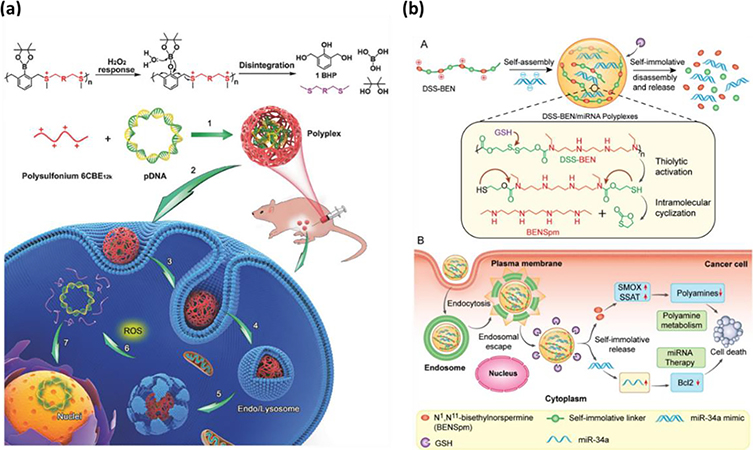
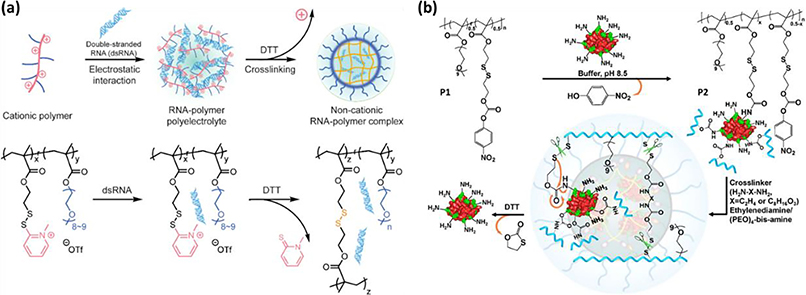
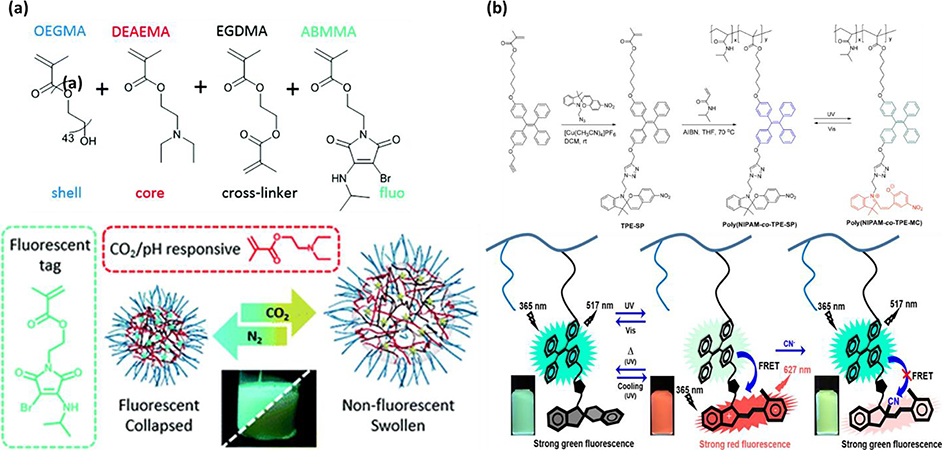
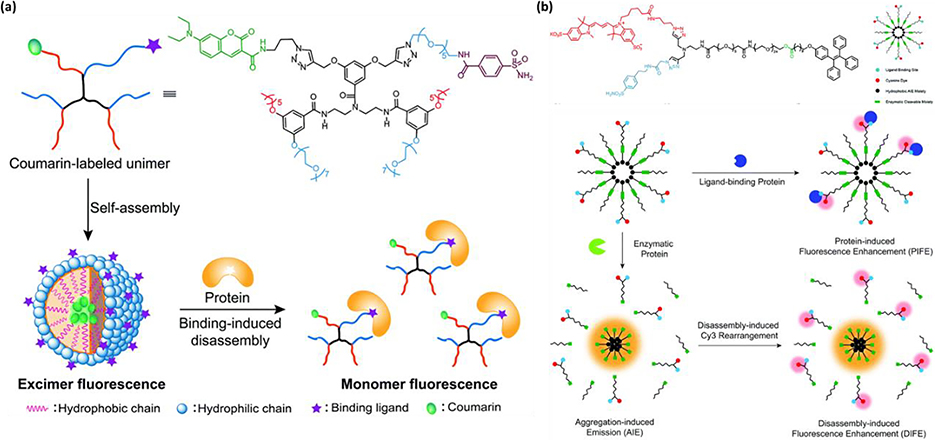
Abstract
Stimuli-responsive nano-assemblies from amphiphilic macromolecules could undergo controlled structural transformations and generate diverse macroscopic phenomenon under stimuli. Due to the controllable responsiveness, they have been applied for broad material and biomedical applications, such as biologics delivery, sensing, imaging, and catalysis. Understanding the mechanisms of the assembly-disassembly processes and structural determinants behind the responsive properties is fundamentally important for designing the next generation of nano-assemblies with programmable responsiveness. In this review, we focus on structural determinants of assemblies from amphiphilic macromolecules and their macromolecular level alterations under stimuli, such as the disruption of hydrophilic-lipophilic balance (HLB), depolymerization, decrosslinking, and changes of molecular packing in assemblies, which eventually lead to a series of macroscopic phenomenon for practical purposes. Applications of stimuli-responsive nano-assemblies in delivery, sensing and imaging were also summarized based on their structural features. We expect this review could provide readers an overview of the structural considerations in the design and applications of nanoassemblies and incentivize more explorations in stimuli-responsive soft matters.
Keywords: Stimuli-responsive, Amphiphilic macromolecules, Structural determinants, Nanoassemblies, Self-assembly, Disassembly, Biomedical applications
Graphical Abstract

1. Introduction
Biological processes generally rely on the adaptive response to biomolecules and environmental stimuli to initiate structural and functional alterations. With myriad of covalent transformations and non-covalent interactions occurring concurrently and in sequence, nature also uses compartmentalization to organize these events and accommodate incompatibilities [1,2]. Mimicking nature by packing functional molecules into compartments to protect the payloads from leakage and damage, while performing on-demand activation in response to changes in the surrounding environment, has been a design target. Artificial macromolecules have been applied for self-organization into nanostructures, taking advantage of their propensity for compartmentalization and controlling the adaptive structural transformations and activity of payloads in response to surrounding stimuli [3,4]. These self-organized nanostructures, a.k.a., nano-assemblies, have drawn significant attention due to their broad materials and biomedical applications.
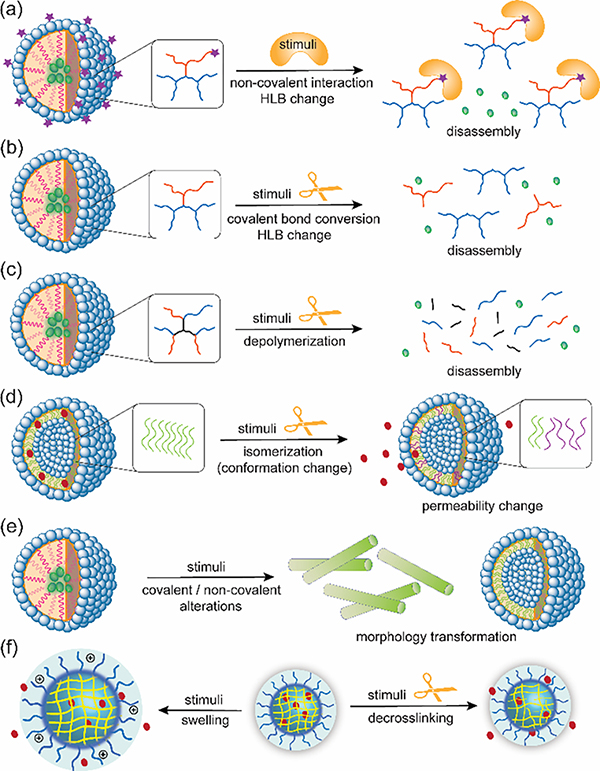
Over the past few decades, there has been extensive research on amphiphilic macromolecules with built-in triggers to control assembly/disassembly behaviors upon exposure to a specific stimulus for delivery, catalysis, agriculture, and sensing/imaging applications [5–11]. Tailoring macromolecules that respond to chemical, physical, and biological stimuli enable structural alterations to activate on-demand functions in nano-assemblies. Molecular design for this purpose often involves stimulus-induced changes at the molecular level, including disruption of hydrophilic-lipophilic balance (HLB), depolymerization, decrosslinking, changes in molecular conformation/configuration, and packing (Fig. 1). These changes subsequently impact factors such as solubility, morphology, size, permeability, and colloidal stability of the assemblies. A wide range of chemistries such as functional group transformations, configurational changes, and self-immolative chain reactions have been developed to this end. Strategic installation of these triggerable moieties into macromolecules to maximize the targeted changes enables the translation of the molecular scale structural transformations to nanoscale assemblies in response to surrounding stimuli and external triggers.
Fig. 1.
Different strategies for stimuli-triggered alterations in nano-assemblies: (a) Disassembly via HLB change by the disruption of non-covalent interactions, (b) disassembly via HLB change by the disruption of covalent bond, (c) disassembly via depolymerization, (d) permeability change via conformation/configuration alterations, (e) stimuli-triggered morphology transformation, (f) stimuli-triggered swelling or decrosslinking of crosslinked nano-assemblies.
Over the course of this review, we will focus on the recent advances in responsive amphiphilic macromolecular nano-assemblies from the perspective of structural determinants in polymers that dictate their assembling/disassembling behaviors. An analysis on structure-property relationships of amphiphilic molecules, classification of stimuli, assembly fidelity in the presence and absence of various stimuli, and areas in which these materials find applications are anticipated to give reader a comprehensive overview of this field and ignite new ideas for designing the next generation “smart” materials using amphiphilic macromolecules. Organizationally, we aim to first categorize the types of stimuli (i.e., chemical, physical, and biomacromolecular stimuli), triggerable moieties, and their responsive behavior. Considering many reviews on this topic [12–20], we herein specifically focus on the structural determinants in nano-assemblies and aim to helping readers to gain a better understanding on trigger-induced processes. We then focus on tactics adopted in nanostructure transformation and provide viewpoints of how structural determinants at molecular level are associated with nano- and macroscopic phenomena. A comprehensive analysis of molecular structure, stimuli-triggered assembly alterations and macroscopic responses will be integrated in the following section. Finally, recent applications in stimuli-responsive nano-assemblies are summarized based on the structural transformation-guided strategies. We believe that the discussion on structural determinants behind alterations at molecular, macromolecular, and nanoscopic levels will help widen the horizon and incentivize advances in macromolecular soft matters.
2. Commonly used stimuli for responsive disassembly
A wide variety of stimuli-responsiveness have been exploited to trigger structural alterations of nano-assemblies, which can be classified into chemical, physical, and biological stimuli on a basis of triggering sources. Extracorporeal physical stimuli such as temperature, light, and mechanical force can induce covalent and non-covalent structural alterations with spatiotemporal precision. Nano-assemblies at the site of interest that receive a specific physical excitement at a certain magnitude cause changes in structural integrity. Macromolecules that are sensitive to chemicals such as reactive oxygen species (ROS), reducing agents, glucose, and pH offer the opportunity for response to specific microenvironments encountered in nature. Finally, nano-assemblies can be programmed to respond to contact with specific biomacromolecules through covalent modifications due to enzymatic reactions or non-covalent alterations such as binding events with proteins. In this section, we give a brief introduction to each of the stimuli and the general opportunities that present to design responsive soft matter.
2.1. Physical stimuli: temperature, light and mechanical force
Temperature
The oft-used response in temperature-responsive macromolecules involves abrupt phase change in aqueous media. These changes are akin to sol-to-gel transition or vice versa at the molecule’s cloud point temperature (Tcp). This transition point is also known as the lower critical solution temperature (LCST) or the upper critical solution temperature (UCST), depending on the direction of phase transition [21]. Macromolecules with LCST character are soluble in aqueous solution below the Tcp, often due to the hydrogen bonds formed between thermoresponsive moieties and water molecules. Upon heating, the hydrogen bonds weaken, causing an increase in the molecular hydrophobicity, which leads to chain collapse and increased turbidity [22,23]. For macromolecules that exhibit an UCST behavior, stronger intra- and inter-chain interactions due to hydrogen bonds or electrostatic interactions result in poor aqueous solubility at lower temperatures. Hence, solubility increases upon heating because of hydration of thermoresponsive moieties by the surrounding water molecules that dissipates the intra- and inter-chain interactions [24]. The tunable Tcp range of polymers can be adjusted by various factors such as monomer structure, chain length, composition, and morphology of polymers, as well as the solvent, polymer concentration, counter ions, salt type and concentration, and additives. The molecular considerations necessary for the design of thermoresponsive nano-assemblies have been extensively discussed and summarized in our previous review [25]. Additional information regarding the design and applications of thermoresponsive materials has also been discussed by other research groups [26,27].
Light
Controlling a chemical process at the site of interest and the appropriate timing offers a tremendous opportunity for precise activation. Light is a versatile trigger with controllable parameters of irradiation time, site, wavelength, and intensity, allowing for structural transition of macromolecular nano-assemblies with spatiotemporal precision. Macromolecules with hydrophobic, photocleavable pendants could undergo hydrophobic-to-hydrophilic transition upon light irradiation [28]. Common photocleavable aryl (methyl)ester groups such as pyrene [29], o-nitrobenzyl [30], coumarin [31], and perylene [32], linked to the polymer backbone provides nano-assemblies with hydrophobicity and structural integrity. Upon bond breakage of the photochromic moieties during photochemical reactions, the resulting hydrophilic (meth)acrylic acids can lead to structural disintegration (Table 1). Photocleavable groups can be repeatedly inserted into the main chain or placed at the head group of self-immolative hydrophobic core-forming blocks [33,34]. In response to light triggers, cascade degradation of self-immolative blocks or simultaneous chain shattering degradation of hydrophobic main chains lead to the disruption of assemblies [35–37]. One major challenge of photo-responsive systems is the limited penetration ability of the light used to trigger the desired responses. In recent years, great advances have been achieved with upconversion materials and photocleavable molecules in response to deep red or near-infrared (NIR) light of phototherapeutic window (650–900 nm) to expand the horizon of feasibility [38–40]. Despite the availability of various light sources, the limited penetration depth of light within a range of millimeters still poses a significant challenge for a wide variety of applications [41].
Table 1.
Photoactive moieties and corresponding behaviors.
| Photochemistry | Responsive moiety | Absorption range | Responsive behavior |
|---|---|---|---|
| Bond cleavage | Pyrenylmethyl ester [29,52] | 300–500 nm |
|
| o-Nitrobenzyl ester [53] | 350–500 nm |
|
|
| Coumarinyl ester [31] | 350–450 nm |
|
|
| Perylene-3-ylmethyl ester [54] | 350-550 nm |
|
|
| Ruthenium complex [55] | 350–500 nm |

|
|
| Boron-dipyrromethene [56] | < 693 nm |
|
|
| Heptamethine cyanine [57,58] | 690–780 nm |
|
|
| Isomerization | Azobenzene [59,60] | (trans to cis) 320–350 nm; (cis to trans) 400–450 nm |
|
| Spiropyran [61] | (SP to MC) 300–400 nm; (MC to SP) 450–650 nm |
|
|
| Bis-dithienylethene [62,63] | (cis to trans) 280–360 nm; (trans to cis) 450–720 nm |

|
|
| Dimerization | Coumarin [64,65] | (unimers to dimer) >310 nm; (dimer to unimers) <260 nm |
|
| Anthracene [51,66] | (unimers to dimer) >350 nm; (dimer to unimers) <300 nm |
|
|
| Styrylpyrene [49,67] | (unimers to dimer) 400–560 nm; (dimer to unimers) ≤360 nm |
|
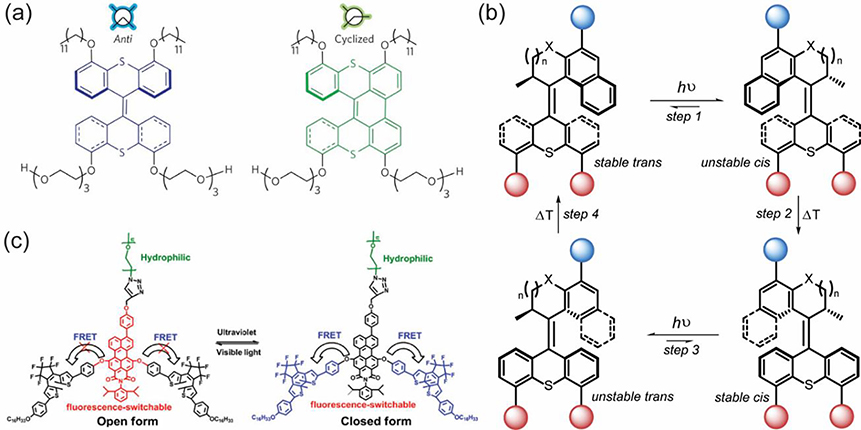
Photoisomerization is a reversible process that changes the hydrophilicity or molecular packing through bond scissoring or rotation, allowing for recurring transitions between structural construction and disruption. While the reversible nature of photoisomerization is useful for controlling structural transitions, the photoinduced reversibility might not be fully achieved due to the photochromic fatigue after several irradiation cycles. For example, trans-cis photoisomerization of phenyl rings on either side of nitrogen-nitrogen double bond provides azobenzene-containing nano-assemblies with the momentum for light-induced disruption [42]. The azobenzene in the apolar trans form (dipole moment ~ 0 D) converted to the polar cis form (dipole moment ~ 4.4 D) upon light irradiation gives rise to the increasing polarity of macromolecules,[43] leading to the disruption of nano-assembles [43]. Moreover, tight stacking of azobenzene in the trans orientation was found to help solidify structural integration while cis form upon light triggering causes distortion of molecular packing and leads to leaky structure. However, the complete disruption of nano-assemblies containing azobenzene is barely achievable due to the photochromic fatigue.
Reversible crosslinking and decrosslinking in response to light provides macromolecular assemblies with a switch to tune the structural integrity on demand. Photoisomerization from hydrophobic, ring-closed spiropyrans (SP) to charged, ring-opened merocyanines (MC) can reversibly trigger hydrophilic-lipophilic imbalance to control the rupture and formation of nanoassemblies [44,45]. Colloidal instability is one of the challenges to be solved for the design of stimuli-responsive systems. Photo-crosslinking can enhance the resilience of nano-assemblies to extreme dilution, while photo-decrosslinking can facilitate the release of entrapped guest molecules. For example, the heavily used coumarin moieties can undergo [2 + 2] photocycloaddition upon light irradiation at λ > 310 nm and photo-cleavage of cyclobutane bridges upon light irradiation at λ < 260 nm [46]. The pendant coumarins provide macromolecules with hydrophobicity for assembly formation and further solidify the structure stability via photodimerization. Enhanced core density upon photodimerization has been utilized to stabilize the encapsulated hydrophobic molecules while enhanced payloads release upon photo-decrosslinking [46–48]. However, similar to photoisomerization, coumarins have been found to undergo photodamage after multiple cycles, resulting in incomplete crosslinking and decrosslinking. The incomplete nature of these processes can also be attributed to the photostationary states of the chromophores at different wavelengths. In contrast to the reversible photo-crosslinking of coumarins, which occurs in the UV region, styrylpyrene [49] and anthracene [50,51] were developed for reversible assembly and disassembly upon light irradiation at visible wavelength λ > 380 nm) Table 1).
Mechanical force
Mechanical force is an effective method for achieving spatiotemporal precision in a triggering event without harming peripheral healthy tissues to the triggering sites. The use of ultrasound in medical applications is intriguing due to its non-invasiveness, absence of ionizing irradiation, and ability to penetrate into deep tissues. Ultrasounds are mechanical waves propagating in a medium through changes in pressure, at frequencies higher than 20 kHz. Frequency and intensity are important parameters in ultrasound, where frequency modulates the depth of tissue penetration and cavitation, and intensity controls the energy transmitted to the site of interests [68]. In general, ultrasound can trigger the transformation of macromolecular assemblies via mechanical and thermal effects. High intensity focused ultrasound (HIFU) at frequency ranging from 600 kHz to 7 MHz generates high hyperthermia (> 43 °C) at the focal point while inertial cavitation induced by low-frequency ultrasound at 20 to 90 kHz cause shear force and free radical formation [69]. HIFU has been used to boost the hydrolysis of 2-tetrahydropyranyl methacrylate (THPMA) copolymer that resides at hydrophobic core of micelles to cause the HLB change and enhance the LCST sensitivity from 25 °C to 42 °C, resulting in the disassembly of macromolecules and payload release [70]. Ultrasound-induced cavitation can transiently enhance permeability of curcumin-loaded particles and recover structural integrity within short time post-irradiation, resulting in spatiotemporal drug release and repetitively triggered drug release on demand [71]. Indirect disintegration of macromolecular assembly in response to ultrasound can be achieved by sonodynamic property under ultrasonic cavitation. Sonosensitizer hypocrellin-loaded poly(ethyleneglycol)-poly(propylenesulfide) micelles underwent disassembly upon ultrasonic irradiation. Hypocrellin molecules generated reactive oxygen species (ROS) that oxidized hydrophobic sulfide to hydrophilic sulfone, giving rise to HLB change and the disassembly of particles [72].
2.2. Chemical stimuli: pH, ROS, redox and diols (glucose)
pH
pH-responsive moieties have been incorporated in nano-assemblies for triggerable structural transformation via several strategies, including HLB change, depolymerization and decrosslinking. The building blocks of the macromolecules are designed to sense changes in pH values via charge shifting or acid-induced bond cleavage. Charge shifting by pH changes could generally lead to alterations in hydrophilicity of polymers, resulting in either disassembly or the swelling/shrinking of particles. Acid-induced bond cleavage on the other hand causes the breakage of acid-labile bonds in macromolecular nano-assemblies, compromising the driving forces of particle formation, such as hydrophobic interactions and chain-chain crosslinking to cause the disassociation of nano-assemblies. When pH-responsive moieties are employed as repeating units on polymer backbones, pH-induced cleavages could cause depolymerization and disintegration of assemblies. Overall, pH-responsive groups are the most widely studied functionalities in macromolecular assemblies, independently or synergistically with other responsive groups to achieve controllable responsiveness. We direct readers to several reviews that summarized comprehensive strategies to design pH-responsive polymeric nano-assembly for diverse applications [73–75].
Redox
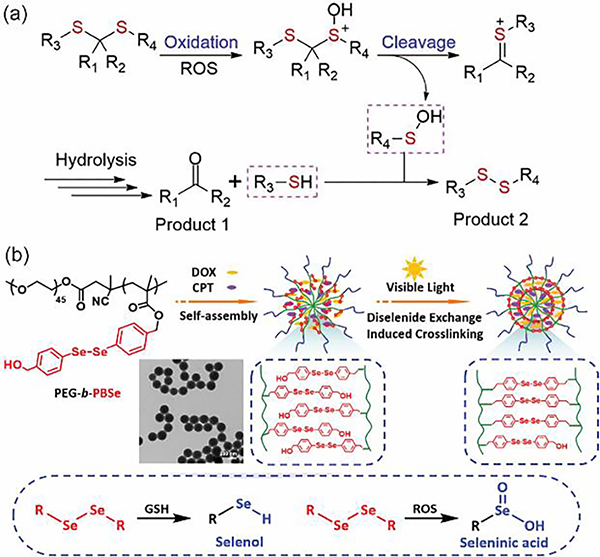
Macromolecular assembly/disassembly transitions in response to redox stimuli (i.e., reductive, and oxidative stimuli) are dictated by the structural determinants with triggered bond breakage and changes in HLB. ROS and reducing agents (e.g., glutathione, GSH) are the two major categories of stimuli in the development of redox-responsive macromolecules, though gas molecules such as reactive nitrogen and sulfur species involved in physiological and pathological pathways are emerging areas for biological applications. For comprehensive discussion on reactive oxygen, nitrogen, and sulfur species responsive macromolecules, we direct the readers to an excellent review focusing on responsive macromolecules and their molecular assemblies for biomedical applications [76]. ROS including radical species of superoxide anion radical (O2•−) and hydroxyl radical (HO•), and non-radical species of hydrogen peroxide (H2O2), singlet oxygen (1O2) and hypochlorous acid (HOCl) are found overexpressed in signal transduction or stress response of inflammation, oxidative stress, and aging [77–80]. ROS-responsive moieties such chalcogen ether including thioether, selenoether and telluroether, thioketal, and arylboronic ester are widely leveraged in the design of macromolecules with triggered disassembly behavior [81,82]. The oxidation of chalcogen ethers built in hydrophobic chains increases the hydrophilicity of amphiphilic macromolecules, altering the HLB to trigger the disruption of nano-assemblies. For example, hydrophobic thioether that can be oxidized into sulfoxide and sulfone with higher water solubility are heavily incorporated into amphiphilic macromolecules for the control of assembly/disassembly transition since an early example of the use of oxidative conversions to destabilize nano-assemblies in 2004 (Table 2) [83]. Thioketal in response to a broad spectrum of ROS including H2O2, HOCl, HO• and O2•− can be degraded into acetone and thiols [84,85]. Incorporation of ROS-responsive thioketal into macromolecules endows nano-assembly with a switch for triggered bond breakage and structural disruption. Arylboronic ester is another commonly used ROS-responsive functional group due to its high selectivity and sensitivity [86]. The oxidation reaction of arylboronic ester with H2O2 results in the formation of boronic acid and phenol species that can further trigger polymer fragmentation via electronic cascade processes-induced bond cleavages, triggering the disruption of macromolecular nano-assemblies.
Table 2.
Redox-responsive moieties and corresponding behaviors in response to stimuli.
| Responsive moiety | Stimulus | Responsive behavior |
|---|---|---|
| Chalcogen ether | ROS |
|
| Thioketal | ROS |
|
| Arylboronic ester | ROS |
|
| Diselenide | ROS and GSH |

|
| Disulfide | GSH |
|
| Dithiomaleimide | GSH |
|
GSH, a tripeptide of glutamate, cysteine, and glycine, is the most abundant and endogenous small thiol molecule that acts as an antioxidant and regulates cellular redox homeostasis [87,88]. The dramatic difference of GSH concentration between intracellular environment (1–10 mM) and extracellular compartment (1–10 μM) provide reduction-responsive polymeric nanoassemblies with a hallmark for triggering structural transformation [89–91]. As a well-studied GSH-responsive functionality, disulfide has been installed at various position of macromolecules (backbones vs. sidechains) which can provide nano-assemblies with distinct disintegration properties upon treatment with GSH [92]. For example, incorporation of multiple disulfide bonds into the main chain of hydrophobic block can cause chain shattering and induce the degradation of nano-assemblies. Single disulfide bond built at the junction point of hydrophobic and hydrophilic blocks, in the core-forming crosslinkers, or in between hydrophobic block can lead to hydrophilic-lipophilic imbalance, inducing the destabilization of nano-assemblies. Recently, poly disulfides) have gained considerable attention due to the triggering degradation of disulfide bonds installed in the main chain of macromolecules [93,94]. Disulfide-containing polymers are typically elaborated via chemical oxidation, photo-polymerization, thermal polymerization, thiolate-initiated ring-opening polymerization, or cryo-polymerization and their applications in responsive assemblies have been discussed in Section 4 and 5. Similar to disulfides, dithiomaleimide (DTM) has been widely used in bioconjugation, responsive polymer materials, drug delivery, and imaging probes given the sensitive responsiveness to GSH [95–100]. The decoration of DTM at the joint of amphiphilic polymers provides nano-assemblies with GSH-responsive cleavage of DTM linker, inducing destabilization and particle disruption [101–105]. Taking advantage of elevated expression level of GSH and ROS in cancer and inflammatory disease, redox-responsive polymer materials have been generally leveraged in the development of nanomedicines and imaging techniques. We direct the readers to the recent report on nanomedicines via redox approach [106]. Furthermore, redox-responsive moieties along with self-immolative chemistry have been widely introduced into the nano-assemblies of prodrug and polyprodrug amphiphiles [13].
Diols (glucose)
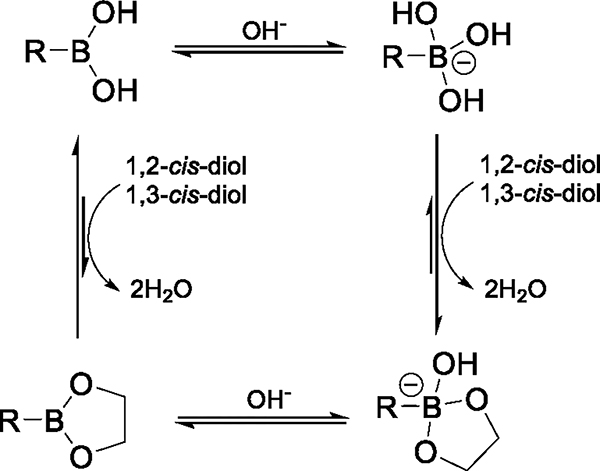
Diol-responsive macromolecules have been widely used in biomedical applications due to the abundant polysaccharides in biosystems. In particular, boronic acid-containing macromolecules show a wide variety of applications, including self-healing hydrogels, drug delivery, biologics delivery, sensor, and boron neutron capture therapy, given the versatile features of boronic acid, such as dynamic covalent bonds, responsive to ROS, pH and molecules containing diol (i.e., sugar, adenosine triphosphate (ATP), and ribose), and cancer-targeting capability [20,86]. Here, we focus on the boronic acid-containing macromolecules in response to molecules containing diol and their chemistry behind the determinants of structural alteration. Boronic acid mainly acts as a Lewis acid due to the vacant p-orbital on the boron center, which allows the formation of reversible boronate ester with 1,2-cis-diols or 1,3-cis-diols. In the neutral form, boronic acid exists as a trigonal planar sp2-hybridized boron, sharing a bond with either an alkyl or an aryl group, and along with two hydroxyl groups, giving rise to six valence electrons on boron center (Fig. 2, top left). In aqueous solution, boronic acids exist in equilibrium between the neutral, hydrophobic form of boronic acid and the hydrophilic, tetrahedral hydroxyboronate anion after complexation with a hydroxide ion (Fig.2, top right). Hence, boronate esterification is favorable at pH values higher than pKa. With manipulation of substituents on boronic acid, the pKa of boronic acids is tunable from 4.0 to 10.5. The binding interaction between boronic acid and diol is highly affected by the substituents on both molecules and pH value of surrounding environment, in which dynamic covalent interaction provides polymeric materials with modulable and predictable response to biological molecules for biomedical application, including glucose-responsive insulin delivery and sensors of monitoring blood glucose level. Detailed utilities of boronic acid/ester-based molecular determinants in stimuli-responsive assemblies are introduced in Section 4 and 5.
Fig. 2.
Esterification equilibrium between boronic acids and diols.
Apart from the above-mentioned stimuli, some gases have also been used as stimuli to trigger assembly alterations. For example, CO2 [107] and SO2 [108] could be used as stimuli to trigger the cleavage of acid-labile bonds and charge shifting of some pH-responsive groups, as the two gases could dissolve in water to form carbonic acid and sulfurous acid respectively, generating an acidic environment and leading to specific alterations. Gases like H2S could act as reducing agents to reduce azide to amines and trigger the corresponding transformation of assemblies [109]. Other gases such as formaldehyde [110] and CO were also utilized as stimuli to initiate fluorescence or polymer degradation [111], but their study in responsive assemblies is still rare. As most of the gases could eventually function as the above-mentioned stimuli (acid, redox or ROS), specific examples would be introduced together with other chemical-based stimuli instead of in an independent section.
2.3. Biomacromolecules: proteins
Structural alterations associated with protein can be generally categorized into enzymatic bond breakage and binding-induced imbalance between unimer and aggregate of macromolecules [76,112–115]. Through enzymatic cleavage, the hydrophobic-to-hydrophilic transition or vice versa can trigger the changes in HLB to initiate the formation or disruption of macromolecular nano-assembly [116–120], to drive particle morphology change [121–123] or to modulate the complexity of nanoparticles [124–128]. Enzyme-initiated depolymerization of polymer segments is also utilized to trigger particle disassembly. Upon enzymatic digestion, deprotecting group can undergo cascade self-immolation via elimination or cyclization, resulting in depolymerization and particle shattering [129,130]. In addition to enzymatic bond cleavage, noncovalent binding of protein (specifically and non-specifically) to nano-assemblies can perturbate HLB and compromise structural integrity [131,132]. For example, polyelectrolyte nano-assemblies have been found to interact with complementarily charged protein, causing structural disruption. Specific recognition between proteins and ligands decorated on nanoassemblies can undergo binding-induced disassembly on demand.
3. Stimuli-triggered interruption of HLB
As the self-assembled structures from amphiphilic molecules rely on the latter’s HLB, stimulus-induced perturbation of this balance is a convenient approach to trigger nano-structural transformations [4]. Side chain or main chain functionalities in the polymer can be triggered to be cleaved or transformed to alter HLB that induces nano-structural destabilization. Similarly, the disassembling behavior can be triggered by attaching biomacromolecules via specific recognition on macromolecular nano-assemblies due to the interruption of HLB.
3.1. Interruption of HLB without polymer fragmentation
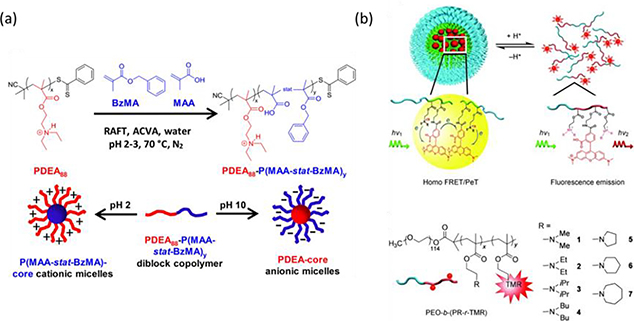
Amphiphilic moieties that are key to the assembly process can be used to trigger changes in hydrophobicity, leading to structural disintegration or morphological transition [73,74,133]. A vast body of literature has been dedicated to investigating how stimuli-responsive polymers undergo changes in hydrophobicity in response to triggers and their implications for interrupting HLB and inducing structural transformations. Here, we focus on describing how polymers respond to different types of stimuli along with a few examples that induce HLB change. pH variations have generated a wide variety of nano-assemblies that can undergo structural transformation due to charge shifting. The hydrophobic-to-hydrophilic transition or vice versa could be attributed to charge shifting of neutral polymers (e.g., amines) that change from hydrophobic to positively-charged/hydrophilic with the decrease in pH, or anionic polymers (e.g., carboxylates) that convert from negatively-charged/hydrophilic to neutral/hydrophobic as pH decreases. For example, amphiphilic block copolymers containing two segments that show oppositely charge-shifting behavior are known to exhibit a schizophrenic character that form a stable micelle at high pH, while converting into reverse micelle as pH decreases [134]. The diblock copolymer, poly(4-vinyl benzoic acid60-block-2-(diethylamino)ethyl methacrylate66) P(VBA60-b-DEA66), can reversibly form stable micelles and reverse micelles by simply switching pH. Here, the stable anionic micelles, with the size of 66 nm, have deprotonated PVBA (pKa = 7.1) anionic shells and hydrophobic PDEA core (pKa = 7.3) at pH 9.2, while the formation of positively-charged reverse micelles, with the size of 36 nm, comprising cationic shells of protonated PDEA and PVBA core was observed at pH 2 [135]. The schizophrenic character of block copolymer was further validated by dual-color self-reporting pH-responsive poly(2-(diethylamino)ethyl methacrylate-block-poly(methacrylic acid-statistical-benzyl methacrylate) (PDEA-P(MAA-stat-BzMA) (Fig. 3a) [134]. A pink copolymer dispersion from rhodamine B methacrylate (RhBMA) incorporated in between PDEA shells was observed at pH 2, whereas fluorescence of fluorescein O-methacrylate (FMA) along with MAA and BzMA core was quenched possibly due to aggregation-indued quenching. At pH 10, FMA was activated at the shells of P(MAA-stat-BzMA) while fluorescence of RhBMA was dormant at the PDEA core. In addition to schizophrenic character, pH-responsive charge shifting was leveraged to develop ultrasensitive, reversible nanoprobes with assembling/disassembling behavior owing to supramolecular cooperativity [136,137]. Reversible assembling/disassembling behaviors between amphiphilic macromolecules in response to pH changes gives rise to cooperative protonation/deprotonation. The all-or-nothing two-state between free polymers, or unimers, and micelles can lead to ultrasensitive pH nanoprobes comprising fluorophores with small Stoke shifts (< 40 nm) along with protonatable block (Fig. 3b) [138]. Tertiary amines with various hydrophobic substituents as ionizable block of PEG block copolymer are used to tune the transition pH values. The degree of substituent hydrophobicity dramatically affects the protonation of micelles with different pKa. Sharp pH response of multicolor nanoprobes can be achieved by introducing various types of fluorophores that showed quenching based on homo F rster resonance energy transfer (FRET) in constrained hydrophobic core. Moreover, mixing the same polymers containing fluorescence quenchers with fluorophore-labeled polymers during the micellization can expand the nanoprobes with broad selection of fluorescence emission, which was previously limited by the use of fluorophores with only homo FRET [139]. Micellization confines the fluorophores and quenchers in the core, leading to dormant fluorescence. Due to ultrasensitive cooperative disassembly, facile all-to-nothing transition of micelles enables to dissociate the quenchers and turn on the fluorescence. Similar pH-induced assembly or aggregation was also adopted in aggregation-induced emission (AIE) nanoprobes [140]. Crosslinking nanoparticles with pH-responsive diethylamino or carboxylic groups were used to tune the on/off state of AIE fluorescence in response to changes in pH values. pH-responsive charge shifting polymers have shown a wide variety of structural transformations dictated by changes in charged property and hydrophobicity. We note that pH-responsive polymers have made significant progress in controlling assembling/disassembling behaviors of nano-assemblies. We direct readers to reviews with a plethora of examples in this context [73,74,133].
Fig. 3.
(a) The copolymerization of methacrylic acid and benzyl methacrylate by RAFT aqueous emulsion polymerization (top panel), and the schizophrenic micellization behavior of PDEA88-P(MAA-stat-BzMA)y diblock copolymers in acidic and basic aqueous solution (bottom panel) [134], Copyright 2017. Adapted with permission from the American Chemical Society. (b) Mechanisms of homo Förster resonance energy transfer and photoinduced electron transfer behind tunable, ultrasensitive pH-responsive nanoparticles, where the neutralized PR segments that could self-assemble into the micelle cores at pH > pKa led to quenching of fluorophores while formation of unimers at pH < pKa lighted on fluorescence (top panel). Structures of the PEO-b-(PR-r-TMR) copolymers (PEO = poly(ethylene oxide), PR = ionizable block, TMR = tetramethyl rhodamine, and R = dialkyl and cyclic substituents) (bottom panel) [138], Copyright 2011. Adapted with permission from John Wiley & Sons Inc.
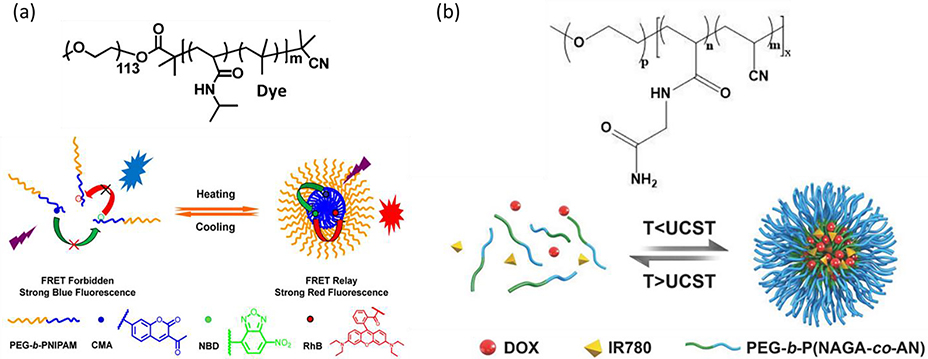
Since the thermal phase transition of infamous poly(N-isopropylacrylamide) (PNIPAM) was reported in 1967 [141], temperature-responsive materials have been heavily investigated as biomedical and smart materials [26,142,143]. Given the LCST around body temperature (32 °C) in water [144], PNIPAM has been widely explored for biomedical applications. For example, block copolymers of poly(ethylene glycol)-block-poly(N-isopropylacrylamide) (PEG-b-PNIPAM) individually labeled with dyes were developed for temperature imaging of live cells by ratiometric fluorescence (Fig. 4a) [145]. The cascade FRET behaviors between dyes were modulated through the transition between unimers and micelles upon heating and cooling, in which the distance between dyes can be manipulated by the degree of aggregation of PNIPAM at various temperatures. To probe the thermal dynamics in cells, a nanothermometer composed of poly(N-isopropylacrylamide-co-tetrabutylphosphoniumstyrenesulfonate) [P(NIPAM-co-TPSS)] decorated with AIE molecules, 3-ethyl-2-[4-(1,2,2-triphenylvinyl)styryl]benzothiazol-3-ium iodide (TPEBT), on TPSS [146]. At the temperature below LCST of PNIPAM, P(NIPAM-co-TPSS) can self-assemble into micelles with the size of 140 nm, comprising PNIPAM shells and PTPSS core. In this aggregation state, TPEBT exhibits strong fluorescence within the PTPSS core. Upon heating in the range of 25–45 °C, a linear correlation between fluorescence and temperature within 32–40 °C was observed. During the heating process, the hydrogen bonds between the amide groups of PNIPAM and water molecules weakened, leading to dehydration of the alkyl group and aggregation of PNIPAM chains. As a result of the interaction between PNIPAM and PTPSS, PTPSS was forced out at the particle shell, forming reverse micelles [147]. TPEBT molecules were gradually revealed at the surface and deactivated the fluorescence emission due to energy dissipation. For a LCST system, heat-triggered hydrogen bond breaking between water and hydrophilic moieties could ultimately lead to the collapse of hydrophilic chains and disturb the intrinsic HLB, triggering the corresponding alterations in assembly level and ultimately macroscopic transformations.
Fig. 4.
(a) General structure of dye-conjugated PEG-b-PNIPAM (top panel) and fluorescence transition from polymeric ratiometric fluorescent thermometers upon heating and cooling (bottom panel) [145], Copyright 2015. Adapted with permission from the American Chemical Society. (b) Structure of PEG-b-P(NAGA-co-AN) (top panel), and UCST-type structural transformation that induced payload release (bottom panel) [154], Copyright 2018. Adapted with permission from John Wiley & Sons Inc.
While polymers with lower critical solution temperature (LCST) thermal behavior have been heavily investigated, those exhibiting upper critical solution temperature (UCST) property can also be used for nanomedicine [148]. While the low mobility of UCST polymers secure the payload stability at the temperature below UCST, the increased solubility of polymers upon heating could enable complete payload release [149]. Poly(N-acryloylglycinamide) was reported to show UCST with the transition temperature of 22–23 °C and a broad hysteresis upon cooling, but the effect of salt concentrations on cloud point limited the application [150,151]. Though poly(acrylamide-co-acrylonitrile) [P(AAm-co-AN)] showed tunable cloud point by varying the content of AN, the cloud point of P(AAm-co-AN) that increases with increasing concentration would also compromise the applicability [152]. Poly(N-acryloylglycinamide-co-acrylonitrile) [P(NAGA-co-AN)] showed the independence of polymer concentration and salt concentration on cloud point with narrow hysteresis while maintaining the tunability of cloud point [153]. The UCST polymer PEG-b-P(NAGA-co-AN) with cloud point of 44 °C was developed to ferry chemo-drug, doxorubicin (DOX), along with photothermal effect for spatiotemporal drug-resistant cancer treatment (Fig. 4b) [154]. PEG-b-P(NAGA-co-AN) can self-assemble into micelle with a size of 110 nm at room temperature while showing a drastic decrease in size of 7.2 nm upon heating over cloud point. The drastic changes in size upon heating enabled the delivery system to show burst drug release upon photothermal administration. However, compared to polymeric nanoparticles with LCST, nanoparticles that can dissipate at temperature above UCST remain underappreciated for biomedical applications due to the scarcity of polymers with suitable responsive temperature and biocompatibility. We have described prominent thermo-responsive polymers with LCST or UCST and provided examples of their transition between hydrophobic and hydrophilic states in response to temperature changes. For readers interested in delving further into this field, we recommend recently published in-depth reviews on the design and applications of these materials [26,27].
Oxidation-responsive polymers that exhibit triggered disintegration due to HLB change have also been explored in the past few years. Previous studies have focused on amphiphilic polymers that contain hydrophobic thioethers capable of being oxidized into sulfoxides and sulfones. Upon oxidation, these polymers increase in hydrophilicity, causing an interruption in HLB that leads to morphological transformation or disassembly. For example, oxidation-responsive block copolymer poly(ethylene glycol)-block-poly(2-(methylthio)ethyl glycidyl ether) (mPEG-b-PMTEGE) and random copolymer mPEG-r-PMTEGE were synthesized via anionic ring opening polymerization with molecular weights ranging from 5,600 to 12,000 g·mol−1 [155]. The amphiphilic copolymers with various copolymer compositions at different molecular weight can self-assemble into micelles with the size ranging 9.3 – 13.7 nm while unimers with the size from 2.3 – 2.6 nm were observed upon treatment with H2O2. Hydrophobic thioether upon treatment with diluted H2O2 were oxidized to sulfoxide after a few minutes and showed full conversion in 2.5 h at 310 K but 6 h in 296 K, increasing the dipole moment and solubility in aqueous solution. Hierarchical structure of poly(oligo(ethylene glycol) methyl ether methacrylate)-block-poly(2-(methylthio)ethyl methacrylate) (POEGMA-b-PMTEMA) nanoassemblies containing photocatalyst, tetraphenylporphine zinc, were obtained via polymerization-induced self-assembly process [156]. Upon light irradiation at 560 nm, the photosensitization of molecular oxygen by tetraphenylporphine into singlet oxygen oxidized the hydrophobic thioether to sulfoxide, rapidly leading to structural destruction. We note the growing interest in developing polymers that can sense oxidative stress and induce structural alterations by undergoing amphiphilic-to-hydrophilic transition for biomedical applications. We direct readers to recent reviews that offer in-depth discussions on the design rationale behind materials capable of responding to ROS in biomedical applications [157,158].
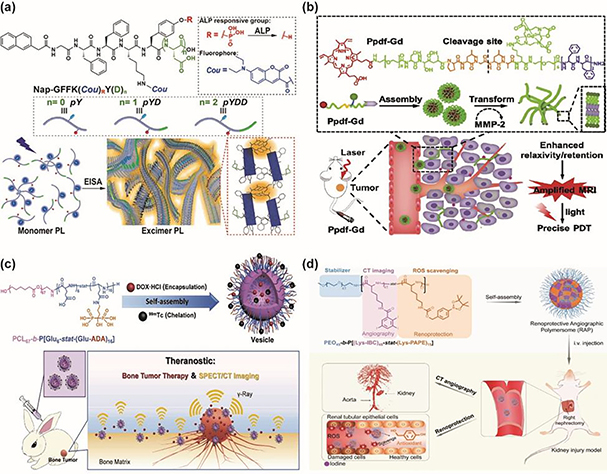
In addition to chemical stimuli, the interaction with biomolecules can perturb HLB and structural integrity of nano-assemblies. Noncovalent binding of protein to nano-assemblies was found to interrupts HLB and disassemble the polyelectrolyte assemblies via electrostatic interaction, leading to payload release for protein sensing [159,160]. The strategy was further applied to amphiphilic dendrimer assemblies that showed specific binding-induced disassembly [161,162]. The biotin ligand decorated on dendron periphery that was recognized by extravidin triggered hydrophilic-lipophilic imbalance, resulting in protein-induced disassembly and release of hydrophobic guests. The location of biotin was found to greatly influence the binding event as well as disassembly efficiency. The ligand placed at the focal point showed less accessibility to extravidin due to steric hindrance while ligand decorated on dendron periphery showed higher accessibility and efficient protein-induced disassembly. Similar strategy was exploited in the disease relevant binding pair of benzenesulfonamide and bovine carbonic anhydrase II (bCA-II) [163]. Biodegradable amphiphilic random polypeptide nano-assemblies with decoration of benzenesulfonamide at the surface underwent specific ligand-protein recognition in the presence of bCA-II, triggering the changes in size from 200 nm to 5 nm within 30 h. The protein-induced disassembly of amphiphilic dendrimer assemblies was exploited a protein concentration-dependent process as determined by ratiometric signal changes in fluorescence of monomer and excimer, where the coumarin moiety incorporated in hydrophobic core emitted excimer fluorescence while the monomer fluorescence was restored upon micellar disassembly [164]. Programmed disassembly in response to two-input AND logic gate was developed to endow selective and spatiotemporal control [165]. Masking the sulfonamide by photocage N-(o-nitrobenzyl) was able to inhibit the binding event between bCA and benzenesulfonamide at the surface of amphiphilic dendrimer nano-assemblies. Upon light treatment, benzenesulfonamide regained the binding affinity to bCA, initiating protein-induced disassembly. Compared to other stimuli-responsive systems, the binding interactions between ligands and proteins are generally very specific and binding affinities could be tuned via the structural modulation of ligands. Considering that the abnormally expressed proteins are important biomarkers for many diseases, this strategy is of great promise to be used for diagnosis and receptor-directed drug delivery.
3.2. Interruption of HLB via polymer fragmentation
3.2.1. Cleavage at hydrophobic-hydrophilic junction
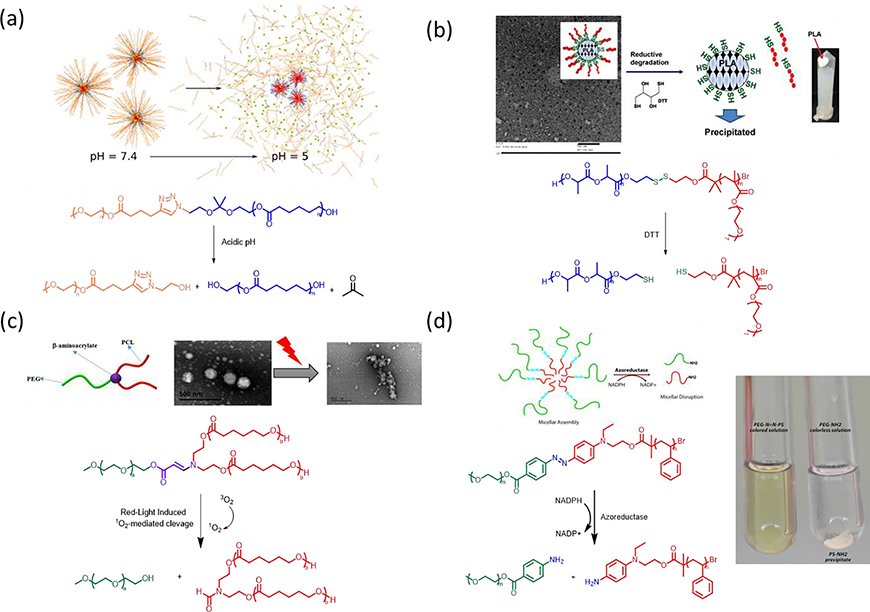
Macromolecular nano-assemblies, composed of a hydrophilic block linked with a hydrophobic block by a trigger-degradable junction, can disintegrate upon stimulation. The bond breakage of a responsive linker could result in the cleavage of hydrophilic and hydrophobic polymer segments, further disrupting the HLB and generally leading to particle disruption in the form of precipitation. Herein, we discuss a few studies in which amphiphilic polymers with built-in responsive junctions that can sense physical, chemical, or biological triggers undergo an interruption in HLB and structural disintegration. Photocleavable group such as o-nitrobenzyl [166] and boron dipyrromethene [167,168] at the junction of hydrophilic and hydrophobic blocks have been developed for on-demand structural disintegration. The acid-labile ketal at the block junction of poly(ethylene oxide monomethyl ether) (MPEO)-b-poly ε-caprolactone) (PCL) that forms micellar nanoparticles showed accelerated hydrolysis of ketal groups at pH 5 compared to pH 7.4. Shedding of PEG chains increased the hydrophobicity, leading to disassembly and aggregation to facilitate the release of encapsulated paclitaxel (Fig. 5a) [169]. Similarly, shell-sheddable PCL and poly(oligo(ethylene glycol) monomethyl ether methacrylate) block copolymer with built-in disulfide bond at the block junction (PCL-SS-POEOMA) was developed by a combination of ring-opening polymerization and atom transfer radical polymerization (Fig. 5b) [170]. Shedding hydrophilic POEOMA corona of core-shell micelle with size of 30 nm, in response to reduction, in the presence of dithiothreitol (DTT), giving rise to the loss of colloidal stability and precipitation of PLA core. AB2 miktoarm block copolymers, poly(ethylene glycol)-b-poly(caprolactone)2 (PEG-b-PCL2), with built-in singlet oxygen (1O2)-labile β-aminoacrylate moiety at the junction were developed for photodynamically triggered drug release (Fig. 5c) [171]. Hydrophobic photosensitizer chlorin e6 (Ce6) encapsulated inside the hydrophobic PLA core can generate singlet oxygen (1O2) under light irradiation, which can induce the cleavage of β-aminoacrylate linkage. This bond cleavage led to the detachment of hydrophilic PEG, ultimately resulting in the dissociation of micellar structure. The cleavable joint of hydrophilic and hydrophobic block in response to enzyme can also initiate disruption of micellar nano-assembly. For example, the azobenzene linkage established at the junction of poly(ethylene glycol)-b-poly(styrene) (PEG-N=N-PS) amphiphilic copolymer has been shown cleavable in the presence of enzyme azoreductase along with coenzyme NADPH (Fig. 5d) [116]. The cleavage of azobenzene at the joint of hydrophilic poly(ethylene glycol) and hydrophobic poly(styrene) gives rise to micellar disruption and precipitation of PS segments. Moreover, responsive moieties built in between hydrophobic chains upon triggering can effectively shorten the hydrophobic chain length and disturb the HLB at which the macromolecules form stable nano-assemblies, leading to particle disassembly. For example, the cleavage of lipophilic units in response to esterase led to the disturbance of HLB amphiphilic dendrimer nano-assemblies, rendering the dendrimers hydrophilic and inducing the particle disassembly [118]. The accessibility of enzymes to cleavable moiety was found to affect the enzymatic response and disassembly kinetics [172,173]. The degree of polymerization and HLB of amphiphilic oligomers can alter the unimer–aggregate equilibrium to tune the accessibility of enzyme to responsive moieties, resulting in tunable disassembling kinetics and controlled payload release [120]. These responsive amphiphilic copolymers are widely applied in the nano-formulation of hydrophobic drugs for drug delivery. The cleavage of hydrophilic and hydrophobic polymer segments in response to breakage of triggerable junctions can result in particle disruption and the release of encapsulated drugs. Nonetheless, the structural disintegration of nano-assemblies in the form of precipitation of hydrophobic segments likely traps a portion of drugs in precipitates, hampering the drug release. Therefore, a thorough assessment of the drug release profile of nanoparticles falling within this category would be imperative to tailor their suitability in real-world applications.
Fig. 5.
Cleavage at hydrophobic and hydrophilic junction led to the disruption of nanoparticles in a form of precipitation. (a) Chemical structure of the acid-labile block copolymer MPEO44-b-PCL17 (top panel) and the schematic illustration of assembly/disassembly transition of nanoparticles at pH ~5.0 (bottom panel) [169], Copyright 2021. Adapted with permission from the authors. (b) Illustration of particle disruption of PCL-SS-POEOMA micelles in response to DTT (top panel) and chemical structure of the reduction-responsive block copolymer PCL-SS-POEOMA (bottom panel) [170], Copyright 2015. Adapted with permission from the American Chemical Society. (c) Schematic illustration of amphiphilic miktoarm PEG-b-PCL2 copolymer with a junction of 1O2-Labile β-aminoacrylate (top panel), and their self-assembly and particle disruption 1O2-mediated dissociation upon red-laser (bottom panel) [171], Copyright 2018. Adapted with permission from the American Chemical Society. (d) Schematic representation (top panel) and a digital picture (right panel) of the micellar assembly from PEG-N=N-PS block copolymer and triggered disruption into PEG and PS homopolymers by the enzyme azoreductase in the presence of NADPH. Chemical structure of the acid-labile block copolymer PEG-N=N-PS (bottom panel) [116], Copyright 2018. Adapted with permission from the American Chemical Society.
3.2.2. Cleavage of sidechains
In addition to the bond breakage at the junction of the hydrophobic and hydrophilic block, the interruption of HLB through the cleavage of sidechains on macromolecules has been extensively studied for responsive structural alterations. In this section, we focus on previous studies that investigated the particle disruption of amphiphilic polymers due to cleavage of hydrophobic pendants upon exposure to physical, chemical, or biological stimuli, leading to structural transformation. The cleavage of bonds between the polymer backbone and hydrophobic pendants can cause the polymer backbone to become hydrophilic, resulting in the disruption of HLB and subsequent changes in the nano-assemblies. For instance, the photo-solvolysis of 1-pyrenylmethyl esters under UV irradiation, leaves carboxylic acids on the substrate [174]. Amphiphilic copolymers consisting of hydrophobic, photocleavable groups rapidly became a mainstream fashion to construct nano-assembly with triggerable degradation [28,175,176]. The bond cleavage of hydrophobic, photocleavable groups, such as pyrene [29], o-nitrobenzyl [30], coumarin [46] and perylene [32] at ultraviolet region, and [Ru(tpy)(biq)(H2O)]2+ tpy = 2,2′:6′,2″-terpyridine and biq = 2,2′-biquinoline) [177,178] at visible wavelength, on amphiphilic polymers induces the transformation from hydrophobicity to hydrophilicity, leading to HLB and the disruption of nano-assembly. Photoresponsive therapeutic delivery based on photocleavage has been significantly developed for precision medicine due to its spatiotemporal precision [33,34]. The use of light in delivery systems enables particles to release payloads at the site of interest on demand and reduces off-target effects [33,34,179–182]. While photoactivation provides high precision in therapeutic delivery, the development of photo-controlled therapeutics is still hampered by issues such as phototoxicity, shallow depth of light penetration, and uncertain timing of the triggering event. Recently, the development of two-photon triggering system [31,183] and photo-upconversion mechanism [184–187] assuage the concerns above mentioned, but further exploration in this field is still necessary for practical applications.
In comparison with photoactivation, ultrasound offers similar spatiotemporal precision but a controlled depth of penetration and the availability of image-guided triggering systems. The availability of advanced medical instruments and systemic administration has made it possible to introduce ultrasound-triggered systems in clinical practice [68,69]. Amphiphilic copolymers composed of water-soluble poly(ethylene oxide) (PEO) block and hydrophobic poly(2-(2-methoxyethoxy)ethyl methacrylate) (PMEO2MA) scrambled with poly(2-tetrahydropyranyl methacrylate) (PTHPMA) was developed, which could self-assemble into micelles with core of PMEO2MA and PTHPMA and PEO corona in water at temperatures above its LCST [70]. HIFU irradiation induced the hydrolysis of THPMA and left hydrophilic methacrylic acid on the polymer backbones, resulting elevated LCST of existing micelles that led to the disruption of particle and payload release (Nile red) at the same temperature. Recently, we introduced PTHPMA into fluoropolymer hydrogels for enhanced 19F MRI imaging [188], which would be potentially a theragnostic delivery system via MRI-guided focused ultrasound. Remarkably, the mechanochemistry area has burst over the past decade [189,190], which brings various new chemistries with distinct responsive properties. Considering the spatiotemporal precision and noninvasiveness of ultrasound as a stimulus, we believe more and more mechanical force-responsive moieties would be introduced into amphiphilic macromolecules and assemblies in the coming decades and bring opportunities in more applications.
Amphiphilic copolymers with acid-labile bonds linking hydrophobic side chains have gained significant attention due to their stable colloidal properties under physiological conditions and their ability to undergo hydrolysis at low pH. These copolymers are especially useful in environments with lower pH levels, such as the tumor microenvironment (pH 7.2–6.8), endosomes (pH 6.8–6.0), and lysosomes (pH < 5) [191–193]. The distinctive lower pH environment provides a niche for pH-responsive polymer materials to serve as delivery systems for cancer treatment. At lower pH, hydrophobic pH-labile groups, which may include drugs or chemical functional groups, undergo hydrolysis, leading to a hydrophilic-lipophilic imbalance and eventual disruption. A majority of acid-labile linkers, such as hydrazone, imine, acetal/ketal, ortho ester, maleic acid amide (MAA), and β-thiopropionate functional groups have been developed and utilized in pH-responsive delivery systems [73,194]. For example, corecrosslinked polymeric micelles (CCPMs) formed by micellization of acid-triggered native active pharmaceutical ingredient (API) in methoxy poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl) methacrylamide-lactate]) (mPEG-b-pHPMAmLacn) followed by covalently crosslinking via free radical polymerization. Acid-labile trityl-based linkers is vulnerable at pH 5.0 or 6.5 but relatively stable at pH 7.4 [195,196]. With variation of substituents on acid-labile trityl linkers, model API compounds such as DMXAA-amine, DOX, and gemcitabine conjugated on CCPMs showed the tunability of trityl degradation as well as drug release in different pH milieu.
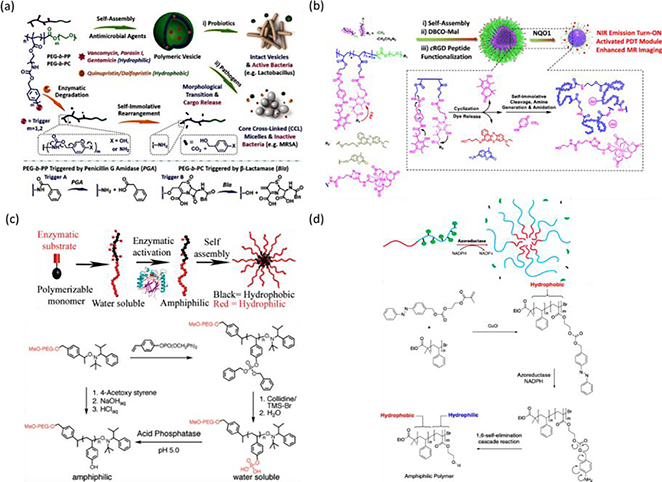
Reactive oxygen species, including hydrogen peroxide (H2O2), superoxide (O2•−), hydroxyl radical •OH), peroxynitrite ONOO−), and hypochlorite OCl−), are known to induce oxidative stress and can be used as triggers for oxidation-responsive or ROS-responsive materials [86,197]. For example, boronic acids/esters such as phenylboronic acid (PBA) are sensitive to ROS. Amphiphilic copolymer bearing hydrophobic PBA pendants from post-polymerization modification of methoxy poly(ethylene glycol)-b-poly[(N-2-hydroxyethyl)-aspartamide] (mPEG113-b-PHEA21) was used to encapsulate chemo-drugs, such as DOX, camptothecin (CPT), epirubicin, and irinotecan via nanoprecipitation and donor–acceptor coordination, self-assembling into drug-loaded micelles [198]. With treatment of hydrogen peroxide, arylboronic ester of PBA was oxidized and cut from polymer side chain after rearrangement to recover hydrophilic nature of mPEG113-b-PHEA21. Apart from boronic ester as a ROS-induced cleavable bond, thioketal bond is susceptible to ROS, forming free thiol alone with acetone as a byproduct [85,199,200]. Amphiphilic block copolymer composed of hydrophilic poly(N,N-dimethylacrylamide) (PDMA) and hydrophobic polyprodrug of ROS-cleavable thioketal-linked CPTs (PDMA-b-PCPTSM) self-assembled into intact micelles with size of 55 nm for responsive cancer treatment [201]. In response to high level of oxidative stress, cleavage of thioketal linkers liberated CPT molecules and restored hydrophilic poly(2-hydroxyethyl methacrylate) after self-immolative cyclization, inducing the particle deconstruction. With a similar strategy, reduction-responsive PEG-b-PCPTM polyprodrug amphiphiles with various repeating units of PCPTM self-assemble into four types of hierarchical nanostructures, including spheres, flower-like large compound vesicles, smooth disks, and staggered lamellae [202]. In response to the highly cytosolic GSH concentration (10 mM), disulfide bonds between the polymer backbone and CPT can be reduced to free thiols, which further undergo cyclization to release CPT in a traceless fashion. In particular, PEG-b-PCPTM nano-assemblies showed shape-modulated drug release kinetics, cellular internalization pathways, degradation rate, and therapeutic efficacy. Cleavage of sidechains of amphiphilic macromolecules in response to enzymes can result in the compromising of HLB and disruption of nano-assemblies. Polymersomes are composed of a hydrophilic poly(ethylene glycol) (PEG) block and a hydrophobic block containing enzyme-cleavable self-immolative side linkages and enzyme-cleavable end-caps. Upon treatment with penicillin G amidase and β-lactamase, recovery of the capping amine or hydroxyl group can undergo cascade self-immolation to initiate particle disruption and morphology transformation, leading to subsequent drug release (Fig. 6a) [203]. Similar strategy was also applied to NAD(P)H: quinone oxidoreductase isozyme 1 (NQO1)-responsive micelles for enhanced magnetic resonance (MR) imaging and fluorescence-guided photodynamic therapy (Fig. 6b) [204].
Fig. 6.
(a) Enzyme-responsive polymeric vesicles for bacterial strain-selective delivery of antibiotics and structural transformation in response to enzyme (top panel). Illustration of bond cleavage of built-in triggers in response to penicillin G amidase, and β-lactamase (bottom panel) [203], Copyright 2016. Adapted with permission from John Wiley & Sons Inc. (b) Self-assembly of amphiphilic BCPs containing quinone trimethyl lock-capped self-immolative side linkages and triggered particle transformation under exposure to NQO1 [204], Copyright 2020. Adapted with permission from the American Chemical Society. (c) The illustration of enzyme-induced self-assembly from hydrophilic block copolymer (top panel) and synthetic approach for the preparation of amphiphilic diblock copolymers (bottom panel) [117], Copyright 2009. Adapted with permission from the American Chemical Society. (d) Schematic illustration of enzyme-induced self-assembly from hydrophobic block copolymers (top panel). Synthesis of hydrophobic precursor of block copolymer, and hydrophobic-to-amphiphilic transition behind the enzyme-induced particle formation (bottom panel) [205], Copyright 2014. Adapted with permission from the American Chemical Society.
Enzyme-responsiveness has been applied not only to induce structural disintegration but also to trigger assembly. Hydrophilic block copolymers composed of a monomethyl ether 5 kDa PEG and 4-vinylphenyl phosphate can respond to acid phosphatase to cleave phosphate moieties and convert into amphiphilic copolymers, triggering self-assembly to form colloidal nanostructures in situ (Fig. 6c) [117]. In a similar fashion, enzyme treatment induced self-assembly behavior to form hydrophobicity-driven micelles with a hydrophobic block copolymer of styrene and hydroxyl methacrylate caged with a hydrophobic, enzyme-sensitive azobenzene (Fig. 6d) [205]. Upon the exposure to azoreductase in the presence of coenzyme NADPH, enzymatic cleavage of the azobenzene linkage triggers a spontaneous elimination reaction to form a hydrophilic hydroxyethyl methacrylate, resulting in amphiphilic block copolymers and subsequent self-assemblies. The breakage of covalent bond in response to triggers has shown the interference of HLB to manipulate the transformation of nanostructures.
4. Stimuli-triggered depolymerization
Nano-assemblies generally form via a variety of covalent and noncovalent interactions, which work synergistically to retain their fidelity. The interactions typically originated from different moieties on polymer chains in multivalent manner; thus, polymer integrity is crucial for maintaining the stability of assemblies. Stimuli-triggered macromolecular fragmentation could break the multivalent interactions, leading to nanostructure disassembly. Therefore, depolymerization is an important strategy to trigger assembly alterations, which could be in cascade (chain unzipping) or parallel (chain scission) manners, depending on the chemistry exploited in molecular design. Stimuli-triggered stepwise or simultaneous cleavage of chemical bonds on macromolecular backbones typically leads to particle disassembly and corresponding macroscopic transformations, which would be summarized in this section.
4.1. Stimuli-triggered chain unzipping
Cascade degradation method has long been studied for stimuli-triggered molecular amplification, with potential applications in sensing, imaging, and drug delivery. Some commonly used cascade degradation chemistry includes bond breakage by electronic cascade over an aromatic structure or cyclization-induced cleavages. Among these chemistries, (aza)quinone methide precursor [206], first proposed as self-immolative connector to design prodrugs [207], have grown to be one of the most widely used degradable moieties for bioconjugation [208], design of chemical probes [209], dendrimers [210], and polymers [37,211] to realize cascade and controllable fragment release from molecular skeletons [212]. As different functional groups can be incorporated into the structures, cascade degradations can be triggered using a variety of stimuli [213].
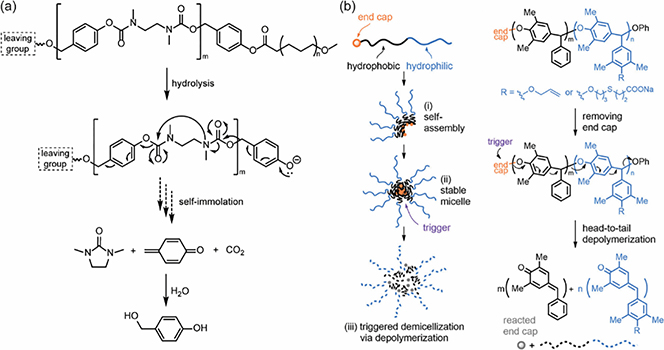
Recently, this chemistry has been employed for the design of amphiphilic macromolecules to fabricate nano-assemblies, utilizing the stimuli-triggerable fragmentation feature for the design of responsive nanostructures, expanding the responsiveness from single molecules to nanostructures and eventually generating macroscopic phenomenon [210,216]. In 2009, an amphiphilic PEG-polycarbamate block copolymer was synthesized, which underwent degradation via an alternating electronic cascade 1,6-elimination and cyclization mechanism (Fig. 7a) [214]. The polymer could form assemblies and encapsulate Nile red as the cargo which was released after a cascade polymer degradation. Despite that the initiation of cascade degradation relies on the spontaneous hydrolysis of an ester linkage between PEG and polycarbamate blocks, it brought a new strategy for the design of stimuli-responsive amphiphilic polymers with (aza)quinone methide precursors. Since then, various stimuli-responsive groups have been functionalized as the caps of polymers for substrate sensing/imaging, triggerable cargo release and other applications. For example, DOTA-Gd and fluorine-rich sidechainstethered amphiphilic polymers were functionalized with acid-sensitive imine and ROS-responsive boronic acid and were fabricated to micellar nanoparticles [216]. Stimuli-triggered polymer fragmentation and particle disassembly at tumor region led to the release of highly reactive (aza)quinone methide-based intermediates which can be trapped by intracellular thiol-relevant substrates and turn on the theragnostic signals for imaging. The utilization of these quinone methide intermediates brings a new sight for the bio-applications of these polymers as the intermediates are generally considered to be toxic and bio-incompatible. Similar strategies have also been reported for quinone methide-based linkers for protein modifications and drug discovery [217,218]. However, due to the triggerable degradation requirements, quinone methide-based polymers are generally synthesized via polycondensations or additions to afford rigid and hydrophobic backbones with limited number of repeating units. To achieve amphiphilicity, the hydrophilic moieties could be installed either as separate blocks or as sidechains on repeating units. If they were modified as sidechains, monomers would possess at least three functional groups (two for polymerization and one as the hydrophilic moiety), which may need tedious synthetic processes.
Fig. 7.
(a) Self-Immolation of polymers via an alternating alternating electronic cascade 1,6-elimination and cyclization mechanism [214]. (b) Poly(benzylether)-based self-immolative polymer for the design of stimuli-responsive assemblies [215], Copyright 2022. Adapted with permission from the American Chemical Society.
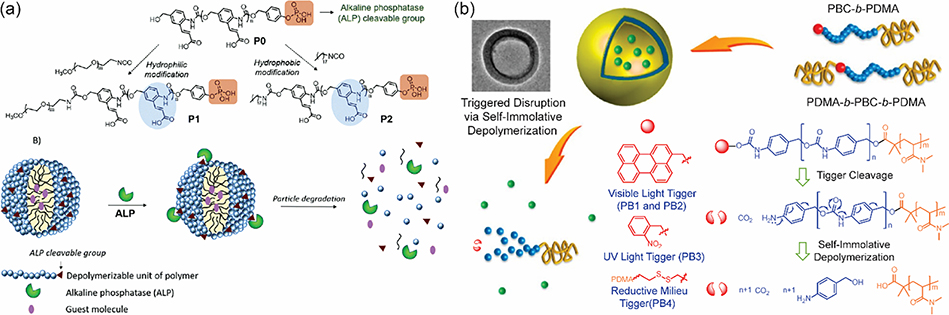
Apart from polycarbonates or polycarbamates, substituted poly(benzylether) were recently designed which could depolymerize via similar degradation mechanisms [211,212]. These amphiphilic polymers can be synthesized by tethering hydrophilic moieties on these polymer chains. The formed micellar nano-assemblies could degrade via a head-to-tail depolymerization and release the encapsulated hydrophobic guest molecules (Fig. 7b) [208]. It should be noted that for degradable poly(benzylether) mentioned above, the leaving group is a phenolic moiety which is quite different from the generally used carbonates and carbamates. Actually, although most quinone methide-based polymers shared similar depolymerization mechanisms, the degradation kinetics could be significantly affected by polymer structures and the accessibility of stimuli in different assemblies. In 2020, our group reported the assembly of alkaline phosphatase (ALP) responsive self-immolative polymers by complexing with charged polyelectrolyte or via an oil-in-water emulsion methodology to form spherical nanoparticles (Fig. 8a) [130]. Interestingly, the two types of particles exhibited very distinct disassembly kinetics, likely due to the different accessibility of ALP to the phosphate caps of polymers. In another study, we found that carboxylate functionalized p-amino-cinnamyl alcohol fabricated self-immolative polymers could undergo both stimuli-triggered chain unzipping and nucleophile-induced chain scission [222]. Depolymerization can be programmed exclusively via either of the two pathways with distinct kinetics by a judicial selection of functional groups and triggers. These fundamental studies motivated us to utilize this chemistry to design stimuli-triggerable soft matters. We formulated vesicle-like morphology nanoparticles by complexing carboxylic acid functionalized polymers with positive charged poly(diallyl)-dimethylammonium chloride, which can encapsulate enzyme in the hydrophilic core [223]. After particle destruction via photoinduced chain unzipping, the encapsulated enzyme was released and catalyzed the construction of hydrogel via the crosslinking of tyrosine-based substrates. Previous studies have demonstrated that the degradation kinetics could be affected by leaving groups and their substitution position (ortho or para), responsive moieties and stimuli, microenvironment (solvent, pH, etc.), aggregation state and accessibility of triggers [212].
Fig. 8.
(a) ALP-responsive self-immolative nano-assemblies [130], Copyright 2020. Adapted with permission from the Royal Society of Chemistry. (b) Self-Immolative Polymersomes from poly(benzylcabomate)-PDMA block copolymers [54], Copyright 2014. Adapted with permission from the American Chemical Society.
Precise control of stimuli-triggered responsiveness is highly demanded in the applications of smart soft materials. To achieve the controllability, dual or multiple triggers have been installed into polymer skeletons via (aza)quinone methide-based degradation mechanisms for programmable responsiveness. For instance, [224]programmable microcapsules were prepared using self-immolative polymers functionalized with different triggerable moieties [225]. An exposure to specific stimulus can trigger the rupture of microcapsules and release of core contents. Sophisticated block copolymers were later designed by incorporating self-immolative polymer blocks with a hydrophilic poly(N,N-dimethylacrylamide) (PDMA) block (Fig. 8b) [54]. These amphiphilic polymers self-assembled into polymersomes with different sizes from 205 nm to 580 nm, depending on polymer topologies and architectures. The assemblies were applied for the co-encapsulation of both hydrophobic and hydrophilic drugs which were efficiently released after treatment with a reductive milieu trigger, glutathione. These polymersomes can be functionalized with various responsive moieties and used for the design of AND, OR and XOR logic gate-type reaction system on the basis of different stimuli. This system provides an excellent example for the control of enzymatic reactions using amphiphilic nano-assemblies by the precise modulation of multiple stimuli. However, the introduction of multiple responsive moieties could obviously bring complexity for polymer synthesis and particle formulations. Additionally, although not investigated, the generated quinone methide intermediates may be trapped by the nucleophiles on enzymes in the system and diminish the enzymatic activity.
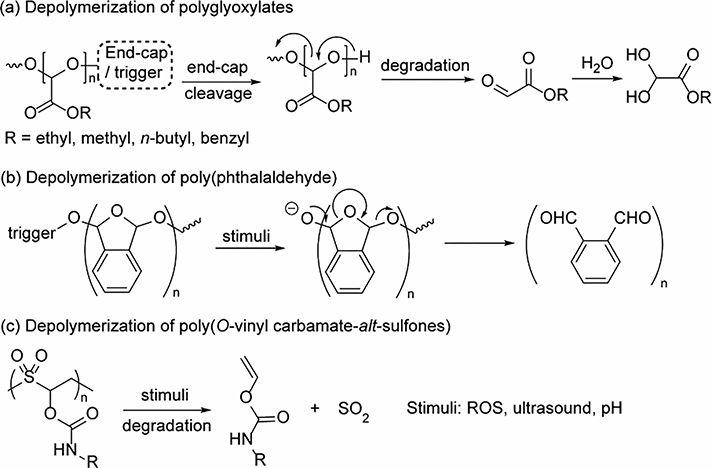
Over the past decades, many other chemistries have been developed as alternative moieties for the design of unzippable polymers. Recently, stimuli-responsive functional groups have been installed into polyglyoxylates, a type of self-immolative polymers which depolymerize in an end-to-end manner to corresponding glyoxylates, and ultimately to alcohols and glyoxylic acid hydrate (Fig. 9a) [226]. The responsive moieties can be functionalized on either side of the linear polymers symmetrically or non-symmetrically, generating homo- and copolymers on the basis of different synthetic strategies [229–231]. Notably, polyglyoxylates can be further modified via an amidation reaction to polyglyoxylamides which exhibited very different physical and chemical properties, including degradative properties [232]. Amphiphilic polyglyoxylates and polyglyoxylamides have been used to prepare nanoparticles and their responsiveness can be tuned by manipulating polymer structures or simply blending two different polymers [229,233]. Poly(phthalaldehyde) (PPA) is another type of well-studied self-immolative polymers [234,235]. Since 2010, stimuli-responsive end caps have been introduced to these polymers, opening up new avenues for the design of triggerable self-immolative PPAs (Fig. 9b) [227]. After exposure to specific stimuli, the PPAs could decompose rapidly to corresponding phthalaldehyde and lead to macroscopic changes. Over the past years, different synthetic methods have been employed for the design of PPA-involved random [236] and block copolymers [237,238]. PEG-tethered amphiphilic PPAs has been formulated to polymeric capsules and encapsulate fluorescein isothiocyanate labeled dextran [239]. The capsules disassembled after fluoride-triggered depolymerization and released the encapsulated guest molecules with tunable kinetics depending on the polymer length and thickness of shell wall. Recently, poly(o-vinyl carbamate-alt-sulfones) were used to formulate nanoparticles with 185 nm size for the encapsulation of guest molecules (Fig. 9c) [228]. These particles are sensitive to pH and ROS, which exhibit different degradation and guest release kinetics under different stimuli. The emergence of new chemistry for amphiphilic macromolecules has brought alternative strategies for the design of stimuli-responsive soft maters with distinct responsive properties. However, the generation of aldehyde intermediates, limited polymer stability and complexity in polymer functionalization are still not satisfactory for many applications, especially in biological areas. Thus, it would be important to explore more synthetic chemistries and degradation mechanisms for responsive macromolecules.
Fig. 9.
Depolymerization of (a) polyglyoxylates [226], (b) poly(phthalaldehyde) [227], and (c) poly(O-vinyl carbamate-alt-sulfones) [228].
4.2. Stimuli-triggered chain scission
Apart from chain unzipping, another depolymerization strategy is stimuli-triggered chain scission. Other than a head-to-tail type degradation in chain unzipping, chain scission happens parallelly for multiple bonds on polymer backbones, independent of each other. This strategy relies on the activation of multiple responsive groups, thus is concentration dependent. Some widely used chemistry includes polydisulfides, poly(aza)quinone methide precursors, and stimuli-triggered cyclable moieties. These amphiphilic polymers have been applied to formulate nanoparticles for diverse applications. Similar to chain unzipping, stimuli-triggered chain scission also leads to polymer fragmentation and ultimately the disruption of nanoparticles.
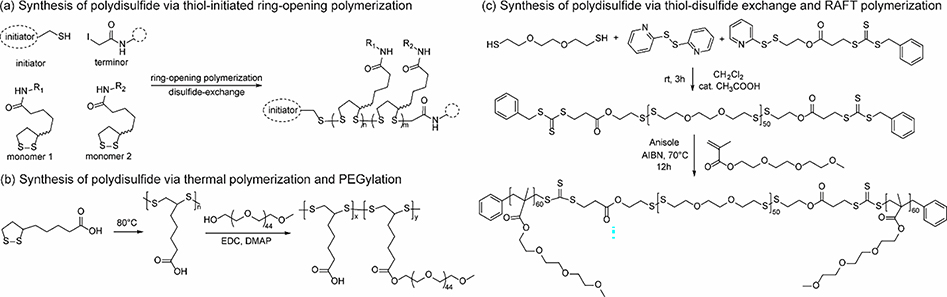
Polydisulfides are polymers with disulfide repeating units on their backbones, which are dynamic and cleavable in response to various stimuli, such as redox, light, and mechanical force [240,241]. Several strategies have been developed for the synthesis of polydisulfides, including ring-opening polymerization of cyclic disulfides, oxidative polymerization of dithiols, thiol-disulfide exchange, and poly-conjugation of monomers with disulfide moieties (disulfides unchanged) [93,240–244]. Recently, substrate-modified cell-penetrating polydisulfides (CPDs) were synthesized via ring-opening disulfide exchange polymerizations (Fig. 10a) [245]. Synthesized polydisulfides were found to penetrate cell membranes rapidly either via endocytosis or direct translocation avoiding endosomal entrapment, depending on polymer structures. Depolymerization could happen within 1 min after reaching cytosol due to the presence of high concentration of GSH. The authors proposed that the direct translocation of polymers across cell membranes was attributed to a combination effect of counterion-mediated and thiol-mediated translocation mechanisms. This study opens a new sight for intracellular delivery of drugs and biomacromolecules, as endosome escape is always a challenging issue in this area [246,247]. Apart from thiol-initiated disulfide-exchange polymerization, polydisulfides can also be afforded by thermal polymerization (Fig. 10b) [248]. The PEGylated amphiphilic polymers self-assembled in water and encapsulated anticancer drug DOX, forming 48 ± 8 nm size spherical nanoparticles. Owing to the disulfide-based backbones and carboxylic acid residues on sidechains, these particles exhibited pH and redox responsive behaviors due to stimuli-triggered charge conversion and polymer fragmentation. Although this work provides a simple method for the synthesis of polydisulfides, the precise control of molecular weight and distribution is challenging.
Fig. 10.
Synthesis of polydisulfide-based random and block copolymers via (a) thiol-initiated ring-opening polymerization [245], (b) thermal polymerization [248], and (c) thiol-disulfide exchange and RAFT polymerization [249].
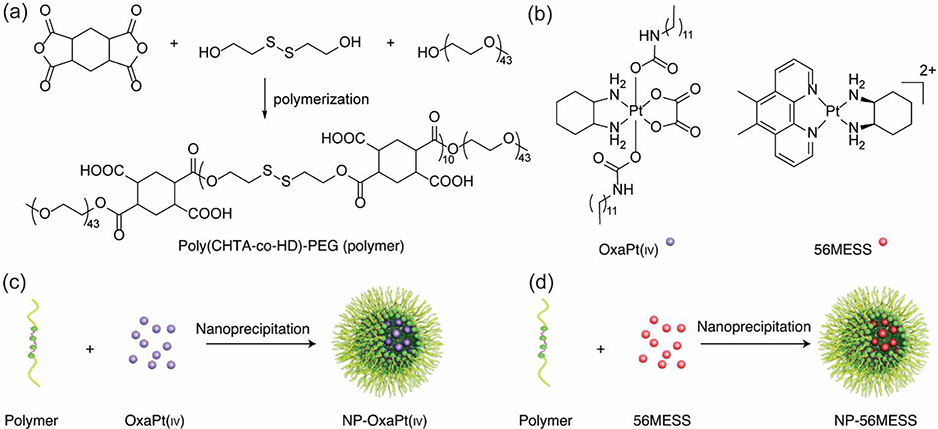
Apart from random copolymers, disulfide-containing block copolymers were also designed for the fabrication of stimuli-responsive nano-assemblies. These polymers could be afforded via different synthetic strategies, including RAFT polymerization (Fig. 10c) [249,250], atom transfer radical polymerization [92,251], and polycondensation. For instance, amphiphilic triblock copolymer poly(1,2,4,5-cyclohexanetetracarboxylic dianhydride-co-hydroxyethyl disulfide)-polyethylene glycol (poly (CHTA-co-HD)-PEG) was recently synthesized via the polycondensation reaction of dianhydride, diol and polyethylene glycol (Fig. 11a) [252]. The polymer was used to formulate spherical nanoparticles by nanoprecipitation, which possessed disulfide linkages and negatively charged carboxylic acid functional groups. These particles could be used to encapsulate a hydrophobic anticancer drug oxaliplatin via hydrophobic interactions and a cationic DNA intercalator 56MESS via electrostatic interactions (Fig. 11b, c & d), which can be released in a reductive tumor environment. Similar investigations have also been performed recently by many other groups, elucidating the great potential of polydisulfides in delivery and their advantages over other materials [253–255]. The well-developed synthetic methods, structure diversity and their responsive degradation toward biological stimuli has made polydisulfide-based amphiphilic macromolecules especially appealing in the design of smart drug delivery systems.
Fig. 11.
(a) Synthesis of poly (CHTA-co-HD)-PEG. (b) Structures of OxaPt(IV) and 56MESS. (c) Formation of NP-OxaPt(IV), and (d) NP-56MESS via nanoprecipitation [252], Copyright 2021. Adapted with permission from the Nature Publishing Group.
Apart from polydisulfides, many other chemistries were also exploited in the design of chain-scissionable polymers (Fig. 12a). For example, diselenide-linked polymers were reported to break down either by oxidizing to selenic acid or reducing to selenol under different environments, causing the disintegration of micellar assemblies and release of encapsulated guest molecules [256]. Monoselenide-linked polymers were found to be degradable via selenoxide elimination, demonstrating the broad responsive properties of selenide-containing materials [257]. However, the broad responsiveness could perturb the stability of polymers and make them sensitive to environment, thus lacking specificity. Polythioacetal-based amphiphilic block copolymers are another class of chain-scissionable polymers [258]. In a recent study, polythioacetal with disulfide linkers were elaborated for programmable drug delivery, which disassembled in the presence of ROS or GSH, releasing the encapsulated hydrophobic drug [259]. In 2017, a palladium-containing block copolymer was synthesized via polycondensation of diisocyanate and palladacycle unit and a subsequential PEGylation [111]. The metallopolymers were found to be sensitive to cell-signaling molecule carbon monoxide (CO) due to a CO-involved cascade insertion–elimination mechanism, breaking the polymer backbones and leading to the fidelity changes of assemblies. The unique responsiveness to this special molecule brings opportunities to design new materials for CO sensing and reporting. However, as the concentration of CO is very low in cells, it would be challenging to design a CO responsive system specifically for biological environment. Recently, charge-altering releasable transporters CA s), a type of biodegradable poly α-aminoester) were synthesized via organocatalytic ring-opening polymerization (Fig. 12b) [260,261]. These cationic amphiphilic polymers can associate with mRNAs and facilitate their intracellular delivery. As the complexes slowly loose proton and encounter charge alterations under physiological environments, they degrade rapidly via intramolecular cyclization and chain scission to form diketopiperazine, liberating the associated mRNAs [262,263]. The presence of these new chemistries has greatly expanded the structural diversity, applicable microenvironment, targeted stimuli, and potential applications of responsive nano-assemblies.
Fig. 12.
(a) Examples of degradable polymers linked by diselenide [256], thioacetal [258] and palladium [111]. (b) Mechanisms for CARTs degradation [260].
Not all the polymer backbone scissions are initiated via the direct cleavage by stimuli, they also occur via stimuli-triggered cascade processes. For example, (aza)quinone methide precursors have been engineered directly on polymer backbones or as protecting groups for the design of chain-scissionable polymers [264]. Different from polymers with head-to-tail degradation mechanisms, they have responsive functionalities exposed at multiple sites in the middle of polymer skeleton instead of terminals [265]. Therefore, bond cleavages occur simultaneously in a parallel fashion at multiple sites in response to stimuli [266,267]. For example, phenylboronic acid-embedded poly(β-aminoesters) were used to complex with proteins via multivalent electrostatic interactions and N-B coordination for cytosolic protein delivery (Fig. 13) [268]. The polymers disintegrated in cancer cells due to the elevated ROS level, leading to the degradation of phenylboronic acid moiety and cascade backbone cleavage. These chemical transformations eventually interrupted the multivalency, broke the assemblies and led to protein dissociation. Compared to a direct backbone cleavage, this type of degradable polymer generally possesses a linker/spacer which could be first activated by stimuli and then break the backbone via a subsequent degradation. The length and structure of linkers could have significant impact on the degradation kinetics.
Fig. 13.
Schematic illustration of the cytosolic protein delivery using ROS-degradable polymer with built-in phenylboronic acid in the backbone and the degradation mechanisms [268], Copyright 2022. Reproduced with permission from John Wiley & Sons Inc.
Due to the structural tunability of quinone methide precursors-based polymers, different functionality can be installed for diverse responsiveness and the generation of multi-stimuli responsive systems [269–271]. For instance, assemblies from azobenzene poly(ester amide) were found to be dual-responsive to both photo-irradiation and reduction (Fig. 14a) [272]. Photo-irradiation triggered the reversible trans-cis isomerization of azobenzene and led to the polarity changes in assembly cores, while hydrazine can irreversibly trigger the polymer backbone cleavage. A synergistic effect of UV light and hydrazine resulted in the significant disruption of assemblies and release of guest molecules. However, the authors didn’t mention the possible applications of this system, likely due to the need to utilize hydrazine, a special chemical which is rare to be used in stimuli-responsive systems. In 2021, an interesting self-propagating degradation behavior was programmed to quinone methide precursor-based block polymers (Fig. 14b) [273]. The polymers were embedded with both external and built-in triggerable functionalities. When applied external stimuli, polymer chain-scissions occurred via the previous mentioned electronic cascade degradation mechanism and liberated primary amines as an amplified second stimulus, triggering the cleavage of built-in functionality and accelerating the degradation of polymers in a cycle amplification fashion. The concept was also tested in nanoparticles which responded to various external stimuli, e.g., reductive milieu, light and esterase, resulting in particle disassembly.
Fig. 14.
(a) Schematic demonstration of the azobenzene-based multi-stimuli responsive system: Reduction leads to irreversible polymer degradation, while photo-triggered isomerization leads to reversible polarity changes in assembly core [272], Copyright 2016. Adapted with permission from the Royal Society of Chemistry. (b) Structures and mechanisms for accelerated backbone degradation of amphiphilic polyurethane nanoparticles in physiologically relevant aqueous media via external stimuli-triggered activation and cascade self-amplification of built-in trigger signals [273], Copyright 2021. Adapted with permission from the American Chemical Society.
Apart from the above-mentioned methods, stimuli-triggered intramolecular cyclization is another strategy for stimuli-triggered chain scission [274,275]. For example, amine-functionalized polycaprolactones recently were engineered with light or enzyme responsive moieties and formulated into nanoparticles. Upon UV light or enzymatic treatment, the liberated sidechain amines could undergo intramolecular cyclization, cleave the polycaprolactones and generate 5-(2-hydroxyethyl)-2-piperidinone fragments, resulting in particle disassembly and guest molecule release [129,276]. Similar strategies were applied for the design of poly(D,L-lactide-co-glycolides) and polycarbonates with deactivated pendant nucleophiles [277,278]. Stimuli-triggered deprotection and subsequently intramolecular cyclization ultimately led to polymer chain scissions and particle disassembly. This chemical strategy was recently adopted for the design of degradable drug carriers (Fig. 15) [279]. In the study, thioketal functionalized polycarbonate-drug conjugates were synthesized and used for the encapsulation of a photosensitizer. Light-induced ROS-generation by photosensitizer could trigger thioketal cleavage which liberated the conjugated DOX and initiated backbone-destruction and carrier disintegration, releasing photosensitizer at the irradiated tissue sites. The photo-irradiation could lead to direct photodynamic therapy and indirect chemotherapy via ROS-triggered DOX release.
Fig. 15.
Schematic illustration of polymer chain-scission via ROS-triggered degradation and cyclization. [279], Copyright 2019. Reproduced with permission from the American Chemical Society.
Overall, stimuli-triggered depolymerization generally leads to a complete or partial fragmentation of macromolecules. The formation of nano-assemblies generally relies on hydrophobic interactions, electrostatic interactions, π-π stacking and other weak interactions. In many cases, these interactions work in synergistic or multivalent manners. The triggered depolymerization can break HLB, interrupt their synergistic effects or disrupt multivalency, eventually disturbing the stability of original assemblies and deconstructing the assemblies completely. These transformations could occur either by chain-unzipping or chain-scission. Polymer chain unzipping usually happens via stimuli-initiated end-cap activation, followed by a head-to-tail type degradation. Some well-studied chemistry includes (aza)quinone methide-based electronic cascade shift and cleavage, degradable polyglyoxylates, PPAs, polysulfones and others. The degradation properties of polymers and corresponding assemblies could be tuned by varying different functionalities, stimuli and blending different polymers to achieve a controllable manner. Moreover, as polymer degradation is relying on end-cap activation, there is only weak or no correlation between stimulus concentration and depolymerization kinetics [222]. However, the degradation of these polymers could generate (aza)quinone methides, aldehydes and other reactive intermediates which could react with nucleophiles within the microenvironment, bringing undesired issues especially for biosystems. Additionally, to achieve cascade degradation, some monomers need tedious protection/deprotection processes and complicated synthetic procedures. Therefore, developing new chemistries overcoming the problems is always in demand. Different from a head-to-tail type degradation, polymer chain-scission occurs parallelly on polymer backbones without relying on the cleavage of adjacent moieties. Stimuli could directly break multiple sites on polymer backbones or activate multiple functionalities which subsequently induces chain-scissions. Compared to chain-unzipping, chain-scissions are initiated via multi-trigger activations, thus the depolymerization kinetics exhibiting close concentration- and reactivity-dependence on stimuli, offering tunability to polymer-degradation and particle-disassembly kinetics [222]. As the backbone-chain breaking is independent of the adjacent bonds, eligible chemistries and stimuli are highly flexible. Compared to other disassembly strategies, stimuli-triggered depolymerization could ideally lead to the complete fragmentation of entire polymer backbones or specific blocks, thus generally causing complete particle disassembly.
5. Stimuli-triggered decrosslinking of nano-assemblies
Crosslinked nanoparticles are nanoplatforms which are stabilized by the connection of different polymer chains via covalent bonds or ionic bonds [280]. Over the past decades, they have been extensively studied as nanocarriers which can physically trap cargo into particle cores. Stimuli-triggered decrosslinking generally leads to the swelling and disintegration of nanoparticles, releasing the entrapped cargo molecules. Currently, several strategies have been developed for the preparation of crosslinked nanoparticles including emulsion polymerization, inverse nanoprecipitation, and post-assembly crosslinking [281]. As the scope of this review is on macromolecular assemblies, we would focus on the crosslinked particles from post-assembly crosslinking and summarize recent advances of stimuli-responsive crosslinked nano-assemblies based on the crosslinkers and corresponding stimuli.
5.1. pH responsive crosslinked nano-assemblies
pH responsive crosslinkers such as acetals [282–284], imines/hydrazones [285–287] and boronic esters [86,288] have been extensively investigated to formulate crosslinked nanoassemblies for diverse applications. Recently, a pH responsive polymer-caged lipoplex (PCL) were prepared via the coating of cholesterol-terminated poly(acrylic acid) to lipid-derived siRNA carriers and then crosslinked with diamine functionalized acetals (Fig. 16a) [289]. The nanoassemblies underwent faster degradation under acidic pHs due to the acid-triggered decrosslinking, leading to guest molecule release (Fig. 16b). Cell experiments demonstrated that acid-triggered decrosslinking led to enhanced siRNA release and protein knockdown efficiency. However, in this work, the gene release kinetics was mainly controlled by pH which is an intrinsic stimulus and lacks tunability in material aspects. The study on structure-property relationship could bring the opportunity to further improve delivery efficiency, as the pH responsiveness and decrosslinking properties could be significantly affected by the structure of linkers. To control the cleavage kinetics of acetal linkers, we systematically investigated the impact of substitutions on their pH sensitivity and applied the results into the design of responsive nanocarriers [290]. By fine-tuning acid-labile moieties (e.g., ketal vs. acetal, inductive effects, substituent effects on aromatic rings), the kinetics can be varied more than 6 orders of magnitude. The same trend was observed on nano-assemblies, demonstrating the potential application in manipulating guest release kinetics via the selection of acid-labile linkers. In a recent study, an amine- and aldehyde-functionalized methoxy-poly(ethylene glycol)-poly(aldehyde ethyl aspartamide) were co-assembled to form imine-crosslinked complex, generating spherical micellar nanoparticles and non-covalently encapsulating hydrophobic drug. Under an acidic environment, the micelles were found to swell from 250 nm to 900 nm and eventually disassemble to release the encapsulated drug [280]. As a well-studied dynamic covalent chemistry, the binding between diols and boronic acids are highly sensitive to pH. Recently, catechol-modified amphiphilic polymers were crosslinked with boronic acid-modified RNase A and used for the codelivery of RNase A and non-covalently encapsulated DOX [291]. The boronic acid-catechol linkages would be cleaved under acidic pH during cellular internalization, dissociating DOX and boronic acid-modified RNase A which was next tracelessly liberated by ROS-triggered degradation of phenylboronic acid. This method provides a new platform for the co-delivery of therapeutic proteins and drugs. However, boronic ester is well known to be diol-responsive and undergo dissociation, which could bring problems for its in vivo applications as the polysaccharide and glucose in circulatory systems and cell surface could compete with catechol and lead to the undesired off-target release.
Fig. 16.
(a) Schematic presentation of the preparation of an acid-degradable siRNA-loaded PCL nanocarrier and its acid-triggered release of siRNAs. (b) The dsDNA release profiles for PCLs under different pH [289], Copyright 2013. Adapted with permission from the American Chemical Society.
Acid-labile moieties were often incorporated with other responsive groups for the design of multi-stimuli responsive systems [162]. For example, benzaldehyde functionalized polymer was covalently modified with an anticancer drug via photocleavable linker at the hydrophobic blocks. he amphiphilic copolymer could assemble in aqueous solution to form core–shell nanostructures with the hydrophobic drug trapped in the core and hydrophilic blocks with benzaldehyde in the outer coronas. The hydrophilic shell could be further stabilized by dihydrazide-functionalized disulfide linkers to form crosslinked nano-assemblies. The photocaged drug could be liberated under photo-irradiation, the kinetics of which could be further modulated by pH- and redox-induced decrosslinking [292]. Similar crosslinking strategy was applied for other polymers to design pH and redox dual responsive nano-assemblies, either covalently conjugated or noncovalently encapsulated drugs in the carriers [293–295]. Recently, imine-linked phenylboronic acid and catechol moieties were separately functionalized to amphiphilic copolymers [296]. The two polymers could form nanoparticles via a cross-linking-induced self-assembly in water and methanol to form spherical and bundle-like assemblies respectively (Fig. 17). This was likely because B-N dative bonds were formed in methanol but largely inhibited in water during the self-assembly processes. The results showed that bundle-like assemblies have faster decrosslinking kinetics under acidic and sugar conditions. Further investigation demonstrated the morphology of assemblies could significantly affect their cellular uptake processes, where assemblies with asymmetrical structure exhibited better cell penetration ability. This work provides a new method for the fabrication of nano-assemblies with distinct morphologies by simply varying the media for assembly formation. However, although the authors hypothesized that the different cell penetrating ability was due to the distinct surface properties and catechol distribution, the potential mechanisms were not investigated.
Fig. 17.
Schematic presentation of nanostructures prepared via the assembly of phenylboronic acid and catechol modified polymers in water and methanol and the corresponding TEM images [296], Copyright 2017. Adapted with permission from the American Chemical Society.
The examples introduced above are mostly acidic pH-triggered decrosslinking and particle disruption. In some cases, pH may not necessarily cause decrosslinking, but changes the hydrophilicity and electrostatic interactions, leading to the swelling of nano-assemblies for stimuli-controlled utilities. For example, crosslinked micelles with acetal pendants were prepared by photo-crosslinking of acrylate residues (Fig. 18) [297]. As the crosslinking was based on non-degradable C-C bond formation, pH changes only led to swelling of particle from 71 to 82 nm instead of disrupting the assemblies. Further investigations demonstrated that the crosslinking significantly increased the stability of assemblies as drug carriers, without affecting their drug release profiles under acidic conditions. Similarly, pH and redox dual responsive assemblies were designed by incorporating acetal moieties as pendants on polymer backbones and disulfides as chemical linkers [298]. Acidic pH conditions led to the cleavage of acetal pendants and changes of assembly hydrophilicity but did not completely destroy the assembly because of the physical crosslinking between polymer chains. In another recent study, crosslinked polymersomes were fabricated by the self-assembly of acetal-terminated triblock copolymers and subsequent chemical crosslinking of fumarate segments [299]. After hydrolysis of the hydrophobic acetal, the sizes of polymersomes significantly expanded from 96 to 200 nm, leading to the alteration of host-guest properties. A similar strategy could be applied for amine and carboxyl-functionalized polymers, as these moieties are considered as pH responsive due to charge conversion under certain pH. For example, zwitterionic nanoparticles with tertiary amines core were constructed by disulfide crosslinking [300]. Upon decreasing the environment pH, the particle sizes could significantly increase from 163 nm at pH 7.4 to 354 nm at pH 5.0 due to the protonation of tertiary amines. Further study on the impact of crosslinking density on particles under different pH confirmed that disulfide-crosslinking significantly restricted the expansion of assemblies and ensured the integrity even after protonation. These examples illustrate that the stimuli-triggered responses of crosslinked nano-assemblies do not always offer complete disassembly, but could cause size and morphology transformations. However, as this strategy can significantly change the surface properties (e.g., charge, hydrophilic) and size of particles, it could still cause significant variations in their host-guest behaviors, thus qualified for controlled molecular release.
Fig. 18.
Schematic illustration of photo-crosslinkable pH-sensitive degradable micelles formation and acid-triggered degradation [297], Copyright 2012. Adapted with permission from Elsevier Science Ltd.
5.2. Disulfide-crosslinked nano-assemblies
Disulfide linkage is well studied for the fabrication of crosslinked nano-assemblies because of the synthetic accessibility, responsiveness toward a bioenvironment-rich stimulus and orthogonality with other functionalities [301–303]. Similar to poly(disulfide) synthesis, disulfide crosslinkers can be installed to nano-assemblies either directly via disulfide formations or indirectly via click chemistry, condensation, or Michael addition (Fig. 19a, b and c) [302–306]. For example, in 2017 we applied disulfide-linked 4-nitrophenyl carbonate as activated functional groups on amphiphilic polymers to conjugate with proteins for delivery [307]. To increase the stability of protein-polymer complexes, the assemblies were crosslinked via substitution reactions of diamines and activated carbonate residues. As the 4-nitrophenyl carbonate was linked via a disulfide bond, the assembly could be disrupted via GSH-induced disulfide cleavage and subsequent self-immolation to tracelessly release the conjugated proteins. However, the strategy also has several limitations, such as relatively low functionalization efficiency, slow conjugation kinetics, and competitive hydrolysis of 4-nitrophenyl carbonate. Thus, we later screened several activated carbonates and found that pentafluorophenyl-containing carbonate possessed excellent balance between amine-conjugation reactivity and hydrolytic degradability (Fig. 19d) [308]. Moreover, the manipulation of polymer charge would facilitate the association with antibody and promote proximity-induced covalent capture, significantly enhancing protein conjugation efficiency. As one of the current issues for protein delivery is that permanent conjugation could irreversibly perturb protein activity, the development of reversible conjugation strategy would on one hand facilitate protein entrapment, on the other hand release them upon stimuli-triggered covalent bond cleavages. These studies provided a general strategy for the design of disulfide-containing self-immolative polymers for therapeutic protein delivery with traceless release features.
Fig. 19.
Disulfide-based polymers formed via (a) Michael addition [305], (b) click chemistry [304], and (c) condensation reactions [306]. (d) Schematic illustration of disulfide-crosslinked nanoparticles for protein conjugation and intracellular delivery via a traceless release manner [308], Copyright 2020. Adapted with permission from John Wiley & Sons Inc.
Apart from the above examples where disulfide linkages were introduced via indirect approaches, a direct disulfide formation is another synthetic strategy for crosslinking. For instance, since it was first explored by us in polymer functionalization, the dynamic exchange chemistry of pyridyl disulfides and free thiols has been widely used in the elaboration of disulfide-containing polymeric materials, especially nano-assemblies [309,310]. The reaction occurs via a SN2-type nucleophilic substitution between a free thiol and pyridyl disulfide, releasing 2-thiopyridone [302]. In 2010, we applied this chemistry for polymer crosslinking, generating disulfide-linked nano-assemblies [311]. The assemblies could noncovalently encapsulate guest molecules and the loading efficiency and capacity could be tuned via changing hydrophobicity, electrostatics and blending of different polymers [312–315]. Further study demonstrated that formation and stability of crosslinked nano-assemblies could be significantly impacted by environment [316,317]. Recently, dithiols were used to crosslink trehalose and activated disulfide copolymers via a direct thiol-disulfide exchange chemistry (Fig. 20a) [318].This direct disulfide linker formation strategy has been employed for the covalent trapping of dithiol-containing glucagon, a peptide for hypoglycemia treatment, into crosslinked nanogel which improved its solubility and stability in solution. After the treatment with TCEP, the embedded glucagon could be released and form fibrils (Fig. 20b and c). In another study, amine and carboxyl group functionalized pyridyl disulfide-containing polymers have been fabricated into vesicular morphology with controllable sizes by tuning the concentration of divalent counterions [319]. Similar as other disulfide-crosslinked assemblies, redox-triggered disulfide cleavage could disturb the permeability of vesicular membranes, leading to protein release and subsequent tryptic digestion. Apart from pyridyl disulfides, poly(S-2-hydroxyethyl-O-ethyl dithiocarbonate methacrylate) was incorporated into an amphiphilic penta-block copolymer for the formulation of crosslinked micelles [320]. After self-assembly, micelles were crosslinked via the aminolysis of dithiocarbonyl moieties to liberate free thiols followed by oxidation to form disulfide linkages (Fig. 20d). The assemblies exhibited excellent colloidal stability under normal physiological conditions and fast drug release profiles in tumor-mimicking environments. The availability of these crosslinking strategies has further expanded the potential applications of disulfide-containing crosslinked nano-assemblies.
Fig. 20.
(a) Structure of PDSMA-co-TrMA and schematic presentation of glucagon–nanogel formation and release. TEM images of PDSMA1-co-TrMA0.8 glucagon nanogels before (b) and after (c) treatment of TECP for 24 h [318], Copyright 2018. Adapted with permission from John Wiley & Sons Inc. (d) Crosslinking via the deprotection and oxidation of free thiols [320].
Disulfide can also be incorporated with other functionalities to design dual and multi-stimuli responsive assemblies. For example, pyridyl disulfide monomer was copolymerized with thermoresponsive PEG moieties and formed crosslinkable copolymers [321]. These polymers exhibited a distinct thermoresponsive profile after crosslinking, demonstrating the significant impact of polymer architecture on their responsive properties. Disulfide-containing polymers could also be incorporated with pH-responsive tertiary amines to form pH and redox dual responsive assemblies. The formed assemblies could undergo charge conversion under acidic pH, as a result manipulating assembly stability, particle-aggregation, surface properties and cellular uptake efficiency [317,322–325]. In a recent study, pyridyl disulfide and pH responsive trimethoxybenzylidene acetals were installed into amphiphilic block copolymers affording a dual responsive polymer, which has been used for the fabrication of drug delivery vehicles with synergistical responsive behaviors under an acidic and GHS-rich environment [326]. Similarly, pH, thermal, and redox triple-responsive crosslinked assemblies were designed as nanocarriers for photodynamic therapy [327]. The assemblies exhibited excellent stability under physiological condition, while swelling remarkably from 108 nm to 1200 nm due to a synergistical responses to the three stimuli (pH, temperature, and reductant). Different from DTT-initiated thiol-disulfide exchange, the crosslinking in this work were relying on tris(2-carboxyethyl)phosphine (TCEP)-triggered disulfide cleavage followed by oxidation to generate new disulfide linkages. The incorporation of multiple stimuli-responsive functionalities brings the opportunity to design programmable AND/OR logic gates in nano-assemblies which would enhance the controllability to the responsiveness.
5.3. Other stimuli-responsive crosslinked nano-assemblies
Apart from the chemistry used above, other common stimuli including ROS, enzyme and light were also exploited for the decrosslinking of nano-assemblies. Examples of ROS-responsive moieties include thioketals and diselenides. Thioketal is a thioether group which degrades in response to ROS, generating acetone and thiol-containing moieties [86]. In 2020, we investigated the degradation mechanism and found that thiol-containing moieties could eventually be converted to disulfides (Fig. 21a) [200]. This moiety was also incorporated for the formation of crosslinked nanocarriers which got destabilized when subjected to ROS, exhibiting guest molecule exchange with adjacent assemblies and ultimately guest molecule-release. Thioketal-crosslinked polycarbonate and PEGylated polyphosphoester micelles were recently reported for drug delivery, which could selectively release anticancer drugs in tumor cells due to ROS-triggered decrosslinking [328,329]. Diselenide-linked materials were found to be redox and ROS dual responsive, generating selenol and selenic acid respectively. Similar to disulfides, diselenides could be installed indirectly via various conjugation methods, e.g., azide-alkyne cycloaddition [330]; or directly via diselenide formation, e.g., the substitution of disodium diselenide with other functional groups on polymer sidechains or photo-triggered diselenide metathesis (Fig. 21b) [331]. These nano-assemblies have been applied as drug delivery systems and exhibited controlled release behaviors under redox or ROS-rich environments.
Fig. 21.
(a) Mechanisms of ROS-triggered thioketal degradation [200], Copyright 2020. Adapted with permission from Elsevier Science Ltd. (b) Schematic illustration of diselenide-crosslinked particle formation, degradation mechanisms and TEM image of the formed assemblies [331], Copyright 2017. Adapted with permission from Elsevier Science Ltd.
Enzymes are biomacromolecules which can catalyze chemical ligations or cleavages. Due to correlations with many biological functions, they have been considered as stimuli for the design of crosslinked assemblies for biomedical applications [332]. Similar to other responsive moieties, enzyme responsive groups can be functionalized to assemblies via different chemical strategies. For example, hydrolytically degradable PLA-attached block copolymers were used for the design of nanoparticles which can crosslink using non-degradable diamines [333]. Enzymatic hydrolysis of PLA could induce the excavation of assembly cores. Interestingly, slower hydrolysis of PLA was observed for crosslinked assemblies due to the poor accessibility of enzyme to the hydrophobic PLA cores. In another study, elastase-responsive peptide was modified to poly(amido amine) and formed 20 nm spherical nanoparticles [334]. These nanoparticles can be crosslinked via oxidation-triggered dimerization of tyrosines on the peptide moieties, generating particles with 50 nm sizes, which disassembled in response to elastase and released the encapsulated drug molecules. To precisely control the encapsulation and release properties of assemblies for specific biological conditions, the incorporation of dual or more responsive moieties in one system is highly desirable. For example, thermoresponsive assemblies were crosslinked using matrix metalloproteinase (MMP)-responsive peptide linkers via azide-alkyne click chemistry [335]. The particle sizes, stability and thermoresponsive properties can be tuned by varying monomer ratios and crosslinking. The platform could be used for protein encapsulation and MMPs-triggered release under certain temperatures. Similarly, redox- and enzyme-activable nanoagents were prepared for combination antitumor therapy (Fig. 22) [336]. The polymers were pre-crosslinked by cathepsin B-cleavable peptide linkers during RAFT polymerization and then dispersed into aqueous solution to form assemblies with uniform sizes. It should be noted that photosensitizer pyropheophorbide was covalently modified to the assemblies via disulfide bonds and poly adenosine diphosphate-ribose polymerase inhibitor AZD2281 was noncovalently encapsulated. The nanoagents could be activated under the treatment of GSH or cathepsin B and exhibited synergistical immunotherapy and photodynamic therapy effects when both stimuli present.
Fig. 22.
Schematic illustration of a redox/enzyme-activatable nanoagent with GFLG peptides and disulfide bonds, after encapsulation of a PARP inhibitor, AZD2281 [336], Copyright 2021. Reproduced with permission from John Wiley & Sons Inc.
Photo-triggered bond formation and cleavage represents another common strategy to control the crosslinking and decrosslinking of assemblies [337]. Among various photo-responsive moieties, coumarin is the most used one which relies on the reversible dimerization under different stimuli [338]. Recently, coumarin functionalized polymers were fabricated to spherical assemblies by solvent evaporation method [339]. Interestingly, spherical morphology could transform to hollow assemblies via interfacial crosslinking. The thickness of crosslinked shells could be modulated by the time of photo-irradiations. In another study, amphiphilic block copolymers were functionalized with photo-cleavable phthalimide esters and crosslinkable coumarin and formed spherical nano-assemblies (Fig. 23) [340]. Under photo-irradiation, hydrophobic phthalimide esters in the assembly cores could be converted into hydrophilic polymethacrylic acid which could prohibit the ability to hold hydrophobic guest molecules and lead to molecular release. In contrast, photo-triggered coumarin crosslinking could retard guest leakage. By manipulating the irradiation wavelength and time, the stability of the assemblies could be tuned to realize a controlled guest molecule release. Apart from coumarin, other photolabile groups were recently utilized for designing crosslinked materials and may potentially be applied for nano-assemblies in the future [341–343]. However, photo-triggered reversible conversions are generally not complete, and the chromophores could be damaged after longtime irradiation, which may perturb their long-term usage. Therefore, it would be of great significance to develop new chemistries with high chemical stability and efficiencies for photochemical reactions.
Fig. 23.
Structure of phthalimide esters and coumarin-functionalized polymer and photo-triggered changes to chemical structures and nano-assemblies [340], Copyright 2017. Reproduced with permission from John Wiley & Sons Inc.
Although most of the crosslinked nano-assemblies rely on covalent bond formation and cleavage, non-covalent bond-based crosslinking is not rare. The interaction could be hydrogen bonding [344], electrostatics [345], metal coordination [346], host-guest interactions [347] and many others [348]. However, as most of them are weak interactions, controlling the stimuli-responsive properties may not be as programmable or even accessible as covalent bonds. In some cases, the interactions could be disturbed by changing pH and the addition of competing reagents which needs to be analyzed case by case. But this strategy could be combined with covalent bond-based stimuli-responsive moieties in design to achieve stable assemblies and controllability in responsiveness.
6. Stimuli-triggered conformation/configuration changes in nano-assemblies
In the previous sections, we summarized stimuli-triggered assembly alterations by HLB change, depolymerization and decrosslinking. However, the alterations can be simply triggered by the conformation/configuration changes of a single bond. Examples of these chemical moieties include azobenzene, aromatic enylidene, dithienylethene and spiropyran [349]. In most of the examples, the conformation/configuration changes are from photo-triggered isomerization or cyclization [338].
For example, we recently reported photo-responsible glassy polymersomes fabricated by azobenzene-linked PEG-PLA block copolymers in aqueous solution and formed 140–150 nm size particles (Fig. 24a) [350]. The vesicles could noncovalently accommodate guest molecules in the hydrophilic cores or hydrophobic bilayer membranes. Interestingly, they could release both the hydrophilic and hydrophobic guest molecules under UV irradiation while conserving them in darkness, exhibiting an out-of-equilibrium actuation behavior (Fig. 24b and c). We presumed that the photo-triggered conformation change of azobenzene could perturb the stability of the interface membrane of the vesicles containing hydrophobic guest molecules which further propagated through the bilayer membrane and led to the leaking of hydrophilic guest molecules. The results demonstrated that the conformation change of a single bond could cause an out-of-equilibrium perturbation of supramolecular assemblies across over 500 chemical bonds. The excellent controllability of membrane permeability and structural simplicity have made the system a potential platform for controllable catalysis. However, the requirement of UV exposure could bring problems for their use in bio-systems as the irradiation could cause severe cell and tissue damage. Thus, the development of new chemistries responding to visible or NIR irradiation would be highly desirable.
Fig. 24.
(a) Structure of azobenzene-linked block copolymer and the illustration of the formed vesicles. (b) and (c) The release profiles of hydrophobic DiI and hydrophilic rhodamine 6G under 360nm UV irradiation and dark conditions [350], Copyright 2017. Adapted with permission from the Nature Publishing Group.
Aromatic enylidenes have been studied as photoswitches to design molecular motors for a long time. Their photo-triggered responsiveness can be reversible or irreversible depending on the molecular structures. In 2011, bis-thiaxanthylidene-based amphiphiles were reported to self-assemble with phospholipid 1,2-dioleoyl-sn-glycero-3-phosphate and formed nanotubes with controllable aspect ratios [351]. Upon UV irradiation λ = 390 nm), these nanotubes disassembled due to the irreversible transformation of anti-folded isomer to the cyclized counterpart, disturbing the original structure packing (Fig. 25a). In another study, overcrowded alkene-based amphiphiles self-assembled into nanotubes which can reversibly change their morphologies to vesicles via photoirradiation or applying heat to trigger the isomerization of double bonds (Fig. 25b) [352]. Recently, dithienylethene were incorporated with PEG and perylenemonoimide to form an amphiphilic photoswitchable imaging agent [353]. The amphiphiles self-assembled to liposomes which fluorescence could be turned on/off via visible light and UV irradiations due to photoswitchable FRET processes (Fig. 25c). The formed liposome could be visualized via super-resolution fluorescent microscopy and provided a comparable size data as TEM and AFM. Different as the above examples, photo-triggered isomerization of spiropyran in nano-assemblies not only leads to configuration changes but also significantly alters their hydrophilicity, leading to the subsequent changes of assemblies [353]. Irreversible chemical transformations could happen completely, but the control of assembly states is also irreversible, and the assemblies are non-reusable; reversible transformations generally is hard to complete and easily affected by external environment, but the assembly states are reversible via the employment of different stimuli. The selection of chemistry dependents on the requirements of specific applications.
Fig. 25.
(a) Structures of bis-thiaxanthylidene-based amphiphiles before and after the irreversible photoisomerization [351], Copyright 2011. Adapted with permission from the Nature Publishing Group. (b) Photo and heat-induced reversible isomerization of overcrowded alkene-based amphiphiles [352], Copyright 2015. Adapted with permission from the American Chemical Society. (c) Photoisomerization of dithienylethene-based imaging agent [353], Copyright 2017. Adapted with permission from the American Chemical Society.
Overall, stimuli-triggered isomerization of chemical joints could lead to molecular conformation/configuration alterations, disturbing the original molecular packing and optical properties of nano-assemblies. These alterations have been exploited for controlled guest molecule release, morphology transformation and super-resolution imaging. However, compared with other strategies, the applications of stimuli-triggered conformation/configuration changes are relatively limited, likely due to the restricted irradiation wavelengths and the disturbance from spontaneously thermo-induced retro-isomerization which perturbed the controllability of responsiveness. Moreover, the synthesis and modification of some responsive moieties are tedious. The bulky molecular size and hydrophobicity further affect their practical utilities in amphiphilic nano-assemblies.
7. Applications of nano-assemblies from amphiphilic macromolecules
7.1. Drug delivery
In the past few decades, stimuli-responsive polymeric nanoparticles have had a tremendous impact on nanomedicine [354,355]. These drug delivery systems are designed to be inactive in the non-target physiological environment but can release small-molecule drugs in response to specific physical, chemical, or biological stimuli at the target microenvironment. For example, acidic tumor microenvironments (pH 6.5–6.8) and endo/lysosomes (pH 4.5–6.5), overexpression of enzymes, and elevated levels of GSH (2–10 mM) and ROS inside cancerous cells are distinguishing features that have been leveraged in the design of stimuli-responsive nanoassemblies [356,357]. Along with emerging hallmarks of cancer [4,358], sophisticated macromolecular engineering has been crafted to create polymeric nanoparticles that can effectively prevent premature drug leakage and reduce adverse effects while increasing drug accumulation at the sites of interest, such as tumor microenvironments and cancerous intracellular milieu, in response to structural alterations upon stimuli-triggering [359–361]. A wide variety of structural determinants built in polymer design have progressively advanced modern medicine by improving the modulation of structural transformations and the complexity of responsiveness.
7.1.1. Drug delivery via the interruption of HLB
Amphiphilic macromolecules have been extensively investigated for their ability to encapsulate small-molecule drugs within the pocket, which prevents drug leakage and enables controlled release at the desired sites. Upon exposure to stimuli, alterations in the HLB can cause particle disruption and subsequent drug release. A wide range of responsive moieties can be engineered into macromolecules to induce HLB interruptions and achieve structural alterations. The ubiquitous acidic tumor microenvironment has been recognized as a universal hallmark of cancer due to the Warburg effect [362,363]. The distinct lower pH in the tumor microenvironment and endo/lysosomes compared to the physiological environment enables pH-responsive polymeric nanoparticles for controlled release of small drug molecules. Charge shifting can cause a hydrophobic-to-hydrophilic transition of amphiphilic macromolecules, eventually inducing structure dissipation. For example, ultra-pH-sensitive micelles (UPSM) of PEG-b-PDPA that showed extraordinary buffer capacity and micelle–unimer phase transition within a narrow pH range (<0.3 pH) in lysosome was developed for effective treatment of oncogene KRAS mutant pancreatic cancer by inhibition of lysosomal catabolism along with chemotherapy in vitro and in vivo (Fig. 26a) [364]. These micelles alone can effectively suppress lysosomal catabolism to reduce cell viability of KRAS mutant PDAC cells and to inhibit tumor development in mice models, whereas non-pH-sensitive micelles (NPSM) showed insignificant effect in vitro and in vivo. Moreover, pH-triggered burst release of triptolide prodrug, triptolide-naphthalene sulfonamide, demonstrated excellent antitumor efficacy while remaining superior safety with low drug leakage. Acidity-triggered ligand-presenting nanoparticle of photodynamic agent (chlorin e6, Ce6)-conjugated ultra-pH-sensitive polymer (PEG-b-PHMA) and acidity-triggered active-targeting prodrug (iRGD-GALGLP-P85-PLGLAG-DOX) were exploited to overcome sequential drug delivery barriers to tumors (Fig. 26b) [365]. In acidic tumor microenvironment, Ce6-conjugated PEG-b-PHMA detached from ATLP nanoparticles driven by amphiphilic to hydrophilic transition due to protonation of PHMA, leaving the nanoparticles solely composed of acidity-triggered active-targeting prodrug that can facilitate tumor penetration and cellular uptake by binding with αvβ3/5 integrin and neuropilin-1 overexpressed in tumor cells. The overexpression of a metalloproteinase-2 (MMP-2) in tumor microenvironment and cancer cells enable acidity-triggered active-targeting prodrug to release DOX via cleavage of MMP-2-labile PLGLAG. Meanwhile, detachment of Ce6-conjugated PEG-b-PHMA activated the photodynamic feature of Ce6 and revealed the increasing fluorescence intensity for in vivo tumor imaging. Upon NIR laser irradiation, significant ROS generation by Ce6 not only induced cancer cell apoptosis but also promoted drug diffusion into cells. By the similar strategy, A nanosized drug delivery system (NDDS) has been developed for multimodal imaging and combinational therapy of drug-resistant tumors. The NDDS was composed of photodynamic agent Ce6-conjugated ultra-pH-sensitive polymer (PEG-b-PDPA), gadolinium-coordinated Ce6-conjugated PEG-b-PDPA, and lysosome-cleavable prodrug. The system utilized fluorescence, magnetic resonance (MR), and photoacoustic imaging to enable precise visualization of the tumor, and combines chemotherapy, photodynamic, and photothermal therapy to enhance the therapeutic effect (Fig. 26c) [366]. Upon cellular internalization, NDDS showed abrupt disruption due to amphiphilic to hydrophilic transition in the early endosome, generating strong fluorescence and activating T1-weighted MR signals for fluorescence and MR imaging, respectively. Meanwhile, prodrugs were liberated upon linker cleavage of Gly-Pro-Leu-Gly tetrapeptide in endo/lysosome. Elevated temperature via NIR irradiation was observed for PA imaging and photothermal therapy while significant ROS was generated for photodynamic therapy and promoting tumor penetration of the chemotherapeutics.
Fig. 26.
(a) Preparation of T-UPSM and T-NPSM (top panel) and illustration of UPSM for drug delivery and blocking of lysosomal acidification in pancreatic cancer treatment (bottom panel) [364], Copyright 2019. Adapted with permission from the American Chemical Society. (b) Preparation of ATLP nanoparticle (top panel) and illustration of ATLP nanoparticles for iRGD-enhanced tumor penetration and imaging-guided combination cancer therapy (bottom panel) [365], Copyright 2017. Adapted with permission from the American Chemical Society. (c) Nano-assembly and chemical structure of the acid-switchable micelles comprising pH-responsive PEG-b-PDPA, gadolinium-coordinated photosensitizer Ce6, and a prodrug of DOX (top panel). Illustration of the NDDS for multimodal imaging and combinational therapy of drug-resistant tumor (bottom panel) [366], Copyright 2016. Adapted with permission from the American Chemical Society. (d) Chemical structure of polymers as building blocks and Illustration of the extracellular click reaction between POLYPROTAC and DBCO-loaded pretargeted NPs in acidic tumor microenvironment (top panel). Schematic illustration of the 58eblock58onal POLY-PROTAC NPs for tumor-specific protein degradation and cascade pathways [367], Copyright 2022. Adapted with permission from the Nature Publishing Group.
To enhance cancer chemotherapy, a combination of gene therapy and chemotherapy was developed. This combination aims to downregulate the anti-apoptotic pathway via RNA interference, thereby sensitizing triple-negative breast cancer cells to chemotherapy. [368]. Poly(ethylene glycol)-block-poly(2-(dimethylamino)ethyl methacrylate)-block-poly(2-(diisopropylamino)ethyl methacrylate) (PEG-b-PDMAEMA-b-PDPA) was found to show efficient drug and gene loading efficacy in comparison to the random copolymer with the same component while the pH-responsive gene and drug release was significant in the intracellular environment to effectively demonstrate the enhanced chemotherapy. Moreover, either drug or gene delivery systems encounter endosomal entrapment via endocytosis and suffer from low efficacy of endosomal escape that leads to inefficient therapy [192,369]. The hydrophobic antifungal drug, amphotericin B (AmB), that was encapsulated in pH-responsive siRNA delivery system demonstrated effective disruption of endosome via membrane poration [370]. Bafilomycin A1, a vacuolar ATPase inhibitor that prevents the acidification of the endocytic organelles, eliminated the improved knockdown efficiency, which supported the synergistic effect on endosomal escape through membrane protonation by AmB together with proton sponge by protonatable polymers. In the in vitro study, siRNA/AmB-loaded poly(2-(dimethylamino)ethyl methacrylate)-block-poly(2-(diisopropylamino)ethyl methacrylate) (PDMA-b-PDPA) micelleplexes showed significant efficiency of endosomal escape for improved gene knockdown. Apart from the delivery of traditional chemo-drugs, polymeric nanotherapeutics that showed programable structural transformations in response to multiple biological stimuli was explored to ferry Proteolysis Targeting Chimeras (PROTACs) molecules for tumor-specific protein degradation (Fig. 26d) [367]. The disassembly of pre-targeting nanoparticles exposed significant amounts of dibenzocyclooctyne (DBCO) molecules in acidic tumor microenvironment, which underwent click reaction with the terminal azide at the surface of POLY-PROTAC, facilitating the intertumoral accumulation. The shedding of PEG corona upon cleavage of GALGLPG by MMP-2 that mitigated the “PEG dilemma” effect improved the efficiency of cellular internalization. In response to the elevated level of GSH in cytoplasm, the cleavage of disulfide bonds liberated PROTAC molecules from polymer backbone, activating the degradation of the bromodomain and extraterminal protein BRD4. The BRD4 degradation in combination with intracellular acid activation of photodynamic therapy of Pheophorbide A synergistically induced notable cell apoptosis in vitro and in vivo.
Conventional drug delivery systems are typically prepared by loading drugs into amphiphilic polymers to form nanoparticles. However, inherent limitations such as low drug loading contents, premature leakage, and tedious preparation and purification compromise their feasibility for utilization [13,373]. Covalent conjugation of hydrophobic drugs into polyprodrug amphiphilic copolymers that assemble into nanoparticles is an effective approach to achieve ultra-high drug loading capacity while reducing drug leakage and adverse effects. Additionally, the stimuli-responsive nature of polyprodrug nanoparticles allows for controlled drug release, which can be achieved through structural dissipation and site-selective activation in response to specific stimuli. For instance, a tailor-made dual pH-sensitive polymer-drug conjugate nanoparticle system has been explored to overcome the limited drug loading capacity in conventional delivery systems and enhance intracellular drug concentration (Fig. 27a) [371]. DOX, a DNA topoisomerase II inhibitor, conjugated on biocompatible polymers showed significant differences in release profiles at different pH values, where the release profile at pH 5.0 was observed 3-fold higher than that at pH 6.8 and 7.4. The degradation of acid-labile hydrazone bonds between DOX molecules and polymer in acidic condition triggered the structural destabilization of nanoparticles and subsequent drug release. Along with the pH-dependent charge-conversional capability, in vitro studies showed the enhanced drug accumulation at pH 6.8 compared with pH 7.4. While polyprodrug amphiphiles are generally formed via post-polymerization modification or copolymerization of drug-modified monomers and hydrophilic monomers, nano-assemblies could also form in situ during the formation of triggered-cleavable bonds between drug molecules and polymers with an ultrahigh drug loading and stimuli-responsive release property. Recently, our group developed reversible click reaction between boronic acids of drugs and salicylhydroxamates of polymers for facile preparation of polymer-drug conjugates with ultrahigh drug loading (25 – 50 wt.%), which triggered in situ formation of nano-assemblies that can liberate drugs in response to various stimuli (Fig. 27b) [372]. Hydrophilic copolymer of salicylhyroxymate and PEG turned into amphiphilic copolymers upon ultrafast and reversible click reaction with bortezomib (BTZ), resulting in reaction-induced self-assemblies. Due to the dynamic covalent feature, the weaker interaction between BTZ and salicylhyroxymate of polymers at acidic condition endowed the in-situ nano-assemblies to release BTZ in a traceless fashion. We expanded the applicability from BTZ other chemo-drugs linked with aryl boronic acids through a self-immolative spacer. Clicked drugs with responsive linkers can be designed to release from the nano-assemblies in redox milieu. In vitro studies showed that the polymer–drug nano-assemblies exhibited competitive therapeutic efficacy compared with free drugs.
Fig. 27.
(a) Chemical structure of the dual pH-responsive polymer–doxorubicin (DOX) conjugate (PPC-Hyd-DOX-DA) and pathway of cancer treatment (top panel), and drug release profile and confocal laser scattering microscopy images (bottom panel) [371], Copyright 2011. Adapted with permission from the American Chemical Society. (b) Chemical structure of polymer and drug for in situ formation of nano-assemblies [372], Copyright 2020. Adapted with permission from John Wiley & Sons Inc.
In addition to pH-responsive drug delivery system, elevated redox (i.e., GSH and ROS) level of cancerous environment could cause structural alteration within stimuli-responsive systems, leading to controlled drug release with minimal leakage in the physiological condition. For example, polyprodrug amphiphilic polymersomes composed of PEO and hydrophobic polyprodrug of indomethacin (IND), a type of nonsteroidal anti-inflammatory drugs, with GSH-responsive disulfide bonds and H2O2-reactive phenylboronic ester linkages can help relieve inflammatory reactions while mitigating the side effects including gastrointestinal damage and cardiovascular toxicity (Fig. 28a) [374]. Polyprodrug amphiphiles with inherently high IND loading contents (> 33 wt.%) showed efficient drug release followed by self-immolation of the linkers and amphiphilic-to-hydrophilic transformation in potent redox milieu, whereas negligible IND release (< 10%) was observed in the presence of GSH and H2O2. Furthermore, redox-responsive polymersomes were shown to effectively reduce the inflammatory reactions induced by lipopolysaccharide in RAW264.7 cells. With similar strategy, ROS-responsive polyprodrug nanoparticles (PNs) with cancer cells and mitochondria dual-targeting capability, or DT-PNs, demonstrated highly potent cancer cell apoptosis due to the upregulated ROS production and self-amplified drug release in mitochondria (Fig. 28b) [201]. DT-PNs composed of amphiphilic polyprodrugs, cancer cell-targeting cRGD-PDMA-b-PCPTSM and mitochondria-targeting TPP-PDMA-b-PCPTSM, showed cascade active targeting to cancer cells through the recognition of αvβ3, a cell surface biomarker of tumor angiogenesis, by cRGD and the interaction between triphenylphosphonium (TPP) and negatively charged phospholipid bilayer of mitochondria. Cleavage of thioketal linkages in response to overexpressed ROS initiated self-immolation triggered structural disintegration and release CPT, a DNA topoisomerase I inhibitor, in mitochondria, upregulating the endogenous mitochondrial ROS that subsequently self-amplifies the CPT release and causes long-term high oxidative stress to induce enhanced cancer cell apoptosis. In the in vivo studies, DT-PNs showed complete tumor eradication while PNs with single targeting moiety or CPT alone revealed limited tumor inhibition.
Fig. 28.
(a) The self-assembly of anti-inflammatory polymersomes of redox-responsive polyprodrug amphiphiles with variations of triggered cleavable linkers. The redox-sensitive disintegration of polymersome and subsequent drug release [374], Copyright 2018. Adapted with permission from Elsevier Science Ltd. (b) Dual-targeting polyprodurg nanoreactors (DT-PNs) for self-amplified drug release with ROS burst in mitochondria [201], Copyright 2019. Adapted with permission from the Nature Publishing Group.
Controlling the structural alterations at the desired sites with spatiotemporal precision allows the drug delivery system to release payloads on demand. For instance, photo-cleavable pendants installed on the sidechain as hydrophobic segments of amphiphilic polymers have been used in controlled drug delivery [175]. Amphiphilic copolymers that spontaneously encapsulate drugs and self-assemble into nanoparticles could undergo amphiphilic-to-hydrophilic transition upon light irradiation, activating drug release upon triggering. Though the limited light penetration depth hampers the applicability of light-responsive delivery, the incorporation of upconversion nanoparticle [184] or utilization of photo-cleavable moieties [177] with responsiveness at wavelength of visible or NIR regions can ameliorate the concern and widen the feasibility. For example, the co-assembly of upconversion nanoparticles (UCNPs), DOX, and UV-light-responsive amphiphilic block copolymer (poly(ethylene glycol)-b-poly(2-nitrobenzylalcohol acrylate)-co-poly(2-nitrobenzylalcohol-amino-N-(2-(2-acryloxyethoxy)ethyl) naphthalimide acrylate (PEG-b-P(NBA-co-NBANA)) into NIR-light-activated ratiometric fluorescent hybrid micelles (RFHM) demonstrated controlled drug delivery and fluorescence alteration for spatiotemporally chemotherapy and bio-imaging (Fig. 29a) [375]. Upon 980 nm laser irradiation, UCNPs in RFHM could convert NIR laser into UV light and induce the cleavage of nitrobenzyl groups, which triggered structural dissipation due to hydrophobic-to-hydrophilic transition and ratiometric alteration of emission from blue to green attributed to internal charge transfer of naphthalimide. The disruption of RFHM, followed by light irradiation for 16 min, induced the drug release with cumulative efficiency of 82% within 30 h whereas negligible leakage (10%) without light irradiation was observed. The spatiotemporal chemotherapy and ratiometric fluorescence transition were further validated in vitro and in vivo. In addition to incorporation into nano-assemblies, installing photo-cleavable groups, that are sensitive to red light or NIR, into polymer sidechain can mitigate the concern regarding the shallow penetration depth of UV irradiation. For example, red-light responsive Ru-containing block copolymer PEG-b-P(CPH-co-RuCHL) was explored for combinational photoactivated chemotherapy and conventional chemotherapy in hypoxic tumors. Amphiphilic polyprodrugs with high content of chlorambucil (CHL)–Ru complex conjugate [Ru(CHLtpy)(biq)(H2O)]2+ ≈ 45 wt. %) could self-assemble into micelles with stable colloidal properties and dormant cytotoxicity. Upon light activation at 650 – 680 nm, cleavage of CHL-Ru complex from polymer backbone resulted in particle degradation and recovery of cytotoxicity in hypoxic cancer cells. In the cellular and animal studies, PEG-b-P(CPH-co-RuCHL) with light irradiation showed enhanced cytotoxicity and significant inhibition of hypoxic tumor growth, which could be attributed to the combinational effect of CHL, a traditional DNA alkylation agent, and Ru complex, a DNA crosslinker, that dramatically induce cell damage (Fig. 29b) [376]. Incorporation of UCNPs or installing photocleavable groups in the photo-responsive drug delivery has shown the deep penetration in vivo for practical applications. However, lack of signal for initiating light-triggering events and timely feedback regulation has proven to be the major hurdle for the development of photo-responsive drug delivery systems. To address the challenge, a few previous findings that leveraged light-on imaging and self-feedback fluorescence alteration showed promise for the precise manipulation of drug release and biological activity in a spatiotemporal precision. For example, amphiphilic block copolymer P(Cy-N-CPT) micelles consisted of hydrophobic, protonatable poly(2-(diisopropylamino)ethyl methacrylate) (PDPA) and NIR-cleavable camptothecin-cyanine conjugates (Cy-N-CPT) showed the on-demand NIR-triggered drug release upon light-on fluorescence signal in acidic tumor, and fine manipulation of photolytic drug release by monitoring dual-channel fluorescence in the hypoxic tumors (Fig. 29c) [377]. The P(Cy-N-CPT) micelle showed drastic particle dissociation due to hydrophobic-to-hydrophilic transition of PDPA with narrow responsive window (ΔpH = 0.3) in acidic conditions, leading to turn-on fluorescence at 810 nm from Cy-N-CPT as second fluorescence channel that enables real-time tracking feature in acidic endocytic organelles of cancer cells. The light-on fluorescence guided the NIR-triggering administration for uncaging chemotherapeutic CPT on demand. Upon NIR irradiation (670 nm), dramatic decrease in fluorescence intensity at 810 nm followed by increased emission at 535 nm was observed, indicating the cleavage of CPT and uncaged cyanine with emission at 535 nm served as second fluorescence channel. The dual-channel fluorescence allowed for real-time tracking of nanoparticles and feedback regulation of photo-triggered drug release in the hypoxic in vivo study. Hierarchical nanostructures have been reported to show the altered interactions between nanoparticles and bio-interfaces, controlling the biological activity from the fate of biodistribution in vivo to cellular interaction [359,379,380]. For instance, hierarchical self-assembled photo-responsive tubisomes showed good biocompatibility, high drug loading content, and rapid drug release upon UV irradiation (Fig. 29d) [378]. Light-responsive amphiphilic block copolymer of hydrophilic pPEGA and hydrophobic, photo-responsive pNBMA linked by cyclic peptide could self-assemble into hierarchical cylindrical micelles, or tubisomes, driven by hydrophobic interactions of pNBMA and hydrogen bond of cyclic peptide. Upon light irradiation, hydrophobic-to-hydrophilic transition of pNBMA triggered complete disruption of tubisomes and subsequent burst drug release. In vitro studies showed the enhanced cytotoxicity due to the drug release in response to photo-triggered disassembly, demonstrating the potent chemotherapy in a spatiotemporal control. Though the recent advances in two-photon irradiation, photon upconversion, and photo-cleavable groups with longer wavelengths, the depth of light penetration is still limited to a range of millimeter. Thus, photoresponsive drug delivery systems would be feasible for cancers on or under the skin, or in the lining of organs.
Fig. 29.
(a) The production of RFHM containing DOX and UCNP via a nanoprecipitation method, and photo-triggered disruption of particle and ratiometric fluorescence (top panel). NIR-induced concurrent chemotherapy and ratiometric fluorescence imaging in intracellular milieu (bottom panel) [375], Copyright 2020. Adapted with permission from John Wiley & Sons Inc. (b) Chemical structure of the PEG-b-P(CPH-co-RuCHL) and its cleaved forms including drug–Ru complex conjugate [Ru(CHLtpy)(biq)(H2O)]2+. Illustration of light administration and the implication of PEG-b-P(CPH-co-RuCHL) in hypoxic tumor environment [376], Copyright 2018. Adapted with permission from John Wiley & Sons Inc. (c) Schematic illustration of P(Cy-N-CPT) micelle and dual-modal PA and dual-channel fluorescence output in response to NIR (top panel). Schematic illustration of light-responsive nanoparticles for real-time tracking and feedback regulation of photo-triggered drug release in the hypoxic in vivo [377], Copyright 2021. Adapted with permission from John Wiley & Sons Inc. (d) Chemical structure of pNBMA25-CP-pPEGA27 (top panel) and an illustration of cellular uptake of DOX-loaded tubisomes, and photo-triggered structural disruption and controlled release (bottom panel) [378], Copyright 2020. Adapted with permission from John Wiley & Sons Inc.
7.1.2. Drug delivery via depolymerization
Stimuli-triggered depolymerization, which forms small-molecule fragments or oligomers upon stimulation, has been utilized to disrupt nanoparticles in response to surrounding stimuli. The trigger-cleavable moiety at the terminal cap of hydrophobic segments can induce a head-to-tail cascade depolymerization of the polymer main chain in response to various types of stimuli via chain unzipping [37]. Self-immolative amphiphilic block copolymer nanoparticles could undergo cascade depolymerization to induce structural dissipation and subsequent drug release. For example, amphiphilic self-immolative polyprodrug (SIP-DOX) containing hydrophilic PEG and hydrophobic, self-immolative backbone with DOX at the side chain could self-assemble into micelles and spontaneously encapsulate the tumor specificity ROS inducer NAD(P)H: quinone oxidoreductase-1 (NQO1)-responsive hemicyanine fluorescent dye (NcyNH2) (Fig. 30a) [381]. The cleavage of terminal phenylboronic ester upon H2O2 that induced self-immolative degradation reactions through elimination triggered depolymerization and cleavage of DOX, resulting in the disruption of the nanoparticles and release of NQO1-responsive NcyNH2. The light-on fluorescence of restoring CyNH2 was utilized to monitor drug activation process in vitro and in vivo. Moreover, CyNH2 could generate ROS to elevate the endogenous ROS, which self-amplifies the ROS-induced particle degradation and enhances the antitumor efficiency with high specificity. The efficiency of depolymerization of self-immolative polymers is heavily dependent on the sensitivity of single triggerable moiety to stimulus, possibly leading to insufficient depolarization. The polymer with multiple triggerable moieties incorporated in the backbone should degrade into small fragments more efficiently upon exposure to stimuli, allowing for multi-site chain scission and faster particle disruption [382]. For example, our group reported that vinylogous polymers based on α-substituted cinnamates that are susceptible to depolymerization via nucleophile-induced chain scission (NICS) and chain unzipping [222]. The NICS pathway was dependent on the concentration of stimulus, whereas the chain unzipping showed weak dependence on stimulus concentration. The kinetic study of depolymerization via NICS was found faster than that by chain unzipping. Depolymerization by multi-site chain scission was introduced into the polymer-drug conjugates or polyprodrugs to achieve ultrahigh drug content and on-demand drug release. For example, chain-shattering polymeric nanotherapeutics containing polymers from condensation polymerization of chemotherapeutics and light-triggerable self-immolative spacers showed the light-induced particle disruption and burst release of >90% drug release efficiency in 2 min in a spatiotemporal precision [383]. The in vitro study revealed significant enhancement in cytotoxicity of chain-shattering polymeric nanotherapeutics upon exposure to UV irradiation. Amphiphilic copolymer with multi-cleavable domains on hydrophobic backbone can self-assemble into nanoparticles, while the particle formation of self-immolative homopolymer is typically formulated with polymer additives. For example, amphiphilic polyprodrugs PEG-P(MTO-ss-CUR) nanoparticles containing predefine ratios of anticancer drugs mitoxantrone (MTO) and curcumin (CUR) showed the predefined ratios of drug release for synergistic effect on cancer chemotherapy (Fig. 30b) [384]. In response to elevated level of GSH in cancer cells, the cleavage of multiple disulfide spacers between MTO and CUR that underwent self-immolation via cyclization gave rise to depolymerization by chain scission, therefore inducing particle disruption and facile drug release. Due to flexible feed ratio of MTO and CUR in polymerization, optimal synergistic effect on combination chemotherapy was observed in drug-resistant MCF-7/ADR cells and animal study.
Fig. 30.
(a) Chemical structure of SIP-DOX and its nano-assembly, and the self-immolation of SIP-DOX that induced disassembly and drug release (top panel). Schematic illustration of self-amplified ROS-responsive drug release nanosystem (SIP-DOX) for cancer therapy (bottom panel) [381] Copyright 2022. Adapted with permission from Elsevier Science Ltd. (b) Illustration of amphiphilic polyprodrugs PEG-P(MTO-ss-CUR) nanoparticles with a predefined ratio of MTO and CUR, and disassembly of nanoparticle along with drug release in response GSH [384], Copyright 2021. Adapted with permission from the American Chemical Society.
7.1.3. Drug delivery via decrosslinking
Crosslinking can greatly enhance the stability of nanoparticles and prevent premature leakage of the hydrophobic cargo from the core, whereas non-crosslinked counterparts often suffer from compromised structural integrity, especially in ultra-diluted physiological environments [385,386]. Post-assembly crosslinking has been widely implemented to stabilize nanoparticles while also endowing them with stimuli-responsiveness for controlled drug release. For example, biodegradable polyphosphoester-based PEBP-b-PBYP-g-PEG could ameliorate the solubility issue of potent chemotherapeutics, paclitaxel (PTX), and exhibit higher aqueous suspension of P X at concentrations up to 4.8 mg/mL in comparison with <2.0 μg/mL of drug alone (Fig. 31a) [387]. With shell-crosslinking via radical-mediated thiol–yne reactions, the shell cross-linked knedel-like nanoparticles exhibited sustained PTX release with 2-fold half-life than that of non-crosslinked micelles. In the animal study, the shell cross-linked knedel-like nanoparticles could prolong the pharmacokinetics with lung extravasation t1/2 of ca. 8 d, whereas the non-crosslinked counterparts showed a t1/2 of ca. 4 d and small-molecule dyes exhibited a t1/2 of 4 h. In addition to shell crosslinking, the core crosslinking followed by formation of nanoparticle can also enhance the structural integrity as well as stability of encapsulated payloads. Installing the trigger-cleavable crosslinkers enables the crosslinked nanoparticle to show stimuli-responsive particle disruption and on-demand payload release. Our group developed PPFPA-r-PPEGMA amphiphilic copolymer containing hydrophilic polyethylene glycol methacrylate (PEGMA) and crosslinkable pentafluorophenyl acrylate (PFPA) that could form surface-modified, crosslinked nanogel by the amination of pentafluorophenyl (PFP)-containing micelles and diamine cross-linkers, followed by surface decoration with molecules of interest [388]. By crosslinking with disulfide-containing crosslinkers, nanogel could undergo particle disruption and payload release via decrosslinking in response to reducing agents such as DTT or GSH. Other than redox stimuli, pH-responsive crosslinked nano-assemblies with acetal- and ketal-based linkers were developed for controlled decrosslinking and payload release [290]. The substituent variation of acetal- and ketal-based crosslinkers was shown to significantly affect the kinetics of pH-induced degradation with difference than 6 orders of magnitude, allowing for fine-tuned kinetics of payload release from crosslinked nanogels in response to pH responsiveness. Photo-responsive decrosslinking that leads to dissociation of nano-assemblies allows for spatiotemporal drug release in a remote control. For example, indocyanine green, a photothermal agent, loaded star polymers linked by thermally labile azo crosslinkers could de-crosslink into polymer precursor in response to photothermal effect upon NIR irradiation [389]. The facile decrosslinking upon NIR irradiation induced the burst release of model dyes ≈ 43%) in an hour whereas the negligible release (< 5%) was observed without NIR treatment. Except for the formation of crosslinked nanoparticles via post-assembly by crosslinkers, in situ formation of crosslinked nanoparticles via formation of disulfide was explored for controlled payload release. For example, our group developed surface-functionalizable self-crosslinked nanogels loaded with hydrophobic chemotherapeutics that showed reduction-responsive drug release capability [311,390]. Amphiphilic copolymers P(PDSMA-co-PEGMA) containing poly(ethylene glycol) methyl ether methacrylate (PEGMA) and pyridyl disulfide methacrylate (PDSMA) that could self-assemble and encapsulate hydrophobic drugs simultaneously formed reduction-responsive crosslinked nanogel via formation of disulfide bonds in situ between pyridyldisulfides upon DTT reduction, while the remaining pyridyldisulfide groups were further modified for the decoration of functional groups. The versatile amphiphilic copolymers containing PSPMA not only allowed for a facile preparation of reduction-responsive drug-loaded nanogels but also the flexible surface modification such as small molecules, peptides, or proteins [391,392]. The platform was utilized to develop an anti-CD4 antibody conjugated mertansine-loaded nanogels, or antibody-nanoparticle conjugates (ANCs), for targeted chemotherapy of primary CD4+ T cells and a CD4high T cell lymphoma (Fig. 31b) [393]. The mertansine drugs were conjugated on P(PDSMA-co-PEGMA) via disulfide exchange, which could form nanogel by self-crosslinking upon DTT treatment followed by surface modification of anti-CD4 antibody by thiol exchange. ANCs not only showed enhanced uptake efficiency in CD4+ T cells among CD4+, CD8+, and CD45R+ cells but also decreased the non-specific uptake in CD4− lymphocytes that was found in nanogels without antibody. ANCs showed five times more potent cytotoxicity against CD4high mT-ALL cells than CD4low mT-ALL cells, while the nanogels alone revealed insignificant differences in cytotoxicity of CD4high mT-ALL cells and CD4low mT-ALL cells. Moreover, ANGs also benefited from intrinsically high drug-to-antibody ratio, showing compelling cytotoxicity compared with relative ADC whose drug-to-antibody ratio was calculated to be 3.
Fig. 31.
(a) Biodegradable polyphosphoester-based PEBP-b-PBYP-g-PEG self-assembly for cancer chemotherapy [387], Copyright 2015. Adapted with permission from the American Chemical Society. (b) Components of ANCs and the selective therapy against CD4high mT-ALL cells [393], Copyright 2020. Adapted with permission from the American Chemical Society.
7.2. Biologics delivery
Biologics, such as genes and proteins, have emerged as a novel therapeutic strategy and are seen as alternatives to small molecules for several diseases due to their target specificity. This is witnessed by the increasing number of biologics license applications approved by the FDA’s Center for Drug Evaluation and Research in the past decade [394,395]. High specificity of biologics not only mitigates potential adverse effects of small-molecule drugs but also enables targeting undruggable, tumorigenic proteins due to shallow pockets that are difficult for small molecule binding. For example, small-interfering RNA (siRNA) therapeutics, an example of effective RNA interference (RNAi), silences disease-related genes and down-regulates expression of associated proteins [396,397], while messenger RNA (mRNA) therapeutics guides cells to generate proteins of interest [398]. Moreover, therapeutic proteins that can specifically block the proteins of target and interference protein-protein interaction hold great promise to treat extracellular and intracellular episodes. Despite the unprecedented opportunities, biological barriers upon systemic administration hamper the efficacy and feasibility of biologics for cancer therapy. Exogenous biologics are vulnerable to enzymatic degradation, leading to rapid clearance from the body and short blood circulation time. The intrinsic hydrophilicity and charged property of biologics result in low permeability through the cell membrane, which limits their capability for biomedical applications. Compartmentalization of therapeutic biologics side polymeric nanoparticles, that alters the interaction of biologics and bio-interface, can greatly enhance the efficacy for systemic cancer therapy [399–402].
Ultra-pH-sensitive polymers that showed sharp micelle-to-unimer transition at acidic environment have been exploited for controlled biologics delivery for cancer therapy. For example, envelope-type nanoplatform composed of MeO-PEG-b-P(DPA-co-GMA-Rn) and ACUPA-PEG-b-PDPA can actively target prostate cancer cells to silence the expression of prohibitin 1 and inhibit tumor growth in vivo (Fig. 32a) [403]. The chain length of oligoarginine grafts randomly dispersed in pH-sensitive hydrophobic poly(2-(diisopropylamino)ethyl methacrylate) (PDPA) segment was found to affect the resulting size of nanoparticles and siRNA encapsulation efficiency, where the increased size and decreased encapsulation efficiency was observed with the increasing of chain length with oligoarginine grafts. The modification of S,S-2-[3-[5-amino-1-carboxypentyl]ureido]pentanedioic acid (ACUPA) ligand enabled the envelope-type nanoplatform to actively bind to prostate specific membrane antigen overexpressed in prostate cancer, enhancing the cellular uptake efficiency and specificity. Upon cellular internalization, the protonation of the sharp pH-responsive PDPA segment in the endosome triggered the particle disassembly due to amphiphilic-to-hydrophilic transformation and exposure of oligoarginine grafts, synergistically facilitating endosomal escape via proton sponge and membrane penetration. The encapsulated siRNA molecules were further liberated to initiate RNA interference of prohibitin 1 for the inhibition of tumor growth. Cytosolic delivery of cyclic dinucleotide (CDN) agonists of stimulator of interferon genes (STING) has recently emerged as a promising class of cancer immunotherapy. Polymer vehicles can shield CDN agonists to circumvent biological barriers such as rapid clearance, and inefficiency penetration through cell membrane, while showing effective delivery and subsequent CDNs release into cytoplasm for the induction of type I interferon (IFN-I)-driven inflammatory program to rejuvenate suppressed immune response [404–407]. STING-activating nanoparticles (STING-NPs) was designed for cytosolic delivery of the endogenous CDN ligand for S ING, 2′3′-cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) for stimulating immunosuppressive tumors to immunogenic (Fig. 32b) [408]. In the design of PEG-b-[DEAEMA-co-BMA], hydrophilic PEG provided STING-NPs with enhanced biocompatibility and colloidal stability for prolong blood circulation while the integration of pH-sensitive 2-(diethylamino) ethyl methacrylate (DEAEMA) and hydrophobic butyl methacrylate (BMA) moieties, and crosslinkable pyridyl disulfide ethyl methacrylate was developed for optimal efficiency of endosomal escape and in-situ crosslinking purposes, respectively. cGAMP is sequestered in the hydrophilic core of polymersome with pH-destabilizing and disulfide-crosslinked bilayer, altering the interaction between bio-interface and cGAMP to enhance the efficiency of cytosolic delivery. Upon endosomal entrapment, protonation of PDEAEMA in response to endolysosomal acidification led to disassembly of polymersome to promote endosomal escape and to release cGAMP for the induction of signaling pathway of IFN-I. STING-NPs stimulated IFN-I-driven innate immune, turning tumor microenvironment from noninflamed cold into T cell-inflamed hot. In combination with immune checkpoint blockade therapy, STING-NPs that can improve immune responses to immune checkpoint blockade in a poorly immunogenic murine melanoma mode significantly inhibited the tumor growth. Melittin, a primary oligopeptide component of bee venom, has demonstrated a wide spectrum of anti-cancer activity due to membrane-lysis feature [409]. However, the practical application in cancer therapy is limited due to non-specific disruption of cell membrane that could cause lethal hemolytic activity and vulnerable to enzymatic degradation [410]. Recent advances in peptide delivery have shown great promise to mitigate the adverse effects and enhance therapeutic efficacy for safe, systemic administration [411]. For example, D-melittin conjugated on ultra pH-sensitive block of amphiphilic polymer that could self-assemble into D-melittin micelles at physiological condition was buried in the hydrophobic core, reducing non-specific hemolysis, and preventing from enzymatic degradation [412]. Abrupt dissociation of D-melittin micelles in acidic endosome restored the membrane-lysis of D-melittin and facilitated the endosomal escape, subsequently inducing immunogenic cell death as witnessed by primary hallmarks including calreticulin surface expression, and ATP and HMGB1 release. D-melittin micelles were found to effectively inhibit the growth of CT26 (colon cancer) and 4T1 (breast cancer) tumors while maintaining minimal side effects. Moreover, in combination with immune checkpoint blockade therapy, D-melittin micelles showed synergistic effect to completely eradicate CT26 tumor.
Fig. 32.
(a) Chemical composition of ultra-pH-sensitive nanoplatform for pH-responsive siRNA delivery (top panel) and the schematic illustration of ultra-pH-sensitive nanoplatform for RNAi in vitro and in vivo [403], Copyright 2017. Adapted with permission from the American Chemical Society. (b) Chemical composition of the STING-NP and formulation strategy for enhanced cytosolic delivery of cGAMP (left panel), and schematic illustration of STING-NP that stimulates immunosuppressive tumors to immunogenic (right panel) [408], Copyright 2019. Adapted with permission from the Nature Publishing Group.
Macromolecules capable of depolymerization can stabilize biologics and form nanoparticles that enhance bioactivity. Furthermore, the release of biologics can be enhanced in response to stimuli, which can recover therapeutic activity at the targeted sites. For example, ROS-responsive plasmid DNA (pDNA) delivery polyplexes that could undergo polymerization in response to H2O2 was explored to deliver tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL)-encoding plasmid (pTRAIL) for a safe and efficacious cancer treatment (Fig. 33a) [267]. The ROS-degradable polysulfoniums containing phenylboronic ester could effectively encapsulate pDNA via electrostatic interaction, while the cleavage of phenylboronic ester triggered depolymerization via multi-site scission to unpack the pDNA and facilitate the transcription upon exposure to ROS in cancer cells. Furthermore, charged polysulfoniums upon degradation led to uncharged thioether fragments, enhancing the biosafety and release efficiency without interference of gene transcription. The bioluminescence from the delivery of luciferase-encoding plasmid was used to evaluate in vivo transfection efficiency, where ROS-degradable polysulfoniums showed prolong and enhanced bioluminescence than gene delivery mediated by conventional polyethyleneimine. The polysulfoniums polyplexes containing pTRAIL showed efficacious inhibition of tumor growth as witnessed by elevated TRAIL expression from western blot analysis, and apoptotic nuclear condensation and shrinkage from Hematoxylin and eosin staining. Apart from gene therapy alone, co-delivery gene therapeutics and anticancer drugs can achieve combinational therapy or synergistic therapy. For example, self-immolative nanoparticles that simultaneously delivered miR-34a mimics and polyamine analog N1,N11-bisethylnorspermine (BENSpm) showed enhanced cell killing capability as well as inhibition of tumor growth for combination cancer therapy (Fig. 33b) [413]. While BENSpm-based biodegradable polycation containing repeating, self-immolative disulfide bonds on the backbone could condense miR-34a to form polyplexes via electrostatic interactions and facilitate the transfection efficiency, the depolymerization via multi-site scission by cyclization of cleaved sulfide in response to elevated level of GSH in cancer cells induced the particle degradation and subsequent BENSpm and miR-34a release. The release of BENSpm, a type of polyamine analogues, was able to upregulate the rate-limiting enzymes in polyamine catabolism, including spermine/spermidine N-acetyltransferase and spermine oxidase, to downregulate the expression of natural polyamines, resulting in inhibition of cancer cell growth and apoptosis. Depolymerization of BENSpm-based biodegradable polycation that decreased the multivalent effect on electrostatic interactions to unpack miR-34a led to the downregulation of Bcl-2 and antiapoptotic pathway to enhance the cell killing capability together with BENSpm.
Fig. 33.
(a) ROS-responsive degradable polysulfoniums and the polyplex for intraperitoneal gene delivery [267], Copyright 2017. Adapted with permission from John Wiley & Sons Inc. (b) GSH-responsive BENSpm-based biodegradable polycation /miR-34a nanoparticles for dual drug delivery via triggered depolymerization [413], Copyright 2016. Adapted with permission Elsevier Science Ltd.
Inter-polymer crosslinking of biologics-loaded particles can effectively enhance the structural integrity and protect biologics from degradation and facile clearance. For example, our group leveraged bait-and-switch supramolecular strategy to develop charge-free RNA delivery system (Fig. 34a) [315]. Double-strain DNA (dsDNA) was first trapped inside the polymer-dsDNA complexes via electrostatic interaction between dsDNA and methylated pyridyl disulfide of cationic copolymer, followed by concurrent disulfide crosslinking and charge removals. The charge-free RNA–polymer complexes showed efficient RNAi of Tuba1a mRNA in 4/8-cell embryos and morula embryos while minimal material cytotoxicity compared to classical cationic delivery systems. In addition to concomitant crosslinking and decationization, the formation of polyplexes with subsequent disulfide crosslinking and decationization by acid-cleavable linker was explored to develop decationized polyplexes [414]. The decationized polyplexes demonstrated the competitive RNAi with reduced toxicity in vitro and in vivo. For effective cytosolic protein delivery, our lab recently developed the templated self-assembly of a covalent polymer network for protein encapsulation and traceless release inside cells (Fig. 34b) [307]. The p-nitrophenylcarbonate moieties on the polymer could react with multiple lysine moieties on protein as a template followed diamine crosslinkers, giving rise to crosslinked protein nanoassemblies surrounded by PEG corona. The cleavage of disulfide bonds embedded in crosslinking network induced self-immolation via cyclization to disrupt the crosslinked particles and release encapsulated proteins in a traceless fashion. Encapsulation of protein inside crosslinked polymers not only effectively prevented proteolytic degradation by protease but also controlled the protein activity. Upon GSH-induced decrosslinking, traceless release of proteins regained biological activity. In vitro study revealed efficient cytosolic protein delivery and dose-dependent therapeutic efficacy.
Fig. 34.
(a) Formation of the noncationic RNA–polymer complex [315], Copyright 2019. Adapted with permission from the American Chemical Society. (b) Formation of crosslinked protein nano-assembly, and reduction-responsive traceless protein release [307], Copyright 2017. Adapted with permission from the American Chemical Society.
Although macromolecule-based drug delivery systems have been extensively investigated in the past decades, most of the current drug and biomacromolecules delivery vehicles are lipid-based nanocarriers. Among approved nanomedicines [415], Genexol®PM, known as Cynviloq™, is the one-of-a-kind polymeric micelle-based nanomedicine as the first-line cancer therapy. The nanomedicine benefits from low drug leakage due to polymeric nano-formulation, showing higher maximum tolerated dose compared with commercially available Taxol® and Abraxane®. Genexol®PM mainly consists of a low molecular weight, biocompatible and biodegradable amphiphilic block copolymer, monomethoxy poly(ethylene glycol)-block-poly(D,L-lactide) (mPEG-b-PDLLA) and chemo-drug, paclitaxel. It can rapidly release paclitaxel via diffusion and/or disassembly in physiological condition due to the degradation of low-molecular-weight PLA [416,417]. It should be noted that while an overwhelming number of stimuli-responsive polymeric nanomedicines have been developed to address the challenges in achieving therapeutic efficacy, to the best of our knowledge, none of pre-clinical candidates have hit the market. Pre-clinical evaluations that can better predict and translate to human patients are required to propel the development of stimuli-responsive polymeric nanomedicines [360].
7.3. Sensing
Polymeric nano-assemblies can undergo structural alterations in response to various physical, chemical, or biological changes in a specific environment, making them excellent platforms for detection and sensing [418]. The on-and-off switch or changes in colors corresponding to structural alterations has been leveraged as indicators for sensing. For example, CO2/pH-responsive crosslinked nanoprobes that showed reversible ON/OFF fluorescence emission could be used as a CO2 sensor (Fig. 35a) [419]. The CO2/pH-responsive nanogel was composed of pH-responsive poly(N,N-diethylaminoethyl methacrylate) (PDEAEMA), environmentally dependent fluorogenic aminobromomaleimides, oligoethylene glycol methacrylate (OEGMA), and ethylene glycol dimethacrylate (EGDMA) crosslinker. The CO2/pH-responsive swelling/shrinking alteration of nanogels enabled environmentally dependent aminobromomaleimides inside the nanogel light on at high pH value or under nitrogen, whereas the loss of fluorescence was observed in the nanogels with treatment of CO2. Protonation of PDEAEMA at acidic conditions or in CO2 triggered the swelling of nanogels and exposed aminobromomaleimides to the protic solvent, leading to the fluorescent quenching possibly due to the formation of an extended hydrogen-bonding network that altered the electronic structure [420]. Due to the reversibly facile swelling/shrinking alteration, ON/OFF cycles of fluorescence were observed with successive CO2/N2 purges. Aside from on-to-off transition, multi-color transition of nanoprobe can enable to detect the analysts of interest in real-time. For instance, amphiphilic random copolymer consisted of hydrophilic NIPAM and bifluorophoric tetraphenylethylene–spiropyran was developed to sense cyanide CN−), a lethal toxic anion, under various conditions such as light irradiation, changes in pH, and temperature (Fig. 35b) [421]. UV irradiation facilitated the photo-isomerization of spiropyrans (SP) to merocyanines (MC), leading to the emission of MC at 627 nm upon excitation of TPE at 365 nm via FRET process. The sensor polymer showed low detection limits and high selectivity for cyanide ions in aqueous media based on the ratiometric fluorescence between MC and TPE AIEgens. Among different analytes, presence of CN− led to the lowest I627/I517 as the CN− ions could react with the MC moiety in the open form of the sensor polymer and reducing the MC emission, which led to the recovery of emission from TPE. Fluorescence-based pH nano-sensor was developed for probing intracellular pH on a basis of amphiphilic copolymer containing hydrophobic pH sensitive TPE -oxazolidine and hydrophilic PEG blocks [422]. Decrease in pH in the acidic organelle upon cell internalization induced the ring opening of dyes of hydrophobic segment and changes in electronic structure, resulting in a red-shift emission. The reversibility of the emission response was validated when shift of emission from red to cyan was observed in the presence of lysosomal activity inhibitors chloroquine and bafilomycin.
Fig. 35.
(a) Chemical structure of monomers in CO2/pH-responsive crosslinked nanoprobes (top panel), and CO2/pH-responsive swelling/shrinking alteration of nanogels with reversible ON/OFF fluorescence emission [419], Copyright 2016. Adapted with permission from the Royal Society of Chemistry. (b) Synthetic route to amphiphilic random copolymer comprising hydrophilic NIPAM and bifluorophoric PE –spiropyran top panel), and color transition along with alterations of chemical structure, under various conditions such as light irradiation, changes in pH, temperature, and cyanide binding [421], Copyright 2020. Adapted with permission from the American Chemical Society.
In addition to the detection and sensing of small molecules, the development of protein sensors is of great interest for disease-related aberrant protein expression and diagnosis. Our group developed the “lock and key” strategy for protein sensing and quantification by ratiometric excimer–monomer fluorescence (Fig.36a) [164,423]. The ratiometric approach circumvented the environmental interference of fluorescence while excimer–monomer transition controlled by supramolecular dissociation in response to protein binding exhibited the high selectivity and quantitative detection toward the protein of interest. The selective recognition of phenyl sulfonamide ligand and bovine carbonic anhydrase (bCA), which induced protein binding-induced imbalance between unimer and aggregate of macromolecules, was found dependent on protein concentration. The coumarin installed in hydrophobic region showed strong excimer fluorescence in a constrained hydrophobic core while the monomer fluorescence was observed to be enhanced upon exposure to bCA. The ratiometric changes in excimer–monomer fluorescence along with supramolecular disassembly was shown as a promising strategy for specific protein sensing. Moreover, multichannel fluorescence nanoprobe was developed for dual protein sensing (Fig. 36b) [424]. Protein–ligand binding and enzymatic cleavage were programmed into nanoprobes composed of Cy3 fluorophore and phenylsulfonamide (a bCA binding ligand) at the surface and TPE AIEgens in the core. This nanoprobe could undergo protein-induced fluorescence enhancement (PIFE) or disassembly-induced fluorescence enhancement (DIFE) upon supramolecular disassembly in response to two specific proteins. In biomimetic environments, the addition of bCA that binds phenylsulfonamide led to the fluorescence enhancement of Cy3 due to PIFE. The addition of porcine liver esterase, that cleaved ester bond and generated decyl TPE, resulted in supramolecular disassembly, leading to the generation of AIE via the aggregation of TPE and the increase of Cy3 fluorescence due to DIFE. The fluorescence enhancement by supramolecular dissociation in response to dual proteins demonstrated their potential applications in multiple protein biomarker detection.
Fig. 36.
(a) Schematic illustration of protein binding-induced disassembly for protein sensing and quantification by ratiometric excimer–monomer fluorescence [164], Copyright 2021. Adapted with permission from the Royal Society of Chemistry. (b) Chemical structure of molecules as multichannel fluorescence nanoprobes for dual protein sensing (top panel), and mechanism of dual protein sensing via AIE, PIFE, and DIFE [424], Copyright 2021. Adapted with permission from the Royal Society of Chemistry.
7.4. Imaging
In the past decades, a number of stimuli-responsive nano-assembly systems have been developed for reliable in vitro and in vivo imaging applications. The techniques in bioimaging are playing a vital and indispensable role in detecting physiological or pathological environments for mapping the distribution of pharmaceutical reagents and designing administered therapy [425–427]. These assemblies can be functionalized with specific targeting ligands, allowing them to selectively bind to specific cells or biomolecules of interest. In response to the specific recognitions, nano-assemblies could generally change their microstructures, activate the imaging signal. Compared with non-specific imaging techniques, these methods could give enhanced contrast and specificity, allowing for more accurate and detailed visualization of biological processes. Additionally, by incorporating different types of imaging agents or modalities into these assemblies, researchers can achieve multimodal imaging. Due to the unique superiorities such as high sensitivity, excellent biocompatibility, diverse molecular design and ease in synthesis, stimuli-responsive nano-assemblies from amphiphilic macromolecules have offered excellent choices for imaging and made great progresses over the past decades [301,428].
A number of multifunctional systems based on stimuli-responsive nano-assemblies have been actively developed for real-time in vivo imaging of malignant tumors [429–431]. Compared with normal tissues, tumors usually feature a distinct bioenvironment such as low pH, hypoxia, redox imbalance or aberrant expression of certain enzymes [432,433]. These features have been adopted for the design of responsive assemblies in tumor studies based on endogenous enzyme- [434–436], pH- [133,437,438], ROS- [429,439], ultrasound- [440], and temperature-responsive platforms [441]. Upon specific activation, the triggered particle aggregation or disassembly could actuate the formation of nanostructures and offer imaging signals [442–444]. To date, nano-assemblies have been applied in several bioimaging types, including fluorescence imaging, magnetic resonance imaging, photoacoustic tomography, and computed tomography [428,445,446]. For example, an enzyme-triggered self-assembly of high ordered luminescent supramolecular assemblies was exploited to efficiently record the spatiotemporal details of alkaline phosphatase (ALP) activity in vitro and in vivo [434]. In this work, a coumarin dye (Cou) was conjugated to short peptides with a hydrophilic L-phosphotyrosine group that was sensitive to ALP (Fig. 37a). ALP in cancer cells (tissues) could cleave the L-phosphotyrosine group and enable the nanoparticles-to-nanofibers structural transition, inducing monomer–excimer transition of Cou and showing the spatiotemporal distribution details and endogenous ALP activity in tumor tissues. This study provided an efficient ALP imaging tool for cancer diagnosis. Improved imaging performance can also be realized by integrating structure transition with enzyme-triggered self-assembly of polymers to construct highly ordered supramolecular assemblies. In 2018, a MMP-2 responsive transformable chimeric peptide PpiX-PEG8-SSSPLGLAK (DOTA)-PEG6-F4 (Ppdf-Gd) has been reported in magnetic resonance imaging (MRI) (Fig. 37b) [435]. The amphiphilic Ppdf-Gd self-assembled spherical nanoparticles in a neutral condition. Once in the tumor site where MMP-2 is overexpressed, the Pro-Leu-Gly-Leu-Ala peptide sequence was recognized and selectively hydrolyzed, generating LAKDOTA(Gd)-PEG6-F4 fragments. As a result, the spherical structures were transformed to long fibers due to the π-π stacking interaction among Phe amino acids and hydrogen bond within the peptide chain. The sphere-to-fiber switch led to a higher relaxation rate of DOTA(Gd) and increased its retention time in tumor tissues, resulting in an amplified MRI signal.
Fig. 37.
Applications of stimuli-responsive nano-assemblies in imaging. (a) Upper: illustration of the molecular structure of Cou conjugating self-assembly peptides with ALP responsive unit. Lower: EISA of peptide–fluorophore conjugates yielded supramolecular nanofibers and enabled the monomer–excimer transition of Cou [434], Copyright 2021. Adapted with permission from John Wiley & Sons Inc. (b) Schematic illustration of MMP-2-triggered transformation of Ppdf-Gd from spherical nanoparticles to nanofibers and the application in photodynamic therapy [435], Copyright 2018. Adapted with permission from Elsevier Science Ltd. (c) Preparation scheme of bone-targeting self-assembly vesicles and their application in simultaneous diagnosis and treatment of malignant bone tumor [430], Copyright 2021, Adapted with permission from Elsevier Science Ltd. (d) Preparation scheme of renoprotective angiographic polymersome (RAP) and its renoprotection behavior in CT angiography [447], Copyright 2020. Adapted with permission from John Wiley & Sons Inc.
The combination of Imaging modalities and therapeutic reagents can often lead to comprehensive advantages in tumor research. For example, an amphiphilic polymeric micelle with H2O2-sensitive benzil and AIE fluorophore TPE units were recently reported [429]. The system could be used to efficiently encapsulate therapeutic agents for tumor treatment and monitor the drug release process using AIE. In detail, nano-sized micelles could form via the self-assembly of TPE-attached amphiphilic polymer TPG1 and encapsulate anti-cancer drug DOX. When reaching tumor sites, the benzil moieties within polymers could be cleaved by the high level of H2O2 via Baeyer-Villiger type reaction, leading to the decomposition of TPG1 micelles and release of DOX. Meanwhile, the degradation product emitted a strong fluorescence signal due to its AIE feature which was used for monitoring the drug release process. Remarkably, this is the first reported benzil-based H2O2-responsive micelles for synergistical therapeutic delivery and imaging applications. In another study, a bone-targeting polymer vesicle system was developed for single photon emission computed tomography/computed tomography (SPECT/CT) imaging and anticancer drug delivery [430]. The vesicles were formed via the self-assembly of PCL67-b-P[Glu6-stat-(Glu-ADA)16] block copolymers. The alendronic acid (ADA) units in polymer chains could chelate 99mTc and endow the vesicles with an excellent bone SPECT/CT imaging ability (Fig. 37c). Similar to the above-mentioned systems, DOX was encapsulated in a vesicular nanostructure during the polymer self-assembly process. Once the vesicles reached acidic tumor environment, the –COO− groups in the PGA coronas of vesicles were protonated, weakening the electrostatic interaction, and leading to the disassembly of nanostructures and the release of drugs. In the investigation of a bone tumor rabbit model, the 99mTc labeled vesicles were intravenously injected and targeted to bone tissues efficiently which were likely facilitated by the chelation of Glu and Glu-ADA in the coronas with divalent calcium ions (Ca2+). Meanwhile, the distribution of DOX drugs in vesicles could be dynamically monitored through SPECT/CT imaging. This study showed a promising multifunctional self-assembly nanoplatform for real-time SPECT/CT diagnosis in imaging-guided bone cancer therapy. Compared to conventional imaging agents and drugs, stimuli-responsive nanoassemblies hold significant promise to achieve comprehensive theragnostic effects, including targeted delivery, highly specific and accurate imaging, and multimodal imaging capabilities.
Apart from the successful application in tumor imaging, stimuli-responsive nano-assemblies have also shown great potential for bioimaging in other diseases such as renal insufficiency [447], bacterial infection [448], and ulcerative colitis [449]. Recently, a renoprotective angiographic polymersomes (RAPs) has been designed for renoprotective contrast agents in computed tomography (CT) angiography [447]. The RAPs are designed based on ROS-responsive self-assembly of poly(ethylene oxide)-block-poly(triiodobenzoic chloride-conjugated polylysine-stat-phenylboronic acid pinacol ester-conjugated polylysine) (PEO45-b-P[(Lys-IBC)45-stat-(Lys-PAPE)15]) (Fig. 37d). The Lys-BIC repeat units in polymersomes are CT imaging reagents and Lys-PAPE segments are capable of scavenging ROS to prevent contrast-induced nephropathyin kidneys. During the CT angiography, the assemblies of RAPs were disrupted by H2O2 produced by contrast agents, generating phenolate which can produce quinone methide. The quinone methide further reacted with H2O to generate hydroxybenzyl alcohol and eliminated hydroxyl radicals and superoxide. In this way, the ROS-sensitive RAPs exhibited dual renoprotective functions and significantly reduced contrast-induced nephropathy during the angiography, thus realizing an improved CT angiography result. Real time monitoring of autophagy in living objects during the therapeutic process is closely related with treatment efficiency in various mammalian diseases such as neurodegenerative disorders, infection and hypoxia [450]. In 2017, an intracellular in situ self-assembly building blocks (DPBP) for monitoring autophagy has been reported [450]. DPBP contained a specific peptide unit that was responsive to an autophagy-specific enzyme. Upon the enzymatic activation of autophagy, bis(pyrene) derivative molecules were released and self-aggregated, which emitted a 30-fold enhanced fluorescence. This intracellular enzyme-responsive self-assembly strategy could be adopted for a quantitative and real-time imaging of autophagy in living systems and the evaluation of therapeutic processes in various diseases. The responsiveness of nanoassemblies to microenvironmental cues can be tailored to match the unique characteristics of different disease states. This adaptability makes them potentially valuable tools for bioimaging beyond the specific examples mentioned above and results in enhanced imaging sensitivity and specificity, which can lead to improved diagnostic accuracy, earlier disease detection, and targeted therapeutic interventions.
Overall, recent years have seen significant and flourishing development of stimuli-responsive amphiphilic macromolecule nano-assemblies in the bioimaging of various diseases. Despite of the great advances achieved, there are still some challenges. For example, the assembly or disassembly behaviors under dynamic and complex in vivo environments still need to be explored. A comprehensive understanding of these fundamental processes would provide us with important information for designing biologically safe, stable, and disease-specific macromolecular materials with better imaging performance, biocompatibility, and biodegradability. Also, multifunctional imaging modalities in these systems are highly demanded to improve bioimaging signals and bring new methods for the theranostics of certain diseases.
8. Summary and Outlook
In this review, we have summarized different responsive strategies for the design of smart nano-assemblies from amphiphilic macromolecules, including alteration of HLB, depolymerization, decrosslinking and conformation/configuration changes. Structural determinants for assembly formation and corresponding responsiveness, e.g., macromolecular architectures, conjugation methods, responsive groups, and corresponding stimuli have been particularly discussed. Stimuli-triggered molecular level alterations could cause a series of changes in particle level, including aggregation, morphology transformations, change of permeability and even disassembly. These changes further lead to various phenomenon, such as guest-molecule release, change of rheology and mechanical properties, actuation of materials and generation of luminescence, which eventually were applied for sensing, imaging, drug delivery, tissue engineering and controllable nanoreactors. The burst development in this area has brought remarkable controllability for their applications in nanomedicine, bioengineering and materials science.
While plethora of studies have contributed to stimuli-responsiveness on controlled structural transformations, analysis of the nanostructure and transformations in response to stimuli has not been meticulously investigated to elucidate these intricate processes. The evaluation of nanostructures in response to stimuli has traditionally relied on the conventional TEM technique. However, observations made on dried, stained samples fail to capture the nanoparticles in their natural state within the solution, potentially resulting in misleading conclusions for structural analysis. In particular, the determination of vesicular structures using the conventional TEM technique can lead to misjudgments and controversial conclusions. Light scattering and advanced microscopic techniques have to come along with conventional TEM scanning of staining nanostructures to provide insights into sophisticated nanostructures [451]. For example, atomic force microscopy (AFM), dynamic and static light scattering (DLS & SLS) measurements, and small angle neutrons scattering (SANS) together with freeze-fracture transmission electron microscopy (FFTEM) were utilized to explore the micellar and vesicular nanoparticles of block copolymers with different chain length [452]. Although these techniques supported that the assemblies could be vesicles/micelles, advanced microscopic characterizations, e.g. Cryo-TEM, may still be a better option for fully understanding the real morphology. Except for the traditional TEM techniques that provide indirect observation from dried, stained samples, recent advances in liquid-phase electron microscopy allow in situ observations of unstaining nanostructures at liquid phase [453]. Self-assembling behaviors and time-lapse evolution of amphiphilic macromolecules were directly monitored in situ by liquid-phase electron microscopy [454]. Very recently, liquid-phase electron microscopy has been exploited to probe thermoresponsive polymerization-induced self-assembly, providing real-time observations of morphological evolution at controlled temperature. Sophisticated, vesicular morphology of amphiphilic macromolecular self-assemblies observed in the liquid phase at consistent temperature via liquid-phase electron microscopy are proven effective to prevent misjudgment from dried, stained artifacts [455]. Despite the limited access to advanced equipment for real-time, in-situ observations poses a hindrance to the advancement of stimuli-responsive nanoparticles, we anticipate that either light scattering or advanced microscopic techniques can be carefully evaluated together with traditional TEM observation on nanostructures and its transformation in response to stimuli.
Apart from characterization techniques, there are several issues to be further improved for the future development in the stimuli-responsive nano-assemblies: (a) Precise control of responsiveness (e.g., micro- and macro- level transformations, alteration kinetics and reversibility) is highly demanded for applications in complex systems. This could be realized by developing more precisely controllable responsive chemistry [456], designing multi-stimuli responsive systems, and engineering “logic gates” in nano-assemblies. (b) Increasing the selectivity of nano-assemblies for specific recognition events, featuring anti-interference properties from surrounding environment, is always desirable for various applications. For example, nano-reactors with controlled substrate permeability, nano-sensors for specific substrate recognition and nano-carriers for targeted cell penetration could be realized by installing nano-assemblies with different ligands and recognition moieties. (c) Biocompatibility of materials is crucial for their biomedical application. Several factors should be considered for this purpose: (i) Low toxicity before and after stimuli-treatment, (ii) biodegradability, (iii) possibility of clearance from bodies, and (iv) accessibility to bio-related stimuli. (d) Precise control of particle morphology, size and stability is important for designing programable assemblies with predictable properties, e.g., drug loading capacity, guest release properties, and cell-penetration behaviors, which is still challenging in this area. This could be improved by using polymers with more controllable structures (narrow polydispersity or dendrimers), particle crosslinking, polymer blending [314], designing hybrid particles and etching solid core to prepare hollow polymeric particles [457]. Overall, although many of the above-mentioned issues are challenging and have existed for a long time, with the development of new chemistry, technology, and fabrication strategies, we believe this area will burst in the next decades and offer the scientific community with more controllable and programmable nanotools for practical utilities.
Acknowledgments
We acknowledge National Institutes of Health (GM-136395 and T32 GM135096), the U.S. Army Research Office (W911NF2310124) for the financial support. H. L. also acknowledges the financial support by State Key Laboratory of Polymer Materials Engineering (Grant No. sklpme2023-2-08) and the Fundamental Research Funds for the Central Universities.
Abbreviation
- ACUPA
S,S-2-[3-[5-amino-1-carboxypentyl]ureido]pentanedioic acid
- ADA
alendronic acid
- AIE
aggregation-induced emission
- ALP
alkaline phosphatase
- AmB
amphotericin B
- ANCs
antibody-nanoparticle conjugates
- API
active pharmaceutical ingredient
- ATLP
acidity-triggered ligand-presenting
- ATP
adenosine triphosphate
- bCA-II
bovine carbonic anhydrase II
- BMA
butyl methacrylate
- BTZ
bortezomib
- CARTs
charge-altering releasable transporters
- CCPMs
core-crosslinked polymeric micelles
- CDN
cyclic dinucleotide
- Ce6
chlorin e6
- cGAMP
2’3’-cyclic guanosine monophosphate–adenosine monophosphate
- CN−
cyanide
- Cou
coumarin dye
- CPDs
cell-penetrating polydisulfides
- CPT
camptothecin
- CPT
camptothecin
- CUR
curcumin
- Cy-N-CPT
camptothecin-cyanine conjugates
- DBCO
dibenzocyclooctyne
- DEAEMA
2-(diethylamino) ethyl methacrylate
- DIFE
disassembly-induced fluorescence enhancement
- DOX
doxorubicin
- dsDNA
double-strain DNA
- DTM
dithiomaleimide
- DTT
dithiothreitol
- EGDMA
ethylene glycol dimethacrylate
- FMA
fluorescein O-methacrylate
- FRET
Förster resonance energy transfer
- GSH
glutathione
- H2O2
hydrogen peroxide
- HIFU
high intensity focused ultrasound
- HLB
hydrophilic-lipophilic balance
- HO•
hydroxyl radical
- HOCl
hypochlorous acid
- IFN-I
type I interferon
- IND
indomethacin
- LCST
lower critical solution temperature
- MAA
maleic acid amide
- MC
merocyanines
- MMP-2
metalloproteinase-2
- MMPs
matrix metalloproteinases
- mPEG113-b-PHEA21
methoxy poly(ethylene glycol)113-b-poly[(N-2-hydroxyethyl)-aspartamide]21
- mPEG-b-pHPMAmLacn
methoxy poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl) methacrylamide-lactate])
- mPEG-b-PMTEGE
poly(ethylene glycol)-block-poly(2-(methylthio)ethyl glycidyl ether)
- MPEO
poly(ethylene oxide monomethyl ether)
- MR
magnetic resonance
- mRNA
messenger RNA
- MTO
mitoxantrone
- NDDS
nanosized drug delivery system
- NICS
nucleophile-induced chain scission
- NIPAM
N-isopropylacrylamide
- NIR
near-infrared
- NPSM
non-pH-sensitive micelles
- NQO1
NAD(P)H:quinone oxidoreductase isozyme 1
- 1O2
singlet oxygen
- O2•−
superoxide anion radical
- OCl−
hypochlorite
- OEGMA
oligoethylene glycol methacrylate
- ONOO−
peroxynitrite
- P AAm-co-AN)]
poly(acrylamide-co-acrylonitrile)
- P(NAGA-co-AN)
Poly(N-acryloylglycinamide-co-acrylonitrile)
- P(NIPAM-co-TPSS)]
poly(N-isopropylacrylamide-co-tetrabutylphosphonium styrenesulfonate)
- P(VBA60-b-DEA66)
poly(4-vinyl benzoic acid60-block-2-diethylamino)ethyl methacrylate66)
- PCL
poly ε-caprolactone)
- PDEAEMA
poly(N,N-diethylaminoethyl methacrylate)
- PDEA-P(MAA-stat-BzMA)
poly(2-(diethylamino)ethyl methacrylate-block-poly(methacrylic acid-statistical-benzyl methacrylate)
- PDMA
poly(N,N-dimethylacrylamide)
- PDMA-b-PDPA
poly(2-(dimethylamino)ethyl methacrylate)-block-poly(2-(diisopropylamino)ethyl methacrylate)
- PDMAEMA
poly(2-(dimethylamino)ethyl methacrylate)
- pDNA
plasmid DNA
- PDPA
poly(2-(diisopropylamino)ethyl methacrylate)
- PDSMA
pyridyl disulfide methacrylate
- PEG-b-P(NBA-co-NBANA)
(poly(ethylene glycol)-b-poly(2-nitrobenzylalcohol acrylate)-co-poly(2-nitrobenzylalcohol-amino-N-(2-(2-acryloxyethoxy)ethyl) naphthalimide acrylate
- PEG-b-PCL2
poly(ethylene glycol)-b-poly(caprolactone)2
- PEG-b-PDMAEMA-b-PDPA
Poly(ethylene glycol)-block-poly(2-(dimethylamino)ethyl methacrylate)-block-poly(2-(diisopropylamino)ethyl methacrylate)
- PEG-b-PNIPAM
poly(ethylene glycol)-block-poly(N-isopropylacrylamide)
- PEGMA
polyethylene glycol methacrylate
- PEG-N=N-PS
poly(ethylene glycol)-b-poly(styrene)
- PEO
poly(ethylene oxide)
- PEO45-b-P[(Lys-IBC)45-stat-(Lys-PAPE)15]
poly(ethylene oxide)-block-poly(triiodobenzoic chloride-conjugated polylysine-stat-phenylboronic acid pinacol ester-conjugated polylysine)
- PFP
pentafluorophenyl
- PFPA
pentafluorophenyl acrylate
- PIFE
protein-induced fluorescence enhancement
- PMEO2MA
poly(2-(2-methoxyethoxy)ethyl methacrylate)
- PNs
polyprodrug nanoparticles
- POEGMA-b-PMTEMA
poly(oligo(ethylene glycol) methyl ether methacrylate)-block-poly(2-(methylthio)ethyl methacrylate)
- poly (CHTA-co-HD)-PEG
poly(1,2,4,5-cyclohexanetetracarboxylic dianhydride-co-hydroxyethyl disulfide)-polyethylene glycol
- PPA
poly(phthalaldehyde)
- PROTACs
Proteolysis Targeting Chimeras
- PTHPMA
poly(2-tetrahydropyranyl methacrylate)
- pTRAIL
(TRAIL)-encoding plasmid
- PTX
paclitaxel
- RAPs
renoprotective angiographic polymersomes
- RFHM
ratiometric fluorescent hybrid micelles
- RhBMA
rhodamine B methacrylate
- RNAi
RNA interference
- ROS
reactive oxygen species
- siRNA
small-interfering RNA
- SP
spiropyrans
- SPECT/CT
single photon emission computed tomography/computed tomography
- STING
stimulator of interferon genes
- STING-NPs
STING-activating nanoparticles
- TCEP
tris(2-carboxyethyl)phosphine
- THPMA
2-tetrahydropyranyl methacrylate
- TMR
tetramethyl rhodamine
- TPE
tetraphenylethylene
- TPEBT
3-ethyl-2-[4-(1,2,2-triphenylvinyl)styryl]benzothiazol-3-ium iodide
- TPP
triphenylphosphonium
- TRAIL
tumor-necrosis-factor-related apoptosis-inducing ligand
- UCNPs
upconversion nanoparticles
- UCST
upper critical solution temperature
- UPSM
ultra-pH-sensitive micelles
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
No data was used for the research described in the article.
References
- [1].Aubert S, Bezagu M, Spivey AC, Arseniyadis S. Spatial and temporal control of chemical processes. Nat Rev Chem 2019;3:706–22. 10.1038/s41570-019-0139-6. [DOI] [Google Scholar]
- [2].Pentinmikko N, Lozano R, Scharaw S, Andersson S, Englund JI, Castillo-Azofeifa D, et al. Cellular shape reinforces niche to stem cell signaling in the small intestine. Sci Adv 2022;8:eabm1847. 10.1126/sciadv.abm1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marguet M, Bonduelle C, Lecommandoux S. Multicompartmentalized polymeric systems: towards biomimetic cellular structure and function. Chem Soc Rev 2012;42:512–29. 10.1039/c2cs35312a. [DOI] [PubMed] [Google Scholar]
- [4].Lutz J-F, Lehn J-M, Meijer EW, Matyjaszewski K. From precision polymers to complex materials and systems. Nat Rev Mater 2016;1:16024. 10.1038/natrevmats.2016.24. [DOI] [Google Scholar]
- [5].Bai Y, Chen J, Zimmerman SC. Designed transition metal catalysts for intracellular organic synthesis. Chem Soc Rev 2018;47:1811–21. 10.1039/c7cs00447h. [DOI] [PubMed] [Google Scholar]
- [6].Xiang S, Hammer B, Kremer K, Müllen K, Weil T. Engineering Surface Amphiphilicity of Polymer Nanostructures. Prog Polym Sci 2021;124:101489. 10.1016/j.progpolymsci.2021.101489. [DOI] [Google Scholar]
- [7].Chagri S, Ng DYW, Weil T. Designing bioresponsive nanomaterials for intracellular self-assembly. Nat Rev Chem 2022;6:320–38. 10.1038/s41570-022-00373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim H, Park Y, Stevens MM, Kwon W, Hahn SK. Multifunctional hyaluronate – nanoparticle hybrid systems for diagnostic, therapeutic and theranostic applications. J Control Release 2019;303:55–66. 10.1016/j.jconrel.2019.04.003. [DOI] [PubMed] [Google Scholar]
- [9].Vinciguerra D, Gelb MB, Maynard HD. Synthesis and Application of Trehalose Materials. JACS Au 2022;2:1561–87. 10.1021/jacsau.2c00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xin X, Judy JD, Sumerlin BB, He Z. Nano-enabled agriculture: from nanoparticles to smart nanodelivery systems. Environ Chem 2020;17:413. 10.1071/en19254. [DOI] [Google Scholar]
- [11].Liu H, Kanjilal P, Thayumanavan S. Self-assembly of polymers from multicomponent reactions. Polym Int 2022;71:562–8. 10.1002/pi.6352. [DOI] [Google Scholar]
- [12].Hu J, Zhang G, Liu S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev 2012;41:5933–49. 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- [13].Deng Z, Liu S. Controlled drug delivery with nanoassemblies of redox-responsive prodrug and polyprodrug amphiphiles. J Control Release 2020;326:276–96. 10.1016/j.jconrel.2020.07.010. [DOI] [PubMed] [Google Scholar]
- [14].Deng Z, Hu J, Liu S. Disulfide-Based Self-Immolative Linkers and Functional Bioconjugates for Biological Applications. Macromol Rapid Commun 2020;41:1900531. 10.1002/marc.201900531. [DOI] [PubMed] [Google Scholar]
- [15].Reineke TM. Stimuli-Responsive Polymers for Biological Detection and Delivery. ACS Macro Lett 2016;5:14–8. 10.1021/acsmacrolett.5b00862. [DOI] [PubMed] [Google Scholar]
- [16].Sikder A, Esen C, O’Reilly RK. Nucleobase-Interaction-Directed Biomimetic Supramolecular Self-Assembly. Acc Chem Res 2022;55:1609–19. 10.1021/acs.accounts.2c00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang M, Gao B, Wang X, Li W, Feng Y. Enzyme-responsive strategy as a prospective cue to construct intelligent biomaterials for disease diagnosis and therapy. Biomater Sci 2022;10:1883–903. 10.1039/d2bm00067a. [DOI] [PubMed] [Google Scholar]
- [18].Beauté L, McClenaghan N, Lecommandoux S. Photo-triggered polymer nanomedicines: From molecular mechanisms to therapeutic applications. Adv Drug Deliver Rev 2019;138:148–66. 10.1016/j.addr.2018.12.010. [DOI] [PubMed] [Google Scholar]
- [19].Sikder A, Pearce AK, Parkinson SJ, Napier R, O’Reilly RK. Recent Trends in Advanced Polymer Materials in Agriculture Related Applications. ACS Appl Polym Mater 2021;3:1203–17. 10.1021/acsapm.0c00982. [DOI] [Google Scholar]
- [20].Brooks WLA, Sumerlin BS. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem Rev 2016;116:1375–97. 10.1021/acs.chemrev.5b00300. [DOI] [PubMed] [Google Scholar]
- [21].Roy D, Brooks WL, Sumerlin BS. New directions in thermoresponsive polymers. Chem Soc Rev 2013;42:7214–43. 10.1039/c3cs35499g. [DOI] [PubMed] [Google Scholar]
- [22].Vancoillie G, Frank D, Hoogenboom R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog Polym Sci 2014;39:1074–95. 10.1016/j.progpolymsci.2014.02.005. [DOI] [Google Scholar]
- [23].Badi N Non-linear PEG-based thermoresponsive polymer systems. Prog Polym Sci 2017;66:54–79. 10.1016/j.progpolymsci.2016.12.006. [DOI] [Google Scholar]
- [24].Niskanen J, Tenhu H.How to manipulate the upper critical solution temperature (UCST)? Polym Chem 2016;8:220–32. 10.1039/c6py01612j. [DOI] [Google Scholar]
- [25].Liu H, Prachyathipsakul T, Koyasseril-Yehiya TM, Le SP, Thayumanavan S. Molecular bases for temperature sensitivity in supramolecular assemblies and their applications as thermoresponsive soft materials. Mater Horizons 2021;9:164–93. 10.1039/d1mh01091c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qiao S-L, Mamuti M, An H-W, Wang H. Thermoresponsive Polymer Assemblies: From Molecular Design to Theranostics Application. Prog Polym Sci 2022;131:101578. 10.1016/j.progpolymsci.2022.101578. [DOI] [Google Scholar]
- [27].Zhao C, Ma Z, Zhu XX. Rational Design of Thermoresponsive Polymers in Aqueous Solutions: A Thermodynamics Map. Prog Polym Sci 2019;90:269–91. 10.1016/j.progpolymsci.2019.01.001. [DOI] [Google Scholar]
- [28].Xiao P, Zhang J, Zhao J, Stenzel MH. Light-induced release of molecules from polymers. Prog Polym Sci 2017;74:1–33. 10.1016/j.progpolymsci.2017.06.002. [DOI] [Google Scholar]
- [29].Jiang J, Tong X, Zhao Y. A New Design for Light-Breakable Polymer Micelles. J Am Chem Soc 2005;127:8290–1. 10.1021/ja0521019. [DOI] [PubMed] [Google Scholar]
- [30].Jiang J, Tong X, Morris D, Zhao Y. Toward Photocontrolled Release Using Light-Dissociable Block Copolymer Micelles. Macromolecules 2006;39:4633–40. 10.1021/ma060142z. [DOI] [Google Scholar]
- [31].Babin J, Pelletier M, Lepage M, Allard J, Morris D, Zhao Y. A New Two-Photon-Sensitive Block Copolymer Nanocarrier. Angew Chem Int Ed 2009;48:3329–32. 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]
- [32].Dong J, Zhang R, Wu H, Zhan X, Yang H, Zhu S, et al. Polymer Nanoparticles for Controlled Release Stimulated by Visible Light and pH. Macromol Rapid Commun 2014;35:1255–9. 10.1002/marc.201400078. [DOI] [PubMed] [Google Scholar]
- [33].Liu J, Kang W, Wang W. Photocleavage-based Photoresponsive Drug Delivery. Photochem Photobiol 2022;98:288–302. 10.1111/php.13570. [DOI] [PubMed] [Google Scholar]
- [34].Choi SK. Photoactivation Strategies for Therapeutic Release in Nanodelivery Systems. Adv Ther 2020;3:2000117. 10.1002/adtp.202000117. [DOI] [Google Scholar]
- [35].Yardley RE, Kenaree AR, Gillies ER. Triggering Depolymerization: Progress and Opportunities for Self-Immolative Polymers.Macromolecules 2019;52:6342–60. 10.1021/acs.macromol.9b00965. [DOI] [Google Scholar]
- [36].Peles-Strahl L, Sasson R, Slor G, Edelstein-Pardo N, Dahan A, Amir RJ. Utilizing Self-Immolative ATRP Initiators To Prepare Stimuli-Responsive Polymeric Films from Nonresponsive Polymers. Macromolecules 2019;52:3268–77. 10.1021/acs.macromol.8b02566. [DOI] [Google Scholar]
- [37].Shelef O, Gnaim S, Shabat D.Self-Immolative Polymers: An Emerging Class of Degradable Materials with Distinct Disassembly Profiles. J Am Chem Soc 2021;143:21177–88. 10.1021/jacs.1c11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xiong H, Xu Y, Kim B, Rha H, Zhang B, Li M, et al. Photo-controllable biochemistry: Exploiting the photocages in phototherapeutic window. Chem 2022;9:29–64. 10.1016/j.chempr.2022.11.007. [DOI] [Google Scholar]
- [39].einstain R, Slanina T, Kand D, Kl n P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem Rev 2020;120:13135–272. 10.1021/acs.chemrev.0c00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Choi PJ, Park TI, Cooper E, Dragunow M, Denny WA, Jose J. Heptamethine Cyanine Dye Mediated Drug Delivery: Hype or Hope. Bioconjugate Chem 2020;31:1724–39. 10.1021/acs.bioconjchem.0c00302. [DOI] [PubMed] [Google Scholar]
- [41].Juzenas P, Juzeniene A, Kaalhus O, Iani V, Moan J. Noninvasive fluorescence excitation spectroscopy during application of 5-aminolevulinic acid in vivo. Photochem Photobiol Sci 2002;1:745–8. 10.1039/b203459j. [DOI] [PubMed] [Google Scholar]
- [42].Wang G, Tong X, Zhao Y. Preparation of Azobenzene-Containing Amphiphilic Diblock Copolymers for Light-Responsive Micellar Aggregates. Macromolecules 2004;37:8911–7. 10.1021/ma048416a. [DOI] [Google Scholar]
- [43].Tong X, Wang G, Soldera A, Zhao Y. How Can Azobenzene Block Copolymer Vesicles Be Dissociated and Reformed by Light? J Phys Chem B 2005;109:20281–7. 10.1021/jp0524274. [DOI] [PubMed] [Google Scholar]
- [44].Lee H, Wu W, Oh JK, Mueller L, Sherwood G, Peteanu L, et al. Light-Induced Reversible Formation of Polymeric Micelles. Angew Chem Int Ed 2007;46:2453–7. 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]
- [45].Tong R, Hemmati HD, Langer R, Kohane DS. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J Am Chem Soc 2012;134:8848–55. 10.1021/ja211888a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang J, Qi B, Lepage M, Zhao Y. Polymer Micelles Stabilization on Demand through Reversible Photo-Cross-Linking. Macromolecules 2007;40:790–2. 10.1021/ma062493j. [DOI] [Google Scholar]
- [47].Kabb CP, O’Bryan CS, Deng CC, Angelini TE, Sumerlin BS. Photoreversible Covalent Hydrogels for Soft-Matter Additive Manufacturing. ACS Appl Mater Inter 2018;10:16793–801. 10.1021/acsami.8b02441. [DOI] [PubMed] [Google Scholar]
- [48].Wang H, Miao W, Wang F, Cheng Y. A Self-Assembled Coumarin-Anchored Dendrimer for Efficient Gene Delivery and Light-Responsive Drug Delivery. Biomacromolecules 2018;19:2194–201. 10.1021/acs.biomac.8b00246. [DOI] [PubMed] [Google Scholar]
- [49].Frisch H, Menzel JP, Bloesser FR, Marschner DE, Mundsinger K, Barner-Kowollik C. Photochemistry in Confined Environments for Single-Chain Nanoparticle Design. J Am Chem Soc 2018;140:9551–7. 10.1021/jacs.8b04531. [DOI] [PubMed] [Google Scholar]
- [50].Frank PG, Tuten BT, Prasher A, Chao D, Berda EB. Intra-Chain Photodimerization of Pendant Anthracene Units as an Efficient Route to Single-Chain Nanoparticle Fabrication. Macromol Rapid Commun 2014;35:249–53. 10.1002/marc.201300677. [DOI] [PubMed] [Google Scholar]
- [51].Damme JV, Prez FD. Anthracene-containing polymers toward high-end applications. Prog Polym Sci 2018;82:92–119. 10.1016/j.progpolymsci.2018.02.002. [DOI] [Google Scholar]
- [52].You J, Yoon JA, Kim J, Huang C-F, Matyjaszewski K, Kim E. Excimer Emission from Self-Assembly of Fluorescent Diblock Copolymer Prepared by Atom Transfer Radical Polymerization. Chem Mater 2010;22. 10.1021/cm1011026. [DOI] [Google Scholar]
- [53].Zhao H, Sterner ES, Coughlin EB, Theato P. o-Nitrobenzyl Alcohol Derivatives: Opportunities in Polymer and Materials Science. Macromolecules 2012;45:1723–36. 10.1021/ma201924h. [DOI] [Google Scholar]
- [54].Liu G, Wang X, Hu J, Zhang G, Liu S. Self-Immolative Polymersomes for High-Efficiency Triggered Release and Programmed Enzymatic Reactions. J Am Chem Soc 2014;136. 10.1021/ja5030832. [DOI] [PubMed] [Google Scholar]
- [55].Zhou H, Chen M, Liu Y, Wu S. Stimuli-Responsive Ruthenium-Containing Polymers. Macromol Rapid Commun 2018;39:1800372. 10.1002/marc.201800372. [DOI] [PubMed] [Google Scholar]
- [56].Huang L, Han G. Near Infrared Boron Dipyrromethene Nanoparticles for Optotheranostics. Small Methods 2018;2:1700370. 10.1002/smtd.201700370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gorka AP, Nani RR, Zhu J, Mackem S, Schnermann MJ. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J Am Chem Soc 2014;136:14153–9. 10.1021/ja5065203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gorka AP, Nani RR, Schnermann MJ. Harnessing Cyanine Reactivity for Optical Imaging and Drug Delivery. Acc Chem Res 2018;51:3226–35. 10.1021/acs.accounts.8b00384. [DOI] [PubMed] [Google Scholar]
- [59].Vapaavuori J, Bazuin CG, Priimagi A. Supramolecular design principles for efficient photoresponsive polymer–azobenzene complexes. J Mater Chem C 2018;6:2168–88. 10.1039/c7tc05005d. [DOI] [Google Scholar]
- [60].Weis P, Wu S. Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared. Macromol Rapid Commun 2018;39:1700220. 10.1002/marc.201700220. [DOI] [PubMed] [Google Scholar]
- [61].Kortekaas L, Browne WR. The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome. Chem Soc Rev 2019;48:3406–24. 10.1039/c9cs00203k. [DOI] [PubMed] [Google Scholar]
- [62].Wigglesworth TJ, Myles AJ, Branda NR. High-Content Photochromic Polymers Based on Dithienylethenes. Eur J Org Chem 2005;2005:1233–8. 10.1002/ejoc.200400623. [DOI] [Google Scholar]
- [63].Luo Q, Cheng H, Tian H. Recent progress on photochromic diarylethene polymers. Polym Chem 2011;2:2435–43. 10.1039/c1py00167a. [DOI] [Google Scholar]
- [64].Trenor SR, Shultz AR, Love BJ, Long TE. Coumarins in Polymers: From Light Harvesting to Photo-Cross-Linkable Tissue Scaffolds. Chem Rev 2004;104:3059–78. 10.1021/cr030037c. [DOI] [PubMed] [Google Scholar]
- [65].Sarmah M, Chutia K, Dutta D, Gogoi P. Overview of coumarin-fused-coumarins: synthesis, photophysical properties and their applications. Org Biomol Chem 2021;20:55–72. 10.1039/d1ob01876k. [DOI] [PubMed] [Google Scholar]
- [66].Yoshizawa M, Catti L. Bent Anthracene Dimers as Versatile Building Blocks for Supramolecular Capsules. Acc Chem Res 2019;52:2392–404. 10.1021/acs.accounts.9b00301. [DOI] [PubMed] [Google Scholar]
- [67].Marschner DE, Frisch H, Offenloch JT, Tuten BT, Becer CR, Walther A, et al. Visible Light [2 + 2] Cycloadditions for Reversible Polymer Ligation. Macromolecules 2018;51:3802–7. 10.1021/acs.macromol.8b00613. [DOI] [Google Scholar]
- [68].Boissenot T, Bordat A, Fattal E, Tsapis N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J Control Release 2016;241:144–63. 10.1016/j.jconrel.2016.09.026. [DOI] [PubMed] [Google Scholar]
- [69].Awad NS, Paul V, AlSawaftah NM, Haar G ter, Allen TM, Pitt WG, et al. Ultrasound-Responsive Nanocarriers in Cancer Treatment: A Review. ACS Pharmacol Transl Sci 2021;4:589–612. 10.1021/acsptsci.0c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].uan J, Boissi re O, Zhao Y, Yan B, Tremblay L, Lacelle S, et al. Ultrasound-Responsive Block Copolymer Micelles Based on a New Amplification Mechanism. Langmuir 2012;28:16463–8. 10.1021/la303946b. [DOI] [PubMed] [Google Scholar]
- [71].Wu P, Jia Y, Qu F, Sun Y, Wang P, Zhang K, et al. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl Mater Inter 2017;9:25706–16. 10.1021/acsami.7b05469. [DOI] [PubMed] [Google Scholar]
- [72].Liu X, Zhao K, Cao J, Qi X, Wu L, Shen S. Ultrasound responsive self-assembled micelles loaded with hypocrellin for cancer sonodynamic therapy. Int J Pharm 2021;608:121052. 10.1016/j.ijpharm.2021.121052. [DOI] [PubMed] [Google Scholar]
- [73].Deirram N, Zhang C, Kermaniyan SS, Johnston APR, Such GK. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol Rapid Commun 2019;40:1800917. 10.1002/marc.201800917. [DOI] [PubMed] [Google Scholar]
- [74].Dai S, Ravi P, Tam KC. pH-Responsive polymers : synthesis, properties and applications. Soft Matter 2008;4:435–49. 10.1039/b714741d. [DOI] [PubMed] [Google Scholar]
- [75].Kocak G, Tuncer C, Bütün V.pH-Responsive polymers. Polym Chem 2016;8:144–76. 10.1039/c6py01872f. [DOI] [Google Scholar]
- [76].Deng Z, Hu J, Liu S. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS)-Responsive Polymersomes for Triggered Drug Release. Macromol Rapid Commun 2017;38:1600685. 10.1002/marc.201600685. [DOI] [PubMed] [Google Scholar]
- [77].Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 2011;7:504–11. 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kong H, Chandel NS. Regulation of redox balance in cancer and T cells. J Biol Chem 2018;293:7499–507. 10.1074/jbc.tm117.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging 2018;13:757–72. 10.2147/cia.s158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Murphy MP, Bayir H, Belousov V, Chang CJ, Davies KJA, Davies MJ, et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metabolism 2022;4:651–62. 10.1038/s42255-022-00591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hsu P-H, Almutairi A Recent progress of redox-responsive polymeric nanomaterials for controlled release. J Mater Chem B 2021;9:2179–88. 10.1039/d0tb02190c. [DOI] [PubMed] [Google Scholar]
- [82].Lee SH, Gupta MK, Bang JB, Bae H, Sung H. Current Progress in Reactive Oxygen Species (ROS)-Responsive Materials for Biomedical Applications. Adv Healthc Mater 2013;2:908–15. 10.1002/adhm.201200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Napoli A, Valentini M, Tirelli N, Müller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater 2004;3:183–9. 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- [84].Shim MS, Xia Y. A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells. Angew Chem Int Ed 2013;52:6926–9. 10.1002/anie.201209633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 2010;9:923–8. 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Stubelius A, Lee S, Almutairi A. The Chemistry of Boronic Acids in Nanomaterials for Drug Delivery. Acc Chem Res 2019;52:3108–19. 10.1021/acs.accounts.9b00292. [DOI] [PubMed] [Google Scholar]
- [87].Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct 2004;22:343–52. 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- [88].Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem-Biol Interact 1998;111:1–14. 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- [89].Yang J, Chen H, Vlahov IR, Cheng J-X, Low PS. Evaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imaging. Proc Natl Acad Sci USA 2006;103:13872–7. 10.1073/pnas.0601455103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Quinn JF, Whittaker MR, Davis TP. Glutathione responsive polymers and their application in drug delivery systems. Polym Chem 2016;8:97–126. 10.1039/c6py01365a. [DOI] [Google Scholar]
- [91].Gao W, Langer R, Farokhzad OC. Poly(ethylene glycol) with Observable Shedding. Angew Chem Int Ed 2010;49:6567–71. 10.1002/anie.201001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Oh JK. Disassembly and tumor-targeting drug delivery of reduction-responsive degradable block copolymer nanoassemblies. Polym Chem 2019;10:1554–68. 10.1039/c8py01808a. [DOI] [Google Scholar]
- [93].Zhang R, Nie T, Fang Y, Huang H, Wu J. Poly(disulfide)s: From Synthesis to Drug Delivery. Biomacromolecules 2022;23:1–19. 10.1021/acs.biomac.1c01210. [DOI] [PubMed] [Google Scholar]
- [94].Zhang Q, Qu D-H, Feringa BL, Tian H. Disulfide-Mediated Reversible Polymerization toward Intrinsically Dynamic Smart Materials. J Am Chem Soc 2022;144:2022–33. 10.1021/jacs.1c10359. [DOI] [PubMed] [Google Scholar]
- [95].Smith MEB, Schumacher FF, Ryan CP, Tedaldi LM, Papaioannou D, Waksman G, et al. Protein Modification, Bioconjugation, and Disulfide Bridging Using Bromomaleimides. J Am Chem Soc 2010;132:1960–5. 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Morais M, Nunes JPM, Karu K, Forte N, Benni I, Smith MEB, et al. Optimisation of the dibromomaleimide (DBM) platform for native antibody conjugation by accelerated post-conjugation hydrolysis. Org Biomol Chem 2017;15:2947–52. 10.1039/c7ob00220c. [DOI] [PubMed] [Google Scholar]
- [97].Chen Z, Boyd SD, Calvo JS, Murray KW, Mejia GL, Benjamin CE, et al. Fluorescent Functionalization across Quaternary Structure in a Virus-like Particle. Bioconjugate Chem 2017;28:2277–83. 10.1021/acs.bioconjchem.7b00305. [DOI] [PubMed] [Google Scholar]
- [98].Jones MW, Strickland RA, Schumacher FF, Caddick S, Baker JamesR, Gibson MI, et al. Polymeric Dibromomaleimides As Extremely Efficient Disulfide Bridging Bioconjugation and Pegylation Agents. J Am Chem Soc 2012;134:1847–52. 10.1021/ja210335f. [DOI] [PubMed] [Google Scholar]
- [99].Xie Y, Arno MC, Husband JT, Torrent-Sucarrat M, O’Reilly RK. Manipulating the fluorescence lifetime at the sub-cellular scale via photo-switchable barcoding. Nat Commun 2020;11:2460. 10.1038/s41467-020-16297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Robin MP, Osborne SAM, Pikramenou Z, Raymond JE, O’Reilly RK. Fluorescent Block Copolymer Micelles That Can Self-Report on Their Assembly and Small Molecule Encapsulation. Macromolecules 2016;49:653–62. 10.1021/acs.macromol.5b02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Robin MP, Jones M, Haddleton DM, O’Reilly RK. Dibromomaleimide End Functional Polymers by RAFT Polymerization Without the Need of Protecting Groups. ACS Macro Lett 2011;1:222–6. 10.1021/mz200164x. [DOI] [PubMed] [Google Scholar]
- [102].Tang S, Olsen BD. Controlling topological entanglement in engineered protein hydrogels with a variety of thiol coupling chemistries. Frontiers Chem 2014;2:23. 10.3389/fchem.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bai T, Du J, Chen J, Duan X, Zhuang Q, Chen H, et al. Reduction-responsive dithiomaleimide-based polymeric micelles for controlled anti-cancer drug delivery and bioimaging. Polym Chem 2017;8:7160–8. 10.1039/c7py01675a. [DOI] [Google Scholar]
- [104].Wang H, Xu M, Xiong M, Cheng J. Reduction-responsive dithiomaleimide-based nanomedicine with high drug loading and FRET-indicated drug release. Chem Comm 2015;51:4807–10. 10.1039/c5cc00148j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhu L, Guo Y, Qian Q, Yan D, Li Y, Zhu X, et al. Carrier-Free Delivery of Precise Drug–Chemogene Conjugates for Synergistic Treatment of Drug-Resistant Cancer. Angew Chem Int Ed 2020;59:17944–50. 10.1002/anie.202006895. [DOI] [PubMed] [Google Scholar]
- [106].Kwon S, Ko H, You DG, Kataoka K, Park JH. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc Chem Res 2019;52:1771–82. 10.1021/acs.accounts.9b00136. [DOI] [PubMed] [Google Scholar]
- [107].Shieh Y-T, Yeh Y-C, Cheng C-C. Two-Way CO2-Responsive Polymer Particles with Controllable Amphiphilic Properties. ACS Omega 2020;5:1862–9. 10.1021/acsomega.9b03319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhu Y, Hu Y, Zeng J, Chen C, Li S, Jiang Y. Rapidly SO2-responsive vesicles with intrinsic fluorescent indicators for membrane structure evolution. Chin Chem Lett 2022;33:5180–3. 10.1016/j.cclet.2022.02.005. [DOI] [Google Scholar]
- [109].Hsu P-H, Kawasaki R, Yamana K, Isozaki H, Kawamura S, Ikeda A, et al. Hydrogen Sulfide-Responsive Self-Assembled Nanogel. ACS Appl Polym Mater 2020;2:3756–60. 10.1021/acsapm.0c00759. [DOI] [Google Scholar]
- [110].Xu H, Xu H, Ma S, Chen X, Huang L, Chen J, et al. Analyte Regeneration Fluorescent Probes for Formaldehyde Enabled by Regiospecific Formaldehyde-Induced Intramolecularity. J Am Chem Soc 2018;140:16408–12. 10.1021/jacs.8b09794. [DOI] [PubMed] [Google Scholar]
- [111].Xu M, Liu L, Hu J, Zhao Y, Yan Q. CO-Signaling Molecule-Responsive Nanoparticles Formed from Palladium-Containing Block Copolymers. ACS Macro Lett 2017;6:458–62. 10.1021/acsmacrolett.7b00042. [DOI] [PubMed] [Google Scholar]
- [112].Shahriari M, Zahiri M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Enzyme responsive drug delivery systems in cancer treatment. J Control Release 2019;308:172–89. 10.1016/j.jconrel.2019.07.004. [DOI] [PubMed] [Google Scholar]
- [113].Mu J, Lin J, Huang P, Chen X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem Soc Rev 2018;47:5554–73. 10.1039/c7cs00663b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhou Q, Si Z, Wang K, Li K, Hong W, Zhang Y, et al. Enzyme-triggered smart antimicrobial drug release systems against bacterial infections. J Control Release 2022;352:507–26. 10.1016/j.jconrel.2022.10.038. [DOI] [PubMed] [Google Scholar]
- [115].Hahn ME, Gianneschi NC. Enzyme-directed assembly and manipulation of organic nanomaterials. Chem Commun 2011;47:11814–21. 10.1039/c1cc15220c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rao J, Khan A. Enzyme Sensitive Synthetic Polymer Micelles Based on the Azobenzene Motif. J Am Chem Soc 2013;135:14056–9. 10.1021/ja407514z. [DOI] [PubMed] [Google Scholar]
- [117].Amir RJ, Zhong S, Pochan DJ, Hawker CJ. Enzymatically Triggered Self-Assembly of Block Copolymers. J Am Chem Soc 2009;131:13949–51. 10.1021/ja9060917. [DOI] [PubMed] [Google Scholar]
- [118].Azagarsamy MA, Sokkalingam P, Thayumanavan S. Enzyme-Triggered Disassembly of Dendrimer-Based Amphiphilic Nanocontainers. J Am Chem Soc 2009;131:14184–5. 10.1021/ja906162u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rosenbaum I, Avinery R, Harnoy AJ, Slor G, Tirosh E, Hananel U, et al. Reversible Dimerization of Polymeric Amphiphiles Acts as a Molecular Switch of Enzymatic Degradability. Biomacromolecules 2017;18:3457–68. 10.1021/acs.biomac.7b01150. [DOI] [PubMed] [Google Scholar]
- [120].Gao J, Wang H, Zhuang J, Thayumanavan S. Tunable enzyme responses in amphiphilic nanoassemblies through alterations in the unimer–aggregate equilibrium. Chem Sci 2019;10:3018–24. 10.1039/c8sc04744h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ku T-H, Chien M-P, Thompson MP, Sinkovits RS, Olson NH, Baker TS, et al. Controlling and Switching the Morphology of Micellar Nanoparticles with Enzymes. J Am Chem Soc 2011;133:8392–5. 10.1021/ja2004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Adamiak L, Touve MA, LeGuyader CLM, Gianneschi NC. Peptide Brush Polymers and Nanoparticles with Enzyme-Regulated Structure and Charge for Inducing or Evading Macrophage Cell Uptake. ACS Nano 2017;11:9877–88. 10.1021/acsnano.7b03686. [DOI] [PubMed] [Google Scholar]
- [123].Chien M, Rush AM, Thompson MP, Gianneschi NC. Programmable Shape-Shifting Micelles. Angew Chem Int Ed 2010;49:5076–80. 10.1002/anie.201000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gordon MR, Zhao B, Anson F, Fernandez A, Singh K, Homyak C, et al. Matrix Metalloproteinase-9-Responsive Nanogels for Proximal Surface Conversion and Activated Cellular Uptake. Biomacromolecules 2018;19:860–71. 10.1021/acs.biomac.7b01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Proetto MT, Callmann CE, Cliff J, Szymanski CJ, Hu D, Howell SB, et al. Tumor Retention of Enzyme-Responsive Pt(II) Drug-Loaded Nanoparticles Imaged by Nanoscale Secondary Ion Mass Spectrometry and Fluorescence Microscopy. ACS Central Sci 2018;4:1477–84. 10.1021/acscentsci.8b00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ringgaard L, Melander F, Eliasen R, Henriksen JR, Jølck RI, Engel TB, et al. Tumor repolarization by an advanced liposomal drug delivery system provides a potent new approach for chemo-immunotherapy. Sci Adv 2020;6:eaba5628. 10.1126/sciadv.aba5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Alemdaroglu FE, Wang J, Börsch M, Berger R, Herrmann A. Enzymatic Control of the Size of DNA Block Copolymer Nanoparticles. Angew Chem Int Ed 2008;47:974–6. 10.1002/anie.200703466. [DOI] [PubMed] [Google Scholar]
- [128].Tang L, Tjong V, Li N, Yingling YG, Chilkoti A, Zauscher S. Enzymatic Polymerization of High Molecular eight DNA Amphiphiles That Self-Assemble into Star-Like Micelles. Adv Mater 2014;26:3050–4. 10.1002/adma.201306049. [DOI] [PubMed] [Google Scholar]
- [129].Park J, Jo S, Lee YM, Saravanakumar G, Lee J, Park D, et al. Enzyme-Triggered Disassembly of Polymeric Micelles by Controlled Depolymerization via Cascade Cyclization for Anticancer Drug Delivery. ACS Appl Mater Inter 2021;13:8060–70. 10.1021/acsami.0c22644. [DOI] [PubMed] [Google Scholar]
- [130].Kumar V, Munkhbat O, Secinti H, Thayumanavan S. Disassembly of polymeric nanoparticles with enzyme-triggered polymer unzipping: polyelectrolyte complexes vs. amphiphilic nanoassemblies. Chem Commun 2020;56:8456–9. 10.1039/d0cc03257c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Slor G, Amir RJ. Using High Molecular Precision to Study Enzymatically Induced Disassembly of Polymeric Nanocarriers: Direct Enzymatic Activation or Equilibrium-Based Degradation? Macromolecules 2021;54:1577–88. 10.1021/acs.macromol.0c02263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kumar V, Koyasseril-Yehiya TM, Thayumanavan S. Molecular Assemblies: Characterization and Applications. ACS Sym Ser 2020:95–107. 10.1021/bk-2020-1355.ch007. [DOI] [Google Scholar]
- [133].Feng Q, Wilhelm J, Gao J. Transistor-like Ultra-pH-Sensitive Polymeric Nanoparticles. Acc Chem Res 2019;52:1485–95. 10.1021/acs.accounts.9b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Canning SL, Neal TJ, Armes SP. pH-Responsive Schizophrenic Diblock Copolymers Prepared by Polymerization-Induced Self-Assembly. Macromolecules 2017;50:6108–16. 10.1021/acs.macromol.7b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Liu S, Armes SP. Polymeric Surfactants for the New Millennium: A pH-Responsive, Zwitterionic, Schizophrenic Diblock Copolymer. Angew Chem Int Ed 2002;41:1413–6. . [DOI] [PubMed] [Google Scholar]
- [136].Hunter CA, Anderson HL. What is Cooperativity? Angew Chem Int Ed 2009;48:7488–99. 10.1002/anie.200902490. [DOI] [PubMed] [Google Scholar]
- [137].Li Y, Zhao T, Wang C, Lin Z, Huang G, Sumer BD, et al. Molecular basis of cooperativity in pH-triggered supramolecular self-assembly. Nat Commun 2016;7:13214. 10.1038/ncomms13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zhou K, ang Y, Huang, Luby-Phelps K, Sumer BD, Gao J. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angew Chem Int Ed 2011;50:6109–14. 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ma X, Wang Y, Zhao T, Li Y, Su L-C, Wang Z, et al. Ultra-pH-Sensitive Nanoprobe Library with Broad pH Tunability and Fluorescence Emissions. J Am Chem Soc 2014;136:11085–92. 10.1021/ja5053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zhao Y, Zhu W, Ren L, Zhang K. Aggregation-induced emission polymer nanoparticles with pH-responsive fluorescence. Polym Chem 2016;7:5386–95. 10.1039/c6py01009a. [DOI] [Google Scholar]
- [141].Scarpa JS, Mueller DD, Klotz IM. Slow hydrogen-deuterium exchange in a non-.alpha.-helical polyamide. J Am Chem Soc 1967;89:6024–30. 10.1021/ja01000a006. [DOI] [Google Scholar]
- [142].Weber C, Hoogenboom R, Schubert US. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog Polym Sci 2012;37:686–714. 10.1016/j.progpolymsci.2011.10.002. [DOI] [Google Scholar]
- [143].Sun H, Kabb CP, Sims MB, Sumerlin BS. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog Polym Sci 2019;89:61–75. 10.1016/j.progpolymsci.2018.09.006. [DOI] [Google Scholar]
- [144].Schild HG. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci 1992;17:163–249. 10.1016/0079-6700(92)90023-r. [DOI] [Google Scholar]
- [145].Hu X, Li Y, Liu T, Zhang G, Liu S. Intracellular Cascade FRET for Temperature Imaging of Living Cells with Polymeric Ratiometric Fluorescent Thermometers. ACS Appl Mater Inter 2015;7:15551–60. 10.1021/acsami.5b04025. [DOI] [PubMed] [Google Scholar]
- [146].Yin N, Lin B, Huo F, Shu Y, Wang J. Nanothermometer with Temperature Induced Reversible Emission for Evaluation of Intracellular Thermal Dynamics. Anal Chem 2022;94:12111–9. 10.1021/acs.analchem.2c02106. [DOI] [PubMed] [Google Scholar]
- [147].Wang G, Wu P. Unusual Phase Transition Behavior of Poly(N-isopropylacrylamide)-co-Poly(tetrabutylphosphonium styrenesulfonate) in Water: Mild and Linear Changes in the Poly(N-isopropylacrylamide) Part. Langmuir 2016;32:3728–36. 10.1021/acs.langmuir.6b00392. [DOI] [PubMed] [Google Scholar]
- [148].Bordat A, Boissenot T, Nicolas J, Tsapis N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliver Rev 2018;138:167–92. 10.1016/j.addr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- [149].Le M, Huang W, Chen K-F, Lin C, Cai L, Zhang H, et al. Upper critical solution temperature polymeric drug carriers. Chem Eng J 2021;432:134354. 10.1016/j.cej.2021.134354. [DOI] [Google Scholar]
- [150].Seuring J, Bayer FM, Huber K, Agarwal S. Upper Critical Solution Temperature of Poly(N-acryloyl glycinamide) in Water: A Concealed Property. Macromolecules 2012;45:374–84. 10.1021/ma202059t. [DOI] [Google Scholar]
- [151].Liu F, Seuring J, Agarwal S. Atom transfer radical polymerization as a tool for making poly( N -acryloylglycinamide) with molar mass independent UCST-type transitions in water and electrolytes. Polym Chem 2013;4:3123–31. 10.1039/c3py00222e. [DOI] [Google Scholar]
- [152].Seuring J, Agarwal S. First Example of a Universal and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Water and Electrolyte Solution. Macromolecules 2012;45:3910–8. 10.1021/ma300355k. [DOI] [Google Scholar]
- [153].Käfer F, Lerch A, Agarwal S. Tunable, concentration-independent, sharp, hysteresis-free UCST phase transition from poly(N-acryloyl glycinamide-acrylonitrile) system. J Polym Sci A Polym Chem 2017;55:274–9. 10.1002/pola.28374. [DOI] [Google Scholar]
- [154].Deng Y, Käfer F, Chen T, Jin Q, Ji J, Agarwal S. Let There be Light: Polymeric Micelles with Upper Critical Solution Temperature as Light-Triggered Heat Nanogenerators for Combating Drug-Resistant Cancer. Small 2018;14:1802420. 10.1002/smll.201802420. [DOI] [PubMed] [Google Scholar]
- [155].Herzberger J, Fischer K, Leibig D, Bros M, Thiermann R, Frey H. Oxidation-Responsive and “Clickable” Poly(ethylene glycol) via Copolymerization of 2-(Methylthio)ethyl Glycidyl Ether. J Am Chem Soc 2016;138:9212–23. 10.1021/jacs.6b04548. [DOI] [PubMed] [Google Scholar]
- [156].Xu S, Ng G, Xu J, Kuchel RP, Yeow J, Boyer C. 2-(Methylthio)ethyl Methacrylate: A Versatile Monomer for Stimuli Responsiveness and Polymerization-Induced Self-Assembly in the Presence of Air. ACS Macro Lett 2017;6:1237–44. 10.1021/acsmacrolett.7b00731. [DOI] [PubMed] [Google Scholar]
- [157].Criado-Gonzalez M, Mecerreyes D. Thioether-based ROS responsive polymers for biomedical applications. J Mater Chem B 2022;10:7206–21. 10.1039/d2tb00615d. [DOI] [PubMed] [Google Scholar]
- [158].El-Mohtadi F, d’Arcy R, Tirelli N. Oxidation-Responsive Materials: Biological Rationale, State of the Art, Multiple Responsiveness, and Open Issues. Macromol Rapid Commun 2019;40:1800699. 10.1002/marc.201800699. [DOI] [PubMed] [Google Scholar]
- [159].Savariar EN, Ghosh S, Gonz lez DC, Thayumanavan S. Disassembly of Noncovalent Amphiphilic Polymers with Proteins and Utility in Pattern Sensing. J Am Chem Soc 2008;130:5416–7. 10.1021/ja800164z. [DOI] [PubMed] [Google Scholar]
- [160].Gonz lez DC, Savariar EN, Thayumanavan S. Fluorescence Patterns from Supramolecular Polymer Assembly and Disassembly for Sensing Metallo- and Nonmetalloproteins. J Am Chem Soc 2009;131:7708–16. 10.1021/ja900579g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Torres DA, Garzoni M, Subrahmanyam AV, Pavan GM, Thayumanavan S. Protein-Triggered Supramolecular Disassembly: Insights Based on Variations in Ligand Location in Amphiphilic Dendrons. J Am Chem Soc 2014;136:5385–99. 10.1021/ja500634u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Azagarsamy MA, Yesilyurt V, Thayumanavan S. Disassembly of Dendritic Micellar Containers Due to Protein Binding. J Am Chem Soc 2010;132:4550–1. 10.1021/ja100746d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Molla MR, Prasad P, Thayumanavan S. Protein-Induced Supramolecular Disassembly of Amphiphilic Polypeptide Nanoassemblies. J Am Chem Soc 2015;137:7286–9. 10.1021/jacs.5b04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Liu H, Westley J, Thayumanavan S. Excimer–monomer fluorescence changes by supramolecular disassembly for protein sensing and quantification. Chem Commun 2021;57:9776–9. 10.1039/d1cc03944j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Gao J, Liu X, Secinti H, Jiang Z, Munkhbat O, Xu Y, et al. Photoactivation of Ligands for Extrinsically and Intrinsically Triggered Disassembly of Amphiphilic Nanoassemblies. Eur J Chem 2018;24:1789–94. 10.1002/chem.201705217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Cabane E, Malinova V, Meier W. Synthesis of Photocleavable Amphiphilic Block Copolymers: Toward the Design of Photosensitive Nanocarriers. Macromol Rapid Commun 2010;211:1847–56. 10.1002/macp.201000151. [DOI] [Google Scholar]
- [167].Patil NG, Basutkar NB, Ambade AV. Visible light-triggered disruption of micelles of an amphiphilic block copolymer with BODIPY at the junction. Chem Commun 2015;51:17708–11. 10.1039/c5cc06820g. [DOI] [PubMed] [Google Scholar]
- [168].Strasser P, Russo M, Stadler P, Breiteneder P, Redhammer G, Himmelsbach M, et al. Green-light photocleavable meso -methyl BODIPY building blocks for macromolecular chemistry. Polym Chem 2021;12:6927–36. 10.1039/d1py01245b. [DOI] [Google Scholar]
- [169].Petrova SL, Jäger E, Jäger A, Höcherl A, Konefał R, Zhigunov A, et al. Development of an Acid-Labile Ketal Linked Amphiphilic Block Copolymer Nanoparticles for pH-Triggered Release of Paclitaxel. Polymers 2021;13:1465. 10.3390/polym13091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sourkohi BK, Cunningham A, Zhang Q, Oh JK. Biodegradable Block Copolymer Micelles with Thiol-Responsive Sheddable Coronas. Biomacromolecules 2011;12:3819–25. 10.1021/bm2011032. [DOI] [PubMed] [Google Scholar]
- [171].Saravanakumar G, Park H, Kim J, Park D, Pramanick S, Kim DH, et al. Miktoarm Amphiphilic Block Copolymer with Singlet Oxygen-Labile Stereospecific β-Aminoacrylate Junction: Synthesis, Self-Assembly, and Photodynamically Triggered Drug Release. Biomacromolecules 2018;19:2202–13. 10.1021/acs.biomac.8b00290. [DOI] [PubMed] [Google Scholar]
- [172].Segal M, Avinery R, Buzhor M, Shaharabani R, Harnoy AJ, Tirosh E, et al. Molecular Precision and Enzymatic Degradation: From Readily to Undegradable Polymeric Micelles by Minor Structural Changes. J Am Chem Soc 2017;139:803–10. 10.1021/jacs.6b10624. [DOI] [PubMed] [Google Scholar]
- [173].Habraken GJM, Peeters M, Thornton PD, Koning CE, Heise A. Selective Enzymatic Degradation of Self-Assembled Particles from Amphiphilic Block Copolymers Obtained by the Combination of N-Carboxyanhydride and Nitroxide-Mediated Polymerization. Biomacromolecules 2011;12:3761–9. 10.1021/bm2010033. [DOI] [PubMed] [Google Scholar]
- [174].Iwamura M, Ishikawa T, Koyama Y, Sakuma K, Iwamura H. 1-Pyrenylmethyl esters, photolabile protecting groups for carboxylic acids. Tetrahedron Lett 1987;28:679–82. 10.1016/s0040-4039(00)95811-8. [DOI] [Google Scholar]
- [175].Zhao Y Light-Responsive Block Copolymer Micelles. Macromolecules 2012;45:3647–57. 10.1021/ma300094t. [DOI] [Google Scholar]
- [176].Gohy J-F, Zhao Y Photo-responsive block copolymer micelles: design and behavior. Chem Soc Rev 2013;42:7117–29. 10.1039/c3cs35469e. [DOI] [PubMed] [Google Scholar]
- [177].Sun W, Parowatkin M, Steffen W, Butt H, Mailänder V, Wu S. Ruthenium-Containing Block Copolymer Assemblies: Red-Light-Responsive Metallopolymers with Tunable Nanostructures for Enhanced Cellular Uptake and Anticancer Phototherapy. Adv Healthc Mater 2016;5:467–73. 10.1002/adhm.201500827. [DOI] [PubMed] [Google Scholar]
- [178].He M, Wang R, Wan P, Wang H, Cheng Y, Miao P, et al. Biodegradable Ru-Containing Polycarbonate Micelles for Photoinduced Anticancer Multitherapeutic Agent Delivery and Phototherapy Enhancement. Biomacromolecules 2022;23:1733–44. 10.1021/acs.biomac.1c01651. [DOI] [PubMed] [Google Scholar]
- [179].Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliver Rev 2012;64:1005–20. 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Olejniczak J, Carling C-J, Almutairi A. Photocontrolled release using one-photon absorption of visible or NIR light. J Control Release 2015;219:18–30. 10.1016/j.jconrel.2015.09.030. [DOI] [PubMed] [Google Scholar]
- [181].Karimi M, Zangabad PS, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light. J Am Chem Soc 2017;139:4584–610. 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Liu G, Liu W, Dong C-M. UV- and NIR-responsive polymeric nanomedicines for on-demand drug delivery. Polym Chem 2013;4:3431–43. 10.1039/c3py21121e. [DOI] [Google Scholar]
- [183].Fomina N, McFearin CL, Almutairi A. Increasing materials’ response to two-photon NIR light via self-immolative dendritic scaffolds. Chem Commun 2012;48:9138–40. 10.1039/c2cc00072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Yan B, Boyer J-C, Branda NR, Zhao Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J Am Chem Soc 2011;133:19714–7. 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- [185].Wang W, Liu Q, Zhan C, Barhoumi A, Yang T, Wylie RG, et al. Efficient Triplet–Triplet Annihilation-Based Upconversion for Nanoparticle Phototargeting. Nano Lett 2015;15:6332–8. 10.1021/acs.nanolett.5b01325. [DOI] [PubMed] [Google Scholar]
- [186].Sun L-D, Wang Y-F, Yan C-H. Paradigms and Challenges for Bioapplication of Rare Earth Upconversion Luminescent Nanoparticles: Small Size and Tunable Emission/Excitation Spectra. Acc Chem Res 2014;47:1001–9. 10.1021/ar400218t. [DOI] [PubMed] [Google Scholar]
- [187].Liu Q, Wang W, Zhan C, Yang T, Kohane DS. Enhanced Precision of Nanoparticle Phototargeting in Vivo at a Safe Irradiance. Nano Lett 2016;16:4516–20. 10.1021/acs.nanolett.6b01730. [DOI] [PubMed] [Google Scholar]
- [188].Munkhbat O, Canakci M, Zheng S, Hu W, Osborne B, Bogdanov AA, et al. 19 F MRI of Polymer Nanogels Aided by Improved Segmental Mobility of Embedded Fluorine Moieties. Biomacromolecules 2018;20:790–800. 10.1021/acs.biomac.8b01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Tu L, Liao Z, Luo Z, Wu Y, Herrmann A, Huo S. Ultrasound-controlled drug release and drug activation for cancer therapy. Exploration 2021;1:20210023. 10.1002/exp.20210023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Zhang Y, Yu J, Bomba HN, Zhu Y, Gu Z. Mechanical Force-Triggered Drug Delivery. Chem Rev 2016;116:12536–63. 10.1021/acs.chemrev.6b00369. [DOI] [PubMed] [Google Scholar]
- [191].Zhou J, Shao Z, Liu J, Duan Q, Wang X, Li J, et al. From Endocytosis to Nonendocytosis: The Emerging Era of Gene Delivery. ACS Appl Bio Mater 2020;3:2686–701. 10.1021/acsabm.9b01131. [DOI] [PubMed] [Google Scholar]
- [192].Pei D, Buyanova M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjugate Chem 2019;30:273–83. 10.1021/acs.bioconjchem.8b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Pei D How Do Biomolecules Cross the Cell Membrane? Acc Chem Res 2022;55:309–18. 10.1021/acs.accounts.1c00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Binauld S, Stenzel MH. Acid-degradable polymers for drug delivery: a decade of innovation. Chem Commun 2012;49:2082–102. 10.1039/c2cc36589h. [DOI] [PubMed] [Google Scholar]
- [195].Timmers M, Weterings J, Geijn M, Bell R, Lenting PE, Rijcken CJF, et al. A New Class of Tunable Acid-Sensitive Linkers for Native Drug Release Based on the Trityl Protecting Group. Bioconjugate Chem 2022;33:1707–15. 10.1021/acs.bioconjchem.2c00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hu Q, Rijcken CJF, Gaal E van, Brundel P, Kostkova H, Etrych T, et al. Tailoring the physicochemical properties of core-crosslinked polymeric micelles for pharmaceutical applications. J Control Release 2016;244:314–25. 10.1016/j.jconrel.2016.07.012. [DOI] [PubMed] [Google Scholar]
- [197].Xu Q, He C, Xiao C, Chen X. Reactive Oxygen Species (ROS) Responsive Polymers for Biomedical Applications. Macromol Biosci 2016;16:635–46. 10.1002/mabi.201500440. [DOI] [PubMed] [Google Scholar]
- [198].Lv S, Wu Y, Cai K, He H, Li Y, Lan M, et al. High Drug Loading and Sub-Quantitative Loading Efficiency of Polymeric Micelles Driven by Donor–Receptor Coordination Interactions. J Am Chem Soc 2018;140:1235–8. 10.1021/jacs.7b12776. [DOI] [PubMed] [Google Scholar]
- [199].Kim JS, Jo SD, Seah GL, Kim I, Nam YS. ROS-induced biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics. J Ind Eng Chem 2015;21:1137–42. 10.1016/j.jiec.2014.05.026. [DOI] [Google Scholar]
- [200].Liu B, Thayumanavan S. Mechanistic Investigation on Oxidative Degradation of ROS-Responsive Thioacetal/Thioketal Moieties and Their Implications. Cell Reports Phys Sci 2020;1:100271. 10.1016/j.xcrp.2020.100271. [DOI] [Google Scholar]
- [201].Zhang W, Hu X, Shen Q, Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat Commun 2019;10:1704. 10.1038/s41467-019-09566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hu X, Hu J, Tian J, Ge Z, Zhang G, Luo K, et al. Polyprodrug Amphiphiles: Hierarchical Assemblies for Shape-Regulated Cellular Internalization, Trafficking, and Drug Delivery. J Am Chem Soc 2013;135:17617–29. 10.1021/ja409686x. [DOI] [PubMed] [Google Scholar]
- [203].Li Y, Liu G, Wang X, Hu J, Liu S. Enzyme-Responsive Polymeric Vesicles for Bacterial-Strain-Selective Delivery of Antimicrobial Agents. Angew Chem Int Ed 2016;55:1760–4. 10.1002/anie.201509401. [DOI] [PubMed] [Google Scholar]
- [204].Yao C, Li Y, Wang Z, Song C, Hu X, Liu S. Cytosolic NQO1 Enzyme-Activated Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Polymeric Vesicles. ACS Nano 2020;14:1919–35. 10.1021/acsnano.9b08285. [DOI] [PubMed] [Google Scholar]
- [205].Rao J, Hottinger C, Khan A. Enzyme-Triggered Cascade Reactions and Assembly of Abiotic Block Copolymers into Micellar Nanostructures. J Am Chem Soc 2014;136:5872–5. 10.1021/ja501632r. [DOI] [PubMed] [Google Scholar]
- [206].Kemp DS, Hoyng CF. New protective groups for peptide synthesis—I the Bic group base and solvent lability of the 5-benzisoxazolylmetheleneoxycarbonylamino function. Tetrahedron Lett 1975;16:4625–8. 10.1016/s0040-4039(00)91036-0. [DOI] [Google Scholar]
- [207].Carl PL, Chakravarty PK, Katzenellenbogen JA. A novel connector linkage applicable in prodrug design. J Med Chem 1981;24:479–80. 10.1021/jm00137a001. [DOI] [PubMed] [Google Scholar]
- [208].Rose DA, Treacy JW, Yang ZJ, Ko JH, Houk KN, Maynard HD. Self-Immolative Hydroxybenzylamine Linkers for Traceless Protein Modification. J Am Chem Soc 2022;144:6050–8. 10.1021/jacs.2c01136. [DOI] [PubMed] [Google Scholar]
- [209].Babin BM, Fernandez-Cuervo G, Sheng J, Green O, Ordonez AA, Turner ML, et al. Chemiluminescent Protease Probe for Rapid, Sensitive, and Inexpensive Detection of Live Mycobacterium tuberculosis. ACS Central Sci 2021;7:803–14. 10.1021/acscentsci.0c01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Gnaim S, Shabat D. Quinone-Methide Species, A Gateway to Functional Molecular Systems: From Self-Immolative Dendrimers to Long-Wavelength Fluorescent Dyes. Acc Chem Res 2014;47:2970–84. 10.1021/ar500179y. [DOI] [PubMed] [Google Scholar]
- [211].Sagi A, Weinstain R, Karton N, Shabat D. Self-Immolative Polymers. J Am Chem Soc 2008;130:5434–5. 10.1021/ja801065d. [DOI] [PubMed] [Google Scholar]
- [212].Alouane A, Labruère R, Saux TL, Schmidt F, Jullien L. Self-Immolative Spacers: Kinetic Aspects, Structure–Property Relationships, and Applications. Angew Chem Int Ed 2015;54:7492–509. 10.1002/anie.201500088. [DOI] [PubMed] [Google Scholar]
- [213].Roth ME, Green O, Gnaim S, Shabat D. Dendritic, Oligomeric, and Polymeric Self-Immolative Molecular Amplification. Chem Rev 2016;116:1309–52. 10.1021/acs.chemrev.5b00372. [DOI] [PubMed] [Google Scholar]
- [214].DeWit MA, Gillies ER. A Cascade Biodegradable Polymer Based on Alternating Cyclization and Elimination Reactions. J Am Chem Soc 2009;131:18327–34. 10.1021/ja905343x. [DOI] [PubMed] [Google Scholar]
- [215].Kim JW, Kim HJ, Park J, Chae JA, Song H-W, Choi E, et al. Self-Immolative and Amphiphilic Poly(benzyl ether)-Based Copolymers: Synthesis and Triggered Demicellization via Head-to-Tail Depolymerization. Macromolecules 2022;55:6140–9. 10.1021/acs.macromol.2c00615. [DOI] [Google Scholar]
- [216].Ding Z, Cen J, Wu Y, Zhong K, Liu G, Hu J, et al. Self-Immolative nanoparticles for stimuli-triggered activation, covalent trapping and accumulation of in situ generated small molecule theranostic fragments. Giant 2020;1:1–13. 10.1016/j.giant.2020.100012. [DOI] [Google Scholar]
- [217].Liu J, Cheng R, Eps NV, Wang N, Morizumi T, Ou W-L, et al. Genetically Encoded Quinone Methides Enabling Rapid, Site-Specific, and Photocontrolled Protein Modification with Amine Reagents. J Am Chem Soc 2020;142:17057–68. 10.1021/jacs.0c06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Huang C, Liu Y, Rokita SE. Targeting duplex DNA with the reversible reactivity of quinone methides. Signal Transduct Target Ther 2016;1:16009. 10.1038/sigtrans.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Liu G, Zhang G, Hu J, Wang X, Zhu M, Liu S. Hyperbranched Self-Immolative Polymers (hSIPs) for Programmed Payload Delivery and Ultrasensitive Detection. J Am Chem Soc 2015;137:11645–55. 10.1021/jacs.5b05060. [DOI] [PubMed] [Google Scholar]
- [220].Yeung K, Kim H, Mohapatra H, Phillips ST. Surface-Accessible Detection Units in Self-Immolative Polymers Enable Translation of Selective Molecular Detection Events into Amplified Responses in Macroscopic, Solid-State Plastics. J Am Chem Soc 2015;137:5324–7. 10.1021/jacs.5b02799. [DOI] [PubMed] [Google Scholar]
- [221].Xiao Y, Li H, Zhang B, Cheng Z, Li Y, Tan X, et al. Modulating the Depolymerization of Self-Immolative Brush Polymers with Poly(benzyl ether) Backbones. Macromolecules 2018;51:2899–905. 10.1021/acs.macromol.8b00208. [DOI] [PubMed] [Google Scholar]
- [222].Addy PS, Shivrayan M, Cencer M, Zhuang J, Moore JS, Thayumanavan S. Polymer with Competing Depolymerization Pathways: Chain Unzipping versus Chain Scission. ACS Macro Lett 2020;9:855–9. 10.1021/acsmacrolett.0c00250. [DOI] [PubMed] [Google Scholar]
- [223].Kumar V, Harris JT, Ribbe A, Franc M, Bae Y, McNeil AJ, et al. Construction from Destruction: Hydrogel Formation from Triggered Depolymerization-Based Release of an Enzymatic Catalyst. ACS Macro Lett 2020;9:377–81. 10.1021/acsmacrolett.0c00023. [DOI] [PubMed] [Google Scholar]
- [224].Yardley RE, Gillies ER. Multi-stimuli-responsive self-immolative polymer assemblies. J Polym Sci A Polym Chem 2018;56:1868–77. 10.1002/pola.29070. [DOI] [Google Scholar]
- [225].Esser-Kahn AP, Sottos NR, White SR, Moore JS. Programmable Microcapsules from Self-Immolative Polymers. J Am Chem Soc 2010;132:10266–8. 10.1021/ja104812p. [DOI] [PubMed] [Google Scholar]
- [226].Fan B, Trant JF, Wong AD, Gillies ER. Polyglyoxylates: A Versatile Class of Triggerable Self-Immolative Polymers from Readily Accessible Monomers. J Am Chem Soc 2014;136:10116–23. 10.1021/ja504727u. [DOI] [PubMed] [Google Scholar]
- [227].Seo W, Phillips ST. Patterned Plastics That Change Physical Structure in Response to Applied Chemical Signals. J Am Chem Soc 2010;132:9234–5. 10.1021/ja104420k. [DOI] [PubMed] [Google Scholar]
- [228].Kumar K, Casta o EJ, eidner AR, Yildirim A, Goodwin AP. Depolymerizable Poly(O-vinyl carbamate-alt-sulfones) as Customizable Macromolecular Scaffolds for Mucosal Drug Delivery. ACS Macro Lett 2016;5:636–40. 10.1021/acsmacrolett.6b00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Gambles MT, Fan B, Borecki A, Gillies ER. Hybrid Polyester Self-Immolative Polymer Nanoparticles for Controlled Drug Release. ACS Omega 2018;3:5002–11. 10.1021/acsomega.8b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Kenaree AR, Gillies ER. Controlled Polymerization of Ethyl Glyoxylate Using Alkyllithium and Alkoxide Initiators. Macromolecules 2018;51:5501–10. 10.1021/acs.macromol.8b01007. [DOI] [Google Scholar]
- [231].Liang X, Gillies ER. Self-immolative Amphiphilic Diblock Copolymers with Individually Triggerable Blocks. ACS Polym Au 2022;2:313–23. 10.1021/acspolymersau.2c00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Sirianni QEA, Kenaree AR, Gillies ER. Polyglyoxylamides: Tuning Structure and Properties of Self-Immolative Polymers. Macromolecules 2019;52:262–70. 10.1021/acs.macromol.8b02616. [DOI] [Google Scholar]
- [233].Sirianni QEA, Wang T, Borecki A, Deng Z, Ronald JA, Gillies ER. Self-immolative polyplexes for DNA delivery. Biomater Sci 2022;10:2557–67. 10.1039/d1bm01684a. [DOI] [PubMed] [Google Scholar]
- [234].Kaitz JA, Diesendruck CE, Moore JS. End Group Characterization of Poly(phthalaldehyde): Surprising Discovery of a Reversible, Cationic Macrocyclization Mechanism. J Am Chem Soc 2013;135:12755–61. 10.1021/ja405628g. [DOI] [PubMed] [Google Scholar]
- [235].Coulembier O, Knoll A, Pires D, Gotsmann B, Duerig U, Frommer J, et al. Probe-Based Nanolithography: Self-Amplified Depolymerization Media for Dry Lithography. Macromolecules 2010;43:572–4. 10.1021/ma9019152. [DOI] [Google Scholar]
- [236].Kaitz JA, Possanza CM, Song Y, Diesendruck CE, Spiering AJH, Meijer EW, et al. Depolymerizable, adaptive supramolecular polymer nanoparticles and networks. Polym Chem 2014;5:3788–94. 10.1039/c3py01690k. [DOI] [Google Scholar]
- [237].DiLauro AM, Robbins JS, Phillips ST. Reproducible and Scalable Synthesis of End-Cap-Functionalized Depolymerizable Poly(phthalaldehydes). Macromolecules 2013;46:2963–8. 10.1021/ma4001594. [DOI] [Google Scholar]
- [238].Pessoni L, inter JD, Surin M, Hergu N, Delbosc N, Lazzaroni R, et al. Synthesis of Polyphthalaldehyde-Based Block Copolymers: Utilization of a Thermo-Sacrificial Segment for an Easy Access to Fine-Tuned Poly(3-hexylthiophene) Nanostructured Films. Macromolecules 2016;49:3001–8. 10.1021/acs.macromol.6b00283. [DOI] [Google Scholar]
- [239].DiLauro AM, Abbaspourrad A, Weitz DA, Phillips ST. Stimuli-Responsive Core–Shell Microcapsules with Tunable Rates of Release by Using a Depolymerizable Poly(phthalaldehyde) Membrane. Macromolecules 2013;46:3309–13. 10.1021/ma400456p. [DOI] [Google Scholar]
- [240].Kishore K, Ganesh K. Polymer Synthesis/Polymer Engineering. Adv Polym Sci 1995;121:81–121. 10.1007/bfb0018579. [DOI] [Google Scholar]
- [241].Bang E-K, Lista M, Sforazzini G, Sakai N, Matile S. Poly(disulfide)s. Chem Sci 2012;3:1752–63. 10.1039/c2sc20098h. [DOI] [Google Scholar]
- [242].Sakai N, Lista M, Kel O, Sakurai S, Emery D, Mareda J, et al. Self-Organizing Surface-Initiated Polymerization: Facile Access to Complex Functional Systems. J Am Chem Soc 2011;133:15224–7. 10.1021/ja203792n. [DOI] [PubMed] [Google Scholar]
- [243].Kim S, Wittek KI, Lee Y. Synthesis of poly(disulfide)s with narrow molecular weight distributions via lactone ring-opening polymerization. Chem Sci 2020;11:4882–6. 10.1039/d0sc00834f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Liu Y, Jia Y, Wu Q, Moore JS. Architecture-Controlled Ring-Opening Polymerization for Dynamic Covalent Poly(disulfide)s. J Am Chem Soc 2019;141:17075–80. 10.1021/jacs.9b08957. [DOI] [PubMed] [Google Scholar]
- [245].Gasparini G, Bang E-K, Molinard G, Tulumello DV, Ward S, Kelley SO, et al. Cellular Uptake of Substrate-Initiated Cell-Penetrating Poly(disulfide)s. J Am Chem Soc 2014;136:6069–74. 10.1021/ja501581b. [DOI] [PubMed] [Google Scholar]
- [246].Du S, Liew SS, Li L, Yao SQ. Bypassing Endocytosis: Direct Cytosolic Delivery of Proteins. J Am Chem Soc 2018;140:15986–96. 10.1021/jacs.8b06584. [DOI] [PubMed] [Google Scholar]
- [247].Laurent Q, Martinent R, Lim B, Pham A-T, Kato T, L pez-Andarias J, et al. Thiol-Mediated Uptake. JACS Au 2021;1:710–28. 10.1021/jacsau.1c00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Yang H, Shen W, Liu W, Chen L, Zhang P, Xiao C, et al. PEGylated Poly(α-lipoic acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy. Biomacromolecules 2018;19:4492–503. 10.1021/acs.biomac.8b01394. [DOI] [PubMed] [Google Scholar]
- [249].Bej R, Sarkar J, Ghosh S. Structural diversity in poly(disulfide)s. J Polym Sci A Polym Chem 2018;56:194–202. 10.1002/pola.28882. [DOI] [Google Scholar]
- [250].Du J, Choi B, Liu Y, Feng A, Thang SH. Degradable pH and redox dual responsive nanoparticles for efficient covalent drug delivery. Polym Chem 2019;10:1291–8. 10.1039/c8py01583j. [DOI] [Google Scholar]
- [251].Basak D, Bej R, Ghosh S. Amphiphilic poly(disulfide) micelles and a remarkable impact of the core hydrophobicity on redox responsive disassembly. Polym Chem 2015;6:6465–74. 10.1039/c5py00969c. [DOI] [Google Scholar]
- [252].Wang Y, Jiang Y, Wei D, Singh P, Yu Y, Lee T, et al. Nanoparticle-mediated convection-enhanced delivery of a DNA intercalator to gliomas circumvents temozolomide resistance. Nat Biomed Eng 2021;5:1048–58. 10.1038/s41551-021-00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Li S, Saw PE, Lin C, Nie Y, Tao W, Farokhzad OC, et al. Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy. Biomaterials 2020;234:119760. 10.1016/j.biomaterials.2020.119760. [DOI] [PubMed] [Google Scholar]
- [254].Wang H, Hu Y, Wang Y, Lu J, Lu H. Doxorubicin@ PEPylated interferon-polydisulfide: A multi-responsive nanoparticle for enhanced chemo–protein combination therapy. Giant 2021;5:100040. 10.1016/j.giant.2020.100040. [DOI] [Google Scholar]
- [255].Zhu Y, Lin M, Hu W, Wang J, Zhang Z, Zhang K, et al. Controllable Disulfide Exchange Polymerization of Polyguanidine for Effective Biomedical Applications by Thiol-Mediated Uptake. Angew Chem Int Ed 2022;61:e202200535. 10.1002/anie.202200535. [DOI] [PubMed] [Google Scholar]
- [256].Ma N, Li Y, Xu H, Wang Z, Zhang X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J Am Chem Soc 2010;132:442–3. 10.1021/ja908124g. [DOI] [PubMed] [Google Scholar]
- [257].Wang L, Zhu K, Cao W, Sun C, Lu C, Xu H. ROS-triggered degradation of selenide-containing polymers based on selenoxide elimination. Polym Chem 2019;10:2039–46. 10.1039/c9py00171a. [DOI] [Google Scholar]
- [258].Xu L, Zhao M, Zhang H, Gao W, Guo Z, Zhang X, et al. Cinnamaldehyde-Based Poly(ester-thioacetal) To Generate Reactive Oxygen Species for Fabricating Reactive Oxygen Species-Responsive Nanoparticles. Biomacromolecules 2018;19:4658–67. 10.1021/acs.biomac.8b01423. [DOI] [PubMed] [Google Scholar]
- [259].Chen D, Zhang G, Li R, Guan M, Wang X, Zou T, et al. Biodegradable, Hydrogen Peroxide, and Glutathione Dual Responsive Nanoparticles for Potential Programmable Paclitaxel Release. J Am Chem Soc 2018;140:7373–6. 10.1021/jacs.7b12025. [DOI] [PubMed] [Google Scholar]
- [260].McKinlay CJ, Vargas JR, Blake TR, Hardy JW, Kanada M, Contag CH, et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc National Acad Sci 2017;114:E448–56. 10.1073/pnas.1614193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Blake TR, Ho WC, Turlington CR, Zang X, Huttner MA, Wender PA, et al. Synthesis and mechanistic investigations of pH-responsive cationic poly(aminoester)s. Chem Sci 2020;11:2951–66. 10.1039/c9sc05267d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].McKinlay CJ, Benner NL, Haabeth OA, Waymouth RM, Wender PA. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc National Acad Sci 2018;115:E5859–66. 10.1073/pnas.1805358115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Haabeth OAW, Blake TR, McKinlay CJ, Waymouth RM, Wender PA, Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc National Acad Sci 2018;115:E9153–61. 10.1073/pnas.1810002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and Near-IR Triggered Release from Polymeric Nanoparticles. J Mater Chem B 2010;132:9540–2. 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Xiao Y, Tan X, Li Z, Zhang K. Self-immolative polymers in biomedicine. J Mater Chem B 2020;8:6697–709. 10.1039/d0tb01119c. [DOI] [PubMed] [Google Scholar]
- [266].Ma L, Baumgartner R, Zhang Y, Song Z, Cai K, Cheng J. UV-responsive degradable polymers derived from 1-(4-aminophenyl) ethane-1,2-diol. J Polym Sci A Polym Chem 2015;53:1161–8. 10.1002/pola.27550. [DOI] [Google Scholar]
- [267].Zhu D, Yan H, Liu X, Xiang J, Zhou Z, Tang J, et al. Intracellularly Disintegratable Polysulfoniums for Efficient Gene Delivery. Adv Funct Mater 2017;27:1606826. 10.1002/adfm.201606826. [DOI] [Google Scholar]
- [268].Liu X, Zhao Z, Wu F, Chen Y, Yin L. Tailoring Hyperbranched Poly(β-amino ester) as a Robust and Universal Platform for Cytosolic Protein Delivery. Adv Mater 2022;34:2108116. 10.1002/adma.202108116. [DOI] [PubMed] [Google Scholar]
- [269].Zhang J, Hao X, Sang W, Yan Q. Hydrogen Polysulfide Biosignal-Responsive Polymersomes as a Nanoplatform for Distinguishing Intracellular Reactive Sulfur Species (RSS). Small 2017;13:1701601. 10.1002/smll.201701601. [DOI] [PubMed] [Google Scholar]
- [270].Zhou Y, Gu Z, Liu C, Yang S, Ma X, Chen Q, et al. A Polymeric Nanobeacon for Monitoring the Fluctuation of Hydrogen Polysulfides during Fertilization and Embryonic Development. Angew Chem Int Ed 2022;61:e202114504. 10.1002/anie.202114504. [DOI] [PubMed] [Google Scholar]
- [271].Liu P, Zhang W, Deng J, Zheng Y, Weng J, Yu F, et al. Chain-shattering polymeric sulfur dioxide prodrug micelles for redox-triggered gas therapy of osteosarcoma. J Mater Chem B 2022;10:5263–71. 10.1039/d2tb00287f. [DOI] [PubMed] [Google Scholar]
- [272].Wong AD, Prinzen AL, Gillies ER. Poly(ester amide)s with pendant azobenzenes: multi-responsive self-immolative moieties for modulating polymer assemblies. Polym Chem 2016;7:1871–81. 10.1039/c5py01824b. [DOI] [Google Scholar]
- [273].Tan J, Deng Z, Song C, Xu J, Zhang Y, Yu Y, et al. Coordinating External and Built-In Triggers for Tunable Degradation of Polymeric Nanoparticles via Cycle Amplification. J Am Chem Soc 2021;143:13738–48. 10.1021/jacs.1c05617. [DOI] [PubMed] [Google Scholar]
- [274].Hsu P-H, Arboleda C, Stubelius A, Li L-W, Olejniczak J, Almutairi A. Highly responsive and rapid hydrogen peroxide-triggered degradation of polycaprolactone nanoparticles. Biomater Sci 2020;8:2394–7. 10.1039/c9bm02019e. [DOI] [PubMed] [Google Scholar]
- [275].Zhang L-J, Deng X-X, Du F-S, Li Z-C. Chemical Synthesis of Functional Poly(4-hydroxybutyrate) with Controlled Degradation via Intramolecular Cyclization. Macromolecules 2013;46:9554–62. 10.1021/ma402191r. [DOI] [Google Scholar]
- [276].Lux C de G, Almutairi A. Intramolecular Cyclization for Stimuli-Controlled Depolymerization of Polycaprolactone Particles Leading to Disassembly and Payload Release. ACS Macro Lett 2013;2:432–5. 10.1021/mz400129h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Sun J, Birnbaum W, Anderski J, Picker M-T, Mulac D, Langer K, et al. Use of Light-Degradable Aliphatic Polycarbonate Nanoparticles As Drug Carrier for Photosensitizer. Biomacromolecules 2018;19:4677–90. 10.1021/acs.biomac.8b01446. [DOI] [PubMed] [Google Scholar]
- [278].Olejniczak J, Chan M, Almutairi A. Light-Triggered Intramolecular Cyclization in Poly(lactic-co-glycolic acid)-Based Polymers for Controlled Degradation. Macromolecules 2015;48:3166–72. 10.1021/acs.macromol.5b00455. [DOI] [Google Scholar]
- [279].Wang M, Zhai Y, Ye H, Lv Q, Sun B, Luo C, et al. High Co-loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019;13:7010–23. 10.1021/acsnano.9b02096. [DOI] [PubMed] [Google Scholar]
- [280].Mavila S, Eivgi O, Berkovich I, Lemcoff NG. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem Rev 2016;116:878–961. 10.1021/acs.chemrev.5b00290. [DOI] [PubMed] [Google Scholar]
- [281].Lages M, Nicolas J. In situ encapsulation of biologically active ingredients into polymer particles by polymerization in dispersed media. Prog Polym Sci 2023;137:101637. 10.1016/j.progpolymsci.2022.101637. [DOI] [Google Scholar]
- [282].Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Fr chet JMJ. Acetal-Derivatized Dextran: An Acid-Responsive Biodegradable Material for Therapeutic Applications. J Am Chem Soc 2008;130:10494–5. 10.1021/ja803947s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Murthy N, Thng YX, Schuck S, Xu MC, Fréchet JMJ. A Novel Strategy for Encapsulation and Release of Proteins: Hydrogels and Microgels with Acid-Labile Acetal Cross-Linkers. J Am Chem Soc 2002;124:12398–9. 10.1021/ja026925r. [DOI] [PubMed] [Google Scholar]
- [284].Griset AP, Walpole J, Liu R, Gaffey A, Colson YL, Grinstaff MW. Expansile Nanoparticles: Synthesis, Characterization, and in Vivo Efficacy of an Acid-Responsive Polymeric Drug Delivery System. J Am Chem Soc 2009;131:2469–71. 10.1021/ja807416t. [DOI] [PubMed] [Google Scholar]
- [285].Wang X, Wang L, Yang S, Zhang M, Xiong Q, Zhao H, et al. Construction of Multifunctionalizable, Core-Cross-Linked Polymeric Nanoparticles via Dynamic Covalent Bond. Macromolecules 2014;47:1999–2009. 10.1021/ma402402p. [DOI] [Google Scholar]
- [286].Jackson AW, Stakes C, Fulton DA. The formation of core cross-linked star polymer and nanogel assemblies facilitated by the formation of dynamic covalent imine bonds. Polym Chem 2011;2:2500–11. 10.1039/c1py00261a. [DOI] [Google Scholar]
- [287].Belowich ME, Stoddart JF. Dynamic imine chemistry. Chem Soc Rev 2012;41:2003–24. 10.1039/c2cs15305j. [DOI] [PubMed] [Google Scholar]
- [288].Ruan C, Liu L, Wang Q, Chen X, Chen Q, Lu Y, et al. Reactive Oxygen Species-Biodegradable Gene Carrier for the Targeting Therapy of Breast Cancer. ACS Appl Mater Inter 2018;10:10398–408. 10.1021/acsami.8b01712. [DOI] [PubMed] [Google Scholar]
- [289].Hong BJ, Chipre AJ, Nguyen ST. Acid-Degradable Polymer-Caged Lipoplex (PCL) Platform for siRNA Delivery: Facile Cellular Triggered Release of siRNA. J Am Chem Soc 2013;135:17655–8. 10.1021/ja404491r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Liu B, Thayumanavan S. Substituent Effects on the pH Sensitivity of Acetals and Ketals and Their Correlation with Encapsulation Stability in Polymeric Nanogels. J Am Chem Soc 2017;139:2306–17. 10.1021/jacs.6b11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Zhang P, Zhang Y, Ding X, Shen W, Li M, Wagner E, et al. A Multistage Cooperative Nanoplatform Enables Intracellular Co-Delivery of Proteins and Chemotherapeutics for Cancer Therapy. Adv Mater 2020;32:2000013. 10.1002/adma.202000013. [DOI] [PubMed] [Google Scholar]
- [292].Hu X, Tian J, Liu T, Zhang G, Liu S. Photo-Triggered Release of Caged Camptothecin Prodrugs from Dually Responsive Shell Cross-Linked Micelles. Macromolecules 2013;46:6243–56. 10.1021/ma400691j. [DOI] [Google Scholar]
- [293].Li L, Li D, Zhang M, He J, Liu J, Ni P. One-Pot Synthesis of pH/Redox Responsive Polymeric Prodrug and Fabrication of Shell Cross-Linked Prodrug Micelles for Antitumor Drug Transportation. Bioconjugate Chem 2018;29:2806–17. 10.1021/acs.bioconjchem.8b00421. [DOI] [PubMed] [Google Scholar]
- [294].Xiong D, Wen L, Peng S, Xu J, Zhang L. Reversible Cross-Linked Mixed Micelles for pH Triggered Swelling and Redox Triggered Degradation for Enhanced and Controlled Drug Release. Pharm 2020;12:258. 10.3390/pharmaceutics12030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Xiong D, Yao N, Gu H, Wang J, Zhang L. Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polymer 2017;114:161–72. 10.1016/j.polymer.2017.03.002. [DOI] [Google Scholar]
- [296].Yuan C, Hong B, Chang Y, Mao J, Li Y, Xu Y, et al. Cross-Linking Induced Self-Organization of Polymers into Degradable Assemblies. ACS Appl Mater Inter 2017;9:14700–8. 10.1021/acsami.7b02252. [DOI] [PubMed] [Google Scholar]
- [297].Wu Y, Chen W, Meng F, Wang Z, Cheng R, Deng C, et al. Core-crosslinked pH-sensitive degradable micelles: A promising approach to resolve the extracellular stability versus intracellular drug release dilemma. J Control Release 2012;164:338–45. 10.1016/j.jconrel.2012.07.011. [DOI] [PubMed] [Google Scholar]
- [298].Yi X-Q, Zhang Q, Zhao D, Xu J-Q, Zhong Z-L, Zhuo R-X, et al. Preparation of pH and redox dual-sensitive core crosslinked micelles for overcoming drug resistance of DOX. Polym Chem 2016;7:1719–29. 10.1039/c5py01783a. [DOI] [Google Scholar]
- [299].Liu X, Yaszemski MJ, Lu L. Expansile crosslinked polymersomes for pH sensitive delivery of doxorubicin. Biomater Sci 2016;4:245–9. 10.1039/c5bm00269a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Huang P, Liu J, Wang W, Li C, Zhou J, Wang X, et al. Zwitterionic Nanoparticles Constructed with Well-Defined Reduction-Responsive Shell and pH-Sensitive Core for “Spatiotemporally Pinpointed” Drug Delivery. ACS Appl Mater Inter 2014;6:14631–43. 10.1021/am503974y. [DOI] [PubMed] [Google Scholar]
- [301].Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S. Multi-stimuli responsive macromolecules and their assemblies. Chem Soc Rev 2013;42:7421–35. 10.1039/c3cs60094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Altinbasak I, Arslan M, Sanyal R, Sanyal A. Pyridyl disulfide-based thiol–disulfide exchange reaction: shaping the design of redox-responsive polymeric materials. Polym Chem 2020;11:7603–24. 10.1039/d0py01215g. [DOI] [Google Scholar]
- [303].Mutlu H, Ceper EB, Li X, Yang J, Dong W, Ozmen MM, et al. Sulfur Chemistry in Polymer and Materials Science. Macromol Rapid Commun 2019;40:1800650. 10.1002/marc.201800650. [DOI] [PubMed] [Google Scholar]
- [304].Cao XT, Kim YH, Park JM, Lim KT. One-pot syntheses of dual-responsive core cross-linked polymeric micelles and covalently entrapped drug by click chemistry. Eur Polym J 2016;78:264–73. 10.1016/j.eurpolymj.2016.03.039. [DOI] [Google Scholar]
- [305].Huang Y, Li Y, Tang Z, Su Q, Liao T, Gu Y, et al. Dual-Responsive Cross-Linked Micelles from Amphiphilic Four-Arm Star Copolymers with Different Block Ratios for Triggering DOX Release. Macromol Res 2020;28:762–71. 10.1007/s13233-020-9094-0. [DOI] [Google Scholar]
- [306].Abandansari HS, Abuali M, Nabid MR, Niknejad H. Enhance chemotherapy efficacy and minimize anticancer drug side effects by using reversibly pH- and redox-responsive cross-linked unimolecular micelles. Polymer 2017;116:16–26. 10.1016/j.polymer.2017.03.062. [DOI] [Google Scholar]
- [307].Dutta K, Hu D, Zhao B, Ribbe AE, Zhuang J, Thayumanavan S. Templated Self-Assembly of a Covalent Polymer Network for Intracellular Protein Delivery and Traceless Release. J Am Chem Soc 2017;139:5676–9. 10.1021/jacs.7b01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Dutta K, Kanjilal P, Das R, Thayumanavan S. Synergistic Interplay of Covalent and Non-Covalent Interactions in Reactive Polymer Nanoassembly Facilitates Intracellular Delivery of Antibodies. Angew Chem Int Ed 2021;60:1821–30. 10.1002/anie.202010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Ghosh S, Basu S, Thayumanavan S. Simultaneous and Reversible Functionalization of Copolymers for Biological Applications †. Macromolecules 2006;39:5595–7. 10.1021/ma061420x. [DOI] [Google Scholar]
- [310].Sui B, Cheng C, Xu P. Pyridyl Disulfide Functionalized Polymers as Nanotherapeutic Platforms. Adv Ther 2019;2:1900062. 10.1002/adtp.201900062. [DOI] [Google Scholar]
- [311].Ryu J-H, Jiwpanich S, Chacko R, Bickerton S, Thayumanavan S. Surface-Functionalizable Polymer Nanogels with Facile Hydrophobic Guest Encapsulation Capabilities. J Am Chem Soc 2010;132:8246–7. 10.1021/ja102316a. [DOI] [PubMed] [Google Scholar]
- [312].Gao J, Le S, Thayumanavan S. Enzyme Catalysis in Non-Native Environment with Unnatural Selectivity Using Polymeric Nanoreactors. Angew Chem Int Ed 2021;60:27189–94. 10.1002/anie.202109477. [DOI] [PubMed] [Google Scholar]
- [313].Wu P, Gao J, Prasad P, Dutta K, Kanjilal P, Thayumanavan S. Influence of Polymer Structure and Architecture on Drug Loading and Redox-Triggered Release. Biomacromolecules 2022;23:339–48. 10.1021/acs.biomac.1c01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Jiang Z, Liu H, He H, Ribbe AE, Thayumanavan S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020;53:2713–23. 10.1021/acs.macromol.9b02595. [DOI] [Google Scholar]
- [315].Jiang Z, Cui W, Prasad P, Touve MA, Gianneschi NC, Mager J, et al. Bait-and-Switch Supramolecular Strategy To Generate Noncationic RNA–Polymer Complexes for RNA Delivery. Biomacromolecules 2019;20:435–42. 10.1021/acs.biomac.8b01321. [DOI] [PubMed] [Google Scholar]
- [316].Li L, Ryu J-H, Thayumanavan S. Effect of Hofmeister Ions on the Size and Encapsulation Stability of Polymer Nanogels. Langmuir 2013;29:50–5. 10.1021/la3033463. [DOI] [PubMed] [Google Scholar]
- [317].Li L, Thayumanavan S. Environment-Dependent Guest Exchange in Supramolecular Hosts. Langmuir 2014;30:12384–90. 10.1021/la502760c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Boehnke N, Kammeyer JK, Damoiseaux R, Maynard HD. Stabilization of Glucagon by Trehalose Glycopolymer Nanogels. Adv Funct Mater 2018;28:1705475. 10.1002/adfm.201705475. [DOI] [Google Scholar]
- [319].Zhuang J, Garzoni M, Torres DA, Poe A, Pavan GM, Thayumanavan S. Programmable Nanoassemblies from Non-Assembling Homopolymers Using Ad Hoc Electrostatic Interactions. Angew Chem Int Ed 2017;56:4145–9. 10.1002/anie.201611688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Lin W, Ma G, Kampf N, Yuan Z, Chen S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016;17:2010–8. 10.1021/acs.biomac.6b00168. [DOI] [PubMed] [Google Scholar]
- [321].Sun W, An Z, Wu P. Revealing the distinct thermal transition behavior between PEGA-based linear polymers and their disulfide cross-linked nanogels. Phys Chem Chem Phys 2017;19:25746–53. 10.1039/c7cp05084d. [DOI] [PubMed] [Google Scholar]
- [322].Yuan C, Raghupathi K, Popere BC, Ventura J, Dai L, Thayumanavan S. Composite supramolecular nanoassemblies with independent stimulus sensitivities. Chem Sci 2014;5:229–34. 10.1039/c3sc52347k. [DOI] [Google Scholar]
- [323].Li L, Raghupathi K, Yuan C, Thayumanavan S. Surface charge generation in nanogels for activated cellular uptake at tumor-relevant pH. Chem Sci 2013;4:3654–60. 10.1039/c3sc50899d. [DOI] [Google Scholar]
- [324].Zhuang J, Thayumanavan S. Triblock–Diblock Composite Nanoassemblies with Sequentially Addressable Host–Guest Properties for Hydrophobics and Hydrophilics. ACS Macro Lett 2020;9:1019–23. 10.1021/acsmacrolett.0c00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Zhuang J, Chacko R, Torres DFA, Wang H, Thayumanavan S. Dual Stimuli–Dual Response Nanoassemblies Prepared from a Simple Homopolymer. ACS Macro Lett 2014;3:1–5. 10.1021/mz400515s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Chen W, Meng F, Cheng R, Deng C, Feijen J, Zhong Z. Facile construction of dual-bioresponsive biodegradable micelles with superior extracellular stability and activated intracellular drug release. J Control Release 2015;210:125–33. 10.1016/j.jconrel.2015.05.273. [DOI] [PubMed] [Google Scholar]
- [327].He H, Cattran AW, Nguyen T, Nieminen A-L, Xu P. Triple-responsive expansile nanogel for tumor and mitochondria targeted photosensitizer delivery. Biomaterials 2014;35:9546–53. 10.1016/j.biomaterials.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Wang D, Wang S, Xia Y, Liu S, Jia R, Xu G, et al. Preparation of ROS-responsive core crosslinked polycarbonate micelles with thioketal linkage. Colloids Surfaces B Biointerfaces 2020;195:111276. 10.1016/j.colsurfb.2020.111276. [DOI] [PubMed] [Google Scholar]
- [329].Zhou J, Sun C, Yu C. Highly-controllable drug release from core cross-linked singlet oxygen-responsive nanoparticles for cancer therapy. RSC Adv 2020;10:19997–20008. 10.1039/d0ra02053b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Siboro SAP, Salma SA, Kim H-R, Jeong YT, Gal Y-S, Lim KT. Diselenide Core Cross-Linked Micelles of Poly(Ethylene Oxide)-b-Poly(Glycidyl Methacrylate) Prepared through Alkyne-Azide Click Chemistry as a Near-Infrared Controlled Drug Delivery System. Materials 2020;13:2846. 10.3390/ma13122846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Zhai S, Hu X, Hu Y, Wu B, Xing D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017;121:41–54. 10.1016/j.biomaterials.2017.01.002. [DOI] [PubMed] [Google Scholar]
- [332].Ding Y, Kang Y, Zhang X. Enzyme-responsive polymer assemblies constructed through covalent synthesis and supramolecular strategy. Chem Commun 2015;51:996–1003. 10.1039/c4cc05878j. [DOI] [PubMed] [Google Scholar]
- [333].Samarajeewa S, Shrestha R, Li Y, Wooley KL. Degradability of Poly(Lactic Acid)-Containing Nanoparticles: Enzymatic Access through a Cross-Linked Shell Barrier. J Am Chem Soc 2012;134:1235–42. 10.1021/ja2095602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Wang Y, Luo Y, Zhao Q, Wang Z, Xu Z, Jia X. An Enzyme-Responsive Nanogel Carrier Based on PAMAM Dendrimers for Drug Delivery. ACS Appl Mater Inter 2016;8:19899–906. 10.1021/acsami.6b05567. [DOI] [PubMed] [Google Scholar]
- [335].Massi L, Najer A, Chapman R, Spicer CD, Nele V, Che J, et al. Tuneable peptide cross-linked nanogels for enzyme-triggered protein delivery. J Mater Chem B 2020;8:8894–907. 10.1039/d0tb01546f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Luo Q, Duan Z, Li X, Gu L, Ren L, Zhu H, et al. Branched Polymer-Based Redox/Enzyme-Activatable Photodynamic Nanoagent to Trigger STING-Dependent Immune Responses for Enhanced Therapeutic Effect. Adv Funct Mater 2022;32:2110408. 10.1002/adfm.202110408. [DOI] [Google Scholar]
- [337].Ruskowitz ER, DeForest CA. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat Rev Mater 2018;3:17087. 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- [338].Wang H, Zhuang J, Thayumanavan S. Functionalizable Amine-Based Polymer Nanoparticles. ACS Macro Lett 2013;2:948–51. 10.1021/mz4004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Kitayama Y, Takeuchi T. Photodegradable Polymer Capsules Fabricated via Interfacial Photocross-linking of Spherical Polymer Particles. ACS Appl Polym Mater 2020;2:3813–20. 10.1021/acsapm.0c00472. [DOI] [Google Scholar]
- [340].Zhang X, Wang Y, Li G, Liu Z, Liu Z, Jiang J. Amphiphilic Imbalance and Stabilization of Block Copolymer Micelles on-Demand through Combinational Photo-Cleavage and Photo-Crosslinking. Macromol Rapid Commun 2017;38:1600543. 10.1002/marc.201600543. [DOI] [PubMed] [Google Scholar]
- [341].LeValley PJ, Neelarapu R, Sutherland BP, Dasgupta S, Kloxin CJ, Kloxin AM. Photolabile Linkers: Exploiting Labile Bond Chemistry to Control Mode and Rate of Hydrogel Degradation and Protein Release. J Am Chem Soc 2020;142:4671–9. 10.1021/jacs.9b11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Rapp TL, Wang Y, Delessio MA, Gau MR, Dmochowski IJ. Designing photolabile ruthenium polypyridyl crosslinkers for hydrogel formation and multiplexed, visible-light degradation. RSC Adv 2019;9:4942–7. 10.1039/c8ra09764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Accardo JV, McClure ER, Mosquera MA, Kalow JA. Using Visible Light to Tune Boronic Acid–Ester Equilibria. J Am Chem Soc 2020;142:19969–79. 10.1021/jacs.0c08551. [DOI] [PubMed] [Google Scholar]
- [344].Chen J, Yan B, Wang X, Huang Q, Thundat T, Zeng H. Core cross-linked double hydrophilic block copolymer micelles based on multiple hydrogen-bonding interactions. Polym Chem 2017;8:3066–73. 10.1039/c7py00210f. [DOI] [Google Scholar]
- [345].Wang J, Wang J, Ding P, Zhou W, Li Y, Drechsler M, et al.A Supramolecular Crosslinker To Give Salt-Resistant Polyion Complex Micelles and Improved MRI Contrast Agents. Angew Chem Int Ed 2018;57:12680–4. 10.1002/anie.201805707. [DOI] [PubMed] [Google Scholar]
- [346].Sill KN, Sullivan B, Carie A, Semple JE. Synthesis and Characterization of Micelle-Forming PEG-Poly(Amino Acid) Copolymers with Iron-Hydroxamate Cross-Linkable Blocks for Encapsulation and Release of Hydrophobic Drugs. Biomacromolecules 2017;18:1874–84. 10.1021/acs.biomac.7b00317. [DOI] [PubMed] [Google Scholar]
- [347].Yhaya F, Binauld S, Kim Y, Stenzel MH. Shell Cross-linking of Cyclodextrin-Based Micelles via Supramolecular Chemistry for the Delivery of Drugs. Macromol Rapid Commun 2012;33:1868–74. 10.1002/marc.201200473. [DOI] [PubMed] [Google Scholar]
- [348].Lin M, Dai Y, Xia F, Zhang X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater Sci Eng C 2021;119:111626. 10.1016/j.msec.2020.111626. [DOI] [PubMed] [Google Scholar]
- [349].Goulet-Hanssens A, Eisenreich F, Hecht S. Enlightening Materials with Photoswitches. Adv Mater 2020;32:1905966. 10.1002/adma.201905966. [DOI] [PubMed] [Google Scholar]
- [350].Molla MR, Rangadurai P, Antony L, Swaminathan S, Pablo JJ de, Thayumanavan S. Dynamic actuation of glassy polymersomes through isomerization of a single azobenzene unit at the block copolymer interface. Nat Chem 2018;10:659–66. 10.1038/s41557-018-0027-6. [DOI] [PubMed] [Google Scholar]
- [351].Coleman AC, Beierle JM, Stuart MCA, Maciá B, Caroli G, Mika JT, et al. Light–induced disassembly of self-assembled vesicle-capped nanotubes observed in real time. Nat Nanotechnol 2011;6:547–52. 10.1038/nnano.2011.120. [DOI] [PubMed] [Google Scholar]
- [352].Dijken DJ van, Chen J, Stuart MCA, Hou L, Feringa BL. Amphiphilic Molecular Motors for Responsive Aggregation in Water. J Am Chem Soc 2016;138:660–9. 10.1021/jacs.5b11318. [DOI] [PubMed] [Google Scholar]
- [353].Liu J-X, Xin B, Li C, Xie N-H, Gong W-L, Huang Z-L, et al. PEGylated Perylenemonoimide-Dithienylethene for Super-Resolution Imaging of Liposomes.ACS Appl Mater Inter 2017;9:10338–43. 10.1021/acsami.6b15076. [DOI] [PubMed] [Google Scholar]
- [354].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2020;20:1–24. 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng 2021;5:1–17. 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- [356].Cheng R, Meng F, Deng C, Zhong Z. Bioresponsive polymeric nanotherapeutics for targeted cancer chemotherapy. Nano Today 2015;10:656–70. 10.1016/j.nantod.2015.09.005. [DOI] [Google Scholar]
- [357].Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev 2016;116:2602–63. 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [359].Poon W, Kingston BR, Ouyang B, Ngo W, Chan WCW. A framework for designing delivery systems. Nat Nanotechnol 2020;15:819–29. 10.1038/s41565-020-0759-5. [DOI] [PubMed] [Google Scholar]
- [360].Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliver Rev 2017;108:25–38. 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- [361].Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020;181:151–67. 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- [362].Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009;324:1029–33. 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater 2013;13:204–12. 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Kong C, Li Y, Liu Z, Ye J, Wang Z, Zhang L, et al. Targeting the Oncogene KRAS Mutant Pancreatic Cancer by Synergistic Blocking of Lysosomal Acidification and Rapid Drug Release. ACS Nano 2019;13:4049–63. 10.1021/acsnano.8b08246. [DOI] [PubMed] [Google Scholar]
- [365].Wang T, Wang D, Liu J, Feng B, Zhou F, Zhang H, et al. Acidity-Triggered Ligand-Presenting Nanoparticles To Overcome Sequential Drug Delivery Barriers to Tumors. Nano Lett 2017;17:5429–36. 10.1021/acs.nanolett.7b02031. [DOI] [PubMed] [Google Scholar]
- [366].Wang T, Wang D, Yu H, Wang M, Liu J, Feng B, et al. Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug-Resistant Tumor. ACS Nano 2016;10:3496–508. 10.1021/acsnano.5b07706. [DOI] [PubMed] [Google Scholar]
- [367].Gao J, Hou B, Zhu Q, Yang L, Jiang X, Zou Z, et al. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumour-specific protein degradation and precise cancer therapy. Nat Commun 2022;13:1–14. 10.1038/s41467-022-32050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Lu H-H, Liu HW, Dinh TK, Huang C-H, Huang H-C, Tseng Y-C, et al. pH-Responsive, two-in-one doxorubicin and Bcl-2 siRNA-loaded micelleplexes for triple-negative breast cancer therapy. Polym Chem 2022;13:5568–78. 10.1039/d2py00246a. [DOI] [Google Scholar]
- [369].Kwon YJ. Before and after Endosomal Escape: Roles of Stimuli-Converting siRNA/Polymer Interactions in Determining Gene Silencing Efficiency. Acc Chem Res 2012;45:1077–88. 10.1021/ar200241v. [DOI] [PubMed] [Google Scholar]
- [370].Yu H, Zou Y, Wang Y, Huang X, Huang G, Sumer BD, et al. Overcoming Endosomal Barrier by Amphotericin B-Loaded Dual pH-Responsive PDMA-b-PDPA Micelleplexes for siRNA Delivery. ACS Nano 2011;5:9246–55. 10.1021/nn203503h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Du J-Z, Du X-J, Mao C-Q, Wang J Tailor-Made Dual pH-Sensitive Polymer–Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J Am Chem Soc 2011;133:17560–3. 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- [372].Liu B, Wu R, Gong S, Xiao H, Thayumanavan S. In Situ Formation of Polymeric Nanoassemblies Using an Efficient Reversible Click Reaction. Angew Chem Int Ed 2020;59:15135–40. 10.1002/anie.202004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Yang K, Yang Z, Yu G, Nie Z, Wang R, Chen X. Polyprodrug Nanomedicines: An Emerging Paradigm for Cancer Therapy. Adv Mater 2022;34:2107434. 10.1002/adma.202107434. [DOI] [PubMed] [Google Scholar]
- [374].Tan J, Deng Z, Liu G, Hu J, Liu S. Anti-inflammatory polymersomes of redox-responsive polyprodrug amphiphiles with inflammation-triggered indomethacin release characteristics. Biomaterials 2018;178:608–19. 10.1016/j.biomaterials.2018.03.035. [DOI] [PubMed] [Google Scholar]
- [375].Chen Y, Ma T, Liu P, Ren J, Li Y, Jiang H, et al. NIR-Light-Activated Ratiometric Fluorescent Hybrid Micelles for High Spatiotemporally Controlled Biological Imaging and Chemotherapy. Small 2020;16:2005667. 10.1002/smll.202005667. [DOI] [PubMed] [Google Scholar]
- [376].Sun W, Wen Y, Thiramanas R, Chen M, Han J, Gong N, et al. Red-Light-Controlled Release of Drug–Ru Complex Conjugates from Metallopolymer Micelles for Phototherapy in Hypoxic Tumor Environments. Adv Funct Mater 2018;28:1804227. 10.1002/adfm.201804227. [DOI] [Google Scholar]
- [377].Zhang Y, Yan C, Zheng Q, Jia Q, Wang Z, Shi P, et al. Harnessing Hypoxia-Dependent Cyanine Photocages for In Vivo Precision Drug Release. Angew Chem Int Ed 2021;60:9553–61. 10.1002/anie.202017349. [DOI] [PubMed] [Google Scholar]
- [378].Yang J, Song J, Song Q, Rho JY, Mansfield EDH, Hall SCL, et al. Hierarchical Self-Assembled Photo-Responsive Tubisomes from a Cyclic Peptide-Bridged Amphiphilic Block Copolymer. Angew Chem Int Ed 2020;59:8860–3. 10.1002/anie.201916111. [DOI] [PubMed] [Google Scholar]
- [379].Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliver Rev 2019;143:68–96. 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- [380].Zhao J, Stenzel MH. Entry of nanoparticles into cells: the importance of nanoparticle properties. Polym Chem 2017;9:259–72. 10.1039/c7py01603d. [DOI] [Google Scholar]
- [381].Wang K, Xiao X, Liu Y, Zong Q, Tu Y, Yuan Y. Self-immolative polyprodrug-based tumor-specific cascade amplificated drug release nanosystem for orchestrated synergistic cancer therapy. Biomaterials 2022;289:121803. 10.1016/j.biomaterials.2022.121803. [DOI] [PubMed] [Google Scholar]
- [382].Wang H-C, Zhang Y, Possanza CM, Zimmerman SC, Cheng J, Moore JS, et al. Trigger Chemistries for Better Industrial Formulations. ACS Appl Mater Inter 2015;7:6369–82. 10.1021/acsami.5b00485. [DOI] [PubMed] [Google Scholar]
- [383].Zhang Y, Yin Q, Yin L, Ma L, Tang L, Cheng J. Chain-Shattering Polymeric Therapeutics with On-Demand Drug-Release Capability. Angew Chem Int Ed 2013;52:6435–9. 10.1002/anie.201300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Yu F, Tu Y, Luo S, Xiao X, Yao W, Jiang M, et al. Dual-Drug Backboned Polyprodrug with a Predefined Drug Combination for Synergistic Chemotherapy. Nano Lett 2021;21:2216–23. 10.1021/acs.nanolett.0c05028. [DOI] [PubMed] [Google Scholar]
- [385].Cuggino JC, Blanco ERO, Gugliotta LM, Igarzabal CIA, Calderón M. Crossing biological barriers with nanogels to improve drug delivery performance. J Control Release 2019;307:221–46. 10.1016/j.jconrel.2019.06.005. [DOI] [PubMed] [Google Scholar]
- [386].Zhao Q, Zhang S, Wu F, Li D, Zhang X, Chen W, et al. Rational Design of Nanogels for Overcoming the Biological Barriers in Various Administration Routes. Angew Chem Int Ed 2021;60:14760–78. 10.1002/anie.201911048. [DOI] [PubMed] [Google Scholar]
- [387].Zhang F, Zhang S, Pollack SF, Li R, Gonzalez AM, Fan J, et al. Improving Paclitaxel Delivery: In Vitro and In Vivo Characterization of PEGylated Polyphosphoester-Based Nanocarriers. J Am Chem Soc 2015;137:2056–66. 10.1021/ja512616s. [DOI] [PubMed] [Google Scholar]
- [388].Zhuang J, Jiwpanich S, Deepak VD, Thayumanavan S. Facile Preparation of Nanogels Using Activated Ester Containing Polymers. ACS Macro Lett 2011;1:175–9. 10.1021/mz200123f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Dai Y, Sun H, Pal S, Zhang Y, Park S, Kabb CP, et al. Near-IR-induced dissociation of thermally-sensitive star polymers. Chem Sci 2017;8:1815–21. 10.1039/c6sc04650a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Ryu J-H, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. Self-Cross-Linked Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. J Am Chem Soc 2010;132:17227–35. 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- [391].Ryu J-H, Bickerton S, Zhuang J, Thayumanavan S. Ligand-Decorated Nanogels: Fast One-Pot Synthesis and Cellular Targeting. Biomacromolecules 2012;13:1515–22. 10.1021/bm300201x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Matsumoto NM, González-Toro DC, Chacko RT, Maynard HD, Thayumanavan S. Synthesis of nanogel– protein conjugates. Polym Chem 2013;4:2464–9. 10.1039/c3py00085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Canakci M, Singh K, Munkhbat O, Shanthalingam S, Mitra A, Gordon M, et al. Targeting CD4+ Cells with Anti-CD4 Conjugated Mertansine-Loaded Nanogels. Biomacromolecules 2020;21:2473–81. 10.1021/acs.biomac.0c00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Mullard A RNAi drugs at “a remarkable period of a renaissance.” Nat Rev Drug Discov 2022;21:12–3. 10.1038/d41573-021-00210-8. [DOI] [PubMed] [Google Scholar]
- [395].Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 2021;6:1–17. 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001;411:494–8. 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- [397].Hamilton A A Species of Small Antisense RNA in Posttranscriptional Gene Silencing in Plants. Science 1999;286:950–2. 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- [398].Cobb M Who discovered messenger RNA? Curr Biol 2015;25:R526–32. 10.1016/j.cub.2015.05.032. [DOI] [PubMed] [Google Scholar]
- [399].Lv J, Fan Q, Wang H, Cheng Y. Polymers for Cytosolic Protein Delivery. Biomaterials 2019;218:119358. 10.1016/j.biomaterials.2019.119358. [DOI] [PubMed] [Google Scholar]
- [400].Li Y, Li P, Li R, Xu Q. Intracellular Antibody Delivery Mediated by Lipids, Polymers, and Inorganic Nanomaterials for Therapeutic Applications. Adv Ther 2020;3:2000178. 10.1002/adtp.202000178. [DOI] [Google Scholar]
- [401].Qin X, Yu C, Wei J, Li L, Zhang C, Wu Q, et al. Rational Design of Nanocarriers for Intracellular Protein Delivery. Adv Mater 2019;31:1902791. 10.1002/adma.201902791. [DOI] [PubMed] [Google Scholar]
- [402].Kumar R, Chalarca CFS, Bockman MR, Bruggen CV, Grimme CJ, Dalal RJ, et al. Polymeric Delivery of Therapeutic Nucleic Acids. Chem Rev 2021;121:11527–652. 10.1021/acs.chemrev.0c00997. [DOI] [PubMed] [Google Scholar]
- [403].Xu X, Wu J, Liu Y, Saw PE, Tao W, Yu M, et al. Multifunctional Envelope-Type siRNA Delivery Nanoparticle Platform for Prostate Cancer Therapy. ACS Nano 2017;11:2618–27. 10.1021/acsnano.6b07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol 2015;15:760–70. 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Li X-D, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal Roles of cGAS-cGAMP Signaling in Antiviral Defense and Immune Adjuvant Effects. Science 2013;341:1390–4. 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Milling L, Zhang Y, Irvine DJ. Delivering safer immunotherapies for cancer. Adv Drug Deliver Rev 2017;114:79–101. 10.1016/j.addr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 2019;18:175–96. 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol 2019;14:269–78. 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [409].Gajski G, Garaj-Vrhovac V. Melittin: A lytic peptide with anticancer properties. Environ Toxicol Phar 2013;36:697–705. 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- [410].Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today 2003;8:259–66. 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- [411].Zhou J, Wan C, Cheng J, Huang H, Lovell JF, Jin H. Delivery Strategies for Melittin-Based Cancer Therapy. ACS Appl Mater Inter 2021;13:17158–73. 10.1021/acsami.1c03640. [DOI] [PubMed] [Google Scholar]
- [412].Lv S, Sylvestre M, Song K, Pun SH. Development of D-melittin polymeric nanoparticles for anti-cancer treatment. Biomaterials 2021;277:121076. 10.1016/j.biomaterials.2021.121076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [413].Xie Y, Murray-Stewart T, Wang Y, Yu F, Li J, Marton LJ, et al. Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J Control Release 2017;246:110–9. 10.1016/j.jconrel.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Novo L, Rizzo LY, Golombek SK, Dakwar GR, Lou B, Remaut K, et al. Decationized polyplexes as stable and safe carrier systems for improved biodistribution in systemic gene therapy. J Control Release 2014;195:162–75. 10.1016/j.jconrel.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Jiang Y, Jiang Z, Wang M, Ma L. Current understandings and clinical translation of nanomedicines for breast cancer therapy. Adv Drug Deliv Rev 2022;180:114034. 10.1016/j.addr.2021.114034. [DOI] [PubMed] [Google Scholar]
- [416].Chen H, Kim S, He W, Wang H, Low PS, Park K, et al. Fast Release of Lipophilic Agents from Circulating PEG-PDLLA Micelles Revealed by in Vivo Förster Resonance Energy Transfer Imaging. Langmuir 2008;24:5213–7. 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- [417].Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release 2001;72:191–202. 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- [418].Zhang Q, Zhang Y, Wan Y, Carvalho W, Hu L, Serpe MJ. Stimuli-Responsive Polymers for Sensing and Reacting to Environmental Conditions. Prog Polym Sci 2021;116:101386. 10.1016/j.progpolymsci.2021.101386. [DOI] [Google Scholar]
- [419].Mabire AB, Brouard Q, Pitto-Barry A, Williams RJ, Willcock H, Kirby N, et al. CO 2/pH-responsive particles with built-in fluorescence read-out. Polym Chem 2016;7:5943–8. 10.1039/c6py01254j. [DOI] [Google Scholar]
- [420].Mabire AB, Robin MP, Quan W-D, illcock H, Stavros VG, O’Reilly RK. Aminomaleimide fluorophores: a simple functional group with bright, solvent dependent emission. Chem Commun 2015;51:9733–6. 10.1039/c5cc02908b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Nhien PQ, Chou W-L, Cuc TTK, Khang TM, Wu C-H, Thirumalaivasan N, et al. Multi-Stimuli Responsive FRET Processes of Bifluorophoric AIEgens in an Amphiphilic Copolymer and Its Application to Cyanide Detection in Aqueous Media. ACS Appl Mater Inter 2020;12:10959–72. 10.1021/acsami.9b21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Qi Q, Li Y, Yan X, Zhang F, Jiang S, Su J, et al. Intracellular pH sensing using polymeric micelle containing tetraphenylethylene-oxazolidine. Polym Chem 2016;7:5273–80. 10.1039/c6py01072e. [DOI] [Google Scholar]
- [423].Liu H, Lionello C, Westley J, Cardellini A, Huynh U, Pavan GM, et al. Understanding functional group and assembly dynamics in temperature responsive systems leads to design principles for enzyme responsive assemblies. Nanoscale 2021;13:11568–75. 10.1039/d1nr02000e. [DOI] [PubMed] [Google Scholar]
- [424].Gao J, Prachyathipsakul T, Thayumanavan S. Multichannel dual protein sensing using amphiphilic supramolecular assemblies. Chem Commun 2021;57:12828–31. 10.1039/d1cc05407d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [425].Wang X, Niu D, Li P, Wu Q, Bo X, Liu B, et al. Dual-Enzyme-Loaded Multifunctional Hybrid Nanogel System for Pathological Responsive Ultrasound Imaging and T 2-Weighted Magnetic Resonance Imaging. ACS Nano 2015;9:5646–56. 10.1021/nn5068094. [DOI] [PubMed] [Google Scholar]
- [426].Mi P Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020;10:4557–88. 10.7150/thno.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Lv R, Yang P, He F, Gai S, Yang G, Dai Y, et al. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials 2015;63:115–27. 10.1016/j.biomaterials.2015.05.016. [DOI] [PubMed] [Google Scholar]
- [428].Yin J, Chen Y, Zhang Z-H, Han X. Stimuli-Responsive Block Copolymer-Based Assemblies for Cargo Delivery and Theranostic Applications. Polymers-Basel 2016;8:268. 10.3390/polym8070268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Dai Y-D, Sun X-Y, Sun W, Yang J-B, Liu R, Luo Y, et al. H 2 O 2 -responsive polymeric micelles with a benzil moiety for efficient DOX delivery and AIE imaging. Org Biomol Chem 2019;17:5570–7. 10.1039/c9ob00859d. [DOI] [PubMed] [Google Scholar]
- [430].Zhou X, Yan N, Cornel EJ, Cai H, Xue S, Xi H, et al. Bone-targeting polymer vesicles for simultaneous imaging and effective malignant bone tumor treatment. Biomaterials 2021;269:120345. 10.1016/j.biomaterials.2020.120345. [DOI] [PubMed] [Google Scholar]
- [431].Qi G, Gao Y, Wang L, Wang H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv Mater 2018;30:1703444. 10.1002/adma.201703444. [DOI] [PubMed] [Google Scholar]
- [432].Khouzam RA, Brodaczewska K, Filipiak A, Zeinelabdin NA, Buart S, Szczylik C, et al. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front Immunol 2021;11:613114. 10.3389/fimmu.2020.613114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [433].Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Medicine 2020;52:15–30. 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Zhong Y, Zhan J, Xu G, Chen Y, Qin Q, Liao X, et al. Enzyme-Instructed Self-Assembly Enabled Monomer–Excimer Transition to Construct Higher Ordered Luminescent Supramolecular Assembly for Activity-based Bioimaging. Angew Chem Int Ed 2021;60:8121–9. 10.1002/anie.202014278. [DOI] [PubMed] [Google Scholar]
- [435].Zhang J, Mu Y-L, Ma Z-Y, Han K, Han H-Y. Tumor-triggered transformation of chimeric peptide for dual-stage-amplified magnetic resonance imaging and precise photodynamic therapy. Biomaterials 2018;182:269–78. 10.1016/j.biomaterials.2018.08.026. [DOI] [PubMed] [Google Scholar]
- [436].Kwek G, Do TC, Lu X, Lin J, Xing B. Scratching the Surface of Unventured Possibilities with In Situ Self-Assembly: Protease-Activated Developments for Imaging and Therapy. ACS Appl Bio Mater 2021;4:2192–216. 10.1021/acsabm.0c01340. [DOI] [PubMed] [Google Scholar]
- [437].Torii Y, Sugimura N, Mitomo H, Niikura K, Ijiro K.pH-Responsive Coassembly of Oligo(ethylene glycol)-Coated Gold Nanoparticles with External Anionic Polymers via Hydrogen Bonding. Langmuir 2017;33:5537–44. 10.1021/acs.langmuir.7b01084. [DOI] [PubMed] [Google Scholar]
- [438].Qian Y, Wang W, Wang Z, Jia X, Han Q, Rostami I, et al. pH-Triggered Peptide Self-Assembly for Targeting Imaging and Therapy toward Angiogenesis with Enhanced Signals. Acs Appl Mater Inter 2018;10:7871–81. 10.1021/acsami.8b00583. [DOI] [PubMed] [Google Scholar]
- [439].Chen W, Li S, Lang JC, Chang Y, Pan Z, Kroll P, et al. Combined Tumor Environment Triggered Self-Assembling Peptide Nanofibers and Inducible Multivalent Ligand Display for Cancer Cell Targeting with Enhanced Sensitivity and Specificity. Small 2020;16:2002780. 10.1002/smll.202002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Rapoport N, Nam K-H, Gupta R, Gao Z, Mohan P, Payne A, et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release 2011;153:4–15. 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [441].Liu F-H, Cong Y, Qi G-B, Ji L, Qiao Z-Y, Wang H. Near-Infrared Laser-Driven in Situ Self-Assembly as a General Strategy for Deep Tumor Therapy. Nano Lett 2018;18:6577–84. 10.1021/acs.nanolett.8b03174. [DOI] [PubMed] [Google Scholar]
- [442].Lin X, Wu J, Liu Y, Lin N, Hu J, Zhang B. Stimuli-Responsive Drug Delivery Systems for the Diagnosis and Therapy of Lung Cancer. Molecules 2022;27:948. 10.3390/molecules27030948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [443].Murugan B, Sagadevan S, Fatimah I, Oh W-C, Hossain MAM, Johan MR. Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine. Nanotechnol Rev 2021;10:933–53. 10.1515/ntrev-2021-0067. [DOI] [Google Scholar]
- [444].Ruiz AL, Ramirez A, McEnnis K. Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery. Pharm 2022;14:421. 10.3390/pharmaceutics14020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [445].Nhien PQ, Wu P-H, Wu C-H, Wu JI, Hue BTB, Du B-W, et al. Multi-stimuli responsive fluorescence of amphiphilic AIEgen copolymers for ultrafast, highly sensitive and selective copper ion detection in water. Sensors Actuators B Chem 2021;344;130241. 10.1016/j.snb.2021.130241. [DOI] [Google Scholar]
- [446].Li Y, Qian Y, Liu T, Zhang G, Liu S.Light-Triggered Concomitant Enhancement of Magnetic Resonance Imaging Contrast Performance and Drug Release Rate of Functionalized Amphiphilic Diblock Copolymer Micelles. Biomacromolecules 2012;13:3877–86. 10.1021/bm301425j. [DOI] [PubMed] [Google Scholar]
- [447].Liu D, Cornel EJ, Du J. Renoprotective Angiographic Polymersomes. Adv Funct Mater 2021;31:2007330. 10.1002/adfm.202007330. [DOI] [Google Scholar]
- [448].Yang C, Ren C, Zhou J, Liu J, Zhang Y, Huang F, et al. Dual Fluorescent- and Isotopic-Labelled Self-Assembling Vancomycin for in vivo Imaging of Bacterial Infections. Angew Chem Int Ed 2017;56:2356–60. 10.1002/anie.201610926. [DOI] [PubMed] [Google Scholar]
- [449].Yan X, Yang C, Yang M, Ma Y, Zhang Y, Zhang Y, et al. All-in-one theranostic nanoplatform based on polymer nanoparticles for BRET/FRET-initiated bioluminescence imaging and synergistically anti-inflammatory therapy for ulcerative colitis.J Nanobiotechnol 2022;20:99. 10.1186/s12951-022-01299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].Lin Y-X, Qiao S-L, Wang Y, Zhang R-X, An H-W, Ma Y, et al. An in Situ Intracellular Self-Assembly Strategy for Quantitatively and Temporally Monitoring Autophagy. ACS Nano 2017;11:1826–39. 10.1021/acsnano.6b07843. [DOI] [PubMed] [Google Scholar]
- [451].Habel J, Ogbonna A, Larsen N, Cherré S, Kynde S, Midtgaard SR, et al. Selecting analytical tools for characterization of polymersomes in aqueous solution. RSC Adv 2015;5:79924–46. 10.1039/c5ra16403f. [DOI] [Google Scholar]
- [452].Houga C, Giermanska J, Lecommandoux S, Borsali R, Taton D, Gnanou Y, et al. Micelles and Polymersomes Obtained by Self-Assembly of Dextran and Polystyrene Based Block Copolymers. Biomacromolecules 2009;10:32–40. 10.1021/bm800778n. [DOI] [PubMed] [Google Scholar]
- [453].Wu H, Friedrich H, Patterson JP, Sommerdijk NAJM, Jonge N. Liquid-Phase Electron Microscopy for Soft Matter Science and Biology. Adv Mater 2020;32:2001582. 10.1002/adma.202001582. [DOI] [PubMed] [Google Scholar]
- [454].Parent LR, Bakalis E, Ram rez-Hern ndez A, Kammeyer JK, Park C, Pablo J de, et al. Directly Observing Micelle Fusion and Growth in Solution by Liquid-Cell Transmission Electron Microscopy. J Am Chem Soc 2017;139:17140–51. 10.1021/jacs.7b09060. [DOI] [PubMed] [Google Scholar]
- [455].Scheutz GM, Touve MA, Carlini AS, Garrison JB, Gnanasekaran K, Sumerlin BS, et al. Probing Thermoresponsive Polymerization-Induced Self-Assembly with Variable-Temperature Liquid-Cell Transmission Electron Microscopy. Matter 2021;4:722–36. 10.1016/j.matt.2020.11.017. [DOI] [Google Scholar]
- [456].Liu H, Lu H-H, Zhuang J, Thayumanavan S. Three-Component Dynamic Covalent Chemistry: From Janus Small Molecules to Functional Polymers. J Am Chem Soc 2021;143:20735–46. 10.1021/jacs.1c08574. [DOI] [PubMed] [Google Scholar]
- [457].Xu X, Asher SA. Synthesis and Utilization of Monodisperse Hollow Polymeric Particles in Photonic Crystals. J Am Chem Soc 2004;126:7940–5. 10.1021/ja049453k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.