Abstract
Cardiac masses are a significant cause of patient morbidity and mortality by virtue of their symptoms and surgical removal. Preoperative diagnosis of a cardiac mass is usually based on clinical correlation and transthoracic echocardiography findings. Myxomas are the most common benign cardiac tumors, commonly occurring in the left atrium attached to the interatrial septum near the fossa ovalis. Although, at times atypical location and unusual morphology may pose a diagnostic dilemma with 2D echocardiography. 3D echocardiography with its multifaceted advantages, including multiplanar cropping abilities and superior imaging quality can help distinguish between a clot and a myxoma.
Keywords: Myxoma, Thrombus, 3D echocardiography
INTRODUCTION
Cardiac masses are uncommon, with an echocardiographic prevalence of 0.15%.[1] Historically, cardiac myxomas have been considered the most common benign cardiac tumor, usually occurring as a sporadic, solitary lesion on the left atrial side of the fossa ovalis (80%). Myxomas have also been reported to arise from the right atrium (RA) in 20% of cases. More rarely, the aorta, pulmonary artery, ventricles, and even the vena cava can be the site of origin.[2] Although benign, myxomas can cause varied symptoms due to hemodynamic impairment (valve obstruction, compression of vessels, and cardiac chambers), arrhythmias, and embolism requiring therapy, either medical or surgical.[3] The imaging appearance of thrombi and sessile myxomas may mimic each other.[4] Conversely, an atrial thrombus in some cases may have a stalk, misleading clinicians into diagnosing it as a myxoma. Differentiating between them can at times become a diagnostic challenge for the intraoperative echocardiographer, which is of paramount importance in tailoring treatment strategies.[5]
CASE DETAILS
A 19-year-old male presented with gradually worsening dyspnea on exertion over 2 years with associated complaints of abdominal distension and pedal edema for 12 months. The patient also had a history of atrial fibrillation. Transthoracic echocardiography revealed a dilated RA with spontaneous echo contrast (SEC), dilated and severely dysfunctional right ventricle (RV), and a sessile solitary mass attached to the RA-free wall. Valvular pulmonary stenosis was also noted. Taking into consideration the clinical and echo findings, the mass was diagnosed as a thrombus. Low-molecular-weight heparin therapy was started, and the patient was planned for clot removal and pulmonary valvotomy. Intraoperative 2D transesophageal echocardiography (TEE) revealed a 3.5 cm × 3.8 cm echodense, homogeneous, ovoid-shaped, sessile, and immobile mass situated below the superior vena cava (SVC) on the RA free wall, suggestive of a thrombus [Figure 1]. 3D echocardiography (3DE) confirmed it to be a solitary, sessile mass; however, the 3D volume data set analysis showed that the mass had a heterogeneous, granular surface texture [Figure 2], few irregular calcifications in crop plane view, and random small areas of echolucency, suggestive of a tumor rather than a thrombus [Figure 3 and Video 1].[6] Surgically excised mass pointed toward it being a myxoma [Figure 4].
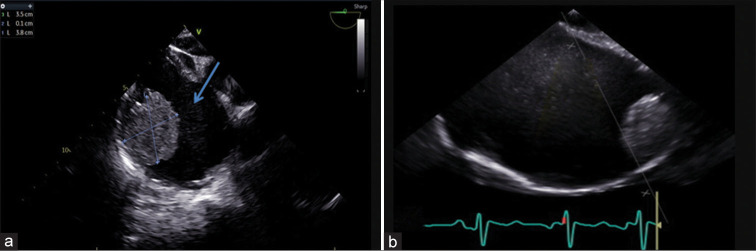
Figure 1:
19-year-old male presented with dyspnea on exertion over 2 years with abdominal distension and pedal edema for 12 months. (a) 2D transesophageal echocardiography (TEE) image in midesophageal 4 chamber right focused view (0°) shows a homogenous mass located on the supero-lateral wall of the right atrium (RA) measuring 3.5 cm × 3.8 cm with spontaneous echo contrast (blue arrow). (b) 2D TEE image in midesophageal bicaval view, showing the mass on the superolateral wall of a dilated right atrium (RA).
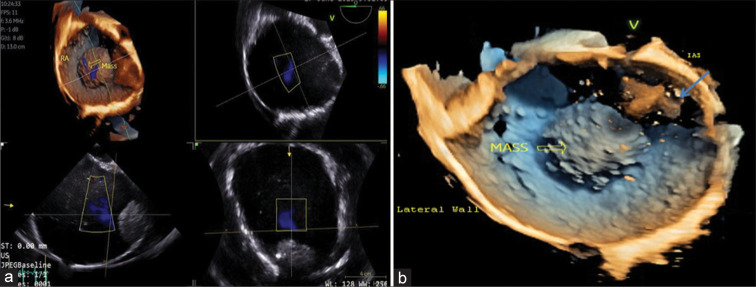
Figure 2:
A 19-year-old male presented with dyspnea on exertion over 2 years with abdominal distension and pedal edema for 12 months. (a) 3D transesophageal echocardiography (TEE) showing multi-planar reconstruction of the mass in the right atrium (RA). (b) 3D TEE view from the right ventricular aspect. The mass (hollow yellow arrow) on the superolateral surface of the RA has a granular surface with the presence of red colored, cross-shaped (blue arrow) artifacts due to spontaneous echo contrast.
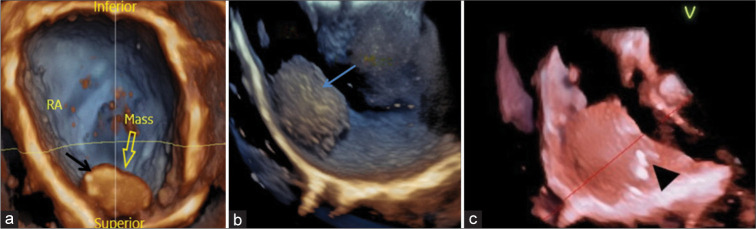
Figure 3:
A 19-year-old male presented with dyspnea on exertion over 2 years with abdominal distension and pedal edema for 12 months. 3D transesophageal echocardiography images using tissue rendering mode: (a) mass viewed from the left atrium with the interatrial septum removed and the image flipped with the inferior portion on top shows areas of echolucency (black arrow) within the mass (hollow yellow arrow). (b) Mass viewed from the lateral wall of the right atrium, showing a rough echotexture (blue arrow). (c) shows a crop plane cutting through the middle of the mass, the random small areas of echolucency and few irregular calcifications (black arrow head) are suggestive of a tumor rather than a thrombus. Right atrium (RA).
Figure 4:
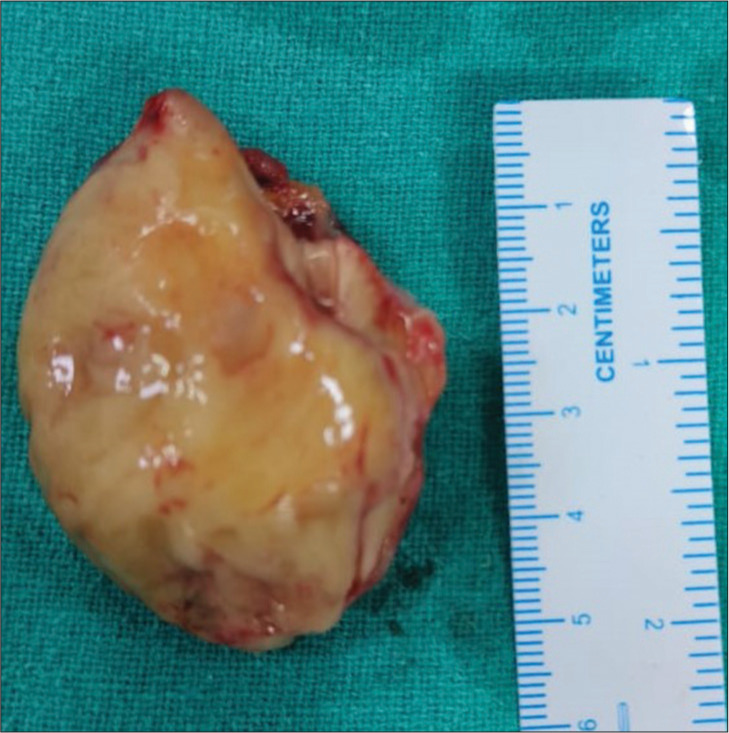
A 19-year-old male presented with dyspnea on exertion over 2 years with abdominal distension and pedal edema for 12 months. Mass after surgical excision is shown above.
DISCUSSION
Primary cardiac tumors, cardiac metastases, vegetations, thrombi, fluid-filled lesions, and artifacts form the differential diagnosis for cardiac masses [Table 1].[6,7] Among them atrial myxomas show the most variability in shape and consistency. Right heart thrombi are often serpiginous, highly mobile, and associated with deep vein thrombosis and pulmonary embolism. Treatment with anticoagulants can help dissolve the thrombus, as was tried in our case.
Table 1:
Echocardiographic features of cardiac tumors and mass.
| Tumor/mass | Characteristics |
|---|---|
| Myxoma | Usually smooth surface, mobile, pedunculated, heterogeneous, and minimal enhancement with contrast echo |
| Papillary fibroelastoma | Mobile pedunculated, homogenous, multiple fibrillar projections, small<1 cm, attached to valvular structures/cords |
| Lipoma | Smooth surface, increased echogenicity, minimal enhancement with echo contrast |
| Rhabdomyoma | Small, well-defined, solitary/multiple homogenous nodules or pedunculated mass, age <1 year |
| Sarcoma | Heterogeneous echogenicity, and hypoechogenic area may be suggestive of tumor necrosis |
| Cardiac hemangioma | Vascular channels with large echolucent areas, and high echogenicity with echo contrast |
| Vegetations | Irregular, lobulated, echo dense, chaotically mobile, can be multiple, attached to the upstream surface of valvular leaflets or mural endocardium |
Echocardiography is the first line of diagnostic tool for the evaluation of intra-cardiac tumors.[8] TEE with better resolution becomes the imaging modality of choice for posteriorly located lesions, in perioperative assessment and guidance for surgical removal. The RA mass in our patient was not suspicious of myxoma, given the absence of stalk, location in RA, attachment site on the RA free wall, immobility along with dilated RA, RV, history of atrial fibrillation, and SEC or “smoke-like” echo, indicating a predisposing stasis, which almost always accompanies a thrombus on 2D echo. Table 2 gives the differentiating features between tumor (myxoma) and thrombi. Although cardiac magnetic resonance imaging (MRI) could have been performed for better tissue characterization of the RA mass,[9] in addition to it being expensive, it is not a part of routine investigation at our institute.
Table 2:
Differentiation between thrombus versus myxoma.
| Characteristics | Thrombus | Tumor (Myxoma) |
|---|---|---|
| Area of origin | LAA or LA (Rarely other chambers) | LA, fossa ovalis Less often in RA or other chambers |
| Mode of attachment | Broader attachment rarely has a stalk | Usually pedunculated, rarely sessile |
| Predisposing conditions | Enlarged atria, atrial fibrillation, Low cardiac output syndrome, and LV aneurysm | Carney’s complex (familial myxoma syndrome) |
| Appearance | Well defined borders | Capsulated myxoma – regular borders, smooth surface Gelatinous myxomas – more irregular, soft, and multilobate surface |
| Response to intervention | Decreased size with thrombolytic therapy and anticoagulation | No response to thrombolysis/anticoagulation |
| Echogenicity | Homogenous, central hypodense zone post thrombolysis | Heterogeneous |
| Multiplicity | Single or multiple | Usually, solitary |
| Contrast Echocardiography | No Enhancement | Myxomas – Partial enhancement Malignant tumors – complete enhancement |
LAA: Left atrial appendage, LA: Left atrium, RA: Right atrium, LV: Left ventricle
3DE can delineate structural details difficult to image with 2D [Table 3].[10,11] 3DE refined the diagnosis as a myxoma in a case of cardiac mass by visualization of the peduncle.[12] A 13-patient study concluded, that 3D TEE is a valuable tool in morphologic imaging, bridges the gap between 2D and anatomy, useful in making decisions and surgical interventions.[13] 3DE was superior even to MRI in confirming a myxoma by identifying the stalk and attachment at the base of the interatrial septum close to the origin of the right inferior pulmonary vein.[14] 3D with its ability to acquire a pyramidal volume of 2D image,[15] which can be rotated and multi-planar cropping[16] capabilities to focus on the region of interest assists in identifying atypical masses allowing better tumor characterization, spatial relations, attachment, differential diagnosis, surgical planning, rationalized use of hospital resources, and eventual patient outcome.
Table 3:
Advantages of 3D echocardiography over 2D.
| Characteristics | 3D | 2D |
|---|---|---|
| Localization | +++ | + |
| Attachment | ++ | + |
| Atypical anatomical features | +++ | + |
| En-face view | +++ | _ |
| Dropout artifacts for thin structures | _ | ++ |
| Spatial relationship | +++ | ++ |
| Mobility | +++ | + |
| Multiplane cropping | +++ | _ |
- No, + fair, ++ good, +++ very good
Intraoperative caution should have been exercised had the mass been a thrombus – during central venous catheterization, bicaval venous cannulation site since the mass was near the SVC-RA junction, to avoid inadvertent dislodgement and iatrogenic pulmonary embolism, which could have increased the cardiopulmonary bypass (CPB) duration and difficult weaning from CPB in a pre-operative dysfunctional RV.
CONCLUSION
An intracardiac mass should be evaluated taking into consideration the clinical setting, age of the patient, tumor location, and echocardiography findings. 3D TEE gives us greater imaging quality and additional information, improving the sensitivity and specificity of diagnosis compared to 2D imaging.
Funding Statement
Financial support and sponsorship
Nil.
Footnotes
How to cite this article: Badge M, Kapoor PM, Thiruselvan T, Francis J. Delineating thrombus versus myxoma: Perioperative 3D transesophageal echocardiography to the rescue! J Clin Imaging Sci. 2024;14:6. doi: 10.25259/JCIS_136_2023
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available online at
References
- 1.Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: A review of methods and clinical applications. Radiology. 2013;268:26–43. doi: 10.1148/radiol.13121239. [DOI] [PubMed] [Google Scholar]
- 2.Al Sergani R, Alamro B, Al Admawi M, Elmahi I, Iannuzzo G, Cittadini A, et al. Three dimensional echocardiographic imaging of multiple recurrent myxomas. Monaldi Arch Chest Dis. 2020;90:17–20. doi: 10.4081/monaldi.2020.1188. [DOI] [PubMed] [Google Scholar]
- 3.Pino PG, Moreo A, Lestuzzi C. Differential diagnosis of cardiac tumors: General consideration and echocardiographic approach. J Clin Ultrasound. 2022;50:1177–93. doi: 10.1002/jcu.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmoud O, Haynos W, Rollor J. Left atrial thrombi masquerading as myxomas: Mini case series and literature review. CASE (Phila) 2020;4:252–9. doi: 10.1016/j.case.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuon E, Kreplin M, Weiss W, Dahm JB. The challenge presented by right atrial myxoma. Herz. 2004;29:702–9. doi: 10.1007/s00059-004-2571-7. [DOI] [PubMed] [Google Scholar]
- 6.Peretz-Larochelle M, Blissett S, Lipes J, Kovacina B, Afilalo J, Rudski L. Clot or not? CASE (Phila) 2018;2:47–50. doi: 10.1016/j.case.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412–9. doi: 10.1016/j.jacc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 8.Zaragoza-Macias E, Chen MA, Gill EA. Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography. 2012;29:207–19. doi: 10.1111/j.1540-8175.2011.01627.x. Erratum in: Echocardiography 2012;29:1. Zaragosa-Macias, Elisa [corrected to Zaragoza-Macias, Elisa] [DOI] [PubMed] [Google Scholar]
- 9.Alyami A, Ajeebi A, Almutairi T, Suliman I. Large right atrial mass, is it tumor or thrombus? A case report. Int J Med Dev Countries. 2020;4:2357–61. doi: 10.24911/IJMDC.51-1598448285. [DOI] [Google Scholar]
- 10.Gadhinglajkar S, Sreedhar R. Intraoperative evaluation of left atrial myxoma using real-time 3D transesophageal echocardiography. Ann Card Anaesth. 2010;13:180–1. doi: 10.4103/0971-9784.62937. [DOI] [PubMed] [Google Scholar]
- 11.Mantegazza V, Gripari P, Tamborini G, Muratori M, Fusini L, Ghulam Ali S, et al. 3D echocardiography in mitral valve prolapse. Front Cardiovasc Med. 2023;9:1050476. doi: 10.3389/fcvm.2022.1050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: Thrombus or myxoma? J Cardiovasc Ultrasound. 2010;18:154–6. doi: 10.4250/jcu.2010.18.4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alamro B, Pergola V, Eltayeb A, Alshammari A, Kholaif N, Alhamshari A, et al. Role of three-dimensional transesophageal echocardiography in cardiac myxomas: an imaging challenge. Monaldi Arch Chest Dis. 2023 doi: 10.4081/monaldi.2023.2768. Doi: 10.4081/monaldi.2023.2768. [DOI] [PubMed] [Google Scholar]
- 14.Galzerano D, Pragliola C, Al Admawi M, Mallardo M, Di Michele S, Gaudio C. The role of 3D echocardiographic imaging in the differential diagnosis of an atypical left atrial myxoma. Monaldi Arch Chest Dis. 2018;88:906. doi: 10.4081/monaldi.2018.906. [DOI] [PubMed] [Google Scholar]
- 15.Mankad R, Herrmann J. Cardiac tumors: Echo assessment. Echo Res Pract. 2016;3:R65–77. doi: 10.1530/ERP-16-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L'Acqua C, Piazzoni N, Muratori M, Mazzanti V. Intraoperative 3D TrueVue transesophageal echo imaging in cardiac mass: Bridge between cardiac anesthesiologist and surgeon. Ann Card Anaesth. 2022;25:241–3. doi: 10.4103/aca.aca_213_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.