Abstract
Background
How diabetes mellitus (DM), race/ethnicity, and sex impact ischemic events following coronary artery stent procedures is unknown.
Methods
Using the PLATINUM Diversity and PROMUS Element Plus Post-Approval Pooled Study (N = 4184), we examined the impact of race/ethnicity, sex, and DM on coronary stent outcomes. Primary outcome was 1-year major adverse cardiac events (MACE) (MACE composite: death, myocardial infarction [MI], and target vessel revascularization).
Results
The study sample included 1437 diabetic patients (501 White men, 470 White women, 246 minority men, 220 minority women) and 2641 patients without medically treated DM (561 minority, 1090 women). Mean age (years) ranged from 61 in minority men to 65 in White women. Diabetic patients had a higher prevalence of atherosclerotic risk factors and comorbidities. Diabetic minority women (DMW; 70% Black, 27% Hispanic) had similar atherosclerotic risk factors to other diabetics, but experienced higher 1-year MACE (14.4% vs 7.5%, P < .01) and MI (4.3% vs 1.6%, P < .01) rates compared with patients without medically treated DM. No other diabetic cohort (White men, White women, minority men) showed an increased risk of MACE vs patients without medically treated DM. The incremental risk of MACE in DMW was associated with insulin use and persisted after risk adjustment (adjusted odds ratio 1.6 vs patients without medically treated DM; 95% CI, 1.0-2.5). Independent predictors of 1-year MACE included insulin use, hyperlipidemia, renal disease, and prior MI.
Conclusions
DMW face the highest risk of ischemic events following coronary stenting, driven, in part, by insulin use. Aggressive secondary prevention and strict glycemic control are imperative in this cohort, and further research is warranted to elucidate the biologic mechanisms underpinning these observations.
Keywords: coronary, diabetes, minority, outcomes, stent, women
Central Illustration

Highlights
-
•
Diabetic minority women face the highest risk of ischemic events following percutaneous coronary intervention (PCI)
-
•
Increase in major adverse cardiac events (MACEs) was most notable after 90 days, driven by target vessel revascularization and myocardial infarction
-
•
The incremental risk was associated with insulin use and persisted after risk adjustment
-
•
No other diabetic cohort (White men, White women, minority men) showed increased MACEs vs. nondiabetics
-
•
Aggressive secondary prevention and strict glycemic control are imperative in diabetic minority women to reduce health disparities post PCI
Introduction
Approximately 10% of adults in the United States suffer from diabetes mellitus (DM), and this prevalence is estimated to increase to 1 in 3 by 2050.1,2 DM is an important risk factor, not only for the development of coronary artery disease (CAD) but also for prognostication in patients with established CAD.3, 4, 5 DM confers a higher risk of ischemic events after myocardial infarction (MI), percutaneous coronary intervention (PCI), and coronary artery bypass graft surgery (CABG)4, 5, 6, 7 and increases the risk of fatal coronary events to a greater degree in women compared with men.8 Minority groups in the United States are disproportionately affected by DM, with its prevalence being 2-fold higher in African Americans and Hispanics than in Whites.9,10 Although race/ethnicity and sex have been shown to influence outcomes in patients undergoing PCI,11, 12, 13, 14, 15, 16, 17, 18, 19 the interplay between these factors and DM in determining ischemic events following PCI is unknown because prior studies have enrolled inadequate numbers of women and/or minorities. The purpose of this study was to evaluate the influence of race/ethnicity and sex on 1-year outcomes in a diverse cohort of patients with DM undergoing PCI, focusing on the comparative outcomes of diabetic minority women (DMW).
Methods
Study design, setting, and participants
The PLATINUM Diversity Study (PD)12 was a prospective, multicenter, observational study that enrolled patients who received ≥1 Promus PREMIER stent (Boston Scientific) and self-identified as having at least 1 of the following characteristics: female sex, Black (of African heritage), Hispanic/Latino, or American Indian/Alaskan Native. There were no exclusion criteria. Patients were enrolled at 52 US sites beginning in October 2014 and followed for 12 months. The primary results of this study have been previously published.12 The PROMUS Element Plus Post-Approval Study (PE Plus) was a prospective, multicenter, open label observational study completed in August 2014 that enrolled an “all-comers” cohort of patients also across 52 US sites who were treated with the PROMUS Element Plus everolimus-eluting stent (Boston Scientific).17 Both the PD and PE Plus studies complied with the Declaration of Helsinki and were approved by each site’s locally appointed ethics committee or institutional review board. Informed consent was required within 24 hours of stent implantation within both studies. The PD and PE Plus studies are registered at www.clinicaltrials.gov under identifiers NCT02240810 and NCT01589978, respectively. The principal investigators had direct access to the primary data from the study. The data and study protocol for these stent registries will be made available to other researchers in accordance with the Boston Scientific Data Sharing Policy (http://www.bostonscientific.com/en-US/data-sharing-requests.html). Both studies were sponsored and funded by Boston Scientific Corporation, who assisted with the study designs, data management, safety monitoring and biostatical analyses of both studies. End point definitions and all clinical end points were adjudicated by an independent clinical events committee. The statistical rationale for pooling these 2 studies has been previously reported.12 During the design of the pooled PD/PE Plus cohort study, there was a prespecified plan to evaluate outcomes in diabetic patients according to race/ethnicity and sex.
Study sample, design, and clinical end points
Patients were categorized into 1 of 4 demographic groups: White men, White women, minority men, and minority women. Medically treated patients with DM were defined as those actively taking either an oral hypoglycemic agent and/or parenteral insulin. For comparison purposes, all other patients, including nondiabetic patients and those self-identifying as diabetics but not taking insulin or oral agents were included in the nondiabetic control group. Diabetic patients were further categorized into those taking parenteral insulin vs those taking only oral hypoglycemic agents. Baseline clinical and angiographic characteristics and 1-year clinical event rates were compared between each diabetic cohort and patients without medically treated DM. The primary end point of this study was the rate of 1-year major adverse cardiac events (MACE): all-cause death, MI, and/or target vessel revascularization (TVR). Additional secondary end points included 1-year death (cardiac and noncardiac), death/MI, MI (stent-related and not stent-related), Academic Research Consortium definite/probable stent thrombosis, and target vessel failure. Angiographic characteristics were reported as described by site investigators. All clinical end points were adjudicated by an independent clinical events committee using the established definitions derived from the PD and PE Plus studies.17,20,21 Multivariate regression was used to risk adjust the primary outcome (MACE) and define independent predictors within the diabetic cohort.
Statistical methods
Two-sided t tests were used to compare continuous variables and χ2 or Fisher exact tests for discrete variables. A χ2 test compared the unadjusted outcomes between groups. Statistical significance was declared if the 2-sided lower 95% CI boundary on the difference between groups was less than 0 and the χ2 P value <.05. Kaplan–Meier curves were used with a log rank test to compare the occurrence of events over time. Odds ratios (ORs) and 95% CIs were generated for 1-year clinical events and risk-adjusted using multivariate logistic regression. Significant baseline clinical, angiographic, and procedural covariates with P value <.1 from the univariate model were used in the multivariate model. The stepwise method was used for selection of covariates in the multivariate model. A variable that was significant at the. 1 level was entered and stayed in the multivariate model. To risk adjust, a ‘group’ variable was forced into the model and the OR determined. Candidate variables included: age, sex, body mass index (BMI), ethnicity, race, insulin use, oral hypoglycemic agent, current smoker, hyperlipidemia, hypertension, prior MI, congestive heart failure, history of PCI, history of CABG, renal disease, history of peripheral vascular disease, angina status, left ventricular ejection fraction, left main disease, multivessel disease, lesion length, reference vessel diameter, bifurcation lesion, chronic total occlusion, calcification, and thrombus. All statistical analyses were performed using SAS System software, version 9.2 or later (SAS Institute Inc).
Results
Study sample
The study sample was drawn from a total of 4184 patients (1501 PD Study and 2683 PE Plus Study patients). Of these, 106 (2.5%) were excluded due to unclear diabetic status or race/ethnicity, leaving 4078 patients, of whom 1437 self-reported as having medically treated DM (501 White men, 470 White women, 246 minority men, 220 minority women) and 2641 who self-reported as not having medically treated DM (1160 White men, 899 White women, 391 minority men, 191 minority women). Twelve-month follow-up was robust (87% to 93%) in all groups. The study flowchart is shown in Figure 1.
Figure 1.
Study flowchart. DM, diabetes mellitus; PE Plus PAS, PROMUS Element Plus Post-Approval Study.
Baseline characteristics
The baseline characteristics of diabetic patients and patients without medically treated DM are shown in Table 1. The mean age of patients ranged from 61 ± 9.6 years in diabetic minority men to 65 ± 11 years in diabetic White women. Blacks comprised approximately two-thirds of all minority patients, Hispanic/Latinos approximately one-third, with very few American Indian/Alaskan Natives. Approximately three-quarters of diabetic patients were taking an oral agent, and insulin use ranged from a low of 37% in diabetic White men to a high of 53% in DMW. Diabetic patients had higher BMIs than patients without medically treated DM and were more likely to present with other comorbidities, including hyperlipidemia, hypertension, congestive heart failure, prior PCI, prior CABG, prior cerebrovascular accidents, renal disease, peripheral vascular disease, and multivessel CAD. Among diabetic patients, women were more likely to have a history of congestive heart failure but less likely to have a history of MI. Minorities were more likely to have a history of cerebrovascular accidents but less likely to have atrial fibrillation. Coronary lesion length, number of stents implanted, reference vessel diameter, and left ventricular ejection fraction were similar across all groups.
Table 1.
Baseline demographics and clinical characteristics.
| Diabetic minority women n=220 patients n=308 lesions |
Diabetic White men n=501 patients n=676 lesions |
Diabetic White women n=470 patients n=655 lesions |
Diabetic minority men n=246 patients n=342 lesions |
Men and women without diabetes n=2641 patients n=3516 lesions |
|
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 64 ± 11 | 64 ± 10 | 65 ± 11 | 61 ± 9.6 | 64 ± 12 |
| Body mass index, kg/m2 | 34 ± 6.9 | 32 ± 5.9 | 34 ± 7.6 | 32 ± 6.6 | 30 ± 6.5 |
| Race/ethnicity∗ | |||||
| Black | 154 (70) | 0 (0) | 0 (0) | 151 (61) | 366 (14) |
| Hispanic/Latino | 60 (27) | 2 (0.4) | 3 (0.6) | 95 (38) | 195 (7.4) |
| Diabetes medications∗ | |||||
| Oral agent | 156 (71) | 402 (80) | 340 (72) | 181 (74) | 0 (0) |
| Insulin | 116 (53) | 186 (37) | 241 (51) | 110 (45) | 0 (0) |
| Medical history | |||||
| Current smoker | 33 (15) | 88 (18) | 75 (16) | 44 (18) | 684 (26) |
| Hyperlipidemia | 181 (82) | 438 (87) | 403 (86) | 213 (87) | 1807 (68) |
| Hypertension | 207 (94) | 449 (90) | 430 (92) | 225 (92) | 1959 (74) |
| Prior MI | 84 (38) | 241 (48) | 182 (39) | 104 (42) | 1193 (45) |
| MI within 72 h of the index procedure | 63 (29) | 111 (22) | 128 (27) | 69 (27) | 908 (34) |
| Congestive heart failure | 55 (25) | 67 (13) | 87 (19) | 42 (17) | 236 (8.9) |
| History of PCI | 95 (43) | 268 (54) | 219 (47) | 124 (50) | 59 (2.2) |
| History of CABG | 34 (16) | 129 (26) | 102 (22) | 47 (19) | 332 (13) |
| History of AF | 9 (4.1) | 55 (11) | 54 (12) | 13 (5.3) | 233 (8.8) |
| History of CVA | 26 (12) | 23 (4.6) | 38 (8.1) | 27 (11) | 133 (5) |
| Renal disease | 55 (25) | 68 (14) | 87 (19) | 73 (30) | 239 (9) |
| History of PVD | 28 (13) | 75 (15) | 80 (17) | 30 (12) | 255 (9.7) |
| Anginal status | |||||
| None | 55 (25) | 136 (27) | 136 (29) | 86 (35) | 738 (28) |
| Stable | 70 (32) | 128 (26) | 127 (27) | 87 (35) | 619 (23) |
| Unstable | 84 (38) | 222 (44) | 184 (39) | 63 (26) | 1156 (44) |
| Unknown | 11 (5) | 15 (3) | 10 (4.9) | 10 (4.1) | 128 (4.8) |
| Cardiogenic shock | 2 (0.9) | 1 (0.2) | 4 (0.9) | 3 (1.2) | 13 (0.5) |
| Angiographic data | |||||
| LVEF, % | 52 ± 12 | 52 ± 11 | 55 ± 12 | 51 ± 13 | 54 ± 12 |
| Left main disease | 12 (5.5) | 48 (9.6) | 38 (8.1) | 26 (11) | 149 (5.6) |
| Multivessel disease | 92 (42) | 279 (56) | 212 (45) | 120 (49) | 949 (36) |
| Lesion length, mm | 19 ± 12 | 17 ± 11 | 17 ± 11 | 19 ± 10 | 18 ± 11 |
| Reference vessel diameter, mm | 2.9 ± 0.56 | 2.9 ± 0.52 | 2.8 ± 0.49 | 2.9 ± 0.50 | 2.9 ± 0.53 |
| No. of stents | 1.1 ± 0.36 | 1.1 ± 0.40 | 1.1 ± 0.35 | 1.1 ± 0.44 | 1.1 ± 0.38 |
| Bifurcation lesion | 10 (3.2) | 53 (7.8) | 48 (7.3) | 14 (4.1) | 260 (7.4) |
| Total occlusion (≤3 mo) | 7 (2.3) | 16 (2.4) | 23 (3.5) | 13 (3.8) | 160 (4.5) |
| CTO (>3 mo) | 8 (2.6) | 4 (0.6) | 18 (2.7) | 6 (1.8) | 62 (1.8) |
| Calcification | 136 (42) | 309 (46) | 327 (50) | 136 (40) | 1480 (42) |
| Thrombus | 16 (5.2) | 52 (7.7) | 46 (7) | 21 (6.1) | 446 (12.7) |
Values are mean ± SD or n (%).
AF, atrial fibrillation; CABG, coronary artery bypass surgery; CTO, chronic total occlusion. CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease.
Not mutually exclusive.
Use and adherence to antiplatelet therapy
Self-reported use of antiplatelet medications in White and minority men and women enrolled in the PD and PE Plus Post-Approval Pooled Study have been previously reported.12 Clopidogrel was the most prescribed P2Y12 inhibitor (65%), followed by prasugrel (18%) and ticagrelor (15%), with rare use of other medications and no differences in use between diabetic cohorts and patients without medically treated DM at discharge. Use of antiplatelet medications in diabetic patients is shown in Supplemental Figure S1. Aspirin and dual antiplatelet therapy (DAPT) use at discharge was high in all diabetic cohorts (93% to 97%). At 1 and 6 months, aspirin and DAPT use remained high and comparable between diabetic cohorts. At 1 year, DAPT use was lower in diabetic White women (82% vs 88%, P = .004) and diabetic minority men (81% vs 88%, P = .01) compared with diabetic White men. DMW showed no difference in DAPT use at 1 year (85% vs 88%, P = .21) compared with diabetic White men.
Unadjusted outcomes according to race/ethnicity and sex
The unadjusted clinical outcomes for each of the 4 diabetic cohorts compared with patients without medically treated DM are shown in Figure 2. DMW experienced higher rates of MACE (14.4 % vs 7.5%, P < .01), MI (4.3% vs 1.6%, P < .01), and TVR (8.5% vs 4.4%, P = .02) than patients without medically treated DM (Figure 2A and B and Central Illustration). The highest risk of MACE (19%) was observed in DMW treated with insulin and ischemic events most pronounced at 90 to 120 days post PCI, driven by a combination of numerically higher rates of death, MI, and TVR (Figure 2D). Although DMW treated with insulin also experienced the highest rate of death numerically, this was not statistically significant compared with patients without medically treated DM (6.6% vs 2.7%, P > .05). In contrast, diabetic White men, diabetic White women, and diabetic minority men all showed similar rates of MACE compared with patients without medically treated DM (9.4%, 9.2%, 8.2%, respectively vs 7.5%; P > .05 for all; Figure 2A and B). However, diabetic White women and White men treated with insulin also experienced higher rates of MACE vs patients without medically treated DM (13.3% and 11.9% vs 7.5%, P < .01, P = .03, respectively; Figure 2C and D). The risk of stent thrombosis was highest in diabetic White women (2.0% vs 0.8%, P = .02 vs patients without medically treated DM). Among diabetic patients taking only oral agents, no diabetic cohort experienced a higher rate of MACE or other secondary outcome compared with patients without medically treated DM (Figure 2E and F). Within the DMW cohort, Black and Hispanic women showed comparable baseline characteristics and similar 1-year outcomes. Although not statistically significant, the rate of MI was numerically higher in Black vs Hispanic diabetic women (medically treated DM: 5.7% vs 1.7%, P = .24; insulin treated DM: 6.9% vs 0%, P = .12).
Figure 2.
Graphs depicting (A) unadjusted clinical outcomes for all medically treated diabetic cohorts vs. nondiabetics, (B) Kaplan–Meier curves comparing MACE for all medically treated diabetic cohorts vs. nondiabetics, (C) unadjusted clinical outcomes for insulin treated diabetic cohorts vs. nondiabetics, (D) Kaplan–Meier curves comparing MACE for insulin treated diabetic cohorts vs. nondiabetics, (E) unadjusted clinical outcomes for oral agent treated diabetic cohorts vs. nondiabetics and (F) Kaplan–Meier curves comparing MACE for oral agent treated diabetic cohorts vs. nondiabetics. IT, insulin treated; MACE, major adverse cardiac event (death/myocardial infarction [MI]/target vessel revascularization [TVR]); OAT, oral agent treated.
∗Non-DM defined as patients without medically treated diabetes.
Central Illustration.
Influence of race/ethnicity and sex on 1-year outcomes after percutaneous coronary intervention in patients with diabetes mellitus. MACE, major adverse cardiac events.
Multivariable predictors of MACE and adjusted outcomes
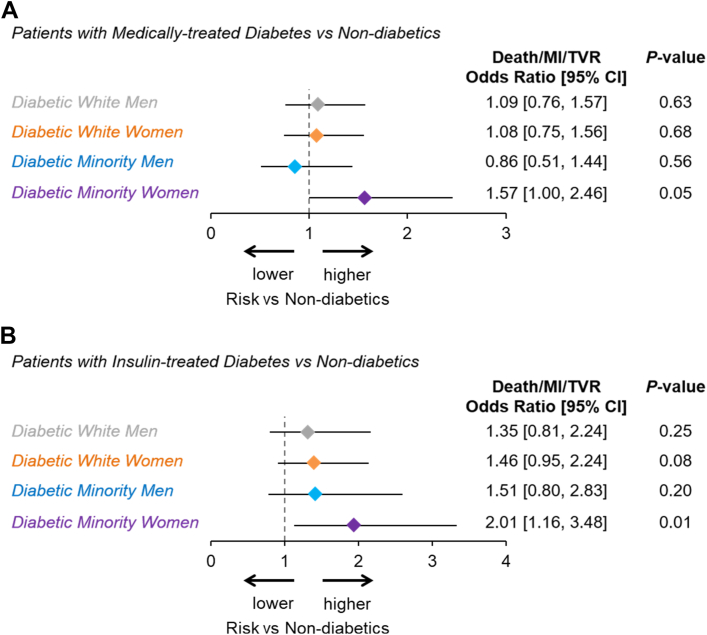
The multivariable predictors of 1-year MACE for medically treated diabetic patients are shown in Table 2. Independent predictors of MACE within diabetics included insulin therapy, hyperlipidemia, renal disease, and previous MI. Minority race/ethnicity and female sex, as individual variables, were not independent predictors of MACE. The risk-adjusted OR for MACE in DMW was 1.6 (95% CI, 1.00-2.46; P = .05) vs patients without medically treated DM and 2.0 (95% CI, 1.16-3.48; P = .01) for DMW treated with insulin vs patients without medically treated DM (Figure 3A). None of the other diabetic cohorts treated with insulin or with an oral agent showed an increased risk of MACE after risk adjustment (Figure 3).
Table 2.
Multivariable predictors of MACE in medically treated diabetic patients.
| Variable | Coefficient | Standard error | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| History of hyperlipidemia | 0.914 | 0.379 | 2.94 (1.19-5.24) | .02 |
| History of renal disease | 0.735 | 0.210 | 2.09 (1.38-3.15) | .005 |
| Insulin treatment | 0.632 | 0.201 | 1.88 (1.27-2.79) | .002 |
| Previous MI | 0.608 | 0.192 | 1.84 (1.26-2.68) | .002 |
| History of PVD | 0.390 | 0.232 | 1.48 (0.94-2.33) | .09 |
| Female vs male | 0.147 | 0.192 | 1.16 (0.80-1.70) | .44 |
| Minority vs White | 0.101 | 0.201 | 1.11 (0.75-1.64) | .61 |
Model derived from patients with medically treated diabetes (N=1437). See methods for description of model. Candidate variables included: age, sex, body mass index, race, ethnicity, insulin use, oral hypoglycemic agent, current smoker, hyperlipidemia, hypertension, prior myocardial infarction (MI), congestive heart failure, history of percutaneous coronary intervention, history of coronary artery bypass graft surgery, renal disease, history of peripheral vascular disease (PVD), angina status, left ventricular ejection fraction, left main disease, multivessel disease, lesion length, reference vessel diameter, bifurcation lesion, chronic total occlusion, calcification, and thrombus.
Figure 3.
Risk-adjusted major adverse cardiac events (MACE) for (A) all medically treated diabetics vs nondiabetics and (B) insulin treated diabetics vs nondiabetics.
Discussion
To our knowledge, this is the first study to examine the interplay between race/ethnicity and sex in diabetic patients undergoing contemporary PCI. Using the most diverse US coronary stent database to date, we observed the following: (1) diabetic patients presented with higher BMIs, more comorbidities, and more severe CAD than patients without medically treated DM, regardless of race, ethnicity, or sex; (2) minority (ie, Black and Hispanic) women with DM experienced a 2-fold higher risk of MACE and 3-fold increased risk of MI compared with patients without medically treated DM in the year following PCI; (3) the incremental risk in DMW was not attributed to variations in antiplatelet therapy, was most evident in patients treated with insulin, and persisted after risk adjustment for baseline characteristics; (4) diabetic White women and men treated with insulin also had a higher rate of MACE than patients without medically treated DM; (5) the independent predictors of MACE in diabetic patients included insulin use, hyperlipidemia, renal disease, and prior MI; and (6) minority race/ethnicity and female sex, as individual variables, were not independent predictors of MACE.
The glycometabolic abnormalities associated with DM induce vascular dysfunction and predispose patients to premature atherosclerosis and adverse thrombotic/ischemic events.1,2 Although PCI success rates in diabetic patients are comparable to those in patients without medically treated DM, several studies have reported that diabetic patients face an increased risk of post PCI cardiac ischemic events.3, 4, 5, 6 A recent meta-analysis of 139,774 patients from 42 coronary stent studies showed that diabetic patients have a 1.5- to 2-fold higher risk of death, MACE, and TVR.7 However, these studies did not examine the influence of race/ethnicity and sex on PCI outcomes in diabetic patients. We are unaware of other studies that have examined these relationships. Our observation that DMW (70% African Americans and 27% Hispanic/Latinos) had the highest risk of MACE and MI compared with patients without medically treated DM highlights a subset of women who are particularly susceptible to ischemic events following PCI.
Why DMW experienced the highest burden of ischemic events in the year following PCI is unclear. The increase in MACE in DMW was most notable after 90 days and driven by TVR and MI. Although self-reported DAPT use decreased slightly during the year post PCI in DMW, it remained comparable to that noted for White men and patients without medically treated DM. Therefore, variations in antiplatelet therapy were unlikely to account for the observed differences in outcomes. However, there are limitations to self-reported medication adherence, so late-filling or a lack of filling of prescriptions must always be ruled out in vulnerable populations. It has also been hypothesized that Black women may be more prone to ischemic events due to increased thrombogenicity post PCI.20 Gurbel et al20 reported that Black women undergoing coronary arteriography show greater platelet–fibrin clot strength compared with other demographic groups, suggesting a more thrombogenic phenotype that corresponded with an increased risk of post PCI ischemic events. Although race/ethnicity and sex alone were not independent predictors of MACE in our study, the combination of Black race or Hispanic ethnicity, female sex, and DM did confer significant incremental ischemic risk. Further research is necessary to understand the biologic underpinnings of these differences. Due to a lack of enrollment of minority women in clinical research, these questions remain heretofore untested and unanswered.
Differences in baseline characteristics also potentially contributed to the increased risk observed, as DMW had higher rates of comorbidities including hypertension, MI, congestive heart failure, and stroke. However, diabetic minority men carried a similar burden of comorbidities without the increment ischemic risk observed in DMW. This suggests that there may exist a complex interplay between DM, race/ethnicity, and sex in determining ischemic events. In our study, DMW had the highest rate of insulin use (53%), which may be a marker of DM duration, severity, and/or control. A recent analysis of outcomes following coronary stenting in an exclusively female population revealed a graded risk such that women taking insulin had the highest risk of events in the 3 years post PCI.21 In that study, diabetic women treated with insulin showed a >2.5-fold higher rate of MACE within 1 year of coronary stent implantation. A similar trend was noted with our data because DMW, diabetic White women, and diabetic men treated with insulin all experienced an increased risk of MACE compared with patients without medically treated DM. Our study is the first to stratify outcomes in diabetic patients according to not only sex but also race/ethnicity. Collectively, these findings suggest that the glycometabolic abnormalities associated with insulin treatment may play a role in the progression of atherosclerosis and/or restenosis in diabetic patients undergoing PCI. Still, other studies have shown mixed results, some revealing an increased risk of adverse cardiovascular events linked to exogenous insulin use in a dose dependent manner22,23 and others postulating that this relationship is influenced by time-dependent factors such as long-term glycemic control and weight gain, as opposed to any direct harm by insulin.24 In the Taxus Element vs Xience Prime in a Diabetic Population (TUXEDO) trial, insulin-treated diabetic patients who underwent coronary stenting were at increased risk of cardiovascular events compared with patients without medically treated DM in unadjusted models; however, this risk was attenuated after adjustment for confounders.25 This contrasts with our study, in which insulin treatment remained an independent predictor of MACE after accounting for other covariates.
There were several variables not accounted for in our study that may have also contributed to our findings, such as control of cardiovascular risk factors prior to or after coronary revascularization. Cardiovascular risk factors have been shown to be less aggressively treated and controlled in women and minorities with DM.26,27 Whether these disparities in risk factor control are related to social determinants of health (access to care, socioeconomic status, education level), gaps in patient awareness, inherent treatment bias, the impact of discrimination, or perhaps biological phenomena is unknown. Insulin resistance, itself, is associated with a host of cardiovascular and metabolic derangements, including dyslipidemia, hypertension, hypercoagulability, atherosclerosis, and heart failure from left ventricular hypertrophy, left ventricular diastolic and systolic dysfunction, myocardial cell death, and cardiac fibrosis.28,29 These major consequences of insulin resistance may have also contributed to our findings. In summary, a better understanding of the genetic, biological, social, and health care-related factors that influence outcomes in DMW following coronary revascularization is necessary to adequately address the excess risk noted in this cohort. In the meantime, practitioners should be aware of DMW as a higher-risk cohort (Central Illustration) so that optimal antiplatelet therapy and adherence, aggressive secondary prevention, and effective control of DM can be particularly emphasized in these patients.
Limitations
There are several limitations to this study. The classification of the DM treatment group was based on self-reporting; therefore, the potential for misclassification exists. We could not account for differences in the severity of DM, as the duration of DM, categorization as type I vs type II DM, indicators of glycemic control (ie, hemoglobin A1C), compliance with treatment, and diabetic complications were not captured. The use of and adherence to antiplatelet therapy were also self-reported and may have been prone to error. However, as previously mentioned, we believe that it is unlikely that this would have influenced intergroup comparisons. The study lacked an angiographic core lab, instead relying on site-determined angiographic lesion characteristics. Completeness of revascularization, which may contribute to PCI outcomes, was not evaluated in this study. In addition, the specific type of MI (ST-elevation MI vs non–ST-elevation MI) was not captured in the PD study, which is a limitation as sex-based differences in treatment are more common in emergent clinical presentations. Finally, our study was statistically powered for MACE but not other secondary end points.
Conclusions
DMW experience a 2-fold increased risk of MACE and 3-fold increased risk of MI following PCI compared with patients without medically treated DM and were the highest-risk diabetic cohort. The incremental risk of ischemic events in the diabetic patients in our study was associated with insulin use and only persisted in DMW after risk adjustment. These findings suggest an interplay between DM, race/ethnicity, and sex in determining post PCI ischemic events. Until further elucidation of the biologic underpinnings of these clinical observations, lifestyle intervention, aggressive secondary prevention, and strict glycemic control should be emphasized in DMW to reduce these health disparities.
Acknowledgments
The authors acknowledge the Dudley Family for their continued contributions and support of the INOVA Dudley Family Center for Cardiovascular Innovation. We thank Kristine Roy, PhD (Boston Scientific Corporation) for her assistance in manuscript preparation and statistical analyses and Songtao Jiang, MSc, and Chuyu Deng, BSc, (Boston Scientific Corporation) for their assistance with statistical analyses. We also thank Nadia Batchelor, BFA, for her assistance in creating the medical illustrations associated with this publication.
Declaration of competing interest
Roxana Mehran reports institutional grant/research support from Abbott Laboratories, AstraZeneca, AUM Cardiovascular, Bayer, Beth Israel Deaconess Medical Center, Bristol-Myers Squibb, CSL Behring, Eli Lilly/Daiichi-Sankyo, Medtronic, Novartis Pharmaceuticals, OrbusNeich, The Medicines Company, and Watermark Research Partners; is a consultant or serves on executive committees for AstraZeneca, Boston Scientific, Cardiovascular Systems, Janssen Pharmaceuticals, Medscape, Merck, Osprey Medical, Sanofi USA, Shanghai BraccoSine Pharmaceutical, The Medicines Company, and Watermark Research Partners (all minor); and holds equity in Claret Medical and Elixir Medical Corporation. Abdulla Damluji reports research funding from Pepper Scholars Program. Behnam Tehrani serves on the speakers bureau and is a consultant for Medtronic. Matthew Sherwood received honoraria from Medtronic. Alexander Truesdell is a consultant and serves on the speakers bureau for Abiomed Inc. John Wang serves as a consultant and is on the executive committee of Boston Scientific. Paul Underwood and Dominic Allocco are full-time employees and stock holders of Boston Scientific Corporation. Wayne Batchelor is a consultant and clinical trial steering committee member for V-Wave; is a consultant, receives research support, and is a clinical trial steering committee member for Boston Scientific; is a speaker and consultant for and receives research support from Abbott; is a consultant and steering committee member for Medtronic; is a consultant and speaker for Edwards and is a consultant and National Lead Investigator for Idorsia. Kelly Epps, Ridhima Goel, David Kandzari, Behnam Tehrani, Scott Davis, Mario Lopez, and Sarabjeet Singh reported no financial interests.
Funding sources
The PLATINUM Diversity study was supported by the Boston Scientific Corporation. The funding organization assisted in the study design and conduct, collection, management, and analysis of the data. Wayne Batchelor and Roxana Mehran had access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics statement and patient consent
Both the PLATINUM Diversity Study and PROMUS Element Plus Post-Approval Study complied with the Declaration of Helsinki and were approved by each site’s locally appointed ethics committee or institutional review board. Informed consent was required within 24 hours of stent implantation within both studies.
Footnotes
Clinical Trial Registration: NCT02240810 (http://clinicaltrials.gov/).
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2023.101053.
Supplementary material
Supplemental Figure S1.
References
- 1.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty J.D., Davidson C.J. Diabetes and coronary revascularization. JAMA. 2005;293(12):1501–1508. doi: 10.1001/jama.293.12.1501. [DOI] [PubMed] [Google Scholar]
- 3.Dangas G.D., Farkouh M.E., Sleeper L.A., et al. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM Trial. J Am Coll Cardiol. 2014;64(12):1189–1197. doi: 10.1016/j.jacc.2014.06.1182. [DOI] [PubMed] [Google Scholar]
- 4.Kaul U., Bangalore S., Seth A., et al. Paclitaxel-eluting versus everolimus-eluting coronary stents in diabetes. N Engl J Med. 2015;373(18):1709–1719. doi: 10.1056/NEJMoa1510188. [DOI] [PubMed] [Google Scholar]
- 5.Moussa I., Leon M.B., Baim D.S., et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109(19):2273–2278. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 6.Kereiakes D.J., Cutlip D.E., Applegate R.J., et al. Outcomes in diabetic and nondiabetic patients treated with everolimus- or paclitaxel-eluting stents: results from the SPIRIT IV clinical trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) J Am Coll Cardiol. 2010;56(25):2084–2089. doi: 10.1016/j.jacc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Zhuo X., Zhang C., Feng J., Ouyang S., Niu P., Dai Z. In-hospital, short-term and long-term adverse clinical outcomes observed in patients with type 2 diabetes mellitus vs non-diabetes mellitus following percutaneous coronary intervention: a meta-analysis including 139,774 patients. Medicine (Baltimore) 2019;98(8) doi: 10.1097/MD.0000000000014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huxley R., Barzi F., Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brancati F.L., Kao W.H., Folsom A.R., Watson R.L., Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 10.Spanakis E.K., Golden S.H. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhter N., Milford-Beland S., Roe M.T., Piana R.N., Kao J., Shroff A. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) Am Heart J. 2009;157(1):141–148. doi: 10.1016/j.ahj.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor W., Kandzari D.E., Davis S., et al. Outcomes in women and minorities compared with white men 1 year after everolimus-eluting stent implantation: insights and results from the PLATINUM Diversity and PROMUS Element Plus Post-Approval Study pooled analysis. JAMA Cardiol. 2017;2(12):1303–1313. doi: 10.1001/jamacardio.2017.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batchelor W.B., Ellis S.G., Ormiston J.A., et al. Racial differences in long-term outcomes after percutaneous coronary intervention with paclitaxel-eluting coronary stents. J Interv Cardiol. 2013;26(1):49–57. doi: 10.1111/j.1540-8183.2012.00760.x. [DOI] [PubMed] [Google Scholar]
- 14.Chandrasekhar J., Baber U., Sartori S., et al. Sex-related differences in outcomes among men and women under 55 years of age with acute coronary syndrome undergoing percutaneous coronary intervention: results from the PROMETHEUS study. Catheter Cardiovasc Interv. 2017;89(4):629–637. doi: 10.1002/ccd.26606. [DOI] [PubMed] [Google Scholar]
- 15.Epps K.C., Holper E.M., Selzer F., et al. Sex differences in outcomes following percutaneous coronary intervention according to age. Circ Cardiovasc Qual Outcomes. 2016;9(2):S16–S25. doi: 10.1161/CIRCOUTCOMES.115.002482. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golomb M., Redfors B., Crowley A., et al. Prognostic impact of race in patients undergoing PCI: analysis from 10 randomized coronary stent trials. J Am Coll Cardiol Intv. 2020;13(13):1586–1595. doi: 10.1016/j.jcin.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Kandzari D.E., Amjadi N., Caputo C., et al. One-year outcomes in “real-world” patients treated with a thin-strut, platinum-chromium, everolimus-eluting stent (from the PROMUS Element Plus US Post-Approval Study [PE-Plus PAS]) Am J Cardiol. 2016;117(4):539–545. doi: 10.1016/j.amjcard.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Lansky A.J., Costa R.A., Mooney M., et al. Gender-based outcomes after paclitaxel-eluting stent implantation in patients with coronary artery disease. J Am Coll Cardiol. 2005;45(8):1180–1185. doi: 10.1016/j.jacc.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 19.Thompson C.A., Kaplan A.V., Friedman B.J., et al. Gender-based differences of percutaneous coronary intervention in the drug-eluting stent era. Catheter Cardiovasc Interv. 2006;67(1):25–31. doi: 10.1002/ccd.20564. [DOI] [PubMed] [Google Scholar]
- 20.Gurbel P.A., Bliden K.P., Cohen E., et al. Race and sex differences in thrombogenicity: risk of ischemic events following coronary stenting. Blood Coagul Fibrinolysis. 2008;19(4):268–275. doi: 10.1097/MBC.0b013e3282ff76ae. [DOI] [PubMed] [Google Scholar]
- 21.Baber U., Stefanini G.G., Giustino G., et al. Impact of diabetes mellitus in women undergoing percutaneous coronary intervention with drug-eluting stents. Circ Cardiovasc Interv. 2019;12(7) doi: 10.1161/CIRCINTERVENTIONS.118.007734. [DOI] [PubMed] [Google Scholar]
- 22.Holden S.E., Jenkins-Jones S., Morgan C.L., Schernthaner G., Currie C.J. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab. 2015;17(4):350–362. doi: 10.1111/dom.12412. [DOI] [PubMed] [Google Scholar]
- 23.Stoekenbroek R.M., Rensing K.L., Bernelot Moens S.J., et al. High daily insulin exposure in patients with type 2 diabetes is associated with increased risk of cardiovascular events. Atherosclerosis. 2015;240(2):318–323. doi: 10.1016/j.atherosclerosis.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Gamble J.M., Chibrikov E., Twells L.K., et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol. 2017;5(1):43–52. doi: 10.1016/S2213-8587(16)30316-3. [DOI] [PubMed] [Google Scholar]
- 25.Bangalore S., Bhagwat A., Pinto B., et al. Percutaneous coronary intervention in patients with insulin-treated and non-insulin-treated diabetes mellitus: secondary analysis of the TUXEDO trial. JAMA Cardiol. 2016;1(3):266–273. doi: 10.1001/jamacardio.2016.0305. [DOI] [PubMed] [Google Scholar]
- 26.Holland A.T., Zhao B., Wong E.C., Choi S.E., Wong N.D., Palaniappan L.P. Racial/ethnic differences in control of cardiovascular risk factors among type 2 diabetes patients in an insured, ambulatory care population. J Diabetes Complications. 2013;27(1):34–40. doi: 10.1016/j.jdiacomp.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wexler D.J., Grant R.W., Meigs J.B., Nathan D.M., Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28(3):514–520. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- 28.Ginsberg H.N. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aroor A.R., Mandavia C.H., Sowers J.R. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8(4):609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]