adults with heart failure: mixed‐methods study. JMIR Aging. 2018;1:e12178. doi: 10.2196/12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Most rural states 2023. World Population Review. 2023. Accessed September 1, 2023. https://worldpopulationreview.com/state‐rankings/most‐rural‐states
- 19. Lorig K. Self‐management of chronic illness: a model for the future. Generations: J Am Soc Aging. 1993;17:11–14. [Google Scholar]
- 20. Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self‐management behaviors? Health Serv Res. 2007;42:1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bandura A, Freeman WH, Lightsey R. Self‐Efficacy: The Exercise of Control. Springer; 1999.
- 22. Gamble J‐M, Eurich DT, Ezekowitz JA, Kaul P, Quan H, McAlister FA. Patterns of care and outcomes differ for urban versus rural patients with newly diagnosed heart failure, even in a universal healthcare system. Circ Heart Fail. 2011;4:317–323. doi: 10.1161/CIRCHEARTFAILURE.110.959262 [DOI] [PubMed] [Google Scholar]
- 23. van der Wal MHL, Jaarsma T, Moser DK, van Gilst WH, van Veldhuisen DJ. Qualitative examination of compliance in heart failure patients in The Netherlands. Heart Lung. 2010;39:121–130. doi: 10.1016/j.hrtlng.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 24. Opasich C, Rapezzi C, Lucci D, Gorini M, Pozzar F, Zanelli E, Tavazzi L, Maggioni AP. Precipitating factors and decision‐making processes of short‐term worsening heart failure despite "optimal" treatment (from the IN‐CHF registry). Am J Cardiol. 2001;88:382–387. doi: 10.1016/s0002-9149(01)01683-6 [DOI] [PubMed] [Google Scholar]
- 25. Michalsen A, König G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–441. doi: 10.1136/hrt.80.5.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennett SJ, Huster GA, Baker SL, Milgrom LB, Kirchgassner A, Birt J, Pressler ML. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7:168–174. doi: 10.4037/ajcc1998.7.3.168 [DOI] [PubMed] [Google Scholar]
- 27. Young L, Barnason S, Kupzyk K. Mechanism of engaging self‐management behavior in rural heart failure patients. Appl Nurs Res. 2016;30:222–227. doi: 10.1016/j.apnr.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young L, Barnason S, Do V. Promoting self‐management through adherence among heart failure patients discharged from rural hospitals: a study protocol. F1000Res. 2014;3:317. doi: 10.12688/f1000research.5998.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dixon A, Hibbard J, Tusler M. How do people with different levels of activation self‐manage their chronic conditions? Patient. 2009;2:257–268. doi: 10.2165/11313790-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 30. Riegel B, Lee CS, Dickson VV. Self care in patients with chronic heart failure. Nat Rev Cardiol. 2011;8:644–654. doi: 10.1038/nrcardio.2011.95 [DOI] [PubMed] [Google Scholar]
- 31. Jiang Y, Shorey S, Seah B, Chan WX, Tam WWS, Wang W. The effectiveness of psychological interventions on self‐care, psychological and health outcomes in patients with chronic heart failure—a systematic review and meta‐analysis. Int J Nurs Stud. 2018;78:16–25. doi: 10.1016/j.ijnurstu.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 32. Anderson M, Perrin A. Tech adoption climbs among older adults. Pew Research Center. 2017. https://www.pewresearch.org/internet/2017/05/17/tech‐adoption‐climbs‐among‐older‐adults/
- 33. Chen X, Orom H, Hay JL, Waters EA, Schofield E, Li Y, Kiviniemi MT. Differences in rural and urban health information access and use. J Rural Health. 2019;35:405–417. doi: 10.1111/jrh.12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brewer LC, Jenkins S, Hayes SN, Kumbamu A, Jones C, Burke LE, Cooper LA, Patten CA. Community‐based, cluster‐randomized pilot trial of a cardiovascular mobile health intervention: preliminary findings of the FAITH! Trial. Circulation. 2022;146:175–190. doi: 10.1161/CIRCULATIONAHA.122.059046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


