Abstract
Background
Anastomotic leakage (AL) following esophagectomy represents a serious complication that often results in prolonged hospitalization and necessitates repeated interventions, including nothing-by-mouth (NPO) restriction, endoscopic vacuum therapy (EVT), or surgical repair. In this study, we evaluated the patterns and outcomes of AL treatment.
Methods
We retrospectively reviewed the medical records of patients who underwent esophagectomy for esophageal cancer at a single center between 2003 and 2020. Of 3,096 examined cases, 181 patients (5.8%) with AL were included in the study 114 patients (63%) with cervical anastomosis (CA) and 67 (37%) with intrathoracic anastomosis (TA).
Results
The incidence of AL was 11.9% in the CA and 3.2% in the TA group (p<0.001). Among patients with CA who developed AL, 87 (76.3%) were managed with NPO, 15 (13.2%) with EVT, and 12 (10.5%) with surgical repair. Over 90% of patients with cervical AL resumed an oral diet by the time of discharge, regardless of treatment method. Among patients with TA and AL, 36 (53.7%) received NPO, 25 (37.7%) underwent EVT, and 6 (9%) required surgery. Of these, 34 patients who were managed with NPO and 19 with EVT could resume an oral diet. However, only 2 patients who underwent surgery resumed an oral diet, and 2 patients required additional EVT.
Conclusion
Although patients with CA displayed a higher incidence of AL, their rate of successful oral intake exceeded that of those with TA, regardless of treatment method. Among patients exhibiting AL with TA, EVT was more commonly employed than in CA cases, and it appears effective.
Keywords: Esophageal neoplasms, Esophagectomy, Anastomotic leak, Endoscopy, Vacuum-assisted closure
Introduction
Esophagectomy and esophagogastrostomy have been adopted among curative treatment options for patients with esophageal cancer [1]. Regardless of the anastomosis site, anastomotic leakage (AL) is a highly troublesome complication, resulting in substantial morbidity, prolonged hospitalization, considerable medical expenses, and elevated mortality rates [2].
AL can arise as a serious complication following esophagectomy [3]. The reported incidence of AL ranges from 11.4% to 21.2% [4-7], and the associated mortality rates span from 7.2% to 35% [2]. Prior research has identified variations in AL incidence based on the location of the anastomosis. Specifically, cervical anastomoses have tended to exhibit a higher incidence of AL than intrathoracic anastomoses [2,3]. The incidence of AL in cases involving cervical anastomosis is reported to be between 6.6% and 17.2%, while for intrathoracic anastomoses, the incidence ranges from 2% to 15.9% [2,8-12].
Both nonoperative and operative approaches are utilized in the treatment of AL following esophagectomy. Nonoperative strategies include conservative management and endoscopic intervention. Conservative management involves a regimen of nothing by mouth (NPO) and nutritional support through either tube feeding via jejunostomy or parenteral nutrition. Endoscopic options include the placement of self-expandable metal stents and the application of endoluminal vacuum-assisted therapy (EVT), as well as the use of endoscopic clips and suturing techniques, alongside other novel approaches. Of the various endoscopic techniques, EVT is gaining prominence due to its excellent reported outcomes. Surgical intervention may be indicated for patients with sepsis or fulminant mediastinitis, or in cases requiring conduit removal and replacement due to AL caused by conduit necrosis. However, the current trend among surgeons is to endeavor to preserve the gastric conduit whenever feasible.
This study was conducted to describe the clinical characteristics of AL in patients who underwent esophagectomy as treatment for esophageal cancer, as well as to document the patterns and outcomes associated with each treatment modality for AL.
Methods
Patient selection
We conducted a retrospective review of patients who underwent esophagectomy, utilizing data from the Registry for Thoracic Cancer Surgery (RTCS) of Samsung Medical Center. The RTCS is a repository of prospectively gathered data regarding all patients who have undergone thoracic surgery at our institution since 1994. Our review focused on patients who underwent esophagectomy between September 2003 and December 2020 (N=3,096). For this analysis, we included patients who experienced AL (n=181, 5.8%) and excluded those with graft necrosis necessitating conduit resection with diversion (n=15, 0.5%). This study adhered to the principles outlined in the Declaration of Helsinki. The requirement for informed consent was waived following the approval of the institutional review board of Samsung Medical Center (IRB approval no., 2023- 07-088-002).
Surgery
Mobilization of the conduit, either stomach or colon, was achieved through an upper midline laparotomy. Most patients underwent esophagectomy via a transthoracic approach. To create the intrathoracic anastomosis, a connection between the conduit and the esophagus was established using a circular stapler just below the thoracic inlet, accessed via right thoracotomy. The cervical anastomosis was established on the left side of the neck through a cervical incision, employing either hand-sewn techniques or circular stapling depending on the surgeon’s preference. The hand-sewn technique was carried out using a continuous running suture for the posterior row of the anastomosis and full-thickness, single-layer interrupted sutures for the anterior row. When the cervical anastomosis was constructed using a stapler, a circular stapler was utilized.
In patients with squamous cell carcinoma of the middle and lower thoracic esophagus who show no evidence of cervical nodal metastasis on preoperative imaging studies, our standard procedure has been to perform esophagectomy with 2-field lymph node dissection (2FL). This 2FL approach encompasses both the mediastinal and abdominal lymph node stations. In contrast, for squamous cell carcinoma located in the upper thoracic esophagus, we have adopted a 3-field lymph node dissection strategy, which involves the resection of lymph nodes from the cervical station in addition to the aforementioned 2 stations.
Anastomotic assessment
All patients underwent endoscopy and/or esophagography to diagnose AL between 4 to 7 days following esophagectomy. AL was defined as a full-thickness gastrointestinal defect involving the esophagus, anastomosis, staple line, or conduit, regardless of the presentation or method of detection, in accordance with the International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy as presented by the Esophageal Complications Consensus Group. Patients with AL were categorized based on the location of the anastomosis: either cervical or intrathoracic.
Patients diagnosed with AL following esophagectomy underwent weekly evaluations using endoscopy. In the absence of AL during these endoscopic examinations, patients were permitted to begin oral intake. When endoscopic findings were inconclusive regarding the presence of AL, esophagography with a radio-contrast medium was performed to ascertain its presence. Those with AL deemed resolved by esophagography were cleared to resume oral intake.
Treatment strategy for anastomotic leakage
Once the presence of AL after esophagectomy was established, the treatment approach for patients diagnosed with AL was determined by the respective surgeon. The criteria for selecting a treatment approach have exhibited minor variations over time. Prior to 2015, conservative management was adopted for small ALs identified by endoscopy that were anticipated to resolve spontaneously, provided the patient remained clinically stable. This conservative approach involved maintaining the patient on NPO while administering enteral or parenteral nutrition. In contrast, if the AL was of a considerable size, or if the patient’s condition worsened due to complications such as sepsis, surgical drainage and primary repair were undertaken.
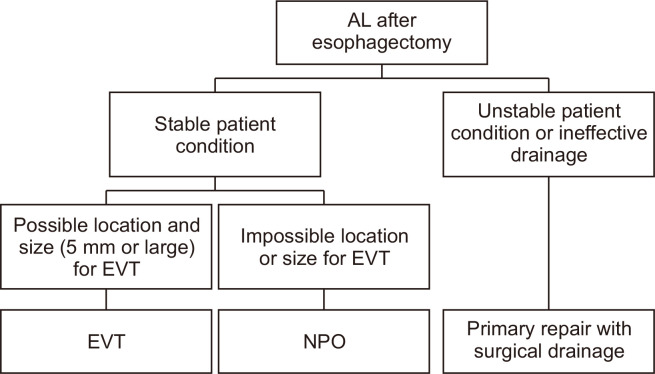
In 2015, we began performing EVT at our institution, prompting changes in the treatment selection criteria for AL. Conservative management was applied if the AL was smaller than 5 mm or if EVT was not feasible due to the location of the lesion. Conversely, for ALs measuring 5 mm or larger, EVT was the preferred treatment. In instances where the drainage catheter had been removed or proved to be ineffective—necessitating surgical drainage—primary repair was performed along with the placement of an effective surgical drainage catheter. Fig. 1 illustrates the treatment selection strategy for AL following the adoption of EVT.
Fig. 1.
Schematic diagram of treatment modality selection for anastomotic leakage (AL) after esophagectomy. EVT, endoscopic vacuum therapy; NPO, nothing by mouth.
Statistical analyses
Patient characteristics and clinical outcomes were compared across treatments using the Student t-test or the Mann-Whitney U test for continuous variables, based on the normality of the distribution. The Pearson chi-square and Fisher exact tests were used for categorical variables, as appropriate. Multivariable Cox regression analysis was performed to identify prognostic factors for AL healing. A p-value of less than 0.05 was considered to indicate statistical significance. Statistical analyses were conducted using R ver. 3.6.1 (The R Project for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
Results
Patient characteristics
Of the 3,096 patients who underwent esophagectomy during the study period, 181 (5.8%) experienced AL. The mean patient age was 64.2±8 years, and male participants predominated at 95.6%. The mean preoperative body mass index was 22.3±3.6 kg/m2. Prior to surgery, 76 patients received treatment, with 73 (40.3%) undergoing concurrent chemoradiation (CCRT) and 3 (1.7%) receiving chemotherapy alone. The distribution of tumor locations was as follows: cervical (6 patients, 4%), upper thoracic (51 patients, 33.6%), mid-thoracic (52 patients, 34.2%), lower thoracic (41 patients, 27%), and esophagogastric junction (2 patients, 1.3%). Regarding the site of anastomosis, cervical anastomosis was performed in 114 patients (63%), while intrathoracic anastomosis was utilized in 67 patients (37%). Pathology reports indicated that R0 resection was achieved in over 90% of cases, while R1 or R2 resections were reported in fewer than 10%. These findings are detailed in Table 1.
Table 1.
Patient characteristics and outcomes by treatment
| Characteristic | Total (N=181) | NPO (n=123) | EVT (n=40) | Surgery (n=18) | p-value |
|---|---|---|---|---|---|
| Age (yr) | 64.24±7.99 | 64.05±8.22 | 64.25±7.30 | 65.56±8.15 | 0.7697 |
| Sex | 0.6148 | ||||
| Female | 8 (4.42) | 7 (5.69) | 1 (2.50) | 0 | |
| Male | 173 (95.58) | 116 (94.31) | 39 (97.5) | 18 (100.00) | |
| Preoperative body mass index (kg/m2) | 22.34±3.61 | 22.58±3.71 | 21.66±3.81 | 22.25±2.11 | 0.4195 |
| Smoking | 0.999 | ||||
| No | 13 (8.55) | 10 (8.85) | 2 (8.33) | 1 (6.67) | |
| Yes | 139 (91.45) | 103 (91.15) | 22 (91.67) | 14 (93.33) | |
| Preoperative therapy | 0.7727 | ||||
| None | 105 (58.01) | 72 (58.54) | 21 (52.50) | 12 (66.67) | |
| Concurrent chemoradiation | 73 (40.33) | 49 (39.84) | 18 (45.00) | 6 (33.33) | |
| Chemotherapy | 3 (1.66) | 2 (1.63) | 1 (2.50) | 0 | |
| Tumor location | 0.225 | ||||
| Cervical | 6 (3.95) | 4 (3.54) | 1 (4.17) | 1 (6.67) | |
| Upper thoracic | 51 (33.55) | 43 (38.05) | 3 (12.50) | 5 (33.33) | |
| Mid-thoracic | 52 (34.21) | 37 (32.74) | 9 (37.50) | 6 (40.00) | |
| Lower thoracic | 41 (26.97) | 27 (23.89) | 11 (45.83) | 3 (20.00) | |
| Esophagogastric junction | 2 (1.32) | 2 (1.77) | 0 | 0 | |
| Site of anastomosis | <0.001 | ||||
| Cervical | 114 (62.98) | 87 (70.73) | 15 (37.50) | 12 (66.67) | |
| Intrathoracic | 67 (37.02) | 36 (29.27) | 25 (62.50) | 6 (33.33) | |
| Resection margin | 0.7056 | ||||
| R0 | 163 (90.05) | 110 (89.43) | 38 (95.00) | 15 (83.33) | |
| R1 | 17 (9.39) | 12 (9.76) | 2 (5.00) | 3 (16.67) | |
| R2 | 1 (0.55) | 1 (0.81) | 0 | 0 | |
| Time between esophagectomy and diagnosis of AL (day) | 11 (7–15) | 10 (7–13) | 15 (13–22) | 11 (9–15.5) | <0.001 |
| Treatment outcome | 0.0017 | ||||
| Failure | 10 (5.52) | 2 (1.63) | 5 (12.50) | 3 (16.67) | |
| Success without additional therapy | 158 (87.29) | 112 (91.06) | 33 (82.50) | 13 (72.22) | |
| Subsequent surgery | 10 (5.52) | 8 (6.5) | 2 (5.00) | 0 | |
| Subsequent EVT | 3 (1.66) | 1 (0.81) | 0 | 2 (11.11) | |
| Oral diet | 0.0034 | ||||
| Failure | 12 (6.63) | 3 (2.44) | 6 (15.00) | 3 (16.67) | |
| Success | 169 (93.37) | 120 (97.56) | 34 (85.00) | 15 (83.33) | |
| Time between esophagectomy and oral diet resumption (day) | 31 (21–52) | 25 (19–38) | 52 (34.5–76.5) | 52 (29–84) | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range). Statistically significant results are marked in bold.
NPO, nothing by mouth; EVT, endoscopic vacuum therapy; AL, anastomotic leakage.
Treatment outcomes
Of the 181 patients with AL, 123 patients (68%) underwent conservative management, 40 patients (22.1%) received EVT, and 18 patients (9.9%) were treated with surgery as the initial intervention. The median duration from esophagectomy to the identification of AL was 11 days (interquartile range [IQR], 7–15 days). Patients who received conservative management experienced a significantly shorter median time of 10 days (IQR, 7–13 days) compared to those who underwent EVT, who had a median time of 15 days (IQR, 13–22 days), and those who received surgical repair, with a median time of 11 days (IQR, 9–15.5 days).
Among the patients initially treated with conservative management, 112 (91.1%) successfully resumed an oral diet without the need for further intervention. Nine patients required additional procedures, with 8 (6.5%) undergoing surgery and 1 (1%) receiving EVT. Overall, 97.6% of the patients in this group were able to achieve oral intake, with a median duration of 25 days (IQR, 13–22 days) after esophagectomy.
Among patients who received EVT as their initial treatment, 33 (82.5%) were able to resume an oral diet without the need for further intervention, while 2 (5%) required subsequent surgery. Consequently, 85% of these patients could resume an oral diet, with a median time of 52 days (IQR, 34.5–76.5 days) following esophagectomy. In the group receiving surgery as initial treatment, 13 (72.2%) were able to successfully resume an oral diet without additional therapy, while 2 (11.1%) required subsequent EVT. Consequently, 83.3% of these patients managed to achieve oral intake, with a median duration of 52 days (IQR, 29–84 days) following esophagectomy.
Treatment patterns and outcomes by anastomotic location
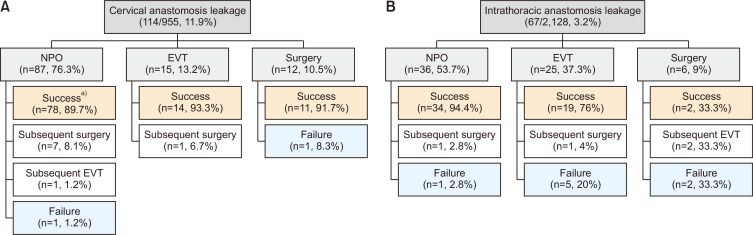
Fig. 2 illustrates the treatment patterns and outcomes based on the location of the anastomosis. Of the 2,128 patients with an intrathoracic anastomosis, 67 (3.2%) experienced AL. Of these patients, conservative management was the initial treatment for 36 (53.7%), EVT for 25 (37.3%), and surgical repair for 6 (9%). Among the 36 patients managed conservatively, 34 (94.4%) were able to resume an oral diet. In contrast, 19 (76%) of the 25 patients who received EVT and only 2 (33.3%) of the 6 patients who underwent surgical repair were able to achieve oral intake.
Fig. 2.
(A, B) Treatment pattern and outcomes by anastomosis site. NPO, nothing by mouth; EVT, endoscopic vacuum therapy. a)Succeeded in achieving oral diet.
Of the 955 patients who underwent cervical anastomosis, 114 (11.9%) developed AL. Of these patients, conservative management was employed for 87 (76.3%), EVT for 15 (13.2%), and primary surgical repair for the remaining 12 (10.5%). Each treatment approach demonstrated superior outcomes relative to those for intrathoracic AL. The rates of successful oral diet resumption were 89.7% for patients treated conservatively, 93.3% for those who received EVT, and 91.7% for those who underwent primary surgical repair.
Prognostic factors for anastomotic leakage healing
Multivariable Cox regression analysis was conducted to identify prognostic factors influencing the healing of AL. The choice of treatment modality for AL has progressed over time and is typically based on the clinical severity of the case. Since many patients in this study received a combination of treatment modalities, this was not included as a variable in our analysis. When considering age, body mass index, preoperative CCRT, and the location of the anastomosis as variables, the odds ratio (OR) for preoperative CCRT was found to be 3.739 (95% confidence interval [CI], 1.004–13.922; p=0.049). The OR for intrathoracic anastomosis was 10.979 (95% CI, 2.059–49.351; p=0.004) (Table 2).
Table 2.
Multivariable Cox regression analysis of factors associated with healing of anastomotic leakage after esophagectomy
| Variable | Odds ratio (95% CI) | p-value |
|---|---|---|
| Age | 1.0295 (0.9469–1.1193) | 0.4960 |
| Preoperative CCRT (vs. upfront surgery) | 3.7391 (1.0042–13.922) | 0.0493 |
| Preoperative BMI | 0.8437 (0.6909–1.0302) | 0.0953 |
| Intrathoracic (vs. cervical) anastomosis | 10.0791 (2.0585–49.351) | 0.0044 |
CI, confidence interval; CCRT, concurrent chemoradiation; BMI, body mass index.
Discussion
In this study, our objective was to gain deeper insight into the treatment patterns and trends in outcomes for AL following esophagectomy as treatment for esophageal cancer. Among the 3,096 patients analyzed, the overall incidence of AL was 5.8%. When we categorized the data based on anastomosis location, the incidence of AL was 3.2% for intrathoracic anastomosis and 11.9% for cervical anastomosis. This finding aligns with other studies that have reported a higher incidence of AL in patients who underwent cervical anastomosis [2,3].
Most patients (68%) who developed AL following esophagectomy received conservative treatment, including an NPO regimen; of these patients, 93.4% successfully resumed an oral diet. Among the patients with intrathoracic anastomosis, conservative management incorporating therapeutic NPO was the predominant treatment approach, achieving a success rate of 94.4%.
Patients with intrathoracic AL were more likely to undergo EVT relative to those with cervical AL. This finding may stem from the challenges associated with re-thoracotomy for surgical intervention and the dissection required due to extensive adhesions resulting from inflammation in the pleural cavity. Additionally, surgical management must account for scenarios where the gastric conduit must be abandoned, necessitating colon or jejunum interposition or the performance of an esophagostomy, followed by prolonged parenteral nutrition, before delayed re-anastomosis can be attempted. Most surgeons are reluctant to choose surgery as the initial treatment, preferring instead to explore options to preserve the gastric conduit. Consequently, we administered EVT approximately 3 times more often for intrathoracic AL than for cervical AL. EVT demonstrated good therapeutic performance, with a treatment success rate of 76%. Given this relatively high success rate and the less invasive nature of the treatment, EVT has been confirmed as a useful therapy for intrathoracic AL, particularly when the surgical approach is complicated. This finding aligns with other research indicating that EVT is commonly employed for intrathoracic AL and has a treatment success rate ranging from 86% to 100% [13].
In patients with cervical AL, surgical intervention was performed more often than among those with intrathoracic AL; this is because the surgical approach is less complex, and endoscopic treatments such as self-expanding metal stents are challenging to implement at the cervical AL site [1]. Surgical treatment demonstrated a markedly higher therapeutic success rate in cervical AL compared to intrathoracic AL (91.7% versus 33.3%). Moreover, EVT displayed good therapeutic performance in cervical AL, achieving a treatment success rate of 93.3%.
In this study, we examined the time interval between esophagectomy and the initiation of an oral diet in cases of AL. We found that the median time to start an oral diet was 31 days across all patients. For those who received only conservative management with therapeutic NPO, the median time was 25 days. In contrast, patients who underwent EVT and surgical intervention had a median time of 52 days. The duration was significantly shorter for the group treated conservatively with therapeutic NPO, presumably because some cases received conservative management due to the anastomotic defect being too small for EVT or the mucosal condition being deemed satisfactory for surgical repair. Consequently, these findings should not be interpreted as evidence of the superiority of conservative treatment.
In this study, we also identified prognostic factors that influenced the healing of AL. The findings showed that neither age nor body mass index had a significant impact on the healing of AL. However, the administration of preoperative CCRT was associated with less successful AL healing. Additionally, AL healing was less favorable when intrathoracic anastomosis was performed, as opposed to cervical anastomosis. These results align with prior research demonstrating an association between preoperative CCRT and an increased risk of anastomotic complications following esophagectomy in patients with esophageal cancer. Studies have reported that patients who received preoperative CCRT faced a higher incidence of AL after esophagectomy [14,15]. Moreover, a study analyzing patients who underwent EVT for AL treatment after esophagectomy found that those who received preoperative CCRT required a longer duration of EVT [16]. This may be attributed to the detrimental effects of radiation on the wound healing process, which in turn contributes to the development of AL after esophagectomy. The location of the AL was also identified as a prognostic factor for healing. In cases of intrathoracic AL, the contaminated contents are likely to disperse widely into the mediastinal and pleural spaces, making it difficult to drain these contents and eradicate sources of infection in the surrounding area. In contrast, cervical AL confines the contaminated contents to a limited space, facilitating easier drainage and maintenance of cleanliness. This likely creates a more conducive microenvironment for the healing of AL.
This study had several limitations. First, as a retrospective observational study, it inherently included potential confounding variables. Additionally, we faced a lack of detailed information regarding the severity of AL, such as the size or extent of the lesion, which precluded the classification of AL severity. This limitation arose because the data were collected through retrospective review of medical records. Consequently, we were unable to propose treatment recommendations tailored to the severity of AL. Second, the study’s findings are based on data from a single institution, which may not be representative of other settings. Variations in patient selection, surgical techniques, and preoperative adjuvant therapy across institutions could yield different outcomes. To address this limitation, future multi-institutional cohort studies are warranted. Third, the inclusion of patients who underwent esophagectomy over a span of 17 years introduces the possibility that changes in the treatment of AL may have occurred during this period. For instance, EVT has only been actively utilized in our hospital in more recent years. Finally, since esophagectomy was performed by several surgeons, the incidence of AL and the choice of treatment methods may vary according to each surgeon’s preferred surgical technique, potentially introducing bias to the results.
In conclusion, the incidence rate of AL was greater among patients with cervical anastomosis; however, the rate of successful oral diet resumption was higher in those with intrathoracic AL, irrespective of the management approach used. Patients with AL following intrathoracic anastomosis were more likely to receive EVT compared to those with cervical anastomosis and AL, and EVT appears to be an effective treatment option.
Funding Statement
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article information
Author contributions
Conceptualization: HWS, YJJ, JHC, HKK, YSC, JIZ, YMS. Data curation: HWS, YJJ, YMS. Writing–original draft: HWS, YJJ. Reviewing the manuscript: JHC, HKK, YSC, JIZ. Reviewing and editing the manuscript: YMS. Final approval of the manuscript: all authors.
Conflict of interest
Hong Kwan Kim was an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
References
- 1.Fabbi M, Hagens ER, van Berge Henegouwen MI, Gisbertz SS. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus. 2021;34:doaa039. doi: 10.1093/dote/doaa039. https://doi.org/10.1093/dote/doaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassis ES, Kosinski AS, Ross P, Jr, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919–26. doi: 10.1016/j.athoracsur.2013.07.119. https://doi.org/10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 3.Jones CE, Watson TJ. Anastomotic leakage following esophagectomy. Thorac Surg Clin. 2015;25:449–59. doi: 10.1016/j.thorsurg.2015.07.004. https://doi.org/10.1016/j.thorsurg.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg. 2019;269:291–8. doi: 10.1097/SLA.0000000000002611. https://doi.org/10.1097/SLA.0000000000002611. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt HM, Gisbertz SS, Moons J, et al. Defining benchmarks for transthoracic esophagectomy: a multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann Surg. 2017;266:814–21. doi: 10.1097/SLA.0000000000002445. https://doi.org/10.1097/SLA.0000000000002445. [DOI] [PubMed] [Google Scholar]
- 6.Seesing MF, Gisbertz SS, Goense L, et al. A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg. 2017;266:839–46. doi: 10.1097/SLA.0000000000002393. https://doi.org/10.1097/SLA.0000000000002393. [DOI] [PubMed] [Google Scholar]
- 7.van Workum F, van der Maas J, van den Wildenberg FJ, et al. Improved functional results after minimally invasive esophagectomy: intrathoracic versus cervical anastomosis. Ann Thorac Surg. 2017;103:267–73. doi: 10.1016/j.athoracsur.2016.07.010. https://doi.org/10.1016/j.athoracsur.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Brown AM, Pucci MJ, Berger AC, et al. A standardized comparison of peri-operative complications after minimally invasive esophagectomy: Ivor Lewis versus McKeown. Surg Endosc. 2018;32:204–11. doi: 10.1007/s00464-017-5660-4. https://doi.org/10.1007/s00464-017-5660-4. [DOI] [PubMed] [Google Scholar]
- 9.Fumagalli U, Baiocchi GL, Celotti A, et al. Incidence and treatment of mediastinal leakage after esophagectomy: insights from the multicenter study on mediastinal leaks. World J Gastroenterol. 2019;25:356–66. doi: 10.3748/wjg.v25.i3.356. https://doi.org/10.3748/wjg.v25.i3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol. 2017;43:258–69. doi: 10.1016/j.ejso.2016.06.394. https://doi.org/10.1016/j.ejso.2016.06.394. [DOI] [PubMed] [Google Scholar]
- 11.Schröder W, Raptis DA, Schmidt HM, et al. Anastomotic techniques and associated morbidity in total minimally invasive transthoracic esophagectomy: results from the EsoBenchmark database. Ann Surg. 2019;270:820–6. doi: 10.1097/SLA.0000000000003538. https://doi.org/10.1097/SLA.0000000000003538. [DOI] [PubMed] [Google Scholar]
- 12.Van Daele E, Van de Putte D, Ceelen W, Van Nieuwenhove Y, Pattyn P. Risk factors and consequences of anastomotic leakage after Ivor Lewis oesophagectomy. Interact Cardiovasc Thorac Surg. 2016;22:32–7. doi: 10.1093/icvts/ivv276. https://doi.org/10.1093/icvts/ivv276. [DOI] [PubMed] [Google Scholar]
- 13.Pines G, Bar I, Elami A, et al. Modified endoscopic vacuum therapy for nonhealing esophageal anastomotic leak: technique description and review of literature. J Laparoendosc Adv Surg Tech A. 2018;28:33–40. doi: 10.1089/lap.2017.0318. https://doi.org/10.1089/lap.2017.0318. [DOI] [PubMed] [Google Scholar]
- 14.Klevebro F, Friesland S, Hedman M, et al. Neoadjuvant chemoradiotherapy may increase the risk of severe anastomotic complications after esophagectomy with cervical anastomosis. Langenbecks Arch Surg. 2016;401:323–31. doi: 10.1007/s00423-016-1409-0. https://doi.org/10.1007/s00423-016-1409-0. [DOI] [PubMed] [Google Scholar]
- 15.Vande Walle C, Ceelen WP, Boterberg T, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys. 2012;82:e513–9. doi: 10.1016/j.ijrobp.2011.05.071. https://doi.org/10.1016/j.ijrobp.2011.05.071. [DOI] [PubMed] [Google Scholar]
- 16.Min YW, Kim T, Lee H, et al. Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg. 2019;19:37. doi: 10.1186/s12893-019-0497-5. https://doi.org/10.1186/s12893-019-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]