To the editor.
Despite advancements in Chimeric Antigen Receptor T-cell (CAR-T) therapy and its notable successes, relapses and non-relapse mortality (NRM) still significantly affect prognosis. These factors contribute to the ongoing complexities in achieving favorable outcomes for patients undergoing CAR-T treatment. Secondary leukemias represent a potential complication that may manifest subsequent to CAR-T treatment. Overall, secondary leukemia may account for mutations in the FLT3 gene. These mutations can induce uncontrolled proliferation of blood cells, thereby fostering the development of aggressive and refractory forms of leukemia.
The onset of clonal hematopoiesis and persistent cytopenias, both preceding and following CAR-T therapy, has been variably reported. Additionally, there is emerging evidence highlighting a heightened incidence of secondary myeloid malignancies subsequent to CAR-T treatment. Our team encountered a case involving a 33-year-old male diagnosed with diffuse large B cell lymphoma (DLBCL), who subsequently developed therapy-related acute myeloid leukemia (t-AML) featuring a FLT3-ITD mutation, which occurred following treatment with multiple lines of therapy and CD19-directed CAR-T.
To the best of our knowledge, there have been no reported cases of FLT3-mutated t-AML following CAR-T therapy up to the present moment.
Previous History and CHIP
The patient was diagnosed with indolent lymphoma at the age of 27 years, with mediastinal bulky disease and without bone marrow involvement, with subsequent evolution in aggressive B cell lymphoma requiring intensive treatment. Overall, the patient received 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), 2 courses of IEV (ifosfamide, epirubicin, and etoposide) consolidated with FEAM-conditioned (fotemustine, cytarabine, etoposide, melphalan) autologous hematopoietic stem cell transplantation and 30 Gy radiating treatment, 3 cycles of R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin), lenalidomide, 6 courses of R-GEMOX (rituximab, gemcitabine, and oxaliplatin) and 6 cycles of pixantrone. Unfortunately, the response to treatment was poor, with a maximal treatment-free remission of only 20 months. The patient was finally proposed for treatment with chimeric antigen T-cell receptor cells (CAR-T) and received CD28-costimulated CAR-T infusion with a high burden bulky refractory disease.
At the time of CAR-T therapy, a bone marrow examination was performed: no signs of dysplasia or lymphoma were described, and cytogenetic analysis documented a normal 46XY karyotype. At that time, Next Generation Sequencing (NGS) tests revealed a clonal hematopoiesis (CH), with CBLp.(Asp460del), DNMT3Ap.(Arg882His), and JAK3p.(Arg582Trp); all showing a VAF >4% (between 5 and 49%) (Figure 1A, APPENDIX A and B). As no cytopenia was present in peripheral blood counts, the patient was characterized as hosting a clonal hematopoiesis of indeterminate potential (CHIP).
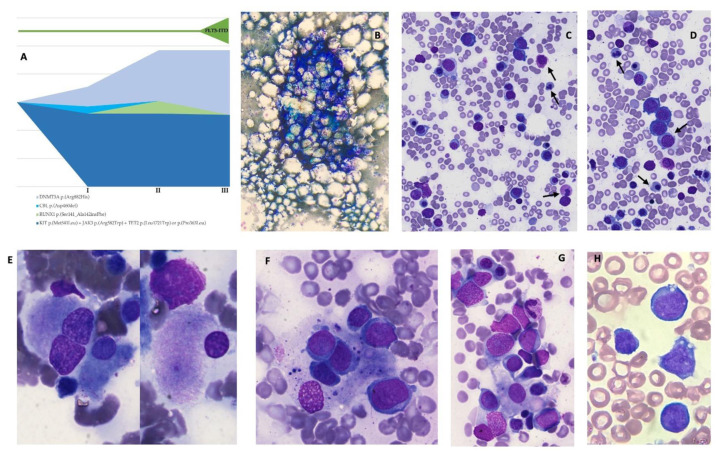
Figure 1. Bone marrow findings during different phases of the myeloid neoplasia.
A: Next Generation Sequencing (NGS) performed on bone marrow samples immediately before CAR-T treatment, at the onset of t-AML with myelodysplasia-related changes, and during the hyperleukocytosis phase with FLT3-ITD mutation. NGS reveals a pre-exhisting condition of CHIP with CBL p.(Asp460del), KIT p.(Met541Leu), DNMT3A p.(Arg882His), JAK3 p.(Arg582Trp), TET2 p.(Leu1721Trp) and TET2 p.(Pro363Leu) being expressed at various VAF. At the onset of t-AML, it is possible to observe the appearance of a RUNX1-mutated clone, which does not persist subsequently, and an expansion of the DNMT3A compound, together with the acquisition of FLT3-ITD mutation. B–C–D: 3 months after CAR-T. Bone marrow examination at the onset of severe trilinear cytopenia. The cellularity appears reduced with age. Granulocyte precursors are extremely rare and with dysmorphic features, resulting in relative erythroid hyperplasia. Erythoid precursors are represented in all maturative phases and show signs of diserytrhorpoiesis, with hemoglobinization defects, occasional binucleation, internuclear bridges, and asynchronous maturation. One macrophage makes contact with erythroid precursors. E–F–G: 6 months after CAR-T. Acute myeloid leukemia with myelodysplastic features and activated macrophages. Dysplastic megakaryocytes can be observed together with a considerable amount of heterogeneous blasts; blasts present with middle-to-large size, open chromatin, up to four nucleoli, and granulated basophilic cytoplasm. A huge number of activated macrophages with images of phagocytosis and cellular detritus can be observed, as well. H: 15 months after CAR-T. Acute myeloid leukemia with hyperleukocytosis and FLT3 mutation. WBC were 160x10^9/L, with large-size blasts with fine chromatin, one or more nucleoli, and basophil cytoplasm with occasional vacuoles.
CAR-T Treatment
During treatment with CAR-T, the following complications occurred: cytokine release syndrome (CRS) of grade 2, neurotoxicity of grade 2, deep vein thrombosis with pulmonary embolism, and consumptive coagulopathy. Supportive therapy and specific treatment included four doses of tocilizumab, dexamethasone, transfusions of red blood cells (RBC) and fresh frozen plasma.
One and up to two months after CAR-T cells, the patient was experiencing a good hematological recovery with no grade 3–4 cytopenias and partial remission of lymphoma at the PET-CT scan.
Phase I: Hypoplasia and Macrophage Activation
Three months after CAR-T, increasing neutropenia was observed, with oscillations of absolute neutrophils count (ANC) and platelets count. At that time, CAR-T cells were still detectable in PB (2 CAR+ cells/microL). Four months after CAR-T, the patient presented at the Emergency Department with severe grade 4 trilinear cytopenia. Major viral infections were excluded, and a bone marrow examination revealed severe hypocellularity with some signs of erythrophagocytosis (Figure 1B–C–D). From that moment on, the patient became dependent on RBC transfusions.
Phase II. Acute Myeloid Leukemia with Clonal Evolution
Two months later, six months after CAR-T infusion, cytopenia persisted, and a bone marrow biopsy was therefore repeated. The lymphoma was persistently in a stable partial response. This time, findings were consistent with therapy-related acute myeloid leukemia (t-AML), with 30% of blasts at the cytomorphological examination; the blasts were mostly medium to large with basophilic cytoplasm. Trilinear dysplasia with the presence of micro megakaryocytes, as well as several images of erythrophagocytosis, was also described (Figure 1E–F–G). Histological examination confirmed abundant cellularity, dyserythropoiesis with megaloblastic aspects and megakaryocyte dysplasia, with microforms and nuclei lobulation defects. Blasts were CD34+ CD117+ HLA-DR+ CD13+ CD33+, with partial aberrant expression of CD7. The cytogenetic analysis showed a partial mosaic karyotype with 40% of cells with monosomy 7 and the presence of a small supernumerary chromosome derived from chromosome 7. Molecular research for FLT3-ITD, NPM1, and core binding factors tests were negative. Macrophage hyperplasia with aspects of hemophagocytosis and MF-3 reticulin fibrosis were also described, together with a modest interstitial T lymphoid component. Still, there was no evidence of lymphomatous infiltration. NGS revealed a more than doubled DNMT3A clone and the appearance of RUNX1 clone with VAF 9% (Figure 1A, Appendix B).
At that point, the patient was treated with two cycles of demethylating agents and BCL2 inhibitors, which were complicated by sepsis and cardiac failure. Those toxicities have been interpreted as a cumulative result of all previous treatments. The patient was then discharged and received palliative care for seven more months.
Phase III: Hyperleukocytosis and FLT3-ITD Gain
Finally, 15 months after CAR-T infusion and 8 months after diagnosis of AML, a new access at the emergency department documented hyperleukocytosis (WBC 160x10^9/L), and a new-onset mutation of FLT3-ITD was found. NGS analysis was not substantially modified, with the exception of the disappearance of the RUNX1 clone and a further increase of the DNMT3A clone (Figure 1A, Appendix B).
The patient was treated with leukocyte apheresis and hydroxyurea but finally died from a subsequent infectious complication.
Discussion
The incidence of CH before CAR-T treatment has been reported in up to 48% of cases.1 In some papers, the presence of CH has been associated with better lymphoma outcomes and worse CRS and neurological toxicities,2 although the data on efficacy have not always been confirmed.
Patients with lymphoma undergoing autologous stem cell transplantation exhibit Clonal Hematopoiesis of Indeterminate Potential (CHIP) with at least one mutation in approximately 30% of cases. In this population, the presence of clonal hematopoiesis predicts the development of therapy-related myeloid neoplasms (t-MDS/AML).
Individuals with CHIP share an increased risk of advancing to hematological malignancies compared to those without mutated clones. This elevated risk seems to correlate with the VAF of the mutated genes. TP53 can significantly influence the development of therapy-related myeloid neoplasms (t-MN) during the clonal evolution of t-MN, while others, such as ASXL1, may contribute to the onset of leukemia. The risk is approximately 11 to 13 times higher in individuals with clonal hematopoiesis, with an overall transformation rate of about 1% per year.3 The cumulative incidence of therapy-related myeloid neoplasms (t-MN) for patients with or without CHIP has been reported as 7.4% versus 1.7% at 5 years and 14.1% versus 4.3% at 10 years, respectively.4 Certain specific alterations, such as TET2, may be more indicative of drug-induced toxicities.5
Secondary myeloid neoplasms have been described in 1–13% of cases after CAR-T cells, according to different reports.1,6,7
When a retroactive analysis was possible, t-AMLs after CAR-T have been proven as derived from a previous clone in some cases,1,7,8 despite there being no prospective data to determine if t-AML tends to arise from a previous CH.
So far, the number of previous lines, together with increased lymphoma-related survival-may be reasonably considered as leading risks for the development of t-AML.
The addition of topoisomerase II inhibitors to alkylating agents has been associated with a shorter latency in the onset of t-AML (mean 6 months).9 Our patient was exposed to an impressive number of alkylating agents and other chemotherapies and developed t-AML seven months after the last chemotherapy and three years after radiating treatment, immediately after prolonged aplasia. Alkhateeb and colleagues report a very short delay between CAR-T infusion and the onset of t-MN (around 9.1 months).7
The hypothesis that an aplastic milieu may favor leukemogenesis or clonal escape in the setting of CAR-T treatment is evocative. However, it has never been properly explored and may need further evidence. On the other side, some t-MN seem not to derive from previous CH, as reported by Alkhateeb.7
Detection of FLT3-ITD mutations has been described in 12–18% therapy-related AML, possibly with lower incidence when compared to de-novo AML (12 vs 24%).10
To the best of our knowledge, this is the first case in which acquired FLT3-ITD mutations have been reported in a t-AML following CAR-T. In this case, we have not identified any FLT3 mutated clone before CAR-T treatment, despite there not being enough evidence to hypothesize a causative correlation between FLT3 and CAR-T.
The presence of CH before CAR-T is a challenging topic in terms of efficacy of treatment, impact on hematological recovery, and onset of subsequent myeloid neoplasms. More integrated and prospective data are needed to frame the risk of potential candidates for CAR-T treatment.
Declarations
The authors acknowledge the support of “Centro di Ricerca sulle Cellule Staminali Emopoietiche e le Terapie Cellulari “Università Cattolica del Sacro Cuore, Roma”.
We want to extend our sincere gratitude to dr Monica Rossi and dr Ilaria Pansini for their invaluable contribution to the coordination and processing of the biological material. Our gratitude also to prof. Maria Teresa Voso and dr Maria Colangelo for their generous availability and willingness to share crucial information and materials essential for this article.
The patient had consented to the use of anonymized data.
For data availability, please contact the corresponding author.
APPENDIX A.
The NGS panel included the following genes: ASXL1, CALR, CBL, CBLB, CEBPA, KIT, CSF3R, CUX1, DNMT3A, EZH2, IDH1, IDH2, IKZF1, JAK2, JAK3, KRAS, MPL, NRAS, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, ZRSR2
APPENDIX B.
NGS was performed before CAR-T, at the onset of t-AML and at the hyperleukocytosis phase. VAF of single clones are showed in the table. KIT p.(Met541Leu), TET2 p.(Leu1721Trp), and TET2 p.(Pro363Leu) are considered as polymorphisms.
| Before CAR-T | 7 months after CAR-T (t-AML) | 15 months after CAR-T (t-AML FLT3-ITD) | |
|---|---|---|---|
| VAF (%) | VAF (%) | VAF (%) | |
| RUNX1 p.(Ser141_Ala142insPhe) | 0 | 9 | 0 |
| CBL p.(Asp460del) | 5 | 0 | 0 |
| KIT p.(Met541Leu) | 52 | 52 | 51 |
| DNMT3A p.(Arg882His) | 14 | 36 | 46 |
| JAK3 p.(Arg582Trp) | 49 | 49 | 51 |
| TET2 p.(Leu1721Trp) | 49 | 49 | 50 |
| TET2 p.(Pro363Leu) | 49 | 49 | 50 |
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Miller PG, Sperling AS, Brea EJ, et al. Clonal hematopoiesis in patients receiving chimeric antigen receptor T-cell therapy. Blood Adv. 2021;5(15):2982–2986. doi: 10.1182/bloodadvances.2021004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teipel R, Kroschinsky F, Kramer M, et al. Prevalence and variation of CHIP in patients with aggressive lymphomas undergoing CD19-directed CAR T-cell treatment. Blood Adv. 2022;6(6):1941–1946. doi: 10.1182/bloodadvances.2021005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabiani E, Cristiano A, Hajrullaj H, Falconi G, Leone G, Voso MT. Therapy-Related Myeloid Neoplasms: Predisposition and Clonal Evolution. Mediterr J Hematol Infect Dis. 2023;15(1) doi: 10.4084/MJHID.2023.064. doi: 10.4084/MJHID.2023.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. Journal of Clinical Oncology. 2017;35(14):1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa U, Castelli G, Pelosi E. Clonal Hematopoiesis: Role in Hematologic and Non-Hematologic Malignancies. Mediterr J Hematol Infect Dis. 2022;14(1) doi: 10.4084/MJHID.2022.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strati P, Varma A, Adkins S, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica. 2021;106(10):2667–2672. doi: 10.3324/haematol.2020.254045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkhateeb HB, Mohty R, Greipp P, et al. Therapy-related myeloid neoplasms following chimeric antigen receptor T-cell therapy for Non-Hodgkin Lymphoma. Blood Cancer Journal 2022 12:7. 2022;12(7):1–5. doi: 10.1038/s41408-022-00707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant. 2020;26(1):26–33. doi: 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fianchi L, Pagano L, Piciocchi A, et al. Characteristics and outcome of therapy-related myeloid neoplasms: Report from the Italian network on secondary leukemias. Am J Hematol. 2015;90(5):E80–E85. doi: 10.1002/ajh.23966. [DOI] [PubMed] [Google Scholar]
- 10.Kayser S, Döhner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]