Abstract
Background:
3-Dimensional (3D) printing has proven its role in various fields. Recently, 3D printing has also been introduced in the otolaryngology domain. The nasopharynx, paranasal sinuses, and the anterior skull base have a complex anatomy. Critical structures must be delicately protected and preserved during a surgical procedure. It is, therefore, very important for the surgeon to have an excellent spatial understanding of the complex surgical field that is being traversed.
Case Description:
Our case is of a 19-year-old male with a 2-month history of recurrent epistaxis, nasal blockage, and headache. Based on the computed tomography scan and the clinical presentation, the patient was diagnosed with juvenile nasopharyngeal angiofibroma. The patient underwent angioembolization of the tumor followed by endoscopic surgical resection. The patient remained stable postoperatively and demonstrated a good recovery in the follow-up visit with no signs of cranial deficits. This case report highlights the use of a patient-specific 3D-printed biomodel to visualize this rare tumor of the nasopharynx. The benefits of using the model in surgical planning, patient education, and resident training are reported. We found that the ability to visualize the tumor on a tangible model, viewing its actual size in relation to the adjacent anatomy and all the structures associated with it, greatly enhances the surgeon’s capacity to tackle such a difficult tumor endoscopically.
Conclusion:
Incorporating 3D-printed biomodels in surgical practice should result in improved outcomes for the patients.
Keywords: 3D printing, Angiofibroma, Biomodel, Simulation, Skull base

INTRODUCTION
Juvenile nasopharyngeal angiofibroma is a rare tumor accounting for approximately 0.05% of head and neck tumors.[14] This benign and highly vascular neoplasm occurs solely in the nasopharynx of adolescent males. It presents mainly with the complaint of recurrent epistaxis and nasal obstruction.[12] The most preferred radiological workup to identify the extension and boundaries of the tumor is a computed tomography (CT) scan. Previously, this tumor was surgically approached through intraoral or external incision, but with advancement in surgical techniques, it is now excised endoscopically. Interventional radiology facilitates the procedure through preoperative embolization of feeding vessels, resulting in minimal bleeding intraoperatively.[12] In our case, besides CT and magnetic resonance imaging (MRI), we also used a 3-dimensional (3D) printed biomodel to understand the complex location of the recurrent tumor and plan our surgical approach and procedure. 3D models have been used to model patient-specific tumors like renal cancer models.[15] According to the surgeons, the 3D models helped with the comprehension of anatomy, helped with the decision on surgical approach, and increased their confidence about their correct planning of the surgery.[15]
In neurosurgery, presurgical planning using 3D-printed brain tumor models ensures safe and successful surgeries, especially for less experienced surgeons.[10,11] Data have shown that the application of 3D printing technology in endoscopic brain tumor surgeries improved surgical outcomes by aiding in reconstruction reducing operation time, blood loss, and complication rate.[3,5] To the best of our knowledge, this is the 1st time a patient-specific 3D model has been used for preand intra-operative planning for a nasopharyngeal tumor. We have used the CARE checklist to report our findings.[4]
CASE PRESENTATION
A 19-year-old male patient presented to our hospital with a chief complaint of recurrent nasal bleeding over two months. He also complained of progressive nasal blockage, continuous nasal discharge, and occasional headaches throughout the past few weeks, with a history of similar complaints two years previously. He had no other comorbidities, nor was he taking any regular medications. His surgical history was significant for radiotherapy of the tumor in 2019 and an attempted resection of a nasal mass in 2020. His family history and physical examination were unremarkable. His initial relevant laboratory workup was reported to be normal.
CT findings
His CT scan showed a lobulated, enhancing soft-tissue mass in the region of the right pterygopalatine fissure and bilateral posterior ethmoid sinuses, anterior to the medial pterygoid plate with extension into both sphenoid sinuses posteriorly and the right maxillary sinus laterally. Arbitrarily, the mass measured approximately 29 mm × 29 mm in the craniocaudal and transverse dimensions. The enhancing soft-tissue mass anterior to the right pterygoid plates measured approximately 18 mm × 12 mm. The right maxillary sinus was not visualized, status-post intervention. The brain showed normal gray and white matter differentiation. No acute intracranial hemorrhage, established infarct, or mass effect was seen. There was no hydrocephalus or shift of the midline structures. Ventricles and basal cisterns appeared normal. His non-contrast CT scan images are shown in Figure 1.
Figure 1:
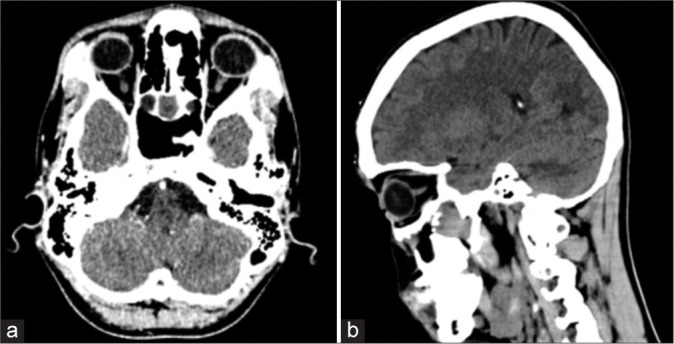
Non-contrast computed tomography of the patient showing the tumor. (a) Axial view, (b) Sagittal view.
The case was thoroughly reviewed, and he was managed along the lines of a recurrent angiofibroma. If not treated, the patient would have suffered from persistent nose bleeds, and the tumor would invade into the cranium. Due to the complex site of the tumor, neurosurgery was also consulted. The family was extensively counseled, and after informed consent, he was planned for an endonasal endoscopic resection of the juvenile angiofibroma post angioembolization.
3D biomodel development
A team of Biomedical Engineers was involved in this case for the development of a patient-specific 3D-printed biomodel [Figure 2]. Detailed information and CT DICOM images were provided to the team. Utilizing “computer-aided design” and slicing tools such as “3D slicer” and “Autodesk Meshmixer,” a 3D biomodel was developed. Consequently, a series of interactive discussion sessions were held between the clinical and biomedical engineering teams to develop the final revised version of the model. Two different types of materials, polylactic acid (PLA) and thermoplastic polyurethane 95A (TPU95A), were used for printing. The skull was printed using PLA material due to its rigidity and stiff nature. For the tumor, TPU95A material was used due to its flexible nature. Dual extrusion printing was used to print this model.
Figure 2:
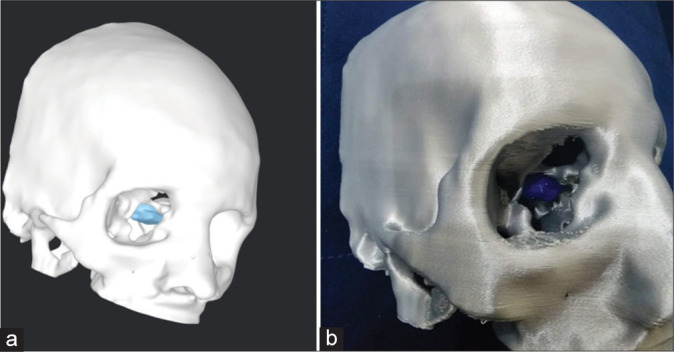
Three dimensional (3D) biomodel (a) on computer software, (b) 3D-printed.
Angioembolization
A 4 French vascular access sheath was placed in the right femoral artery, and through this access, bilateral external carotid arteries were cannulated, and angiograms were performed. The angiogram revealed a vascular blush in the posterior nasal region corresponding to the lesion seen on diagnostic imaging. This was predominantly supplied by bilateral internal maxillary artery branches (right>Left). The supplying branches were selectively cannulated and embolized using polyvinyl alcohol particles through a microcatheter. Post embolization run showed a significant reduction in the arterial supply of the lesion.
Tumor resection
Two days after angioembolization, the patient underwent endoscopic endonasal excision of the angiofibroma under general anesthesia with a cover of hypotensive drugs to minimize bleeding. The 3D biomodel was used by the surgical team, preoperatively [Figure 3] and intraoperatively, to visualize the tumor and plan the optimal approach. During the procedure, a big anteroseptal defect was visualized secondary to his previous surgery. The tumor was visible, extending into the right pterygoid and pterygopalatine fossa. The tumor was shaved off and removed using reticular dissection. Careful dissection was done while separating the tumor from the fibrous tissue. On reaching the distal edge, the tumor was found to adhere to the dura mater. It was carefully excised and revealed to be a blood-filled cyst.
Figure 3:
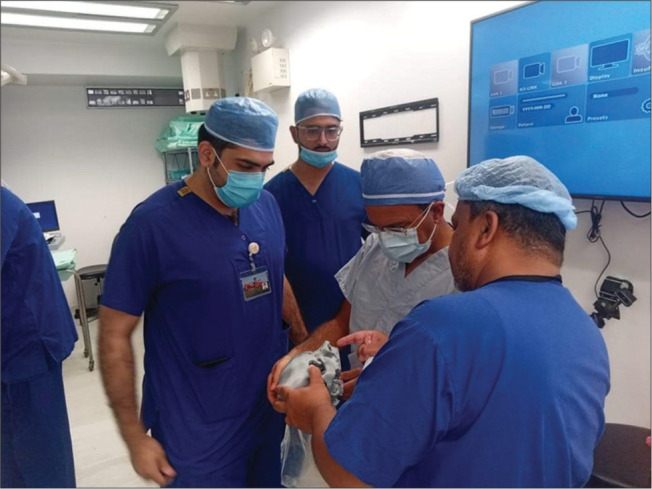
Three-dimensional printed biomodel being used in surgical planning.
Postoperative care
The patient was closely monitored for the next two days postoperatively. He remained vitally and clinically stable. Postoperatively, his extraocular movements, pupillary reflex, and higher mental functions were thoroughly examined and found to be normal. On a follow-up visit to the clinic, he showed promising recovery with no signs of any cranial deficits. The summary of the patient’s management is described in Figure 4.
Figure 4:
Order of the management of the patient.
DISCUSSION
In the 5th century BC, Hippocrates first described the tumor which was later termed “angiofibroma” in 1940 by Friedberg. The tumor, over time, has gained the name “Juvenile Nasopharyngeal Angiofibroma” due to its predisposition in the adolescent age group.[1] It has a higher bias toward the male population.[1] Although the tumor itself has a very low reported incidence of 1:5,000–1:60,000 in otolaryngology patients, it is still the most common head and neck tumor to date in the young male population. Angiofibroma is predominantly a benign tumor arising mostly from the posterolateral wall of the nasopharynx.[1] It has a rich supply of vascular networks, which make it susceptible to a major life-threatening bleed if engaged.[2] It can expand, extend, and infiltrate the surrounding bony structures, causing various presenting complaints. The patient may present with symptoms of recurrent epistaxis, nasal obstruction, mass effect in the nasal passage that may block sinus drainage pathways resulting in secondary sinusitis, and recurrent otitis media and conductive hearing loss secondary to eustachian tube blockage. In advanced cases, it can cause diplopia, proptosis, and anosmia and affect the second and sixth cranial nerves secondary to superior orbital fissure involvement.
A CT scan, MRI, and angiography are the main radiological tools used to determine the position, size, extent, erosion into the surrounding bony structures, and invasion through the various foramina and fissures. Bony involvement is best demonstrated on CT scan, intracranial spread on MRI, and feeding vessels can be best identified on angiography, which can also be used for preoperative angioembolization to reduce hemorrhagic complications. The arterial supply of the tumor is largely from the distal internal maxillary artery branches, namely, the sphenopalatine, descending palatine, and posterior superior alveolar branches.[1]
The ability to obtain a 3D-printed model where a detailed knowledge of patient-specific anatomy can be physically observed aids in planning the best route of surgery. It not only gives the surgeon a better modality to plan for the best surgical approach but can also enhance safety by minimizing the risk of a complication during the actual surgical procedure. In our case, the surgeon was able to successfully use the tactile dimensions of the model and plan the endoscopic approach for the angiofibroma. Due to the recurrent nature and complexity of the tumor in our case, this added modality in the planning phase of the surgery significantly improved our capacity to resect the tumor completely without any residual tissue, which could have resulted in a recurrence.
In a collaborative attempt between Otolaryngology and Neurosurgery to remove this complex angiofibroma, which was adhered to the dura mater, a new modality was utilized. Nowadays, 3D-printed models are utilized not only for orthopedics surgeries but also play a major role in skull base tumor resections, reconstructive maxillofacial surgeries, and cardiac surgeries as well. Multiple different types of 3D-printed models allow us to differentiate the normal tissue from the tumor, thus allowing the surgeon to review the tumor margins more thoroughly with its anatomical surrounding structures. 3D-printed models specific to the patient mimic the complex and distorted anatomy of the patient. Numerous workshops with 3D-printed skulls have been carried out and it is found to be a useful tool in individualized surgical planning for endoscopic surgeries.[9]
Preoperative planning
A 3D model may help increase the accuracy and reduce the duration of surgery, which would, in turn, reduce intraoperative risks and decrease anesthesia time and exposure to ionizing radiation. Studies have demonstrated benefits in patient outcomes with the incorporation of 3D models.[2,9]
According to a case report on using 3D-printed models in endoscopic sinus surgery, the patient-specific 3D model aided in improved visualization of tumor size and its relation to the surrounding anatomy.[8] This allowed the surgeon to perform a less invasive surgery, which resulted in a faster recovery for the patient and reduced the risk of complications.[8] Preoperative manipulation of 3D models allowed surgeons to perform endoscopic surgeries more confidently and efficiently and placed them in a better position to assess different surgical approaches for increased safety, better exposure, and shorter surgical times.[7] Surgical planning using the 3D model helped to reduce not only the OR time but also the hospital stay, thereby reducing the overall medical cost.[8]
Patient education
Good patient satisfaction requires that patients have all their concerns addressed. This often means that they want to understand their pathology. 3D-printed models serve as an excellent tool to serve that purpose. In the case of tumors, these models help patients understand where the tumor lies and why a particular intervention is being carried out in a particular manner. Sander et al.[13] compared 3D-printed models with standard two-dimensional diagrams and charts in a set of 100 patients planned for endoscopic sinus surgery. These patients were educated on their anatomy, disease state, and treatment options. Their study found statistically significant improvement in patients’ self-rated understanding of the anatomy, disease process, and visualization in the arm where 3D models were used.[7] Another study used patient-specific 3D models in preoperative counseling of parents of pediatric patients undergoing endoscopic endonasal skull base surgery.[6] Almost all of the ten parents included in the study agreed or strongly agreed that the 3D model was helpful in explaining to them the pathology, the surgical planning, and the possible complications associated with the procedure.[6]
Surgical teaching
With advances in technology, there is a natural and parallel increase in the complexity, accuracy, and approaches to surgical planning. Effective surgical training is enhanced when utilizing simulation on 3D models for junior trainees to learn the essential skills. Surgery of the paranasal sinuses and the anterior skull base requires expertise in protecting critical structures such as the optic nerve, brainstem, brain, and carotid arteries. A workshop conducted by Narayanan et al., found that simulating endoscopic sinus and skull base surgeries helped trainees learn the crucial steps of the procedure, such as image-guided navigation and the use of power tools for endoscopic drilling.[9] The participants found the models very realistic as the technology allowed the models to replicate the anatomy of actual patients. Trainees were also able to develop their hand-eye coordination on these models, a skill that is important while performing endoscopic procedures in the skull base region. Finally, these simulations are excellent for allowing teams of surgeons from different specialties to practice working together in a coordinated manner.[9]
CONCLUSION
Juvenile nasopharyngeal angiofibroma is a rare, benign tumor that is mainly prevalent in the adolescent male population. The main presentation of the affected individual varies from unilateral to bilateral nasal blockage to severe epistaxis. A contrast CT scan is the first and most accurate radiological method to check for the tumor’s size and extension, helping in staging the tumor to denote the best possible surgical approach. Angiography, along with angioembolization, is the best collateral procedure done before surgery to minimize blood loss and consequently reduce fatal risk factors. In our case, we created and utilized a 3D-printed biomodel to further enhance 3D visualization of the tumor, allowing us to practice and identify the best approach for surgery, thereby minimizing operating time complications and ensuring faster recovery and reduced hospital stay for our patient. It is important to note that although not 100% accurate, the 3D-printed biomodel enables the surgeon to plan the approach for tumor excision with high accuracy, thereby minimizing unfavorable consequences for the excision of a tumor with a high risk of bleeding.
Acknowledgments
We express our gratitude to the Ideas and Innovation Division at the Department of Surgery and the Technology Innovation Support Centre for providing the necessary resources to carry out this study.
Footnotes
How to cite this article: Zahid F, Memon A, Siddiqui M, Deewani M, Asif O, Javer A, et al. Successful use of a patient specific 3D-printed biomodel as surgical guide for excision of juvenile nasopharyngeal angiofibroma extending to skull base: A case report. Surg Neurol Int. 2024;15:44. doi: 10.25259/SNI_743_2023
Contributor Information
Fahad Zahid, Email: fahad.zahid22@alumni.aku.edu.
Ayesha Memon, Email: ayesha.memon21@alumni.aku.edu.
Moghira Siddiqui, Email: moghira.siddqui@aku.edu.
Muhammad Hammad Deewani, Email: m.hammadwahid@gmail.com.
Osama Asif, Email: osama.asif@aku.edu.
Amin Javer, Email: sinussurgeon@drjaver.com.
Ahsan Ali Khan, Email: ahsanali.khan@aku.edu.
Authors’ contributions
AM wrote the initial draft of the manuscript. FZ co-wrote and edited the manuscript. MS, MHD, and AAK conceptualized and designed the framework of the study. OA provided technical expertise with regard to generating a 3D patient-specific biomodel on the computer and then printing it using a 3D printer. AJ led the surgical team. MHD was involved in managing the patient. MS, AJ, and AAK participated in the surgery and provided key edits to the manuscript based on their expertise. All authors reviewed and approved the final manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the present study.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Atalar MH, Solak O, Müderris S. Juvenile nasopharyngeal angiofibroma: Radiologic evaluation and pre-operative embolization. KBB Forum. 2006;5:56–61. [Google Scholar]
- 2.Budu V, Bulescu I, Mogoantă CA. Particular aspects in endoscopic surgery for juvenile nasopharyngeal angiofibromas. Case reports and review of literature. Rom J Morphol Embryol. 2013;54:867–70. [PubMed] [Google Scholar]
- 3.Essayed WI, Unadkat P, Hosny A, Frisken S, Rassi MS, Mukundan S, et al. 3D printing and intraoperative neuronavigation tailoring for skull base reconstruction after extended endoscopic endonasal surgery: Proof of concept. J Neurosurg. 2018;130:248–55. doi: 10.3171/2017.9.JNS171253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang X, Liu Z, Wang X, Li XD, Cheng K, Zhou Y, et al. A small 3D-printing model of macroadenomas for endoscopic endonasal surgery. Pituitary. 2019;22:46–53. doi: 10.1007/s11102-018-0927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez JE, Shaffer AD, Hammersley E, Ghodadra A, Stapleton AL. Use of patient-specific 3D printed models in pre-operative counseling for pediatric skull base surgery. Int J Pediatr Otorhinolaryngol. 2023;171:111655. doi: 10.1016/j.ijporl.2023.111655. [DOI] [PubMed] [Google Scholar]
- 7.Low CM, Morris JM, Price DL, Matsumoto JS, Stokken JK, O’Brien EK, et al. Three-dimensional printing: Current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33:770–81. doi: 10.1177/1945892419866319. [DOI] [PubMed] [Google Scholar]
- 8.Mallon A, Farnan T. A case report detailing the use of 3D printing technology in surgical planning and decision making in ENT surgery-an axial 3D first in Northern Ireland. Int J Surg Case Rep. 2021;87:106407. doi: 10.1016/j.ijscr.2021.106407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayanan V, Narayanan P, Rajagopalan R, Karuppiah R, Rahman ZA, Wormald PJ, et al. Endoscopic skull base training using 3D printed models with pre-existing pathology. Eur Arch Otorhinolaryngol. 2015;272:753–7. doi: 10.1007/s00405-014-3300-3. [DOI] [PubMed] [Google Scholar]
- 10.Oishi M, Fukuda M, Yajima N, Yoshida K, Takahashi M, Hiraishi T, et al. Interactive presurgical simulation applying advanced 3D imaging and modeling techniques for skull base and deep tumors. J Neurosurg. 2013;119:94–105. doi: 10.3171/2013.3.JNS121109. [DOI] [PubMed] [Google Scholar]
- 11.Panesar SS, Magnetta M, Mukherjee D, Abhinav K, Branstetter BF, Gardner PA, et al. Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg Focus. 2019;47:E12. doi: 10.3171/2019.9.FOCUS19511. [DOI] [PubMed] [Google Scholar]
- 12.Rogers DJ, Bevans SE, Harsha WJ. Endoscopic resection of juvenile nasopharyngeal angiofibroma. Adv Otorhinolaryngol. 2012;73:132–6. doi: 10.1159/000334470. [DOI] [PubMed] [Google Scholar]
- 13.Sander IM, Liepert TT, Doney EL, Leevy WM, Liepert DR. Patient education for endoscopic sinus surgery: Preliminary experience using 3D-printed clinical imaging data. J Funct Biomater. 2017;8:13. doi: 10.3390/jfb8020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlăescu AN, Ioniţă E, Ciolofan MS, Mogoantă CA, Voiosu C, Rusescu A, et al. Current approach of juvenile nasopharyngeal angiofibroma: A case series. Rom J Morphol Embryol. 2022;63:105–11. doi: 10.47162/RJME.63.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wake N, Rude T, Kang SK, Stifelman MD, Borin JF, Sodickson DK, et al. 3D printed renal cancer models derived from MRI data: Application in pre-surgical planning. Abdom Radiol (NY) 2017;42:1501–9. doi: 10.1007/s00261-016-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the present study.



