Abstract
Background:
Dementia with Lewy bodies (DLB) is characterized neuropathologically by brainstem and cortical Lewy bodies and Lewy neurites, neuronal loss in brainstem nuclei, and Alzheimer disease (AD) pathology. Previous studies have suggested that spongiform change in the entorhinal cortex may also be a pathologic feature; however, this change has not been well characterized.
Design/Method:
An autopsy series of 40 subjects with DLB and 40 subjects with AD were matched on age, sex, and last Mini Mental State Examination before death. Using semistereological methods on representative sections through the transentorhinal and perirhinal cortices, quantitative counts and semiquantitative grading of vacuolization were performed by 1 rater (A.S.) blinded to subjects’ diagnoses. In addition, electron microscopy of representative sections was performed.
Results:
Vacuolization was 4- to 5-fold more prominent in the perirhinal, as compared with transentorhinal, cortex. Moderate to severe vacuolization was found in 57.5% of DLB, but only 7.5% of AD subjects. There were statistically significant differences between mean numbers of vacuoles in the perirhinal (DLB mean = 27.91; AD mean = 2.35; P < 0.001) and transentorhinal (DLB mean = 5.92; AD mean = 0.5; P < 0.001) cortices in DLB as well as AD cases. Electron microscopy revealed both axonal and dendritic pathology, with dilatation, vacuole formation, and abnormal membranous profiles.
Conclusions:
Although the exact mechanism remains to be elucidated, vacuolization seems to be more specific for DLB than AD, with disproportionate involvement of the perirhinal cortex.
Keywords: dementia with Lewy bodies, spongiform change, vacuolization, Alzheimer disease, entorhinal cortex, perirhinal cortex, semiquantitative grading of vacuolization, Braak stage, amyloid plaques, tangles
Although still debated, dementia with Lewy bodies (DLB) is perhaps the second most common cause of dementia after Alzheimer disease (AD).1 The clinical presentation is characterized by fluctuating cognitive impairment, attention deficits, and visuospatial dysfunction, along with well-formed complex visual hallucinations and mild parkinsonian symptoms.1,2 Yet, in the majority of cases, the distinction between DLB and AD can be subtle, making it very difficult to diagnose in life.3–7 Neuropathologic studies have demonstrated the presence of Lewy bodies in both subcortical and cortical regions of the brain, neuronal loss in brainstem nuclei, and spongiform change in the temporal lobes1 of patients with DLB. Although vacuolization has been identified in a number of neurodegenerative diseases,1,8–12 2 studies have suggested that vacuolization, especially temporal lobe vacuolization, may be an important neuropathologic feature of Lewy body disease.8,13 A previous study has suggested that the spongiform change or vacuolization may result from degeneration of terminal axons of large pyramidal neurons; however, this unique finding has not been well characterized.14 Thus, the objective of the current study was to examine the nature and degree of temporal lobe spongiform change in a large series of well-characterized, autopsy-confirmed DLB and AD patients and to explore potential associations with vacuole distribution and density.
METHODS
Subjects
Subjects included in the study were followed clinically at the University of California San Diego (UCSD) Alzheimer Disease Research Center and came to autopsy between 1990 and 2006 with a pathologic diagnosis of DLB or AD. Subjects had been evaluated at the UCSD Alzheimer Disease Research Center with standardized medical, neurological, neuropsychological, and laboratory examinations. To be included in the study, subjects had to have no other confounding pathologic diagnoses and have a Mini Mental State Examination (MMSE) score > 0 within 3 years of their death.
There were 40 autopsy-proven DLB patients who met all the requirements for inclusion. A random sample of AD cases, matched on the basis of age, sex, last MMSE before death, and interval from last MMSE to death, was obtained using the SAS macro gmatch.15
Tissue Examination
All autopsies were performed according to a standardized protocol.16 The left hemibrain was fixed by immersion in 10% formalin for 5 to 7 days. The paraffin-embedded blocks from mid-frontal, rostral superior temporal, and inferior parietal areas of neocortex, anterior cingulate gyrus, posterior cingulate gyrus, hippocampus, entorhinal cortex, basal ganglia/substantia innominata, mesencephalon, and pons were cut at 7mm thickness for hematoxylin and eosin and thioflavin-S staining. All slides had a unique numeric identifier, assigned by the Department of Pathology, UCSD. Total plaque, neuritic plaque, and neurofibrillary tangle counts were determined by the same examiner (L.H.) with the same criteria used consistently. Lewy body pathology was graded on a semiquantitative scale following the current criteria of the DLB Consortium.17
All of the DLB cases included in this study met DLB criteria for a pathologic diagnosis of DLB, based on the presence of brainstem and cortical Lewy bodies identified by hematoxylin and eosin and antiubiquitin immunostaining, as recommended by the Consortium on DLB.1 The neuropathologic diagnosis of AD was based on both National Institutes of Health and Consortium to Establish a Registry for Alzheimer’s Disease criteria for AD, as well as on the exclusion of brainstem and cortical Lewy bodies. Most of the AD patients displayed significant numbers of neurofibrillary tangles in each of the neocortical regions examined (Braak stages V or VI), thereby fulfilling National Institutes of Health-Reagan guidelines for a high likelihood that their dementia was attributable to AD pathology. As mentioned above, DLB or AD cases with significant coexistent vascular (>10mL of infarcted brain tissue, 2 or more cortical microinfarcts, 2 or more lacunes, or hippocampal sclerosis) or other pathology (that could by itself cause dementia) were excluded from analyses.
Quantification of the vacuoles was performed using a video camera (Sony DCX-390; Tokyo, Japan) and software program (Bioquant Nova Prime system; Bioquant Image Analysis Corporation, Tennessee) that controlled the motorized stage (ASI-XYZ; ASI Inc., Eugene, OR) on a BH-2 microscope (Olympus, Melville, NY). Figure 1 represents coronal section of the left hemibrain with delineation of the regions of interest (entorhinal and perirhinal cortices). Entorhinal/perirhinal structures were circumscribed using a marker, which was then traced and delineated by the program (Fig. 2). A (75 × 750μm) grid was overlaid on each section and manually examined for vacuoles. Vacuoles were defined as orbicular spaces, ranging from 5 to 15μm without evidence of any cellular or vascular remnants (Fig. 3). Depending on the size of the entorhinal/perirhinal section, there were 50 to 150 high-power (×10) grids to be viewed for quantification. Vacuole density within each dissector box on the grid was scored on a 0 to 4 scale. Boxes containing 2 or less vacuoles were scored as 0; boxes containing 3 to 10 vacuoles were scored as 1; boxes containing 11 to 20 vacuoles were scored as 2; boxes containing 21 to 30 vacuoles were scored as 3, and boxes containing 31 or more vacuoles were scored as 4. The scorer (A.S.) was blinded to the patient clinical information and pathologic presentation. The dissector box scores were then summed and overall vacuole pathology score calculated using the categories “none” (sum = 0), “mild” (sum = 1 to 9), “moderate” (sum = 10 to 49), and “severe” (sum > 50). Once scoring of the vacuoles was complete in all the grids, the program created a 2-dimensional depiction of vacuole density across the circumscribed tissue (Fig. 2).
FIGURE 1.
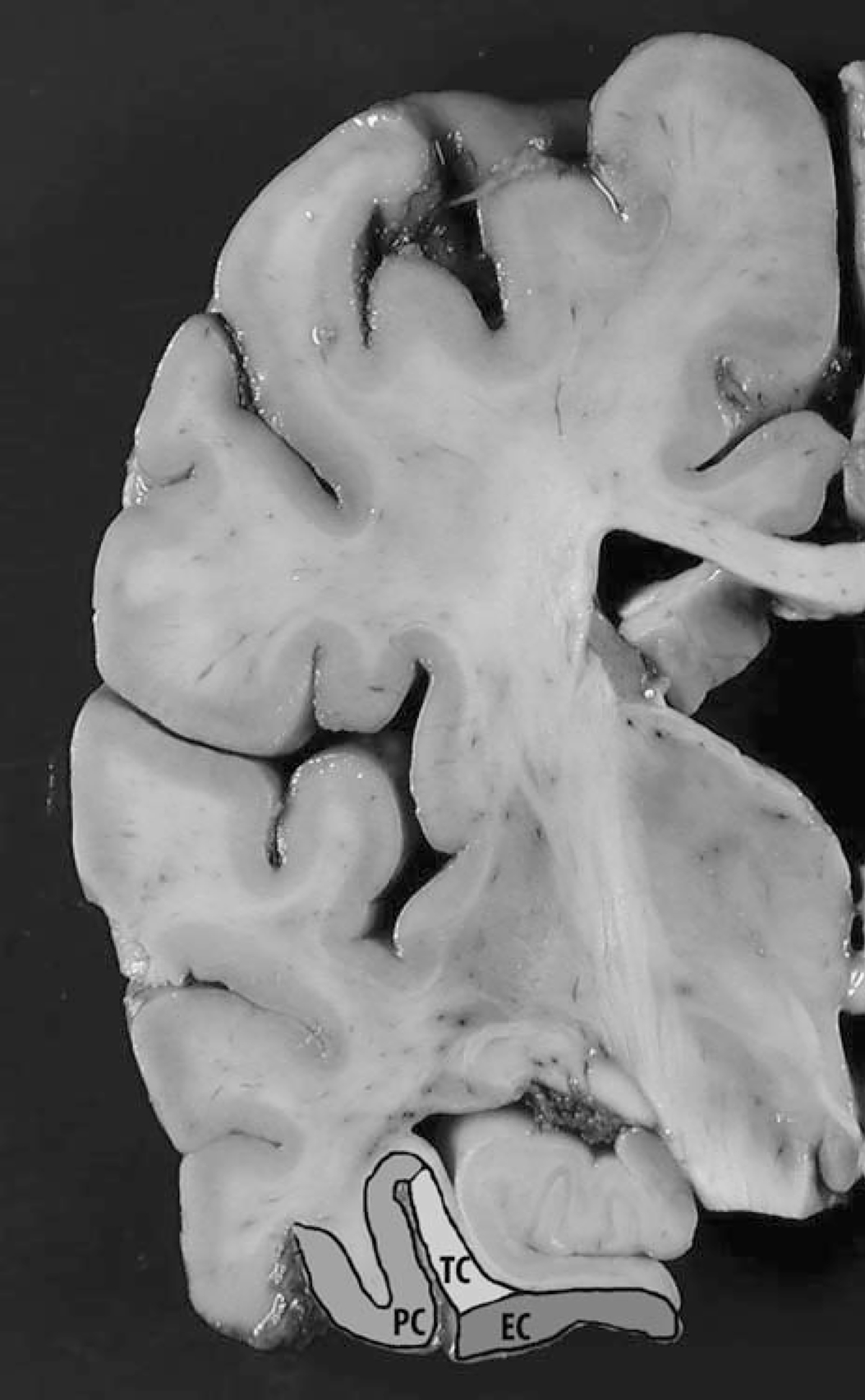
Coronal section of the cerebral hemisphere showing regions of interest (EC and PC). EC indicates entorhinal cortex; PC, perirhinal cortex; TC, transentorhinal cortex.
FIGURE 2.
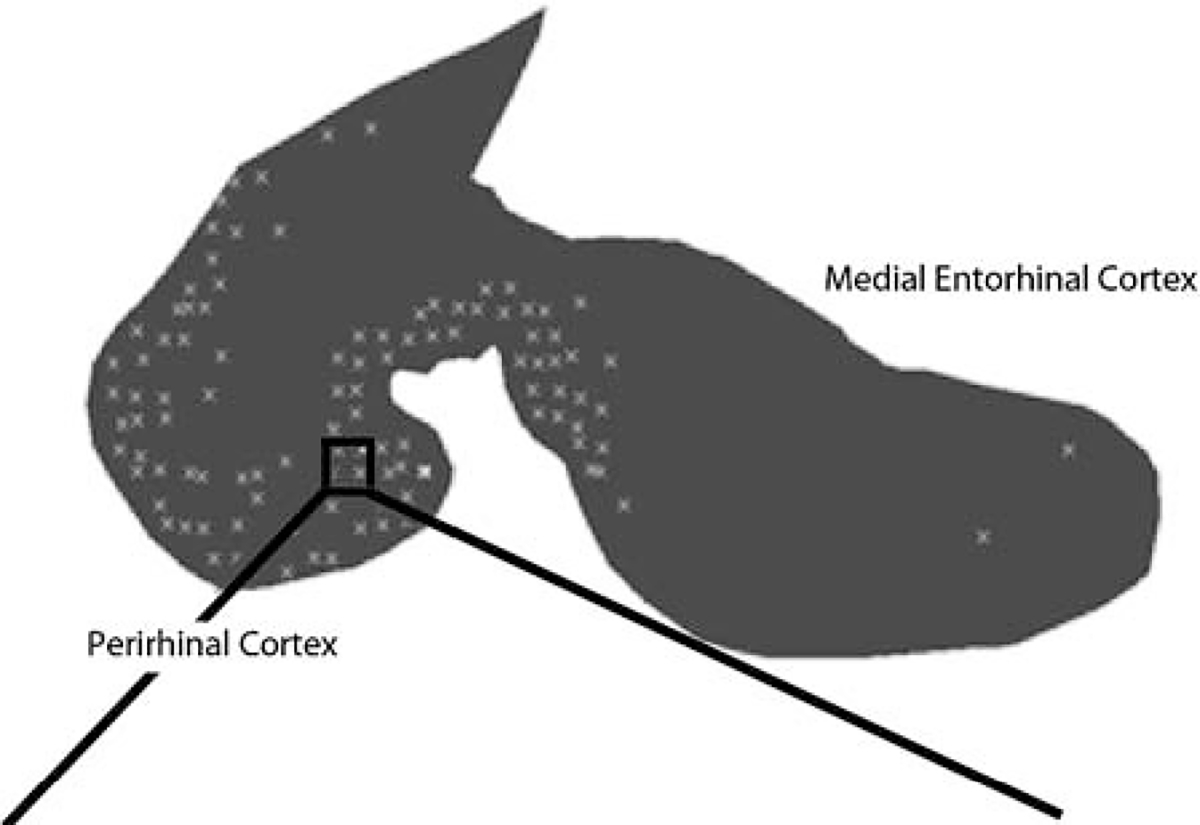
Line tracing of entorhinal and perirhinal cortex vacuolization.
FIGURE 3.
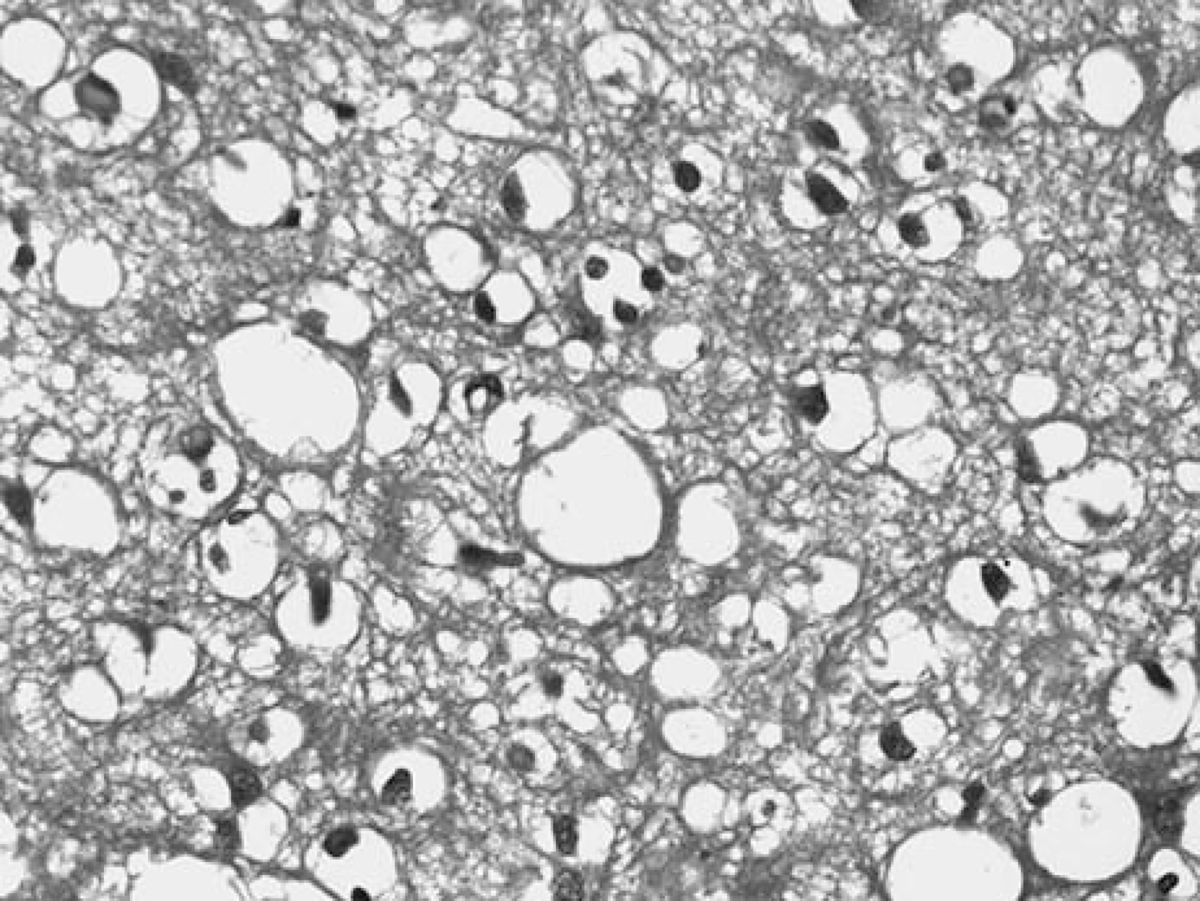
Vacuoles in perirhinal cortex at × 400 magnification, dementia with Lewy bodies.
Electron Microscopy (EM)
An entorhinal cortex section was fixed overnight in 2% glutaraldehyde at 4°C immediately before embedding for EM. The section was then washed twice for 3 minutes each in 0.1M sodium cacodylate buffer pH 7.4, fixed in 1% osmium tetroxide for 15 minutes, rinsed in distilled water twice for 3 minutes each, quickly rinsed in 50% ethanol, en bloc stained for 20 minutes to 1 hour with ethanol-uranyl acid solution, and then dehydrated through graded ethanol solutions. The section was then placed in 1:1 propylene oxide/100% ethanol for 4 minutes, propylene oxide for 5 minutes twice, and then a 1:1 mixture of Epon and propylene oxide for 30 minutes. The brain regions of interest (hippocampus and entorhinal cortex) were subsequently cut out, positioned in a BEEM capsule, covered with fresh Epon, allowed to stand for 30minutes to 1 hour, and finally polymerized for 24 to 48 hours in a 65°C oven. The resulting blocks containing the regions of interest were then trimmed, cut on a Reichert Ultracut E ultramicrotome, viewed on a Zeiss 10B transmission electron microscope, and photographed.
Statistical Analysis
The distribution of continuous matching variables was compared in cases versus controls using a t test. Plaque counts, tangle counts, and inclusion counts were compared between groups using Wilcoxon matched pairs rank-sum test. Categorical variables were compared in cases versus controls using Fisher exact test. Association between semiquantitative measures of plaque, tangle, and inclusion body levels was tested using Spearman rank correlation coefficients.
RESULTS
The demographic and clinical characteristics of the patient groups are shown in Table 1. There were no group differences with regard to age at death (DLB mean = 79.12 years; AD mean = 78.68 years; P < 0.76), sex (DLB mean = 75; AD mean = 75; P = 1), global severity of dementia on the MMSE at last evaluation (DLB mean = 5.8; AD mean = 6.2; P < 0.77), or interval from last examination to death (DLB mean = 1.19y; AD mean = 1.12y; P < 0.73) (Table 1). Plaque and tangle densities were consistently higher in AD, and Lewy body pathology was higher in DLB, consistent with the neuropathologic diagnoses (Table 2).
TABLE 1.
Clinical and Demographic Matching Variables
| AD (N = 40) | DLB (N = 40) | P * | |
|---|---|---|---|
|
| |||
| Age at death mean (range) | 78.7 (66–98) | 79.1 (66–96) | 0.76 |
| Sex (% female) | 75 | 75 | 1 |
| Last MMSE (mean) | 6.2 | 5.8 | 0.77 |
| Interval to death (y) | 1.12 | 1.19 | 0.73 |
Continuous variables were compared using a t test.
AD indicates Alzheimer disease; DLB, dementia with Lewy bodies; MMSE, Mini Mental State Examination.
TABLE 2.
Pathologic Variables
| AD (N = 40)† | DLB (N = 40)† | P * | |
|---|---|---|---|
|
| |||
| Diffuse plaques | |||
| MF | 46.97 (50, 20–50) | 41.72 (49, 7–50) | 0.08 |
| IP | 45.61 (50, 21–50) | 38.41 (40, 17–50) | < 0.01 |
| ST | 45.05 (50, 20–50) | 34.88 (33, 8–50) | < 0.001 |
| HP | 19.42 (17.5, 4–44) | 13.55 (11.5, 0–39) | < 0.01 |
| Amyloid | |||
| MF | 1.68 (2, 0–4) | 1.44 (1, 0–4) | 0.40 |
| IP | 1.24 (1, 0–4) | 1.38 (1, 0–4) | 0.65 |
| ST | 0.74 (0, 0–3) | 1.07 (0, 0–4) | 0.19 |
| HP | 0.92 (1, 0–4) | 1.03 (0, 0–4) | 0.68 |
| Neuritic plaques | |||
| MF | 35.11 (43.5, 2–50) | 22.34 (19, 4–50) | < 0.01 |
| IP | 35.22 (42, 5–50) | 24.53 (22.5, 2–50) | 0.02 |
| ST | 27.03 (23, 3–50) | 20.74 (16, 4–107) | 0.07 |
| HP | 12.42 (11.5, 2–33) | 7.38 (4, 0–25) | 0.01 |
| Tangles | |||
| MF | 4.39 (3, 0–22) | 1.31 (0, 0–11) | < 0.001 |
| IP | 6.08 (4, 0–18) | 1.82 (1, 0–9) | < 0.001 |
| ST | 6.76 (5.5, 0–19) | 2.88 (1, 0–14) | < 0.001 |
| HP | 20.05 (19, 2–66) | 9.41 (4, 0–41) | < 0.001 |
| Lewy bodies | 0.03 (0, 0–1) | 1 (1, 1–1) | < 0.001 |
| Braak stage | 5.47 (6, 3–6) | 3.7 (4, 0–6) | < 0.001 |
| Vacuolization | |||
| Entorhinal | 0.5 (0, 0–5) | 5.92 (1, 0–38) | < 0.001 |
| Perirhinal | 2.35 (0, 0–16) | 27.91 (13, 0–141) | < 0.001 |
Wilcoxon Matched-Paired Rank Sum Test.
Mean (Median, Range).
AD indicates Alzheimer disease; DLB, dementia with Lewy bodies; HP, hippocampus; IP, inferior parietal; MF, mid-frontal; ST, superior temporal.
In both AD and DLB, vacuolization was 4- to 5-fold more prominent in the perirhinal, as compared with entorhinal, cortex (P < 0.001) (Table 2). Vacuolization was found to a variable extent in 94% of DLB subjects, but only 46% of AD subjects. Of the 40 AD subjects, 26 had no vacuolization, whereas only 7 of the DLB subjects had no vacuolization; 11 of the AD and 10 of the DLB had mild vacuolization; 3 of the AD as opposed to 13 of the DLB subjects had moderate vacuolization; and none of the AD but 10 of the DLB had severe vacuolization (overall P < 0.001) (Table 3).
TABLE 3.
Percentage of Subjects With Given Severity of Spongiform Change
| AD (N = 40) | DLB (N = 40) | |
|---|---|---|
|
| ||
| None | 65 | 18 |
| Mild | 28 | 25 |
| Moderate | 7 | 32 |
| Severe | 0 | 25 |
AD indicates Alzheimer disease; DLB, dementia with Lewy bodies.
The mean number of vacuoles per examined section was significantly higher in DLB cases compared with AD cases in the perirhinal (27.91 vs. 2.35; P < 0.001) and entorhinal (5.92 vs. 0.50; P < 0.001) cortices. Although most of the vacuoles in the entorhinal cortex were seen in the transentorhinal cortex, the perirhinal cortex demonstrated the highest number of vacuoles, as illustrated in the representative section on Figure 3.
Although there were statistically significant correlations (P < 0.01) between perirhinal vacuolization scores and numbers of superior temporal diffuse and neuritic plaques, superior temporal tangles, and mid-frontal tangles in AD cases, there were no statistically significant correlations between entorhinal or perirhinal vacuolization scores and neuropathologic features in DLB cases. There were also no statistically significant correlations between degree of vacuolization and numbers of Lewy bodies within either group (Table 4). Furthermore, there were no statistically significant correlations between degree of vacuolization and sex, age at onset, or duration of disease from onset (data not presented).
TABLE 4.
Correlations Between Entorhinal and Perirhinal Vacuolization Scores and Neuropathologic Features in Dementia With Lewy Bodies (DLB) and Alzheimer Disease (AD) Cases
| AD (N = 40) |
DLB (N = 40) |
|||
|---|---|---|---|---|
| EC | PC | EC | PC | |
|
| ||||
| GLB | 0.347 | 0.000 | 0.193 | −0.012 |
| MF plaques | 0.047 | 0.295 | 0.191 | 0.056 |
| IP plaques | 0.035 | 0.316 | 0.069 | 0.083 |
| ST plaques | 0.121 | 0.518** | 0.097 | 0.396 |
| HP plaques | 0.039 | 0.099 | 0.150 | 0.303 |
| MF NP | 0.206 | 0.420 | − 0.082 | − 0.004 |
| IP NP | 0.283 | 0.530** | 0.141 | 0.146 |
| ST NP | 0.244 | 0.570** | − 0.05 | 0.072 |
| HP NP | 0.021 | 0.217 | 0.036 | 0.251 |
| MF tangles | 0.127 | 0.573** | 0.147 | 0.12 |
| IP tangles | 0.174 | 0.437 | 0.11 | 0.172 |
| ST tangles | 0.072 | 0.574** | 0.028 | 0.275 |
| HP tangles | 0.015 | 0.36 | − 0.205 | 0.247 |
| MF amyloid | − 0.196 | − 0.144 | 0.127 | 0.345 |
| IP amyloid | − 0.155 | − 0.232 | 0.051 | 0.125 |
| ST amyloid | −0.037 | 0.244 | −0.047 | 0.175 |
| HP amyloid | − 0.126 | − 0.117 | − 0.097 | 0.268 |
| Braak stage | 0.395 | 0.436 | 0.127 | 0.315 |
P < 0.01.
AD indicates Alzheimer disease; DLB, dementia with Lewy body; EC, entorhinal cortex; GLB, global Lewy body score; HP, hippocampal; IP, inferior parietal; MF, mid-frontal; NP, neuritic plaque; PC, perirhinal cortex; ST, superior temporal.
EM demonstrated the neuropil vacuolization to be at the expense of the neuritic processes. Most of the vacuolization could be seen in the postsynatic regions, namely the proximal branches of the dendrites (Fig. 4). Closer analysis of the dendrites in the DLB cases suggested that the vacuolization was commonly in the smooth endoplasmic reticulum (ER). The cisterns of the ER were distended irregularly, and the ER membranes were disrupted.
FIGURE 4.
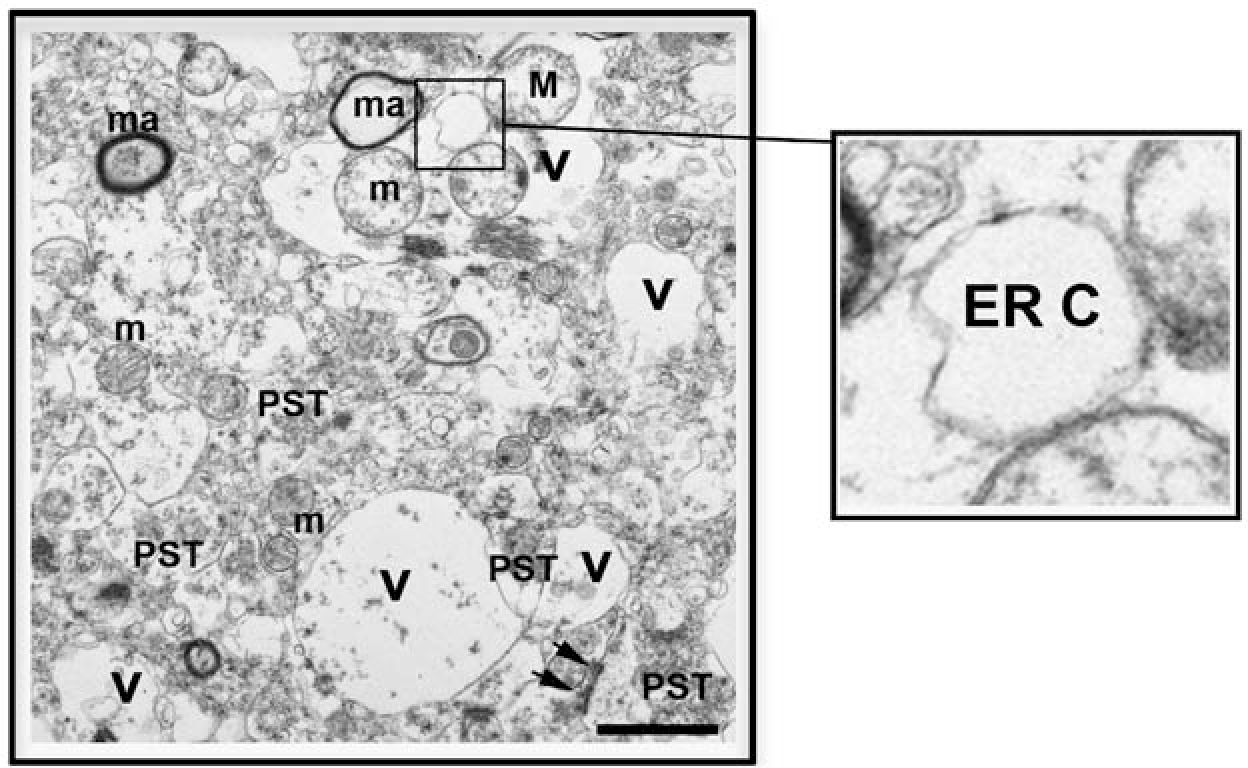
Ultrastructural analysis of vacuoles in the temporal cortex of dementia with Lewy bodies case. Electron micrograph of layer V of perirhinal cortex illustrating the vacuolization (V) of the neuropil involving dendrites. PST= presynaptic terminals. The postsynaptic density is marked with arrows. The vacuolized dendrites contain mitochondria (m). The myelinated axons (ma) are preserved. The vacuoles in the dendrites display dilated endoplasmic reticulum cisterns (ER C), details shown in inset. Bar= 1 μm.
DISCUSSION
In this study, we found a consistent pattern of greater temporal lobe vacuolization in the DLB, as compared with AD, subjects; in addition, the perirhinal cortex was afflicted with spongiform change to a significantly greater extent than the entorhinal cortex. Vacuolization did not seem to be a marker of clinical severity, duration of disease, or patient age but was strongly correlated with Lewy body pathology, consistent with previous studies.13 Although vacuolization in DLB, as compared with AD subjects, has previously been reported,13,18 to our knowledge, the preponderance of perirhinal vacuolization, as compared with entorhinal involvement, is a novel finding that has not previously been described, yet may provide important clues to the pathophysiology of this dementia syndrome.
Although there is limited information regarding the mechanism or the significance of these vacuoles, previous observations have linked degree of spongiform change with loss of the large pyramidal neurons in the entorhinal cortex19 and with the disappearance of ubiquitin-positive granular structures.20,21 The occurrence of spongiform change without significant gliosis may suggest that the large pyramidal neurons in the transentorhinal and perirhinal cortex degenerate more rapidly in DLB than in AD. Our EM findings further suggest that there is postsynaptic, specifically proximal dendritic involvement. This is consistent with previous studies supporting a postsynaptic origin for the neuropil vacuolization in DLB.8 The mechanisms for such postsynaptic pathology in the DLB cases is unclear; however, the ER plays a role in calcium storage regulation and stress response to protein misfolding—a pathway that is severely affected in disorders with a-synuclein accumulation.22
The pattern and extent of vacuolization in DLB may also shed light on why DLB subjects experience greater visuoperceptual abnormalities than AD subjects, as previous studies have suggested that perirhinal cortex might play a role, not only in declarative memory, but also in visual perception and “object identification.”23–28 Thus, lesions in perirhinal cortex could lead to difficulty representing visual aspects of object information, and these defective visual perceptions may play a role in the development of visual hallucination, delusional misidentification, visual agnosia, and visuoconstructive aberrancies often observed in DLB.29,30 In support of this notion is the finding that DLB patients with hallucinations, as compared with those without hallucinations, demonstrate hypometabolism in visual association areas, including the perirhinal cortex.31
This study is not without limitations. This was a convenience sample of cases from 1 academic medical center, and the vacuolization was rated using a semiquantitative technique.
In summary, we sought to investigate the nature and degree of temporal lobe spongiform change in DLB and AD. We found that spongiform change was significantly more prevalent in DLB as compared with AD, specifically in the perirhinal as opposed to entorhinal cortices. Although the exact mechanism of this phenomenon remains to be elucidated, findings on EM suggest that the vacuolization may be related to alterations of smooth ER involved in packaging and transport of defective proteins and that, along with Lewy bodies and Lewy neurites, vacuolization should be included as an important histopathologic hallmark of Lewy body disease.1,32,33
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 2.Perry R, McKeith I, Perry E. Lewy body dementia—clinical, pathological and neurochemical interconnections. J Neural Transm Suppl. 1997;51:95–109. [DOI] [PubMed] [Google Scholar]
- 3.Crystal HA, Dickson DW, Lizardi JE, et al. Antemortem diagnosis of diffuse Lewy body disease. Neurology. 1990;40:1523–1528. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Fairbairn AF, Bothwell RA, et al. An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type. Neurology. 1994;44:872–877. [DOI] [PubMed] [Google Scholar]
- 5.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luis CA, Barker WW, Gajaraj K, et al. Sensitivity and specificity of three clinical criteria for dementia with Lewy bodies in an autopsy-verified sample. Int J Geriatr Psychiatry. 1999;14:526–533. [PubMed] [Google Scholar]
- 7.Mok W, Chow TW, Zheng L, et al. Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. Am J Alzheimers Dis Other Demen. 2004;19:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen LA, Masliah E, Terry RD, et al. A neuropathological subset of Alzheimer’s disease with concomitant Lewy body disease and spongiform change. Acta Neuropathol. 1989;78:194–201. [DOI] [PubMed] [Google Scholar]
- 9.Brun A, Passant U. Frontal lobe degeneration of non-Alzheimer type. Structural characteristics, diagnostic criteria and relation to other frontotemporal dementias. Acta Neurol Scand Suppl. 1996;168:28–30. [PubMed] [Google Scholar]
- 10.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. [DOI] [PubMed] [Google Scholar]
- 11.Smith TW, Anwer U, DeGirolami U, et al. Vacuolar change in Alzheimer’s disease. Arch Neurol. 1987;44:1225–1228. [DOI] [PubMed] [Google Scholar]
- 12.Masters CL, Kakulas BA, Alpers MP, et al. Preclinical lesions and their progression in the experimental spongiform encephalopathies (kuru and Creutzfeldt-Jakob disease) in primates. J Neuropathol Exp Neurol. 1976;35:593–605. [DOI] [PubMed] [Google Scholar]
- 13.Fujino Y, Dickson DW. Limbic lobe microvacuolation is minimal in Alzheimer’s disease in the absence of concurrent Lewy body disease. Int J Clin Exp Pathol. 2008;1:369–375. [PMC free article] [PubMed] [Google Scholar]
- 14.Iseki E, Marui W, Akiyama H, et al. Degeneration process of Lewy bodies in the brains of patients with dementia with Lewy bodies using alpha-synuclein-immunohistochemistry. Neurosci Lett. 2000;286:69–73. [DOI] [PubMed] [Google Scholar]
- 15.Holdstock JS. The role of the human medial temporal lobe in object recognition and object discrimination. Q J Exp Psychol B. 2005;58:326–339. [DOI] [PubMed] [Google Scholar]
- 16.Terry RD, Peck A, DeTeresa R, et al. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. [DOI] [PubMed] [Google Scholar]
- 17.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 18.Hansen LA, Samuel W. Criteria for Alzheimer’s disease and the nosology of dementia with Lewy bodies. Neurology. 1997; 48:126–132. [DOI] [PubMed] [Google Scholar]
- 19.Iseki E, Marui W, Kosaka K, et al. Degenerative terminals of the perforant pathway are human alpha-synuclein-immuno-reactive in the hippocampus of patients with diffuse Lewy body disease. Neurosci Lett. 1998;258:81–84. [DOI] [PubMed] [Google Scholar]
- 20.Iseki E, Odawara T, Li F, et al. Age-related ubiquitin-positive granular structures in non-demented subjects and neurodegenerative disorders. J Neurol Sci. 1996;142:25–29. [DOI] [PubMed] [Google Scholar]
- 21.Iseki E, Li F, Kosaka K. Close relationship between spongiform change and ubiquitin-positive granular structures in diffuse Lewy body disease. J Neurol Sci. 1997;146:53–57. [DOI] [PubMed] [Google Scholar]
- 22.Jiang P, Gan M, Ebrahim AS, et al. ER stress response plays an important role in aggregation of alpha-synuclein. Mol Neurodegener. 2010;5:56. (article no.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 1994;6:1466–1478. [DOI] [PubMed] [Google Scholar]
- 24.Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36:535–546. [DOI] [PubMed] [Google Scholar]
- 25.Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. [DOI] [PubMed] [Google Scholar]
- 26.Murray EA, Bussey TJ, Hampton RR, et al. The para hippocampal region and object identification. Ann N Y Acad Sci. 2000;911:166–174. [DOI] [PubMed] [Google Scholar]
- 27.Tyler LK, Stamatakis EA, Bright P, et al. Processing objects at different levels of specificity. J Cogn Neurosci. 2004;16: 351–362. [DOI] [PubMed] [Google Scholar]
- 28.Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q J Exp Psychol B. 2005;58:202–217. [DOI] [PubMed] [Google Scholar]
- 29.Mori E, Shimomura T, Fujimori M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–493. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005; 65:1232–1238. [DOI] [PubMed] [Google Scholar]
- 31.Perneczky R, Drzezga A, Boecker H, et al. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25: 531–538. [DOI] [PubMed] [Google Scholar]
- 32.McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53:902–905. [DOI] [PubMed] [Google Scholar]
- 33.Hohl U, Tiraboschi P, Hansen LA, et al. Diagnostic accuracy of dementia with Lewy bodies. Arch Neurol. 2000;57:347–351. [DOI] [PubMed] [Google Scholar]


