Abstract
Hematological cancers include leukemia, myeloma and lymphoma and up to 178.000 new cases are diagnosed with these tumors each year. Different kinds of treatment including radiotherapy, chemotherapy, immunotherapy and stem cell transplantation have been employed in the therapy of hematological cancers. However, they are still causing death among patients. On the other hand, curcumin as an anti-cancer agent for the suppression of human cancers has been introduced. The treatment of hematological cancers using curcumin has been followed. Curcumin diminishes viability and survival rate of leukemia, myeloma and lymphoma cells. Curcumin stimulates apoptosis and G2/M arrest to impair progression of tumor. Curcumin decreases levels of matrix metalloproteinases in suppressing cancer metastasis. A number of downstream targets including VEGF, Akt and STAT3 undergo suppression by curcumin in suppressing progression of hematological cancers. Curcumin stimulates DNA damage and reduces resistance of cancer cells to irradiation. Furthermore, curcumin causes drug sensitivity of hematological tumors, especially myeloma. For targeted delivery of curcumin and improving its pharmacokinetic and anti-cancer features, nanostructures containing curcumin and other anti-cancer agents have been developed.
Keywords: Curcumin, Hematological cancers, Nanoparticles, Targeted delivery, Molecular pathway
Graphical abstract
Abbreviations
- MMm
Multiple myeloma
- AML
Acute myeloid leukemia
- allo-SCT
Allogeneic stem cell transplant
- HL
Hodgkin's lymphoma
- NHL
Non-Hodgkin's lymphoma
- ROS
Reactive oxygen species
- HSP90
Heat shock protein 90
- mRNA
Messenger RNA
- lncRNAs
Long non-coding RNAs
- HHT
Homoharringtonine
- SLNs
Solid lipid nanoparticles
- P-gp
P-glycoprotein
1. Introduction
Annually, a high number of people with hematological malignancies are diagnosed and based on estimates, this number is close to 178 000 new cases for lymphoma, leukemia and multiple myeloma (MM).1 Compared to other kinds of cancers, the patients survived from hematological malignancies have been higher, showing improvement in this case. In respect to progresses in field of biology that has revealed more factors involved in progression of hematological malignancies, the treatment approaches for these cancers are altering.1 The acute myeloid leukemia (AML) is one of the most common hematological malignancies around the world that in recent years, we have witnessed a remarkable advance in the treatment of patients with AML and providing better prognosis because of understanding biology and pathophysiology of this disease.2, 3, 4, 5, 6 In the late 1970s, a gold standard regimen including 3 days of daunorubicin and 7 days of cytarabine was used for therapy of AML and this regimen provided 5-year survival of 30–35 % for young people and 10–15 % for elder patients.6 AML is considered as a clonal disease in which immature myeloid cells in the bone marrow undergo rapid proliferation and their differentiation status is not completed.7 Although intensive chemotherapy has been utilized for AML treatment, the relapse of disease commonly occurs that results in death in many patients. There is no treatment for patients undergoing relapse; however, allogeneic stem cell transplant (allo-SCT) may be used and in some patients, it has demonstrated promising results. As it was mentioned, the prognosis of AML is elder patients is undesirable that is maybe due to poor risk cytogenetics and abnormal changes at molecular level as well as presence of concurrent comorbidities.5
Another kind of hematological malignancies is lymphoma that is categorized into two distinct classes including HL and NHL based on pathological profile. It has been reported that lymphoma accounts for 3.2 % of all new cases of tumor around the world and most of the cases of NHL (87.5 %) rather than HL (12.5 %).8,9 The chemotherapy is also used for treatment of lymphoma and it is different in HL and NHL. The anti-cancer agents including doxorubicin, bleomycin, vinblastine and dacarbazine are extensively applied in treatment of HL, while vincristine, cyclophosphamide and prednisone are employed for treatment of NHL. The high-dose chemotherapy and stem cell transplantation are also options for lymphoma therapy. In spite of use of aforementioned therapies for lymphoma patients, the mortality rate of this malignant disease has been stable around the world.10,11
The abnormal growth of neoplastic plasma cells is considered as the factor responsible for development of MM.12,13 Based on the estimates, MM is the second most common blood cancer and its incidence rate is different based on the region. For instance, up to 140 000 new cases are annually diagnosed worldwide that 1876 cases are related to Australia.14 In spite of significant effort in developing novel therapeutic strategies for MM, its 10-year survival rate is still as low as 17 %.15 The malignant transformation of plasma cells for development of MM emanates from genetic and epigenetic alterations. Then, abnormal proliferation of cells in bone marrow occurs and high amount of non-functional monoclonal antibody releases into bloodstream.16,17 The hypercalcaemia, renal dysfunction and osteolytic bone lesions are a number of clinical manifestations of MM. Recently, proteasome inhibitors and immmunomodlatory drugs have been employed for treatment of MM to improve prognosis of patients.18 However, MM treatment is still a challenge for physicians.
In recent years, the main focus of the papers has been on the solid tumors. However, hematological tumors are considered as main reasons of death among patients. There is a need for an updated review to investigate the curcumin-mediated suppression of hematological cancers that is purpose of current review. Here, it is discussed that curcumin intereferes hematological cancer progression based on regulation of biological events and molecular pathways. The main mechanisms including apoptosis, cell cycle arrest, autophagy and drug efflux transporters, among others are evaluated. Moreover, the regulation of molecular pathways including MEK/ERK, STAT3 and Akt, among others by curcumin is shown. The major hallmarks of hematological tumors including proliferation, metastasis and angiogenesis can be suppressed by curcumin. To this end, we first summarize function of curcumin in tumor therapy and then, the function of curcumin in suppressing different types of hematological tumors is evaluated.
2. Review methodology
The search in literature and finding suitable papers for the current review were performed based on the databases including Pubmed, Sciencedirect and Googlescholar. The following keywords were used to search literature and collect relevant references including “curcumin”, “cancer”, “lymphoma”, “myeloma”, “leukemia”, “cancer therapy” and “natural products in cancer therapy”.
3. Curcumin and cancer therapy
Curcumin is a crystalline compound with yellow color and molecular weight of 368.7. The solubility of curcumin is various based on the alkaline or acidic states; it has been shown that curcumin is in enol form in alkaline state with red color, while it occurs as insoluble keto form in acidic or neutral state.19 The studies demonstrate partial solubility or insolubility of curcumin in water, while it is soluble in alcohol. THe presence of curcuminoid phytochemicals that curcumin is among them cause the yellow color of curcumin20. Curcumin has a long history of being used in the life and people living in south Asia and India have employed it as a flavoring agent for their food. The popularity of turmeric is due to its health-promoting impacts.21 The popularity of turmeric has shown an increase in recent years and one of the reasons for its popularity is presence of diferuloylmethane or curcumin. There are several health-promoting impacts for curcumin such as lowering fat levels, slowing sclerosis progression, depression treatment, preventing neurological disorder progression and detoxifying liver.22 Curcumin's health promoting activities can be summarized as anti-oxidant, anti-inflammatory, anti-cancer and anti-angiogenesis, among others.23, 24, 25, 26, 27, 28
Curcumin shows both chemoprotection and chemo-sensitive activities, and furthermore it is capable of causing radiosensitivity.29, 30, 31 Curcumin augments chemotherapy through ROS generation and apoptosis induction.32 Curcumin-mediated p53 upregulation also causes apoptosis.33 Curcumin interferes with proliferation and metastasis of colorectal cancer and diminishes tumor burden.34 The curcumin analog known PAC has capacity of triggering apoptosis in oral tumor cells and decreasing viability via Wnt and NF-κB inhibition, among others. Furthermore, PAC stimulates autophagy and reduces ROS generation in decreasing oral cancer cell survival.35 Co-application of curcumin and ginsenoside 20(S)-Rg3 suppresses breast tumor growth and elevates radio-sensitivity.36 Furthermore, curcumin and carnosic acid impaired proper function of mitochondria, and induces defects in oxidative phosphorylation mechanism in mitochondria.37 Curcumin has capacity of regulating Wnt and STAT3 molecular pathways in exerting its anti-cancer activity.38 In an experiment, nano-assemblies for delivery of curcumin and tannic acid were used and by increasing tumor uptake, a significant increase occurred in anti-proliferative activity of these valuable compounds in suppressing breast tumor progression.39 The liposomal nanocarriers are capable of co-delivery of curcumin and ruthenium (II) to facilitate ROS levels, and to suppress survival of cervical tumor.40 In respect to overexpression folate receptor on cervical tumor cells, folate-targeted nanocarriers of curcumin can reduce cancer viability.41
4. Curcumin and leukemia
4.1. Curcumin and regulation of molecular pathways
Table 1 displays curcumin use in leukemia treatment. The activation of Akt signaling increases progression of AML. Curcumin has capacity of inactivating Akt signaling in AML. Curcumin stimulates death in AML and these anti-cancer activities of curcumin were abrogated using SC-79 as an Akt activator, while they were boosted using afuresertib as Akt inhibitor. The AML xenograft mouse model highlighted the function of curcumin in improving survival rate of animal models and suppressing tumor growth.42 One of the challenges of leukemia treatment of dormancy that provides sufficient time for tumor cells to proliferate and ensure their colonization. In a recent experiment, a derivative of curcumin known as C212 was used to suppress leukemia progression. Compared to curcumin, C212 demonstrates more capacity in triggering cell death and G2/M arrest, and suppressing growth rate. Furthermore, quiescent leukemia cells are resistance to conventional therapies and C212 has capacity of removing these tumor cells. Besides, curcumin is able to reduce levels of HSP90 in mediating protein degradation and aggregation, and suppressing progression rate of leukemia cells.43 BCAT1 and mTOR are related to apoptosis in leukemia. Curcumin-mediated inhibition of BCAT1 and mTOR triggers apoptosis in leukemia cells.44 Although most of the studies have focused on apoptosis as a kind of cell death regulated by curcumin in affecting leukemia progression, autophagy is also associated with cell death. It has been shown that curcumin and tetrahydrocurcumin have capacity of inducing both autophagy and apoptosis in reducing progression of leukemia cells and decreasing their survival rate.45 Autophagy may also exert pro-survival role in tumor cells and its regulation by curcumin in leukemia treatment requires more investigation.
Table 1.
The capacity of curcumin in leukemia treatment.
| In vitro/In vivo | Cell line/Animal model | Study design | Molecular taregts | Biological mechanism | Remarks | Refs |
|---|---|---|---|---|---|---|
| In vitro | SHI-1 cells | 6.25, 12.5 and 25 μM | MMP-2 MMP-9 |
Apoptosis induction Suppressing metastasis of tumor cells Down-regulation of MMP-2 and MMP-9 |
76 | |
| Apoptosis | ||||||
| Metastasis | ||||||
| In vitro In vivo |
HL60 cells | 5, 10, 25, and 50 μM | NF-κB | Apoptosis | Apoptosis induction | 77 |
| Xenograft model | Inhibiting NF-κB signaling pathway | |||||
| In vitro In vivo |
K562, MV4-11, HL-60, ML-1, Kasumi-1 and THP-1 cells | 3 and 10 μM | DNMT1, p65 and Sp1 | Apoptosis and cell progression | Decreased expression levels of DNMT1, p65 and Sp1 Apoptosis stimulation Cell cycle arrest |
78 |
| Mice | ||||||
| In vitro In vivo |
K562 and LAMA84 cells CML mouse xenograft |
5, 10, 20 and 40 μM | miR-196b | – | miRNA-196b upregulation by curcumin in reducing expression level of Bcr-Abl Reduced tumor size in vivo |
79 |
| In vitro In vivo |
SUP-B15 cells Mouse model |
5, 10, 15 and 20 μM | Akt/mTOR and ABL/STAT5 | Apoptosis and proliferation | Apoptosis induction Inhibition of Akt/mTOR and ABL/STAT5 signaling pathways Proliferation inhibition |
80 |
| In vitro | SKM-1 and KG1a cells | 0–80 μM | Caspase-3 PARP |
Apoptosis | Apoptosis induction Upregulation of caspase-3 and mediating cleavage of PARP Survivin protein down-regulation |
81 |
| In vitro | HL60, Kasumi, NB4, and KG1 cells | 0–40 μM | FoxM1 VEGF MMP-2 MMP-9 |
Apoptosis | FoxM1 down-regulation by curcumin and subsequent decrease in expression levels of cyclin B1, CDK2, Cdc25B, Bcl-2, survivin, VEGF, MMP-2 and MMP-9 Decreasing survival rate of tumor cells Apoptosis induction |
82 |
| In vitro | WEHI-3 cells | 0–20 μM | – | Apoptosis ROS production DNA damage |
Triggering apoptosis in tumor cells via mitochondrial and endoplasmic reticulum pathways ROS overgeneration DNA damage induction |
83 |
| In vitro | K562 cells | 0–30 μM | Caspase-3 and -9 | Cell cycle arrest Cell death |
Cell cycle arrest at G2/M phase Cell death induction via overexpression of caspase-3 and -9 |
84 |
| In vitro | HL60, K562, MOLT4 and KG1 cells | 0–50 μM | DR4 and DR5 | – | Enhancing expression level of DR4 and DR5 Down-regulation of Mcl-1, Bcl-xL and XIAP Increasing sensitivity of tumor cells to TRAIL inhibitors |
85 |
| In vitro | KG-1 and U937 cells | 0–100 μM | VEGF | Apoptosis | Apoptosis induction Decreased proliferation of tumor cells Reduction in expression level of VEGF |
86 |
| In vitro | KG-1a, KG-1, and EoL-1 cells | 0–50 μg/mL | – | Proliferation | Suppressing proliferation rate of tumor cells | 87 |
| In vitro | SUP-B15 cells | 10 μM | RAF/MEK/ERK | Autophagy | Induction of RAF/MEK/ERK pathway to stimulate autophagy and suppress progression of tumor cells | 88 |
| In vitro | 697, REH, SupB15, and RS4; 11 cells | 0–40 μM | Bax/Bcl-2 Caspase-8 |
Apoptosis | Increased Bax/Bcl-2 ratio Loss of mitochondrial membrane potential and subsequent release of cytochrome C Caspase-8 activation and truncation of BID |
89 |
Upregulation of STAT3 causes leukemia progression. AML secrete extracellular vesicles enriched with miRNA-1246 that induces STAT3 in promoting survival of leukemia stem cells.46 LINC00265 sponges miRNA-4500 to mediate STAT3 in proliferation and metastasis of leukemia cells.47 STAT3 upregulates MYC to facilitate viability of leukemia stem cells.48 Based on these examples, STAT3 signaling activation causes tumorigenesis. Curcumin and thalidomide decreases expression level of STAT3 and Bcl-xL to stimulate death.49 C1206 as a derivative of curcumin can reduce HSP90 level and suppress Akt, MEK, ERK and C-RAF. This new derivative of curcumin (C1206) elevates mitochondrial-mediated apoptosis in leukemia.50 The major molecular pathways that here have been discussed by studies is limited to mTOR, Akt, STAT3, miRNAs and lncRNAs. However, there are other molecular targets participating in progression of leukemia that can be targeted by curcumin. A pathway that impairs Akt axis is PTEN and further studies should evaluate if curcumin is able to increase PTEN expression in Akt suppression. Moreover, STAT3 has close interaction with EMT in cancer metastasis and curcumin-mediated regulation of STAT3/EMT axis in affecting metastasis of leukemia still needs investigation. The studies have been limited to the regulation of growth and invasion of leukemia cells by curcumin upon modulation of molecular targets, but response to therapy should be also highlighted.
4.2. Curcumin in combination cancer therapy
Based on the discussions, curcumin has high capacity in suppressing progression of leukemia cells. However, there is also a potential way to accelerate tumor-suppressing activity of curcumin and that is use of combination therapy. Quercetin is another plant derived-natural compound that causes apoptosis in leukemia and diminishes their migration and invasion.51,52 Both curcumin or quercetin alone are able to stimulate apoptosis; noteworthy, co-application of curcumin and quercetin stimulates more apoptosis in tumor cells compared to curcumin or quercetin alone, and these anti-cancer agents suppress NF-κB signaling, while they upregulate expression level of p53.53 Curcumin and quercetin enhance levels of ROS, while it decreases GSH levels to mediate loss of MMP. Curcumin and quercetin induce cytochrome C release from mitochondria and they mediate cleavage of PARP and caspase-9. Therefore, quercetin and curcumin are able to stimulate apoptosis in leukemia through ROS overgeneration.54 Another experiment demonstrates that a combination of curcumin, quercetin and cannabidiol causes loss of MMP in leukemia therapy.55 Another potent anti-tumor agent derived from nature is piperine that can trigger apoptosis in leukemia cells via caspase-3 and -9 increase, and Bcl-2 decrease.56,57 The drug resistance commonly occurs in leukemia cells that prevents cytotoxicity of chemotherapeutic agents.58,59 Both curcumin and piperine suppress proliferation of leukemia cells and their IC50 value has reported to be 30 μM and 25 μM, respectively. These anti-cancer agents have capacity of inducing apoptosis in leukemia cells via mitochondrial pathway. Besides, curcumin and piperine induce autophagy and mediate S arrest.60 The current section clearly demonstrated that curcumin and its combination with other therapies can suppress leukemia progression. However, a gap is lack of emphasis on the combination of curcumin with chemotherapy drugs, since experiments are mainly about combination of curcumin with other phytochemicals.
4.3. Curcumin, drug resistance and stemness
The lncRNA HOTAIR causes Adriamycin insensitivity of leukemia. Curcumin administration decreases expression level of lncRNA HOTAIR to enhance miRNA-20a-5p levels, downregulating WT1 and subsequent apoptosis induction in leukemia cells. Furthermore, curcumin suppresses invasion of leukemia.61 Curcumin has capacity of affecting cancer stem cell markers in leukemia. Curcumin administration suppresses colony formation in leukemia cells and decreases expression levels of Gli-1, Notch-1 and Cyclin D1 to impair stem cell markers.50 The first limitation is the focus only Adriamycin, but there are also other chemotherapy drugs such as cisplatin, paclitaxel and docetaxel that function of curcumin in increasing drug response of leukemia to these compound should be evaluated. Moreover, there are other stem cells markers such as ALDH and CDs that their regulation by curcumin in leukemia should be evaluated.
4.4. Curcumin and pyroptosis
A lytic cell death mechanism that is dependent on the function of caspase-1/-4/-5/-11 is known as pyroptosis and its activation occurs as a response to inflammasomes.62,63 Inflammasomes are considered as complexes that have been comprised of PRR, ASC and effector caspases. The stimulation of caspases is performed by inflammasomes and they participate in cleaving and activating the certain members of pore-forming gasdermins. The cleavage of gasdermin participates in plasma membrane pore formation and this causes dysfunction of membrane. Then, cytosolic protein release occurs to mediate cell death. The downregulation of pyroptoic inflammasomes and gasdermins is observed in human cancers.64,65 The stimulation of pyroptosis by drugs can accelerate inflammatory cell death and it impairs growth and metastasis of tumor cells.66,67 Curcumin has potential of pyroptosis induction in leukemia. The upregulation of AIM2, IFI16 and NLRC4 inflammasomes is mediated by curcumin that is dependent on elevation in expression level of ISG3. Then, stimulation of caspase 1 occurs to enhance cleavage of GSDMD and mediate pyroptosis.68 The regulation of pyroptosis by curcumin has been only mentioned, while there are other types of cell death pathways especially ferroptosis and necroptosis that their modulation by curcumin in leukemia therapy should be mentioned.
4.5. Curcumin, poor bioavailability and application of nanoparticles
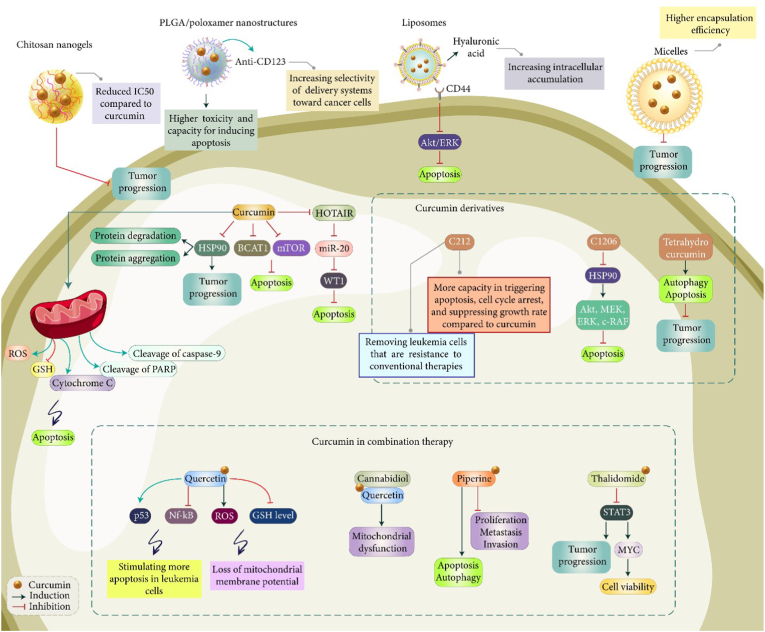
The poor bioavailability is a significant problem of curcumin and application of nanoparticles can increase potential of curcumin in suppressing leukemia progression. For this purpose, chitosan nanogels have been prepared using 1 % acetic acid and then, cross-linked with EDC and NHS. The curcumin-loaded nanogels demonstrated particle size of 150 nm and they were beneficial in reducing IC50 value of curcumin; so that, curcumin alone has IC50 of 50 μg/ml, while curcumin-loaded nanogels showed IC50 of 25 μg/ml that is half of curcumin alone. The viability assay revealed that curcumin-mediated apoptosis in leukemia cells occurs by concentration of 25 μg/ml, while curcumin-loaded nanogels stimulate apoptosis at concentration of 12.5 μg/ml. Therefore, application of chitosan-based nanogels is advantageous in reducing IC50 of curcumin69. CD123 shows overexpression on leukemia and anti-CD123-functionalized nanoparticles can increase selectivity towards tumor cells. In this case, PLGA/poloxamer nanostructures were prepared for curcumin delivery and they were modified with anti-CD123. Compared to curcumin alone, drug-loaded nanoparticles demonstrated higher cytotoxicity and induced more apoptosis in leukemia cells (KG-1a cells).70 Another cell surface marker of leukemia is CD44 with overexpression, while its expression is at low level in normal cells. The most well-known factor targeting CD44 is hyaluronic acid that there have been much efforts in modification of nanoparticles with this agent.71 The hyaluronic acid-modified liposomal delivery of curcumin causes leukemia suppression. This surface modification significantly enhances tumor uptake and suppresses growth rate of tumor cells. Furthermore, hyaluronic acid-modified curcumin-loaded liposomes suppressed Akt/ERK axis to induce apoptosis in leukemia cells.72 The curcumin-embedded micelles demonstrated particle size of 50 with high encapsulation efficiency (75 %). They prudentially accumulated in leukemia cells and suppressed progression of tumor cells.73 Therefore, curcumin delivery by nanoparticles can significantly enhance ability in cancer therapy (Fig. 1).74,75 There are two important limitations with the current studies. The first limitation is related to lack of focus on the role of carbon and metal nanoparticles for the delivery of curcumin in leukemia therapy. The second limitation is lack of focus on the multifunctional nanoparticles such as pH-, redox- and light-sensitive carriers for specific curcumin delivery. However, the current studies clearly highlight the fact that nanocarriers can potentially improve efficacy of curcumin in leukemia suppression. Table 1 provides a summary of experiments using curcumin in treatment of leukemia.
Fig. 1.
Curcumin administration in treatment of leukemia. The apoptosis regulation by curcumin reduces tumorigenesis. Increase in cytochrome c release, upregulation of caspases and cleavage of PARP by curcumin can induce apoptosis. The curcumin derivatives have also shown potential in regulation of apoptosis and autophagy in cancer therapy. Curcumin can be employed with quercetin, canabidiol, piperine and thalidomide in treatment of leukemia. Moreover, polymeric nanoparticles and chitosan nanostructures have delivered curcumin in leukemia therapy.
5. Curcumin and lymphoma
5.1. Curcumin and non-coding RNAs
The miRNAs are carcinogenic regulators. miRNA function is to bind to mRNA for gene expression reduction. The abnormal profile of miRNAs leads to cancer initiation and development.90,91 miRNA-21 is a new emerging miRNA in lymphoma and it exerts tumor-promoting function. STAT5 promotes miRNA-21 levels and it causes unfavorable prognosis in lymphoma.92, 93, 94 Curcumin has ability of targeting miRNA-21 in impairing progression of lymphoma. Curcumin administration accelerates apoptosis and disrupts the growth and metastasis. Curcumin suppresses miRNA-21 to elevate VHL levels in exerting its tumor-suppressor activity.95 Curcumin suppresses progression and survival of lymphoma cells.96 miRNA-28–5p is another factor involved in regulating progression of lymphoma. miRNA-28–5p has poor expression in lymphoma and its upregulation suppresses tumor progression via YWHAZ down-regulation.97 Furthermore, miRNA-28–5p stimulates and its level undergoes down-regulation by CDK12/MYC to elevate tumorigenesis.98 Curcumin suppresses lymphoma cell viability and this anti-cancer impact reduces by miRNA-28–5p down-regulation. Curcumin promotes miRNA-28–5p expression to induce apoptosis via caspase-3 upregulation. By increasing miRNA-28–5p expression, curcumin reduces BECN1 expression and enhances p62 expression.96 Curcumin-mediated lncRNA regulation has been also reported. Briefly, lncRNAs are similar to miRNAs in terms of inability in encoding proteins and both of them have regulatory functions in cells. However, lncRNAs can be present in the cytoplasm and nucleus.99,100 LncRNA H19 enhances proliferation rate of lymphoma cells via Akt signaling activation and its polymorphism is associated with cancer susceptibility.101,102 Regarding to curcumin ability in lncRNAs modulation in cancer therapy,103,104 this interaction can be evaluated in lymphoma therapy. This section highlighted the regulation of ncRNAs by curcumin in lymphoma therapy. However, the major focus is on the miRNAs. Moreover, there is no report on lncRNAs and circRNAs control by curcumin in lymphoma. However, the studies highlight the role of lncRNAs105 and circRNAs106 in modulation of tumorigenesis in lymphoma and since curcumin is an epigenetic drug, further studies in this case is required.
5.2. Curcumin, STAT3 and NF-κB
One of the signaling networks involved in progression of lymphoma cells is STAT3 pathway that has an oncogenic function and it prevents apoptosis, autophagy and ferroptosis in lymphoma.107 The overexpression of STAT3 prevents apoptosis in lymphoma cells108 and its inhibition promotes drug sensitivity.109 Plus to STAT3, NF-κB also plays an oncogenic function in lymphoma. NF-κB stimulates STAT3 to suppress ferroptosis in lymphoma.110 Simultaneous reduction of both NF-κB and STAT3 can pave the way for suppressing progression of lymphoma cells.111 Notably, curcumin has demonstrated capacity in regulating both NF-κB and STAT3 in lymphoma treatment. Curcumin downregulates STAT3, Bcl-2 and surviving and it impairs NF-κB in lymphoma therapy.112 Hyperactivation of NF-κB and STAT3 occurs in lymphoma to ensure their survival and progression. Curcumin triggers apoptosis in lymphoma cells. Curcumin reduces levels of c-Myc, survivin, XIAP, c-IAP1, Bcl-2 and Bcl-xL via suppressing NF-κB and STAT3, causing apoptosis and decreased proliferation of lymphoma cells.113 The in vivo experiment on lymphoma-bearing mice has shown that curcumin suppresses NF-κB signaling in preventing tumor growth.114 Based on these studies, curcumin has capacity of regulating two important oncogenic networks in lymphoma progression including STAT3 and NF-κB. According to the literature, STAT3115 and NF-κB116 are critical regulators of tumorigenesis in lymphoma and ideal targets. However, the first note is that they are not the only regulators of lymphoma progression and there are also other molecular targets including PTEN and PI3K/Akt. Furthermore, since STAT3 and NF-κB have interaction, the regulation of their interaction by curcumin in lymphoma therapy requires investigation.
5.3. Curcumin and combination cancer therapy
In order to increase capacity of curcumin in lymphoma treatment, its combination with other drugs has been performed. Omacetaxine causes apoptosis and cycle arrest in lymphoma therapy. Furthermore, omacetaxine diminishes telomerase activity and enhances cell differentiation in lymphoma in impairing tumor progression.117 Curcumin and omacetaxine suppress growth and metastasis of lymphoma and preventing angiogenesis.118 Due to carcinogenic function of Akt, its suppression disrupts the hallmarks of lymphoma.119,120 A combination of curcumin and omacetaxine reduces VEGF levels to downregulate Akt. Furthermore, curcumin and omacetaxine decrease levels of MMP-2 and MMP-9 in reducing tumorigenesis.118 Another experiment has focused on co-administration of curcumin and homoharringtonine (HHT) in treatment of lymphoma. Curcumin and HHT suppress progression of lymphoma and cause apoptosis in Raji cells via upregulation of Bax, caspase-3 and caspase-9. The tumor-suppressor function of curcumin and HHT in lymphoma is attributed to inhibition of TGF-β/Smad3 axis.121 The current evidences are advocators of using curcumin in combination with other agents in lymphoma therapy. However, the limitation is that there are a variety of synthetic and natural compounds capable of combination lymphoma therapy that still requires investigation. For instance, combination of curcumin with quercetin, resveratrol and kaempferol, among others, requires more investigation.
5.4. Curcumin, apoptosis and stemness
In addition to in vitro experiments, in vivo studies have also confirmed function of curcumin in suppressing lymphoma progression. Curcumin suppresses progression of Raji cells based on time and dose. Curcumin stimulates apoptosis in lymphoma via upregulation of Bax, Bid and cytochrome C, and decreases level of c-Myc. Furthermore, curcumin stimulates cleavage of PARP in causing cell death in lymphoma. Therefore, curcumin stimulates apoptosis via mitochondrial pathway in lymphoma cells. Curcumin administration is beneficial in decreasing tumor burden.118 Furthermore, curcumin-mediates stemness suppression is based on downregulation of Gli-1, Notch-1 and cyclin D1.50 Taking everything together, curcumin can disrupt lymphoma progression and it can suppress proliferation and invasion. Besides, curcumin is beneficial in reducing stemness. However, curcumin-mediated reversal of chemoresistance in lymphoma requires attention. There is a significant gap in the current studies related to curcumin-mediated regulation of cell death pathways. The fact is that apoptosis is not the only mechanism in lymphoma. Based on the literature, autophagy,122 necroptosis123 and ferroptosis124 are other mechanisms dysregulated in lymphoma and curcumin impact on them needs further investigation.
5.5. Curcumin and nano-scale delivery systems
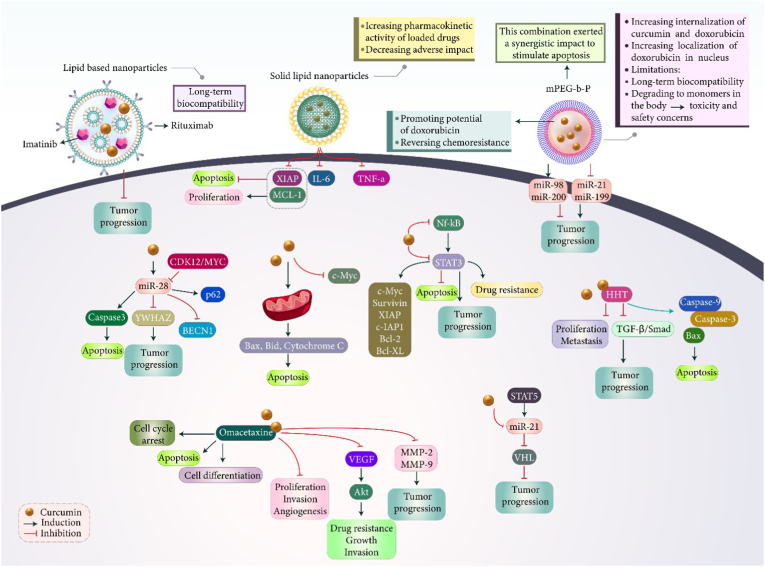
For improving capacity of curcumin in lymphoma suppression, different kinds of nanostructures have been developed. mPEG-b-P (Glu-co-Phe polymeric nanoparticles were developed to provide a platform for co-delivery of curcumin and doxorubicin.125 Doxorubicin is a chemotherapeutic agent and due to development of resistance, its delivery by nanostructures is performed. Furthermore, anti-cancer agents such as curcumin are used for promoting potential of doxorubicin in cancer therapy and reversing chemoresistance.126,127 Polymeric nanostructures significantly enhanced internalization of curcumin and doxorubicin in lymphoma cells, and doxorubicin was localized in nucleus. This combination exerted a synergistic impact and stimulated apoptosis in lymphoma cells more than curcumin or doxorubicin alone. Doxorubicin- and curcumin-loaded polymeric nanoparticles reduced expression level of miRNAs including miRNA-21, -199a and promoted expression levels of miRNA-98 and -200c in impairing lymphoma progression. In vivo experiment on mice demonstrated potential of doxorubicin- and curcumin-loaded polymeric nanoparticles in suppressing tumor growth and improving survival time of animal models.125 One of the limitations related to polymeric nanoparticles is their long-term biocompatibility. In fact, polymers can be degraded to monomers in body and more studies should be performed in understanding toxicity of polymeric nanoparticles in body. In order to solve problem related to toxicity and safety concerns, lipid-based nanoparticles have been developed for co-delivery of curcumin and imatinib in lymphoma that demonstrate long-term biocompatibility, even in clinical trials. CD20 receptor undergoes overexpression on lymphoma cells and in order to increase selectivity of nanocarriers towards cancer cells, their modification with rituximab has been performed to target CD20 receptor. Compared to non-targeted nanostructures, curcumin- and imatinib-loaded lipid nanostructures demonstrated higher cytotoxicity. The drug-loaded nanostructures suppressed lymphoma progression and use of nanoparticles enabled decrease in IC50 of curcumin and imatinib.128
Compared to conventional polymeric nanoparticles, solid lipid nanoparticles (SLNs) have obtained much attention in recent years. These nanostructures are comprised of a solid lipid core that can encapsulate lipophilic drugs such as curcumin for adjusted release. They can improve pharmacokinetic activity of loaded drug and by targeted delivery at tumor site, significantly decrease adverse impacts.129, 130, 131, 132, 133 In an experiment, SLNs were used for delivery of curcumin in lymphoma treatment. These nanoformulations reduced tumor growth in animal models up to 50 %, while curcumin alone decreased tumor growth by 35.8 %. The expression level of XIAP and Mcl-1 decreased by curcumin-loaded SLNs to suppress proliferation and to induce apoptosis in lymphoma cells. Furthermore, a significant reduction in levels of IL-6 and TNF-α were observed upon treatment of lymphoma by curcumin-loaded SLNs.101 Based on these studies, nanocarriers are capable of targeted delivery of curcumin at tumor site and they suppress lymphoma proliferation by inducting apoptosis.134 More experiments are required to investigate role of other nanoparticles in delivery of curcumin for lymphoma suppression. Since curcumin potential in lymphoma suppression has been improved significantly through application of nanoparticles, a gap in the field is lack of studies about co-delivery of curcumin with siRNA, shRNA or CRISPR in synergistic lymphoma inhibition. Table 2 and Fig. 2 provide a summary of curcumin use in lymphoma suppression.
Table 2.
The use of curcumin in treatment and suppression of lymphoma.
| In vitro/In vivo | Cell line/Animal model | Study design | Molecular target | Biological mechanism | Remarks | Refs |
|---|---|---|---|---|---|---|
| In vitro In vivo |
Raji cells Xenograft model |
10 μM | VEGF Akt MMP-2 and -9 |
Proliferation Metastasis |
A combination of curcumin and omacetaxine suppresses proliferation and metastasis of cancer cells Inhibition of VEGF/Akt signaling Down-regulation of MMP-2 and MMP-9 |
118 |
| In vitro In vivo |
U937 and Raji cells Xenograft model |
0–80 μM | TGF-β/Smad | Proliferation | A combination of curcumin and HHT inhibits proliferation of lymphoma cells Inhibition of TGF-β/Smad axis Increasing E-cadherin levels Reducing N-cadherin levels |
121 |
| In vitro | ut-78, HH, MJ, My-La CD4+ and My-La CD8+ cells | 12–24 μM | – | Apoptosis DNA damage Autophagy |
Apoptosis induction Activation of caspase cascade DNA fragmentation Inducing dephosphorylation of Akt Autophagy induction |
135 |
| In vitro | PEL cells | 0–80 μM | JAK1 and STAT3 | Apoptosis Proliferation |
Apoptosis induction Decreasing proliferation of tumor cells Mediating loss of mitochondrial membrane potential Cytochrome C release Activation of caspase-3 Inhibition of JAK1 and STAT3 signaling |
136 |
| In vitro | MJ, Hut78, and HH cells | 5–20 μM | STAT3 Bcl-2 Survivin NF-kappaB Caspase-3 |
Apoptosis | Apoptosis induction Down-regulation of STAT3, Bcl-2 and survivin Inhibition of NF-kappaB signaling Activation of caspase-3 |
112 |
| In vivo | Lymphoma bearing mice | 50, 100 and 150 mg/kg | IL-1α and IL-1β | Inflammation | Inhibiting tumorigenesis via down-regulation of IL-1α and IL-1β Preventing inflammation |
137 |
| In vivo | Lymphoma bearing mice | 1.5, 3 and 4.5 mg/kg | IL-6 and TNF-α NF-kappaB |
– | Down-regulation of IL-6 and TNF-α Inhibition of NF-kappaB signaling |
138 |
| In vitro | H-RS and Jurkat cells | 2.5–100 μM | STAT3 and NF-kappaB | Apoptosis | Apoptosis induction Cell cycle arrest Suppressing STAT3 and NF-kappaB molecular pathways Down-regulation of Bcl-2, Bcl-xL, XIAP and survivin |
113 |
| In vitro | CH12F3 lymphoma cells | 0–6.5 μM | Caspase-3 | Apoptosis DNA damage |
Sensitizing cancer cells to DNA damage Apoptosis induction in a caspase-3 dependent manner |
139 |
| In vitro | Namalwa, Ramos and Raji cells | 2, 10 and 20 μmol/L | mTOR | Cell cycle progression | Cell cycle arrest at G2/M phase Increasing sensitivity of lymphoma cells to radiation Inhibiting mTOR phosphorylation |
140 |
| In vitro In vivo |
Lymphoma cells NOD/SCID mice |
0–40 μM 200 mg/kg |
Akt/mTOR PPARγ |
Apoptosis Cell cycle progression |
Apoptosis induction Cell cycle arrest at G2 phase PPARγ overexpression Inhibition of Akt/mTOR signaling |
141 |
| In vitro | AS283A, KK124, Pa682PB, BML895, and CA46 cells | 10 or 20 μmol/L | NF-kappaB Bax |
Apoptosis | Down-regulation of NF-kappaB Overexpression of Bax Apoptosis induction Reducing viability of tumor cells |
142 |
| In vitro | JeKo-1, Mino, SP-53, and Granta 519. JeKo-1 cells | 10, 25 and 50 μM | NF-kappaB | Apoptosis | Apoptosis induction Cell cycle arrest at G1/S phase Proliferation inhibition Inhibition of NF-kappaB signaling pathway |
143 |
Fig. 2.
Curcumin use in treatment of lymphoma. The lipid-based nanoparticles are common structures for delivery of curcumin in lymphoma therapy and co-delivery with other compounds such as imatinib. Proliferation suppression and apoptosis acceleration can be obtained through application of curcumin. The upregulation of miR-98 and miR-200 and downregulation of miR-21 and miR-199 mediate the anti-cancer activity of curcumin. Upregulation of Bax and Bad, and release of cytochrome C can mediate apoptosis in tumor cells. Furthermore, cancer metastasis can be reduced due to downregulation of MMP-2 and MMP-9.
6. Curcumin and multiple myeloma
6.1. Curcumin, STAT3, JNK and NF-κB pathways
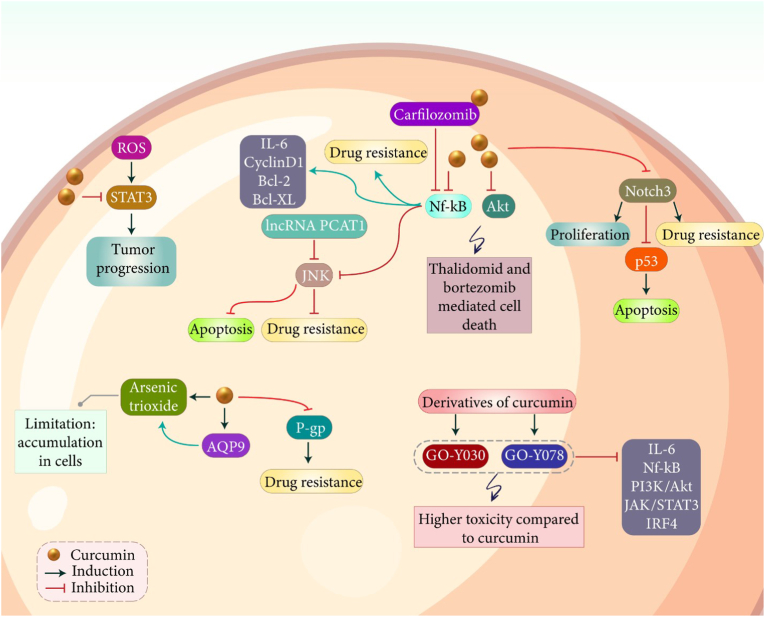
Similar to other types of hematological cancers, curcumin has demonstrated capacity in suppressing progression of myeloma cells. Noteworthy, curcumin targets various molecular pathways in impairing progression of myeloma cells. STAT3 signaling shows oncogenic function in multiple myeloma and its inhibition leads to apoptosis and cell cycle arrest.144 Overgeneration of ROS results in activation of STAT3 signaling to enhance growth and viability of myeloma cells.145 Various pharmacological compounds such as arctiin and others that are mainly phytochemicals have been used for STAT3 signaling inhibition and subsequent decrease in progression of myeloma cells.146,147 Curcumin can regulate expression level of STAT3 in affecting myeloma progression. High levels of IL-6 stimulate STAT3 signaling in increasing progression of myeloma cells. Curcumin administration suppresses proliferation of myeloma cells via preventing phosphorylation of STAT3.148 However, only one experiment has evaluated potential of curcumin in targeting STAT3 signaling in myeloma and more studies to understand related molecular pathways and their impact on tumor progression are required. Another oncogenic signaling in multiple myeloma is NF-κB pathway that S1PR2 down-regulation leads to a significant increase in growth and invasion of myeloma cells via NF-κB stimulation.149 Magnolol promotes expression level of miRNA-129 to suppress NF-κB signaling, resulting in apoptosis induction and remarkable reduction in growth and metastasis of tumor cells.150 Furthermore, NF-κB signaling participates in drug resistance in myeloma and its inhibition decreases stemness and chemoresistance features of tumor cells.151,152 A combination of curcumin and carfilzomib is beneficial in suppressing progression of myeloma cells. Both curcumin and carfilzomib have capacity of suppressing NF-κB signaling in inhibiting myeloma progression and this impact is potentiated by their combination. In fact, the combination of these drugs demonstrates more capacity in preventing nuclear translocation of NF-κB compared to their use alone. Furthermore, curcumin and carfilzomib combination stimulate cell cycle arrest at G0/G1 phase and promotes expression level of p53/p21 in suppressing myeloma progression.153 It appears that proliferation and apoptosis of myeloma cells mainly depend on activation of NF-κB signaling. Curcumin administration decreases phosphorylation of IkappaBalpha and suppresses activation of IKK. Furthermore, curcumin decreases NF-κB expression and inhibits expression of its downstream targets including Bcl-2, Bcl-xL, cyclin D1 and interleukin-6. Then, a significant decrease occurs in proliferation of myeloma cells and cell cycle arrest at G1/S is observed. Curcumin also stimulates expression of caspase-7, caspase-9 and PARP in reducing viability of myeloma cells. It has been shown that down-regulation of NF-κB by curcumin is beneficial in increasing drug sensitivity of myeloma cells.154 One of the downstream targets of NF-κB signaling in multiple myeloma is JNK.155 In contrast to NF-κB, JNK signaling has a tumor-suppressor activity and enhancing its expression is of importance in cancer therapy. Activation of JNK signaling by disulfiram/copper results in apoptosis in myeloma cells.156 Down-regulation of lncRNA PCAT-1 leads to activation of JNK signaling and subsequent drug sensitivity of myeloma cells.157 Based on these studies, activation of JNK signaling stimulates apoptosis, diminishes progression of tumor cells and promotes drug sensitivity.158,159 It has been reported that curcumin promotes ability of bortezomib in myeloma suppression. Curcumin reduces expression level of NF-κB to induce JNK signaling, resulting in an increase in anti-cancer activity of bortezomib against myeloma cells.155 A strength point of current studies related to myeloma is evaluation of using curcumin with chemotherapy drugs in tumor suppression. However, the limitation point is the main focus on a few molecular targets including NF-κB, STAT3 and JNK. There are other dysregulated pathways such as PTEN, lncRNAs, miRNAs and circRNAs in myeloma. Moreover, the role of curcumin in affecting chromosomal instability, histone modification and acetylation, as other factors participating in tumorigenesis in myeloma should be highlighted.
6.2. Curcumin, drug resistance and accumulation of anti-cancer agents
One of the problems in effective chemotherapy of myeloma is low accumulation of anti-cancer agents in tumor cells. Curcumin is suggested to be involved in enhancing intracellular accumulation of anti-cancer drugs in boosting toxicity against myeloma cells. A recent experiment has shown that curcumin and arsenic trioxide exert synergistic impact in myeloma therapy.160 Arsenic trioxide is a new emerging anti-cancer agent in myeloma treatment and it can induce apoptosis in tumor cells and exerts synergistic impact with bortezomib in myeloma therapy.161, 162, 163 One of the problems related to arsenic trioxide is its accumulation in myeloma cells. Curcumin administration promotes levels of AQP9 at mRNA and proteins levels to promote intracellular accumulation of arsenic trioxide in myeloma cells. Furthermore, a reduction occurs in expression level of anti-apoptotic proteins such as Mcl-1 and Bcl-2, while expression level of Bax and caspase-3 enhances. Based on this experiment, curcumin has capacity of increasing accumulation of arsenic trioxide in U266 cells.160 Another factor involved in drug resistance is P-glycoprotein (P-gp)164,165 and studies should investigate how curcumin regulates its expression in increasing cytotoxicity of anti-cancer agents myeloma cells. Curcumin has capacity of regulating expression and activity of P-gp and by suppressing its activity, curcumin prevents drug resistance in tumor cells.166, 167, 168 Therefore, curcumin and P-gp association in myeloma should be examined in future studies.
The in vivo experiment has revealed potential of curcumin in reversing drug resistance in vivo. Curcumin has capacity of impairing progression and growth of myeloma cells, and it promotes sensitivity to dexamethasone, doxorubicin and melphalan. Curcumin reduces expression level of NF-κB and Akt to promote thalidomide- and bortezomib-mediated cell death. Then, a decrease occurs in expression levels of cyclin D1, Bcl-xL, Bcl-2, TRAF1, cIAP-1, XIAP, survivin and VEGF. Furthermore, in vivo experiment on nude mice model demonstrated capacity of curcumin in promoting anti-cancer activity of bortezomib and reducing expression of CD31 and VEGF.169 The first important viewpoint is related to the role of curcumin in downregulation of P-gp to increase accumulation of drugs in myeloma. The second strength point is the combination cancer therapy by curcumin along with bortezomib or thalidomide. However, a gap in the field of lack of application of nanoparticles for co-delivery of drugs in myeloma therapy. Moreover, nanoparticles can facilitate the bypass from drug efflux transporters for better cancer therapy.
6.3. Curcumin, PI3K/Akt and Notch pathways
Another important signaling pathway involved in progression of myeloma is PI3K/Akt signaling. IGF-1 stimulates PI3K/Akt signaling to induce EMT in increasing progression and metastasis of myeloma cells.170 miRNA-215–5p suppresses progression of myeloma cells via inhibition of PI3K/Akt signaling to induce apoptosis and cell cycle arrest.171 Anti-cancer agents suppressing PI3K/Akt signaling are of importance in impairing progression of myeloma cells.172,173 An experiment has developed two derivatives of curcumin known as GO-Y030 and GO-Y078 that demonstrate higher cytotoxicity against myeloma cells compared to curcumin (7-12-fold increase in anti-cancer activity). These analogs prevent generation of IL-6 and suppressed related molecular pathways including NF-κB, PI3K/Akt, JAK/STAT3 and IRF4 in myeloma suppression.174 Another important molecular pathway in myeloma is Notch signaling. Increasing evidence demonstrates oncogenic function of Notch signaling in myeloma and its association with proliferation and drug resistance. Inhibition of Notch signaling stimulates apoptosis and promotes chemo-sensitivity of myeloma cells.175, 176, 177 Overexpression of Notch3 is in favor of myeloma progression and prevents apoptosis in tumor cells. It has been reported that curcumin decreases survival rate of tumor cells in a concentration-dependent manner. Curcumin stimulates apoptosis via Notch3 signaling inhibition to promote expression level of p53.178 Overall, studies highlight the fact that curcumin is a potent anti-cancer agent against myeloma (Fig. 3 and Table 3).179, 180, 181, 182, 183, 184, 185 Notably, studies have investigated potential of curcumin in regulating signaling networks involved in myeloma progression. However, there is no experiment using nanoparticles for targeted delivery of curcumin to myeloma cells that can be focus of future studies.
Fig. 3.
Curcumin administration in treatment of myeloma. The downregulation of STAT3, Akt and Notch3 can significantly reduce the progression of tumor cells. Upregulation of p53 by curcumin induces apoptosis. Moreover, GO-Y030 and GO-Y078 s derivatives of curcumin downregulate IL-6, PI3K/Akt, STAT3 and IRF4 in suppressing tumorigenesis. Moreover, P-gp suppression by curcumin reduces chemoresistance.
Table 3.
Application of curcumin in suppressing myeloma progression.
| In vitro/In vivo | Cell line/Animal model | Study design | Molecular target | Biological mechanism | Remarks | Refs |
|---|---|---|---|---|---|---|
| In vitro In vivo |
Nude mice | 5 and 10 μM | NF-κB cyclin D1, Bcl-xL, Bcl-2, TRAF1, cIAP-1, XIAP and survivin VEGF |
Proliferation | Proliferation impairment Enhanced sensitivity to doxorubicin, melphalan and dexamethasone Inhibition of NF-κB and Akt signaling pathways Down-regulation of cyclin D1, Bcl-xL, Bcl-2, TRAF1, cIAP-1, XIAP and survivin VEGF inhibition Suppressing tumor progression in vivo |
169 |
| In vitro | U266 cells | 0–20 μM | NF-κB | – | A combination of curcumin and CFZ suppresses NF-κB signaling and impairs progression of tumor cells | 153 |
| In vitro | U266 cells | 0–5 μmol/L | AQP9 | Apoptosis | Apoptosis induction Suppressing proliferation of tumor cells Overexpression of AQP9 at mRNA and protein levels Promoting intracellular accumulation of arsenic trioxide |
160 |
| In vitro | U266, MM.1S, RPMI 8226, and MM.1R cells | 50 μM | IL-6/STAT3 | Proliferation | Proliferation inhibition Suppressing IL-6/STAT3 signaling |
148 |
| In vitro | U266, MM.1, and MM.1R cells | 0–50 μM | NF-κB | Apoptosis Growth |
Apoptosis induction Growth inhibition Suppressing NF-κB signaling Down-regulation of Bcl-2, Bcl-xL, cyclin D1 and interleukin-6 Cell cycle arrest at G1/S phase |
154 |
| In vitro | RPMI8226 cells | 2 and 20 μM | NF-κB, PI3K/AKT, JAK/STAT3, and IRF4 | – | GO-Y030 and GO-Y078 as curcumin derivatives demonstrate higher cytotoxicity compared to curcumin Reducing IL-6 production Suppressing NF-κB, PI3K/AKT, JAK/STAT3, and IRF4 pathways |
174 |
| In vitro | U266 cells | 2.5, 5 and 10 μM | Bcl-2 Bax |
Apoptosis | Decreasing number of cancer cells Apoptosis induction G0/G1 cell cycle arrest Bcl-2 down-regulation Bax upregulation |
186 |
| In vitro | P3X63Ag8 cells | 0–80 μM | P53 Notch3 |
Apoptosis | Apoptosis induction Promoting p53 expression via Notch3 signaling inhibition |
178 |
| In vitro | RPMI8226 cells | – | Capase-3 | Apoptosis | Proliferation inhibition Apoptosis induction Suppressing Notch1 signaling Upregulation of caspase-3 |
187 |
| In vitro | H929 cells | – | Bcl-2 and cyclin D1 Bax NF-kappaB |
Apoptosis Proliferation |
Synergistic impact between curcumin and bortezomib Down-regulation of Bcl-2 and cyclin D1 Bax upregulation Inhibition of NF-kappaB signaling Apoptosis induction Proliferation inhibition |
188 |
| In vitro | RPMI-8226 and NCI–H929 cells | 0–20 μM | mTOR DNMT3a DNMT3b |
– | Curcumin induces hypermethylation of mTOR promoter Overexpression of DNMT3a and DNMT3b |
189 |
| In vitro | RPMI8226 cells | 12.15 μmol/L and 4.9 μmol/L | Bax Bcl-2 |
Apoptosis | Apoptosis induction Cell cycle arrest at G2/M phase Bcl-2 down-regulation Bax upregulation Inhibiting proliferation of tumor cells |
190 |
| In vitro | MOLP-2/R cells | 10 μmol/L | FA/BRCA | Apoptosis | Apoptosis induction G2/M cell cycle arrest Inhibiting FA/BRCA pathway |
191 |
| In vitro | H929 cells | – | NF-kappaB JNK |
– | Inhibition of NF-kappaB pathway to induce JNK signaling, leading to increased cytotoxicity of bortezomib | 155 |
7. Conclusion and remarks
This summary of curcumin's impact on treatment of hematological cancers demonstrated that the different biological mechanisms including proliferation, metastasis, EMT, apoptosis, autophagy and cell cycle arrest are highly regulated. Moreover, the DNA damage is induced by curcumin to disrupt progression of hematological tumors. The function of curcumin in regulation of major pathways in hematological cancers including NF-κB, PI3K/Akt, STAT3 and Notch confirmed the reduction in expression level of oncogenic factors. However, a gap in current studies is ignoring on evaluation of RNA modification by curcumin in treatment of hematological cancers, especially the non-coding RNAs that play a vital role in tumorigenesis. Therefore, curcumin-mediated regulation of RNAs in treatment of hematological cancers requires more attention.
The current studies have shown that curcumin is a regulator of apoptosis and pyroptosis in various hematological cancers. However, there are other types of cell death mechanisms that their control by curcumin can exert its anti-tumor function. Since curcumin has pleiotropic function, it has ability of regulating other types of cell death pathways. Previous studies have shown that curcumin is a regulator of ferroptosis,192 cuproptosis193 and necroptosis.194 However, there is no report about their regulation by curcumin in hematological tumors. Therefore, curcumin impact on these cell death pathways should be evaluated. Moreover, curcumin-mediated regulation of efferocytosis in cancers and hematological tumors should be evaluated. It is also noteworthy curcumin can modulate autophagy as another cell death mechanism in hematological tumors. However, autophagy has a dual function in cancer and can be protective or lethal.195 In spite of autophagy control by curcumin in hematological cancers, the future studies should evaluate its dual role and the need for application of autophagy inhibitors when it has protective function and application of autophagy inducers, when the function of autophagy is lethal.
An important issue that has been of interest in recent years is the sex and gender evaluation of diseases. Currently, there are reports showing that there are differences about diseases in males and females and therapies based on gender can be developed.196,197 This is also true for cancer and there are differences in cancer incidence rate in males and females.198 Because of wide application of curcumin in cancer therapy, the future studies are encouraged to evaluate gender-based response to curcumin.
In spite of significant evaluation of using curcumin in treatment of hematological cancers, there are a number of limitations that should be addressed. The first limitation is related to the design of studies. Currently, the studies evaluate the curcumin's use in treatment of hematological studies based on in vitro results and sometimes, in vivo. However, a significant problem related to the in vitro studies is lack of evaluation of curcumin's bioavailability. Moreover, the in vivo studies are only limited to the anti-cancer activity of curcumin based on tumor size and weight. To improve knowledge towards the anti-cancer activities of curcumin, the use of 3D-culture and ex vivo application is suggested. The current studies have significantly shown the role of curcumin stimulation of apoptosis, cell cycle arrest and downregulation of oncogenic factors. However, the application of curcumin in combination with other conventional therapies for synergistic tumor removal has not been evaluated. The most popular conventional therapy for hematological cancers is chemotherapy. The resistance of tumor cells to apoptosis, upregulation of alternative molecular pathways and development of drug resistance can lead to failures in treatment of hematological cancers. Therefore, chemoresistance threatens life of cancer patients. Owing to multifunctional role of curcumin in treatment of hematological tumors, the co-administration of curcumin with other therapies is suggested to increase potential of conventional treatments in hematological cancer suppression. Curcumin can promote apoptosis and cell cycle arrest in sensitizing to chemotherapy. Moreover, since drug efflux transporters participate in chemoresistance and curcumin has shown inhibitory impact of P-gp as a transporter, it can prevent drug resistance development. Therefore, the upcoming studies should put more attention on the role of curcumin in combination cancer therapy and reduction in drug resistance development. A major problem towards application of curcumin in clinical trials is the poor pharmacokinetic profile of this compound. In fact, rapid metabolism of curcumin causes its poor bioavailability and low half-time, thereby limiting pharmacological activities of this compound. Moreover, after oral administration of curcumin, there are significant barriers towards its absorbance and it is currently low in intestines. A significant limitation of current studies is lack of attention to the use of nanoparticles for the delivery of curcumin in treatment of hematological cancers. The nanoparticles can improve the pharmacokinetic profile of curcumin including increase in half-time, reduction in metabolism, enhancement in blood circulation time, elevation in tumor tissue accumulation and increase in anti-cancer activity. Therefore, it is highly suggested to employ curcumin-loaded nanostructures in treatment of hematological cancers. Various classes of nanoparticles including polymeric, carbon, lipid and metal nanostructures are significantly used for curcumin delivery in targeted cancer suppression. Therefore, it is suggested to use such nanoparticles for the delivery of curcumin. However, only improvement in the anti-cancer activity of curcumin through application of nanoparticles can not endow the confirmation for use in clinical trials. In addition to high potential in tumor suppression, the nanoformulations of curcumin should have high biocompatibility. Currently, the lipid nanoparticles such as liposomes are being used in clinical trials and their long-term safety has been confirmed in human. Therefore, the suggestion to the future studies is to focus on application of lipid nanoparticles in curcumin delivery and acceleration of anti-cancer activity.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Atrash S., et al. CAR-T treatment for hematological malignancies. 2020;68(5):956–964. doi: 10.1136/jim-2020-001290. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H. Acute myeloid leukemia--major progress over four decades and glimpses into the future. Am J Hematol. 2016;91(1):131–145. doi: 10.1002/ajh.24246. [DOI] [PubMed] [Google Scholar]
- 3.Short N.J., Rytting M.E., Cortes J.E.J.T.L. Acute myeloid leukaemia. 2018;392(10147):593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadia T.M., et al. Toward individualized therapy in acute myeloid leukemia: Contemp Rev. 2015;1(6):820–828. doi: 10.1001/jamaoncol.2015.0617. [DOI] [PubMed] [Google Scholar]
- 5.Döhner H., Weisdorf D.J., Bloomfield C.D. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H.M., et al. Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach. 2021;127(8):1186–1207. doi: 10.1002/cncr.33477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isidori A., et al. 2021. Immunotherapy in Acute Myeloid Leukemia: Where We Stand; p. 1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., et al. Stem Cell as Vehicles of Antibody in Treatment of Lymphoma: A Novel and Potential Targeted Therapy. 2021;17(3):829–841. doi: 10.1007/s12015-020-10080-z. [DOI] [PubMed] [Google Scholar]
- 10.Shanbhag S., Ambinder R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi: 10.3322/caac.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Barta S.K. Diffuse large B‐cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–616. doi: 10.1002/ajh.25460. [DOI] [PubMed] [Google Scholar]
- 12.Podar K., Leleu X. Relapsed/refractory multiple myeloma in 2020/2021 and beyond. Cancers. 2021;13(20):5154. doi: 10.3390/cancers13205154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson H.N. Immunotherapy of multiple myeloma: Promise and challenges. ImmunoTargets Ther. 2021;10:343–371. doi: 10.2147/ITT.S306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Röllig C., Knop S., Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208. doi: 10.1016/S0140-6736(14)60493-1. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S.K., et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan G.J., Walker B.A., Davies F.E. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 17.Wallington-Beddoe C.T., et al. Resistance to proteasome inhibitors and other targeted therapies in myeloma. Br J Haematol. 2018;182(1):11–28. doi: 10.1111/bjh.15210. [DOI] [PubMed] [Google Scholar]
- 18.Wallington-Beddoe C.T., Mynott R.L. Prognostic and predictive biomarker developments in multiple myeloma. J Hematol Oncol. 2021;14(1) doi: 10.1186/s13045-021-01162-7. 151-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esatbeyoglu T., et al. Curcumin—from molecule to biological function. 2012;51(22):5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 20.Lestari M., Indrayanto G.J.V. Profiles Drug Subst Excipients Relat Methodol. 2014;39:113–204. doi: 10.1016/B978-0-12-800173-8.00003-9. [DOI] [PubMed] [Google Scholar]
- 21.Akbar M.U., et al. Critical review on curcumin as a therapeutic agent: From traditional herbal medicine to an ideal therapeutic agent. 2018;28(1) doi: 10.1615/CritRevEukaryotGeneExpr.2018020088. [DOI] [PubMed] [Google Scholar]
- 22.Karthika C., et al. Curcumin as a great contributor for the treatment and mitigation of colorectal cancer. Exp Gerontol. 2021;152 doi: 10.1016/j.exger.2021.111438. [DOI] [PubMed] [Google Scholar]
- 23.Tomeh M.A., Hadianamrei R., Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci. 2019;20(5) doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin S.J., et al. Curcumin and its multi-target function against Pain and inflammation: an update of pre-clinical Data. Curr Drug Targets. 2021;22(6):656–671. doi: 10.2174/1389450121666200925150022. [DOI] [PubMed] [Google Scholar]
- 25.Singh L., et al. Curcumin as a natural Remedy for Atherosclerosis: a pharmacological review. Molecules. 2021;26(13) doi: 10.3390/molecules26134036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabczyk M., et al. Curcumin in metabolic health and disease. Nutrients. 2021;13(12) doi: 10.3390/nu13124440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel S.S., et al. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr. 2020;60(6):887–939. doi: 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]
- 28.Pulido-Moran M., et al. Curcumin and health. Molecules. 2016;21(3):264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., et al. Current clinical developments in curcumin-based therapeutics for cancer and chronic diseases. 2021;35(12):6768–6801. doi: 10.1002/ptr.7264. [DOI] [PubMed] [Google Scholar]
- 30.Abadi A.J., et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. 2022;36(1):189–213. doi: 10.1002/ptr.7305. [DOI] [PubMed] [Google Scholar]
- 31.Ashrafizadeh M., et al. Curcumin therapeutic modulation of the wnt signaling pathway. 2020;21(11):1006–1015. doi: 10.2174/1389201021666200305115101. [DOI] [PubMed] [Google Scholar]
- 32.Patra S., et al. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin Cancer Biol. 2021;73:310–320. doi: 10.1016/j.semcancer.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Zoi V., et al. The role of curcumin in cancer treatment. Biomedicines. 2021;9(9):1086. doi: 10.3390/biomedicines9091086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrero de la Parte B., et al. Curcumin reduces colorectal cancer cell proliferation and migration and Slows in vivo growth of liver Metastases in Rats. Biomedicines. 2021;9(9) doi: 10.3390/biomedicines9091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semlali A., et al. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-90754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Changizi V., Gharekhani V., Motavaseli E. Co-Treatment with ginsenoside 20(S)-Rg3 and curcumin increases Radiosensitivity of MDA-MB-231 cancer cell line. Iran J Med Sci. 2021;46(4):291–297. doi: 10.30476/ijms.2020.83977.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ossikbayeva S., et al. Curcumin and carnosic acid cooperate to inhibit proliferation and alter mitochondrial function of metastatic prostate cancer cells. Antioxidants. 2021;10(10) doi: 10.3390/antiox10101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashrafizadeh M., et al. Potential therapeutic effects of curcumin mediated by JAK/STAT signaling pathway: Review. 2020;34(8):1745–1760. doi: 10.1002/ptr.6642. [DOI] [PubMed] [Google Scholar]
- 39.Sunoqrot S., et al. Curcumin-tannic acid-poloxamer nanoassemblies enhance curcumin's uptake and bioactivity against cancer cells in vitro. Int J Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121255. [DOI] [PubMed] [Google Scholar]
- 40.Lakshmi B.A., et al. Ruthenium(II)-curcumin liposome nanoparticles: synthesis, characterization, and their effects against cervical cancer. Colloids Surf B Biointerfaces. 2021;204 doi: 10.1016/j.colsurfb.2021.111773. [DOI] [PubMed] [Google Scholar]
- 41.Azarian M., et al. 2022. Folic Acid-Adorned Curcumin-Loaded Iron Oxide Nanoparticles for Cervical Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H., et al. Curcumin promotes cell cycle arrest and apoptosis of acute myeloid leukemia cells by inactivating AKT. Oncol Rep. 2021;45(4) doi: 10.3892/or.2021.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu B., et al. Curcumin derivative C212 inhibits Hsp90 and eliminates both growing and quiescent leukemia cells in deep dormancy. Cell Commun Signal. 2020;18(1):159. doi: 10.1186/s12964-020-00652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng Y.H., et al. Curcumin induces apoptosis by inhibiting BCAT1 expression and mTOR signaling in cytarabine-resistant myeloid leukemia cells. Mol Med Rep. 2021;24(2) doi: 10.3892/mmr.2021.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng Y.H., et al. Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother Res. 2019;33(4):1199–1207. doi: 10.1002/ptr.6316. [DOI] [PubMed] [Google Scholar]
- 46.Chen L., et al. microRNA-1246-containing extracellular vesicles from acute myeloid leukemia cells promote the survival of leukemia stem cells via the LRIG1-meditated STAT3 pathway. Aging (Albany NY) 2021;13(10):13644–13662. doi: 10.18632/aging.202893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao D., et al. LINC00265/miR-4500 Axis accelerates acute lymphoblastic leukemia progression by enhancing STAT3 signals. Cancer Manag Res. 2021;13:8147–8156. doi: 10.2147/CMAR.S274590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amaya M.L., et al. The STAT3-MYC axis promotes survival of leukemia stem cells by regulating SLC1A5 and oxidative phosphorylation. Blood. 2022;139(4):584–596. doi: 10.1182/blood.2021013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammadi Kian M., et al. Curcumin combined with thalidomide reduces expression of STAT3 and bcl-xL, leading to apoptosis in acute myeloid leukemia cell lines. Drug Des Dev Ther. 2020;14:185–194. doi: 10.2147/DDDT.S228610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan Y.J., et al. C1206, a novel curcumin derivative, potently inhibits Hsp90 and human chronic myeloid leukemia cells in vitro. Acta Pharmacol Sin. 2018;39(4):649–658. doi: 10.1038/aps.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva S.V.S., et al. Structural design, synthesis and antioxidant, antileishmania, anti-inflammatory and anticancer activities of a novel quercetin acetylated derivative. Molecules. 2021;26(22) doi: 10.3390/molecules26226923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torello C.O., Alvarez M.C., Olalla Saad S.T. Polyphenolic flavonoid compound quercetin effects in the treatment of acute myeloid leukemia and myelodysplastic syndromes. Molecules. 2021;26(19) doi: 10.3390/molecules26195781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mutlu Altundağ E., et al. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells: II. Signal transduction pathways involved. Nutr Cancer. 2021;73(4):703–712. doi: 10.1080/01635581.2020.1767167. [DOI] [PubMed] [Google Scholar]
- 54.Mutlu Altundağ E., et al. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells. Nutr Cancer. 2018;70(1):97–108. doi: 10.1080/01635581.2018.1380208. [DOI] [PubMed] [Google Scholar]
- 55.Olivas-Aguirre M., et al. Phenolic compounds cannabidiol, curcumin and quercetin cause mitochondrial dysfunction and suppress acute lymphoblastic leukemia cells. Int J Mol Sci. 2020;22(1) doi: 10.3390/ijms22010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banerjee S., et al. Black pepper and piperine induce anticancer effects on leukemia cell line. Toxicol Res. 2021;10(2):169–182. doi: 10.1093/toxres/tfab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitra S., et al. Anticancer applications and pharmacological properties of piperidine and piperine: a comprehensive review on molecular mechanisms and therapeutic perspectives. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.772418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usman R.M., et al. Role and mechanism of autophagy-regulating factors in tumorigenesis and drug resistance. Asia Pac J Clin Oncol. 2021;17(3):193–208. doi: 10.1111/ajco.13449. [DOI] [PubMed] [Google Scholar]
- 59.Floren M., Gillette J.M. Acute myeloid leukemia: therapy resistance and a potential role for tetraspanin membrane scaffolds. Int J Biochem Cell Biol. 2021;137 doi: 10.1016/j.biocel.2021.106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li N., et al. Antiproliferative potential of piperine and curcumin in drug-resistant human leukemia cancer cells are mediated via autophagy and apoptosis induction, S-phase cell cycle arrest and inhibition of cell invasion and migration. J buon. 2020;25(1):401–406. [PubMed] [Google Scholar]
- 61.Liu J.M., et al. Curcumin attenuates Adriamycin-resistance of acute myeloid leukemia by inhibiting the lncRNA HOTAIR/miR-20a-5p/WT1 axis. Lab Invest. 2021;101(10):1308–1317. doi: 10.1038/s41374-021-00640-3. [DOI] [PubMed] [Google Scholar]
- 62.Ta A., Vanaja S.K. Inflammasome activation and evasion by bacterial pathogens. Curr Opin Immunol. 2021;68:125–133. doi: 10.1016/j.coi.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Fang Y., et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109595. [DOI] [PubMed] [Google Scholar]
- 65.Xia X., et al. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death Dis. 2019;10(9):650. doi: 10.1038/s41419-019-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruan J., Wang S., Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem Biol Interact. 2020;323 doi: 10.1016/j.cbi.2020.109052. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y.Y., Liu X.L., Zhao R. Induction of pyroptosis and its implications in cancer management. Front Oncol. 2019;9:971. doi: 10.3389/fonc.2019.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y., et al. Curcumin activates NLRC4, AIM2, and IFI16 inflammasomes and induces pyroptosis by up-regulated ISG3 transcript factor in acute myeloid leukemia cell lines. Cancer Biol Ther. 2022;23(1):328–335. doi: 10.1080/15384047.2022.2058862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khatamsaz S., Hashemi M. Curcumin and curcumin-loaded nanogel induce apoptosis activity in K562 chronic myelogenous leukemia cells. Galen Med J. 2018;7:e921. doi: 10.22086/gmj.v0i0.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nirachonkul W., et al. CD123-Targeted nano-curcumin molecule enhances cytotoxic efficacy in leukemic stem cells. Nanomaterials. 2021;11(11) doi: 10.3390/nano11112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashrafizadeh M., et al. Hyaluronic acid-based nanoplatforms for Doxorubicin: a review of stimuli-responsive carriers, co-delivery and resistance suppression. Carbohydr Polym. 2021;272 doi: 10.1016/j.carbpol.2021.118491. [DOI] [PubMed] [Google Scholar]
- 72.Sun D., et al. Novel curcumin liposome modified with hyaluronan targeting CD44 plays an anti-leukemic role in acute myeloid leukemia in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9(20):16857–16868. doi: 10.1021/acsami.7b02863. [DOI] [PubMed] [Google Scholar]
- 73.Tima S., et al. FLT3-specific curcumin micelles enhance activity of curcumin on FLT3-ITD overexpressing MV4-11 leukemic cells. Drug Dev Ind Pharm. 2019;45(3):498–505. doi: 10.1080/03639045.2018.1562462. [DOI] [PubMed] [Google Scholar]
- 74.Leung M.H.M., Shen A.Q. Microfluidic assisted nanoprecipitation of PLGA nanoparticles for curcumin delivery to leukemia jurkat cells. Langmuir. 2018;34(13):3961–3970. doi: 10.1021/acs.langmuir.7b04335. [DOI] [PubMed] [Google Scholar]
- 75.Tima S., et al. Stable curcumin-loaded polymeric micellar formulation for enhancing cellular uptake and cytotoxicity to FLT3 overexpressing EoL-1 leukemic cells. Eur J Pharm Biopharm. 2017;114:57–68. doi: 10.1016/j.ejpb.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 76.Zhu G.H., et al. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm Biol. 2016;54(8):1303–1311. doi: 10.3109/13880209.2015.1060508. [DOI] [PubMed] [Google Scholar]
- 77.Nagy L.I., et al. Curcumin and its analogue induce apoptosis in leukemia cells and have additive effects with bortezomib in cellular and xenograft models. BioMed Res Int. 2015;2015 doi: 10.1155/2015/968981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu J., et al. Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taverna S., et al. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget. 2015;6(26):21918–21933. doi: 10.18632/oncotarget.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Y., et al. Curcumin potentiates the anti-leukemia effects of imatinib by downregulation of the AKT/mTOR pathway and BCR/ABL gene expression in Ph+ acute lymphoblastic leukemia. Int J Biochem Cell Biol. 2015;65:1–11. doi: 10.1016/j.biocel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Zeng Y., et al. Curcumin reduces the expression of survivin, leading to enhancement of arsenic trioxide-induced apoptosis in myelodysplastic syndrome and leukemia stem-like cells. Oncol Rep. 2016;36(3):1233–1242. doi: 10.3892/or.2016.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J.R., et al. Inactivation of FoxM1 transcription factor contributes to curcumin-induced inhibition of survival, angiogenesis, and chemosensitivity in acute myeloid leukemia cells. J Mol Med (Berl) 2014;92(12):1319–1330. doi: 10.1007/s00109-014-1198-2. [DOI] [PubMed] [Google Scholar]
- 83.Huang A.C., et al. Induction of apoptosis by curcumin in murine myelomonocytic leukemia WEHI-3 cells is mediated via endoplasmic reticulum stress and mitochondria-dependent pathways. Environ Toxicol. 2013;28(5):255–266. doi: 10.1002/tox.20716. [DOI] [PubMed] [Google Scholar]
- 84.Martínez-Castillo M., et al. Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells. Oncol Lett. 2018;15(5):6777–6783. doi: 10.3892/ol.2018.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Surapally S., Jayaprakasam M., Verma R.S. Curcumin augments therapeutic efficacy of TRAIL-based immunotoxins in leukemia. Pharmacol Rep. 2020;72(4):1032–1046. doi: 10.1007/s43440-020-00073-7. [DOI] [PubMed] [Google Scholar]
- 86.Salemi M., et al. Anti-vascular endothelial growth factor targeting by curcumin and thalidomide in acute myeloid leukemia cells. Asian Pac J Cancer Prev APJCP. 2017;18(11):3055–3061. doi: 10.22034/APJCP.2017.18.11.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chueahongthong F., et al. Co-treatments of edible curcumin from turmeric rhizomes and chemotherapeutic drugs on cytotoxicity and FLT3 protein expression in leukemic stem cells. Molecules. 2021;26(19) doi: 10.3390/molecules26195785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo Y., et al. The autophagy induced by curcumin via MEK/ERK pathway plays an early anti-leukemia role in human Philadelphia chromosome-positive acute lymphoblastic leukemia SUP-B15 cells. J Cancer Res Therapeut. 2018;14(Supplement):S125–s131. doi: 10.4103/0973-1482.172111. [DOI] [PubMed] [Google Scholar]
- 89.Kuttikrishnan S., et al. Curcumin induces apoptotic cell death via inhibition of PI3-kinase/AKT pathway in B-precursor acute lymphoblastic leukemia. Front Oncol. 2019;9:484. doi: 10.3389/fonc.2019.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang A., et al. LncRNA UCA1 promotes development of gastric cancer via the miR-145/MYO6 axis. Cell Mol Biol Lett. 2021;26(1):33. doi: 10.1186/s11658-021-00275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paskeh M.D.A., et al. Revealing the role of miRNA-489 as a new onco-suppressor factor in different cancers based on pre-clinical and clinical evidence. Int J Biol Macromol. 2021;191:727–737. doi: 10.1016/j.ijbiomac.2021.09.089. [DOI] [PubMed] [Google Scholar]
- 92.Li J., et al. miR-21 expression predicts prognosis in diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2015;8(11):15019–15024. [PMC free article] [PubMed] [Google Scholar]
- 93.Gu L., et al. Inhibition of miR-21 induces biological and behavioral alterations in diffuse large B-cell lymphoma. Acta Haematol. 2013;130(2):87–94. doi: 10.1159/000346441. [DOI] [PubMed] [Google Scholar]
- 94.Lindahl L.M., et al. STAT5 induces miR-21 expression in cutaneous T cell lymphoma. Oncotarget. 2016;7(29):45730–45744. doi: 10.18632/oncotarget.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L., et al. Curcumin inhibits the proliferation, migration, invasion, and apoptosis of diffuse large B-cell lymphoma cell line by regulating MiR-21/VHL Axis. Yonsei Med J. 2020;61(1):20–29. doi: 10.3349/ymj.2020.61.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang T., et al. MiR-28-5p mediates the anti-proliferative and pro-apoptotic effects of curcumin on human diffuse large B-cell lymphoma cells. J Int Med Res. 2020;48(7) doi: 10.1177/0300060520943792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan S., et al. hsa-MicroRNA-28-5p inhibits diffuse large B-cell lymphoma cell proliferation by downregulating 14-3-3ζ expression. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/4605329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Q.H., et al. Cancer Gene Ther; 2022. CDK12 Activates MYC to Repress miR-28-5p/EZH2 and Amplifies Tonic BCR Signaling to Promote the Development of Diffuse Large B-Cell Lymphoma. [DOI] [PubMed] [Google Scholar]
- 99.Mohan C.D., et al. Paradoxical functions of long noncoding RNAs in modulating STAT3 signaling pathway in hepatocellular carcinoma. Biochim Biophys Acta Rev Cancer. 2021;1876(1) doi: 10.1016/j.bbcan.2021.188574. [DOI] [PubMed] [Google Scholar]
- 100.Mirzaei S., et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: inhibiting or promoting carcinogenesis? Cancer Lett. 2021;509:63–80. doi: 10.1016/j.canlet.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 101.Guorgui J., et al. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin's lymphoma in mice. Arch Biochem Biophys. 2018;648:12–19. doi: 10.1016/j.abb.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J., et al. Correlation between lncRNA H19 rs2839698 polymorphism and susceptibility to NK/T cell lymphoma in Chinese population. J buon. 2021;26(2):587–591. [PubMed] [Google Scholar]
- 103.Liu T., et al. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 2017;631:29–38. doi: 10.1016/j.gene.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 104.Wang W.H., et al. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Pharmazie. 2018;73(7):402–407. doi: 10.1691/ph.2018.8402. [DOI] [PubMed] [Google Scholar]
- 105.Zhao L., et al. LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large B cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint. Cell Death Dis. 2019;10(10):731. doi: 10.1038/s41419-019-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin Z., et al. The role of circular RNAs in hematological malignancies. Genomics. 2020;112(6):4000–4008. doi: 10.1016/j.ygeno.2020.06.051. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y., et al. Artesunate induces apoptosis, autophagy and ferroptosis in diffuse large B cell lymphoma cells by impairing STAT3 signaling. Cell Signal. 2021;88 doi: 10.1016/j.cellsig.2021.110167. [DOI] [PubMed] [Google Scholar]
- 108.Xu L., et al. The acetyltransferase KAT5 inhibitor NU 9056 promotes apoptosis and inhibits JAK2/STAT3 pathway in extranodal NK/T cell lymphoma. Anti Cancer Agents Med Chem. 2021 doi: 10.2174/1871520621666210908103306. [DOI] [PubMed] [Google Scholar]
- 109.Gao M., et al. Interleukin-6 reverses Adriamycin resistance in nasal NK/T-cell lymphoma via downregulation of ABCC4 and inactivation of the JAK2/STAT3/NF-κB/P65 pathway. Environ Toxicol Pharmacol. 2021;85 doi: 10.1016/j.etap.2021.103639. [DOI] [PubMed] [Google Scholar]
- 110.Schmitt A., et al. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood. 2021;138(10):871–884. doi: 10.1182/blood.2020009404. [DOI] [PubMed] [Google Scholar]
- 111.Maeta T., et al. Dimethyl fumarate induces apoptosis via inhibiting NF-κB and STAT3 signaling in adult T-cell leukemia/lymphoma cells. Anticancer Res. 2022;42(5):2301–2309. doi: 10.21873/anticanres.15709. [DOI] [PubMed] [Google Scholar]
- 112.Zhang C., et al. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs: potential role for STAT-3 and NF-kappaB signaling. J Invest Dermatol. 2010;130(8):2110–2119. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 113.Mackenzie G.G., et al. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin's lymphoma cells. Int J Cancer. 2008;123(1):56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 114.Das L., Vinayak M. Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-κB signalling in lymphoma-bearing mice. Biosci Rep. 2012;32(2):161–170. doi: 10.1042/BSR20110043. [DOI] [PubMed] [Google Scholar]
- 115.Liu N., et al. Pulsatilla saponin A Inhibits proliferation and induces apoptosis of diffuse large B-cell lymphoma through the JAK2/STAT3 signaling pathway. Anti Cancer Agents Med Chem. 2023 doi: 10.2174/1871520623666230727104849. [DOI] [PubMed] [Google Scholar]
- 116.Zeng X.X., et al. REGγ promotes mantle cell lymphoma cell apoptosis by downregulating NF-κB signaling. Transl Cancer Res. 2023;12(2):310–320. doi: 10.21037/tcr-22-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L., et al. Omacetaxine mepesuccinate induces apoptosis and cell cycle arrest, promotes cell differentiation, and reduces telomerase activity in diffuse large B-cell lymphoma cells. Mol Med Rep. 2016;13(4):3092–3100. doi: 10.3892/mmr.2016.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y., et al. Curcumin in combination with omacetaxine suppress lymphoma cell growth, migration, invasion, and angiogenesis via inhibition of VEGF/Akt signaling pathway. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.656045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao Y., Ding X. miR-145-5p exerts anti-tumor effects in diffuse large B-cell lymphoma by regulating S1PR1/STAT3/AKT pathway. Leuk Lymphoma. 2021;62(8):1884–1891. doi: 10.1080/10428194.2021.1894642. [DOI] [PubMed] [Google Scholar]
- 120.Huang S., et al. PIK-75 overcomes venetoclax resistance via blocking PI3K-AKT signaling and MCL-1 expression in mantle cell lymphoma. Am J Cancer Res. 2022;12(3):1102–1115. [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y., et al. Curcumin in combination with homoharringtonine suppresses lymphoma cell growth by inhibiting the TGF-β/Smad3 signaling pathway. Aging (Albany NY) 2021;13(14):18757–18768. doi: 10.18632/aging.203319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zheng Z., et al. Autophagy and lymphoma. Adv Exp Med Biol. 2020;1207:615–623. doi: 10.1007/978-981-15-4272-5_44. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Q., et al. Identification and assessment of necroptosis-related genes in clinical prognosis and immune cells in diffuse large B-cell lymphoma. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.904614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong Y., et al. APR-246 triggers ferritinophagy and ferroptosis of diffuse large B-cell lymphoma cells with distinct TP53 mutations. Leukemia. 2022;36(9):2269–2280. doi: 10.1038/s41375-022-01634-w. [DOI] [PubMed] [Google Scholar]
- 125.Guo W., et al. Co-Delivery of doxorubicin and curcumin with polypeptide nanocarrier for synergistic lymphoma therapy. Sci Rep. 2020;10(1):7832. doi: 10.1038/s41598-020-64828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Namkaew J., et al. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH-SY5Y human neuroblastoma cells. In Vitro Cell Dev Biol Anim. 2018;54(9):629–639. doi: 10.1007/s11626-018-0288-9. [DOI] [PubMed] [Google Scholar]
- 127.Lai X., et al. Glutathione-responsive PLGA nanocomplex for dual delivery of doxorubicin and curcumin to overcome tumor multidrug resistance. Nanomedicine. 2021;16(16):1411–1427. doi: 10.2217/nnm-2021-0100. [DOI] [PubMed] [Google Scholar]
- 128.Varshosaz J., et al. Co-delivery of rituximab targeted curcumin and imatinib nanostructured lipid carriers in non-Hodgkin lymphoma cells. J Liposome Res. 2021;31(1):64–78. doi: 10.1080/08982104.2020.1720718. [DOI] [PubMed] [Google Scholar]
- 129.Mehnert W., Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 130.Fundarò A., et al. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after iv administration to rats. 2000;42(4):337–343. doi: 10.1006/phrs.2000.0695. [DOI] [PubMed] [Google Scholar]
- 131.Zara G.P., et al. Intravenous administration to rabbits of non-stealth and stealth doxorubicin-loaded solid lipid nanoparticles at increasing concentrations of stealth agent: pharmacokinetics and distribution of doxorubicin in brain and other tissues. 2002;10(4):327–335. doi: 10.1080/10611860290031868. [DOI] [PubMed] [Google Scholar]
- 132.Jain S.K., et al. Development and characterization of 5-FU bearing ferritin appended solid lipid nanoparticles for tumour targeting. 2008;25(5):289–297. doi: 10.1080/02652040701799598. [DOI] [PubMed] [Google Scholar]
- 133.Reddy L.H., et al. Tumoricidal effects of etoposide incorporated into solid lipid nanoparticles after intraperitoneal administration in Dalton's lymphoma bearing mice. 2006;8(2):E254–E262. doi: 10.1007/BF02854895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trochopoulos A.G.X., et al. Antineoplastic effect of a novel nanosized curcumin on cutaneous T cell lymphoma. Oncol Lett. 2020;20(6):304. doi: 10.3892/ol.2020.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yosifov D.Y., et al. Alkylphosphocholines and curcumin induce programmed cell death in cutaneous T-cell lymphoma cell lines. Leuk Res. 2014;38(1):49–56. doi: 10.1016/j.leukres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 136.Uddin S., et al. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24(47):7022–7030. doi: 10.1038/sj.onc.1208864. [DOI] [PubMed] [Google Scholar]
- 137.Das L., Vinayak M. Curcumin attenuates carcinogenesis by down regulating proinflammatory cytokine interleukin-1 (IL-1α and IL-1β) via modulation of AP-1 and NF-IL6 in lymphoma bearing mice. Int Immunopharm. 2014;20(1):141–147. doi: 10.1016/j.intimp.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 138.Das L., Vinayak M. Long-term effect of curcumin down-regulates expression of tumor necrosis factor-α and interleukin-6 via modulation of E26 transformation-specific protein and nuclear factor-κB transcription factors in livers of lymphoma bearing mice. Leuk Lymphoma. 2014;55(11):2627–2636. doi: 10.3109/10428194.2014.889824. [DOI] [PubMed] [Google Scholar]
- 139.Zhao Q., et al. Curcumin sensitizes lymphoma cells to DNA damage agents through regulating Rad51-dependent homologous recombination. Biomed Pharmacother. 2018;97:115–119. doi: 10.1016/j.biopha.2017.09.078. [DOI] [PubMed] [Google Scholar]
- 140.Qiao Q., Jiang Y., Li G. Curcumin enhances the response of non-Hodgkin's lymphoma cells to ionizing radiation through further induction of cell cycle arrest at the G2/M phase and inhibition of mTOR phosphorylation. Oncol Rep. 2013;29(1):380–386. doi: 10.3892/or.2012.2091. [DOI] [PubMed] [Google Scholar]
- 141.Zhang W., et al. Curcumin exerts anti-tumor effects on diffuse large B cell lymphoma via regulating PPARγ expression. Biochem Biophys Res Commun. 2020;524(1):70–76. doi: 10.1016/j.bbrc.2019.12.129. [DOI] [PubMed] [Google Scholar]
- 142.Hussain A.R., et al. Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt's lymphoma cell lines. Mol Cancer Therapeut. 2008;7(10):3318–3329. doi: 10.1158/1535-7163.MCT-08-0541. [DOI] [PubMed] [Google Scholar]
- 143.Shishodia S., et al. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70(5):700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 144.Zhao Y., et al. Induction of cell cycle arrest and apoptosis by CPUC002 through stabilization of p53 and suppression of STAT3 signaling pathway in multiple myeloma. Cell Biol Toxicol. 2021;37(1):97–111. doi: 10.1007/s10565-020-09565-x. [DOI] [PubMed] [Google Scholar]
- 145.Lin L., et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia. 2019;33(6):1475–1486. doi: 10.1038/s41375-018-0331-6. [DOI] [PubMed] [Google Scholar]
- 146.Lee J.H., et al. Arctiin is a pharmacological inhibitor of STAT3 phosphorylation at tyrosine 705 residue and potentiates bortezomib-induced apoptotic and anti-angiogenic effects in human multiple myeloma cells. Phytomedicine. 2019;55:282–292. doi: 10.1016/j.phymed.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 147.Fu Y., et al. Physalis alkekengi var. franchetii extracts exert antitumor effects on non-small cell lung cancer and multiple myeloma by inhibiting STAT3 signaling. OncoTargets Ther. 2021;14:301–314. doi: 10.2147/OTT.S282334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bharti A.C., Donato N., Aggarwal B.B. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171(7):3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 149.Pang M., et al. S1PR2 knockdown promotes migration and invasion in multiple myeloma cells via NF-κB activation. Cancer Manag Res. 2020;12:7857–7865. doi: 10.2147/CMAR.S237330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jin W., et al. Magnolol suppressed cell migration and invasion and induced cell apoptosis via inhibition of the NF-κB signaling pathway by upregulating microRNA-129 in multiple myeloma. Neoplasma. 2021;68(2):404–415. doi: 10.4149/neo_2020_200923N1010. [DOI] [PubMed] [Google Scholar]
- 151.Tsubaki M., et al. Overexpression of HIF-1α contributes to melphalan resistance in multiple myeloma cells by activation of ERK1/2, Akt, and NF-κB. Lab Invest. 2019;99(1):72–84. doi: 10.1038/s41374-018-0114-8. [DOI] [PubMed] [Google Scholar]
- 152.Yi H., et al. Albendazole inhibits NF-κB signaling pathway to overcome tumor stemness and bortezomib resistance in multiple myeloma. Cancer Lett. 2021;520:307–320. doi: 10.1016/j.canlet.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 153.Allegra A., et al. Curcumin ameliorates the in vitro efficacy of carfilzomib in human multiple myeloma U266 cells targeting p53 and NF-κB pathways. Toxicol Vitro. 2018;47:186–194. doi: 10.1016/j.tiv.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 154.Bharti A.C., et al. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101(3):1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 155.Bai Q.X., Zhang X.Y. Curcumin enhances cytotoxic effects of bortezomib in human multiple myeloma H929 cells: potential roles of NF-κB/JNK. Int J Mol Sci. 2012;13(4):4831–4838. doi: 10.3390/ijms13044831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu Y., et al. Disulfiram/copper markedly induced myeloma cell apoptosis through activation of JNK and intrinsic and extrinsic apoptosis pathways. Biomed Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110048. [DOI] [PubMed] [Google Scholar]
- 157.Shen X., et al. Knockdown of long non-coding RNA PCAT-1 inhibits myeloma cell growth and drug resistance via p38 and JNK MAPK pathways. J Cancer. 2019;10(26):6502–6510. doi: 10.7150/jca.35098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L., et al. Gossypol induces apoptosis of multiple myeloma cells through the JUN-JNK pathway. Am J Cancer Res. 2020;10(3):870–883. [PMC free article] [PubMed] [Google Scholar]
- 159.Su Z., et al. Ciclopirox and bortezomib synergistically inhibits glioblastoma multiforme growth via simultaneously enhancing JNK/p38 MAPK and NF-κB signaling. Cell Death Dis. 2021;12(3):251. doi: 10.1038/s41419-021-03535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Han D., et al. Curcumin synergistically enhances the cytotoxicity of arsenic trioxide in U266 cells by increasing arsenic uptake. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/3083041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu J., et al. Arsenic trioxide inhibits the proliferation of myeloma cell line through notch signaling pathway. Cancer Cell Int. 2013;13(1):25. doi: 10.1186/1475-2867-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elmahi A.Y., et al. Effects of arsenic trioxide alone and in combination with bortezomib in multiple myeloma RPMI 8266 cells. Asian Pac J Cancer Prev APJCP. 2013;14(11):6469–6473. doi: 10.7314/apjcp.2013.14.11.6469. [DOI] [PubMed] [Google Scholar]
- 163.McCafferty-Grad J., et al. Arsenic trioxide uses caspase-dependent and caspase-independent death pathways in myeloma cells. Mol Cancer Therapeut. 2003;2(11):1155–1164. [PubMed] [Google Scholar]
- 164.Ganesan M., et al. Phytochemicals reverse P-glycoprotein mediated multidrug resistance via signal transduction pathways. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111632. [DOI] [PubMed] [Google Scholar]
- 165.Pulukuri A.J., et al. Acquired drug resistance enhances imidazoquinoline efflux by P-glycoprotein. Pharmaceuticals. 2021;14(12) doi: 10.3390/ph14121292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao L., et al. Reversal of P-glycoprotein-mediated multidrug resistance by novel curcumin analogues in paclitaxel-resistant human breast cancer cells. Biochem Cell Biol. 2020;98(4):484–491. doi: 10.1139/bcb-2019-0377. [DOI] [PubMed] [Google Scholar]
- 167.Sagnou M., et al. Novel curcumin derivatives as P-glycoprotein inhibitors: molecular modeling, synthesis and sensitization of multidrug resistant cells to doxorubicin. Eur J Med Chem. 2020;198 doi: 10.1016/j.ejmech.2020.112331. [DOI] [PubMed] [Google Scholar]
- 168.Lopes-Rodrigues V., Sousa E., Vasconcelos M.H. Curcumin as a modulator of P-glycoprotein in cancer: challenges and perspectives. Pharmaceuticals. 2016;9(4) doi: 10.3390/ph9040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sung B., et al. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol Cancer Therapeut. 2009;8(4):959–970. doi: 10.1158/1535-7163.MCT-08-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peng Y., et al. IGF-1 promotes multiple myeloma progression through PI3K/Akt-mediated epithelial-mesenchymal transition. Life Sci. 2020;249 doi: 10.1016/j.lfs.2020.117503. [DOI] [PubMed] [Google Scholar]
- 171.Liu S., et al. miR-215-5p is an anticancer gene in multiple myeloma by targeting RUNX1 and deactivating the PI3K/AKT/mTOR pathway. J Cell Biochem. 2020;121(2):1475–1490. doi: 10.1002/jcb.29383. [DOI] [PubMed] [Google Scholar]
- 172.Chen P., et al. PI3K/Akt inhibitor LY294002 potentiates homoharringtonine antimyeloma activity in myeloma cells adhered to stromal cells and in SCID mouse xenograft. Ann Hematol. 2018;97(5):865–875. doi: 10.1007/s00277-018-3247-3. [DOI] [PubMed] [Google Scholar]
- 173.He W., et al. Diallyl thiosulfinate enhanced the anti-cancer activity of dexamethasone in the side population cells of multiple myeloma by promoting miR-127-3p and deactivating the PI3K/AKT signaling pathway. BMC Cancer. 2021;21(1):125. doi: 10.1186/s12885-021-07833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kudo C., et al. Novel curcumin analogs, GO-Y030 and GO-Y078, are multi-targeted agents with enhanced abilities for multiple myeloma. Anticancer Res. 2011;31(11):3719–3726. [PubMed] [Google Scholar]
- 175.Zhang Z., et al. A Gli inhibitor GANT61 suppresses cell proliferation, promotes cell apoptosis and induces G1/G0 cycle retardation with a dose- and time-dependent manner through inhibiting Notch pathway in multiple myeloma. Cell Cycle. 2020;19(16):2063–2073. doi: 10.1080/15384101.2020.1792686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ding Y., Shen Y. Notch increased vitronection adhesion protects myeloma cells from drug induced apoptosis. Biochem Biophys Res Commun. 2015;467(4):717–722. doi: 10.1016/j.bbrc.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 177.Nefedova Y., et al. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111(4):2220–2229. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Y., et al. Apoptosis of mouse myeloma cells induced by curcumin via the Notch3-p53 signaling axis. Oncol Lett. 2019;17(1):127–134. doi: 10.3892/ol.2018.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gomez-Bougie P., et al. Curcumin induces cell death of the main molecular myeloma subtypes, particularly the poor prognosis subgroups. Cancer Biol Ther. 2015;16(1):60–65. doi: 10.4161/15384047.2014.986997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Park J., et al. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol Oncol. 2008;2(4):317–326. doi: 10.1016/j.molonc.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mujtaba T., et al. Sensitizing human multiple myeloma cells to the proteasome inhibitor bortezomib by novel curcumin analogs. Int J Mol Med. 2012;29(1):102–106. doi: 10.3892/ijmm.2011.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li W., et al. A preliminary study of the effect of curcumin on the expression of p53 protein in a human multiple myeloma cell line. Oncol Lett. 2015;9(4):1719–1724. doi: 10.3892/ol.2015.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xiao H., et al. Reversal of multidrug resistance by curcumin through FA/BRCA pathway in multiple myeloma cell line MOLP-2/R. Ann Hematol. 2010;89(4):399–404. doi: 10.1007/s00277-009-0831-6. [DOI] [PubMed] [Google Scholar]
- 184.Wu C., et al. Effect and mechanism of curcumin on EZH2 - miR-101 regulatory feedback loop in multiple myeloma. Curr Pharmaceut Des. 2018;24(5):564–575. doi: 10.2174/1381612823666170317164639. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y.D., Hu Y., Sun C.Y. [Inhibitory effect of curcumin on angiogenesis induced by brain derived neurotrophic factor from multiple myeloma cells] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14(1):70–74. [PubMed] [Google Scholar]
- 186.Ghoneum M., Gollapudi S. Synergistic apoptotic effect of arabinoxylan rice bran (MGN-3/Biobran) and curcumin (turmeric) on human multiple myeloma cell line U266 in vitro. Neoplasma. 2011;58(2):118–123. doi: 10.4149/neo_2011_02_118. [DOI] [PubMed] [Google Scholar]
- 187.Ge X.P., et al. [Curcumin increases the chemosensitivity of multiple myeloma to bortezomib by inhibiting the Notch1 signaling pathway] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(2):464–471. doi: 10.19746/j.cnki.issn.1009-2137.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 188.Zhang X.Y., et al. [Effect of curcumin in combination with bortezomib on proliferation and apoptosis of human multiple myeloma cell line H929 and its mechanism] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19(3):684–688. [PubMed] [Google Scholar]
- 189.Chen J., et al. Curcumin-induced promoter hypermethylation of the mammalian target of rapamycin gene in multiple myeloma cells. Oncol Lett. 2019;17(1):1108–1114. doi: 10.3892/ol.2018.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu B., et al. [Effect of curcumin on expression of survivin, Bcl-2 and Bax in human multiple myeloma cell line] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15(4):762–766. [PubMed] [Google Scholar]
- 191.Xiao H., Zhang K.J., Zuo X.L. [Reversal of multidrug resistance of the drug resistant human multiple myeloma cell line MOLP-2/R by curcumin and its relation with FA/BRCA pathway] Zhonghua Xue Ye Xue Za Zhi. 2009;30(1):33–37. [PubMed] [Google Scholar]
- 192.Tang X., et al. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac Cancer. 2021;12(8):1219–1230. doi: 10.1111/1759-7714.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu Z., Ma H., Lai Z. The role of ferroptosis and cuproptosis in curcumin against hepatocellular carcinoma. Molecules. 2023;28(4) doi: 10.3390/molecules28041623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee Y.J., Park K.S., Lee S.H. Curcumin targets both apoptosis and necroptosis in acidity-tolerant prostate carcinoma cells. BioMed Res Int. 2021;2021 doi: 10.1155/2021/8859181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qin Y., et al. Autophagy and cancer drug resistance in dialogue: pre-clinical and clinical evidence. Cancer Lett. 2023;570 doi: 10.1016/j.canlet.2023.216307. [DOI] [PubMed] [Google Scholar]
- 196.Campesi I., et al. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol Res. 2017;119:195–207. doi: 10.1016/j.phrs.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 197.Tadic M., et al. Gender-specific therapeutic approach in arterial hypertension – challenges ahead. Pharmacol Res. 2019;141:181–188. doi: 10.1016/j.phrs.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 198.DeCosse J., et al. European journal of cancer prevention; 1993. Gender and Colorectal Cancer; pp. 105–115. [DOI] [PubMed] [Google Scholar]