Abstract
Nonhealing wounds possess elevated numbers of pro-inflammatory M1 macrophages, which fail to transition to anti-inflammatory M2 phenotypes that promote healing. Hemoglobin (Hb) and haptoglobin (Hp) proteins, when complexed (Hb-Hp), can elicit M2-like macrophages through the heme oxygenase-1 (HO-1) pathway. Despite the fact that nonhealing wounds are chronically inflamed, previous studies have focused on non-inflammatory systems, and do not thoroughly compare the effects of complexed vs individual proteins. We aimed to investigate the effect of Hb/Hp treatments on macrophage phenotype in an inflammatory, lipopolysaccharide (LPS)-stimulated environment, similar to chronic wounds. Human M1 macrophages were cultured in vitro and stimulated with LPS. Concurrently, Hp, Hb, or Hb-Hp complexes were delivered. The next day, 27 proteins related to inflammation were measured in the supernatants. Hp treatment decreased a majority of inflammatory factors, Hb increased many, and Hb-Hp had intermediate trends, indicating that Hp attenuated overall inflammation to the greatest extent. From this data, Ingenuity Pathway Analysis software identified high motility group box 1 (HMGB1) as a key canonical pathway—strongly down-regulated from Hp, strongly up-regulated from Hb, and slightly activated from Hb-Hp. HMGB1 measurements in macrophage supernatants confirmed this trend. In vivo results in diabetic mice with biopsy punch wounds demonstrated accelerated wound closure with Hp treatment, and delayed wound closure with Hb treatment. This work specifically studied Hb/Hp effects on macrophages in a highly inflammatory environment relevant to chronic wound healing. Results show that Hp—and not Hb-Hp, which is known to be superior in noninflammatory conditions—reduces inflammation in LPS-stimulated macrophages, and HMGB1 signaling is also implicated. Overall, Hp treatment on M1 macrophages in vitro reduced the inflammatory secretion profile, and also exhibited benefits in in silico and in vivo wound-healing models.
1 |. INTRODUCTION
Chronic wounds are stalled in the first stage of wound healing—inflammation—and have difficulty in progressing to the final two stages of proliferation and remodeling, when tissue regeneration and wound closure occur.1 A major driver of inflammation in chronic wounds is the prolonged presence of pro-inflammatory macrophages, generally referred to as M1 macrophages.2 M1 macrophages phagocytose dead cells and bacteria and produce high levels of reactive oxygen species. Stimulation with pro-inflammatory cytokines or bacterial components promotes the M1 macrophage phenotype, which secretes tumor necrosis factor α (TNF-α), interleukin 8 (IL-8), IL-6, and IL-1β, to name a few.3 In healing wounds, macrophage populations transition to having an M2-like anti-inflammatory phenotype, while the M1 phenotype is attenuated. M2 macrophages produce increased levels of growth factors, such as vascular endothelial growth factor (VEGF), and fibroblast growth factor to support angiogenesis, cell proliferation, and tissue regeneration.4,5 An increase in interleukin 10 (IL-10) is also typically associated with the resolution of inflammation, and accumulation of M2 macrophages. The prolonged presence of M1 macrophages, and a delayed switch to the M2 phenotype, is associated with chronic wound conditions. Therefore, therapeutic methods that promote the transition from M1 to M2 macrophages have been attempted to stimulate healing of chronic wounds.2
There is a body of research that has investigated the potential of hemoglobin (Hb) and haptoglobin (Hp) complexes (Hb-Hp) in eliciting anti-inflammatory macrophages, via the monocyte/macrophage specific receptor CD163 and heme oxygenase-1 pathway (HO-1).6–10 Hb-Hp bind to CD163 on monocytes and macrophages with high affinity and are endocytosed. Intracellularly, the HO-1 enzyme converts heme into Fe2+, carbon monoxide, and bilirubin.6,11,12 Carbon monoxide and bilirubin have been shown to have anti-inflammatory effects, and a strong up-regulation of IL-10 is also associated with this pathway.7,13,14 This pathway is thought to be active in vivo in regions of vascular hemorrhage in atherosclerotic plaques, where high levels of iron were concurrent with M2 macrophage markers, such as high CD206, CD163, and low levels of the M1 marker, TNF-α.8,15
The Hb-Hp/CD163/HO-1/IL-10 pathway may also have potential benefits in wound healing. Expression of CD163 is desirable as it is up-regulated as inflammation is resolved, and wounds begin to enter the proliferation stage.6,7 Furthermore, deletion or inhibition of HO-1 results in delayed wound healing.16 Diabetic mice also have lower HO-1 expression than nondiabetic mice, which may partially explain their delayed healing. Lastly, IL-10 is a potent anti-inflammatory cytokine and is associated with M2-like macrophages that support wound-healing activities.3,17 It is important to note that iron/Hb overload of macrophages is of particular concern in venous ulcers18,19 and promotes an unrestrained pro-inflammatory M1 macrophage population. Therefore, promotion of the Hb-Hp/CD163/HO-1/IL-10 pathway by introduction of Hp or Hb-Hp complexes may mediate Hb toxicity and promote anti-inflammatory macrophage responses, leading to improved chronic wound healing for both diabetic and venous ulcers.
In the current study, our goal was to characterize and predict the therapeutic potential of Hb-Hp complexes on macrophages to ultimately test their potential in improving healing of diabetic and venous ulcers. To create a highly inflammatory in vitro system characteristic of chronic wounds, macrophages were stimulated with lipopolysaccharide (LPS; 1 μg/mL). Stimulation with LPS is known to increase several pro-inflammatory factors, such as interleukin-8 (IL-8), interferon gamma (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF), to name a few.20 Additionally, chronic wounds are often infected, making LPS an appropriate inflammatory stimulus for this model. As controls, Hp and Hb were also delivered individually, in addition to Hb-Hp complexes. The Hb-Hp group was predicted to elicit the strongest anti-inflammatory effects, since the complex has the highest affinity with the CD163 receptor compared to the individual proteins.6,11,12 Furthermore, the individual proteins are usually not reported to have benefits, especially in comparison to Hb-Hp.7,8 Few studies have reported results of this pathway with highly inflammatory, LPS-stimulated macrophages, and those that did, did not thoroughly report results with Hb only or Hp only controls. Furthermore, few studies report cytokine secretion results beyond IL-10. We expanded this characterization as there are many more pro- and anti-inflammatory factors that affect biological outcomes, specifically in regard to wound healing.
Surprisingly, we found that Hp alone was the strongest inhibitor of inflammatory mediator secretion, followed by Hb-Hp. Hb alone significantly increased secretion of many pro-inflammatory factors. Hb-Hp treatment displayed trends similar to Hb for some factors, but also trends similar to Hp for others, including IL-10. Based on current literature, we hypothesize that Hp alone serves to sequester free HMBG1 that is generated by macrophages stimulated with LPS.21–23 As a whole, this work demonstrates the potential of Hp in counteracting inflammatory signaling in macrophages, not only in response to Hb, but in response to LPS as well. This work also demonstrates that Hp can lead to benefits in wound healing through: (a) an in silico model that compared current in vitro cytokine trends with published cytokine trends from human chronic venous ulcers and (b) in vivo biopsy punch wounds in diabetic mice.
2 |. MATERIALS AND METHODS
2.1 |. Reagents
Low endotoxin level Hp (mixed phenotype) purified from human plasma was purchased from Athens Research Technology (Athens, Georgia). LPS from Escherichia coli was purchased from Sigma Aldrich (Saint Louis, Missouri). Human Hb dissolved in lactated Ringer’s solution was provided by Andre Palmer’s lab, following hypotonic lysis of red blood cells and subsequent purification using tangential flow filtration, in a process similar to that reported in Elmer et al.24
2.2 |. Monocyte isolation and macrophage differentiation
Human blood donations/buffy coats were received from New York Blood Center (New York City, New York). Primary monocytes were isolated using Ficoll-Paque density gradient centrifugation and CD14+ magnetic bead separation (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s protocol, in a process similar to that reported in Faulknor et al.25 CD14+ cells were cultured at 37°C at 5 × 105 cells/mL with 5 ng/mL GM-CSF (R&D Systems, Inc, Minneapolis, Minnesota) for 7 days in complete media—Advanced RPMI 1640 (Life Technologies, Carlsbad, California) containing 10% fetal bovine serum, 1% penicillin-streptomycin (P/S) and 4 mM L-glutamine. After 7 days, cells that attached (macrophages) were trypsinized for 15 minutes, frozen down with 1% dimethyl sulfoxide, and stored in liquid nitrogen until use for experiments.
2.3 |. Cell culture and metabolic activity measurement
Macrophages were cultured in complete media at 1 × 105 cells/well in black, glass-bottom 24-well plates (Cellvis, Sunnydale, California) and allowed to attach for 24 hours. Then, cells were activated with 1 μg/mL of LPS in serum-free media (advanced RPMI 1640, 1% P/S, 4 mM L-glutamine). Concurrently, 0.2 mg/mL of Hp, Hb, or Hb-Hp was added to cells. After 24 hours, supernatants were collected and stored at −80°C until analysis for secreted factors. Alamar Blue Cell Viability Reagent (Life Technologies Corporation) for measuring cellular metabolic activity was added to media with 1 μg/mL LPS at a 1:10 ratio. Once an hour up to 4 hours, fluorescence measurements (excitation 535 nm; emission 595 nm) were performed on a DTX 880 Multimode Detector plate reader with Multimode Detection Software (Beckman Coulter, Brea, California).
2.4 |. Multiplex immunoassay and net secretion scoring
Cellular supernatants were used in the Bio-Plex Pro Human Cytokine 27-plex Assay (BIO-RAD, Hercules, California)—a magnetic-bead based immunoassay that detects 27 cytokines, chemokines, and growth factors related to inflammation. The list of all 27 factors can be found on the heatmap in Figure 2. The assay was carried out according to the manufacturer’s instructions, with addition of 0.05% bovine serum albumin to serum-free supernatants. Results were obtained using a Bio-Plex 200 System (BIO-RAD). Results are represented as fold changes. Raw secretion concentrations were normalized to media baseline values. Then, the fold change was determined by taking the log2 of the media-normalized value.
FIGURE 2.
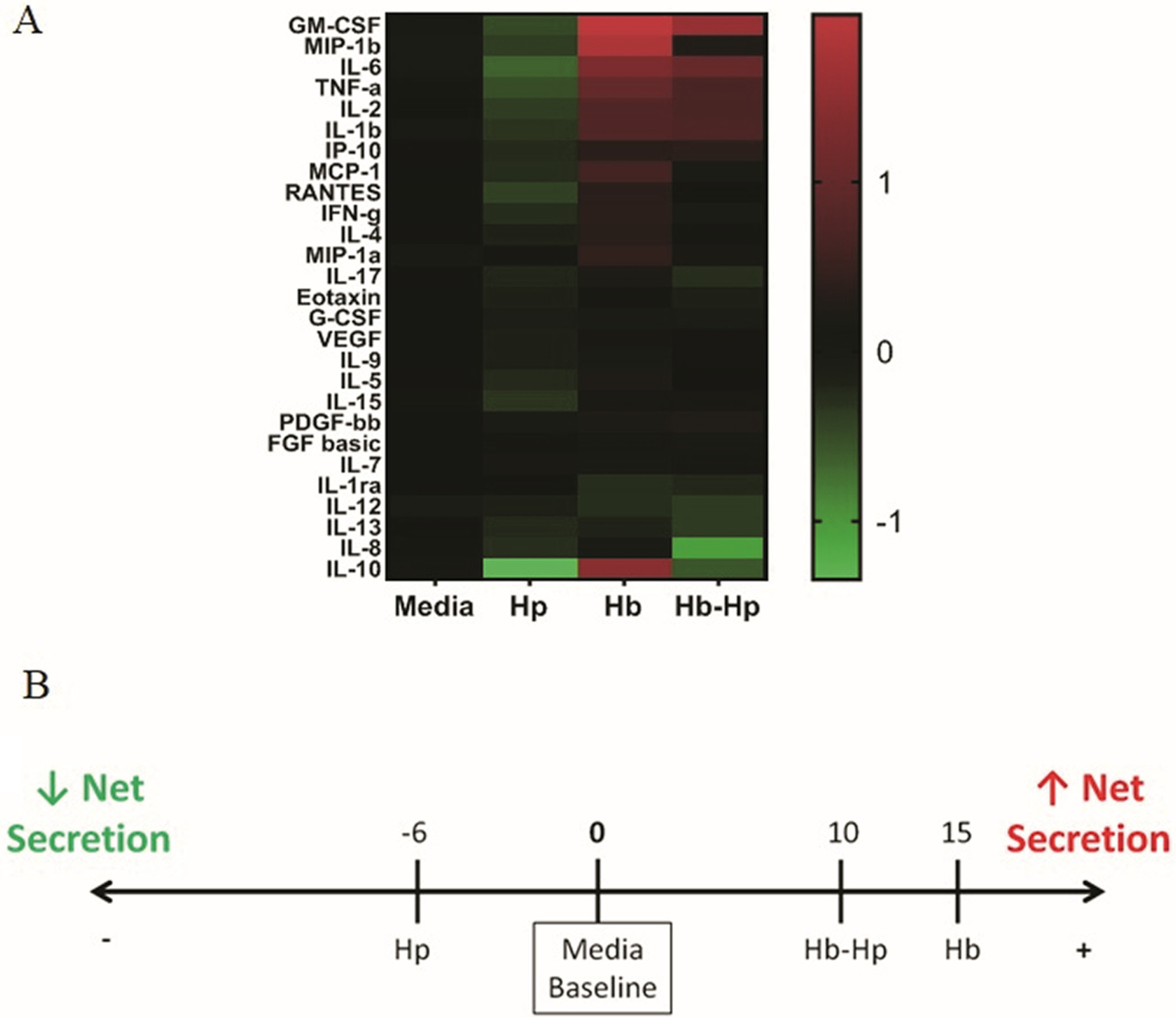
Inflammatory secretion profiles of macrophages treated with Hb/Hp/Hb-Hp. Macrophages were treated with 0.2 mg/mL of Hp, Hb or Hb-Hp, concurrently with 1 μg/mL of LPS. After 24 hours of treatment, supernatants were collected. Cytokine/chemokine/growth factor secretion was measured using a BIO-RAD multiplex assay. A, Heatmap of secretion profile of macrophage supernatants. For each respective factor, results are represented as fold change above (red; positive values) or below (green; negative values) media baseline (black; value of 0). B, Net secretion scoring based off of secretion profile. Fold change values across all secreted factors were summed and rounded to yield a final score. Scores are depicted on the number line, with media baseline (zero) at the center
Net secretion scoring was determined by summing the fold change values across all factors for each treatment, and rounding to the nearest whole number. Media baseline is represented as a score of zero.
2.5 |. HMGB1 ELISA
HMGB1 levels in supernatants were measured used an HMGB1 ELISA (Biomatik Corporation, Ontario, Canada). The procedure was performed following manufacturer’s protocol, and absorbance at 450 nm was measured using a DTX 880 Multimode Detector plate reader with Multimode Detection Software (Beckman Coulter).
2.6 |. Ingenuity Pathway Analysis
Ingenuity Pathway Analysis (IPA; Qiagen, Venlo, the Netherlands) version 01–13 was used to interpret secretion profile results. Average fold changes from three experiments for each condition were ran in an expression core analysis with no mutations, including direct and indirect relationships, interaction and causal networks, all node types and data sources, experimentally observed and high (predicted) confidence, restricted to macrophage cell lines and primary human cells within epidermis, dermis, and skin organs, including endothelial cells, keratinocytes, fibroblasts, macrophages, mononuclear leukocytes, and peripheral blood mononuclear cells. z-Scores assigned a value to the predicted up/down-regulation of HMGB1 from the treatment compared to media baseline conditions. z-Scores represent the predicted activation state of upstream regulators using the expression patterns of the downstream factors, based on relationships published in the literature.
2.7 |. In silico wound-healing scoring
Pro-wound-healing trends were identified from an article by Ligi et al that found significant differences for cytokine/chemokine/growth factor levels between human chronic venous ulcers that were in the inflammatory state vs those showing signs of healing in the proliferative state.26 In that study, the same multiplex immunoassay from BIO-RAD was used to perform measurements in chronic wound fluid. Nine significant pro-wound-healing trends were identified in the paper. The trends observed with the Hp /Hb/Hb-Hp treatment secretion profiles were compared to these pro-wound-healing trends for each of the nine factors and incorporated into a scoring system to predict which secretion profile is most likely to promote healing.
Raw secretion data from the current study was normalized to media baseline values. Then, fold change was determined by taking the log2 of the media-normalized value. If the experimental trend was consistent with the pro-wound-healing trend for a specific factor and had an absolute value fold change of 0.1 to 1, +1 point was awarded. If the trend was consistent and had an absolute value fold change of >1, +2 points were awarded. If the trends were inconsistent (ie, experimental trend increased a factor whereas the pro-wound-healing trend decreased that factor), points were subtracted rather than added. If the fold change value was between >−0.1 and <0.1, no points were awarded.
2.8 |. In vivo wound-healing studies
2.8.1 |. Biopsy punch study
Animal studies were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Rutgers University. Genetically modified diabetic male mice (BKS. Cg-Dock7m+/+ Leprdb/J) were purchased from Jackson Laboratory (Bar Harbor, Maine). All mice were used at the age of 10 weeks. A day prior to surgery, mice were anesthetized by isoflurane (Henry Schein, Melville, New York) inhalation and the back of mice was shaved using a clipper and Nair cream (Church & Dwight Co, Inc; Ewing, New Jersey). On the day of surgery, the mice were anesthetized, and betadine scrub and 70% ethanol were applied alternatively three times to prepare the dorsum for wounding. A biopsy punch (Integra LifeSciences; Princeton, New Jersey) was then used to create a 5 mm diameter circular full-thickness skin wound on the dorsum. The mice were divided into three groups and given different treatments (100 μg/mouse of either Hb or Hp, or plain PBS). In brief, Hp was reconstituted in sterile deionized water to yield a 10 mg/mL stock solution. For the Hb group, human Hb dissolved in lactated Ringer’s solution (100 mg/mL) was diluted in sterile water at 1:10 ratio to prepare a final concentration of 10 mg/mL. A 10 μL of either the Hp or Hb stock was applied to the wound area, and was then covered with Tegaderm (3 M; Saint Paul, Minnesota). A 10 μL of the Hb stock was applied to the wound area and the wound area was covered with Tegaderm. Similarly, 10 μL of PBS was applied to the control group wounds. The wound area was photographed weekly over a period of 28 days. The percent wound closure rate was analyzed using the ImageJ software (NIH) and calculated as .
On post-wounding Day 28, mice were sacrificed and skin samples around the wound area (including the scar area) were excised. The collected tissues were processed for histology as previously described.27 Briefly, the tissues were fixed in 10% neutral buffered formalin (VWR; Radnor, Pennsylvania) for 48 hours and stored in 70% at 4°C ethanol until processing for histology. Tissues were paraffin-embedded and sectioned (5 μm thickness). The sectioned tissues were stained with Masson’s trichrome stain and imaged using a light microscope.
2.8.2 |. Larger excisional wound study
A subsequent animal study was performed to investigate larger wounds and include four experimental groups: PBS controls, or 100 μg of Hps, Hb, or Hb-Hp (1:1). Male diabetic mice were prepped in the same way as above, except instead of a biopsy punch, they received 1 cm × 1 cm square-shaped full thickness excisional wounds, as described previously.27 Treatments were applied to wound areas and covered with Tegaderm. Images were taken of the healing wounds, and percent wound closure was calculated as described above.
2.9 |. Statistics
GraphPad Prism Version 8.1.1 (330) (GraphPad Software, San Diego, California) was used to generate plots and perform statistical analyses. For box and whisker plots, lines are shown at the median value, boxes denote lower and upper quartiles, and bars denote lowest and highest observations. Statistical analysis was performed on a total of three or more independent experiments per group. Raw secretion data were normalized to media baseline values. Then, fold change was determined by taking the log2 of the media-normalized value. Positive values represent up-regulation and negative values represent down-regulation, with zero representing media baseline levels.
One-way analysis of variance (ANOVA) was used for all datasets except for the multiplex data, in which two-way ANOVA was used. Tukey’s post hoc analysis was used to identify groups that displayed significant trends. * denotes significance vs media groups. + denotes significance vs Hp group. # denotes significance vs groups indicated by brackets. Increasing numbers of symbols indicate increasing levels of significance. For example, * denotes P < .05, ** denotes P < .01, *** denotes P < .001, and **** denotes P < .0001. The same holds true for increasing numbers of + and # symbols.
3 |. RESULTS
3.1 |. Effect of treatments on cellular metabolic activity
In order to assess the general effect of Hb/Hp/Hb-Hp treatments on macrophages and to provide more context for functional characterization, metabolic activity per well was measured (Figure 1). This assay was performed on macrophages after 24 hours of exposure to 1 μg/mL of LPS, together with 0.2 mg/mL Hp, Hb, or Hb-Hp complexes (1:1 mass ratio of Hb:Hp). Results were compared to those of media controls. No difference was detected among groups, indicating that net metabolic function of macrophages per well was unaffected by the treatments. For this reason, measured secretion profiles were directly compared among groups, in all subsequent studies.
FIGURE 1.
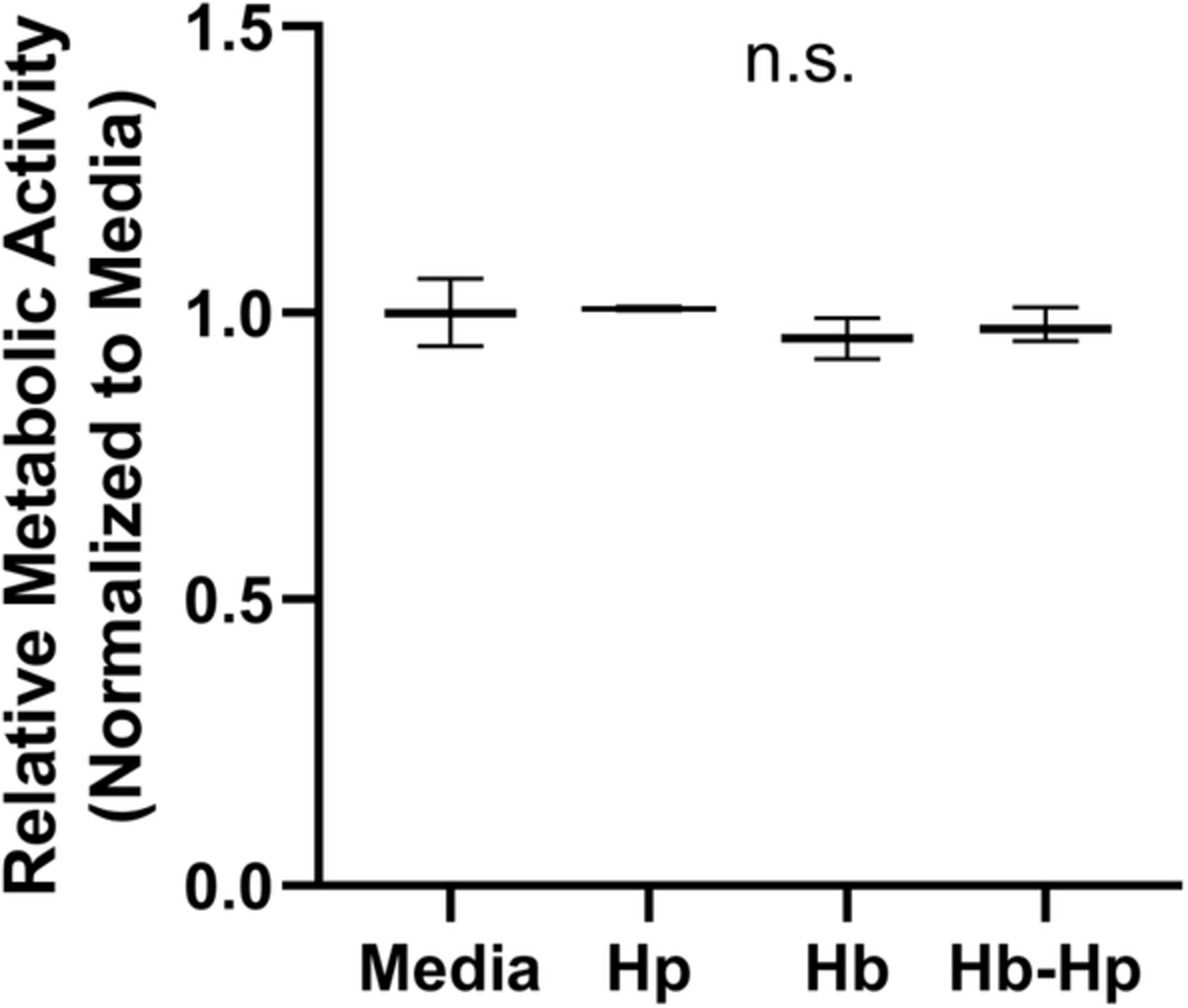
Metabolic activity of macrophages following Hp/Hb/Hb-Hp treatments. Macrophages were treated with or without 0.2 mg/mL of Hp, Hb, or Hb-Hp complexes, concurrently with 1 μg/mL of LPS. After 24 hours of treatment, metabolic activity was measured using the Alamar Blue assay. Fluorescence was measured after 2 hours on a plate reader and results were normalized to the media group. n.s., no significance between all groups
3.2 |. Overall inflammatory secretion profiles
A multiplex, bead-based immunoassay, of 27 different factors related to inflammation was used to characterize the supernatants of treated macrophages (Figure 2). The heatmap shows levels for each factor in each treatment group normalized to the respective baseline level measured in the media control. Up-regulation is indicated by shades of red and down-regulation by shades of green. Most measured factors were down-regulated by Hp treatment. In stark contrast, Hb-treated groups had a strong up-regulation in several factors. Results for Hb-Hp were intermediate—there were several factors that were up-regulated, down-regulated, or remained close to baseline. The numbered linear scale depicts the net secretion score across all measured factors for each treatment, taken by summing the fold change values for each measured factor (Figure 2B). Hp resulted in a net secretion score of −6, reflecting a down-regulation of many factors compared to media baseline. Hb was on the opposite extreme, strongly up-regulating many of the measured factors, yielding a net secretion score of +15. Hb-Hp also had a positive net secretion score of +10.
3.3 |. Most significant secretion trends
Measurements for individual factors that exhibited significant changes among treatment groups are shown in Figure 3. In general, several factors were down-regulated by Hp treatment, and strongly up-regulated by Hb treatment. Hb-Hp treatment also lead to up-regulation, which was less than that of Hb alone, but not significantly different. Factors demonstrating these trends were GM-CSF, IL-6, TNF-α, and IL-2 (Figure 3A). For all of these factors, resulting levels from Hb and Hb-Hp treatments were significantly higher than media baseline and Hp groups. Hp decreased secretion of these factors below media, significantly for IL-6. In this group of factors, there was no significant difference between Hb and Hb-Hp. For MIP-1β (macrophage inflammatory protein 1β; aka CCL4), IL-10, and IL-8 (aka CXCL8), Hb and Hb-Hp had significantly different levels (Figure 3B). For these three factors, Hb-Hp had significantly lower secretion compared to Hb. For MIP-1β, Hb-Hp resulted in levels close to baseline, with Hb significantly higher, and Hp slightly lower (but not significantly). IL-10 had striking trends as a result of treatments. Hp significantly decreased IL-10 levels compared to media baseline (P < .0001). Hb had significantly higher IL-10 compared to media and Hp groups. Hb-Hp decreased IL-10 compared to baseline, significantly lower than Hb (P < .0001), as well as slightly higher than Hp alone (P < .05). For IL-8, Hb-Hp resulted in significantly lower values compared to all other groups. The remaining groups had IL-8 levels close to media baseline, and were not significantly different from each other.
FIGURE 3.
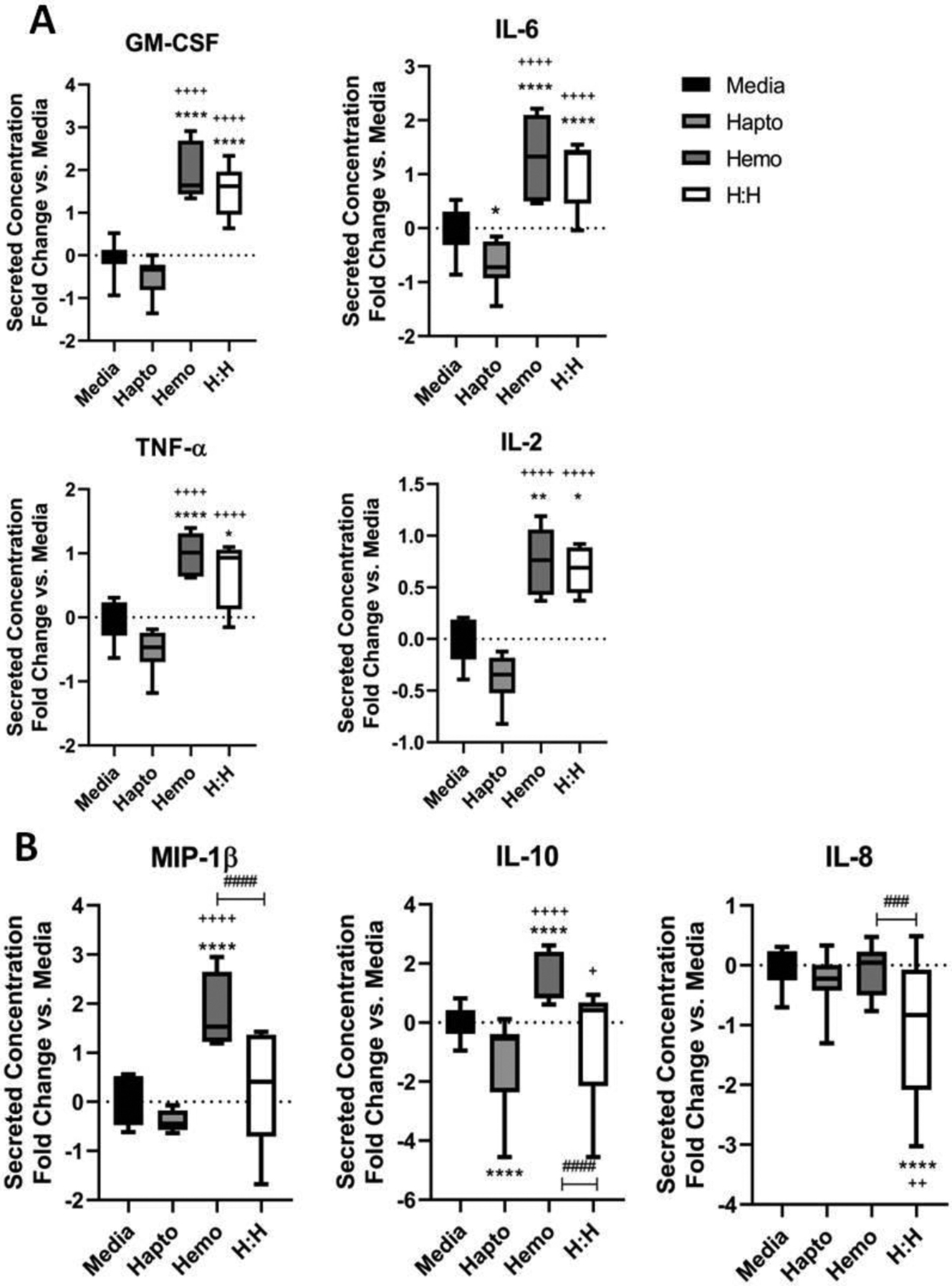
Significant trends for specific factors from the multiplex immunoassay. Results are shown as fold change compared to media baseline (0). Lines within boxes show median values. * denotes significance compared to media and + denotes significance compared to Hp group. # denotes significance between groups in associated brackets. A, Secretion results for a group of four factors that have similar trends: GM-CSF, IL-6, TNF-α, and IL-2. Here, Hp has secretion below media baseline (significant for IL-6). Hb and Hb-Hp have secretion higher than baseline, with Hb typically higher than Hb-Hp. Hb and Hb-Hp are all significantly higher than both media and Hp groups for all factors. B, Secretion results for a group of three factors, MIP-1β, IL-10, and IL-8, in which levels resulting from Hb-Hp are significantly different than Hb treatment
3.4 |. IPA and HMGB1 signaling
Multiplex secretion data were input into IPA in order to identify canonical pathways that may be responsible for the observed secretion trends. The HMGB1 pathway was identified as likely down-regulated by Hp treatment (z-score = −3.606) and up-regulated with Hb treatment (z-score = 1.941; Figure 4A). Slight activation of HMGB1 was predicted with Hb-Hp treatment (z-score = 0.832). The list of factors that are implicated in HMGB1 that this prediction was based on is listed in the chart, along with their trends. Of the measured factors, 14 out of 27 contributed to this prediction. To verify this prediction, HMGB1 levels from the supernatants were measured by ELISA (Figure 4B). IPA-predicted trends matched experimentally measured trends. More specifically, Hp had the lowest HMGB1 levels, below media baseline and other groups. Hb resulted in significantly higher HMGB1 levels than Hp. Hb-Hp resulted in intermediate levels, significantly higher than Hp alone, but still less than Hb on average.
FIGURE 4.
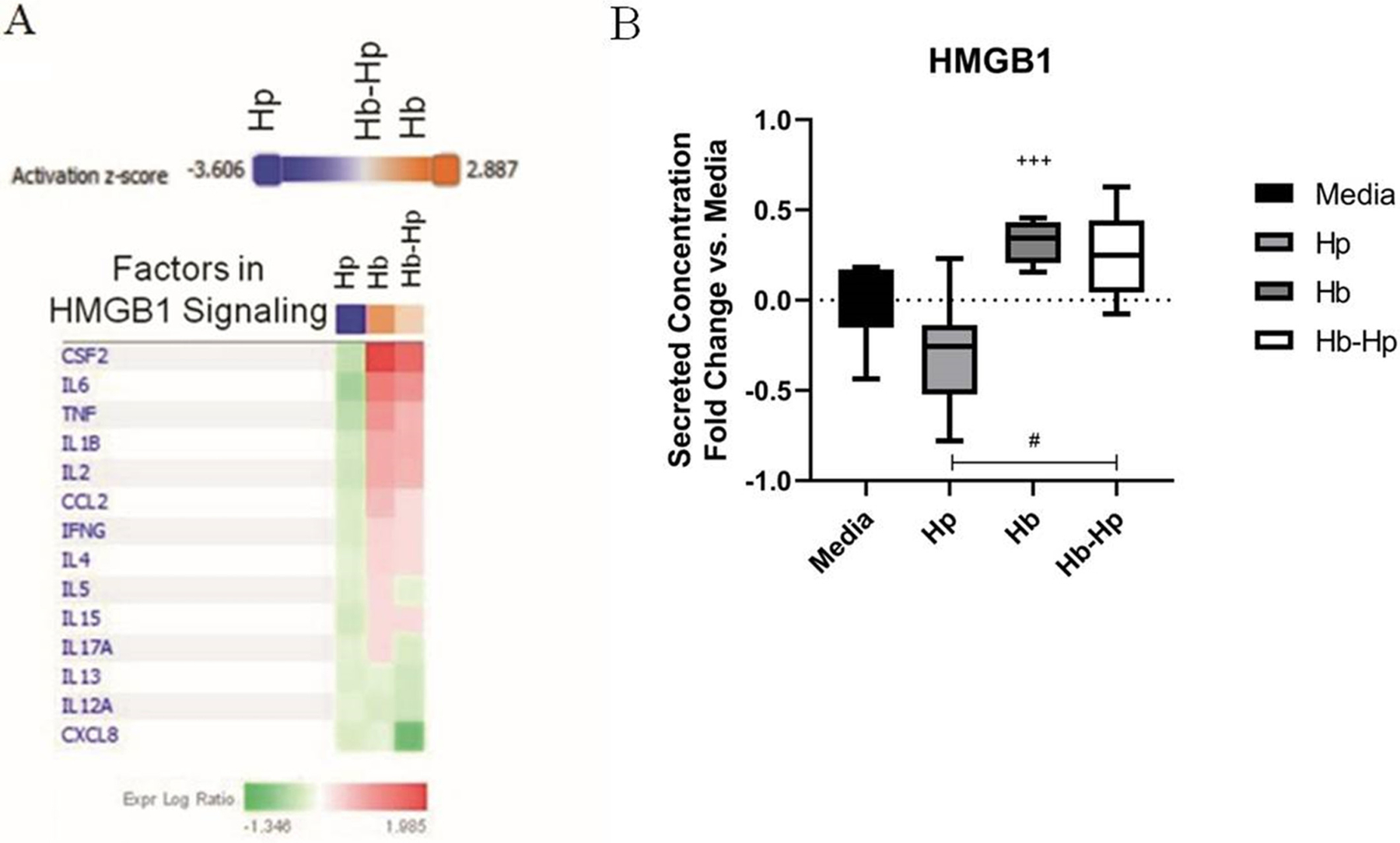
High motility group box 1 (HMGB1) IPA predictions and ELISA measurements from Hb/Hp /Hb-Hp treated macrophages. Secretion profile results were analyzed using Ingenuity Pathway Analysis (IPA), which identified the HMGB1 pathway to be strongly up/down-regulated by these treatments. A, List of the factors in the secretion profile dataset involved in HMGB1 signaling is shown in the heatmap. The treatments are listed in each column. For each factor, the intensity of red indicates how much it is up-regulated due to each treatment, compared to media baseline. Shades of green indicate down-regulation. IPA integrates this information to predict whether HMGB1 signaling, as a whole, is likely to be up- (orange) or down-(blue) regulated, and assigns a z-score to represent the confidence of this prediction. Hp group is predicted to be strongly down-regulated, whereas Hb is predicted to be strongly up-regulated, and slight activation for Hb-Hp. B, Results from HMGB1 ELISA measured from supernatants from previous studies treated with LPS and 0.2 mg/mL of Hp, Hb, or Hb-Hp complexes. Results are normalized to media baseline, and presented as fold changes. Lines within boxes show median values. + denotes significance vs Hp. # denotes significance between indicated groups
3.5 |. In silico prediction of wound healing effects in chronic venous ulcers
We wanted to interpret the inflammatory secretion results within a chronic wound-healing context in order to assess which treatment may yield a macrophage secretion profile that can promote chronic wound healing, particularly in chronic venous ulcers. Ligi et al used the same BIO-RAD assay to measure inflammatory factors in the wound fluid of human venous ulcers.26 Samples were taken from wounds in both the inflammatory stage and granulating/proliferation stage of wound healing. Factor levels were compared between wounds in the two stages, and nine significant trends were identified. These trends are listed in the table in Figure 5. To compare the Hp/Hb/Hb-Hp treatments to these pro-wound-healing trends, a scoring system was developed, based on experimental trends compared to the media group. Points were awarded to treatments that followed the pro-wound-healing trend for the specific factor, and deducted if the trend was not consistent. The total wound-healing score was determined by tallying up the number of points awarded to each treatment. Total wound-healing scores are shown on the number line. Hp had the highest score of +5, exhibiting the most pro-wound-healing trends. Hb-Hp had a slightly lower score, of +3. Hb had a net negative score of −1, indicating that its secretion profile had the fewest pro-wound-healing trends, as observed in Ligi et al. This comparison of our experimental data with published pro-wound-healing secretion trends in chronic venous ulcers predicts that Hp treatment is likely to be more effective in promoting wound healing than Hb-Hp or Hb treatments.
FIGURE 5.
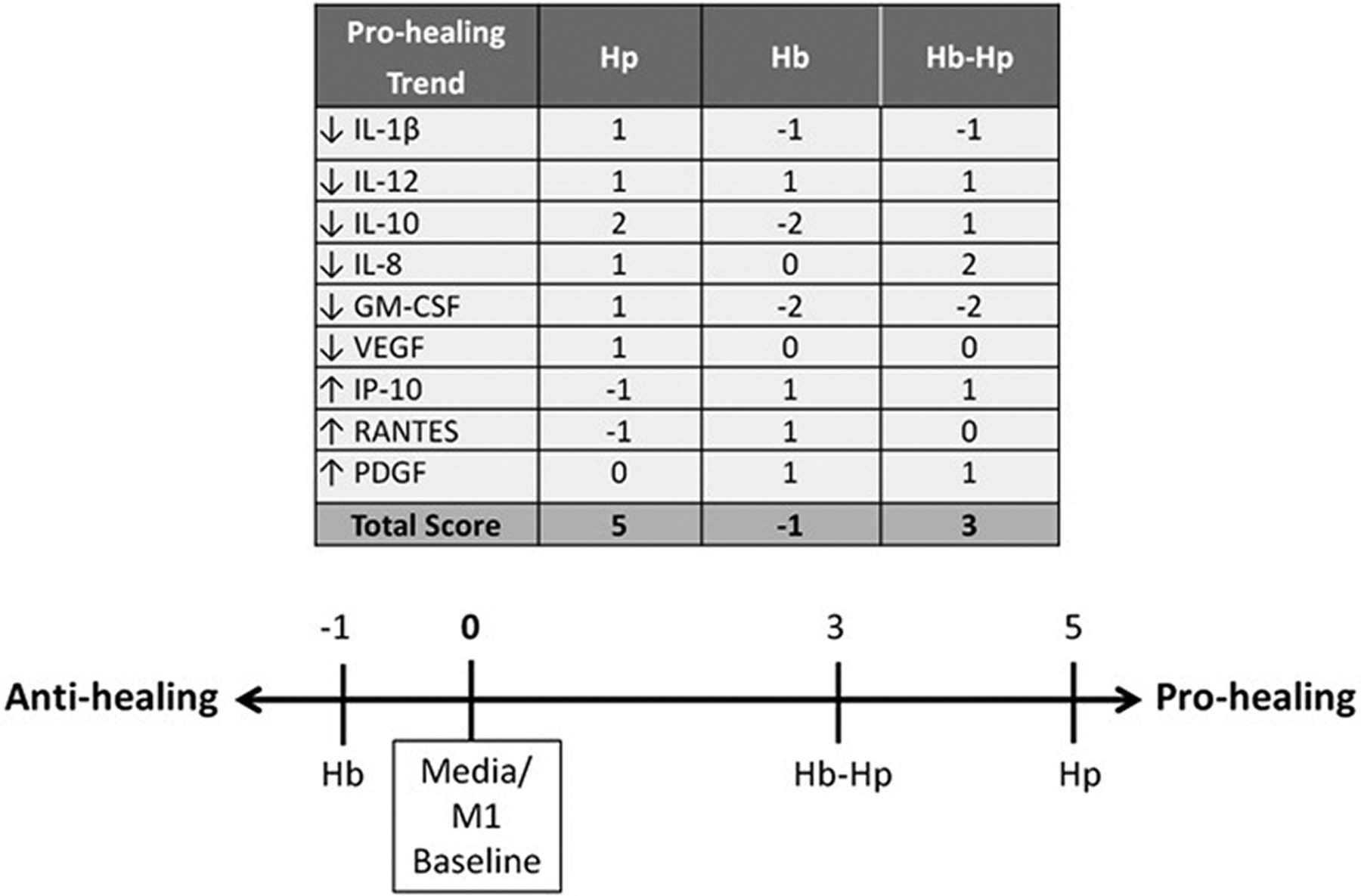
Predicted secretion profile implications in chronic wound healing. Wound-healing scoring based off of secretion profile measured from the multiplex immunoassay, compared to data from healing chronic wound fluid in Ligi et al.24 The authors also used the same BIO-RAD Multiplex assay to perform measurements of the same 27 inflammatory factors. Significant pro-wound-healing trends for nine factors were identified as human venous ulcers began healing. The resulting secretion profiles/fold changes from Hp, Hb, or Hb-Hp in the current study were compared to media baseline to determine the experimental trend. The treatment trends were then compared to the pro-wound-healing trends from Ligi et al. If the experimental trend was consistent with the pro-wound-healing trend and had an absolute value fold change of 0.1 to 1, +1 point was awarded. If the trend was consistent and had an absolute value fold change of >1, +2 points were awarded. If the trends were inconsistent, points were subtracted rather than added. If the fold change value was between >−0.1 and <0.1, no points were awarded. The table shows the score for each treatment for each pro-wound-healing trend, and the number line shows the total score for each treatment
3.6 |. In vivo wound-healing studies
To test the effect of Hp and Hb treatments in an in vivo chronic wound-healing model, an initial pilot study was performed using a biopsy punch wound in diabetic mice. Figure 6A shows representative images of wounds over time. Wounds from each treatment group appear similar until Day 14, at which point the Hp-treated wound is visibly smaller in size compared to the other groups. This is also apparent in the Day 21 image, as the Hp-treated wound is nearly closed whereas the other wounds remain open. Percent wound closure over time is quantified in Figure 6B. Here, the wound-healing curves are separated, with Hp consistently having faster wound closure and Hb consistently having slower wound closure compared to controls. The separation between groups is large at Day 14, at which point Hp-treated wounds are 85% closed, control wounds are 62% closed, and Hb are only 47% closed. Again, on Day 21, Hp-treated wounds are nearly closed (96%), control wounds are larger (85%), and Hb-treated wounds continue to delay healing (79%). Figure 6C shows wound tissues harvested on Day 28, stained with trichrome. The arrows indicate the epidermal and dermal layers. Control wounds appear to have thinner dermal layers compared to Hb- and Hp-treated wounds. Figure 6D shows results from a subsequent study that increased the wound size to a 1 cm × 1 cm excisional wound and also expanded the experimental groups to include mice treated with Hb-Hp complexes. Results from Days 7 and 21 are shown. Similar to the previous model, treatment with Hb slowed wound healing compared to controls. Hb-treated wounds were the largest compared to controls on Day 7 (−29.0% closed vs −12.8% closed) and on Day 21 (0.1% closed vs 42.1% closed). Also, Hp-treated wounds were the smallest on average at both time points (−9.4% closed and 50.4% closed on Days 7 and 21), although closure rates were generally similar between Hp, Hb-Hp, and control mice (no significant difference). Overall, Hb-treated wounds consistently exhibited delayed wound healing in diabetic mice. Hp treatment significantly accelerated wound healing in the biopsy punch wounding model, but optimization and further experimentation must be done to broaden these results to different wounding models.
FIGURE 6.
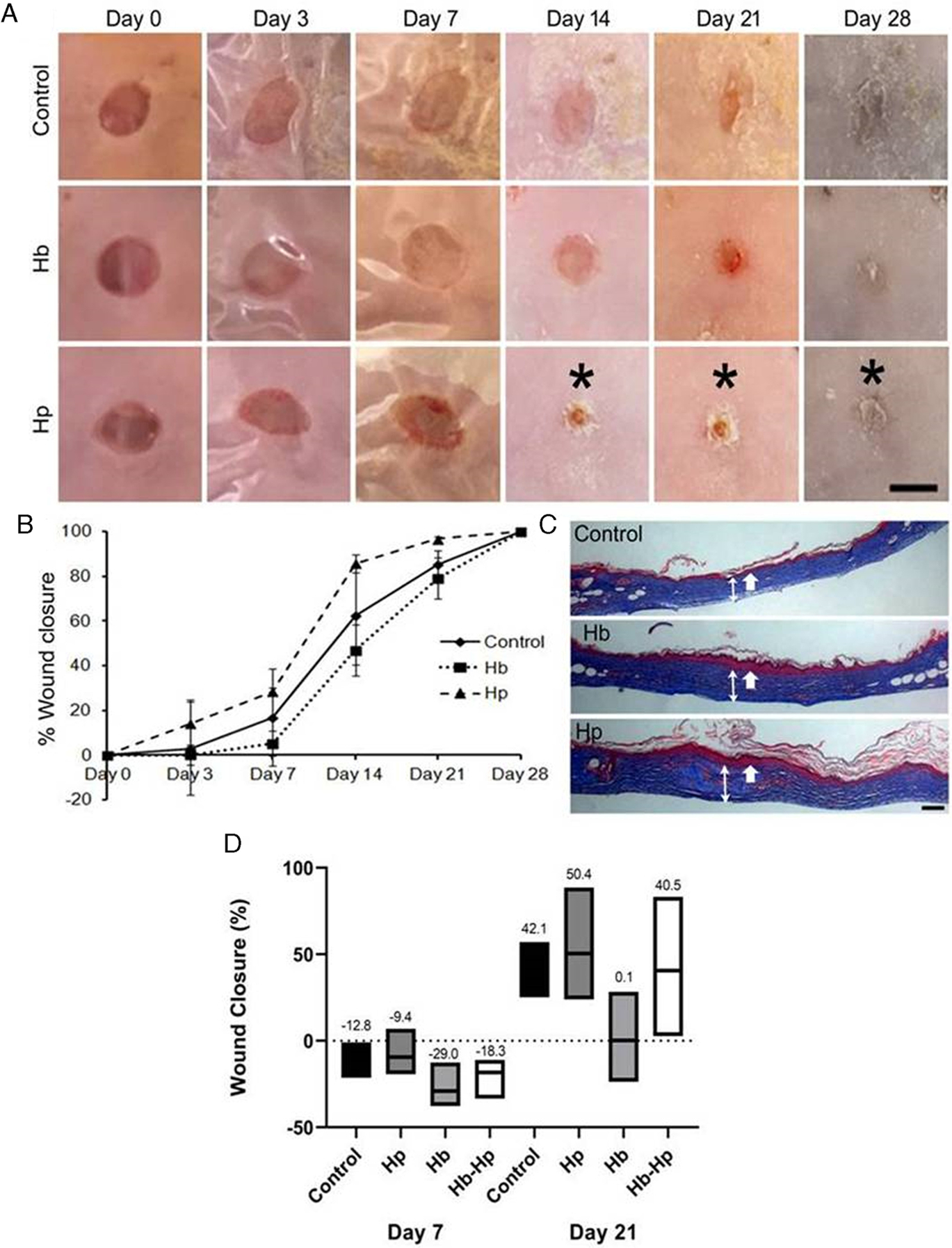
Investigation of Hb and Hp treatments on wound healing in diabetic mice. Mice were wounded with either a 5 mm biopsy punch (A-C), or a 1 cm × 1 cm excisional wound (D). Treatments included single, Day 0 doses of 100 μg/mouse of Hb and Hp (A-C) and Hb-Hp (D), compared to PBS controls. A, Representative images of biopsy punch skin wounds on different days. * denotes visibly smaller wounds in the Hp wounds vs the control and Hb groups. B, Percent wound closure over time of the biopsy punch wounds. Over the entire time course, there is a significant improvement in wound healing with Hp treatment compared to controls and Hb, as determined by ANOVA followed by Tukey’s HSD post hoc analysis (Hp vs control; F = 13.99; P < .0001, Hp vs Hb; F = 55.89; P < .0001, control vs Hb; F = 12.43; P < .0001). Data are represented as mean ± SEM (n = 3). C, Representative images of skin morphology of biopsy punch wounds excised post-sacrifice on Day 28. Tissues were stained with trichrome stain. Thick, single-headed white arrows identify the epidermal layers, and thinner double-headed white arrows span the dermal layers. D, Percent wound closure of excisional wounds treated with controls, Hp, Hb, or Hb-Hp on Days 7 and 21. Boxed edges represent the range of observations per group, and lines within the boxes represent the mean value, which is also written above the boxes
4 |. DISCUSSION
Hb and Hp form complexes (Hb-Hp) that modulate macrophage behavior and promote the M2-like phenotype, by activating the HO-1 pathway and up-regulating IL-10. Previous studies that observed this behavior have focused on in vitro cell culture systems that were not highly inflammatory, and therefore did not contain LPS.7,10,15 We were specifically interested in determining if Hb-Hp treatment could yield an anti-inflammatory secretion profile in macrophages that could counteract the inflammatory environment encountered in chronic wounds, in order to promote healing. Hence, as an inflammatory stimulus for our in vitro cell culture, we used LPS to mimic bacterial infection and inflammation that is commonly found in chronic wounds. We wanted to determine if Hb-Hp could promote anti-inflammatory macrophages in this highly inflammatory, LPS-stimulated environment, and compare the results to macrophages treated only with Hb or Hp. We specifically expanded our investigation into chronic venous ulcers in an in silico model that compared in vitro secretion results to published literature, and diabetic wounds in an in vivo diabetic mouse model.
The measured secretion profile of macrophages included 27 factors related to inflammation. Compared to media baseline levels, Hp reduced secretion of many of the inflammatory mediators (eg, IL-6, TNF-α, IL-1β). Hb alone had the opposite effect, and significantly increased many factors (eg, GM-CSF, IL-6, TNF-α, IL-2). Hb-Hp treatment decreased some factors (eg, IL-8, IL-17, IL-13), and increased others (eg, GM-CSF, IL-6), although to a lesser extent than Hb alone. Overall, Hp reduced inflammatory secretion, Hb increased it, and Hb-Hp resulted in intermediate effects.
Surprisingly, the anti-inflammatory cytokine IL-10 did not increase for macrophages treated with Hb-Hp, in contrast to what other studies have reported.7–9,15 In fact, treatment with Hb-Hp decreased IL-10 secretion compared to baseline, whereas Hp decreased IL-10 levels even more (Figure 3B). The distinction between previously published systems and the current study is the inclusion of LPS as a pro-inflammatory stimulus. LPS is a potent stimulator of many cytokines in human macrophages, including iNOS, TNF, IL-1β, IL-10, and others.17,20,28 The fact that LPS strongly up-regulates IL-10—the prototypical M2 marker—is counterintuitive, because LPS is recognized as an M1 inducer. Our observation of decreased IL-10, along with numerous pro-inflammatory cytokines, has been reported a few times in similar systems using LPS, and Hp/Hb-based treatments. For example, Arredouani et al used LPS-stimulated human macrophages, treated them with Hp at increasing concentrations, and measured cytokine levels 72 hours later.29 A significant decrease in IL-10 was observed, beginning at 250 μg/mL Hp. At the same time, several other pro-inflammatory factors, such as TNF-α and IL-12p70, were also decreased with Hp treatment. Another example of decreased IL-10 secretion, in conjunction with a decrease in other pro-inflammatory factors from macrophages in an LPS-stimulated system, was reported by Roach et al.30 Macrophages were pre-incubated with a Hb-based oxygen carrier (HBOC) called PolyHeme to induce HO-1, followed by LPS treatment.30 Pre-incubation with HBOC, followed by LPS stimulation, resulted in decreased levels of IL-10, as well as TNF-α, MCP-1, and IL-6. Similar to these two studies, treatment with Hp in our LPS-stimulated system, resulted in a decrease in IL-10 along with many other pro-inflammatory cytokines.
One possible explanation to the observed decrease, rather than increase, in IL-10 may be due to a self-regulated negative feedback loop that results in signaling that ultimately inhibits p38 phosphorylation and decreases IL-10 production.28,31 LPS-stimulation in these studies may initially drastically increase IL-10 levels, which activates the negative feedback loop with Hp and Hb-Hp treatment. In contrast, a self-regulated positive feedback loop also exists that affects the extracellular signal-regulated kinase (ERK) pathways, and results in IL-10 up-regulation.28 These positive and negative IL-10 feedback loops, implicated with ERK and p38 signaling, respectively, may be key in explaining different trends observed between previous literature and this study, which specifically used an LPS-stimulated system. This warrants further investigation, as well as extending observation time, as IL-10 levels can rise and fall depending on the specific time point assayed.32
We also used IPA to identify any canonical pathways that could explain the observed secretion profiles, and identified HMGB1 as a pathway with strong predictions that Hp treatment would down-regulate the pathway, while Hb treatment would up-regulate it (Figure 4A). It also predicted that Hb-Hp treatment would only slightly increase HMGB1 compared to the media baseline. Consistent with these predictions, HMGB1 secretion levels were decreased by Hp in LPS-stimulated macrophages. Hb had the highest levels of HMGB1, and Hb-Hp was in-between, both significantly different than Hp alone. Thus, each experimental treatment yielded different inflammatory secretion profiles/trends that closely correlated with different HMGB1 levels.
Recently, a link between Hp, HMGB1, and HO-1 has been made in the literature, which helps explain the findings in the current study. Yang et al inadvertently discovered that free Hp binds to HMGB1 and the HMGB1-Hp complex can be uptaken by CD163+ macrophages, which increased HO-1 and IL-10 expression, and counteracted the inflammatory effects of HMGB1.22,23 They had intended to use Hp-conjugated beads to remove extracellular Hb from the blood of septic rats, however, found that significant amounts of HMGB1 was also captured. Further experiments demonstrated the link between HMGB1-Hp, CD163, HO-1, and IL-10. HMGB1-Hp also led to a decrease in pro-inflammatory factors such as TNF-α and IL-8 in human macrophages, similar to trends seen in the current study with Hp treatment.
The overall trends and results seen between LPS/treatment, HMGB1, and inflammatory secretion outcome are summarized in Figure 7. In vitro macrophages were stimulated with LPS, leading to high HMGB1 production.21 Hp alone is free to bind to HMGB1 and reduce its pro-inflammatory effects. Hb does not bind with HMGB1 to sequester it. In fact, Hb amplifies the pro-inflammatory effects of LPS, so HMGB1 levels are further increased, leading to heightened levels of pro-inflammatory factors.22 In the Hb-Hp group, these complexes have extremely high affinity (Kd on the order of 10−15 M).22 In theory, there is no free Hp for HMGB1-Hp complexes to form, so there is nothing to counteract the additional LPS/HMGB1 challenge, and overall pro-inflammatory factor levels remain high, although to a lesser extent than Hb alone. It is noteworthy that Hb-Hp mixtures were formed at a 1:1 mass ratio. Since Hb and the mixed phenotype Hp used in this study bind at a 1:1 M ratio, using this mass ratio likely yielded excess unbound Hb, which may explain why the secretion profile of Hb-Hp was more similar to Hb, rather than Hp. Moving forward, Hb-Hp complexes will be formed at a 1:1 M ratio, followed by chromatographic separation to remove any noncomplexed Hb and Hp. Furthermore, comparison of activation of HO-1 in the system is pertinent, as both Hb-Hp complexes, and HMBG1-Hp have been reported to up-regulate the pathway.8,9,15,22,23 Thorough dose response of LPS, Hp, and Hb-Hp, and measurement of HO-1 activation could lead to better understanding of these trends.
FIGURE 7.
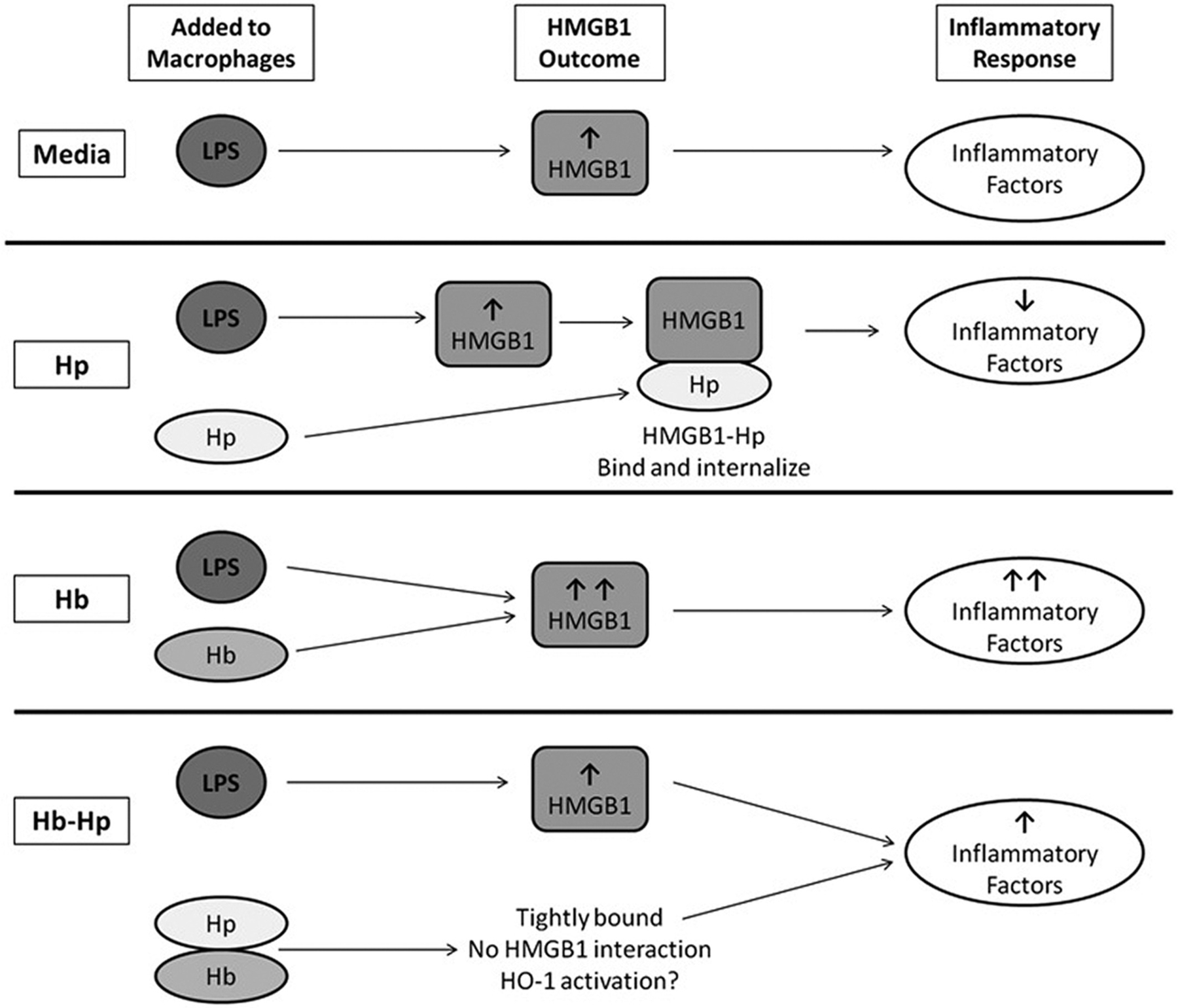
A diagram summarizing the hypothesized interactions between treatments, LPS, HMGB1, and inflammatory factors in the in vitro macrophage culture system. All four experimental groups included culturing macrophages in the presence of 1 μg/mL of LPS. In media alone, LPS leads to an increase in HMGB1 production, which activates several inflammatory factors. In the second row, the Hp treatment is able to bind free HMGB1 and internalize it, thereby leading to a decrease in the level of inflammatory factors. The Hb group, along with LPS, both increase HMGB1, leading to a strong, pro-inflammatory effect. In the last panel, macrophages are treated with LPS and Hb-Hp complexes, which bind tightly. As usual, LPS leads to the increase in HMGB1. As Hb and Hp are bound very tightly, they do not dissociate, and as a complex, Hb-Hp does not bind HMGB1. Therefore, there is an intermediate net inflammatory result, as the HMGB1 challenge is not resolved
Beyond the HMGB1 and general inflammatory secretion trends, our goal was to understand the impact of Hp/Hb/Hb-Hp treatment on human chronic wound healing. Ligi et al took wound fluid samples from human chronic venous ulcers that were either in the inflammatory or the granulating phases of wound healing.26 They used the same 27-plex immunoassay in the current study to identify significant trends in changes of cytokine levels as healing progressed. The significant pro-wound-healing trends observed include decreased IL-1β, IL-12, IL-10, IL-8, GM-CSF, and VEGF, and increased IP-10, RANTES, and platelet-derived growth factor. We compared the secretion profiles of our study to those, as these significant trends have desirable effects in healing (Figure 5). Points were awarded if the treatment (Hp, Hb, or Hb-Hp) followed the pro-wound-healing trend, subtracted if it did not, and summed across all factors to yield a final wound-healing score. The secretion profile from Hp alone resulted in the most pro-wound-healing potential. Hb-Hp had less pro-wound-healing trends, and Hb was not predicted to support wound healing. An interesting note is that Ligi et al measured a significant decrease in IL-10 levels between inflammatory and granulating wounds.26 Thus, IL-10 increase does not necessarily correlate with better healing, as is often assumed with its designation as an M2 marker. A decrease in IL-10 is observed in the Hp group (as well as Hb-Hp) of the current study, suggesting that this may be a beneficial wound-healing therapy.
Overall, decreased HMGB1 levels, as seen with the Hp group, may also be desirable in a wound-healing context. HMGB1 is a damage-associated molecular pattern.33,34 It can act intracellularly as a transcription factor, but it is also released extracellularly during injury. Downstream HMGB1 signaling activates nuclear factor-κB (NF-κB), increasing the secretion of pro-inflammatory factors, such as TNF-α, IL-8, MCP-1, and others. Higher levels of HMGB1 are also found in individuals with type 2 diabetes,35 and therefore may play a role in the development of diabetic ulcers. In wound healing specifically, lower HMGB1 results in minimal scarring, higher wound-breaking strength and wound-collagen content.33,34 Therefore, Hp treatment, which decreases HMGB1 and other pro-inflammatory cytokines in an LPS-stimulated system, may yield benefits in chronic wound healing.
To supplement the in vitro and in silico studies, we performed pilot in vivo wound-healing studies in diabetic mice, which exhibit delayed wound healing (Figure 6). Two wounding models were used: a biopsy punch and an excisional wound, both receiving one-time, topically applied treatments on Day 0. Both models demonstrated delayed wound healing with Hb-treatment compared to controls. The biopsy punch wounds demonstrated accelerated wound healing with Hp treatment compared to controls. The excisional wounds also trended toward improved closure with Hp treatment, however this was not significant, and wounds closed at similar rates with Hb-Hp treatment as well. There are several potential explanations as to why these differences may have been observed between the wound-healing models. First, biopsy punch wounds may heal through different mechanisms than excisional wounds. This is suggested by the fact that biopsy punch wounds consistently reduced in size following wounding (Figure 6B), whereas excisional wounds initially exhibit a large increase in size at Day 7 (negative closure; Figure 6D), which is typical in this model.27,36 Also important to note is the difference in original wound size between the biopsy and excisional wounds (5 mm diameter vs 1 cm × 1 cm, respectively). All wounds received the same dose of treatment, regardless of wound area, so the smaller biopsy punch wounds received more treatment per area than the larger excisional wounds. This may explain the difference in results seen between the two models, specifically the loss of benefit with Hp alone in the larger excisional wounds. Despite this, the general trends of disadvantages in wound healing with Hb, and advantages in wound healing with Hp, were consistent between the in vitro and in vivo work. Future in vivo studies include optimization of Hp/Hb/Hb-Hp treatments on the basis of dose per wound area. Furthermore, a deeper investigation into the treatment effects on macrophage M1/M2 marker expression and cytokine/growth factor expression during wound healing would provide an in vivo context to other key in vitro findings of this manuscript.
Another interesting avenue for future investigation is the effect and potency of different forms of Hp on the observed anti-inflammatory effects. Human Hp has three phenotypes: Hp1–1, Hp2–1, and Hp2–2. The difference in the α subunit of Hp2–1 and Hp2–2 allows for the formation of different sized polymers, and therefore the overall molecular weight can be quite different (89–900 kDa).23,37,38 As Hp phenotype can serve as a predictor for diabetic vascular complications,38 it may be interesting to investigate the abilities of each to bind HMGB1 or Hb, and compare resulting HO-1 activation and inflammatory secretion.
In conclusion, this study revealed that in an inflammatory environment typical of chronic wounds, Hp treatment attenuated pro-inflammatory factor production to the greatest extent with predicted benefits in wound healing. Hp also decreased HMGB1 levels, as predicted by IPA software analysis and measured experimentally via ELISA. A recent, and relatively unknown discovery by Yang et al, found that in addition to binding Hb with high affinity, free Hp also binds to HMGB1 with high, but lesser affinity.22,35 This binding interaction and sequestration of HMGB1 may explain the decrease in associated pro-inflammatory signaling in LPS-stimulated macrophages with Hp treatment. Taken as a whole, these results suggest that Hp on its own can have anti-inflammatory effects on macrophages by affecting HMGB1 signaling, which may have potential in chronic wound-healing applications.
ACKNOWLEDGEMENTS
We would like to thank Alison Acevedo and Dr Ioannis Androulakis for providing their bioinformatics expertise to help interpret results and trends from the multiplex immunoassay. P. K. was funded by a Graduate Assistance in Areas of National Need Fellowship (award number P200A150131) provided by the Department of Education, and by an NIH-funded Biotechnology Training Fellowship (NIH T32 GM008339). Additional funding was provided by NIH grants R01HL126945, R01HL138116, and R01EB021926.
Abbreviations:
- CCL2
see MCP-1
- CCL4
see MIP-1β
- CSF2
see GM-CSF
- CXCL8
IL-8
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor, aka CSF2
- Hp
haptoglobin
- HBOC
hemoglobin-based oxygen carrier
- Hb
hemoglobin
- Hb-Hp
hemoglobin-haptoglobin complex
- HO-1
heme oxygenase 1
- HMGB1
high motility group box 1
- IFN-g
interferon-γ
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1, aka CCL2
- MIP-1β
macrophage inflammatory protein 1β, aka CCL4
- NF-κB
nuclear factor-κB
- P/S
penicillin-streptomycin
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4:560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle). 2012;1:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. [DOI] [PubMed] [Google Scholar]
- 5.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 6.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 7.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. [DOI] [PubMed] [Google Scholar]
- 8.Boyle JJ, Harrington HA, Piper E, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle JJ. Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol. 2012;23:453–461. [DOI] [PubMed] [Google Scholar]
- 10.Ugocsai P, Barlage S, Dada A, Schmitz G. Regulation of surface CD163 expression and cellular effects of receptor mediated hemoglobin-haptoglobin uptake on human monocytes and macrophages. Cytometry A. 2006;69:203–205. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. [DOI] [PubMed] [Google Scholar]
- 12.Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res. 2006;99:943–950. [DOI] [PubMed] [Google Scholar]
- 13.Kapitulnik J Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004;66:773–779. [DOI] [PubMed] [Google Scholar]
- 14.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86: 583–650. [DOI] [PubMed] [Google Scholar]
- 15.Finn AV, Nakano M, Polavarapu R, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grochot-Przeczek A, Lach R, Mis J, et al. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS One. 2009;4:e5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wlaschek M, Singh K, Sindrilaru A, Crisan D, Scharffetter-Kochanek K. Iron and iron-dependent reactive oxygen species in the regulation of macrophages and fibroblasts in non-healing chronic wounds. Free Radic Biol Med. 2019;133:262–275. [DOI] [PubMed] [Google Scholar]
- 20.Lu HL, Huang XY, Luo YF, Tan WP, Chen PF, Guo YB. Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci Rep. 2018;38:BSR20171555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lolmede K, Campana L, Vezzoli M, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–787. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Wang H, Levine YA, et al. Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight. 2016;1:e85375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Wang H, Wang Y, et al. The haptoglobin beta subunit sequesters HMGB1 toxicity in sterile and infectious inflammation. J Intern Med. 2017;282:76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elmer J, Harris DR, Sun G, Palmer AF. Purification of hemoglobin by tangential flow filtration with diafiltration. Biotechnol Prog. 2009;25:1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faulknor RA, Olekson MA, Ekwueme EC, Krzyszczyk P, Freeman JW, Berthiaume F. Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2 transition. Technology. 2017;5:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease - part I: inflammatory biomarkers in wound healing. Biochim Biophys Acta. 1862;2016:1964–1974. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Yarmush ML, Dash BC, Hsia HC, Berthiaume F. Impact of complete spinal cord injury on healing of skin ulcers in mouse models. J Neurotrauma. 2018;35:815–824. [DOI] [PubMed] [Google Scholar]
- 28.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. [DOI] [PubMed] [Google Scholar]
- 29.Arredouani MS, Kasran A, Vanoirbeek JA, Berger FG, Baumann H, Ceuppens JL. Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology. 2005;114:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach JP, Moore EE, Partrick DA, et al. Heme oxygenase-1 induction in macrophages by a hemoglobin-based oxygen carrier reduces endotoxin-stimulated cytokine secretion. Shock. 2009;31:251–257. [DOI] [PubMed] [Google Scholar]
- 31.Hammer M, Mages J, Dietrich H, et al. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur J Immunol. 2005;35:2991–3001. [DOI] [PubMed] [Google Scholar]
- 32.Li JY, Wang N, Khoso MH, et al. FGF-21 elevated IL-10 production to correct LPS-induced inflammation. Inflammation. 2018;41:751–759. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, O’Hearn S, Kavalukas SL, Barbul A. Role of high mobility group box 1 (HMGB1) in wound healing. J Surg Res. 2012;176: 343–347. [DOI] [PubMed] [Google Scholar]
- 34.Dardenne AD, Wulff BC, Wilgus TA. The alarmin HMGB-1 influences healing outcomes in fetal skin wounds. Wound Repair Regen. 2013;21: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhong J, Zhang X, et al. The role of HMGB1 in the pathogenesis of type 2 diabetes. J Diabetes Res. 2016;2016:2543268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeboah A, Cohen RI, Faulknor R, Schloss R, Yarmush ML, Berthiaume F. The development and characterization of SDF1alpha-elastin-like-peptide nanoparticles for wound healing. J Control Release. 2016;232:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galicia G, Ceuppens JL. Haptoglobin function and regulation in autoimmune diseases. Acute Phase Proteins- Regulation and Functions of Acute Phase Proteins; Veas Francisco, IntechOpen, 2011. Available from: https://www.intechopen.com/books/acute-phase-proteins-regulation-and-functions-of-acute-phase-proteins/haptoglobin-function-and-regulation-in-autoimmune-diseases. [Google Scholar]
- 38.Szafranek T, Marsh S, Levy AP. Haptoglobin: a major susceptibility gene for diabetic vascular complications. Exp Clin Cardiol. 2002;7: 113–119. [PMC free article] [PubMed] [Google Scholar]


