Abstract
PURPOSE
Standard curative-intent chemoradiotherapy for human papillomavirus (HPV)–related oropharyngeal carcinoma results in significant toxicity. Since hypoxic tumors are radioresistant, we posited that the aerobic state of a tumor could identify patients eligible for de-escalation of chemoradiotherapy while maintaining treatment efficacy.
METHODS
We enrolled patients with HPV-related oropharyngeal carcinoma to receive de-escalated definitive chemoradiotherapy in a phase II study (ClinicalTrials.gov identifier: NCT03323463). Patients first underwent surgical removal of disease at their primary site, but not of gross disease in the neck. A baseline 18F-fluoromisonidazole positron emission tomography scan was used to measure tumor hypoxia and was repeated 1-2 weeks intratreatment. Patients with nonhypoxic tumors received 30 Gy (3 weeks) with chemotherapy, whereas those with hypoxic tumors received standard chemoradiotherapy to 70 Gy (7 weeks). The primary objective was achieving a 2-year locoregional control (LRC) of 95% with a 7% noninferiority margin.
RESULTS
One hundred fifty-eight patients with T0-2/N1-N2c were enrolled, of which 152 patients were eligible for analyses. Of these, 128 patients met criteria for 30 Gy and 24 patients received 70 Gy. The 2-year LRC was 94.7% (95% CI, 89.8 to 97.7), meeting our primary objective. With a median follow-up time of 38.3 (range, 22.1-58.4) months, the 2-year progression-free survival (PFS) and overall survival (OS) rates were 94% and 100%, respectively, for the 30-Gy cohort. The 70-Gy cohort had similar 2-year PFS and OS rates at 96% and 96%, respectively. Acute grade 3-4 adverse events were more common in 70 Gy versus 30 Gy (58.3% v 32%; P = .02). Late grade 3-4 adverse events only occurred in the 70-Gy cohort, in which 4.5% complained of late dysphagia.
CONCLUSION
Tumor hypoxia is a promising approach to direct dosing of curative-intent chemoradiotherapy for HPV-related carcinomas with preserved efficacy and substantially reduced toxicity that requires further investigation.
A novel trial of PET-guided de-escalation for definitive chemoRT to 30Gy for HPV+ oropharyngeal cancer
INTRODUCTION
Human papillomavirus (HPV)–related oropharyngeal cancers are distinct from tobacco-related cancers and constitute one of the most common head and neck cancers in the United States.1 Definitive chemoradiotherapy or surgery followed by postoperative radiotherapy with or without chemotherapy are two standard treatments.2 While HPV-related oropharyngeal cancers have favorable oncologic outcomes after standard therapy, patients experience long-term side effects, that is, dysphagia and dental complications.3,4 These toxicities have prompted various de-escalation strategies aimed at reducing morbidity.5 Unfortunately, phase III studies substituting cisplatin with cetuximab failed to decrease toxicity and, in fact, demonstrated worse oncologic outcomes.3,4,6 Similarly, phase II studies involving accelerated radiation, induction or concurrent de-escalated chemoradiotherapy, or surgical de-escalation have modest toxicity reductions from standard chemoradiation and some had inferior oncologic outcomes.5,7-14 The outcome differences in these trials could be attributed to de-escalated therapy on the basis of only traditional clinical features.
CONTEXT
Key Objective
Numerous phase II clinical trials have investigated disease control outcomes in patients with human papillomavirus (HPV)–positive cancers when treated with various de-escalation strategies, but high-dose cisplatin concomitant with 70 Gy radiation remains the standard of care. Chemoradiotherapy is highly effective but associated with significant acute and long-term toxicities.
A phase II clinical trial was designed to investigate an hypothesis that measurement of tumor hypoxia with 18F-fluoromisonidazole (FMISO) positron emission tomography (PET) could identify patients eligible for de-escalation from standard 70 Gy to 30 Gy.
Knowledge Generated
Of the 152 eligible patients, 128 received 30 Gy. The 2-year locoregional control and overall survival rates were 94.7% and 100%, respectively. FMISO PET may be able to identify HPV-related oropharyngeal cancers that can receive significant de-escalation of chemoradiotherapy.
Relevance (M.L. Gillison)
-
Although disease control rates appear promising for patients without baseline or early on treatment hypoxia treated with 30 Gy, this approach remains investigational and warrants prospective comparison to the standard of care.*
*Relevance section written by JCO Associate Editor Maura L. Gillison, MD.
Tumor hypoxia diminishes the effectiveness of chemoradiotherapy by reducing radiation-induced free radical production, leading to decreased DNA damage. Consequently, tumor hypoxia is associated with poor outcomes after radiotherapy15,16 and might explain why some tumors require a higher dose of radiation for locoregional control (LRC).17-19 Hypoxia has been measured clinically using 18F-fluoromisonidazole (FMISO) positron emission tomography (PET)20 and associated with poor outcomes in head and neck cancer by multiple groups.18,21,22 Interestingly, baseline levels of hypoxia measured by FMISO PET are similar in HPV-related and HPV-negative tumors.23 Although the majority of hypoxia work has involved HPV-negative disease,18,19,21 emerging evidence suggests that it may also be prognostically important in HPV-related oropharyngeal cancers as well.24,25 Swartz et al24 identified that hypoxia measured by HIF-1α immunohistochemistry in HPV-related oropharyngeal carcinoma decreases overall survival. Furthermore, an individual patient meta-analysis of hypoxia imaging suggests that it may be associated with worse outcomes in HPV-related cancers, albeit with a small number of patients.25
Given hypoxia's role in mediating radiation resistance and its association with outcomes in HPV-related oropharyngeal carcinoma,24,25 we hypothesized that tumors without hypoxia can be treated with a significantly lower radiation dose. We conducted a pilot study26 in 19 patients with HPV-related oropharyngeal cancer who underwent surgical removal of their primary tumor followed by de-escalated chemoradiotherapy to gross nodal disease. Tumors without hypoxia on FMISO PET were de-escalated to 30 Gy, a curative dose traditionally used for HPV-related anal cancers.27 All de-escalated patients had a planned neck dissection at 4 months post-treatment, and 87% were observed to have a major pathologic response (<10% viable tumor).
On the basis of these data and the unmet need to reduce toxicity while maintaining efficacy, we conducted a phase II study personalizing HPV-related oropharyngeal cancer treatment by using FMISO PET. We evaluated the efficacy of 30 Gy in controlling gross nodes in the neck, without a planned neck dissection. However, given the radical radiation dose reduction, the difficulty in salvaging primary tumors, and the desire to conduct de-escalation trials in a stepwise manner, we continued to incorporate surgical removal of the primary site for this protocol.
METHODS
Study Design
The study was designed, sponsored by the Memorial Sloan Kettering Cancer Center, and approved by its institutional review board (ClinicalTrials.gov identifier: NCT03323463, cohort A). Between October 2017 and December 2020, patients were consented, enrolled, and treated at seven different locations within our network. Eligible patients were 18 years and older; had HPV-related oropharyngeal cancer (tonsil, base of tongue, unknown primary), Eastern Cooperative Oncology Group 0-2, and clinical stage T0-2/N1-2c/M0 (American Joint Committee on Cancer, seventh edition); and were able to receive high-dose cisplatin or carboplatin/5-fluorouracil (5FU). HPV status was determined by either positive p16 expression (70% nuclear and cytoplasm expression; Ventana Medical Systems) or mRNA HPV in situ hybridization (RNAscope 2.5 HD Reagent kit [Advanced Cell Diagnostics, Inc, Hayward, CA]).
Before starting chemoradiotherapy, patients underwent primary tumor resection (T1, T2) using a method at the discretion of the surgeon including but not limited to robotic surgery. Microscopic positive margins were permitted. All patients had intact gross nodal disease. Radiation planning occurred approximately 3 weeks postsurgery (median, 2.76 weeks [range, 0.56-3.43 weeks]). FDG PET/computed tomography (CT) radiation simulation, diagnostic magnetic resonance imaging (MRI), and/or CT scans were performed followed by a FMISO PET. Patients with baseline hypoxia underwent repeated FMISO PET 1-2 weeks intratreatment. Only one baseline FMISO PET was performed given its excellent reproducibility on the basis of our previous work.20 Hypoxia status was determined by our established highly reproducible hybrid method on the basis of both a qualitative binary assessment of four standardized image characteristics and a quantitative tumor to background ratio (TBR) on late (150 minutes postinjection) static PET images.28 Typically, there was agreement between the qualitative and quantitative interpretations for the positive scans when using the previous recommended ratio of >1.3, but when there was a disagreement, the visual assessment prevailed (Fig 1A; Data Supplement, Methods [online only]). Pharmacokinetic modeling of dynamic FMISO PET was also performed (Data Supplement, Methods).
FIG 1.
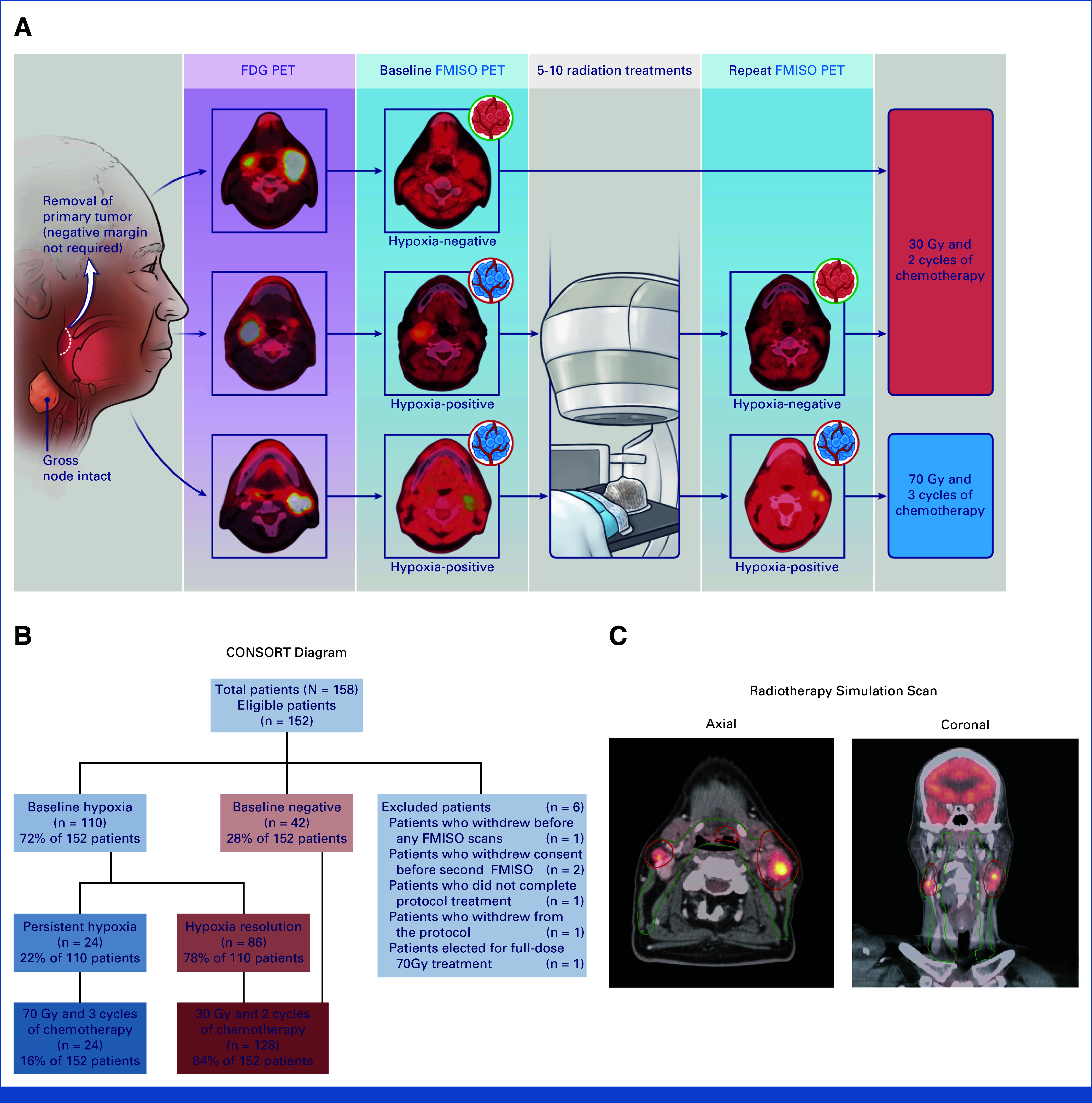
A phase II clinical trial of personalized radiotherapy for oropharyngeal cancer (30-ROC). (A) Protocol schema illustrating typical positive and negative FMISO PET scans pre- and repeated intratreatment along with which patients received 30 Gy versus 70 Gy. (B) CONSORT diagram of all enrolled patients (n = 158), illustrating excluded patients (n = 6) and aggregate statistics for FMISO PET pre- and repeated intratreatment results. (C) Radiotherapy volumes (PTVs) for a typical case on study in a patient with a T1N2c base of tongue tumor. Note that the green contour is PTV that targets microscopic disease and receives 30 Gy regardless of hypoxia status. Red contour highlighting gross nodal disease will receive 30 or 70 Gy depending on hypoxia status. FDG PET, fluorodeoxyglucose positron emission tomography; FMISO PET, 18F-fluoromisonidazole positron emission tomography; PTV, planning target volume; ROC, reduction in oropharyngeal carcinoma.
Personalized Chemoradiotherapy and Follow-Up
Patients without hypoxia on their baseline FMISO PET scan and patients who had hypoxia resolution on their intratreatment FMISO PET received a total dose of 30 Gy at 2 Gy/fraction/d. Patients also received concurrent chemotherapy with two cycles of cisplatin 100 mg/m2 or two cycles carboplatin AUC of 5 and 5FU of 2,400 mg/m2 over 4 days, given on weeks 1 and 4 (second cycle after radiation therapy completion; Fig 1A; Data Supplement, Table 1). All patients received IMRT, targeting the postoperative primary site, gross neck nodes, and potential areas of microscopic spread (typically bilateral necks), all uniformly receiving 30 Gy. For tonsil cancers, the ipsilateral pterygoid plate was included, whereas the pre-epiglottic space was included for base of tongue cancers. Primary site margin did not influence radiotherapy dosing. Well-lateralized tonsil cancer with a single gross node received ipsilateral radiation. For unknown primary cases, the entire oropharyngeal axis was treated. These patients also had a full workup including ipsilateral tonsillectomies. Patients whose tumors exhibited persistent hypoxia on intratreatment FMISO PET received a boost of 40 Gy/2 Gy/fraction to gross nodal disease to a total of 70 Gy with chemotherapy (Figs 1A and 1C). Each patient had two plans: a plan to 30 Gy and a subsequent cone-down plan to 70 Gy. If the patient had hypoxia resolution, the 70 Gy plan was not delivered. Departmental guidelines on radiation target delineations and planning guidelines were followed and reviewed by two radiation oncologists (N.Y.L. and N.R.).
TABLE 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | 30 Gy (n = 128), No. (%) | 70 Gy (n = 24), No. (%) | Total (n = 152), No. (%) |
|---|---|---|---|
| Sex | |||
| Male | 115 (89.8) | 22 (91.7) | 137 (90.1) |
| Female | 13 (10.2) | 2 (8.3) | 15 (9.9) |
| Age, years | |||
| ≤49 | 15 (11.7) | 4 (16.7) | 19 (12.5) |
| 50-59 | 55 (43.0) | 12 (50.0) | 67 (44.1) |
| 60-69 | 49 (38.3) | 8 (33.3) | 57 (37.5) |
| ≥70 | 9 (7.0) | 0 (0) | 9 (5.9) |
| Race | |||
| White | 117 (91.4) | 23 (95.8) | 140 (92.1) |
| Asian/Far East/Indian subcontinent | 2 (1.5) | 0 (0) | 2 (1.3) |
| Black or African American | 1 (0.8) | 0 (0) | 1 (0.7) |
| Unknown or not reported | 8 (6.3) | 1 (4.2) | 9 (5.9) |
| Smoking | |||
| Never | 74 (57.8) | 6 (25.0) | 80 (52.6) |
| ≤10 pack-years | 28 (21.9) | 9 (37.5) | 37 (24.3) |
| >10 pack-years | 26 (20.3) | 9 (37.5) | 35 (23.0) |
| ECOG performance status | |||
| 0 | 101 (78.9) | 20 (83.3) | 121 (79.6) |
| 1 | 27 (21.1) | 4 (16.7) | 31 (20.4) |
| Primary site | |||
| Tonsil | 72 (56.3) | 12 (50.0) | 84 (55.2) |
| BOT | 36 (28.1) | 5 (20.8) | 41 (27.0) |
| Unknown | 20 (15.6) | 7 (29.2) | 27 (17.8) |
| HPV RNA-ISH | |||
| Positive | 123 (96.1) | 23 (95.8) | 146 (96.1) |
| Unknown | 5 (3.9) | 1 (4.2) | 6 (3.9) |
| p16 status | |||
| Positive | 128 (100) | 24 (100) | 152 (100) |
| Negative | 0 (0) | 0 (0) | 0 (0) |
| T class | |||
| 0 | 20 (15.6) | 7 (29.2) | 27 (17.8) |
| 1 | 64 (50.0) | 10 (41.7) | 74 (48.6) |
| 2 | 44 (34.4) | 7 (29.2) | 51 (33.6) |
| N class | |||
| 1 | 14 (10.9) | 3 (12.5) | 17 (11.2) |
| 2a | 11 (8.5) | 3 (12.5) | 14 (9.2) |
| 2b | 76 (59.4) | 16 (66.7) | 92 (60.5) |
| 2c | 27 (21.1) | 2 (8.3) | 29 (19.1) |
| Grouping | |||
| III | 14 (10.9) | 3 (12.5) | 17 (11.2) |
| IVA | 114 (89.1) | 21 (87.5) | 135 (88.8) |
| RTOG risk group | |||
| Low | 107 (83.6) | 19 (79.2) | 126 (82.9) |
| Intermediate | 21 (16.4) | 5 (20.8) | 26 (17.1) |
| Chemotherapy regimen | |||
| Cisplatin | 110 (85.9) | 22 (91.7) | 132 (86.8) |
| Carbo/5FU | 18 (14.1) | 2 (8.3) | 20 (13.2) |
| Margin status | |||
| Negative | 17 (15.7) | 4 (23.5) | 21 (16.8) |
| Close (<2 mm) | 57 (52.8) | 7 (41.2) | 64 (51.2) |
| Positive (on ink) | 34 (31.4) | 6 (35.3) | 40 (32.0) |
Abbreviations: 5FU, fluorouracil; BOT, base of tongue; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; ISH, in situ hybridization; RTOG, Radiation Therapy Oncology Group.
Additional once weekly MRIs and blood samplings were performed. Standard surveillance was performed but in addition included PET, MRI, and/or CT scans at 3-4 months and 1 and 2 years post-treatment.2
Primary and Secondary End Points
The primary end point was 2-year LRC for patients treated with personalized chemoradiotherapy on the basis of FMISO PET. Locoregional failure was defined as any recurrence at the primary tumor site at any time or a neck recurrence >140 days from the end of chemoradiation using definitions adopted from NRG Oncology.3,9 Recurrence was confirmed pathologically. Secondary end points were distant metastasis (DM), progression-free survival (PFS), overall survival (OS), and patient-reported quality-of-life (PRO) outcomes. Acute/late toxicities were assessed by using National Cancer Institute Common Terminology Criteria for Adverse Events, v4.0 from the start of all therapies. PROs were collected using MD Anderson Dysphagia Inventory,29 European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire,30 and Financial Toxicity (COST-FACIT) surveys.31 Objective speech/swallowing function assessments were performed with a modified barium swallow (Data Supplement, Methods). Correlative biomarkers included pretreatment and weekly intratreatment diffusion-weighted (DW) MRIs; tumor-informed assay was performed to assess cell-free circulating tumor DNA (ctDNA) in blood samples obtained before and 2 weeks into chemoradiation (Data Supplement, Methods). As a proxy for cost, we priced the hospital/physician services used by the patients at Medicare service fee rates.
Statistical Analysis
Our primary objective was to determine if the 2-year LRC was comparable with our historical control of 95% with standard chemoradiotherapy,3,4 with a 7% noninferiority margin. To allow for 5% attrition, we enrolled 158 patients where the primary end point was based on the first 150 eligible patients and secondary end points included all eligible patients. A prespecified statistical analysis plan was developed using a one-sided one-sample proportion test for the hypotheses H0: P ≤88% versus H1: P >88%, where P represents the 2-year LRC. With 150 patients, we had a >0.85 power for detecting whether personalized chemoradiation on the basis of the FMISO PET results would result in a 2-year LRC of 95% with an alpha of .025. Secondary end points included Kaplan-Meier estimates to assess PFS and OS, whereas cumulative incidence functions were used to assess DM and LRC. Acute/late toxicities and PRO scores with the specified time points were tabulated/summarized. The exploratory objectives involved correlation analyses of binary factors (ie, ctDNA detection using Fischer’s exact test) and continuous variables (ie, volume and mean ADCs derived from DW-MRI using the Wilcoxon rank-sum test) to identify candidates for de-escalated therapy.
RESULTS
Patients
One hundred fifty-two patients were eligible for analysis (Fig 1B). Clinical characteristics are presented in Table 1. Baseline FMISO PET scans were positive in 110 (72%) patients (median TBR, 1.6 [range, 0.82-3.43]) and negative in 42 (28%) patients (median TBR, 0.97 [range 0.69-1.31]; Appendix Table A1). Of the 110 patients with pretreatment tumor hypoxia, 86 eventually showed hypoxia resolution on repeat intratreatment FMISO PET (TBR, 1.0 [range, 0.63-1.33]). Thus, 24 (16%) patients had nodal disease with persistent hypoxia (median TBR, 1.32 [range, 0.94-2.00]) and treated with 70 Gy, whereas the other 128 (84%) patients received 30 Gy. Of the 128 de-escalated patients, only three were selected as negative on the basis of visual assessment of FMISO-PET despite a TBR of >1.3. The median TBR of 30 Gy patients was lower than that of 70 Gy patients (Data Supplement, Fig S1A, online only). Compartmental analysis from dynamic PET was consistent with TBR derived from late, static FMISO PET images (Data Supplement, Figs S1B and S1C).
Clinical characteristics were not significantly associated with baseline hypoxia status (Data Supplement, Table S1). There was no difference in baseline hypoxia status among never smokers, ≤10 pack-year smokers, and >10 pack-year smokers, and six were current smokers. Smokers were three times more likely to have persistent hypoxia during therapy versus never smokers (P = .02; Data Supplement, Table S1). Among the 128 de-escalated patients, 110 patients initially received two cycles of cisplatin and 18 received carboplatin/5FU. All patients received chemoradiation per protocol.
Efficacy
At the time of data cutoff, the median follow-up was 38.3 months (range, 22.1-58.4 months). The study met its primary objective with a 2-year LRC of 94.7% (CI, 89.8 to 97.7; Fig 2A). The 2-year PFS and OS rates for the entire study were 94% and 99%, respectively. The 2-year PFS and OS were 94% and 100% for the 30-Gy cohort and 96% and 96% for the 70-Gy cohort (Figs 2B and 2C), respectively. There was one death from pulmonary embolus in the 70-Gy cohort. Despite an approximately 60% radiation dose reduction, only eight patients had nodal recurrences (median, 8.1 months [range, 6.8-22.4 months]); all underwent successful limited neck dissections. Four patients had persistent nodal disease for <140 days (Data Supplement, Table S10). No 70 Gy patients experienced a nodal failure. No patients failed in the primary site nor a primary emerged in patients who presented with carcinoma of unknown primary. A single patient in the 30-Gy cohort developed DM. The 2-year disease-specific survival for the entire cohort was 100%. There were no associations between clinical factors and the probability of locoregional recurrence (Data Supplement, Table S2).
FIG 2.

Oncologic outcomes. (A) Cumulative incidence of LRR for the entire cohort using competing risk analysis. (B) PFS by treatment group. (C) OS by treatment group. LRR, locoregional recurrence; OS, overall survival; PFS, progression-free survival.
Adverse Events
Across both cohorts, for ≥grade 3 acute toxicities, <9% of patients had radiation-related and <55% had chemotherapy-related. For late ≥grade 3 toxicities, <0.7% of the patients had radiation-related and none had chemotherapy-related (Data Supplement, Table S3). Fifty-six percent of patients did not require narcotics for pain relief. Regarding late toxicities in the entire cohort, we observed 2.6% grade 2 xerostomia, 5.2% grade 2 dysgeusia, and 1.4% grade 2-3 dysphagia (Data Supplement, Table S3). Ninety-six percent of patients treated with 70 Gy experienced acute dysphagia versus 57% in the 30-Gy cohort (P < .001; Table 2). Acute toxicities were significantly reduced favoring 30 Gy versus 70 Gy (Table 2). Greater than ninety percent of the reported radiation-related late toxicities were grade 1 (Data Supplement, Table S3). Late toxicity in the 70-Gy cohort in this study also compared favorably with other contemporary series treating with 70 Gy, likely because of a significantly reduced subclinical dose of 30 Gy from historical practice of treating with 50-63 Gy (Fig 1C).
TABLE 2.
Most Common Investigator-Reported Adverse Events for the 30-Gy and 70-Gy Cohort
| Acute Toxicity | 30 Gy, % | 70 Gy, % | P | ||
|---|---|---|---|---|---|
| Any | Grade 3-4 | Any | Grade 3-4 | ||
| RT-related | |||||
| Dermatitis | 47.6 | 0.0 | 95.8 | 4.2 | <.001 |
| Dry mouth | 89.1 | 0.0 | 100.0 | 0.0 | .16 |
| Dysphagia | 57.0 | 0.7 | 95.8 | 8.3 | <.001 |
| Oral mucositis | 78.9 | 0.0 | 95.8 | 4.2 | <.001 |
| Dysgeusia | 93.8 | 0.0 | 100.0 | 0.0 | <.001 |
| Hypothyroidism | 6.3 | 0.0 | 12.5 | 0.0 | .38 |
| Chemotherapy-related | |||||
| Neutropenia | 57.0 | 29.7 | 79.2 | 45.8 | .15 |
| Anemia | 85.9 | 0.7 | 95.8 | 4.2 | .003 |
| Thrombocytopenia | 73.4 | 0.0 | 79.1 | 0.0 | .01 |
| Nausea | 44.5 | 0.7 | 66.7 | 4.2 | .08 |
| Vomiting | 9.4 | 1.5 | 12.5 | 0.0 | .67 |
| Neuropathy | 3.9 | 0.0 | 8.3 | 0.0 | .31 |
| Acute kidney injury | 42.9 | 0.7 | 62.5 | 4.2 | .07 |
| Hearing | 8.6 | 0.0 | 8.3 | 0.0 | 1 |
| Late Toxicity | 30 Gy, % | 70 Gy, % | P | ||
|---|---|---|---|---|---|
| Any | Grade 3-4 | Any | Grade 3-4 | ||
| RT-related | |||||
| Dermatitis | 0.0 | 0.0 | 0.0 | 0.0 | 1 |
| Dry mouth | 81.3 | 0.0 | 95.5 | 0.0 | .25 |
| Dysphagia | 11.7 | 0.0 | 18.1 | 4.5 | .22 |
| Oral mucositis | 3.1 | 0.0 | 0.0 | 0.0 | 1 |
| Dysgeusia | 73.4 | 0.0 | 86.4 | 0.0 | .23 |
| Hypothyroidism | 12.5 | 0.0 | 31.8 | 0.0 | .05 |
| Chemotherapy-related | |||||
| Neutropenia | 3.9 | 0.0 | 0.0 | 0.0 | 1 |
| Anemia | 31.3 | 0.7 | 40.9 | 0.0 | .49 |
| Thrombocytopenia | 16.4 | 0.0 | 36.6 | 0.0 | .03 |
| Nausea | 0.7 | 0.0 | 4.5 | 0.0 | .28 |
| Vomiting | 0.0 | 0.0 | 0.0 | 0.0 | 1 |
| Neuropathy | 6.3 | 0.0 | 9.1 | 0.0 | .65 |
| Kidney injury | 18.0 | 0.0 | 36.3 | 0.0 | .09 |
| Hearing | 12.5 | 0.0 | 9.1 | 0.0 | 1 |
Abbreviation: RT, radiation therapy.
PROs and Dysphagia
PROs for swallowing were assessed with MDADI,29 with a mean baseline global score of 87.47 (95% CI, 84.12 to 90.82) to 92.28 (95% CI, 89.58 to 94.99) at 4 months and 94.52 (95% CI, 91.99 to 97.04) at 1 year after chemoradiation (Data Supplement, Fig S2 and Tables S4-S8). These results were consistent with alternative instruments for PRO assessment (EORTC Questionnaire; Data Supplement, Table S9).30 The results from PRO assessments were concordant with objective assessments of dysphagia using modified barium swallow testing, in which none had moderate dysphagia at 1 year after chemoradiation (Fig 3). The patient-reported COST FACIT financial toxicity instrument31 did not identify any patient with composite scores lower than baseline, during or after treatment. This suggests that financial distress was likely higher before treatment and gradually decreased after treatment completion (Data Supplement, Fig S3).
FIG 3.
Toxicity and dysphagia-related outcomes. Modified barium swallow objective assessments using DIGEST score (0 is within normal limits, and 4 denotes profound dysphagia) for the 30-Gy cohort.
Exploratory Biomarkers
MRI scans were obtained in a subset of consented patients (n = 95) to evaluate tumor volume and ADC, with the latter as a proxy for the burden of total tumor cells obtained from DW-MRI. Neither pretreatment tumor volume nor mean ADCs, nor a change in these metrics 2 weeks intratreatment accurately identified the 128 patients who were eligible for 30 Gy on the basis of FMISO PET (Data Supplement, Figs S4 and S5). Moreover, ctDNA was persistently detectable in 64% of patients 2 weeks into chemoradiotherapy, suggesting that this marker could not accurately select all 128 patients on the basis of FMISO PET eligible for 30 Gy de-escalation (Data Supplement, Fig S6). The high levels of persistent ctDNA early during chemoradiation are consistent with those of other groups.32,33
DISCUSSION
The ability to direct radiotherapy dose on the basis of biologic features in clinical practice is absent for most cancers.34-36 Studies attempting to de-escalate therapy for HPV-related oropharyngeal carcinoma have led to inferior oncologic outcomes3,4,6,12 without significant toxicity reduction.7-9 Notably, toxicity in aggregate is also not dramatically reduced in surgical de-escalation studies where patients still received a high radiation dose of 50 Gy-60 Gy (60% of the patients) or chemoradiotherapy to 66 Gy with cisplatin (30% of the patients).8 Without selection, modest dose de-escalation to 60 Gy leads to inferior oncologic outcomes.12 Here, we used FMISO PET to personalize radiotherapy dose to 30 Gy26 and we report a 2-year LRC of 95% with a favorable toxicity profile.18,21-23,25,37,38 It should be noted that 12 of 128 (9%) patients who received 30 Gy required neck dissections versus 2%-8% in other series.3,4,6,7,9
PET imaging with various radiotracers has been used for risk stratification and treatment modification in a variety of diseases.39,40 We used FMISO PET response to direct radiation dose because multiple groups have shown that a positive FMISO scan is a poor prognostic biomarker of radiotherapy cancer outcomes including HPV-related oropharyngeal carcinoma.18,21-23,25,37,38 Despite its lower TBR versus other PET imaging agents, our hybrid qualitative/quantitative method of interpreting FMISO PET has excellent interobserver agreement in identifying hypoxia (κ = 0.859; 95% CI, 0.761 to 0.944) between five nuclear medicine physicians with various levels of experience28 (Appendix Table A1). This interpretation method is similar to other established PET criteria, that is, Lugano criteria in lymphoma37 and PROMISE PSMA PET method for prostate cancer.38 The consistency of FMISO reads, combined with its commercial availability and the ability to produce it with a cyclotron, facilitates its adoption across institutions. However, we acknowledge that using FMISO PET in a multicenter randomized trial to tailor radiotherapy dose presents challenges for implementation into clinical practice. We have tested the feasibility of using FMISO PET to de-escalate HPV-related oropharyngeal cancers enrolling patients from other centers (n = 10) in a subsequent study that completed accrual.
Patients who received 30 Gy had significantly lower rates of acute toxicity versus 70 Gy. None of the 30 Gy patients had moderate dysphagia 1 year after chemoradiation versus the historical rates of 30% at 3-6 months and 15% 2 years after chemoradiation.41,42 Overall, toxicity with our de-escalated approach compares favorably with standard chemoradiotherapy observed on contemporary randomized trials.3,4 Although our patients had slightly less advanced disease than those in RTOG 10-16 and DeEscalate, similar toxicities were observed in the 60-Gy arm of NRG HN-002 as those two studies.9 Notably, NRG HN-002 trial included patients with less advanced neck disease than ours, suggesting that the toxicity differences are mainly due to changes in radiotherapy dose.
Previous work has illustrated that MRI-derived parameters are predictive of treatment response after 70 Gy of chemoradiation for head and neck cancer43-46; however, its role in de-escalation remains an active area of investigation. We were not able to predict hypoxia resolution on the basis of MRI parameters. Further work will be required to elucidate which MR imaging modality or if a multimodal imaging approach may be optimal to identify patients for de-escalation. The attenuated radiation dose also reduced financial toxicity for our patients. Furthermore, using expected Medicare hospital and physician reimbursement as a proxy, we found a 63% decrease in the overall direct cost of health care services among patients who underwent 30 Gy versus 70 Gy.
Limitations of this study are that we have not comprehensively evaluated the performance of FMISO PET as a biomarker for radical de-escalation and that FMISO PET has a limited amount of evidence as a prognostic biomarker for HPV-related oropharyngeal carcinoma.25 At the time of initiation of this study (2017), chemoradiotherapy to 30 Gy in an unselected population would be considered unethical, rendering a definitive evaluation of the performance of FMISO PET as a biomarker not feasible. Emerging data, however, suggest that biomarker selection is necessary for de-escalation of chemoradiotherapy in HPV-related OPC.12 Additional limitations are that this is a single-arm study with relatively short follow-up (median slightly >3 years) in a selected subset of HPV-related oropharyngeal carcinomas with primary tumors amenable to surgery and that the toxicities associated with surgical resection of the primary were not accounted for.
In conclusion, to our knowledge, we report the first personalized phase II trial using functional PET imaging as an integral biomarker to markedly de-escalate definitive chemoradiotherapy in head and neck cancer. This approach significantly decreased adverse events, while producing oncologic outcomes comparable with the standard full-dose radiotherapy. Although such results are promising, further evaluation is required before it can be considered for routine use in clinical practice. A phase III trial is currently being planned to compare this precision radiotherapy approach with the current standard of care, building on the tenets of personalized targeted therapy to usher a new paradigm of biomarker-directed therapy for patients with HPV-related oropharyngeal cancer.
ACKNOWLEDGMENT
We would like to thank Alex Ionescu, Vanessa Wu, Gloria Wasilewski, and Adviti Sarang for their assistance with conduct of the study. We would also like to acknowledge Daniel Gorovets and Elaine Duck for their help with Medicare Reimbursement and Financial Toxicity. We also acknowledge financial support for this trial from National Institutes of Health R01 CA238392-02A1 and R01 CA157770-01A1, National Cancer Institute Cancer Center Support Grant P30 CA008, DIMON HPV Foundation, Serra Initiative on the Management of Head and Neck Cancer Side Effects, and James A. Rowen Precision Radiotherapy Fund.
APPENDIX
TABLE A1.
Detailed Patient Characteristics Including Details on FMISO PET Scan
| Patient | T Stage | N Stage | Primary Site | Smoking Status | Margin Status | Chemotherapy Regimen | Pretreatment FMISO PET Result | Pretreatment TMR | On-Treatment FMISO PET Result | On-Treatment TMR | Locoregional Recurrence | Any Neck Dissection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.133333333 | Negative | 0.944444444 | No | No |
| 2 | T2 | N2b | BOT | Never | Positive | Cisplatin | Hypoxic | 1.714285714 | Negative | 0.9375 | No | No |
| 3 | T1 | N1 | Tonsil | Never | Close | Cisplatin | Negative | 1 | NA | NA | No | No |
| 4 | T2 | N2b | Tonsil | <10 pack-years | Positive | Both | Hypoxic | 1.428571429 | Hypoxic | 1 | No | No |
| 5 | T1 | N2b | BOT | Never | Close | Cisplatin | Hypoxic | 1.6 | Negative | 1.0625 | No | No |
| 6 | T1 | N2b | BOT | Never | Negative | Cisplatin | Negative | 1 | NA | NA | No | No |
| 7 | T1 | N2b | BOT | >10 pack-years | Negative | Cisplatin | Hypoxic | 1.705882353 | Hypoxic | 1.05 | No | No |
| 8 | T2 | N2c | BOT | >10 pack-years | Negative | Cisplatin | Hypoxic | 1.642857143 | Negative | 0.761904762 | No | No |
| 9 | T0 | N2b | Unknown | Never | NA—unknown primary | Cisplatin | Hypoxic | 1.058823529 | Negative | 1.133333333 | No | No |
| 10 | T1 | N2b | BOT | Never | Close | Cisplatin | Hypoxic | 1.5 | Negative | 1 | No | No |
| 11 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.714285714 | Negative | 1.0625 | No | No |
| 12 | T1 | N2b | Tonsil | Never | Positive | Cisplatin | Hypoxic | 2.230769231 | Negative | 1 | No | No |
| 13 | T1 | N2b | Tonsil | Never | Positive | Cisplatin | Hypoxic | 1.333333333 | Negative | 1.066666667 | No | No |
| 14 | T1 | N2c | BOT | Never | Negative | Cisplatin | Hypoxic | 1.214285714 | Negative | 1 | No | No |
| 15 | T1 | N2b | BOT | Never | Negative | Carbo/5FU | Hypoxic | 1.333333333 | Negative | 1.058823529 | No | No |
| 16 | T1 | N2b | BOT | <10 pack-years | Close | Cisplatin | Hypoxic | 1.333333333 | Negative | 0.928571429 | No | No |
| 17 | T1 | N2b | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 2 | Negative | 0.8125 | No | No |
| 18 | T1 | N2b | Tonsil | Never | Positive | Cisplatin | Negative | 1.176470588 | NA | NA | No | No |
| 19 | T2 | N2b | Tonsil | Never | Positive | Carbo/5FU | Negative | 0.823529412 | NA | NA | No | No |
| 20 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 3.090909091 | Negative | 1.076923077 | No | No |
| 21 | T2 | N2c | Tonsil | <10 pack-years | Positive | Cisplatin | Hypoxic | 2.125 | Negative | 1 | No | No |
| 22 | T1 | N2b | Tonsil | Never | Close | Carbo/5FU | Hypoxic | 1.5 | Negative | 1.066666667 | No | No |
| 23 | T2 | N2c | Tonsil | Never | Positive | Carbo/5FU | Negative | 1.214285714 | NA | NA | No | No |
| 24 | T1 | N2c | Tonsil | <10 pack-years | Close | Cisplatin | Hypoxic | 2.333333333 | Hypoxic | 1.615384615 | No | No |
| 25 | T1 | N2c | BOT | <10 pack-years | Positive | Cisplatin | Negative | 0.941176471 | NA | NA | No | No |
| 26 | T2 | N2b | BOT | Never | Negative | Cisplatin | Hypoxic | 1.625 | Negative | 1.0625 | No | No |
| 27 | T1 | N2c | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.923076923 | Hypoxic | 1.333333333 | No | No |
| 28 | T0 | N2a | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.578947368 | Hypoxic | 1.526315789 | No | No |
| 29 | T1 | N2b | BOT | Never | Negative | Cisplatin | Hypoxic | 1.25 | Negative | 0.882352941 | No | No |
| 30 | T2 | N2c | Tonsil | <10 pack-years | Close | Carbo/5FU | Negative | 0.714285714 | NA | NA | No | No |
| 31 | T2 | N2b | BOT | Never | Positive | Cisplatin | Hypoxic | 1 | Negative | 1.066666667 | No | No |
| 32 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Negative | 1.071428571 | NA | NA | No | No |
| 33 | T1 | N1 | BOT | Never | Negative | Cisplatin | Negative | 0.882352941 | NA | NA | No | No |
| 34 | T1 | N1 | BOT | Never | Positive | Cisplatin | Negative | 0.6875 | NA | NA | No | No |
| 35 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.4375 | Negative | 1 | No | No |
| 36 | T2 | N2c | Tonsil | >10 pack-years | Close | Cisplatin | Negative | 0.947368421 | NA | NA | No | No |
| 37 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.25 | Negative | 1 | No | No |
| 38 | T1 | N2b | Tonsil | <10 pack-years | Close | Carbo/5FU | Hypoxic | 1.73 | Hypoxic | 1.733333333 | No | No |
| 39 | T2 | N1 | Tonsil | Never | Positive | Cisplatin | Negative | 1.230769231 | NA | NA | No | No |
| 40 | T2 | N2b | BOT | >10 pack-years | Positive | Cisplatin | Hypoxic | 1.375 | Hypoxic | 1.3125 | No | No |
| 41 | T0 | N2b | Unknown | Never | NA—unknown primary | Cisplatin | Hypoxic | 1.2 | Negative | 0.941176471 | Yes | Yes |
| 42 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.133333333 | Negative | 0.625 | Yes | Yes |
| 43 | T1 | N2b | BOT | >10 pack-years | Negative | Cisplatin | Hypoxic | 2.307692308 | Hypoxic | 1.769230769 | No | No |
| 44 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Carbo/5FU | Hypoxic | 1.266666667 | Negative | 1 | No | No |
| 45 | T1 | N2c | Tonsil | >10 pack-years | Close | Cisplatin | Negative | 0.8 | NA | NA | No | No |
| 46 | T2 | N2b | BOT | <10 pack-years | Positive | Cisplatin | Hypoxic | 1.533333333 | Hypoxic | 1.176470588 | No | No |
| 47 | T2 | N2b | Tonsil | >10 pack-years | Positive | Cisplatin | Negative | 1 | NA | NA | Yes | Yes |
| 48 | T0 | N2b | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.538461538 | Negative | 0.882352941 | No | No |
| 49 | T1 | N2b | Tonsil | Never | Negative | Cisplatin | Hypoxic | 1.642857143 | Negative | 1 | No | No |
| 50 | T1 | N2c | Tonsil | Never | Positive | Carbo/5FU | Negative | 1.076923077 | NA | NA | No | No |
| 51 | T2 | N2c | Tonsil | Never | Positive | Carbo/5FU | Hypoxic | 2.857142857 | Negative | 0.894736842 | No | No |
| 52 | T2 | N1 | BOT | 10 pack-years | Close | Cisplatin | Hypoxic | 1.230769231 | Negative | 1.083333333 | No | No |
| 53 | T1 | N2b | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.3125 | Negative | 1.333333333 | No | No |
| 54 | T2 | N2b | Tonsil | Never | Positive | Cisplatin | Hypoxic | 2.176470588 | Negative | 0.944444444 | No | No |
| 55 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Negative | 0.857142857 | NA | NA | No | No |
| 56 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Carbo/5FU | Hypoxic | 2.428571429 | Negative | 1 | No | No |
| 57 | T0 | N2b | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.733333333 | Negative | 1 | No | No |
| 58 | T1 | N2b | Tonsil | Never | Close | Carbo/5FU | Negative | 1.2 | NA | NA | No | No |
| 59 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 2.076923077 | Negative | 1.214285714 | No | No |
| 60 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.875 | Hypoxic | 1.357142857 | No | No |
| 61 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1 | Negative | 0.888888889 | No | No |
| 62 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.285714286 | Hypoxic | 1.230769231 | No | No |
| 63 | T0 | N2b | Unknown | Never | NA—unknown primary | Cisplatin | Negative | 1.307692308 | NA | NA | No | No |
| 64 | T2 | N1 | Tonsil | Never | Positive | Carbo/5FU | Negative | 0.92 | NA | NA | No | No |
| 65 | T1 | N1 | Tonsil | Never | Close | Cisplatin | Negative | 0.733333333 | NA | NA | No | No |
| 66 | T1 | N2a | Tonsil | Never | Close | Cisplatin | Hypoxic | 1 | Negative | 1 | No | No |
| 67 | T1 | N2b | BOT | Never | Positive | Cisplatin | Negative | 1.058823529 | NA | NA | No | No |
| 68 | T0 | N2b | Unknown | Never | NA—unknown primary | Carbo/5FU | Negative | 0.8 | NA | NA | No | No |
| 69 | T1 | N2c | BOT | <10 pack-years | Positive | Cisplatin | Negative | 1.058823529 | NA | NA | No | No |
| 70 | T1 | N2b | Tonsil | <10 pack-years | Close | Cisplatin | Negative | 1.0625 | NA | NA | No | No |
| 71 | T2 | N2b | Tonsil | <10 pack-years | Close | Carbo/5FU | Hypoxic | 1.166666667 | Negative | 0.736842105 | Yes | Yes |
| 72 | T2 | N2b | Tonsil | Never | Positive | Cisplatin | Hypoxic | 1.0625 | Negative | 1.125 | No | No |
| 73 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 2.071428571 | Negative | 1.0625 | No | No |
| 74 | T1 | N2c | Tonsil | Never | Negative | Cisplatin | Hypoxic | 2.153846154 | Negative | 1 | No | No |
| 75 | T1 | N2a | Tonsil | >10 pack-years | Positive | Cisplatin | Negative | 0.928571429 | NA | NA | No | No |
| 76 | T1 | N2b | BOT | >10 pack-years | Close | Cisplatin | Hypoxic | 1.8125 | Negative | 1 | No | No |
| 77 | T1 | N2b | BOT | >10 pack-years | Positive | Carbo/5FU | Hypoxic | 2 | Negative | 1.105263158 | Yes | Yes |
| 78 | T0 | N2b | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 2 | Hypoxic | 1.182352941 | No | No |
| 79 | T1 | N2c | Tonsil | Never | Close | Cisplatin | Negative | 0.823529412 | NA | NA | No | No |
| 80 | T2 | N1 | BOT | Never | Positive | Cisplatin | Hypoxic | 1.125 | Negative | 1.0625 | No | No |
| 81 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.684210526 | Hypoxic | 1.529411765 | No | No |
| 82 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Negative | 1 | NA | NA | No | No |
| 83 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Negative | 1 | NA | NA | No | No |
| 84 | T1 | N2c | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.928571429 | Negative | 0.941176471 | No | No |
| 85 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 2.357142857 | Negative | 1 | No | No |
| 86 | T0 | N2a | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 2 | Negative | 1 | No | No |
| 87 | T1 | N2a | Tonsil | <10 pack-years | Positive | Cisplatin | Hypoxic | 1.357142857 | Negative | 1.058823529 | No | No |
| 88 | T2 | N2b | BOT | <10 pack-years | Close | Carbo/5FU | Negative | 0.94 | NA | NA | No | No |
| 89 | T1 | N1 | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.5 | Negative | 1 | No | No |
| 90 | T2 | N2b | Tonsil | 10 pack-years | Positive | Cisplatin | Hypoxic | 1.55 | Hypoxic | 0.94 | No | No |
| 91 | T0 | N1 | Unknown | Never | NA—unknown primary | Cisplatin | Hypoxic | 0.821428571 | Negative | 0.842105263 | No | No |
| 92 | T1 | N2a | BOT | Never | Close | Cisplatin | Hypoxic | 1.6 | Negative | 0.947368421 | No | Yes |
| 93 | T0 | N2b | Unknown | Never | NA—unknown primary | Cisplatin | Hypoxic | 2.357142857 | Hypoxic | 1.294117647 | No | No |
| 94 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.625 | Negative | 1 | No | No |
| 95 | T0 | N2b | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Negative | 0.94 | NA | NA | No | No |
| 96 | T0 | N2c | Unknown | Never | NA—unknown primary | Cisplatin | Negative | 1.0625 | NA | NA | No | No |
| 97 | T1 | N2b | Tonsil | <10 pack-years | Close | Cisplatin | Negative | 0.9375 | NA | NA | No | No |
| 98 | T1 | N2a | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.8 | Negative | 1 | No | No |
| 99 | T0 | N2a | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.188 | Negative | 1 | No | No |
| 100 | T0 | N2b | Unknown | Never | NA—unknown primary | Cisplatin | Negative | 0.833333333 | NA | NA | No | No |
| 101 | T2 | N2b | Tonsil | >10 pack-years | Negative | Cisplatin | Hypoxic | 1.894736842 | Negative | 1.052631579 | No | No |
| 102 | T1 | N2c | BOT | <10 pack-years | Negative | Cisplatin | Hypoxic | 2.692307692 | Negative | 1 | Yes | Yes |
| 103 | T2 | N2b | Tonsil | <10 pack-years | Close | Cisplatin | Hypoxic | 1.4 | Negative | 1 | No | No |
| 104 | T2 | N2b | Tonsil | >10 pack-years | Close | Cisplatin | Negative | 1 | NA | NA | No | No |
| 105 | T1 | N1 | BOT | >10 pack-years | Negative | Cisplatin | Negative | 1 | NA | NA | No | No |
| 106 | T1 | N2b | BOT | <10 pack-years | Close | Carbo/5FU | Hypoxic | 1.25 | Negative | 0.888888889 | No | No |
| 107 | T2 | N2b | BOT | >10 pack-years | Positive | Cisplatin | Hypoxic | 1.666666667 | Negative | 0.9375 | No | No |
| 108 | T1 | N1 | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.8 | Hypoxic | 1.538461538 | No | No |
| 109 | T0 | N1 | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.6 | Hypoxic | 1.357142857 | No | No |
| 110 | T2 | N2b | Tonsil | >10 pack-years | Positive | Cisplatin | Hypoxic | 2.428571429 | Negative | 1 | No | No |
| 111 | T1 | N2c | Tonsil | Never | Positive | Cisplatin | Hypoxic | 1.357142857 | Negative | 0.823529412 | No | No |
| 112 | T1 | N1 | BOT | Never | Close | Cisplatin | Hypoxic | 1.307692308 | Negative | 0.769230769 | No | No |
| 113 | T2 | N2b | BOT | >10 pack-years | Positive | Both | Hypoxic | 1.866666667 | Negative | 1 | No | No |
| 114 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.928571429 | Hypoxic | 2 | No | No |
| 115 | T2 | N1 | Tonsil | >10 pack-years | Positive | Cisplatin | Negative | 0.933333333 | NA | NA | No | No |
| 116 | T2 | N2b | Tonsil | <10 pack-years | Negative | Cisplatin | Hypoxic | 1.2 | Negative | 1 | No | No |
| 117 | T1 | N2b | BOT | >10 pack-years | Close | Cisplatin | Hypoxic | 1.727272727 | Negative | 1.176470588 | No | No |
| 118 | T1 | N2c | Tonsil | >10 pack-years | Close | Cisplatin | Negative | 0.75 | NA | NA | No | No |
| 119 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 2.714285714 | Negative | 1 | No | No |
| 120 | T2 | N2b | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.285714286 | Negative | 1.066666667 | No | No |
| 121 | T2 | N2b | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.470588235 | Negative | 1.105263158 | No | No |
| 122 | T1 | N2b | Tonsil | <10 pack-years | Close | Cisplatin | Negative | 0.888888889 | NA | NA | No | Yes |
| 123 | T2 | N2b | Tonsil | >10 pack-years | Close | Cisplatin | Hypoxic | 1.470588235 | Negative | 1.058823529 | No | No |
| 124 | T2 | N1 | Tonsil | Never | Positive | Cisplatin | Hypoxic | 1.333333333 | Hypoxic | 1.133333333 | No | No |
| 125 | T2 | N2c | BOT | Never | Positive | Both | Hypoxic | 2 | Negative | 0.941176471 | Yes | Yes |
| 126 | T2 | N2a | BOT | >10 pack-years | Positive | Cisplatin | Hypoxic | 2.214285714 | Negative | 1 | No | No |
| 127 | T2 | N2c | Tonsil | Never | Positive | Cisplatin | Hypoxic | 1.789473684 | Negative | 1.055555556 | No | No |
| 128 | T0 | N2b | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.2 | Negative | 1 | No | No |
| 129 | T1 | N2a | Tonsil | Never | Negative | Cisplatin | Hypoxic | 1.45 | Hypoxic | 1.777777778 | No | No |
| 130 | T1 | N2a | BOT | Never | Close | Cisplatin | Hypoxic | 2.066666667 | Negative | 0.875 | No | No |
| 131 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1 | Negative | 0.739130435 | No | No |
| 132 | T2 | N2b | BOT | Never | Close | Carbo/5FU | Negative | 1.142857143 | NA | NA | No | No |
| 133 | T2 | N2b | Tonsil | Never | Positive | Carbo/5FU | Hypoxic | 1.733333333 | Hypoxic | 1.307692308 | No | No |
| 134 | T1 | N2b | Tonsil | Never | Close | Cisplatin | Hypoxic | 1.625 | Negative | 1.307692308 | Yes | Yes |
| 135 | T0 | N2a | Unknown | >10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 3.428571429 | Hypoxic | 1.9375 | No | No |
| 136 | T2 | N2b | Tonsil | <10 pack-years | Close | Cisplatin | Hypoxic | 1.647058824 | Hypoxic | 1.176470588 | No | No |
| 137 | T1 | N2c | Tonsil | <10 pack-years | Positive | Cisplatin | Hypoxic | 2.944444444 | Negative | 0.9 | No | No |
| 138 | T2 | N2b | Tonsil | Never | Close | Cisplatin | Negative | 1.055555556 | NA | NA | No | No |
| 139 | T1 | N2b | Tonsil | Never | Positive | Cisplatin | Negative | 0.947368421 | NA | NA | No | No |
| 140 | T2 | N2c | Tonsil | Never | Close | Cisplatin | Hypoxic | 3.142857143 | Negative | 1 | No | No |
| 141 | T1 | N2b | Tonsil | <10 pack-years | Close | Carbo/5FU | Hypoxic | 1.285714286 | Negative | 0.933333333 | No | No |
| 142 | T1 | N1 | Tonsil | 10 pack-years | Negative | Cisplatin | Hypoxic | 1.428571429 | Negative | 1.125 | No | No |
| 143 | T1 | N2b | Tonsil | <10 pack-years | Close | Cisplatin | Hypoxic | 1.692307692 | Negative | 1.181818182 | No | No |
| 144 | T0 | N2c | Unknown | Never | NA—unknown primary | Cisplatin | Hypoxic | 1.117647059 | Negative | 1.066666667 | No | Yes |
| 145 | T2 | N2b | Tonsil | Never | Negative | Cisplatin | Hypoxic | 1.066666667 | Negative | 0.875 | No | No |
| 146 | T1 | N2a | BOT | Never | Close | Cisplatin | Hypoxic | 1.5 | Negative | 1 | No | No |
| 147 | T0 | N2c | Unknown | Never | NA—unknown primary | Cisplatin | Hypoxic | 1.8 | Negative | 1 | No | No |
| 148 | T1 | N2c | BOT | Never | Negative | Cisplatin | Hypoxic | 1.3125 | Negative | 1 | No | Yes |
| 149 | T1 | N2a | Tonsil | Never | Positive | Cisplatin | Negative | 1.105263158 | NA | NA | No | No |
| 150 | T1 | N2b | BOT | <10 pack-years | Negative | Cisplatin | Hypoxic | 1.866666667 | Hypoxic | 1.214285714 | No | No |
| 151 | T2 | N2c | BOT | Never | Negative | Cisplatin | Hypoxic | 1.1875 | Negative | 1.142857143 | No | No |
| 152 | T0 | N2c | Unknown | <10 pack-years | NA—unknown primary | Cisplatin | Hypoxic | 1.133333333 | Negative | 0.933333333 | No | No |
Abbreviations: 5FU, fluorouracil; BOT, base of tongue; FMISO PET, 18F-fluoromisonidazole positron emission tomography; N, nodal; NA, not available; T, tumor; TMR, tumor to muscle ratio.
Nancy Y. Lee
Consulting or Advisory Role: Merck, Merck Serono, Mirati Therapeutics, Elsie Pharmaceuticals, Galera Therapeutics, Nanobiotix, Regeneron
Speakers' Bureau: Varian Medical Systems, Yingming Consulting, Shanghai Joanne Medical Ltd
Eric J. Sherman
Consulting or Advisory Role: Eisai, UpToDate, Lilly, Blueprint Medicines, Exelixis, AffyImmune Therapeutics
Research Funding: Regeneron (Inst), Lilly (Inst), HUTCHMED (Inst), Novartis (Inst), Fore Biotherapeutics (Inst)
Bhuvanesh Singh
Employment: Memorial Sloan-Kettering Cancer Center, New York University (NYU)
Consulting or Advisory Role: CinR
Patents, Royalties, Other Intellectual Property: Methods and composition of inhibiting DCN1-UBC12 interaction (US patent, 9,447,156, September 20, 2016) (Inst), Methods and compositions of inhibiting DCN1-UBC12 interaction (United States Patent 10,525,048; January 7, 2020; United States Patent 11,116,757; September 14, 2021) (Inst)
Louise Cunningham
Employment: Memorial Sloan-Kettering Cancer Center
Bill H. Diplas
Patents, Royalties, Other Intellectual Property: Royalties for licensed cancer mutation detection platform
Brandon S. Imber
Honoraria: GT Medical Technologies
Consulting or Advisory Role: Ono Pharmaceutical, Telix Pharmaceuticals
Research Funding: AstraZeneca (Inst), Kazia Therapeutics (Inst), GT Medical Technologies (Inst)
Khoi Pham
Employment: Memorial Sloan-Kettering Cancer Center
Yao Yu
Research Funding: EMD Serono (Inst)
Patents, Royalties, Other Intellectual Property: Springer Author Royalties
Sean M. McBride
Consulting or Advisory Role: Janssen, AstraZeneca
Research Funding: Genentech, AstraZeneca
C. Jillian Tsai
Employment: Princess Margaret Cancer Centre
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Varian Medical Systems
Daphna Y. Gelblum
Research Funding: Merck/Schering Plow.
Jennifer R. Cracchiolo
Honoraria: Medscape
Luc G.T. Morris
Patents, Royalties, Other Intellectual Property: I am an inventor on IP owned by Memorial Sloan Kettering Cancer Center and licensed to PGDx
Lara A. Dunn
Consulting or Advisory Role: Merck
Research Funding: Regeneron, CUE Biopharma, Nektar, Replimune, Seagan
Loren S. Michel
Consulting or Advisory Role: KisoJi Biotechnology
Research Funding: Exelixis (Inst)
Uncompensated Relationships: AbbVie
David G. Pfister
Consulting or Advisory Role: MeiraGTx, Nykode Therapeutics
Research Funding: AstraZeneca (Inst), Novartis (Inst), MedImmune (Inst), Merck (Inst), Lilly (Inst), Bayer (Inst), Eisai (Inst), Regeneron (Inst), Atara Biotherapeutics (Inst), MeiraGTx (Inst), Hookipa Pharma (Inst)
Alan L. Ho
Stock and Other Ownership Interests: Rgenta
Honoraria: physicans' Education Resource, Endocrine Society (Clinical Endocrinology Update), Chinese American Hematology and Oncology Network
Consulting or Advisory Role: Eisai, Merck, Ayala Pharmaceuticals, Prelude Therapeutics, Kura Oncology, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia Biotech, InxMed, Remix Therapeutics, Elevar Therapeutics, ExpertConnect, Coherus Biosciences
Research Funding: Genentech/Roche, AstraZeneca, Bayer, Eisai, Bristol Myers Squibb, Astellas Pharma, Novartis, Merck, Ayala Pharmaceuticals, Elevar Therapeutics, Kura Oncology, OncC4, BioAtla, Poseida
Patents, Royalties, Other Intellectual Property: Lesional dosimetry methods for tailoring targeted radiotherapy in cancer (Serial number 63/193700, filed 5/27/21)
Simon N. Powell
Consulting or Advisory Role: Varian Medical Systems, Philips Healthcare, Rain Therapeutics, AstraZeneca
Research Funding: Philips Healthcare, Varian Medical Systems
Travel, Accommodations, Expenses: AstraZeneca
Bob T. Li
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Amgen (Inst), Lilly (Inst), MORE Health (Inst), Bolt Biotherapeutics (Inst), Ambrx (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst), Karger Publishers—Book royalty, Shanghai Jiao Tong University Press—Book royalty
Travel, Accommodations, Expenses: MORE Health, Jiangsu Hengrui Medicine, Amgen
Uncompensated Relationships: Amgen, AstraZeneca, Genentech, Lilly, Boehringer Ingelheim, Daiichi Sankyo
Jorge S. Reis-Filho
Leadership: Grupo Oncoclinicas
Stock and Other Ownership Interests: Repare Therapeutics, Paige.AI
Consulting or Advisory Role: Genentech/Roche, Invicro, Ventana Medical Systems, Volition RX, Paige.AI, Goldman Sachs, Novartis, Repare Therapeutics, Personalis, SAGA Diagnostics, Bain Capital Life Sciences
Luis A. Diaz
Leadership: Personal Genome Diagnostics, Jounce Therapeutics, Epitope Diagnostics
Stock and Other Ownership Interests: Personal Genome Diagnostics, Jounce Therapeutics, Zydecom, Thrive Earlier Detection Corp, Neophore, Amgen, Four Paws, Seer, Kinnate Biopharma, Delfi Diagnostics, Epitope Diagnostics
Consulting or Advisory Role: Merck, Personal Genome Diagnostics, Zydecom, Neophore, Four Paws, Seer, Kinnate Biopharma
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: US-2010041048-A1—Circulating Mutant DNA to Assess Tumor Dynamics, US-2015344970-A1—Personalized Tumor Biomarkers, WO-2010118016-A2—Digital quantification of DNA methylation, US-2005202465-A1—Thymidylate synthase gene and metastasis, US-2014227271-A1—Somatic mutations in atrx in brain cancer, WO-2012094401-A2—Genes frequently altered in pancreatic neuroendocrine tumors, US-2013323167-A1—Detecting and treating solid tumors through selective disruption of tumor vasculature, EP-2912468-B1—Papanicolaou test for ovarian and endometrial cancers, US-9976184-B2—Mutations in pancreatic neoplasms, US-2017267760-A1—Checkpoint Blockade and Microsatellite Instability, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018086832-A1—HLA-restricted epitopes encoded by somatically mutated genes, US-2018258490-A1—Assaying ovarian cyst fluid, US-2016208340-A1—TERT Promoter Mutations in Urothelial Neoplasia, US-2015252415-A1—Arid1b and neuroblastoma, WO-2018071796-A2—Compositions and methods for identifying functional anti-tumor T cell responses, EP-3322824-A1—Detection of tumor-derived DNA in cerebrospinal fluid, US-2016273049-A1—Systems and methods for analyzing nucleic acid (Inst), US-2018135044-A1—Non-unique barcodes in a genotyping assay (Inst), US-2017016075-A1—Neoantigen analysis (Inst)
Other Relationship: Innovatus Capital Partners, Blackstone
Open Payments Link: https://openpaymentsdata.cms.gov/physician/211856
Nadeem Riaz
Honoraria: PeerView
Consulting or Advisory Role: Mirati Therapeutics, Repare Therapeutics
Speakers' Bureau: Illumina
Research Funding: Bristol Myers Squibb, Pfizer, Repare Therapeutics, Paige.AI
Travel, Accommodations, Expenses: Varian Medical Systems
No other potential conflicts of interest were reported.
DISCLAIMER
National Institutes of Health/patient donations did not play any role in manuscript submission decision for publication nor data analysis.
PRIOR PRESENTATION
Presented at the ASCO annual meeting, Chicago, IL, June 4-8, 2021.
CLINICAL TRIAL INFORMATION
N.Y.L. and E.J.S. contributed equally to this work as cofirst authors.
AUTHOR CONTRIBUTIONS
Conception and design: Nancy Y. Lee, Eric J. Sherman, Rick Wray, Bhuvanesh Singh, Zhigang Zhang, Jatin P. Shah, Jennifer R. Cracchiolo, David G. Pfister, John L. Humm, Jorge S. Reis-Filho, Nadeem Riaz
Provision of study materials or patients: Eric J. Sherman, Jay O. Boyle, Louise Cunningham, Yao Yu, Sean M. McBride, Daphna Y. Gelblum, Jatin P. Shah, Luc G.T. Morris, James V. Fetten, David G. Pfister, Richard J. Wong, Nadeem Riaz
Collection and assembly of data: Nancy Y. Lee, Eric J. Sherman, Rick Wray, Bhuvanesh Singh, Milan Grkovski, Ramesh Paudyal, Louise Cunningham, Bill H. Diplas, James Han, Yao Yu, C. Jillian Tsai, Linda C. Chen, Daphna Y. Gelblum, Luc G.T. Morris, Lara A. Dunn, Loren S. Michel, James V. Fetten, David G. Pfister, Amita Shukla-Dave, Nadeem Riaz
Data analysis and interpretation: Nancy Y. Lee, Eric J. Sherman, HeiKo Schöder, Rick Wray, Jay O. Boyle, Bhuvanesh Singh, Milan Grkovski, Ramesh Paudyal, Louise Cunningham, Zhigang Zhang, Vaios Hatzoglou, Nora Katabi, Bill H. Diplas, James Han, Brandon S. Imber, Khoi Pham, Kaveh Zakeri, Sean M. McBride, C. Jillian Tsai, Linda C. Chen, Daphna Y. Gelblum, Ian Ganly, Marc A. Cohen, Jennifer R. Cracchiolo, Luc G.T. Morris, Lara A. Dunn, David G. Pfister, Alan L. Ho, Amita Shukla-Dave, John L. Humm, Simon N. Powell, Bob T. Li, Jorge S. Reis-Filho, Luis A. Diaz, Richard J. Wong, Nadeem Riaz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hypoxia-Directed Treatment of Human Papillomavirus–Related Oropharyngeal Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nancy Y. Lee
Consulting or Advisory Role: Merck, Merck Serono, Mirati Therapeutics, Elsie Pharmaceuticals, Galera Therapeutics, Nanobiotix, Regeneron
Speakers' Bureau: Varian Medical Systems, Yingming Consulting, Shanghai Joanne Medical Ltd
Eric J. Sherman
Consulting or Advisory Role: Eisai, UpToDate, Lilly, Blueprint Medicines, Exelixis, AffyImmune Therapeutics
Research Funding: Regeneron (Inst), Lilly (Inst), HUTCHMED (Inst), Novartis (Inst), Fore Biotherapeutics (Inst)
Bhuvanesh Singh
Employment: Memorial Sloan-Kettering Cancer Center, New York University (NYU)
Consulting or Advisory Role: CinR
Patents, Royalties, Other Intellectual Property: Methods and composition of inhibiting DCN1-UBC12 interaction (US patent, 9,447,156, September 20, 2016) (Inst), Methods and compositions of inhibiting DCN1-UBC12 interaction (United States Patent 10,525,048; January 7, 2020; United States Patent 11,116,757; September 14, 2021) (Inst)
Louise Cunningham
Employment: Memorial Sloan-Kettering Cancer Center
Bill H. Diplas
Patents, Royalties, Other Intellectual Property: Royalties for licensed cancer mutation detection platform
Brandon S. Imber
Honoraria: GT Medical Technologies
Consulting or Advisory Role: Ono Pharmaceutical, Telix Pharmaceuticals
Research Funding: AstraZeneca (Inst), Kazia Therapeutics (Inst), GT Medical Technologies (Inst)
Khoi Pham
Employment: Memorial Sloan-Kettering Cancer Center
Yao Yu
Research Funding: EMD Serono (Inst)
Patents, Royalties, Other Intellectual Property: Springer Author Royalties
Sean M. McBride
Consulting or Advisory Role: Janssen, AstraZeneca
Research Funding: Genentech, AstraZeneca
C. Jillian Tsai
Employment: Princess Margaret Cancer Centre
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Varian Medical Systems
Daphna Y. Gelblum
Research Funding: Merck/Schering Plow.
Jennifer R. Cracchiolo
Honoraria: Medscape
Luc G.T. Morris
Patents, Royalties, Other Intellectual Property: I am an inventor on IP owned by Memorial Sloan Kettering Cancer Center and licensed to PGDx
Lara A. Dunn
Consulting or Advisory Role: Merck
Research Funding: Regeneron, CUE Biopharma, Nektar, Replimune, Seagan
Loren S. Michel
Consulting or Advisory Role: KisoJi Biotechnology
Research Funding: Exelixis (Inst)
Uncompensated Relationships: AbbVie
David G. Pfister
Consulting or Advisory Role: MeiraGTx, Nykode Therapeutics
Research Funding: AstraZeneca (Inst), Novartis (Inst), MedImmune (Inst), Merck (Inst), Lilly (Inst), Bayer (Inst), Eisai (Inst), Regeneron (Inst), Atara Biotherapeutics (Inst), MeiraGTx (Inst), Hookipa Pharma (Inst)
Alan L. Ho
Stock and Other Ownership Interests: Rgenta
Honoraria: physicans' Education Resource, Endocrine Society (Clinical Endocrinology Update), Chinese American Hematology and Oncology Network
Consulting or Advisory Role: Eisai, Merck, Ayala Pharmaceuticals, Prelude Therapeutics, Kura Oncology, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia Biotech, InxMed, Remix Therapeutics, Elevar Therapeutics, ExpertConnect, Coherus Biosciences
Research Funding: Genentech/Roche, AstraZeneca, Bayer, Eisai, Bristol Myers Squibb, Astellas Pharma, Novartis, Merck, Ayala Pharmaceuticals, Elevar Therapeutics, Kura Oncology, OncC4, BioAtla, Poseida
Patents, Royalties, Other Intellectual Property: Lesional dosimetry methods for tailoring targeted radiotherapy in cancer (Serial number 63/193700, filed 5/27/21)
Simon N. Powell
Consulting or Advisory Role: Varian Medical Systems, Philips Healthcare, Rain Therapeutics, AstraZeneca
Research Funding: Philips Healthcare, Varian Medical Systems
Travel, Accommodations, Expenses: AstraZeneca
Bob T. Li
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Amgen (Inst), Lilly (Inst), MORE Health (Inst), Bolt Biotherapeutics (Inst), Ambrx (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst), Karger Publishers—Book royalty, Shanghai Jiao Tong University Press—Book royalty
Travel, Accommodations, Expenses: MORE Health, Jiangsu Hengrui Medicine, Amgen
Uncompensated Relationships: Amgen, AstraZeneca, Genentech, Lilly, Boehringer Ingelheim, Daiichi Sankyo
Jorge S. Reis-Filho
Leadership: Grupo Oncoclinicas
Stock and Other Ownership Interests: Repare Therapeutics, Paige.AI
Consulting or Advisory Role: Genentech/Roche, Invicro, Ventana Medical Systems, Volition RX, Paige.AI, Goldman Sachs, Novartis, Repare Therapeutics, Personalis, SAGA Diagnostics, Bain Capital Life Sciences
Luis A. Diaz
Leadership: Personal Genome Diagnostics, Jounce Therapeutics, Epitope Diagnostics
Stock and Other Ownership Interests: Personal Genome Diagnostics, Jounce Therapeutics, Zydecom, Thrive Earlier Detection Corp, Neophore, Amgen, Four Paws, Seer, Kinnate Biopharma, Delfi Diagnostics, Epitope Diagnostics
Consulting or Advisory Role: Merck, Personal Genome Diagnostics, Zydecom, Neophore, Four Paws, Seer, Kinnate Biopharma
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: US-2010041048-A1—Circulating Mutant DNA to Assess Tumor Dynamics, US-2015344970-A1—Personalized Tumor Biomarkers, WO-2010118016-A2—Digital quantification of DNA methylation, US-2005202465-A1—Thymidylate synthase gene and metastasis, US-2014227271-A1—Somatic mutations in atrx in brain cancer, WO-2012094401-A2—Genes frequently altered in pancreatic neuroendocrine tumors, US-2013323167-A1—Detecting and treating solid tumors through selective disruption of tumor vasculature, EP-2912468-B1—Papanicolaou test for ovarian and endometrial cancers, US-9976184-B2—Mutations in pancreatic neoplasms, US-2017267760-A1—Checkpoint Blockade and Microsatellite Instability, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018086832-A1—HLA-restricted epitopes encoded by somatically mutated genes, US-2018258490-A1—Assaying ovarian cyst fluid, US-2016208340-A1—TERT Promoter Mutations in Urothelial Neoplasia, US-2015252415-A1—Arid1b and neuroblastoma, WO-2018071796-A2—Compositions and methods for identifying functional anti-tumor T cell responses, EP-3322824-A1—Detection of tumor-derived DNA in cerebrospinal fluid, US-2016273049-A1—Systems and methods for analyzing nucleic acid (Inst), US-2018135044-A1—Non-unique barcodes in a genotyping assay (Inst), US-2017016075-A1—Neoantigen analysis (Inst)
Other Relationship: Innovatus Capital Partners, Blackstone
Open Payments Link: https://openpaymentsdata.cms.gov/physician/211856
Nadeem Riaz
Honoraria: PeerView
Consulting or Advisory Role: Mirati Therapeutics, Repare Therapeutics
Speakers' Bureau: Illumina
Research Funding: Bristol Myers Squibb, Pfizer, Repare Therapeutics, Paige.AI
Travel, Accommodations, Expenses: Varian Medical Systems
No other potential conflicts of interest were reported.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2. Caudell JJ, Gillison ML, Maghami E, et al. NCCN Guidelines Insights: Head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20:224–234. doi: 10.6004/jnccn.2022.0016. [DOI] [PubMed] [Google Scholar]
- 3. Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang JJ, Yu Y, Chen L, et al. Consensuses, controversies, and future directions in treatment deintensification for human papillomavirus-associated oropharyngeal cancer. CA Cancer J Clin. 2023;73:164–197. doi: 10.3322/caac.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rischin D, King M, Kenny L, et al. Randomized trial of radiation therapy with weekly cisplatin or cetuximab in low-risk HPV-associated oropharyngeal cancer (TROG 12.01)—A Trans-Tasman Radiation Oncology Group study. Int J Radiat Oncol Biol Phys. 2021;111:876–886. doi: 10.1016/j.ijrobp.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 7. Chera BS, Amdur RJ, Green R, et al. Phase II trial of de-intensified chemoradiotherapy for human papillomavirus-associated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2019;37:2661–2669. doi: 10.1200/JCO.19.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferris RL, Flamand Y, Weinstein GS, et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: An ECOG-ACRIN Cancer Research Group trial (E3311) J Clin Oncol. 2022;40:138–149. doi: 10.1200/JCO.21.01752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002) J Clin Oncol. 2021;39:956–965. doi: 10.1200/JCO.20.03128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma DM, Price K, Moore EJ, et al. MC1675, a phase III evaluation of de-escalated adjuvant radiation therapy (DART) vs. standard adjuvant treatment for human papillomavirus associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2021;111:1324. [Google Scholar]
- 11. Palma DA, Prisman E, Berthelet E, et al. Assessment of toxic effects and survival in treatment deescalation with radiotherapy vs transoral surgery for HPV-associated oropharyngeal squamous cell carcinoma: The ORATOR2 phase 2 randomized clinical trial. JAMA Oncol. 2022;8:1–7. doi: 10.1001/jamaoncol.2022.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.nrgoncology.org/Clinical-Trials/Protocol/nrg-hn005?filter=nrg-hn005 NRG Oncology: NRG-HN005: A randomized phase II/III trial of de-intensified radiation therapy for patients with early-stage, p16-positive, non-smoking associated oropharyngeal cancer.
- 13. Chen AM, Felix C, Wang PC, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017;18:803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swisher-McClure S, Lukens JN, Aggarwal C, et al. A phase 2 trial of alternative volumes of oropharyngeal irradiation for de-intensification (AVOID): Omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus-related squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 2020;106:725–732. doi: 10.1016/j.ijrobp.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 15. Busk M, Overgaard J, Horsman MR. Imaging of tumor hypoxia for radiotherapy: Current status and future directions. Semin Nucl Med. 2020;50:562–583. doi: 10.1053/j.semnuclmed.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 16. Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 17. Sørensen BS, Busk M, Olthof N, et al. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother Oncol. 2013;108:500–505. doi: 10.1016/j.radonc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 18. Thorwarth D, Welz S, Mönnich D, et al. Prospective evaluation of a tumor control probability model based on dynamic (18)F-FMISO PET for head and neck cancer radiotherapy. J Nucl Med. 2019;60:1698–1704. doi: 10.2967/jnumed.119.227744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wegge M, Dok R, Nuyts S. Hypoxia and its influence on radiotherapy response of HPV-positive and HPV-negative head and neck cancer. Cancers (Basel) 2021;13:5959. doi: 10.3390/cancers13235959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nehmeh SA, Lee NY, Schröder H, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:235–242. doi: 10.1016/j.ijrobp.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 22. Rajendran JG, Schwartz DL, O'Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trinkaus ME, Hicks RJ, Young RJ, et al. Correlation of p16 status, hypoxic imaging using [18F]-misonidazole positron emission tomography and outcome in patients with loco-regionally advanced head and neck cancer. J Med Imaging Radiat Oncol. 2014;58:89–97. doi: 10.1111/1754-9485.12155. [DOI] [PubMed] [Google Scholar]
- 24. Swartz JE, Pothen AJ, van Kempen PM, et al. Poor prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinomas that overexpress hypoxia inducible factor-1α. Head Neck. 2016;38:1338–1346. doi: 10.1002/hed.24445. [DOI] [PubMed] [Google Scholar]
- 25. Zschaeck S, Löck S, Hofheinz F, et al. Individual patient data meta-analysis of FMISO and FAZA hypoxia PET scans from head and neck cancer patients undergoing definitive radio-chemotherapy. Radiother Oncol. 2020;149:189–196. doi: 10.1016/j.radonc.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 26. Riaz N, Sherman E, Pei X, et al. Precision radiotherapy: Reduction in radiation for oropharyngeal cancer in the 30 ROC trial. J Natl Cancer Inst. 2021;113:742–751. doi: 10.1093/jnci/djaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nigro ND, Seydel HG, Considine B, et al. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–1829. doi: 10.1002/1097-0142(19830515)51:10<1826::aid-cncr2820511012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28. Wray R, Mauguen A, Michaud L, et al. Development and validation of diagnostic interpretation criteria for evaluation of F-18 fluoromisonidazole hypoxia PET/CT—Inter-reader reliability and accuracy. Int J Radiat Oncol Biol Phys. 2022;114:e324. [Google Scholar]
- 29. Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: The M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–876. [PubMed] [Google Scholar]
- 30. Ho KF, Farnell DJ, Routledge JA, et al. Comparison of patient-reported late treatment toxicity (LENT-SOMA) with quality of life (EORTC QLQ-C30 and QLQ-H&N35) assessment after head and neck radiotherapy. Radiother Oncol. 2010;97:270–275. doi: 10.1016/j.radonc.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 31. Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68:153–165. doi: 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chera BS, Kumar S, Beaty BT, et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res. 2019;25:4682–4690. doi: 10.1158/1078-0432.CCR-19-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung E, Han K, Zou J, et al. HPV sequencing facilitates ultrasensitive detection of HPV circulating tumor DNA. Clin Cancer Res. 2021;27:5857–5868. doi: 10.1158/1078-0432.CCR-19-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall WA, Bergom C, Thompson RF, et al. Precision Oncology and genomically guided radiation therapy: A report from the American Society for Radiation Oncology/American Association of Physicists in Medicine/National Cancer Institute Precision Medicine Conference. Int J Radiat Oncol Biol Phys. 2018;101:274–284. doi: 10.1016/j.ijrobp.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 35. Rieckmann T, Tribius S, Grob TJ, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242–246. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 36. Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eiber M, Herrmann K, Calais J, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. doi: 10.2967/jnumed.117.198119. [DOI] [PubMed] [Google Scholar]
- 39. Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 41. Kamal M, Mohamed ASR, Volpe S, et al. Radiotherapy dose–volume parameters predict videofluoroscopy-detected dysphagia per DIGEST after IMRT for oropharyngeal cancer: Results of a prospective registry. Radiother Oncol. 2018;128:442–451. doi: 10.1016/j.radonc.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gharzai LA, Li P, Schipper MJ, et al. Characterization of very late dysphagia after chemoradiation for oropharyngeal squamous cell carcinoma. Oral Oncol. 2020;111:104853. doi: 10.1016/j.oraloncology.2020.104853. [DOI] [PubMed] [Google Scholar]
- 43. Wong KH, Panek R, Dunlop A, et al. Changes in multimodality functional imaging parameters early during chemoradiation predict treatment response in patients with locally advanced head and neck cancer. Eur J Nucl Med Mol Imaging. 2018;45:759–767. doi: 10.1007/s00259-017-3890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shukla-Dave A, Lee NY, Jansen JF, et al. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol Biol Phys. 2012;82:1837–1844. doi: 10.1016/j.ijrobp.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joint Head and Neck Radiotherapy-MRI Development Cooperative. Mohamed ASR, Abusaif A, et al. Prospective validation of diffusion-weighted MRI as a biomarker of tumor response and oncologic outcomes in head and neck cancer: Results from an observational biomarker pre-qualification study. Radiother Oncol. 2023;183:109641. doi: 10.1016/j.radonc.2023.109641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gharzai LA, Rosen BS, Mittal B, et al. Magnetic resonance guided radiotherapy for head and neck cancers. J Clin Med. 2022;11:1388. doi: 10.3390/jcm11051388. [DOI] [PMC free article] [PubMed] [Google Scholar]



