Abstract
Background:
Pregnant and postpartum women in Sub-Saharan Africa are at high risk of HIV acquisition. We evaluated a person-centered dynamic choice intervention for HIV prevention (DCP) among women attending antenatal and postnatal care.
Setting:
Rural Kenya and Uganda.
Methods:
Women (aged 15 years or older) at risk of HIV acquisition seen at antenatal and postnatal care clinics were individually randomized to DCP vs. standard of care (SEARCH; NCT04810650). The DCP intervention included structured client choice of product (daily oral pre-exposure prophylaxis or postexposure prophylaxis), service location (clinic or out of facility), and HIV testing modality (self-test or provider-administered), with option to switch over time and person-centered care (phone access to clinician, structured barrier assessment and counseling, and provider training). The primary outcome was biomedical prevention coverage—proportion of 48-week follow-up with self-reported pre-exposure prophylaxis or postexposure prophylaxis use, compared between arms using targeted maximum likelihood estimation.
Results:
Between April and July 2021, we enrolled 400 women (203 intervention and 197 control); 38% were pregnant, 52% were aged 15–24 years, and 94% reported no pre-exposure prophylaxis or postexposure prophylaxis use for ≥6 months before baseline. Among 384/400 participants (96%) with outcome ascertained, DCP increased biomedical prevention coverage 40% (95% CI: 34% to 47%; P < 0.001); the coverage was 70% in intervention vs. 29% in control. DCP also increased coverage during months at risk of HIV (81% in intervention, 43% in control; 38% absolute increase; 95% CI: 31% to 45%; P < 0.001).
Conclusion:
A person-centered dynamic choice intervention that provided flexibility in product, testing, and service location more than doubled biomedical HIV prevention coverage in a high-risk population already routinely offered access to biomedical prevention options.
Key Words: HIV, PrEP, PEP, antenatal care, postnatal care, person-centered
INTRODUCTION
Pregnant and postpartum women in Sub-Saharan Africa are at high risk of HIV acquisition,1–4 despite widespread rollout of pre-exposure prophylaxis (PrEP) in sub-Saharan Africa and its established safety and efficacy during pregnancy.5 In addition to impact on the health of the women infected, acute HIV infection in pregnancy and postpartum also elevates the risk of mother to child transmission of HIV.5 A recent estimate attributes an estimated 20%–25% of perinatal transmission to acute maternal HIV infection.6
Antenatal and postnatal care (ANC) clinics may provide an underutilized opportunity for reaching and delivering HIV biomedical prevention to women during this high-risk period.7 Antiretroviral therapy delivery for women with HIV is routinely and successfully integrated in ANC clinics.8,9 However, HIV prevention service delivery remains largely restricted to HIV clinics, which may exacerbate access and stigma barriers for women accessing ANC services. Integration of HIV biomedical prevention delivery within routine ANC clinics provides an opportunity to leverage existing infrastructure to reduce these barriers.8,10,11
Optimizing HIV prevention coverage among pregnant and postpartum women will also require innovative delivery models able to meet women's varied and changing needs during this dynamic period. Providing structured client choice in products and services has been shown to improve the coverage of contraception among women,12 and a robust literature documenting varying client-stated preferences suggests that choice may also be key to optimizing HIV prevention coverage.13–15 However, little is known about the actual selections made by pregnant and postpartum women when offered structured choices in HIV prevention products and services nor about the impacts of a delivery model offering such choices on biomedical prevention coverage.
METHODS
Study Design
We conducted an individually randomized trial (SEARCH; NCT04810650) to evaluate the impact of a person-centered model for offering women dynamic choices in HIV biomedical prevention product (PrEP or postexposure prophylaxis [PEP]), visit location (ANC clinic or out of facility), and HIV testing modality (self-test or provider administered) vs. standard of care on HIV biomedical prevention coverage among women attending an ANC clinic in rural East Africa.
Study Setting and Participants
Between April and July 2021, we enrolled pregnant and postpartum women seen at 4 public sector ANC clinics located in rural Kenya (Homa Bay and Migori Counties) and Uganda (Mbarara and Bushenyi districts). Clinics were located at subcounty hospitals in Kenya and level IV facilities in Uganda. In addition to antenatal, postnatal, and maternity services, all 4 facilities provide a range of outpatient and inpatient services, including laboratory, pharmacy, and HIV/AIDS comprehensive care. ANC and postnatal services are offered by nurses/midwives and peer mothers.
All clients attending the ANC clinics were screened for eligibility by the study nurse/midwife. Inclusion criteria were HIV negative status by country standard HIV testing algorithm, age 15 years or older, and current or anticipated risk of HIV acquisition. HIV risk was assessed using both country-specific Ministry of Health PrEP screening tools16,17 and 2 questions on self-assessment of current risk or anticipated risk in the coming 3 months. Persons who screened at risk using either tool were eligible. Both women receiving antenatal services and women receiving postnatal services, regardless of birth outcome or breastfeeding status, were eligible for the study, as were women reporting current or recent use or PrEP or PEP at baseline. Exclusion criteria were unable to provide consent or concurrently participating in another SEARCH trial.
Randomization and Procedures
Consented participants were randomized 1:1 to intervention or control by selecting a sequentially numbered scratch card, revealing the arm only when scratched by the participant. The computer-generated randomization sequence, provided by an independent statistician, was stratified on country and pregnancy status using a stratified block design with random block sizes of 2 and 4. Participants were not blinded to randomization arm, but the study statistician (L.B.B.) was blinded until trial completion and analysis.
Participant demographics, previous self-reported PrEP and PEP use, and partner status were assessed at study baseline (time of randomization). At weeks 24 and 48, a structured monthly survey was administered in both arms to retrospectively assess self-report of PrEP and PEP use (ie, pill ingestion) and perceived risk of HIV acquisition over each of the previous 6 months.
The survey was administered electronically by study staff after a detailed training on how to assess self-report of PreP/PEP use and self-assessed potential HIV exposure from participants without stigmatizing them. The surveys were conducted at either facility or off-site location, depending on the participant's choice; for participants who were unable to be seen physically, the survey was completed through phone.
After the survey, HIV rapid tests and HIV RNA testing (GeneXpert, Cepheid) were conducted among participants in both arms. A 1-month supply of PrEP was provided to all participants at baseline. In the control arm, participants were referred to the HIV clinic for standard HIV prevention services. Women in the intervention arm were offered services through the dynamic choice intervention for HIV prevention (DCP) model.
Study Intervention
We developed the DCP intervention as a theory-driven, person-centered model focused on offering structured dynamic choices in HIV prevention products and services. The intervention was developed using the PRECEDE framework for health promotion strategies to address “predisposing” factors (knowledge, attitudes, or beliefs that affect behavior), “enabling” factors to facilitate behavior, and “reinforcing” factors that include consequences of following a behavior18 and was designed based on the principles of a person-centered care approach that is sensitive and responsive to individual client choices and preferences.19 Before study initiation, intervention components were refined based on qualitative and survey data.
The DCP intervention included (1) structured client choice of biomedical prevention product, HIV test modality, and location of service delivery, with the option to switch between these choices over time (enabling and reinforcing) and (2) person-centered care, including provider training (predisposing, enabling, and reinforcing), structured barrier assessment (reinforcing), counseling (predisposing, enabling, and reinforcing), and phone access to a clinician (reinforcing).
The DCP intervention was integrated within antenatal (ANC)/postnatal (PNC) clinics within public health centers and delivered by study midwives and nurses in the ANC clinics or preferred service location choice. Client visits for intervention delivery occurred at weeks 0, 4, 12, 24, and 36. At each visit, clients were offered structured choices in the following domains: (1) biomedical prevention product—daily oral PrEP [tenofovir disoproxyl fumarate (TDF)/lamivudine (FTC) or TDF/emtricitabine (3TC)] or PEP with tenofovir/lamivudine/dolutegravir provided from the Ministry of Health supplies (with condoms as an additional option); (2) HIV testing—choice of blood-based rapid test or oral HIV self-test (OraQuick); and (3) service location for the next visit—health facility or out of facility in a community setting of the participant's choice. PrEP and PEP were given based on country guidelines.16,17 For participants selecting PrEP, up to a 3-month supply was provided at each visit. PEP was offered with a “pill-in-pocket” option of 3–5 pills to have on hand for unanticipated HIV exposures, as well as an HIV self-test (HIVST) kit. Participants were instructed to take PEP as soon as possible after an exposure and to contact the study midwife/nurse for rapid HIV testing and the remaining supply of PEP.
All providers were trained on person-centered delivery before the study launch. Training explicitly focused on the principle of offering choices without imposing the provider's own views on what might be best for the client and included case studies to illustrate how providers can support agency in client decision-making. The training emphasized delivery of warm patient-friendly services to foster provider–client trust in discussing HIV risk and the best available option without fear of feeling judged. During the study, providers met monthly to share insights and experiences, and 6 scheduled 60-minute on-job booster trainings were held.
Person-centered care offered at each intervention visit included structured assessment of barriers to PrEP/PEP start and adherence, with personalized plans developed in response, and psychological support for trauma. Participants were also provided with a phone contact of a provider to consult and ask any questions, available 24 hours a day, 7 d/wk. In addition, staff contacted all participants who initiated PrEP or PEP by phone to assess adherence and any other concerns every 2 weeks in the first month and monthly thereafter.
Standard Of Care
At enrollment, participants were referred to standard HIV prevention services, including screening for PrEP eligibility at the adjacent HIV clinic. Among persons initiating PrEP, PrEP was provided for a 1-month supply at baseline and for a 1-month to 3-month supply at follow-up visits, with standard rapid blood-based HIV testing, at the health facility. PEP was not routinely available except in cases of sex-based violence or occupational exposure (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/C217).
Outcomes
The primary outcome was 48-week biomedical prevention coverage, defined as the number of months during which a participant reported using either PrEP or PEP (ie, pill ingestion) divided by the number of months assessed. Biomedical prevention coverage during periods of retrospectively self-assessed HIV risk was defined as a prespecified secondary outcome. HIV seroconversion, defined as any reactive HIV self-test or HIV rapid test (per national testing algorithm) with confirmatory HIV RNA testing, was assessed and reported in both arms. Within the intervention arm, we also reported choice of DCP components over follow-up.
Statistical Analyses
The target sample size of 400 persons was designed to ensure 80% power to detect at least a 10% absolute increase in biomedical prevention coverage in the intervention versus control arm of the trial (see Statistical Analysis Plan, Supplemental Digital Content, http://links.lww.com/QAI/C218). Primary and secondary outcomes were compared between arms using targeted minimum loss estimation and adaptive prespecification, which uses machine learning to optimize precision through flexible adjustment for baseline covariates while preserving Type 1 error;20,21 adjustment variables included pregnancy, age, country, and prior use of PrEP/PEP. Prespecified subgroup analyses included pregnancy, age, alcohol use, and prior use of PrEP or PEP. Analyses were conducted using R (version 4.2.1).22
Ethical Approval
We obtained approval from the ethics committee of the University of California; San Francisco Committee on Human Research (San Francisco, CA); the Kenya Scientific and Ethics Review Unit; the National Commission for Science, Technology, and Innovation (Nairobi, Kenya); Ugandan National Council for Science and Technology; and Makerere University School of Medicine Research and Ethics Committee (Kampala, Uganda). All participants aged 18 years or older provided written consent to participate in this study. Participants aged 15–17 years who did not meet the definition of emancipated minors required an adult witness to consent for their participation.
RESULTS
Study Participants
Of 1095 women aged 15 years or older screened for eligibility, we enrolled 400 women (203 intervention and 197 control) (Fig. 1). Among enrolled women, 52% (N = 208) were aged 15–24 years, the median age was 24 years (IQR: 20–29), 78% (N = 310) were married or cohabiting, 38% (N = 154) were pregnant, and 94% (N = 378) reported at least 1 partner during the previous 6 months with HIV positive or unknown status (Table 1). Only 6% (N = 25) of participants reported using PrEP or PEP in the 6 months before enrollment.
FIGURE 1.
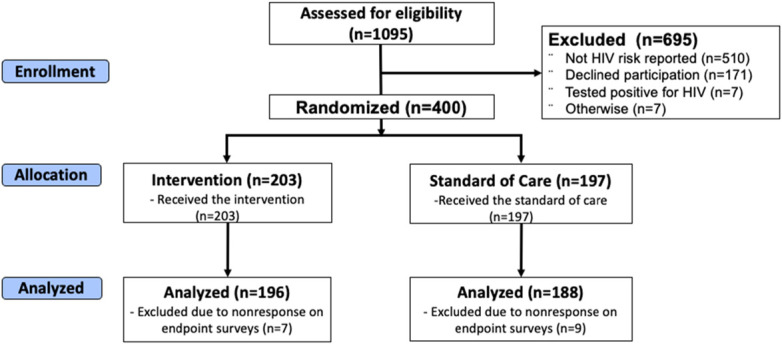
CONSORT diagram.
TABLE 1.
Baseline Characteristics of Study Participants By Arm and Overall
| Intervention (N = 203), (%) | Control (N = 197), (%) | Total (N = 400), (%) | |
| Aged 15–24 yrs | 106/203 (52) | 102/197 (52) | 208/400 (52) |
| Country | |||
| Kenya | 103/203 (51) | 98/197 (50) | 201/400 (50) |
| Uganda | 100/203 (49) | 99/197 (50) | 199/400 (50) |
| Marital status | |||
| Single (never married) | 49/203 (24) | 39/197 (20) | 88/400 (22) |
| Married/cohabitating | 154/203 (76) | 156/197 (79) | 310/400 (78) |
| Divorced/separated/widowed | 0/203 (0) | 2/197 (1) | 2/400 (0) |
| Occupation | |||
| Farmer | 64/202 (32) | 64/197 (32) | 128/399 (32) |
| Housewife | 33/202 (16) | 24/197 (12) | 57/399 (14) |
| Shopkeeper/market vendor | 26/202 (13) | 20/197 (10) | 46/399 (12) |
| Student | 18/202 (9) | 21/197 (11) | 39/399 (10) |
| No job | 14/202 (7) | 16/197 (8) | 30/399 (8) |
| Manual labor/construction | 1/202 (0) | 1/197 (1) | 2/399 (1) |
| Fishing/fishmonger | 4/202 (2) | 4/197 (2) | 8/399 (2) |
| Other | 42/202 (21) | 47/197 (24) | 89/399 (22) |
| HIV risk enrollment criteria | |||
| Ministry of Health only | 3/203 (1) | 1/197 (1) | 4/400 (1) |
| Self-assessed (current/anticipated) only | 8/203 (4) | 9/197 (5) | 17/400 (4) |
| Ministry of Health and self-assessed | 192/203 (95) | 187/197 (95) | 379/400 (95) |
| HIV risk by sexual partners | |||
| Partner with HIV or unknown status (any, past 6 mo) | 192/203 (95) | 186/197 (94) | 378/400 (94) |
| Primary partner with HIV* | 34/101 (34) | 37/99 (37) | 71/200 (36) |
| Primary partner with HIV on ART† | 31/32 (97) | 32/32 (100) | 63/64 (98) |
| Alcohol use (any, prior 3 mo) | 14/203 (7) | 12/197 (6) | 26/400 (6) |
| Pregnant | 80/203 (39) | 74/197 (38) | 154/400 (38) |
| PrEP or PEP use (any, prior 6 mo) | 11/203 (5) | 14/197 (7) | 25/400 (6) |
Among participants reporting a primary partner.
Among participants reporting their primary partner is living with HIV.
Implementation of DCP Intervention
At week 4 after enrollment, 84% of eligible intervention participants (171/203) were seen and offered person-centered care and structured choice of product, test modality, and location for their next visit. This increased to 95% of participants (192/203) at 12 weeks, 92% (186/203) at 24 weeks, and 93% (188/203) at 36 weeks.
At baseline visit, 98% of intervention participants (198/203) chose PrEP, declining to 69% (129/188) at week 36. Across intervention visits, 10% of participants (20/203) chose PEP at least once during the follow-up (Fig. 2A; see Table S2, Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/C217). The number of participants selecting none of the prevention options increased from 0% at baseline to 22% (42/188) at week 36. At baseline, 22% of participants (44/203) chose an out-of-facility location for their next visit; this increased to 46% (87/188) at week 36; 76% of intervention participants (155/203) chose out-of-facility delivery at least once (Fig. 2B). Selection of self-testing ranged from 18% (34/192) to 57% (106/186) across intervention visits; 82% of intervention participants (167/203) chose self-testing at least once (Fig. 2C).
FIGURE 2.
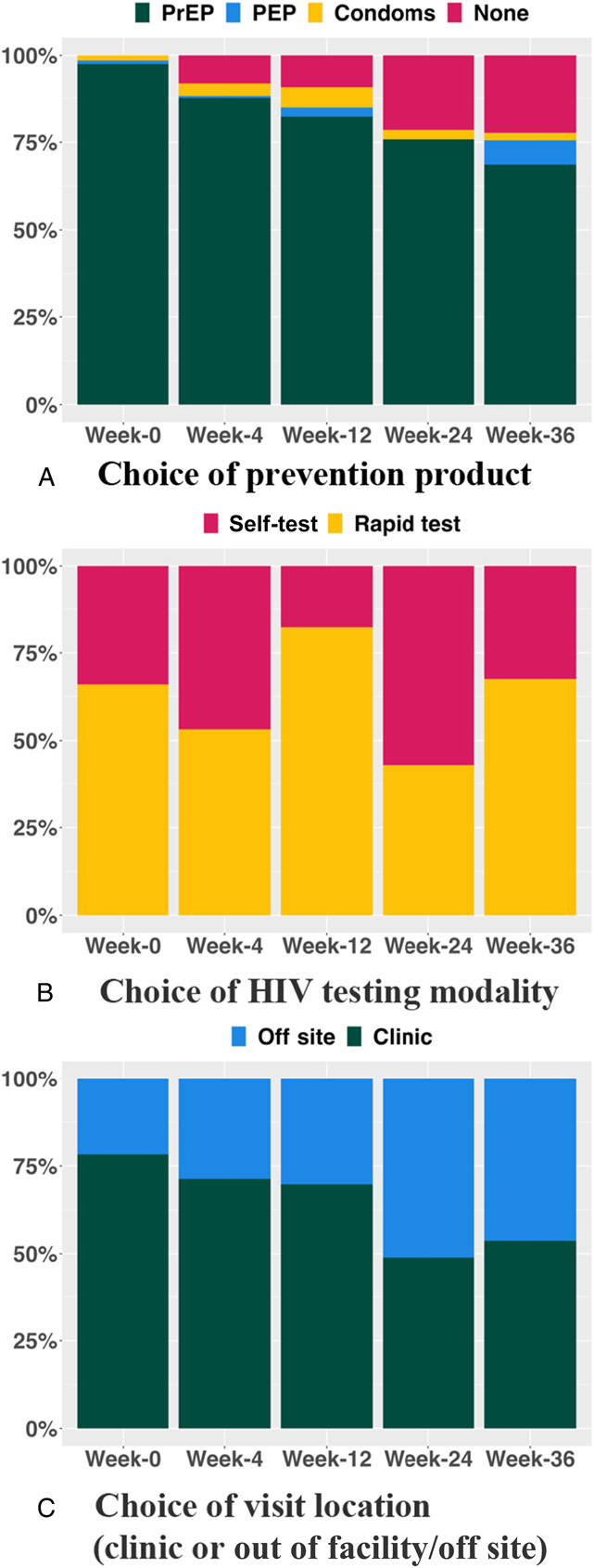
Choice of DCP intervention components over time. A, Choice of prevention product. B, Choice of HIV testing modality. C, Choice of visit location (clinic or out of facility/off site).
Biomedical Prevention Coverage
The primary outcome was ascertained in 384/400 women (96%), 97% (196/203) in the intervention arm and 95% (188/197) in the control arm. The intervention increased biomedical prevention coverage by 40% (absolute percentage point increase; 95% CI: 34% to 47%; P < 0.001). A mean of 70% (95% CI: 65% to 74%) of follow-up time was covered by biomedical prevention in the intervention arm and 29% (95% CI: 25% to 34%) in the control arm (Fig. 3A). Similar effect sizes were seen in subgroups defined by country, age, baseline pregnancy status, partner HIV status, and alcohol use. Among the control arm participants, all of whom were referred to standard-of-care HIV prevention services after baseline risk screen, 28% (56/197) reported no use of PrEP or PEP during follow-up, and only 10% reported 100% biomedical prevention coverage during follow-up. By contrast, only 9% (18/203) of the intervention arm reported no use of PrEP or PEP during follow-up, and 37% of intervention participants (76/203) reported 100% coverage.
FIGURE 3.
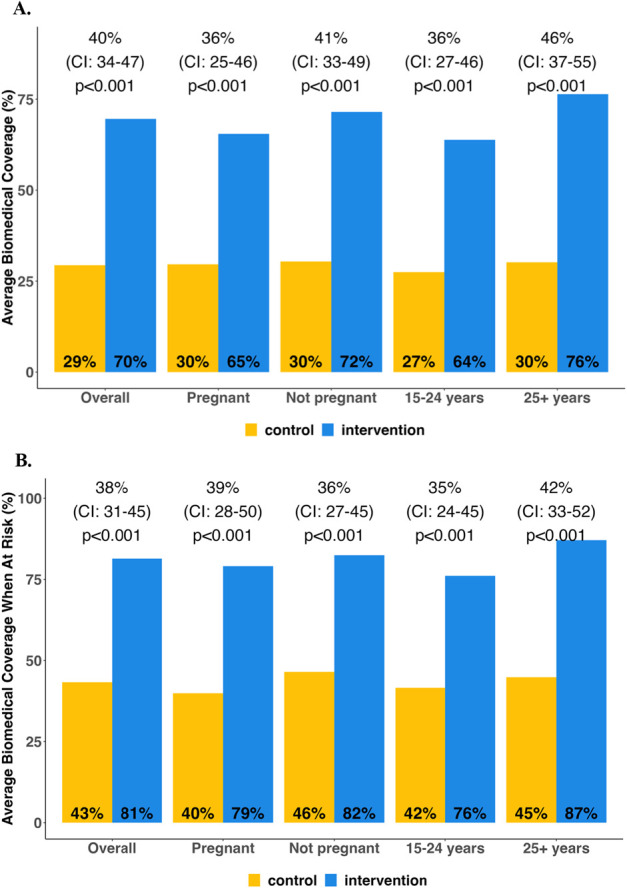
Effect of intervention on proportion of follow-up time with self-reported use of PrEP or PEP, overall and by subgroups. A, Biomedical prevention coverage (primary outcome). B, Biomedical prevention coverage restricted to months with self-reported risk of HIV acquisition. Effect estimates regarding the difference in average biomedical prevention coverage between intervention and control arms.
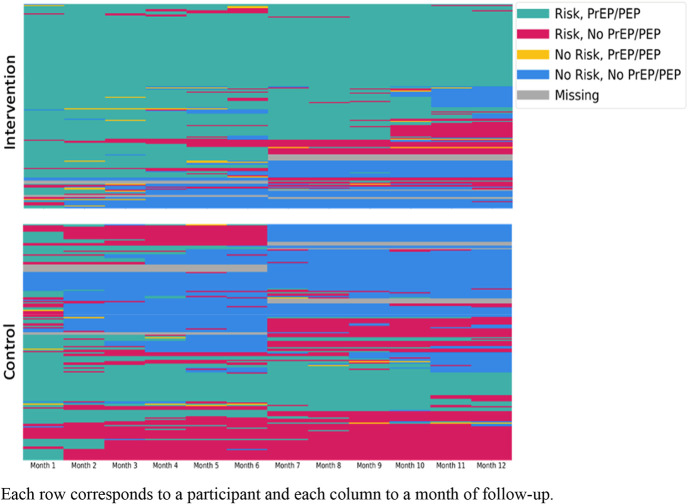
Although all participants reported current or anticipated HIV risk at baseline, participants' self-reported experience of HIV risk (assessed retrospectively at weeks 24 and 48), as well as their use of PrEP and PEP, varied over time (Fig. 4). When follow-up time was restricted to periods of retrospectively self-assessed risk, biomedical prevention coverage was higher in both the intervention arm (mean 81% of at-risk follow-up time covered by biomedical prevention) and the control arm (43% of at-risk follow-up time covered). The intervention increased biomedical prevention coverage during time at-risk by 38% (absolute increase; 95% CI: 31% to 45%; P < 0.001) (Fig. 3B).
FIGURE 4.
Heatmaps of self-reported HIV risk and use of biomedical prevention, by arm over time.
HIV Seroconversion
Three participants, 1 in the control arm and 2 in the intervention arm, seroconverted during follow-up. All 3 participants were aged 24 to 29 years and had a partner or husband of unknown HIV status. In the intervention arm, 1 participant who seroconverted chose PrEP but reported missing 10 doses because of travel around the time of delivery, while the second participant reported no use of PrEP. The 1 participant who seroconverted in the control arm reported no use of PrEP during follow-up. Both women who reported no use of PrEP cited partner or peer disapproval as a barrier to starting PrEP.
DISCUSSION
In this randomized trial, we implemented a DCP intervention that offered clients structured choice of PrEP or PEP, HIV testing type, and visit location using a person-centered approach. The intervention more than doubled HIV biomedical prevention coverage (based on self-reported use) among pregnant and postpartum women compared with standard of care, with similar effect sizes among both younger and older women and during the antenatal and postnatal periods. Coverage was higher in both study arms during periods when participants retrospectively assessed themselves to have been at risk of HIV acquisition, suggesting that participants may have modified their biomedical prevention use based on anticipated risk. However, the intervention also more than doubled biomedical prevention coverage when follow-up was restricted to periods of risk.
Previous work has demonstrated that offering women choices in product and delivery can improve contraception coverage.12 A range of discrete choice experiments have further documented that clients' stated preferences for delivery of HIV biomedical prevention services differ,12–14,23 suggesting a key role for client choice in optimizing HIV prevention coverage. Our study is among the first to document the choices actually made (ie, revealed preferences) by clients when offered structured choices in HIV prevention. It is also among the first to demonstrate that an intervention offering structured dynamic client choices can substantially increase HIV biomedical prevention coverage.
The DCP intervention we evaluated was anchored on a foundation of person-centered care. A core component of the intervention was provider training on how to offer choices in a way that maximized client agency and in the context of warm and respectful interactions and accessibility to address questions or concerns. The DCP intervention offers 1 possible well-specified model for integrating structured dynamic client choices into prevention delivery. When choices were offered in this context, women made a range of choices. In particular, they differed in their selection of self-testing versus provider-administered HIV testing and in their selection of clinic versus out-of-facility delivery, and these selections evolved over time.
Our findings strengthen previous reports of the unmet need for effective models for delivery of biomedical HIV prevention services to pregnant and postpartum women in this rural East African setting. A recent programmatic study in Kenya in 2022 found that only 32% of women retested for HIV during pregnancy and <30% retested postpartum.24 PrEP uptake and retention also remain low in this priority population.23 In our study, despite eligibility for PrEP and active referral to existing standard-of-care services, PrEP coverage among women in the control arm was also low: 28% of control participants reported no use of PrEP or PEP during 48 weeks of follow-up.
By contrast, uptake and retention on PrEP seen in our intervention arm was higher than both the standard of care and that reported in other studies conducted among groups, such as young women, with higher rates of new HIV diagnoses.25,26 Furthermore, over 90% of women in the intervention arm retested during intervention delivery visits that spanned the antenatal and postnatal periods, with almost all women retesting multiple times. The option to select self-testing and to select out-of-facility service delivery may have contributed to these results. In our study, 76% of women chose out-of-facility HIV prevention service delivery of PrEP/PEP refills and 82% of women chose a self-test at least once during follow-up. Both options avoid the need for travel to clinic, which may be particularly helpful during the postpartum period when women are caring for 1 or more newborn infants. These results align with previous work reporting high uptake of self-testing among pregnant women attending ANC27 and documenting the use of self-testing to support the successful delivery of PrEP.28–30 Interestingly, however, only 18%–57% of women in our study opted for self-testing at any given visit, and only 22%–46% of women selected off-site location for any given visit. This heterogeneity of client preferences suggests that any one-size-fits-all option is likely to fall short and highlights the key role of offering client choice, including the option to switch over time, in HIV prevention service delivery.
By contrast to the heterogeneity in clients' choices of testing and location of services, 98% of women in this study initially selected PrEP for HIV prevention, and PrEP remained the most chosen prevention product at all intervention visits. Nonetheless, 10% of women chose PEP at least once during follow-up. Further study is needed to understand the optimal use of PEP in this priority population and its potential to serve as a bridge to other prevention options.31
Importantly, although the intervention facilitated client choice and increased prevention coverage, both selection of any biomedical prevention option (PrEP or PEP) and participant-reported use declined over the course of the study in the intervention arm, and overall biomedical prevention coverage remained suboptimal. Additional biomedical prevention products such as long-acting injectable cabotegravir32,33 may help to overcome remaining barriers to PrEP use and further optimize biomedical prevention coverage. The DCP intervention we evaluated is explicitly designed to facilitate the integration of such novel products as they become available.
Our study supports previous work documenting the potential for HIV biomedical prevention services to be directly integrated into ANC, to augment existing services for the prevention of perinatal transmission.23 A recent mathematical modeling study suggests that such integration has the potential to reduce not only HIV incidence among pregnant and postpartum women and meaningfully reduce perinatal transmission of HIV but also to affect overall HIV incidence.7 Furthermore, in contrast to the current standard practice in these rural settings of offering PrEP at the HIV clinic, ANC clinics offer an HIV-status–neutral setting for delivery of prevention services, which may improve PrEP retention through reduction of stigma and convenience of a “one-stop shop”.34 Our study demonstrates that integration of HIV prevention delivery in a routine ANC setting in regions with high HIV prevalence using a person-centered, choice-based model is both feasible and effective for increasing biomedical prevention coverage. The DCP intervention delivered at scale would be incorporated into the regular structured visits at public facilities to facilitate choice, with additional training to providers on barrier assessment within existing facilities.
Our study has limitations. Our primary outcome of biomedical prevention coverage was assessed through self-report. Participant-assessed retrospective risk of HIV acquisition may have been differential by arm, if, for example, person-centered care led to increased disclosure in the intervention arm; for this reason, prevention coverage during periods of retrospectively assessed risk was prespecified as a secondary rather than primary outcome. Finally, this pilot trial was not powered to assess the impact of the DCP intervention on HIV incidence. The effect of the DCP intervention delivered at scale as part of a multicomponent intervention is being evaluated in an ongoing large cluster randomized trial.
CONCLUSIONS
In conclusion, a person-centered DCP intervention that provided flexibility in prevention product, testing, and service location more than doubled biomedical HIV prevention coverage compared with the standard care among pregnant and postpartum women in rural East Africa, a high-risk population already routinely offered access to biomedical prevention options. This randomized trial represents one of the first studies to systematically offer a structured intervention for biomedical prevention options using a theory-based, person-centered dynamic choice model that adapted services based on HIV exposure risk and life circumstances over time.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this manuscript was supported by the US National Institute of Allergy and Infectious Diseases (NIAID); the National Heart, Lung, and Blood Institute (NHLBI); and the National Institute of Mental Health (NIMH) and co-funded under award number U01AI150510. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The SEARCH study gratefully acknowledges the Ministry of Health of Kenya; Ministry of Health of Uganda; our research teams and administrative teams in San Francisco, Kenya, and Uganda; collaborators and advisory boards; and especially all communities and participants involved.
Footnotes
Supported by the US National Institute of Allergy and Infectious Diseases (NIAID); the National Heart, Lung, and Blood Institute (NHLBI); and the National Institute of Mental Health (NIMH) and cofunded under award number U01AI150510. This research was also supported by NIAID under Award number R01AI167753.
Presented at the Conference on Retroviruses and Opportunistic Infections (CROI); February 19–23, 2023; Seattle, Washington.
C.A.K. has received grant support paid to the University of California, San Francisco from Gilead Sciences. The remaining authors have no funding or conflicts of interest to disclose.
A complete deidentified patient data set sufficient to reproduce the study findings will be made available approximately 1 year after completion of the ongoing trial (NCT04810650) after approval of a concept sheet summarizing the analyses to be performed. Further inquiries can be directed to the SEARCH Scientific Committee at douglas.black@ucsf.edu.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Catherine A. Koss, Email: Catherine.Koss@ucsf.edu.
Helen Sunday, Email: hsunday@idrc-uganda.org.
Edith Biira, Email: ebiira@idrc-uganda.org.
Marilyn Nyabuti, Email: Mnyanduko@gmail.com.
Laura B. Balzer, Email: laura.balzer@berkeley.edu.
Shalika Gupta, Email: shalika@berkeley.edu.
Gabriel Chamie, Email: Gabriel.Chamie@ucsf.edu.
James Ayieko, Email: jimayieko@gmail.com.
Elijah Kakande, Email: ellykax@gmail.com.
Melanie C. Bacon, Email: mbacon@niaid.nih.gov.
Diane Havlir, Email: Diane.Havlir@ucsf.edu.
Moses R. Kamya, Email: mkamya@idrc-uganda.org.
Maya Petersen, Email: mayaliv@berkeley.edu.
REFERENCES
- 1.Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egbe TO, Tazinya R-MA, Halle-Ekane GE, et al. Estimating HIV incidence during pregnancy and knowledge of prevention of mother-to-child transmission with an ad hoc analysis of potential cofactors. J Pregnancy. 2016;2016:7397695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake AL, Wagner A, Richardson B, et al. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi BH, Mbori‐Ngacha D, Essajee S, et al. Accelerating progress towards the elimination of mother‐to‐child transmission of HIV: a narrative review. J Int AIDS Soc. 2020;23:e25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS . In Danger: UNAIDS Global AIDS Update 2022, Geneva Joint United Nations Programme on HIV/AIDS. Geneva: United Nations; 2022. [Google Scholar]
- 6.Irshad U Mahdy H,Tonismae T. HIV in Pregnancy. StatPearls Publishing; 2023. Updated 2022 Sept 20. Available at: https://www.ncbi.nlm.nih.gov/books/NBK558972/. Accessed May 31, 2023. [PubMed] [Google Scholar]
- 7.Joseph Davey DL, Bekker L-G, Gomba Y, et al. Modelling the potential impact of providing preexposure prophylaxis in pregnant and breastfeeding women in South Africa. AIDS. 2019;33:1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller A-B, Petzold M, Chou D, et al. Early antenatal care visit: a systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob Health. 2017;5:e977–e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LF, Stinson K, Newell M-L, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2012;59:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinh T-H, Delaney KP, Goga A, et al. Impact of maternal HIV seroconversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4-8 weeks postpartum in South Africa 2011-2012: a national population-based evaluation. PLoS One. 2015;10:e0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson KB, Leone PA, Fiscus SA, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007;21:2303–2308. [DOI] [PubMed] [Google Scholar]
- 12.Gray AL Smit JA Manzini N, et al. Systematic review of contraceptive medicines “Does choice make a difference?”. Reprod Heal HIV Res Unit of Witwatersrand, South Africa. 2006;(October). [Google Scholar]
- 13.Quaife M, Eakle R, Cabrera Escobar MA, et al. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med Decis Making. 2018;38:120–133. [DOI] [PubMed] [Google Scholar]
- 14.Terris-Prestholt F, Hanson K, MacPhail C, et al. How much demand for new HIV prevention technologies can we really expect? Results from a discrete choice experiment in South Africa. PLoS One. 2013;8:e83193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minnis AM, Browne EN, Boeri M, et al. Young women's stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. 2019;80:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uganda Ministry of Health . Consolidated Guidelines for Prevention and Treatment of HIV and AIDS in Uganda. Uganda Ministry of Health; 2020. [Google Scholar]
- 17.Ministry of Health National AIDS and STI Control Programme . Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018. Kenya Ministry of Health; 2018. [Google Scholar]
- 18.Green L, Kreuter MW. Health Program Planning: An Educational and Ecological Approach. Boston: McGraw-Hill Higher Education; 2005. [Google Scholar]
- 19.Havlir DV, Balzer LB, Charlebois ED, et al. HIV testing and treatment with the use of a community health approach in rural Africa. New Engl J Med. 2019;381:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balzer LB, van der Laan MJ, Petersen ML, et al. Adaptive pre‐specification in randomized trials with and without pair‐matching. Stat Med. 2016;35:4528–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balzer LB, Cai E, Garraza LG, et al. Adaptive Selection of the Optimal Strategy to Improve Precision and Power in Randomized Trials; 2022. arXiv preprint: arXiv:221017453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Fondation for Statistical Computing; 2023. Available at: https://www.R-project.org/. Accessed May 31, 2023. [Google Scholar]
- 23.Kinuthia J, Pintye J, Abuna F, et al. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: results from an implementation programme. Lancet HIV. 2020;7:e38–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penumetsa M, Neary J, Farid S, et al. Implementation of HIV retesting during pregnancy and postpartum in Kenya: a cross-sectional study. Glob Health Sci Pract. 2022;10:e2100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mudau DO, Mulaudzi FM, Sepeng NV, et al. Assessing HIV pre-exposure prophylaxis uptake and retention amongst young females in Gauteng province. AIDS Behav. 2022;27:1182–1187. [DOI] [PubMed] [Google Scholar]
- 26.Mayanja Y, Kamacooko O, Lunkuse JF, et al. Oral pre‐exposure prophylaxis preference, uptake, adherence and continuation among adolescent girls and young women in Kampala, Uganda: a prospective cohort study. J Int AIDS Soc. 2022;25:e25909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoagland B, Torres TS, Bezerra DR, et al. High acceptability of PrEP teleconsultation and HIV self-testing among PrEP users during the COVID-19 pandemic in Brazil. Braz J Infect Dis. 2021;25:101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngure K, Heffron R, Mugo N, et al. Feasibility and acceptability of HIV self‐testing among pre‐exposure prophylaxis users in Kenya. J Int AIDS Soc. 2017;20:21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mujugira A, Nakyanzi A, Kasiita V, et al. HIV self‐testing and oral pre‐exposure prophylaxis are empowering for sex workers and their intimate partners: a qualitative study in Uganda. J Int AIDS Soc. 2021;24:e25782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngure K, Ortblad KF, Mogere P, et al. Efficiency of 6-month PrEP dispensing with HIV self-testing in Kenya: an open-label, randomised, non-inferiority, implementation trial. Lancet HIV. 2022;9:e464–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayieko J, Petersen ML, Kamya MR, et al. PEP for HIV prevention: are we missing opportunities to reduce new infections? J Int AIDS Soc. 2022;25:e25942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022;399:1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. New Engl J Med. 2021;385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green LW, Kreuter MW. The precede–proceed model. Health promotion planning: an educational approach. 3rd ed. Mountain View (CA): Mayfield Publishing Company; 1999:32–43. [Google Scholar]