Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned coprimary or secondary analyses are not yet available. Clinical trial updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
We report the long-term results of the frontline trial with dasatinib and blinatumomab in induction/consolidation (GIMEMA LAL2116, D-ALBA) for adult Philadelphia-positive ALL (Ph+ ALL), which enrolled 63 patients of all ages. At a median follow-up of 53 months, disease-free survival, overall survival, and event-free survival are 75.8%, 80.7%, and 74.6%, respectively. No events have occurred among early molecular responders. A significantly worse outcome was recorded for IKZF1plus patients. Twenty-nine patients—93.1% being in molecular response (ie, complete molecular response or positive nonquantifiable) after dasatinib/blinatumomab—never received chemotherapy/transplant and continued with a tyrosine kinase inhibitor only; 28 patients remain in long-term complete hematologic response (CHR). An allogeneic transplant was carried out in first CHR mainly in patients with persistent minimal residual disease; 83.3% of patients are in continuous CHR. The transplant-related mortality was 12.5% for patients transplanted in first CHR and 13.7% overall. Nine relapses and six deaths have occurred. ABL1 mutations were found in seven cases. The final analysis of the D-ALBA study shows that a chemotherapy-free induction/consolidation regimen on the basis of a targeted strategy (dasatinib) and immunotherapy (blinatumomab) is effective in inducing durable long-term hematologic and molecular responses in adult Ph+ ALL, paving the way for a new era in the management of these patients.
INTRODUCTION
In Philadelphia-positive ALL (Ph+ ALL), tyrosine kinase inhibitors (TKIs) with or without chemotherapy, generally followed by an allogenic stem cell transplant (SCT), have led to survival rates approaching 50% at 1-5 years.1–15 Since 2000, the GIMEMA group pioneered a chemotherapy-free induction strategy for patients with newly diagnosed adult Ph+ ALL.16–20 This approach led to complete hematologic responses (CHRs) in 94%-100% of cases and virtually no deaths in induction, irrespective of age. A further advancement has come by adding blinatumomab. In the D-ALBA (GIMEMA LAL2116, ClinicalTrials.gov identifier: NCT02744768) frontline protocol for adult Ph+ ALL with no upper age limit, dasatinib plus steroids was administered in induction followed by at least two blinatumomab cycles as consolidation.21–23 The initial report21 showed 60% of molecular responses (52% by intention to treat) at the primary end point (after two cycles of blinatumomab) and an overall survival (OS) and disease-free survival (DFS) of 95% and 88%, respectively, at 18 months. Postconsolidation treatment was left to the investigators' choice (Data Supplement, Fig S1 [online only]). We report the long-term follow-up of the study.
PATIENTS AND METHODS
The treatment received after blinatumomab consolidation and the long-term follow-up were collected in the GIMEMA LAL2217 (ClinicalTrials.gov identifier: NCT03318770) ancillary trial after patients signed a written informed consent. The trial was approved by the Sapienza University Institutional Review Board. Demographics of the 63 patients enrolled (median age 54 years, 24-82), genomic characterization, response criteria, minimal residual disease (MRD) determination, and statistical analyses are detailed in the Data Supplement.
RESULTS
Molecular Responses and Survival
Molecular responses increased over time, being 75%, 77%, 70.1%, 85%, 90%, and 93% at 2, 4, 6, 8, 10, and 12 months, respectively, from the last blinatumomab cycle. The percentage of patients with molecular responses increased further at a later follow-up, with the caution that molecular findings were often lacking for allografted patients or those who were lost to follow-up (not shown).
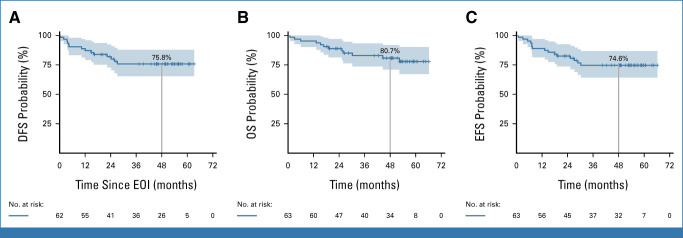
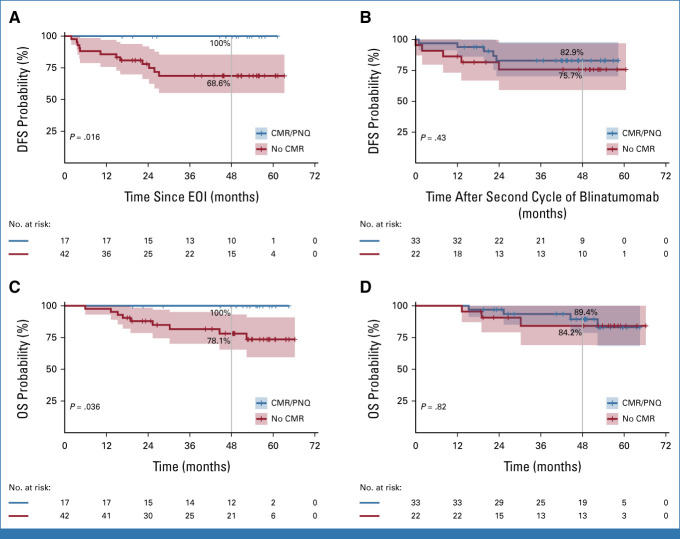
At a median follow-up of 53 months, DFS, OS, and event-free survival (EFS) were 75.8%, 80.7% and 74.6%, respectively (Fig 1). A significantly better DFS was observed in the 17 molecular responders at the end of induction (EOI; 100%) versus nonmolecular responders (n = 42; 68.6%; P = .018). After two blinatumomab cycles, the difference in DFS and OS was no longer significant between molecular responders (n = 33) and MRD+ patients (n = 22): 82.9% versus 75.7% and 89.4% versus 84.2%, respectively (Fig 2).
FIG 1.
(A) DFS, (B) OS, and (C) EFS calculated from the EOI (d +85) and diagnosis/treatment initiation. The shadings of each curve show the 95% CI at a median follow-up of 53 months. DFS, disease-free survival; EFS, event-free survival; EOI, end of induction; OS, overall survival.
FIG 2.
(A and B) DFS and (C and D) OS according to molecular response. DFS was calculated from (A) EOI (d +85) and after the (B) second cycle of blinatumomab (primary end point); OS was calculated from diagnosis/treatment initiation stratifying patients according to molecular response at the (C) EOI (d +85) and (D) after the second cycle of blinatumomab. Blue line: molecular responders; red line: nonmolecular responders. The shadings of each curve show the 95% CI. CMR, complete molecular response; DFS, disease-free survival; EOI, end of induction; OS, overall survival; PNQ, positive nonquantifiable.
A significantly worse DFS was recorded for IKZF1plus patients (n = 11, 45.5%) compared with those without IKZF1 deletion (n = 25, 82.3%) or with IKZF1 only deletions (n = 13, 74%; P = .029). OS results were similar (Data Supplement, Fig S2; Table S1).
Postconsolidation Treatment
Twenty-nine patients (median age 58 years, 30-70) neither received chemotherapy nor transplant and remained on TKI treatment. Twenty-seven patients (93.1%) had obtained a molecular response (ie, complete molecular response or positive nonquantifiable) after dasatinib/blinatumomab, 20 after two blinatumomab cycles, and seven after further blinatumomab cycles. Twenty-eight patients (96.5%) remain in persistent CHR at a median follow-up of 48 months (22-64). Further details are given in the Data Supplement (Fig S1).
Twenty-four patients were allografted in first CHR (median age 51.5 years, 24-67; Data Supplement, Fig S3). Thirteen patients (54.2%) were nonmolecular responders at the primary end point; only two became molecularly negative after further blinatumomab cycles. One patient relapsed 1 month after SCT, and three died of complications. The remaining 20 patients (83.3%) are alive in CHR at a median follow-up of 49 (17-66) months.
Six patients were transplanted in second CHR; three died while three are alive in second CHR at 30, 40, and 52 months, respectively, from the second CHR.
The transplant-related mortality (TRM) in first CHR was 12.5% and 13.7% overall.
Relapses, Deaths, and Toxicity
Overall, nine relapses occurred—four hematologic, four involving the CNS, and one nodal (Table 1)—at a median of 4.4 (1.9-25.8) months. Three patients are alive after salvage treatment and SCT. The IKZF1plus signature was present at diagnosis in four of eight evaluable cases; ABL1 mutations, tested at relapse or at a concomitant MRD increase in extra-hematologic recurrences, were found in seven patients (six T3151I and one E255K). Four patients harbored both the IKZF1plus signature and T315I mutation. Although relapses occurred at a similar rate in p190 and p210 cases (14.6% and 13.6%), time to recurrence was shorter in p190 versus p210 cases (3.9 v 24.3 months). Five deaths occurred in first CHR (one pneumonia during induction, one endocarditis in a mechanical aortic valve carrier, and three after SCT). Toxicity is reported in the Data Supplement (Table S2).
TABLE 1.
Clinical and Biological Features of Relapsed Patients
| Age, Years | WBC (109/L)a | Type of Relapse | Fusion Type | IKZF1 | Mutation | Time From CHR to Relapse, ms | Allo-SCT | Last Follow-Up | Previously Reported in Puzzolo et al24 |
|---|---|---|---|---|---|---|---|---|---|
| 71 | 157.9 | Hematologic | p190 | IKZF1 plus | T315I | 1.9 | No | Dead | Yes |
| 52 | 0.7 | Hematologic | p190 | ND | T315I | 3.6 | Second CHR | Dead | Yes |
| 54 | 11.2 | Nodal | p190 | IKZF1 plus | T315I | 3.6 | Second CHR | Dead | Yes |
| 30 | 35.9 | CNS + molecular | p190 | IKZF1 loss | E255K | 4.2 | Second CHR | Alive | Yes |
| 40 | 10.5 | CNS + molecular | p190 | IKZF1 plus | T315I | 4 | Second CHR | Alive | Yes |
| 81 | 4.9 | Hematologic | p190 | No | T315I | 12 | No | Dead | Yes |
| 53 | 7.2 | CNS + molecular | p210 | No | NE | 24.3 | Second CHR | Dead | No |
| 45 | 23.9 | CNS + molecular | p210 | IKZF1 loss | wt | 25.8 | Second CHR | Alive | No |
| 67 | 2.9 | Hematologic | p210 | IKZF1 plus | T315I | 14.9 | First CHR | Dead | No |
Abbreviations: CHR, complete hematologic response; ND, not done; NE, not evaluable; SCT, allogenic stem cell transplant; wt, wild-type.
At diagnosis.
DISCUSSION
Our group showed for the first time the advantage of treating adult/elderly Ph+ ALL frontline with dasatinib followed by blinatumomab.21–23 Similar results were recently reported combining ponatinib and blinatumomab.25 The final analysis of the D-ALBA study shows that at a median follow-up of 53 months DFS and OS are 75.8% and 80.7%, respectively, which is significantly better than in our previous dasatinib-based trial (Data Supplement, Figs S4-S5).18 DFS is 100% in molecular responders at the EOI, confirming the prognostic role of an early molecular response in a chemotherapy-free setting.26 After two blinatumomab cycles, no significant long-term survival differences between molecular and nonmolecular responders were found, indicating that blinatumomab is effective in preventing a relapse also in patients with a suboptimal early molecular response, possibly also through a host immune modulation.21,24
At a prolonged follow-up, IKZF1plus patients continue to show a significantly worse outcome (Data Supplement, Fig S2), particularly when associated with T315I mutations.
Half of our patients remained on treatment only with a TKI; most (93.3%) were in molecular response after dasatinib/blinatumomab and 96.5% are in CHR at 4 years. Instead, most patients transplanted in first CHR were MRD+, and 83.3% are alive in CHR (median follow-up 49 months). Although the trial was not designed to define the role of SCT, a clear benefit was seen in MRD+ patients while virtually all MRD– patients remain in long-term CHR without SCT. At a prolonged follow-up, we confirm the low TRM, likely due to the lack of systemic chemotherapy and good general conditions of patients undergoing transplant.
These long-term results compare favorably with more intensive treatments combining ponatinib and hyper-cyclophosphamide, vincristine, doxorubicin,27 with an estimated 6-year OS of 75% and EFS of 65% in a younger population (median 46 years) and with the PONALFIL trial,28 where younger patients (18-60 years) received ponatinib, chemotherapy, and transplant, with a 3-year OS and EFS of 96% and 70%, respectively.
Few relapses occurred (9): four had an IKZF1plus signature at presentation and developed a T3151 mutation during treatment, confirming our previous suggestion that IKZF1plus might lead to an increased genomic instability. It appears that IKZF1plus, if not coupled with the T315I mutation, may not per se induce a relapse. This has prompted to investigate T315I mutations by digital droplet polymerase chain reaction.29 T315I mutations are a detrimental event, whose effect should be abrogated by using ponatinib, as explored by the PETHEMA28 and GIMEMA20 groups and the MD Anderson Cancer Center.25 These trials did not report T315I mutations, except for one case.20 Further results will come from the ongoing GIMEMA ALL2820 protocol, where ponatinib is used in place of dasatinib in the experimental arm. An emerging issue is represented by CNS relapses, recorded in four of nine cases. Although it is reassuring that three patients were salvaged by alternative treatments followed by transplant, a signature predictive of a CNS localization/recurrence is being investigated.30
The role of p190 and p210 has been widely discussed: p210 cases generally show a slower MRD clearance, but this does ultimately not affect outcome. Although the noninferior outcome is confirmed in our study (not shown), in p210 cases, relapses occurred at later time points.
The treatment was also well tolerated in the long-term follow-up, with only six patients requiring a TKI switch: Pleural effusion was the most common adverse events.
Taken together, the final analysis of the D-ALBA study (1) confirms the very high DFS and OS rates; (2) documents that early molecular responders are likely not to relapse; (3) shows that patients can remain in sustained long-term CHR without systemic chemotherapy and transplant; (4) shows that the protocol is associated with few relapses, predominantly in cases with an IKZF1plus signature and T315I mutations; (5) points to the beneficial effect of transplant in MRD+ patients; and (6) confirms the low TRM in allografted patients. These results support the design of the ongoing GIMEMA ALL2820 trial where dasatinib is substituted by ponatinib, CNS prophylaxis has been strengthened, MRD has been further refined, and transplant allocation relies on the genetic profile at diagnosis and by the depth/persistence of molecular response.
ACKNOWLEDGMENT
The authors wish to thank Associazione Italiana per la Ricerca sul Cancro (AIRC) 5x1000, Special Program Metastases (21198), Milan (Italy) to RF, Progetti di Rilevante Interesse Nazionale (PRIN) Italia, 2017PPS2X4 project, to S.C., Finanziamento Medi Progetti Universitari 2021 (Sapienza University of Rome) to S.C.
Robin Foà
Consulting or Advisory Role: Autolus Therapeutics
Speakers' Bureau: Amgen, Novartis, Janssen
Renato Bassan
Honoraria: Amgen
Consulting or Advisory Role: Amgen
Alfonso Piciocchi
Travel, Accommodations, Expenses: Abbvie
Antonino Mulè
Consulting or Advisory Role: Abbvie, Pfizer Hellas, Incyte
Massimiliano Bonifacio
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Novartis Italy, Clinigen Healthcare, Incyte, Amgen
Research Funding: Novartis
Nicola Fracchiolla
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals, incyte, Abbvie
Travel, Accommodations, Expenses: Pfizer
Alessandro Rambaldi
Consulting or Advisory Role: Amgen, Omeros, Novartis, Astellas Pharma, Jazz Pharmaceuticals, Roche, Abbvie, Janssen, Pfizer, Incyte, Kite/Gilead
Sabina Chiaretti
Consulting or Advisory Role: Incyte, Gilead Sciences, Amgen, Abbvie
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the at the European Hematology Association meeting, Vienna, Austria, June 9-12, 2022.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Alfonso Piciocchi, Alessandro Rambaldi, Sabina Chiaretti, Renato Bassan, Robin Foà
Provision of study materials or patients: Renato Bassan, Stefano Soddu, Felicetto Ferrara, Antonino Mulè, Massimiliano Bonifacio, Nicola Fracchiolla, Prassede Salutari, Alessandro Rambaldi, Sabina Chiaretti, Robin Foà
Collection and assembly of data: Renato Bassan, Loredana Elia, Felicetto Ferrara, Monia Lunghi, Antonino Mulè, Massimiliano Bonifacio, Nicola Fracchiolla, Prassede Salutari, Anna Guarini, Alessandro Rambaldi, Sabina Chiaretti, Robin Foà, Paola Fazi
Data analysis and interpretation: Alfonso Piciocchi, Stefano Soddu, Monica Messina, Sabina Chiaretti, Robin Foà
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robin Foà
Consulting or Advisory Role: Autolus Therapeutics
Speakers' Bureau: Amgen, Novartis, Janssen
Renato Bassan
Honoraria: Amgen
Consulting or Advisory Role: Amgen
Alfonso Piciocchi
Travel, Accommodations, Expenses: Abbvie
Antonino Mulè
Consulting or Advisory Role: Abbvie, Pfizer Hellas, Incyte
Massimiliano Bonifacio
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Novartis Italy, Clinigen Healthcare, Incyte, Amgen
Research Funding: Novartis
Nicola Fracchiolla
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals, incyte, Abbvie
Travel, Accommodations, Expenses: Pfizer
Alessandro Rambaldi
Consulting or Advisory Role: Amgen, Omeros, Novartis, Astellas Pharma, Jazz Pharmaceuticals, Roche, Abbvie, Janssen, Pfizer, Incyte, Kite/Gilead
Sabina Chiaretti
Consulting or Advisory Role: Incyte, Gilead Sciences, Amgen, Abbvie
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wassmann B, Pfeifer H, Goekbuget N, et al. : Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 108:1469-1477, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Delannoy A, Delabesse E, Lheritier V, et al. : Imatinib and methylprednisolone alternated with chemotherapy improve the outcome of elderly patients with Philadelphia-positive acute lymphoblastic leukemia: Results of the GRAALL AFR09 study. Leukemia 20:1526-1532, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Yanada M, Takeuchi J, Sugiura I, et al. : High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: A phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol 24:460-466, 2006 [DOI] [PubMed] [Google Scholar]
- 4.de Labarthe A, Rousselot P, Huguet-Rigal F, et al. : Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: Results of the GRAAPH-2003 study. Blood 109:1408-1413, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Ottmann OG, Wassmann B, Pfeifer H, et al. : For the GMALL Study Group: Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Cancer 2007:109:2068-2076 [DOI] [PubMed] [Google Scholar]
- 6.Gruber F, Mustjoki S, Porkka K: Impact of tyrosine kinase inhibitors on patient outcomes in Philadelphia chromosome-positive acute lymphoblastic leukaemia. Br J Haematol 145:581-597, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Ribera JM, Oriol A, Gonzalez M, et al. : Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica 95:87-95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassan R, Rossi G, Pogliani EM, et al. : Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol 28:3644-3652, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Ravandi F, O'Brien S, Thomas DA, et al. : First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood 116:2070-2077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thyagu S, Minden MD, Gupta V, et al. : Treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia with imatinib combined with a paediatric-based protocol. Br J Haematol 158:506-514, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. : Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: A GRAALL study. Biol Blood Marrow Transplant 19:150-155, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Fielding AK, Rowe JM, Buck G, et al. : UKALLXII/ECOG2993: Addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood 123:843-850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D-Y, Joo YD, Lim SN, et al. : Nilotinib combined with multi-agent chemotherapy for adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia: Interim results of Korean Adult ALL Working Party Phase 2 Study. Blood 126:746-756, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Yhim HY, Kwak JY, et al. : Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol 27:1081-1088, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Rousselot P, Coudé MM, Gokbuget N, et al. ; European Working Group on Adult ALL (EWALL) group: Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood 128:774-782, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vignetti M, Fazi P, Cimino G, et al. : Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: Results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood 109:3676-3678, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Foà R, Vitale A, Vignetti M, et al. : Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 118:6521-6528, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Chiaretti S, Vitale A, Vignetti M, et al. : A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: Final results of the GIMEMA LAL 0904 study. Haematologica 101:1544-1552, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiaretti S, Ansuinelli M, Vitale A, et al. : A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: Final results of the GIMEMA LAL1509 protocol. Haematologica 106:1828-1838, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinelli G, Papayannidis C, Piciocchi A, et al. : INCB84344-201: Ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv 6:1742-1753, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foà R, Bassan R, Vitale A, et al. : Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med 383:1613-1623, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Foà R, Chiaretti S: Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med 386:2399-2411, 2022 [DOI] [PubMed] [Google Scholar]
- 23.Chiaretti S, Bassan R, Vitale A, et al. : Forty Months Update of the GIMEMA LAL2116 (D-ALBA) Protocol and Ancillary LAL2217 Study for Newly Diagnosed Adult Ph+ ALL. EHA meeting, Vienna: June 9-12, 2022, P353 [Google Scholar]
- 24.Puzzolo MC, Radice G, Peragine N, et al. : Host immune system modulation in Ph+ acute lymphoblastic leukemia patients treated with dasatinib and blinatumomab. Blood 138:2290-2293, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Jabbour E, Short NJ, Jain N, et al. : Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: A US, single-centre, single-arm, phase 2 trial. Lancet Haematol 10:e24-e34, 2023 [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Kim DW, Cho BS, et al. : Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 26:2367-2374, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Short NJ, Jain N, et al. : Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am J Hematol 98:493-501, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribera JM, García-Calduch O, Ribera J, et al. : Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv 6:5395-5402, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardinali D, Beldinanzi M, Ansuinelli M, et al. : Digital droplet PCR for T315I BCR::ABL1 KD mutation assessment in adult Ph-positive acute lymphoblastic leukemia with a minimal residual disease increase. Leuk Lymphoma 64:1884-1887, 2023 [DOI] [PubMed] [Google Scholar]
- 30.Sapienza MR, Chiaretti S, Cardinali D, et al. : A five-gene signature may associate with central nervous system dissemination in adult acute lymphoblastic leukemia. Hematol Oncol 41:789-791, 2023 [DOI] [PubMed] [Google Scholar]