Abstract
Background
To evaluate the therapeutic effectiveness and safety of a neurofeedback wearable device for stress reduction.
Methods
A randomized, double-blind, controlled study was designed. Participants had psychological stress with depression or sleep disturbances. They practiced either neurofeedback-assisted meditation (n = 20; female, 15 [75.0%]; age, 49.40 ± 11.76 years) or neurofeedback non-assisted meditation (n = 18; female, 11 [61.1%]; age, 48.67 ± 12.90 years) for 12 minutes twice a day for two weeks. Outcome variables were self-reported questionnaires, including the Korean version of the Perceived Stress Scale, Beck Depression Inventory-II, Insomnia Severity Index, Pittsburgh Sleep Quality Index, and State Trait Anxiety Index, quantitative electroencephalography (qEEG), and blood tests. Satisfaction with device use was measured at the final visit.
Results
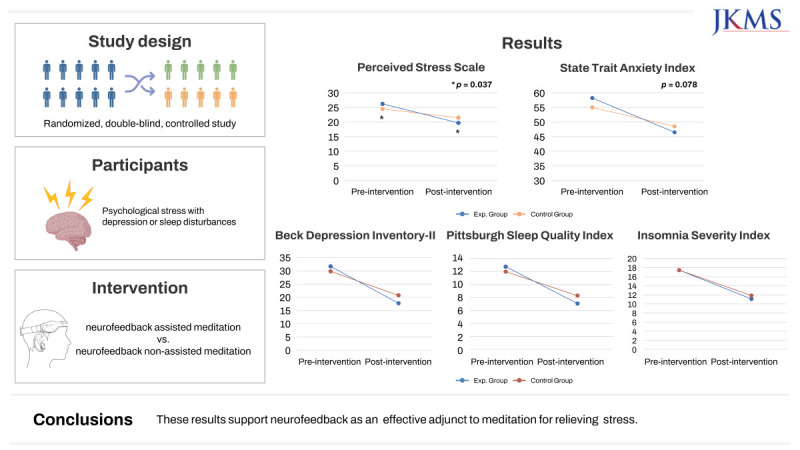
The experimental group had a significant change in PSS score after two weeks of intervention compared with the control group (6.45 ± 0.95 vs. 3.00 ± 5.54, P = 0.037). State anxiety tended to have a greater effect in the experimental group than in the control group (P = 0.078). Depressive mood and sleep also improved in each group, with no significant difference between the two groups. There were no significant differences in stress-related physiological parameters, such as stress hormones or qEEG, between the two groups. Subjective device satisfaction was significantly higher in the experimental group than in the control group (P = 0.008).
Conclusion
Neurofeedback-assisted meditation using a wearable device can help improve subjective stress reduction compared with non-assisted meditation. These results support neurofeedback as an effective adjunct to meditation for relieving stress.
Trial Registration
Clinical Research Information Service Identifier: KCT0007413
Keywords: Meditation, Neurofeedback, Stress Reduction, Wearable Device
Graphical Abstract
INTRODUCTION
The stress response is an adaptive reaction that occurs in stressful situations. It is mediated by the “stress system,” which includes the hypothalamus, autonomic nervous system, and organs.1 In case of chronic stress, long-term activation of the adrenal glands can release excess cortisol (a stress hormone).2 Elevated cortisol levels can increase the risk of many health problems, including anxiety, depression,3 immune disorders,4 heart disease, high blood pressure,5 and diabetes.6 In clinical practice, three main self-regulation strategies are currently used in stress reduction therapy, including meditation, relaxation, and biofeedback.7 Over the last few decades, numerous physiological and psychological benefits of meditation practice have been demonstrated, including tension relaxation, improved concentration, and effects on emotional and cognitive function. In particular, meditation can help relieve stress.8,9 Some studies have found that meditation can induce plastic changes in the brain, thereby creating new neurons and circuits.10,11
Novice meditators can find it difficult to meditate. Without understanding whether they are doing it right, they often stop treatment before any meaningful effects are observed. Therefore, the application of machine-aided learning may offer alternatives for beginners struggling to maintain regular meditation practice. Recent studies have shown that technology-assisted mindfulness meditation practice through mobile applications improves stress management and cognitive efficiency, such as working memory.12,13 Neurofeedback measures brain waves, a physiological phenomenon that is difficult for humans to recognize, and converts them into easy-to-understand information. During this process, a person directly observes the signal from his or her body displayed on the computer, experiences the kind of effort needed to show the increase or decrease of a specific brain wave, and can directly control it.14 The feedback stimuli may be visual, auditory, or other sensory stimuli. The stimuli can inform the user of brain activity so that the user can recognize a response, thus increasing the level of self-control and self-awareness.15 This enhances the plasticity of the brain by strengthening its self-regulatory ability. As in the principle of learning theory, it involves learning the optimal state by looking at the success signal of one’s body.16 Neurofeedback, one of the techniques for training brain self-regulation, has the potential to increase the effectiveness of meditation.17
There are five biomarkers that have been most studied to evaluate stress in electroencephalography (EEG) signals; alpha, theta, beta, delta and asymmetry of alpha. Among them, many studies show changes in brain waves related to stress condition, including a reduction in alpha band power, an increase in beta activity, and a change in theta value.18,19 Overall, in stressful situations, the brain shows a trend in which stable brain wave activity decreases and brain waves related to mental load become more activated. The average alpha and theta power of people with anxiety gradually increased after neurofeedback mindfulness control.20 There was also a study that conducted neurofeedback training to enhance alpha, strengthen theta, and reduce beta.21 The most relevant and consistent EEG finding is that it can increase theta and alpha power.22 Other studies have suggested that neurofeedback can mediate the effects of mindfulness meditation.23,24 A study on neurofeedback training for stress mitigration confirmed the efficacy using a two-channel device at the Fp1 and Fp2 positions.25
This study was designed to evaluate the safety and therapeutic effects of a neurofeedback wearable device (MAVE®) on stress reduction. The meditation content of the device was scientifically verified, and feasibility was assessed by experts.26,27,28 We hypothesized that neurofeedback-assisted meditation could effectively improve stress, depression, and sleep compared with meditation without neurofeedback assistance. In addition, when neurofeedback-assisted meditation was performed using a wearable device, the efficacy and safety of the device were evaluated.
METHODS
Participants
The present study was conducted at the sleep clinic of Seoul National University Bundang Hospital. Participants were recruited between May 2021 and October 2021 through advertising in the hospital and local community. All participants were aged 19–65 years and had psychological stress and symptoms of depression or sleep disturbance. Enrolled participants underwent screening tests at their first visit. A psychiatrist conducted a clinical interview to evaluate the inclusion and exclusion criteria. We included those who met both of the following criteria: 1) Perceived Stress Scale (PSS) score ≥ 14 and 2) either Beck Depression Inventory-II (BDI-II) score ≥ 20 or Insomnia Severity Index (ISI) score ≥ 8. Individuals were excluded if they had other major psychiatric disorders, any sleep disorders based on the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) or International Classification of Sleep Disorders (ICSD)-3, major medical conditions, or neurological abnormalities. We also excluded those who had recently received steroid and hormone therapy, those who suffered from chronic pain, and those who had acute severe stressful life events within the last month, as these conditions could cause an increase in stress hormone levels.
Procedure
Neurofeedback-assisted meditation intervention
Eligible participants were randomly assigned in a 1:1 ratio to either the experimental group practicing neurofeedback-assisted meditation or the control group practicing neurofeedback non-assisted meditation for two weeks. Random assignment was performed via the stratified permuted block randomization method by a single person not directly involved in this clinical trial. Both participants and investigators were blinded to the treatment conditions. A device with the same configuration was used, thus it was impossible to distinguish between the experimental and control groups. Demographic, medical, and anthropometric data were collected after the screening test. Quantitative electroencephalography (qEEG), questionnaires, heart rate variability tests, and blood tests were then conducted for each participant.
Participants were asked to perform meditation using the device twice a day for a total of two weeks (14 days, 12 minutes per session). The MAVE® device is designed to measure EEG signals through two channels of EEG sensors on the forehead and pulse signals through one channel that measures pulse signals (photoplethysmography) in the right earlobe (Fig. 1A). The MAVE® device is composed of a measurement unit and analysis software. In order to process artifacts, signal processing is performed in the analysis software. Independent component analysis (ICA) is used to remove artifacts caused by eye-rolling. Additionally, an adaptive filtering method is employed to eliminate eye blink-induced artifacts, and muscle activity that affects the entire frequency range of the electromyography (EMG) signal is excluded. Since MAVE® is a 2-channel system, independent component analysis (ICA) is not used in its software. The measurement stack uses a 12-bit analog-to-digital converter (ADC) with a sampling rate of 250 samples per second. The MAVE® application provides mindfulness-based meditation content and guides meditation with voice guidance. Brain waves were measured while the participant was meditating and undergoing a signal processing procedure (qEEG spectral analysis). The brain waves returned auditory signals according to the threshold condition of the neurofeedback protocol algorithm. Therefore, the user received both the meditation content guide and neurofeedback as sound stimulation. When the meditation was completed, the measured bio-signals and the feedback signal log were stored in the user’s smartphone. User identifiers (ID) and access log information were also saved.
Fig. 1. MAVE® device and Neurofeedback protocol using MAVE® device and mobile application. (A) MAVE® device consists of a headband with 2 channels of EEG sensor and one channel of PPG sensor. (B) A 2-min resting EEG measurement was performed prior to meditation to determine baseline EEG. The relative power of the target frequency bands (alpha, theta, and high beta bands) was determined. Based on the baseline value, it was set to reach the threshold when all formulas were simultaneously satisfied in the alpha, theta, and high beta bands.
EEG = electroencephalography, PPG = photoplethysmography.
The neurofeedback protocol algorithm is illustrated in Fig. 1B. It consisted of 2 minutes of resting EEG measurement and 10 minutes of neurofeedback combined with meditation for a total of 12 minutes per session. Resting EEG measurements were performed for 2 minutes prior to meditation using the application with eye closed. This was used to determine the baseline EEG. The relative power of the target frequency bands, including alpha, theta, and high beta bands, were also determined. Based on the baseline value determined in this manner, the protocol was set to reach the threshold when alpha and theta waves, which are brain waves to be strengthened, and high beta waves, which are brain waves to be suppressed, were simultaneously satisfied, as shown in the formula. This setting condition was established based on EEG changes known to be common when meditation is effectively performed (Fig. 1B).29,30
When the condition of the above three band values was satisfied, the auditory stimulus signal was provided as output along with feedback. Through this, the participant could know whether his or her meditation state was progressing effectively. The neurofeedback signal uses the sound of a cricket (1 second) in a high-frequency band that does not interfere with meditation. The experimental group meditated while receiving feedback on their EEG status. The control group used the same mindfulness-based meditation content provided by the application. However, the feedback auditory signal was provided at a random time point, independent of the neurofeedback protocol output.
Approximately one week after the second visit, participants were enquired over phone to check if they were using the device properly (number of days of use per week [%]) and whether there were any adverse reactions. The final visit took place two weeks after the initiation of the intervention. Vital signs, physical examination, questionnaire, qEEG, heart rate variability test, and blood tests (cortisol, adrenocorticotropic hormone [ACTH], interleukin-6 [IL-6], tumor necrosis factor-alpha [TNF-α], and brain-derived neurotrophic factor [BDNF]) were evaluated or performed after using the device. Treatment compliance was assessed twice during the study period: a telephonic interview on day 8 of the intervention and a final assessment at the end of the two-week intervention. We collected data on participants’ satisfaction with the device via a questionnaire at the final visit on a scale of 0 (lowest degree) to 10 (highest degree) scale. The participants were enquired if they experienced any side effects in an open-ended manner.
Measures
Questionnaires
All participants were asked to complete self-report questionnaires on the symptoms of stress, depression, and anxiety before and after the intervention. The PSS was adopted to estimate the degree of perceived stress in daily life. The PSS, a classic stress assessment instrument developed in 1983, is widely used today.31 This tool helps understand how different situations affect an individual’s feelings and their perceived stress over the past month. The PSS consists of 10 items with total scores ranging from 0 to 40, with a higher score indicating more severe stress symptoms.32 Symptoms of depression and anxiety were evaluated using the BDI-II and State-Trait Anxiety Inventory (STAI), respectively. The STAI has the advantage of being able to measure both trait anxiety, which is considered stable over time, and state anxiety, which is affected by stressful situations. Participants were administered the Pittsburgh Sleep Quality Index (PSQI) and ISI to evaluate subjective sleep quality and insomnia symptoms, respectively. The PSQI consists of 19 questions, with a total index of over five indicating a subjective sleep complaint.33 The ISI consists of seven questions. It is a 28-point test, with higher scores indicating more severe insomnia.34 The World Health Organization Quality of Life (WHOQOL-BREF) scale has been widely used as a quality-of-life assessment tool. It consists of 26 items in the domains of physical health, psychological health, social relationships, and environment.35 Subjective device satisfaction was evaluated on a scale of 1 (very dissatisfied) to 10 (very satisfied) using a questionnaire at the final visit after 14 days of device use.
Blood biomarkers
The levels of ACTH, cortisol, BDNF, and inflammatory biomarkers, such as IL-6 and TNF-α, were measured. Cortisol follows a robust circadian rhythm that peaks 30 minutes after waking and gradually declines throughout the day.36,37 Therefore, blood tests for biomarkers before and after the intervention were performed at designated times in the afternoon (from 1 PM to 5 PM) as the initial and follow-up assessments for each participant.38 Blood samples were collected from the antecubital vein, processed according to the protocol, and transferred to Seoul Clinical Laboratories (Yongin, Korea), a global clinical testing laboratory.
qEEG
All participants underwent qEEG twice, that is, before and after the intervention. Waking EEG was recorded with participants in a sitting position for 15 minutes, during which they were instructed to close their eyes and relax. Electrodes were placed according to the extended international 10–20 system. The EEG signal was amplified and digitized with 64-channel Neuroscan Synamps (Compumedics, Charlotte, NC, USA) at a sampling rate of 1 kHz. The acquired EEG data were processed using NeuroGuide (Applied Neuroscience, Inc., St. Petersburg, FL, USA). The high-pass filter was set to 100 Hz and the low-pass filter was set to 0.3 Hz. Each EEG was visually inspected to exclude artifacts caused by small body movements, eyelid movements, or fine sleep. Spectral analysis was performed with fast Fourier transform for six bands (delta: 1.0–4.0 Hz; theta: 4.0–8.0 Hz; alpha: 8.0–12.0 Hz; beta: 12.0–25.0 Hz; high beta: 25.0–30.0 Hz; and gamma: 30.0–40.0 Hz). The electrodes were grouped into five cerebral regions to calculate the average power value of each region: frontal (FP1, FP2, F3, F4, F7, and F8), temporal (T3, T4, T5, and T6), central (C3 and C4), parietal (P3 and P4), and occipital (O1 and O2).
Statistical analysis
Continuous variables were presented as means ± standard deviations, while categorical variables were expressed as proportions, unless otherwise specified. All statistical analyses were performed using SPSS Statistics version 16.0 (SPSS Inc., Chicago, IL, USA). For continuous variables (e.g., age and body mass index), a two-sample t-test was used to compare the values obtained between the two groups. For categorical variables, such as sex and medication use, the χ2 test was used. To investigate within-group differences between pre- and post-intervention assessments, including questionnaires, blood tests, and qEEG, a paired t-test or Wilcoxon signed-rank test was adopted. Repeated measures analysis of variance (ANOVA) was used to assess differences between the groups before and after treatment. Generalized estimating equations were used to analyze qEEG changes between groups by region over time. Bonferroni correction was used for post-hoc multiple comparisons and independent samples.
Ethics statement
All participants provided informed consent prior to participating in the study. This study was reviewed and approved in April 2021 by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (B-2103/670-002). It was registered at the Clinical Research Information Service, Korea (CRIS registry number, KCT0007413), and the first registration date was June 17, 2022. All methods used in this study were performed in accordance with relevant guidelines and regulations.
RESULTS
Baseline demographics and clinical characteristics
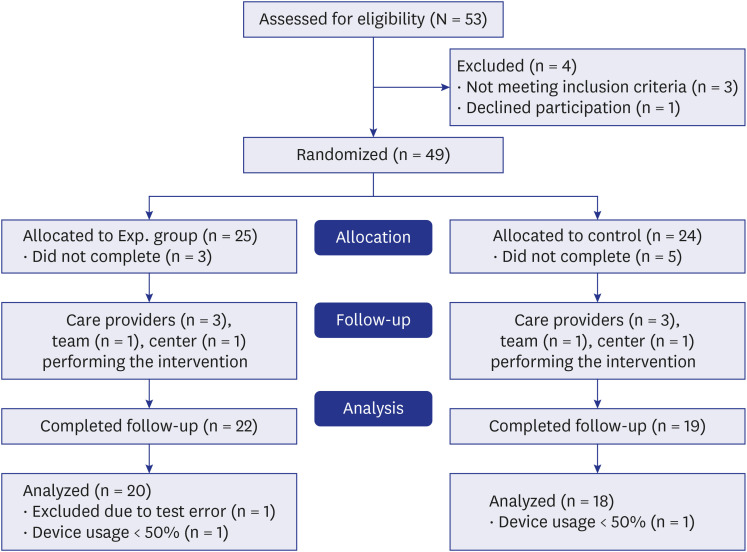
The planned sample size of 80 participants was reduced to 53 due to the coronavirus disease 2019 situation and time constraint of the city-funded study. A total of 53 participants were screened in this study, of which 49 were randomly assigned to two groups, after excluding three who did not meet the PSS or BDI-II/ISI criteria and one who revealed the intention to withdraw immediately after screening (Fig. 2). Therefore, 25 and 24 participants were assigned to the experimental and control groups, respectively. In the experimental group, three people, including one who complained of earlobe discomfort, dropped out. In the control group, five people, including three who complained of side effects, such as headache and earlobe pain, expressed their intention to drop out. The dropout rate was 12.0% (3/25) in the experimental group and 20.8% (5/24) in the control group, with no significant difference between the two groups (P = 0.403). Participants who completed the test at the last visit after the intervention but used the device for less than 90% of the time were excluded from the analysis. Finally, 38 participants who completed all the study protocols were included in the analysis (Fig. 2). When demographics and clinical characteristics, including questionnaires and blood tests, were compared, no statistically significant difference was found between the experimental and control groups in the baseline evaluation. The mean age was 49.1 ± 12.15 years. The majority (26/38, 68.4%) of the participants were females. The demographic and clinical characteristics of the study population are shown in Table 1.
Fig. 2. Flow chart showing the selection of study participants.
Experimental group: neurofeedback-assisted meditation group; Control group: neurofeedback non-assisted meditation group.
Table 1. Demographics and clinical characteristics of the study population.
| Characteristics | Experimental group (n = 20) | Control group (n = 18) | P value | |
|---|---|---|---|---|
| Sex (female) | 15 (75.0) | 11 (61.1) | 0.489 | |
| Age, yr | 49.40 ± 11.76 | 48.67 ± 12.90 | 0.856 | |
| Married | 14 (70.0) | 12 (66.7) | 0.976 | |
| Education, yr | 15.50 ± 2.14 | 15.72 ± 3.18 | 0.942 | |
| BMI, kg/m2 | 23.32 ± 2.19 | 23.61 ± 2.71 | 0.715 | |
| Medication use (present) | 6 (30.0) | 3 (23.7) | 0.627 | |
| Smoker | 2 (10.0) | 1 (5.6) | 0.612 | |
| Alcohol | 10 (50.0) | 10 (55.6) | 0.732 | |
| Questionnaires, score | ||||
| PSS | 25.85 ± 4.97 | 24.22 ± 5.17 | 0.329 | |
| BDI-II | 31.70 ± 10.14 | 29.72 ± 10.09 | 0.551 | |
| ISI | 17.40 ± 5.84 | 17.22 ± 5.14 | 0.922 | |
| STAI-S | 58.45 ± 9.68 | 55.06 ± 10.83 | 0.346 | |
| STAI-T | 59.65 ± 10.28 | 56.78 ± 9.29 | 0.374 | |
| PSQI | 11.95 ± 3.65 | 11.83 ± 3.19 | 0.917 | |
| WHOQOL | 69.70 ± 12.95 | 67.61 ± 11.19 | 0.600 | |
| Blood test | ||||
| IL-6, pg/mL | 1.35 ± 0.88 | 1.20 ± 0.75 | 0.707 | |
| BDNF, ng/mL | 27.21 ± 6.84 | 30.46 ± 6.34 | 0.139 | |
| TNF-α, pg/mL | 0.70 ± 0.22 | 0.69 ± 0.24 | 0.613 | |
| Cortisol, μg/dL | 8.21 ± 3.47 | 8.58 ± 2.80 | 0.723 | |
| ACTH, pg/mL | 13.49 ± 5.61 | 16.41 ± 7.56 | 0.196 | |
Data are presented as mean ± standard deviation for numerical data and as number (percentage) for categorized data.
BMI = body Mass Index, PSS = Perceived Stress Scale, BDI-II = Beck Depression Inventory-II, ISI = Insomnia Severity Index, STAI = State-Trait Anxiety Inventory, PSQI = Pittsburgh Sleep Quality Index, WHOQOL = World Health Organization Quality of Life assessment instrument, IL-6 = interleukin-6, BDNF = brain-derived neurotrophic factor, TNF-α = tumor necrosis factor-alpha, ACTH = adrenocorticotropic hormone.
Effects of MAVE® intervention
Table 2 shows the results of the primary and secondary outcomes measured before and after the intervention period in the experimental and control groups. The difference in PSS scores before and after two weeks of intervention was significantly different between the two groups. In the experimental group, the PSS score decreased considerably from 25.85 ± 4.97 at baseline to 19.40 ± 4.56 after the intervention over two weeks, which was statistically significant (P < 0.0001). In the control group, the PSS level decreased by 3.00 (from 24.22 ± 5.17 at a baseline to 21.22 ± 4.60) after two weeks of intervention; however, the decrease was not statistically significant. Therefore, the neurofeedback-assisted meditation group had a more pronounced subjective stress reduction, indicating that the difference in PSS scores between the groups over time was significant as shown by repeated-measures ANOVA (P = 0.037). Regarding state anxiety, there was a significant change within the experimental and control groups after two weeks of intervention. Although statistical significance was not reached for the difference between the two groups, the effect was found to be greater in the experimental group than in the control group (11.95 ± 10.46 vs. 6.50 ± 7.69, P = 0.078). Depressive mood and sleep improved in both the groups. There were no significant differences between the two groups. As a result of the blood test, both the experimental and control groups showed a decrease in serum BDNF levels after the intervention, although no significant difference was observed before and after treatment between the groups. Serum cortisol and ACTH levels also decreased after the intervention in both groups. However, there were no significant differences within or between groups before and after the intervention (Table 2). Table 3 shows the device-related scores for the control and experimental groups. Satisfaction of the device was significantly higher in the experimental group than in the control group (7.85 ± 1.31 vs. 6.72 ± 1.07, P = 0.008). There was no significant difference in the number of days of device use frequency between the groups.
Table 2. Primary and secondary outcome measures before and after 2 weeks of intervention.
| Variables | Experimental group (n = 20) | Control group (n = 18) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | P | Pre-intervention | Post-intervention | P | |||
| Questionnaires, score | ||||||||
| PSS | 25.85 ± 4.97 | 19.40 ± 4.56 | < 0.001*** | 24.22 ± 5.17 | 21.22 ± 4.60 | 0.318 | 0.037* | |
| BDI-II | 31.70 ± 10.14 | 17.55 ± 10.58 | < 0.001*** | 29.72 ± 10.09 | 20.22 ± 8.10 | < 0.001*** | 0.200 | |
| ISI | 17.40 ± 5.84 | 11.45 ± 5.41 | 0.003** | 17.22 ± 5.17 | 11.83 ± 5.88 | 0.001** | 0.810 | |
| STAI-S | 58.45 ± 9.67 | 46.50 ± 11.45 | < 0.001*** | 55.06 ± 10.83 | 48.56 ± 7.56 | 0.002** | 0.078 | |
| STAI-T | 59.65 ± 10.28 | 48.50 ± 12.09 | 0.001** | 56.78 ± 9.29 | 50.44 ± 8.02 | < 0.001*** | 0.132 | |
| PSQI | 11.95 ± 3.65 | 8.50 ± 3.80 | 0.001** | 11.83 ± 3.19 | 8.33 ± 2.35 | < 0.001*** | 0.963 | |
| WHOQOL | 69.70 ± 12.95 | 76.90 ± 23.12 | 0.011* | 67.61 ± 11.19 | 73.56 ± 12.23 | 0.034* | 0.833 | |
| Blood tests | ||||||||
| IL-6, pg/mL | 1.35 ± 0.88 | 1.37 ± 0.89 | 0.540 | 1.20 ± 0.75 | 1.31 ± 0.87 | 0.831 | 0.856 | |
| BDNF, ng/mL | 27.21 ± 6.84 | 24.52 ± 8.60 | 0.027* | 30.46 ± 6.34 | 25.96 ± 8.62 | 0.010* | 0.318 | |
| TNF-α, pg/mL | 0.70 ± 0.22 | 0.70 ± 0.32 | 0.354 | 0.69 ± 0.24 | 0.67 ± 0.22 | 0.663 | 0.730 | |
| Cortisol, μg/dL | 8.21 ± 3.47 | 7.49 ± 3.23 | 0.442 | 8.58 ± 2.80 | 7.02 ± 2.97 | 0.105 | 0.529 | |
| ACTH, pg/mL | 13.49 ± 5.61 | 13.18 ± 8.86 | 0.895 | 16.41 ± 7.56 | 14.67 ± 6.01 | 0.983 | 0.657 | |
Data are presented as mean ± standard deviation.
PSS = Perceived Stress Scale, BDI-II = Beck Depression Inventory-II, ISI = Insomnia Severity Index, STAI = State-Trait Anxiety Inventory, PSQI = Pittsburgh Sleep Quality Index, WHOQOL = World Health Organization Quality of Life assessment instrument, IL-6 = interleukin-6, BDNF = brain-derived neurotrophic factor, TNF-α = tumor necrosis factor-alpha, ACTH = adrenocorticotropic hormone.
Bold face P values denote statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3. Device-related scores.
| Variables | Experimental group (n = 20) | Control group (n = 18) | P |
|---|---|---|---|
| Days of device use, days | 13.80 ± 0.41 | 13.61 ± 1.50 | 0.573 |
| Number of times the device was used, No. | 26.10 ± 2.55 | 26.33 ± 2.89 | 0.718 |
| Device satisfaction, score | 7.85 ± 1.31 | 6.72 ± 1.07 | 0.008** |
| Side effects of using the device | 2 (10.0) | 0 (0.00) | 0.168 |
Data are presented as mean ± standard deviation for numerical data and as number (percentage) for categorized data.
Bold face P values denote statistical significance: **P < 0.01.
qEEG
The group-by-region over time effect was not significant for the absolute EEG power in all bands, except for the alpha band, which had a significant effect (χ2 = 10.643, P = 0.031). However, differences in brain regions were not found in the post hoc test. There was no significant change in the absolute power of any band in any brain region within or between groups. With respect to the relative EEG power, the group-by-region interaction over time was not significant for most bands. Regarding the relative power, only in the delta band was a Group × Region × Time effect (χ2 = 9.912, P = 0.042). However, there were no regional differences in the post hoc tests. In addition, independent analyzes were conducted for each channel between groups before and after the intervention, but no significant results were obtained.
Safety reports
During the study, side effects were reported by three (12.0%) participants in the neurofeedback-assisted meditation group and three (15.8%) in the non-assisted meditation group. Of these six participants, four expressed their intention to drop out and two completed the use of the device for two weeks. Two participants complained of headaches while using the device, three reported redness or pain in the earlobe, and two reported chest discomfort (feeling of stinging). The relevance and severity of the side effects reported by the participants were evaluated, and only earlobe discomfort was considered relevant. All side effects were mild, and the symptoms were temporary. In addition, there was no significant difference between the experimental and control groups in the incidence rate of adverse events among completers (10.0% [2/20] vs. 0% [0/18], P = 0.168).
DISCUSSION
In this study, the efficacy and safety of neurofeedback-assisted meditation therapy were confirmed for those experiencing symptoms of psychological stress. We hypothesized that neurofeedback could enhance meditation performance, resulting in a reduction in subjective stress, depression and anxiety, and physiological changes as effects of meditation. Compared to the non-assisted meditation treatment group, the neurofeedback-assisted meditation group showed a significant improvement in subjective stress levels. The neurofeedback-assisted group also showed a greater improvement in state anxiety than the control group, although the difference was not statistically significant. No significant decrease in serum cortisol level was observed in either the experimental or control groups. Regarding safety, no serious adverse events occurred during the study period. The risk of adverse events using the MAVE® device was low. There were no significant differences between the two groups. In the evaluation of device satisfaction, subjective device satisfaction was significantly higher in the experimental group than in the control group.
In this study, a significant change in the PSS score indicating stress level was observed after two weeks of meditation intervention. Compared with the control group, in which no statistically significant change was observed, the neurofeedback-assisted meditation group showed a remarkable decrease in the PSS score, which was meaningful as it confirmed the effect of neurofeedback-assisted meditation treatment over time.39 This is consistent with the results of previous studies showing that practicing mindfulness meditation had a positive effect on stress reduction. This implies that the effect of neurofeedback assistance can further maximize the effect of meditation over a short period of time. After two weeks of intervention, the experimental group showed significantly higher satisfaction than the control group in the subjective satisfaction questionnaire of device use. This may be because, first, in the neurofeedback-assisted meditation group, effects of stress reduction and tension relief were greater. Thus, the comfort of the device itself can be high. Alternatively, this could be related to a feedback signal. In this study, auditory cues were used to investigate the effects of neurofeedback. In the experimental group, the measured EEG was calculated according to a predetermined protocol, and an auditory signal was provided when changes beyond the preset level were shown. In contrast, in the control group, the auditory signal was provided randomly by dividing it into three sections during the 10-minute meditation time. Sensitive individuals might feel that they have no control over their feedback because the randomly set feedback signal cannot be ruled out. However, if no signal is given in the control group, the participants can easily recognize the group to which they are assigned.
According to a study on the effect of a biofeedback-assisted meditation program in diabetic patients with depression, stress improvement was shown in those who participated in a 90-minute weekly meditation program with a researcher for six weeks.40 We hypothesized that wearable neurofeedback-assisted meditation could also lead to significant positive changes in physiological measures of relaxation and stress. However, these results do not support our expectations. No significant differences were found in stress-related physiological variables, such as serum cortisol between neurofeedback-assisted meditation and non-assisted meditation. This suggests that the intervention period was too short to achieve physiological changes, unlike subjective stress changes. In addition, in this study, the type and degree of stress were limited, and various physical diseases that could affect cortisol levels were excluded. Many exclusion criteria for physical condition might have influenced the degree of change in serum in the results of this study. Previous studies have shown that meditation intervention has a significant, large effect on cortisol in a sample of a patient with physical illness or in those experiencing a large amount of stress.40 Moreover, there were no significant differences between the groups in the evaluation of most other questionnaires, including BDI-II and PSQI. One possible explanation for the significant improvements in both groups could be the effect of meditation itself. The difference between the two groups was relatively small, regardless of whether neurofeedback was assisted, suggesting that the effect of meditation was strong. Even the non-assisted meditation group may have experienced a sense of accomplishment from completing the meditation practice, contributing to their improvement. Another possible explanation is the placebo effect. The act of participating in a study and receiving some form of intervention can lead to positive response or improvement in symptoms.
Neurofeedback has been introduced to smartphones and mobile applications.12 With the development of wearable devices, the measurement and monitoring of physiological signals (respiration, pulse, etc.) has become simple and accessible to the public. Therefore, it is easy and convenient to provide feedback on a user’s physiological signals immediately. Attempts to apply this to biofeedback are increasing. The wearable device processes the measured signal (input) and provides audible or visual feedback (output). This feedback allows the user to focus on their state and further adjust their behavior, thereby forming a loop. With the development of dry electrodes, it is now possible to conveniently measure EEG without restrictions in place.41,42 In addition, an EEG measuring device designed to be lightweight wirelessly while reducing the number of electrodes is being developed into a portable wearable device.41 Therefore, not only biofeedback using breathing and pulse but also neurofeedback, which monitors and feeds back brain waves, can be a natural direction for the development of wearable devices. In fact, most participants gave positive feedback about the devices, stating that they could be used conveniently anytime and anywhere, which shows high compliance and a high number of days of using the device. Only three (7.2%) of the 41 participants who completed all pre- and post-intervention tests did not achieve 50% of the device use. All 38 patients included in the analysis showed more than 90% compliance. There have been studies that have observed the effects of neurofeedback-mediated meditation.13,43 Our study has a strength in that mindfulness meditation was performed both in sham and neurofeedback-assisted intervention to demonstrate the neurofeedback effect.
This study has some limitations. First, there is no single EEG pattern associated with meditation, although there are various approaches to meditation. Thus, some studies have shown that each meditation style affects a specific area of the brain.44,46,46 However, mindfulness may be the most appropriate meditation practice to manage stress and anxiety, as it requires a much more relaxed form of observation than other meditation styles, such as focus meditation, open heart, and quiet mind.29 Second, this study had a short intervention period, a small sample size and lack of analysis of the correlation between the EEG and the clinical scale changes, which may have limited the ability to detect significant physiological alterations. Furthermore, the absence of a follow-up phase to assess the durability of the neurofeedback training effects over time is another limitation. Therefore, future studies with a longer intervention period, larger sample size, and a follow-up phase are warranted to further investigate the long-term effectiveness and sustainability of the observed effects. Third, most participants in this study were beginner meditators who had never experienced meditation. If they were not fully engaged in meditation, they were likely to think about themselves or something related to them, which could be expressed as a decrease in alpha amplitude or an increase in beta or gamma in the default mode network as a result.47 However, it was confirmed that not only experienced meditators but also beginners could control their experience in relation to the feedback signal.48 Therefore, this might be a reasonable selection of participants, considering the purpose of this study and MAVE® device is to increase access to neurofeedback and meditation. To compensate for these limitations, an education session was conducted so that the participants could use the device and perform meditation after the screening test. In addition, this study protocol was retrospectively registered in the clinical trial registry because the Clinical Research Information Service, a Korean clinical trial registration platform, allows retrospective registration for clinical trials that have already been conducted. However, registration should be completed in advance to meet international standards.
Our randomized, double-blind, and controlled study in individuals with symptoms of psychological stress revealed that neurofeedback-assisted meditation with a portable wearable device could be used anytime and anywhere to help improve subjective stress compared with unassisted meditation. The MAVE® device can be considered a convenient and safe adjuvant treatment option in line with this new trend. However, it is unclear whether its effects persisted over time. This is a worthwhile area for future research. Large-scale samples and long-term longitudinal studies are needed in the future. This study is meaningful in that few studies have directly compared the portable neurofeedback device, which is simple and can measure EEG without any location or time restriction, depending on whether neurofeedback is assisted.
ACKNOWLEDGMENTS
To conduct this study, it is acknowledged that the MAVE® device and software were provided by Meddiction Co., Ltd (Guro-gu, Seoul, Republic of Korea), a medical device manufacturing company.
Footnotes
Funding: This study was supported by a research grant from the Seoul Business Agency Cooperative Project (G Valley ICT convergence and integration technology commercialization support project, grant number: CG200007).
Disclosure: The authors have no potential conflicts of interest to disclose.
Data Availability Statement: The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
- Conceptualization: Hong JK, Yoon IY.
- Data curation: Lee E, Hong JK.
- Formal analysis: Lee EY.
- Funding acquisition: Yoon IY.
- Investigation: Lee E, Hong JK, Choi H.
- Methodology: Lee E, Hong JK.
- Software: Lee E, Yoon IY.
- Validation: Lee E, Choi H, Yoon IY.
- Visualization: Lee E.
- Writing - original draft: Lee E.
- Writing - review & editing: Lee E, Choi H, Yoon IY.
References
- 1.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 2.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 3.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 5.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol. 2000;35(7) Suppl 4:S1–7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd C, Smith J, Weinger K. Stress and diabetes: a review of the links. Diabetes Spectr. 2005;18(2):121–127. [Google Scholar]
- 7.Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 8.Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 9.Jo JM. EEG brainwave analysis for research on meditation influence to the concentration. J Korea Inst Electron Commun Sci. 2014;9(12):1421–1426. [Google Scholar]
- 10.Ahn SK. The Effect of Neurofeedback Training on Attention and School Achievement Motivation of primary. J Korea Acad Ind Coop Soc. 2011;12(12):5525–5530. [Google Scholar]
- 11.Lardone A, Liparoti M, Sorrentino P, Rucco R, Jacini F, Polverino A, et al. Mindfulness meditation is related to long-lasting changes in hippocampal functional topology during resting state: a magnetoencephalography study. Neural Plast. 2018;2018:5340717. doi: 10.1155/2018/5340717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balconi M, Fronda G, Crivelli D. Effects of technology-mediated mindfulness practice on stress: psychophysiological and self-report measures. Stress. 2019;22(2):200–209. doi: 10.1080/10253890.2018.1531845. [DOI] [PubMed] [Google Scholar]
- 13.Crivelli D, Fronda G, Venturella I, Balconi M. Stress and neurocognitive efficiency in managerial contexts: a study on technology-mediated mindfulness practice. Int J Workplace Health Manag. 2019;12(2):42–56. [Google Scholar]
- 14.Brandmeyer T, Delorme A. Meditation and neurofeedback. Front Psychol. 2013;4:688. doi: 10.3389/fpsyg.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teplan M. Fundamentals of EEG measurement. Meas Sci Rev. 2002;2(2):1–11. [Google Scholar]
- 16.Sherlin LH, Arns M, Lubar J, Heinrich H, Kerson C, Strehl U, et al. Neurofeedback and basic learning theory: implications for research and practice. J Neurother. 2011;15(4):292–304. [Google Scholar]
- 17.Nieto-Vallejo AE, Ramírez-Pérez OF, Ballesteros-Arroyave LE, Aragón A. Design of a neurofeedback training system for meditation based on EEG technology. Rev Fac Ing. 2021;30(55):e12489 [Google Scholar]
- 18.Alonso JF, Romero S, Ballester MR, Antonijoan RM, Mañanas MA. Stress assessment based on EEG univariate features and functional connectivity measures. Physiol Meas. 2015;36(7):1351–1365. doi: 10.1088/0967-3334/36/7/1351. [DOI] [PubMed] [Google Scholar]
- 19.Attar ET. Review of electroencephalography signals approaches for mental stress assessment. Neurosciences. 2022;27(4):209–215. doi: 10.17712/nsj.2022.4.20220025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Xiao X, Belkacem AN, Lu L, Wang X, Yi W, et al. Efficacy evaluation of neurofeedback-based anxiety relief. Front Neurosci. 2021;15:758068. doi: 10.3389/fnins.2021.758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheon EJ, Koo BH, Choi JH. The efficacy of neurofeedback in patients with major depressive disorder: an open labeled prospective study. Appl Psychophysiol Biofeedback. 2016;41(1):103–110. doi: 10.1007/s10484-015-9315-8. [DOI] [PubMed] [Google Scholar]
- 22.Lomas T, Ivtzan I, Fu CH. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci Biobehav Rev. 2015;57:401–410. doi: 10.1016/j.neubiorev.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Navarro Gil M, Escolano Marco C, Montero-Marín J, Minguez Zafra J, Shonin E, García Campayo J. Efficacy of neurofeedback on the increase of mindfulness-related capacities in healthy individuals: a controlled trial. Mindfulness. 2018;9(1):303–311. [Google Scholar]
- 24.Sas C, Chopra R. MeditAid: a wearable adaptive neurofeedback-based system for training mindfulness state. Pers Ubiquitous Comput. 2015;19(7):1169–1182. [Google Scholar]
- 25.Hafeez Y, Ali SS, Mumtaz W, Moinuddin M, Adil SH, Al-Saggaf UM, et al. Investigating neurofeedback protocols for stress mitigation: a comparative analysis of different stimulus contents. IEEE Access. 2019;7:141021–141035. [Google Scholar]
- 26.Treleaven DA. Trauma-Sensitive Mindfulness: Practices for Safe and Transformative Healing. New York, NY, USA: WW Norton & Company; 2018. [Google Scholar]
- 27.Kim J. Quality grading measure for online digital contents vitalization. J Digit Contents Soc. 2014;15(2):309–317. [Google Scholar]
- 28.Yoo K. The development of internet contents evaluation criteria for early childhood education. J Early Child Educ. 2006;26(5):287–306. [Google Scholar]
- 29.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 30.Lee DJ, Kulubya E, Goldin P, Goodarzi A, Girgis F. Review of the neural oscillations underlying meditation. Front Neurosci. 2018;12:178. doi: 10.3389/fnins.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EH. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res. 2012;6(4):121–127. doi: 10.1016/j.anr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Shin C, Ko YH, Lim J, Joe SH, Kim S, et al. The reliability and validity studies of the Korean version of the Perceived Stress Scale. Korean J Psychosom Med. 2012;20(2):127–134. [Google Scholar]
- 33.Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16(3):803–812. doi: 10.1007/s11325-011-0579-9. [DOI] [PubMed] [Google Scholar]
- 34.Cho YW, Song ML, Morin CM. Validation of a Korean version of the Insomnia Severity Index. J Clin Neurol. 2014;10(3):210–215. doi: 10.3988/jcn.2014.10.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 36.Akerstedt T, Levi L. Circadian rhythms in the secretion of cortisol, adrenaline and noradrenaline. Eur J Clin Invest. 1978;8(2):57–58. doi: 10.1111/j.1365-2362.1978.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 37.Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]
- 38.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Yu X, Cohen ZP, Tsuchiyagaito A, Cochran G, Aupperle RL, Stewart JL, et al. Neurofeedback-augmented mindfulness training elicits distinct responses in the subregions of the insular cortex in healthy adolescents. Brain Sci. 2022;12(3):363. doi: 10.3390/brainsci12030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koncz A, Demetrovics Z, Takacs ZK. Meditation interventions efficiently reduce cortisol levels of at-risk samples: a meta-analysis. Health Psychol Rev. 2021;15(1):56–84. doi: 10.1080/17437199.2020.1760727. [DOI] [PubMed] [Google Scholar]
- 41.Bhayee S, Tomaszewski P, Lee DH, Moffat G, Pino L, Moreno S, et al. Attentional and affective consequences of technology supported mindfulness training: a randomised, active control, efficacy trial. BMC Psychol. 2016;4(1):60. doi: 10.1186/s40359-016-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polich G, Gray S, Tran D, Morales-Quezada L, Glenn M. Comparing focused attention meditation to meditation with mobile neurofeedback for persistent symptoms after mild-moderate traumatic brain injury: a pilot study. Brain Inj. 2020;34(10):1408–1415. doi: 10.1080/02699052.2020.1802781. [DOI] [PubMed] [Google Scholar]
- 43.Crivelli D, Fronda G, Balconi M. Neurocognitive enhancement effects of combined mindfulness–neurofeedback training in sport. Neuroscience. 2019;412:83–93. doi: 10.1016/j.neuroscience.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 44.Anand BK, Chhina GS, Singh B. Some aspects of electroencephalographic studies in Yogis. Electroencephalogr Clin Neurophysiol. 1961;13(3):452–456. [Google Scholar]
- 45.Tarrant JM. Handbook of Clinical QEEG and Neurotherapy. 1st ed. New York, NY, USA: Routledge; 2016. Neuromeditation: an introduction and overview; pp. 96–113. [Google Scholar]
- 46.Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19(4):1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Lutterveld R, Houlihan SD, Pal P, Sacchet MD, McFarlane-Blake C, Patel PR, et al. Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation. Neuroimage. 2017;151:117–127. doi: 10.1016/j.neuroimage.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]