Abstract
Rotational thromboelastometry (ROTEM) is a global hemostasis assay. The diagnosis added value of ROTEM in congenital dysfibrinogenemia remains to be established.
The aim of this study was to analyze clot formation by ROTEM in a cohort of dysfibrinogenemic patients and to establish correlations with genotype, clinical features, and coagulation parameters.
The study included genetically confirmed congenital dysfibrinogenemia cases (n = 63) and healthy controls (n = 50). EXTEM, INTEM, FIBTEM tests were used to measure ROTEM parameters, that is, clotting time (CT), clot formation time (CFT), maximal clot firmness (MCF) and amplitude 10 min after CT (A10). The ISTH bleeding assessment tool was used to determine bleeding episodes.
CT (INTEM) was statistically significantly shorter in congenital dysfibrinogenemia patients compared to controls while CFT (EXTEM) was prolonged. Patients's MCF in EXTEM, INTEM, and FIBTEM were similar to controls while A10 (FIBTEM) was statistically significantly lower. Fibrinogen activity was positively correlated with fibrinogen antigen, A10 and MCF in all three assays. Bleeding phenotypes were observed in 23 (36.5%) patients. Only CFT in EXTEM and CT in INTEM were statistically different in patients with bleeding phenotype versus controls. Carriers of the FGA mutation p.Arg35His had a CT (EXTEM) slightly prolonged and a reduced A10 (FIBTEM) compared to controls.
Some ROTEM parameters were able to distinguish congenital dysfibrinogenemia patients from controls, and patients with a bleeding phenotype. Prolonged CFT in EXTEM were associated with congenital dysfibrinogenemia and bleeding phenotype. Bleeding episodes in most patients were generally mild and prevalence of thrombosis was very low.
Keywords: bleeding, diagnosis, dysfibrinogenemia, genotype, ROTEM, thrombosis
Introduction
Congenital dysfibrinogenemia is a fibrinogen disorder characterized by low fibrinogen activity and normal fibrinogen antigen [1]. Congenital dysfibrinogenemia is an autosomal dominant disorder caused in the majority of cases by a missense mutation in the coding region of one of the three fibrinogen genes, FGA, FGB, or FGG[2]. The clinical phenotype is highly heterogeneous, from asymptomatic to bleeding tendency, thrombosis, or a combination of both clinical manifestations [3]. However, in most cases, neither standard coagulation assays nor determination of the causative mutation can predict the clinical course of congenital dysfibrinogenemia [4]. Global hemostasis assays may be helpful to better assess the patient's clinical phenotype [5]. Among these, rotational thromboelastometry (ROTEM) is an established viscoelastic whole blood assay assessing clot formation and strength, and fibrinolysis [6,7]. This assay is particlarly interesting because it combines both functional and structural aspects of the blood clot and provides values for some characteristics of fibrin network. One advantage of ROTEM which does not require blood centrifugation is the rapid turnaround time. To date, ROTEM is commonly used to determine the appropriate use of blood products in trauma, liver transplantation or cardiac surgery. ROTEM-guided hemostatic therapy with fibrinogen concentrate is key in coagulation management in the perioperative period and in trauma [8]. In patients with afibrinogenemia and hypofibrinogenemia, ROTEM has been primarily used for rapid assessment of fibrinogen levels and monitoring the response to fibrinogen concentrate therapy [9,10]. So far, only limited data on the diagnostic and predictive value of thromboelastometry in dysfibrinogenemia are available [11–15]. The aim of the present study was to analyze ROTEM parameters in a large cohort of patients with dysfibrinogenemia, and establish whether ROTEM parameters correlate with clinical phenotype and coagulation parameters at diagnosis or genotype, in particular the hotspot FGA mutation (p.Arg35His).
Materials and methods
The study was approved by the ethics committee of Comenius University, Jessenius Faculty of Medicine in Martin, Slovakia, and conducted according to the second declaration of Helsinki. All patients gave written informed consent at study inclusion. We included all consecutive patients with congenital dysfibrinogenemia examined during routine clinical visits from January 2020 to September 2021 in the National Centre Hemostasis and Thrombosis in Martin, Slovakia were included. At the time of blood drawing, all patients were without clinical signs of acute bleeding and thrombosis. None of the included patients had other known hematological diseases, active bleeding episodes, inflammatory conditions or were under anticoagulant therapy.
The diagnosis of congenital dysfibrinogenemia was established based on low fibrinogen activity and normal fibrinogen antigen and genotype confimed by Sanger sequencing [16]. Clinical features, including bleeding, thrombosis, and pregnancy history, were collected by the physician. The ISTH bleeding assessment tool (BAT) was used to determine the severity of bleeding episodes [17], and data reported as ISTH BAT. The control group consisted of 50 healthy blood donors with a physiological blood count and normal levels of fibrinogen activity and antigen with similar ages (median: 43 versus 40.5 years) and sex to the patient group.
Rotational thromboelastometry and standard laboratory assays
Blood samples were collected in the morning. For controls, samples were obtained before application of a transfusion set. Blood was collected into 3.8% sodium citrate tubes by antecubital venepuncture.
ROTEM analysis was performed on a ROTEM delta platform (Werfen, Bedford, USA) with whole citrated blood 20 min after collection. The samples were processed within 1 h after collection, and heated at 37°C directly in the ROTEM. The parameters of ROTEM thromboelastometry analysis were the clotting time (CT), which represents the time from start of the measurement until initiation of clotting in seconds; clot formation time (CFT), which represents the time from initiation of clotting until a clot firmness of 20 mm in seconds; the maximum clot firmness (MCF), which indicates the maximum firmness of the clot in mm; and the A10 which is the clot strength after 10 min from CT in mm. Ellagic acid was used to activate the intrinsic coagulation pathway in INTEM, while recombinant tissue factor was used to activate the extrinsic coagulation pathway in EXTEM. FIBTEM was used to assess fibrinogen contribution to blood clot formation, which contains cytochalasin D as a platelet inhibitor. ROTEM reference ranges were provided by the manufacturer. Measurements were performed for 1 h with each sample.
Coagulation parameters (fibrinogen activity and fibrinogen antigen) were examinated after twice centrifugation of blood plasma at 2700 rpm for 15 min. Fibrinogen activity was measured by the Clauss method (IL: Instrumentation Laboratory, Bedford, Massachusetts, USA). The fibrinogen antigen assay was performed using turbidimetric latex immunoassay (LIA) (Hyphen BioMed, West Chester, Ohio, USA). Hematocrit and platelet count was performed on DxH 900 hematology analyzer (Beckaman Coulter, Miami, Florida, USA).
Statistical analysis
Descriptive statistics were expressed as median (interquartile range). Mann--Whitney tests were used to compare patients and controls group when parameters were not normally distributed. Unpaired t-tests with Welch's correction were used for normally distributed parameters. A P value of 0.05 or less was considered statistically significant. Spearman correlation tests were used to analyze the associations between the different variables or categories. The correlation between MCF by EXTEM and INTEM with hematocrit and platelet count was assessed after normalization of MCF data of and platelets count by simple scaling of patients. Data analyses were performed with GraphPad Prism 8.01 software, and receiver operating analysis (ROC) with Mathlab.
Results
Demographic, genetic, and biological data
Demographic, genetic, and biological data of patients and controls are summarized in Table 1. Overall, we included 63 patients with congenital dysfibrinogenemia (44 women) from 32 unrelated families with a mean age of 40.5 ± 25.5 years with range 4–75 years. One large family was composed of 16 affected members. The median level of fibrinogen activity was 0.57 g/l (normal range: 1.8–4.2 g/l). The median fibrinogen antigen concentration was 2.7 g/l (normal range: 1.8–4.2 g/l). As expected, congenital dysfibrinogenemia patients had decreased fibrinogen actitivy (P < 0.0001) compared to controls, and the ratio between fibrinogen activity and antigen was less than 0.7.
Table 1.
Demographic and biological data of patients and controls
| Median (IQR) Range (min – max) |
Sex (F/M) | Age years |
Fg F (g/l) | Fg Ag (g/l) | Fg F/Fg Ag | Hematocrit (L/l) | Platelet (109/l) | ISTH BAT | EXTEM CT (s) |
EXTEM CFT (s) |
EXTEM MCF (mm) |
INTEM CT (s) |
INTEM CFT (s) |
INTEM MCF (mm) |
FIBTEM A10 (mm) |
FIBTEM MCF (mm) |
| Patients Controls |
44/19 22/28 |
40.5 (25.5) 4 – 75 43 (28) 21-63 P = 0.87 |
0.57 (0.295) 0.20 – 1.3 2.64 (2.25) 1.86 – 4.51 P < 0.0001 |
2.7 (1.1) 1.8 – 4.9 |
0.20 (0.09) 0.07–0.66 |
0.41 (0.06) 0.31 – 0.9 0.43 (0.04) 0.37 – 0.51 P = 0.006 |
235 (72) 88 – 404 253 (76) 157 – 379 P = 0.24 |
0 (1) 0 – 5 |
79 (23.5) 40 – 109 73.5 (11.5) 57 – 96 P = 0.095 |
90 (30.5) 37 – 263 77.5 (26) 43 – 148 P = 0.002 |
63 (28) 49 – 77 63.5 (7.25) 55 – 105 P = 0.48 |
191 (217) 114 – 331 236 (125.5) 144 – 427 P = 0.0004 |
98 (108.5) 36 – 188 96 (50.25) 48 – 214 P = 0.54 |
61 (6) (48 – 78) 60 (7) 50 – 134 P = 0.68 |
13 (7.5) 6 – 33 15 (4) 9 – 26 P = 0.018 |
15 (7.5) 7 – 35 17 (5.5) 10 – 29 P = 0.062 |
| FGA | 36/16 | 40.5 (24.5) 7 - 67 |
0.57 (0.25) 0.2 – 1.3 |
2.9 (1.03) 1.9 – 4.9 |
0.19 (0.08) 0.07–0.41 |
0.41 (0.05) 0.31 – 0.7 |
234 (57) (88 – 381) |
0 (1) 0 - 5 |
80.5 (17.75) 40 - 109) |
88.5 (25.5) 37 – 154 |
64.5 (5) 52 – 77) |
191.5 (42.8) 114 - 331 |
91.50 (26.25) 36 - 157 |
64 (4.5) 53 - 78 |
13 (8) 6 – 33) |
15 (8.25) 7 – 35 |
| FGB | 5/3 | 44 (22.75) 30 – 75 |
0.34 (0.36) 0.3 – 0.99 |
2.3 (0.93) 1.8 – 3.8 |
0.16 (0.13) 0.11–0.32 |
0.44 (0.17) 0.39 – 0.9 |
239 (149) 141 - 404 |
1 (1) 0 - 2 |
52 (12.25) 47 - 61 |
147 (62.5) 60 -263 |
55 (6) 49 - 63 |
164 (20.8) 148 - 192 |
126 (50) 64 - 188 |
51.5 (4) 48 - 61 |
15 (4.5) 12 – 20) |
15 (4.25) 13 – 20 |
| FGG | 3/0 | 27 (13.5) 4 – 31 |
1.3 (0.11) 1.1 – 1.3 |
2.1 (0.1) 2.0 – 2.2 |
0.59 (0.07) 0.52–0.66 |
0.4 (0.03) 0.37 – 0.4 |
274 (88) 187 - 275 |
0 (1.5) 0 - 3 |
80 (4.5) 7 – 87 |
135 (8.5) 123 – 140 |
59 (0.5) 58 – 59 |
216 (17) 191 – 225 |
164 (26) 114 – 166 |
56 (1) 54 - 56 |
9 (7) 9 – 23 |
10 (10.5) 10 – 31 |
FGA mutations include Arg35His (n = 46), Gly32Glu (n = 5) and Arg35Cys (n = 1); FGB mutations include Arg196Cys (n = 8); FGG mutations include Tyr306Cys (n = 3).
p, probability from controls and patients’ comparison.
Twenty-three (36.5%) patients had a personal history of at least one bleeding episode (median ISTH BAT = 1) with epistaxis 2/63 (3.2%), easy bruising 3/63 (4.7%), oral cavity bleeding 1/63 (3.9%), bleeding from minor wound 2/63 (3.2%), tooth extraction bleeding 3/63 (4.7%), bleeding after trauma or surgery 4/63 (6.4%), hematuria 1/63 (1.5%), menorrhagia 11/44 (25.0% of women), gynecology and obstetric bleeding 6/44 (13.6% of women). In 10 patients, ISTH BAT was greater than 1 (15.5%).
Three (4.7%) patients had arterial and venous thrombotic complications (one stroke, one myocardial infarction, and one deep venous thrombosis) and three (6.8% of women) patients’ miscarriage in the first trimester.
All patients had heterozygous mutations, 52 in FGA, eight in FGB, and three in FGG. The majority were carriers of a common hotspot mutation at the Aα thrombin cleavage site, i.e. FGA p.Arg35His (n = 46; 22 families) and p.Arg35Cys (n = 1). Other mutations in FGA were near the thrombin binding site; i.e p.Gly32His (n = 2, one family) and p.Gly32Glu (n = 3, 1 family). Only one mutation was identified in FGB (p.Arg196Cys, n = 8, five families), and one in FGG (p.Tyr306Cys, n = 3, one family).
Comparison of rotational thromboelastometry parameters in congenital dysfibrinogenemia patients and controls
Fibrinogen activity and antigen were positively correlated, A10 (FIBTEM), and MCF in FIBTEM, EXTEM and INTEM (Table 2). In congenital dysfibrinogenemia patients CT (INTEM) was significantly shorter (P = 0.0004) and the CFT (EXTEM) prolonged (P = 0.002) than contols. In addition, patients’ amplitude 10 min after CT (A10) of FIBTEM was significantly lower than controls (P = 0.018). With Receiver Operating analysis (ROC), the area under the curve (AUC) was 0.69 for CT (INTEM), 0.64 CFT (EXTEM), and 0.63 A10 (FIBTEM). The AUC is an effective way to summarize the overall diagnostic accuracy of the test, where 0.5 suggests no discrimination (i.e., ability to diagnose patients with and without the disease or condition based on the test). A value of 0.7 is acceptable, so based on this value only CT (INTEM) apply, with a cutoff value of 232, the sensitivity is 0.87 and specificity 0.52. Although the specificity is low, as around half of the normal population will be false positive.
Table 2.
Correlations between fibrinogen activity and antigen values with the different quantitative variables by Spearman correlation test
| Rho (P) | Fg Ag | EXTEM CT | EXTEM CFT | EXTEM MCF | INTEM CT | INTEM CFT | INTEM MCF | FIBTEM A10 | FIBTEM MCF |
| Fg F | 0.31 (0.01) | 0.07 (0.96) | −0.20 (0.11) | 0.33 (0.007) | 0.06 (0.67) | −0.22 (0.08) | 0.31 (0.01) | 0.32 (0.010) | 0.34 (0.006) |
| Fg Ag | – | 0.07 (0.58) | −0.23 (0.06) | 0.45 (<0.001) | 0.01 (0.92) | −0.36 (0.004) | 0.5 (<0.001) | 0.42 (<0.001) | 0.42 (<0.001) |
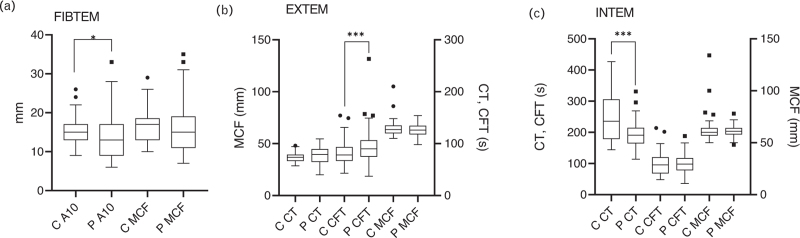
Patients’ MCF of FIBTEM, EXTEM, and INTEM were similar to controls (Fig. 1).
Fig. 1.
Comparison of INTEM parameters between patients (P) and controls (C) group. The box plot spans from 25th and 75th quartiles (interquartile range, IQR), and the line iside the box indicates the median value. Whiskers are calculated by Tukey method (lowest values close to the 25th percentile minus 1.5 IQR and higher values close to the 75th percentile and 1.5. (a) FIBTEM. (b) EXTEM. (c) IQR).
Analyses of ROTEM parameters in the 46 patients with the FGA p.Arg35His hotspot mutation (Table 3) showed a slightly prolonged CT-EXTEM (80.5 s versus 73.5 s, P = 0.004), shortened CT INTEM (188.5 s versus 235.5 s P = 0.001), and A10-FIBTEM (14.0 s versus 15.0 s, P = 0.027) compared with controls.
Table 3.
Comparison of Rotem parameters in patients carriers of the FGA mutation p.Arg35His and controls
| Median (IQR) | p.Arg35His (n = 46) | Controls (n = 50) | P |
| Age (years) | 41 (28) | 43 (11) | 0.67 |
| PLT (109/l) | 235.0 (56.8) | 252.5 (75.8) | 0.49 |
| HT (L/l) | 0.41 (0.05) | 0.43 (0.04) | 0.11 |
| Fg F(g/l) | 0.57 (0.24) | 2.64 (0.86) | <0.0001 |
| EXTEM | |||
| CT (s) | 80.5 (17.5) | 73.5 (11.5) | 0.004 |
| CFT (s) | 87.0 (26.0) | 77.5 (26.0) | 0.066 |
| MCF (mm) | 65.5 (6.0) | 63.5 (7.3) | 0.36 |
| INTEM | |||
| CT (s) | 188.5 (43.5) | 235.5 (125.5) | <0.0001 |
| CFT (s) | 88.5 (30.8) | 95.5 (51.1) | 0.27 |
| MCF (mm) | 64.0 (5.5) | 60.0 (7.0) | 0.08 |
| FIBTEM | |||
| A10 (mm) | 14.0 (7.8) | 15.0 (4.0) | 0.027 |
| MCF (mm) | 15.0 (8.3) | 17.0 (5.5) | 0.12 |
Fg F, fibrinogen activity; P, probability.
The correlation of ROTEM parameters with patients bleeding phenotype (n = 23) showed that only CFT EXTEM and CT INTEM were statistically different from controls (P = 0.035 and P = 0.0014, respectively) (Table 4).
Table 4.
Correlations ROTEM parametrers between patients with bleeding phenotypes and controls
| Median (IQR) Range (min – max) |
n | ISTH BAT | EXTEM CT (s) |
EXTEM CFT (s) |
EXTEM MCF (mm) |
INTEM CT (s) |
INTEM CFT (s) |
INTEM MCF (mm) |
FIBTEM A10 (mm) |
FIBTEM MCF (mm) |
| Patients with bleeding phenotypes Controls |
23 50 |
1 (1.5) 1 – 5 |
79 (21) 47 – 96 73.5 (11.5) 57 – 96 P = 0.390 |
90 (43) 60– 263 77.5 (26) 43 – 148 P = 0.035 |
63 (8.5) 49 – 75 63.5 (7.25) 55 – 105 P = 0.589 |
175 (44) 143 – 254 236 (125.5) 144 – 427 P = 0.0014 |
89 (45) 58– 188 96 (50.25) 48 – 214 P = 1 |
61 (7.5) (48 – 72) 60 (7) 50 – 134 P = 0.834 |
14 (11) 8 – 24 15 (4) 9 – 26 P = 0.217 |
15 (9.5) 8 – 26 17 (5.5) 10 – 29 P = 0.227 |
Discussion
We evaluated the potential of ROTEM parameters to distinguish congenital dysfibrinogenemias from healthy controls and patients’ clinical phenotypes. At present, there are limited data on the diagnostic value of ROTEM in assessing hereditary bleeding disorders [18], and if ROTEM can be used for dysfibrinogenemia diagnosis value. Indeed, rotational thromboelastometry can be envisaged as a useful additional assay for clinical management of patients since this test combines the study of functional and structural aspects of the blood clot.
Previously, Szanto et al.[12] demostrated that ROTEM tests have high sensitivity for the diagnosis of hypofibrinogenemia. At the same time, studies have shown that MCF, and especially FIBTEM, may help to discriminate patients with hypofibrinogenemia or dysfibrinogenemia [12,19]. However, in our study, congenital dysfibrinogenemia patients MCF and A10 (FIBTEM) were within the reference intervals.
In several studies, correlations between fibrinogen activity and A10, MCF in EXTEM and FIBTEM, have been confirmed in for healthy controls [7,20]. In our study, there were moderate correlations between fibrinogen assays (activity and antigen) and MCF-EXTEM of congenital dysfibrinogenemia patients (P = 0.33 P = 0.007, and P = 0.45 P < 0.001). These findings are opposite to those published by Zhou et al.[15] that not significant correlation between congenital dysfibrinogenemia fibrinogen activity and maximum signal amplitude (MA) parameters (equivalent to MCF in ROTEM) were found. One explanation for this discrepancy may be the fact that in our study there was less genetic heterogeneity, as more than 80% of causative mutations were at the thrombin cleavage site of fibrinogen Aα-chains, and in study by Zhou et al. [15], only 44% patients were heterozygous for hotspot mutations in FGA exon 2. The FGA p.Arg35His mutation leads to delayed release of fibrinopeptide A which in turn causes altered fibrin network structure [21]. Furthermore, in our study, A10-FIBTEM was reduced in the 46 patients with the FGA mutation (p.Arg35His), while the MCF values of all three ROTEM assays were almost within the normal range, comparable to results by Zhou et al. [15].
The fibrin clot structure is influenced by several factors. In addition to mutations in the fibrinogen genes, other common genetic polymorphisms may affect the balance of activating/inhibiting the coagulation or fibrinolysis pathway [22]. The results of our study were similar to those of Treliński et al.[19]: dysfibrinogenemic patients had a lower amplitude than controls at 10 min in FIBTEM, which normalized after fibrin polymerization completion, as MCFs were similar to controls in EXTEM and INTEM. Thus, A10 FIBTEM appears to be a good parameter for detecting fibrin polymerization abnormalities. In addition, it has been published that in hypofibrinogenemia, MCF (EXTEM, INTEM, and FIBTEM), including A10-FIBTEM, were directly proportional to the decreased fibrinogen activity. It appears that MCF could be helpful in distinguishing between qualitative and quantitative fibrinogen disorders [14,23].
An unexpected result was the shortened patients CT (INTEM) and normal aPTT (median: 29.7 s, normal range 22.0–32.0 s) in patients compared with controls, that is, it should be associated with a shortened CFT (the time from initiation of clotting until a clot firmness of 20 mm is detected) and increased MCF [24]. However, CFT and MCF in INTEM were comparable to controls.
Tissue factor is used for activation and assessment of the extrinsic pathway [25] reflected in the EXTEM assay. This test is influenced by extrinsic coagulation factors, platelets, and fibrinogen [26]. Clot formation via the extrinsic pathway occurs more rapidly than the intrinsic pathway [27]. In our study, prolonged CT in EXTEM suggests that the extrinsic pathway are not functioning normally, and initiation of clotting is impaired in dysfibrinogemetic patients with mutations in exon 2 of FGA. It is possible that the impairment in the extrinsic pathway is partially compensated by the intrinsic pathway as shown by the shortening of CT in INTEM.
CFT is more impacted by an impaired fibrin polymerization than MCF [28]. In our study, dysfibrinogemic patients had higher CFT in EXTEM than controls. Interestingly, most of the 23 dysfibrinogenemic patients with bleeding phenotype had significantly prolonged CFT values in EXTEM (P = 0.035). This result is similar to the study of Wei et al. [13] who reported that a higher k value in TEG (the equivalent of CFT) can predict risk of bleeding in patients with congenital dysfibrinogenemia [15].
Congenital dysfibrinogenemiais well known that a major difficulty for clinical management of patients with congenital dysfibrinogenemia is their highly heterogeneous clinical phenotype. Studies typically describe a 25–42% incidence of abnormal bleeding in congenital dysfibrinogenemia. In our study, only 15.5% dysfibrinogenemic patients had a bleeding score at least 1. This is lower than the prevalence of bleeding episodes in a previous study by Shapiro et al.[29] (bleeding phenotype: n = 12 [34%] of 35 patients with congenital dysfibrinogenemia). This is probably because we included all patients from our center regardless of clinical phenotype. The bleeding manifestations were mostly mild, and spontaneous life-threatening hemorrhages were rare [3,30]. The most common bleeding symptom was menorrhagia (n = 11 [25.0%] of 44 women). Similar results were reported by Casini et al. (n = 20 [29.4%] of 68 women) [30]. In addition to bleeding manifestions, thrombotic and pregnancy-related complications also occur [3]. Dysfibrinogenemia is a relatively rare cause of thrombophilia [31]. The prevalence of thrombotic complications in our patient cohort was very low, with a similar incidence as a large study of 102 Chinese patients with dysfibrinogenemia [15].
The main purpose of this study was to determine if at least some parameters of ROTEM can help to identify dysfibrinogenemic patients at higher risk of bleeding or thrombotic complications. For example, cases with elevated MCF in EXTEM and FIBTEM compared to normal controls could have a higher risk of developing thrombotic complications. Hincker et al.[32] suggested that MCF can predict thromboembolic complications since in 313 patients with thromboembolic complications they observed a significantly higher MCF and elevated fibrinogen activity. Galanakis et al.[11] reported three dysfibrinogenemic patients with a thrombotic history. Two out of three fibrin clots showed reduced maximum signal amplitude, in the range of 31–25% of normal control values. One dysfibrinogenemic patient with a history of both thrombosis and bleeding had a normal MA value (100%). However, in our study, all three patients with a thrombotic history (stroke, myocardial infarction, and deep venous thrombosis) had median MCF values in FIBTEM identical to controls (17 mm). Among the 23 dysfibrinogenemic patients with history of hemorrhage, median MCF values in FIBTEM were lower than healthy controls (15 versus 17 mm). These values are similar to the MA in FIBTEM reported by Galanakis et al.[11] in patients with bleeding history.
In conclusion, there are still only limited data on the diagnostic value of ROTEM in assessing congenital qualitative fibrinogen disorders. In contrast to routine analyses which only provide information about the initiation phase of clot formation, ROTEM also assesses the strength of the formed clot. EXTEM, INTEM, and FIBTEM are not tests that can clearly discriminates dysfunctional fibrinogen from controls. ROC analysis showed that only CT (INTEM) had acceptable sensitivity but low specificity in distinguishing patients with dysfunctional fibrinogen from controls. However, even in this relatively large cohort of patients, the number of symptomatic dysfibrinogenemic patients was insufficient to determine which ROTEM parameters may be useful to reliably identify patients at risk of bleeding or thrombotic complications.
Acknowledgements
The authors would like to thank the support of projects Vega 1/0436/21, Vega 1/0479/21, Agency for the Support of Research and Development APVV-16-0020, Comenius University grants: 67/2023, 151/2023 and research grant from CSL Behring.
Research performed by T.S., M.D., I.S., J.Z., Z.K., D.K., M.B., K.M.B., and J.S. Study was designed by T.S., M.D. Data were analyzed by T.S., R.M., A.C., and M.N.R. Article was written by T.S. and reviewed by all authors.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Casini A, Undas A, Palla R, Thachil J, de Moerloose P. Subcommittee on Factor XIII and Fibrinogen. Diagnosis and classification of congenital fibrinogen disorders: communication from the SSC of the ISTH. J Thromb Haemost 2018; 16:1887–1890. [DOI] [PubMed] [Google Scholar]
- 2.Casini A, Neerman-Arbez M, Ariëns RA, de Moerloose P. Dysfibrinogenemia: from molecular anomalies to clinical manifestations and management. J Thromb Haemost 2015; 13:909–919. [DOI] [PubMed] [Google Scholar]
- 3.Simurda T, Zolkova J, Kolkova Z, Loderer D, Dobrotova M, Skornova I, et al. Comparison of clinical phenotype with genetic and laboratory results in 31 patients with congenital dysfibrinogenemia in northern Slovakia. Int J Hematol 2020; 111:795–802. [DOI] [PubMed] [Google Scholar]
- 4.Casini A, de Moerloose P. How I treat dysfibrinogenemia. Blood 2021; 138:2021–2030. [DOI] [PubMed] [Google Scholar]
- 5.Undas A. How to assess fibrinogen levels and fibrin clot properties in clinical practice? Semin Thromb Hemost 2016; 42:381–388. [DOI] [PubMed] [Google Scholar]
- 6.Carll T, Wool GD. Basic principles of viscoelastic testing. Transfusion 2020; 6:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Amgalan A, Allen T, Othman M, Ahmadzia HK. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women's SSC of the ISTH. J Thromb Haemost 2020; 18:1813–1838. [DOI] [PubMed] [Google Scholar]
- 8.Erdoes G, Koster A, Levy JH. Viscoelastic coagulation testing: use and current limitations in perioperative decision-making. Anesthesiology 2021; 135:342–349. [DOI] [PubMed] [Google Scholar]
- 9.Ross CR, Subramanian S, Navarro-Puerto J, Subramanian K, Kalappanavar NK, Khayat CD, et al. Pharmacokinetics, surrogate efficacy and safety evaluations of a new human plasma-derived fibrinogen concentrate (FIB Grifols) in adult patients with congenital afibrinogenemia. Thromb Res 2021; 199:110–118. [DOI] [PubMed] [Google Scholar]
- 10.Simurda T, Asselta R, Zolkova J, Brunclikova M, Dobrotova M, Kolkova Z, et al. Congenital afibrinogenemia and hypofibrinogenemia: laboratory and genetic testing in rare bleeding disorders with life-threatening clinical manifestations and challenging management. Diagnostics (Basel) 2021; 11:2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanakis DK, Neerman-Arbez M, Brennan S, Rafailovich M, Hyder L, Travlou O, et al. Thromboelastographic phenotypes of fibrinogen and its variants: clinical and nonclinical implications. Thromb Res 2014; 133:1115–1123. [DOI] [PubMed] [Google Scholar]
- 12.Szanto T, Lassila R, Lemponen M, Lehtinen E, Neerman-Arbez M, Casini A. Whole blood thromboelastometry by ROTEM and thrombin generation by genesia according to the genotype and clinical phenotype in congenital fibrinogen disorders. Int J Mol Sci 2021; 22:2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei A, Liao L, Xiang L, Yan J, Yang W, Nai G, et al. Congenital dysfibrinogenaemia assessed by whole blood thromboelastography. Int J Lab Hematol 2018; 40:459–465. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Xin Y, Ding Q, Jiang L, Chen Y, Dai J, et al. Thromboelastography predicts risks of obstetric complication occurrence in (hypo)dysfibrinogenemia patients under nonpregnant state. Clin Exp Pharmacol Physiol 2016; 43:149–156. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Ding Q, Chen Y, Ouyang Q, Jiang L, Dai J, et al. Clinical features and molecular basis of 102 Chinese patients with congenital dysfibrinogenemia. Blood Cells Mol Dis 2015; 55:308–315. [DOI] [PubMed] [Google Scholar]
- 16.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 1977; 74:5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost 2010; 8:2063–2065. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt DE, Majeed A, Bruzelius M, Odeberg J, Holmström M, Ågren A. A prospective diagnostic accuracy study evaluating rotational thromboelastometry and thromboelastography in 100 patients with von Willebrand disease. Haemophilia 2017; 23:309–318. [DOI] [PubMed] [Google Scholar]
- 19.Treliński J, Pachniewska K, Matczak J, Robak M, Chojnowski K. Assessment of selected ROTEM parameters, kinetics of fibrinogen polymerization and plasmin amidolytic activity in patients with congenital fibrinogen defects. Adv Clin Exp Med 2016; 25:1255–1263. [DOI] [PubMed] [Google Scholar]
- 20.de Vries JJ, Veen CSB, Kruip MJHA, de Maat MPM. FIBTEM clot firmness parameters correlate well with the fibrinogen concentration measured by the Clauss assay in patients and healthy subjects. Scand J Clin Lab Invest 2020; 80:600–605. [DOI] [PubMed] [Google Scholar]
- 21.Sugo T, Endo H, Matsuda M, Ohmori T, Madoiwa S, Mimuro J, et al. A classification of the fibrin network structures formed from the hereditary dysfibrinogens. J Thromb Haemost 2006; 4:1738–1746. [DOI] [PubMed] [Google Scholar]
- 22.Mannucci PM, Franchini M. Classic thrombophilic gene variants. Thromb Haemost 2015; 114:885–889. [DOI] [PubMed] [Google Scholar]
- 23.Khunakanan S, Akaraborworn O, Sangthong B, Thongkhao K. Correlation between maximum clot firmness in FIBTEM and fibrinogen level in critical trauma patients. Crit Care Res Pract 2019; 2019:2756461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multicentre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis 2005; 16:301–310. [DOI] [PubMed] [Google Scholar]
- 25.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007; 27:1687–1693. [DOI] [PubMed] [Google Scholar]
- 26.Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol 2014; 89:228–232. [DOI] [PubMed] [Google Scholar]
- 27.Smith SA, Travers RJ, Morrissey JH. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol 2015; 50:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heim C, Schoettker P. Viscoelastic tests of hemostasis. In: Marcucci C, Schoettker P, editors. Perioperative hemostasis. Berlin, Heidelberg: Springer; 2015. [DOI] [Google Scholar]
- 29.Shapiro SE, Phillips E, Manning RA, Morse CV, Murden SL, Laffan MA, et al. Clinical phenotype, laboratory features and genotype of 35 patients with heritable dysfibrinogenaemia. Br J Haematol 2013; 160:220–227. [DOI] [PubMed] [Google Scholar]
- 30.Casini A, Blondon M, Lebreton A, Koegel J, Tintillier V, de Maistre E, et al. Natural history of patients with congenital dysfibrinogenemia. Blood 2015; 125:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korte W, Poon MC, Iorio A, Makris M. Thrombosis in inherited fibrinogen disorders. Transfus Med Hemother 2017; 44:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major noncardiac surgery. Crit Care 2014; 18:549. [DOI] [PMC free article] [PubMed] [Google Scholar]