Abstract
Evidence suggests that aspirin use reduces the occurrence of colorectal neoplasia. Few studies have investigated the association among Black Americans, who are disproportionately burdened by the disease. We assessed aspirin use in relation to colorectal adenoma among Black women. The Black Women’s Health Study is a prospective cohort of self-identified Black American women established in 1995. Participants reported regular aspirin use on baseline and follow-up questionnaires. Beginning in 1999, participants reported undergoing a colonoscopy or sigmoidoscopy, the only procedures through which colorectal adenomas can be diagnosed. Multivariable logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for associations between aspirin use and colorectal adenoma among 34,397 women who reported at least 1 colonoscopy or sigmoidoscopy. From 1997 through 2018, 1,913 women were diagnosed with an adenoma. Compared to non-aspirin users, regular users had a 14% (OR=0.86, 95% CI 0.78–0.95) lower odds of adenoma. The odds of adenoma decreased with increasing duration of aspirin use (≥10 years: OR=0.80, 95% CI 0.66–0.96). Initiating aspirin at a younger age was associated with a reduced adenoma occurrence (age <40 years at initiation: OR=0.69, 95% CI 0.55–0.86). Regular aspirin use was associated with a decreased odds of colorectal adenoma in this study of Black women. These findings support evidence demonstrating a chemopreventive impact of aspirin on colorectal neoplasia and suggest that aspirin may be a useful prevention strategy among US Black women.
Keywords: Aspirin, NSAIDs, Colorectal adenoma, Black Women
Graphical Abstract
INTRODUCTION
In the United States (US), Black Americans have the highest colorectal cancer (CRC) incidence and mortality rates of any racial/ethnic group, and are more likely to be diagnosed at a younger age and after the disease has spread beyond the colon.1 Colorectal adenoma is the primary premalignant lesion of CRC.2 Black Americans have a high prevalence of colorectal adenoma3 and are more likely than White Americans to be diagnosed with larger, more advanced adenomas,4 which have greater potential to progress into cancer. Thus, new strategies for primary prevention of CRC are imperative.
In randomized clinical trials and observational studies, regular use of aspirin or non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) has been inversely associated with adenoma5–19 and CRC.6,20–31 In 2016, the US Preventive Services Task Force (USPSTF) recommended use of low-dose aspirin for chronic disease prophylaxis, including CRC prevention, in adults aged 50–59 with increased risk of cardiovascular disease (CVD).32 However, in April 2022, the USPSTF changed their recommendation on aspirin use based on an updated assessment of evidence.33 While the USPSTF concluded that there is insufficient evidence that aspirin reduces CRC incidence and mortality, they acknowledged that evidence surrounding CRC outcomes was limited by the number of reporting studies, duration of follow-up, and power, suggesting that further research is needed.33 Moreover, many of the trials evaluated included majority White populations or did not report on race/ethnicity.33 Investigating associations by race/ethnicity may reveal certain high-risk populations, such as Black Americans, for whom the benefits of aspirin may outweigh the harms.
Few studies have assessed the association between aspirin and colorectal neoplasia among Black American populations.8,25,31,34 In a cross-sectional study, a non-significant inverse association was observed between NSAID use and adenoma prevalence among Black American participants.8 In a case-control study, NSAID use was associated with a 60% reduced risk of CRC in Black Americans,25 but this was not replicated in a larger extension of that study.34 In a prospective cohort study with sufficient power to examine associations by race and sex, aspirin use was associated with a 29% reduced risk of CRC in Black American men, but not women.31
In light of the updated recommendations for use of aspirin, the limited data among Black Americans, and the high burden of colorectal neoplasia experienced by Black Americans, it is critical that the impact, if any, of aspirin on colorectal neoplasia be established and quantified. Because the chemopreventive benefits of aspirin may be observed for colorectal adenoma occurrence after a shorter duration of use compared to colorectal cancer incidence,5,6,8–10,13,16 and most colorectal cancers develop from adenomas,2 in the present study, we examined the association between aspirin use and colorectal adenoma in a cohort of Black American women.
MATERIALS AND METHODS
Study Population.
In 1995, 59,000 self-identified Black American women aged 21–69 years were enrolled in the Black Women’s Health Study (BWHS) by returning a completed baseline questionnaire. After enrollment, BWHS participants were followed through biennial questionnaires that collected data on demographics, medication use, lifestyle and behavioral factors, and medical diagnoses. Follow-up is complete for approximately 85% of potential person-years. On the 1999 questionnaire and every subsequent questionnaire, BWHS participants reported whether they had undergone a colonoscopy or flexible sigmoidoscopy procedure (hereafter referred to as lower endoscopy) in the past two years. Endoscopy history prior to 1997 was unknown. Thus, women who reported an endoscopy during the study period may have undergone the procedure for screening or surveillance purposes. Because colorectal adenomas can only be identified through lower endoscopy, BWHS participants had to report at least one lower endoscopy in order to be eligible for the present analysis (n=36,875). Participants were excluded if they had cancer (n=2,431) or a colorectal adenoma (n=47) at baseline; 34,397 participants were included in the analytic sample.
Adenomas can grow indolently for many years before detection by endoscopy. Consequently, some adenomas may have developed prior to aspirin use—assessed two years prior to adenoma occurrence—violating the assumption of temporality. To reduce temporality concerns, in secondary analyses, we restricted the analytic sample to participants who reported two or more lower endoscopies during the study period and were adenoma-free at the first lower endoscopy (n=23,230). These secondary analyses were a proxy for “incident” colorectal adenoma, as women were less likely to have an adenoma at the time of aspirin assessment. Moreover, women with an adenoma diagnosis prior to baseline were excluded from the analytic sample.
Exposure Ascertainment.
Aspirin use was the primary exposure of interest in this analysis. On the 1995 baseline questionnaire and all follow-up questionnaires, participants reported current regular aspirin use, defined as use for ≥3 days/week. Current users at baseline also reported duration of use (1, 2, 3–4, ≥5 years). Non-aspirin NSAID use was assessed as a secondary exposure in this analysis. In 2009 and on each subsequent questionnaire, participants reported current regular use of non-aspirin NSAIDs. Common brand names were included on questionnaires to help participants recall medication use.
Medication use was assessed at the questionnaire cycle immediately prior to lower endoscopy report. We treated medication use as time-varying and modeled it as two separate exposures for each medication: ever use (non-use, ever use) and current use (non-use, past, current use). Current medication users were re-classified as past users if they no longer reported current medication use on subsequent questionnaires. Among aspirin users, duration of aspirin use was calculated by summing the duration of use reported on the 1995 questionnaire and every two-year period in which a participant reported current aspirin use. Duration of aspirin use was categorized as non-use, <5, 5–9, ≥10 years. Duration of non-aspirin NSAID use was calculated by summing every two-year period in which current non-aspirin NSAID use was reported, starting in 2009, and was examined categorically (non-use, 1–2, 3–4, ≥5 years). To estimate the odds of adenoma associated with a 5-year increase in aspirin or non-aspirin NSAID use, we assessed duration of aspirin use and duration of non-aspirin NSAID use as continuous variables. We also considered age at aspirin initiation since recommendations by the USPSTF target specific age groups.33 Using data on duration of aspirin use reported on the 1995 questionnaire and participants’ age at the questionnaire cycle in which they first reported aspirin use, we estimated age at aspirin initiation and classified it into five categories (non-use, <40, 40–59, 60–64, ≥65 years) in primary analyses and into three categories (non-use, <60, ≥60 years) in stratified analyses.
Outcome Ascertainment.
First colorectal adenoma occurrence [during the study period] was the outcome of interest. Participants could contribute only one adenoma occurrence to this analysis. Beginning in 1999 and on each subsequent follow-up questionnaire, participants self-reported new colon or rectal polyp diagnoses that had occurred in the prior two years, as well as the year of diagnosis. After obtaining participant permission to access medical records, study personnel reviewed pathology reports to confirm polyp diagnoses and determine whether confirmed polyps were adenomas. Among participants who self-reported a polyp diagnosis and for whom medical records were abstracted, 76.1% had a pathologically confirmed polyp. Any adenoma (i.e., tubular, tubulovillous, villous, traditional serrated, sessile serrated) that was confirmed through medical record review was included as an adenoma case in this study. Hyperplastic and disconfirmed polyps were not included as adenoma cases and were, thus, considered non-cases.
Black Americans are more likely to be diagnosed with large adenomas4 and adenomas in the proximal colon,3 which are more difficult to visualize endoscopically and more likely to be missed.35 Given these disparities, data on adenoma location (proximal colon, distal colon, rectum) and size (<1, ≥1 cm) were also abstracted from pathology reports and were examined as secondary outcomes. If more than one adenoma was present at lower endoscopy, the largest adenoma size was used for classification in size-specific analyses. If a participant presented with an adenoma in more than one location, she was included in each relevant location-specific analysis. Because flexible sigmoidoscopy procedures do not advance to and screen the proximal colon, location-specific analyses were restricted to participants who underwent a colonoscopy.
Statistical Analysis.
For the analysis, the data were structured in Anderson-Gill format according to each 2-year questionnaire cycle,36 which enabled participants to contribute a new record at each questionnaire cycle in which they reported a lower endoscopy. Participants contributed multiple records if they underwent more than one lower endoscopy. For example, if a woman reported a lower endoscopy in 2001, 2010, and 2015, with a first adenoma detected in 2010, she contributed a record to the 2001 and 2010 cycles and was censored after her diagnosis in 2010. On average, participants reported 2.3 (standard deviation=1.4, range, 1–9) lower endoscopies during the study period. Participants were censored at first colorectal adenoma diagnosis, cancer diagnosis (including colorectal cancer), death, or the end of the study period in 2018, whichever occurred first.
Logistic regression for longitudinal data using generalized estimating equations was used to account for multiple lower endoscopies per participant and to handle time-varying exposures and covariates. Age- and multivariable-adjusted odds ratios (OR) and 95% confidence intervals were estimated for the associations between medication use and colorectal adenoma, according to adenoma location and size. Established and hypothesized risk factors for colorectal adenoma were assessed as candidate covariates. Factors associated with both the exposures and outcome in age-adjusted regression models were selected for inclusion in multivariable models: age (continuous), family history of CRC (yes, no), smoking status (never, past, current), alcohol consumption (non-current, current 1–6, current ≥7 drinks/week), vigorous exercise (none, <5, ≥5 hours/week), red meat consumption (grams/day in quartiles), type 2 diabetes (yes, no), and postmenopausal hormone use (yes, no). Other candidate covariates, including body mass index (BMI), educational attainment, and fiber intake, were not included in multivariable models, as they were not associated with both exposures and outcome in the study sample.
Since current recommendations on aspirin use by the USPSTF target populations with increased CVD risk, we examined whether associations between aspirin and colorectal adenoma vary by strata of ideal cardiovascular health status (poor, intermediate, ideal cardiovascular health), based on the American Heart Association definition.37 Age and selected components of ideal cardiovascular health status (BMI, smoking status, and type 2 diabetes) were also assessed as potential effect measure modifiers.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary NC).
RESULTS
During the 1997–2018 study period, 1,913 women were diagnosed with a colorectal adenoma. Of these, 1,041 were located in the proximal colon, 515 in the distal colon, 110 in the rectum, 213 in multiple locations, and 34 were missing adenoma location data. Among the 1,865 cases that had available information on adenoma size, 1,413 adenomas were <1 cm in size and 452 were ≥1 cm. In secondary analyses exploring incident colorectal adenoma, 859 women were diagnosed with an incident adenoma. At the questionnaire cycle immediately prior to the time of first reported lower endoscopy, 34.4% of BWHS participants reported using aspirin ≥3 days a week. Participants who were diagnosed with an adenoma were more likely to have a family history of CRC and have a history of smoking (Table 1).
Table 1.
Age-standardized characteristics of the study population at the questionnaire cycle prior to first lower endoscopy since study enrollment, according to colorectal adenoma occurrence.
| Colorectal adenoma case | ||
|---|---|---|
| No (n=32,484) |
Yes (n=1,913) |
|
|
| ||
| Age | 51.3 (8.5) | 52.2 (7.3) |
| Family history of colorectal cancer, % | 12 | 20 |
| Body mass index, kg/m2 | 30.3 (6.8) | 30.2 (6.9) |
| Current smoking status | ||
| Never smoker, % | 61 | 57 |
| Past smoker, % | 26 | 29 |
| Current smoker, % | 12 | 14 |
| Alcohol consumption | ||
| Non-drinker, % | 62 | 66 |
| Current drinker, 1–6 drinks/week, % | 26 | 25 |
| Current drinker, ≥7 drinks/week, % | 4 | 6 |
| Vigorous exercise | ||
| None, % | 43 | 48 |
| <5 hours/week, % | 37 | 39 |
| ≥5 hours/week, % | 7 | 7 |
| Red meat consumption | ||
| Quartile 1 (low consumption), % | 24 | 22 |
| Quartile 2, % | 23 | 24 |
| Quartile 3, % | 23 | 25 |
| Quartile 4 (high consumption), % | 24 | 25 |
| Type 2 diabetes, % | 13 | 10 |
| Ever menopausal hormone use, % | 32 | 35 |
| Ideal cardiovascular health | ||
| Poor, % | 19 | 17 |
| Intermediate, % | 57 | 58 |
| Ideal, % | 15 | 18 |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
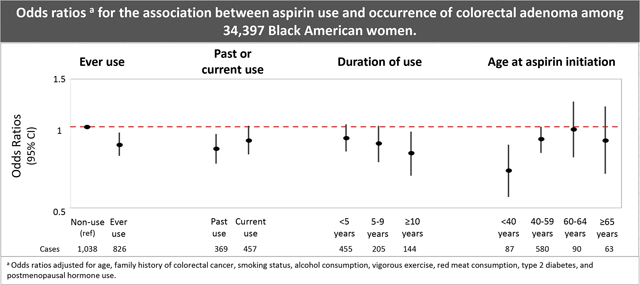
Regular aspirin use was associated with a lower odds of colorectal adenoma in both age- and multivariable-adjusted models, with little evidence of confounding by covariates included in the multivariable model (Table 2). Compared to non-users, women who ever used aspirin regularly had an estimated 14% (OR=0.86, 95% CI 0.78–0.95) lower odds of colorectal adenoma. The ORs for past and current aspirin use were 0.83 (95% CI 0.73–0.94) and 0.89 (95% CI 0.79–1.01), respectively. Longer duration of aspirin use was associated with a lower odds of adenoma, such that for every 5-year increase in duration of use, the odds of adenoma decreased by 8% (OR=0.92, 95% CI 0.86–0.98). The OR associated with at least 10 years of use vs. non-use was 0.80 (95% CI: 0.66, 0.96). Compared to non-users, initiating aspirin use at a younger age was associated with a lower odds of colorectal adenoma (age <40 at aspirin initiation: OR=0.69, 95% CI 0.55–0.86). Associations were slightly attenuated in secondary analyses examining aspirin use in relation to incident adenoma (Supplemental Table 1).
Table 2.
Odds ratios for the association between aspirin use and occurrence of colorectal adenoma.
| Aspirin use | Adenoma cases | Lower Endoscopiesa | Age-adjusted OR (95% CI) | MV-adjusted OR (95% CI)b |
|---|---|---|---|---|
|
| ||||
| Non-usec | 1,038 | 44,026 | 1.00 (ref) | 1.00 (ref) |
| Ever | 826 | 40,622 | 0.85 (0.77–0.93) | 0.86 (0.78–0.95) |
| Past | 369 | 19,025 | 0.81 (0.72–0.92) | 0.83 (0.73–0.94) |
| Current | 457 | 21,597 | 0.88 (0.78–0.99) | 0.89 (0.79–1.01) |
| Duration of use | ||||
| <5 years | 455 | 21,003 | 0.91 (0.81–1.01) | 0.91 (0.81–1.02) |
| 5–9 years | 205 | 9,902 | 0.86 (0.73–1.00) | 0.87 (0.74–1.01) |
| ≥10 years | 144 | 7,455 | 0.79 (0.66–0.95) | 0.80 (0.66–0.96) |
| Per 5-year increase in duration | 0.91 (0.86–0.98) | 0.92 (0.86–0.98) | ||
| Age at aspirin initiation | ||||
| <40 years | 87 | 5,407 | 0.68 (0.55–0.85) | 0.69 (0.55–0.86) |
| 40–59 years | 580 | 27,678 | 0.88 (0.79–0.98) | 0.90 (0.80–1.00) |
| 60–64 years | 90 | 3,968 | 0.95 (0.75–1.20) | 0.98 (0.77–1.24) |
| ≥65 years | 63 | 3,061 | 0.85 (0.64–1.13) | 0.89 (0.67–1.19) |
OR, Odds ratio; CI, Confidence interval; MV, Multivariable.
Total number of lower endoscopies among the 34,397 participants with at least one lower endoscopy reported. Some participants reported more than one lower endoscopy over the study period.
Odds ratios adjusted for age (continuous), family history of colorectal cancer (yes, no), smoking status (never, past, current), alcohol consumption (non-current, current 1–6, current ≥7 drinks/week), vigorous exercise (none, <5, ≥5 hours/week), red meat consumption (quartiles), type 2 diabetes (yes, no), and postmenopausal hormone use (yes, no).
Non-users are defined as participants who did not report regular aspirin use during the study period.
Ever using aspirin was associated with a similar decreased odds of proximal (OR=0.85, 95% CI 0.75–0.96), distal (OR=0.86, 95% CI 0.73–1.01), and rectal (OR=0.82, 95% CI 0.58–1.16; Table 3) adenoma. In analyses according to adenoma size, aspirin use was associated with a reduced odds of small (OR=0.82, 95% CI 0.73–0.92) but not large adenoma.
Table 3.
Odds ratios for the association between aspirin and colorectal adenoma according to adenoma location and size.
| Aspirin use | Adenoma location | Adenoma size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Proximal colon | Distal colon | Rectum | <1 cm | ≥1 cm | ||||||
|
|
|
|
|
|
||||||
| Adenoma cases | MV-adjusted OR (95% CI)a | Adenoma cases | MV-adjusted OR (95% CI)a | Adenoma cases | MV-adjusted OR (95% CI)a | Adenoma cases | MV-adjusted OR (95% CI)a | Adenoma cases | MV-adjusted OR (95% CI)a | |
|
|
|
|
|
|
|
|||||
| Non-useb | 654 | 1.00 (ref) | 380 | 1.00 (ref) | 89 | 1.00 (ref) | 775 | 1.00 (ref) | 239 | 1.00 (ref) |
| Ever | 539 | 0.85 (0.75–0.96) | 292 | 0.86 (0.73–1.02) | 66 | 0.82 (0.58–1.16) | 602 | 0.82 (0.73–0.92) | 202 | 0.97 (0.79–1.19) |
OR, Odds ratio; CI, Confidence interval; MV, Multivariable.
Odds ratios adjusted for age (continuous), family history of colorectal cancer (yes, no), smoking status (never, past, current), alcohol consumption (non-current, current 1–6, current ≥7 drinks/week), vigorous exercise (none, <5, ≥5 hours/week), red meat consumption (quartiles), type 2 diabetes (yes, no), and postmenopausal hormone use (yes, no).
Non-users are defined as participants who did not report regular aspirin use during the study period.
At study entry, 21%, 62%, and 17% of participants were classified as having poor, intermediate, and ideal cardiovascular health, respectively. Among women with poor, intermediate, and ideal cardiovascular health, 19.5%, 33.4%, and 52.8% reported ever having used aspirin, respectively. Based on stratified analyses, ideal cardiovascular health status appeared to modify the association between aspirin use and adenoma (pinteraction=0.07). Among BWHS participants with poor cardiovascular health, ever having used aspirin was associated with a 31% (OR=0.69, 95% CI 0.51–0.94; Table 4) lower odds of adenoma. Weaker inverse associations were observed among women with intermediate (OR=0.89, 95% CI 0.78–1.02) and ideal (OR=0.91, 95% CI 0.74–1.11) cardiovascular health. Similar inverse estimates were observed in women with poor cardiovascular health regardless of age at aspirin initiation (age <60 at initiation: OR=0.71, 95% CI 0.52–0.97; age ≥60 at initiation: OR=0.62, 95% CI 0.28–1.39), albeit imprecise. We observed weaker inverse associations in women of intermediate cardiovascular health (regardless of age at initiation) and women of ideal cardiovascular health who initiated aspirin use before age 60. Further investigation of ideal cardiovascular health status components suggested that effect modification by ideal cardiovascular health status may be driven, in part, by smoking status (ever aspirin use vs. non-use among never smokers: OR=0.90, 95% CI 0.79–1.03; among past smokers: OR=0.90, 95% CI 0.75–1.07; among current smokers: OR=0.66, 95% CI 0.50–0.87; Supplemental Table 2).
Table 4.
Odds ratios for the association between aspirin use and colorectal adenoma stratified by ideal cardiovascular health status.
| Aspirin use | Poor cardiovascular health | Intermediate cardiovascular health | Ideal cardiovascular health | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Adenoma cases | Age-adjusted OR (95% CI) | MV-adjusted OR (95% CI)a | Adenoma cases | Age-adjusted OR (95% CI) | MV-adjusted OR (95% CI)a | Adenoma cases | Age-adjusted OR (95% CI) | MV-adjusted OR (95% CI)a | |
|
|
|
|
|
||||||
| Non-useb | 207 | 1.00 (ref) | 1.00 (ref) | 585 | 1.00 (ref) | 1.00 (ref) | 168 | 1.00 (ref) | 1.00 (ref) |
| Everc | 58 | 0.70 (0.52–0.95) | 0.69 (0.51–0.94) | 447 | 0.87 (0.76–0.99) | 0.89 (0.78–1.02) | 270 | 0.87 (0.71–1.06) | 0.91 (0.74–1.11) |
| Age at aspirin initiationd | |||||||||
| <60 years | 51 | 0.72 (0.53–0.99) | 0.71 (0.52–0.97) | 363 | 0.88 (0.77–1.01) | 0.90 (0.78–1.03) | 212 | 0.83 (0.67–1.03) | 0.87 (0.70–1.07) |
| ≥60 years | 7 | 0.63 (0.28–1.40) | 0.62 (0.28–1.39) | 82 | 0.85 (0.65–1.11) | 0.89 (0.68–1.16) | 55 | 1.22 (0.85–1.77) | 1.28 (0.89–1.86) |
OR, Odds ratio; CI, Confidence interval; MV, Multivariable.
Odds ratios adjusted for age (continuous), family history of colorectal cancer (yes, no), smoking status (never, past, current), alcohol consumption (non-current, current, 1–6, current ≥7 drinks/week), vigorous exercise (none, <5, ≥5 hours/week), red meat consumption (quartiles), diabetes (yes, no), and menopausal hormone use (yes, no).
Non-users are defined as participants who did not report regular aspirin use during the study period.
pinteraction=0.07
pinteraction=0.15
Non-aspirin NSAID use was first ascertained in 2009. At the first subsequent questionnaire cycle immediately prior to lower endoscopy, 24% of participants reported regular non-aspirin NSAID use. Use of non-aspirin NSAIDs over the 2009–2018 study period was not associated with odds of colorectal adenoma (Supplemental Table 3).
DISCUSSION
Within this cohort of Black American women, regular aspirin use was associated with 14% reduced odds of colorectal adenoma. The inverse association was present for all adenoma locations, although estimates were less precise for rectal adenoma. Regular aspirin use was associated with a lower odds of small, but not large adenoma. Both past and current aspirin users experienced an estimated reduction in adenoma occurrence. However, the association was more pronounced among past users. Longer duration of aspirin use and initiating aspirin use at a younger age were associated with greater reductions in the odds of adenoma. The association between aspirin use and colorectal adenoma varied by strata of ideal cardiovascular health status, such that the greatest estimated reduction in adenoma was observed in women classified as having poor cardiovascular health.
Results from the present analysis are consistent with prior studies that observed an inverse association between aspirin use and colorectal adenoma. Several randomized controlled trials (N=272–1,121) reported that regular use of low- or high-dose aspirin decreased the risk of adenoma by 17–36% among high-risk populations, such as individuals with a history of adenoma11,12,17 or a history of CRC.14 Observational studies among majority White populations have also demonstrated an inverse association among both high-risk populations15 and low-risk populations without a history of adenoma.5,6,8–10,13,16 However, not all randomized trials38 or observational studies36 have observed an association. Although estimates of association from the present analysis are somewhat weaker than those in prior studies, they are consistent with a moderate estimated reduction in risk and support previous findings that showed longer duration of aspirin use was associated with greater reductions in adenoma occurrence.7,10,16 Furthermore, our findings from this well-powered prospective cohort study support results from the few prior studies that observed an inverse association between aspirin use and colorectal neoplasia among Black Americans.8,25,31
Given potential adverse side effects of aspirin use, the USPSTF recently revised their previous 2016 recommendation that low-dose aspirin could be used to prevent both CVD and CRC in high-risk adults aged 50–59; their current statement advises that the decision to initiate aspirin for CVD prevention be an individual one for adults aged 40–59.33 The USPSTF noted that the current body of evidence used to draw conclusions about the effect of aspirin on CRC was limited by a small number of reporting studies and short follow-up. Further, racial/ethnic minorities have been largely excluded from the majority White evidence base despite being disproportionately affected by CVD and CRC. Herein, we show that among Black women, aspirin use is associated with a reduced occurrence of colorectal adenoma, the CRC precursor. Our results also indicate that CVD risk is an important consideration in the aspirin-adenoma association, as stratified analyses showed inverse associations to be strongest among women with poor cardiovascular health and weaker among women with ideal cardiovascular health. These results align with the 2016 USPSTF recommendations33 and suggest that Black women at increased risk of CVD may experience the greatest chemopreventive benefits of aspirin. While it is possible that these results may be influenced by the fact that individuals with poor cardiovascular health are often more likely to be prescribed aspirin for primary or secondary CVD prevention, in this study, we found that participants with poor cardiovascular health were less likely to report aspirin use compared to those with intermediate or ideal cardiovascular health. Additional research and replication of our findings in Black and other minority populations is still needed to help racial/ethnic minority groups weigh their individual risks and benefits of initiating or continuing an aspirin regimen.
It is hypothesized that the chemopreventive benefits of aspirin may be age-dependent, such that the inverse association between aspirin and colorectal neoplasia is present only among younger adults who initiate aspirin.39 In the present study, the estimated reduction in adenoma occurrence became attenuated with increasing age at initiation and disappeared when aspirin was initiated at ages ≥60 years. These findings suggest that it is important to consider age at initiation when examining the association. Additionally, these findings add to growing evidence suggesting that age may influence the chemopreventive effects of aspirin. In the ASPirin in Reducing Events in the Elderly (ASPREE) study, a randomized controlled trial (RCT) examining the impact of aspirin use in older individuals (≥70 years), aspirin use was associated with increased cancer mortality, including from colorectal cancer.40 This unexpected finding contrasts with prior RCTs demonstrating the chemopreventive effect of aspirin in younger populations (<65 years).30,41,42 However, among participants who were regular aspirin users before the ASPREE study, aspirin was associated with a non-significant lower risk of all-cause mortality.40 Additionally, for Black Americans, the ASPREE study suggested a benefit of aspirin use for all-cause mortality40 and all-cancer incidence.43 Nonetheless, the results were not statistically significant, and the sample size was small (n=901 Black participants). In a prospective, observational study of men and women aged ≥70 years, aspirin was associated with a reduced risk of CRC only among individuals who initiated aspirin use before age 70.44 Further research is needed to increase understanding of potential interactions with age, mechanisms through which these interactions may operate, and how they impact early versus late stages of the colorectal cancer continuum. This research will improve identification of subpopulations who are more or less likely to benefit from aspirin use.
Because the proximal colon, distal colon, and rectum differ in embryologic origin, function, physiology, and genetics,45 we assessed the aspirin-adenoma association according to adenoma location. Although estimates were not statistically significant for distal and rectal adenomas, an inverse association was found for all adenoma locations, suggesting that the impact of aspirin does not vary by site. Prior studies examining associations according to adenoma location have provided inconsistent results, with some reporting similar associations across locations,8,11,16 and others reporting a stronger association with distal adenoma.9,36
Studies reporting on the association by adenoma size have also provided mixed findings. In some studies, aspirin use was similarly associated with both small and large adenomas.8,12,16 In another study, aspirin was more strongly associated with large adenomas.9 In the present study, aspirin use was associated with a reduced odds of small, but not large adenomas. One potential explanation for our finding is that participants taking aspirin may have been under greater medical surveillance, which may have increased the chance of detecting adenomas early, before they progressed to a larger size. Other explanations are that aspirin may play a greater role in preventing adenoma initiation versus progression or that our results are a chance finding.
We found no association between use of non-aspirin NSAIDs and colorectal adenoma in the present analysis, which is inconsistent with reports of an inverse association in prior studies.6,8,9,13,46,47 Reasons for this difference are not clear. However, non-aspirin NSAIDs were only queried beginning in 2009. Thus, this analysis is based on a relatively small number of adenoma cases and we may have been underpowered to detect a modest association.
Laboratory and experimental studies of aspirin and non-aspirin NSAIDs demonstrate potential mechanisms through which these medications may reduce colorectal neoplasia. For example, aspirin and non-aspirin NSAIDs inactivate enzymes, cyclooxygenase-1 (COX-1) and COX-2, that catalyze the production of prostaglandins, which have been shown to increase cell proliferation, decrease apoptosis, and promote inflammation, motility, invasion and angiogenesis.48–50 By inactivating COX enzymes, aspirin and non-aspirin NSAIDs can inhibit downstream tumorigenic effects. This COX mechanism is supported by experimental studies that showed COX-1 and COX-2 to be overexpressed in colorectal polyps51 and rectal tumors.52Aspirin and non-aspirin NSAIDs may also influence colorectal neoplasia through COX-independent mechanisms. For example, aspirin promotes apoptosis by downregulating anti-apoptotic proteins53 and reduces cell proliferation by increasing the expression of proteins involved in cell cycle arrest.50 These anti-cancer properties have been demonstrated in colon cancer cell lines.54
A limitation of the present analysis is that we were unable to assess frequency of medication use or medication dose, which would have clinical importance for informing recommendations for chemopreventive use. However, to be classified as a regular user of aspirin or non-aspirin NSAIDs, participants had to take the medication at least three times a week. Additionally, these results are consistent with prior randomized trials that reported inverse associations for daily aspirin use at various doses.11,12,17 Another limitation is that data on non-aspirin NSAIDs was not assessed until 2009, which reduced the analytic sample size. Sample size and statistical power were limited in rectal adenoma and stratified analyses. There are multiple potential pathways for CRC development, including the most common adenoma–carcinoma pathway of chromosomal instability.55 More recently, a serrated pathway has also been recognized. The serrated pathway is thought to originate from hyperplastic polyps that transition to serrated adenomas or sessile serrated lesions before developing into dysplasia and invasive CRC.56 However, due to changing definitions of serrated polyps over time, confirmation of sessile serrated lesions from medical records prior to 2010 is extremely challenging.57 Additionally, only a small proportion (6–19%) of hyperplastic polyps are sessile serrated lesions, with roughly half presenting with synchronous adenomas.57 Thus, examining sessile serrated lesions as a distinct polyp category, with sufficient power, was not be feasible in the current study.
Risk factors for colorectal cancer that act early in disease onset (i.e., initiation or promotion) may differ from those factors that act later in the multistage cancer progression pathway (i.e., progression and conversion).58 Thus, understanding risk factors for adenoma may offer opportunities to intervene and prevent colorectal cancer progression. Nonetheless, there are some challenges to examining adenomas as an endpoint instead of colorectal cancer. First, determining adenoma incidence can be challenging, as adenomas can grow for many years before being detected by endoscopy. However, when we restricted our analysis to women who had at least two endoscopies and were not diagnosed with an adenoma at the first endoscopy, results were similar to those from the primary analysis. Second, adenomas are not a reportable condition like cancer. There is likely to be some non-differential misclassification of the outcome due to women not reporting a polyp diagnosis, but this would bias the results toward the null. We were able to confirm 76.1% of self-reported polyps via medical records, and we only included adenomas in the study that were confirmed by medical records. Third, detection of adenomas is dependent on the type of endoscopy procedure performed, as adenomas in the proximal colon are less likely to be detected during sigmoidoscopies. In adenoma location-specific analyses, we restricted the analytic sample to women undergoing colonoscopies. Inclusion of women who underwent sigmoidoscopy procedures in a sensitivity analysis yielded similar results.
A final limitation of the present study is that temporality between aspirin exposure and adenoma occurrence may have been compromised in primary analyses since adenomas can develop and grow undetected for many years prior to the assessment of aspirin use. To mitigate potential violations of temporality, we conducted secondary analyses among women with at least two endoscopy procedures and who were adenoma-free at first endoscopy. Findings from this “incident” adenoma analysis were similar to those in the primary analysis. Furthermore, women with an adenoma diagnosis at baseline were excluded from the present analysis, further reducing temporality concerns.
The large study size and focus on Black American women, who are often underrepresented in research, are two primary strengths of the present analysis. Colorectal adenoma diagnoses were confirmed through medical record review, permitting the ascertainment of detailed information on adenoma location and size. In addition, data on covariates and medication use were updated at each questionnaire cycle. Lastly, examination of the aspirin-adenoma association according to age at initiation and ideal cardiovascular health status are strengths, as these analyses confirmed subsets of the population that may be most likely to experience benefits from aspirin use.
In this study of Black American women, regular aspirin use, especially use initiated before age 60 and for longer durations, was associated with a reduced odds of colorectal adenoma. These findings support a risk reduction effect of aspirin on colorectal adenoma and suggest that aspirin may be a useful prevention strategy for Black Americans, who have a disproportionately high risk of colorectal neoplasia. Moreover, this research demonstrates the importance of studying the aspirin-neoplasia association in racial/ethnic minority groups in order to identify high-risk populations for whom the benefit of aspirin may outweigh the harms.
Supplementary Material
Novelty and Impact:
Racial/ethnic minorities have been largely excluded from studies of aspirin use and colorectal neoplasia, despite being disproportionately affected by the disease. In a cohort of Black women, we found regular aspirin use, particularly use for ≥10 years, was associated with a reduced occurrence of colorectal adenoma. Women who initiated aspirin use at age <40 years experienced the greatest reduction. Findings suggest aspirin may be a useful prevention strategy among Black women.
ACKNOWLEDGEMENTS
Data on colorectal cancer pathology were obtained from several state cancer registries (AZ, CA, CO, CT, DE, DC, FL, GA, IL, IN, KY, LA, MD, MA, MI, NJ, NY, NC, OK, PA, SC, TN, TX, VA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health or the state cancer registries. We thank participants and staff of the BWHS for their contributions.
FUNDING
This work was supported by the National Institutes of Health (R01CA058420 and U01CA164974) and Boston University Peter Paul Career Development Professorship (JL Petrick).
Abbreviations list:
- BMI
body mass index
- BWHS
Black Women’s Health Study
- COX
cyclooxygenase
- CVD
cardiovascular disease
- NSAID
non-steroidal anti-inflammatory drugs
- US
United States
- USPSTF
United States Preventive Services Task Force
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no potential conflicts of interest.
ETHICS STATEMENT
The study protocol for the BWHS was approved by the Boston University Medical Center IRB and the IRBs of participating cancer registries, as required.
DATA AVAILABILITY STATEMENT
The data will be shared on reasonable request to the corresponding author.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi: 10.3322/caac.21601 [DOI] [PubMed] [Google Scholar]
- 2.Conteduca V, Sansonno D, Russi S, Dammacco F. Precancerous colorectal lesions (Review). Int J Oncol. 2013;43(4):973–984. doi: 10.3892/ijo.2013.2041 [DOI] [PubMed] [Google Scholar]
- 3.Corley DA, Jensen CD, Marks AR, et al. Variation of Adenoma Prevalence by Age, Sex, Race, and Colon Location in a Large Population: Implications for Screening and Quality Programs. Clinical Gastroenterology and Hepatology. 2013;11(2):172–180. doi: 10.1016/j.cgh.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening of asymptomatic Black and White Patients. JAMA. 2008;300(12):1417–1422. doi: 10.1001/jama.300.12.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin Use and the Risk for Colorectal Cancer and Adenoma in Male Health Professionals. Ann Intern Med. 1994;121(4):241. doi: 10.7326/0003-4819-121-4-199408150-00001 [DOI] [PubMed] [Google Scholar]
- 6.Peleg II, Lubin MF, Cotsonis GA, Clark WS, Wilcox CM. Long-term use of nonsteroidal antiinflammatory drugs and other chemopreventors and risk of subsequent colorectal neoplasia. Dig Dis Sci. 1996;41(7):1319–1326. doi: 10.1007/BF02088554 [DOI] [PubMed] [Google Scholar]
- 7.Grau M V, Sandler RS, McKeown-Eyssen G, et al. Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: Observational follow-up of a randomized study. J Natl Cancer Inst. 2009;101(4):267–276. doi: 10.1093/jnci/djn484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CC, Hayes RB, Schoen RE, Gunter MJ, Huang WY. Non-steroidal anti-inflammatory drug use and colorectal polyps in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Gastroenterol. 2010;105(12):2646–2655. doi: 10.1038/ajg.2010.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murff HJ, Shrubsole MJ, Chen Z, et al. Non-Steroidal Anti-inflammatory Drug Use and Risk of Adenomatous and Hyperplastic Polyps Harvey. Cancer Prev Res (Phila). 2011;4(11):1799–1807. doi:doi: 10.1158/1940-6207.CAPR-11-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuer-Katschinski B, Nemes K, Rump B, et al. Long-term use of nonsteroidal antiinflammatory drugs and the risk of colorectal adenomas. Digestion. 2000;61(2):129–134. doi: 10.1159/000007745 [DOI] [PubMed] [Google Scholar]
- 11.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. [DOI] [PubMed] [Google Scholar]
- 12.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: One-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. doi: 10.1016/S0016-5085(03)00887-4 [DOI] [PubMed] [Google Scholar]
- 13.Rahme E, Barkun AN, Toubouti Y, Bardou M. The cyclooxygenase-2-selective inhibitors rofecoxib and celecoxib prevent colorectal neoplasia occurrence and recurrence. Gastroenterology. 2003;125(2):404–412. doi: 10.1016/S0016-5085(03)00880-1 [DOI] [PubMed] [Google Scholar]
- 14.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. [DOI] [PubMed] [Google Scholar]
- 15.Tangrea JA, Albert PS, Lanza E, et al. Non-steroidal anti-inflammatory drug use is associated with reduction in recurrence of advanced and non-advanced colorectal adenomas (United States). Cancer Causes and Control. 2003;14(5):403–411. doi: 10.1023/A:1024990617158 [DOI] [PubMed] [Google Scholar]
- 16.Chan AT, Giovannucci EL, Schernhammer ES, et al. A Prospective Study of Aspirin Use and the Risk for Colorectal Adenoma. Ann Intern Med. 2004;140(30):157–167. [DOI] [PubMed] [Google Scholar]
- 17.Logan RFA, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and Folic Acid for the Prevention of Recurrent Colorectal Adenomas. Gastroenterology. 2008;134(1):29–38. doi: 10.1053/j.gastro.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang FC, Wang YJ. The efficacy and safety of non-steroidal anti-inflammatory drugs in preventing the recurrence of colorectal adenoma: A meta-analysis and systematic review of randomized trials. Colorectal Disease. 2015;17(3):188–196. doi: 10.1111/codi.12838 [DOI] [PubMed] [Google Scholar]
- 19.Veettil SK, Lim KG, Ching SM, Saokaew S, Phisalprapa P, Chaiyakunapruk N. Effects of aspirin and non-aspirin nonsteroidal anti-inflammatory drugs on the incidence of recurrent colorectal adenomas: A systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. BMC Cancer. 2017;17(1):1–13. doi: 10.1186/s12885-017-3757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Stolley PD, Shapiro S. A Hypothesis: Nonsteroidal Anti-Inflammatory Drugs Reduce the Incidence of Large-Bowel Cancer. J Natl Cancer Inst. Published online 1991:355–358. doi: 10.1093/jnci/83.5.355 [DOI] [PubMed] [Google Scholar]
- 21.Peleg II, Maibach HT, Brown SH, Wilcox CM. Aspirin and nonsteroidal anti-inflammatory drug use and the risk of subsequent colorectal cancer. Arch Intern Med. 1994;154:394–399. [PubMed] [Google Scholar]
- 22.Qiao Y, Yang T, Gan Y, et al. Associations between aspirin use and the risk of cancers: A meta-analysis of observational studies. BMC Cancer. 2018;18(288). doi: 10.1186/s12885-018-4156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: A systematic review and meta-analysis. Cancer Epidemiol. 2019;58(July 2018):52–62. doi: 10.1016/j.canep.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer. JAMA: The Journal of the American Medical Association. 2005;294(8):914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sansbury LB, Millikan RC, Schroeder JC, Moorman PG, North KE, Sandler RS. Use of nonsteroidal antiinflammatory drugs and risk of colon cancer in a population-based, case-control study of African Americans and Whites. Am J Epidemiol. 2005;162(6):548–558. doi: 10.1093/aje/kwi248 [DOI] [PubMed] [Google Scholar]
- 26.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99(8):608–615. doi: 10.1093/jnci/djk132 [DOI] [PubMed] [Google Scholar]
- 28.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin Dose and Duration of Use and Risk of Colorectal Cancer in Men. Gastroenterology. 2008;134(1):21–28. doi: 10.1053/j.gastro.2007.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RE, Beebe-Donk J, Alshafie GA. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2008;8(237):1–6. doi: 10.1186/1471-2407-8-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. The Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7 [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Wilkens LR, Kolonel LN, Monroe KR, Haiman CA, Marchand L Le. Exploring Differences in the Aspirin – Colorectal Cancer Association by Sex and Race / Ethnicity : The Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017;26(2):162–169. doi: 10.1158/1055-9965.EPI-16-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bibbins-Domingo K, Force on behalf of the USPST. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836–845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 33.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA - Journal of the American Medical Association. 2022;327(16):1577–1584. doi: 10.1001/jama.2022.4983 [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Martin C, Galanko J, et al. Use of nonsteroidal antiinflammatory drugs and distal large bowel cancer in whites and African Americans. Am J Epidemiol. 2008;168(11):1292–1300. doi: 10.1093/aje/kwn255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon K Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–976. doi: 10.2147/CIA.S109285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology. 2018;155(2):355–373.e18. doi: 10.1053/j.gastro.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 38.Burn J, Bishop DT, Mecklin JP, et al. Effect of Aspirin or Resistant Starch on Colorectal Neoplasia in the Lynch Syndrome. N Engl J Med. 2008;359(24):2567–2578. doi: 10.1056/nejmoa0801297 [DOI] [PubMed] [Google Scholar]
- 39.Chan AT, McNeil J. Aspirin and Cancer Prevention in the Elderly: Where Do We Go From Here? Gastroenterology. 2019;156(3):534–538. doi: 10.1053/j.gastro.2018.11.063 [DOI] [PubMed] [Google Scholar]
- 40.McNeil JJ, Nelson MR, Woods RL, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. New England Journal of Medicine. 2018;379(16):1519–1528. doi: 10.1056/nejmoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burn J, Gerdes AM, MacRae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. The Lancet. 2011;378(9809):2081–2087. doi: 10.1016/S0140-6736(11)61049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: Long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85. doi: 10.7326/0003-4819-159-2-201307160-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mcneil JJ, Gibbs P, Orchard SG, et al. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J Natl Cancer Inst. 2021;113(3). doi: 10.1093/jnci/djaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo CG, Ma W, Drew DA, et al. Aspirin Use and Risk of Colorectal Cancer among Older Adults. JAMA Oncol. 2021;7(3):428–435. doi: 10.1001/jamaoncol.2020.7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Fying, Lai M de. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10(3):219–229. doi: 10.1631/jzus.B0820273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandler RS, Galanko JC, Murray SC, Helm JF, Woosley JT. Aspirin and nonsteroidal anti-inflammatory agents and risk for colorectal adenomas. Gastroenterology. 1998;114(3):441–447. doi: 10.1016/S0016-5085(98)70526-8 [DOI] [PubMed] [Google Scholar]
- 47.Chudy-Onwugaje K, Huang WY, Su LJ, et al. Aspirin, ibuprofen, and reduced risk of advanced colorectal adenoma incidence and recurrence and colorectal cancer in the PLCO Cancer Screening Trial. Cancer. 2021;127(17):3145–3155. doi: 10.1002/cncr.33623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 Increases Growth and Motility of Colorectal Carcinoma Cells. Journal of Biological Chemistry. 2001;276(21):18075–18081. doi: 10.1074/jbc.M009689200 [DOI] [PubMed] [Google Scholar]
- 49.Sonoshita M, Takaku K, Sasaki N, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in ApcΔ716 knockout mice. Nat Med. 2001;7(9):1048–1051. doi: 10.1038/nm0901-1048 [DOI] [PubMed] [Google Scholar]
- 50.Ferrández Á, Piazuelo E, Castells A. Aspirin and the prevention of colorectal cancer. Best Pract Res Clin Gastroenterol. 2012;26(2):185–195. doi: 10.1016/j.bpg.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 51.Takeda H, Sonoshita M, Oshima H, et al. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer Res. 2003;63(16):4872–4877. doi: 10.1158/0008-5472.can-05-1949 [DOI] [PubMed] [Google Scholar]
- 52.Dimberg J, Samuelsson A, Hugander A, Söderkvist P. Differential expression of cyclooxygenase 2 in human colorectal cancer. Gut. 1999;45(5):730–732. doi: 10.1136/gut.45.5.730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KM, Song JJ, An JY, Kwon YT, Lee YJ. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. Journal of Biological Chemistry. 2005;280(49):41047–41056. doi: 10.1074/jbc.M503713200 [DOI] [PubMed] [Google Scholar]
- 54.Pathi S, Jutooru I, Chadalapaka G, Nair V, Lee SO, Safe S. Aspirin Inhibits Colon Cancer Cell and Tumor Growth and Downregulates Specificity Protein (Sp) Transcription Factors. PLoS One. 2012;7(10):e48208. doi: 10.1371/journal.pone.0048208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14(8):16365–16385. doi: 10.3390/ijms140816365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1–10. doi: 10.1016/j.humpath.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 57.Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157(4):949–966.e4. doi: 10.1053/j.gastro.2019.06.041 [DOI] [PubMed] [Google Scholar]
- 58.Morrison AS. Sequential pathogenic components of rates. Am J Epidemiol. 1979;109(6). doi: 10.1093/oxfordjournals.aje.a112734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be shared on reasonable request to the corresponding author.


