Abstract
Papillary muscle rupture is usually caused by myocardial infarction although rare cases of non-ischemic etiology have also been described. Among these, infective endocarditis represents an important cause. Herein, we report a case due to Streptococcus agalactiae involving the posteromedial papillary muscle.
Learning objective
Non-ischemic papillary muscle rupture should be suspected when there is no evidence of atherosclerotic coronary artery disease. In the febrile patient, infective endocarditis should be considered in the differential diagnosis.
Keywords: Streptococcus agalactiae, Papillary muscle rupture, Infective endocarditis, Acute mitral regurgitation, Echocardiography
Introduction
Papillary muscle rupture and acute severe mitral regurgitation are rare complications of infective endocarditis. Herein we present a case in a patient with Streptococcus algalactiae bacteremia.
Case report
A 42-year-old male in otherwise good health was admitted to the hospital with complaints of fever and low back pain of 3 days' duration. Examination revealed temperature of 38.30C, blood pressure of 110/70 mm Hg, and pulse of 92 bpm. There were no murmurs, crackles, or lower extremity edema. Electrocardiography showed sinus rhythm at 90 bpm and there were no ischemic ST-T changes. Chest x-ray was unremarkable. The white blood cell count was elevated to 32 × 103/mcL. Blood cultures were obtained and antibiotic treatment with vancomycin and cefepime was started.
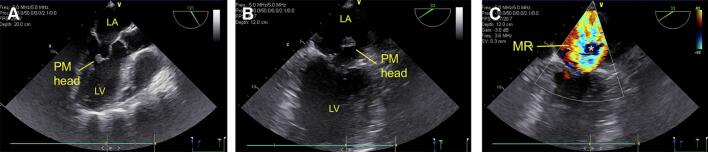
On the second day of hospitalization, the patient became dyspneic and began to deteriorate hemodynamically. Rales were audible over both lung fields although no murmur was appreciated. Transthoracic echocardiographic imaging was performed however image quality was suboptimal due to inadequate acoustic windows and the patient was referred for transesophageal echocardiography. This revealed a ruptured posteromedial papillary muscle (PM) and severe mitral regurgitation (Fig. 1; Video 1, Video 2). All of the heart valves were free of vegetations. The left ventricle was hypercontractile. Pre-operative coronary angiography revealed no evidence of coronary artery disease. The patient was referred for surgery.
Fig. 1.
Transesophageal echocardiographic images of ruptured papillary muscle (PM). (A) Diastolic frame revealing ruptured head of the posteromedial PM in the left ventricle (LV). (B) Systolic frame demonstrating prolapse of the PM head into left atrium (LA). (C) Color flow Doppler image showing severe mitral regurgitation (MR). The asterisk indicates the ruptured PM head.
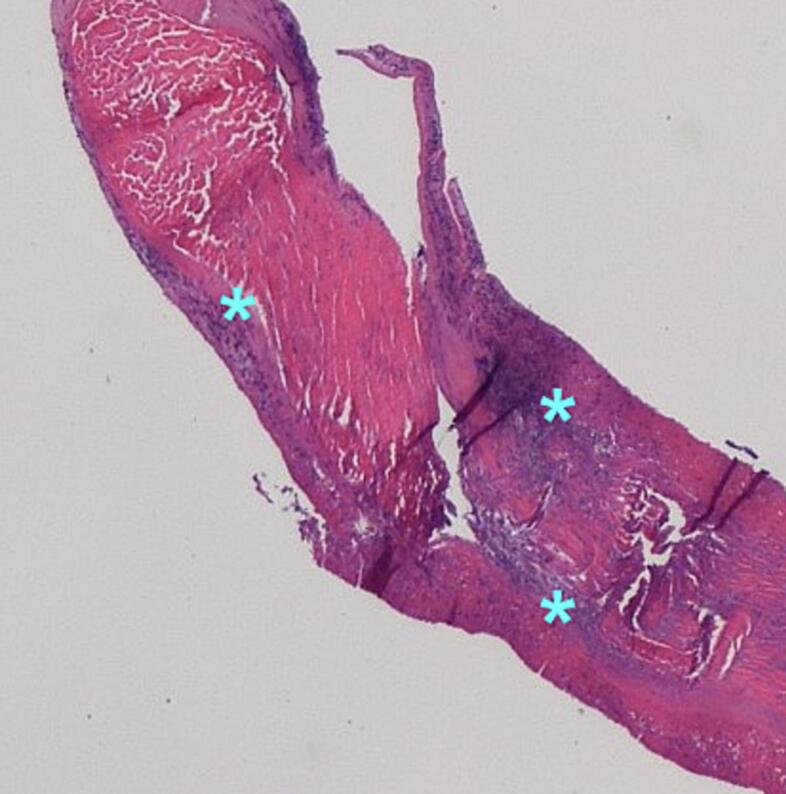
On surgical inspection, the posteromedial PM was ruptured. On-X mitral prosthesis (CryoLife, Kennesaw, GA, USA) was inserted. Histopatholologic examination of the ruptured PM and attached chordae demonstrated numerous foci of neutrophilic infiltration, fibrin deposition, and necrosis consistent with infective endocarditis (Fig. 2). Tissue Gram's stain revealed Gram-positive cocci. Streptococcus agalactiae was eventually isolated from blood cultures obtained on admission. The patient's post-operative course through discharge remained unremarkable and he was continued on antibiotics after leaving the hospital.
Fig. 2.
Histologic section (hematoxylin and eosin stain) of a chord attached to the ruptured papillary muscle head. Note the presence of multiple foci of neutrophilic infiltration (asterisks).
Discussion
Pathogenesis
Most cases of PM rupture are ischemic in etiology. Non-ischemic rupture is rare and has been described in patients with Ehlers-Danlos syndrome type IV, extensive mitral annular calcification, stress cardiomyopathy, myocarditis, and infective endocarditis [1]. Among those with endocarditis, PM rupture may come about through several mechanisms. As in our case, rupture and acute severe mitral regurgitation may result from direct PM invasion. Previous reports attributed direct PM invasion to infection with virulent organisms such as Staphylococcus aureus [2]. To the best of our knowledge, ours represents the first reported case caused by a non-staphylococcal species. PM endocarditis and rupture may also occur when an aortic regurgitation jet originating from an infected aortic valve strikes a PM and establishes a secondary focus of infection [3]. Last, PM rupture may result from embolization of a vegetation fragment into a coronary artery that supplies a PM [4]. This may be recognized angiographically by the presence of abrupt vessel cut-off.
Echocardiographic findings
The infectious etiology of PM rupture was not apparent echocardiographically in our patient. In some, echocardiographic examination may reveal a vegetation attached to a ruptured PM head [5] although this may elude detection due to rapid to-and-fro motion of the detached fragment. Sometimes, PM vegetations do not come to light until surgical exploration [6]. In some patients with PM endocarditis, echocardiographic examination demonstrates vegetations on the aortic valve along with varying amounts of aortic regurgitation [3,6]. Finally, unlike ischemic PM rupture which characteristically affects the posteromedial PM (and is accompanied by abnormal contractility of the posterior wall), rupture due to endocarditis may involve either PM [2,4].
Surgical considerations
Treatment for PM rupture whether due to ischemia or infection requires surgical intervention to restore valve competence. Medical therapy alone carries a high mortality. While the majority of patients with ischemic PM rupture undergo valve replacement, mitral valve repair has been shown to confer a better early prognosis [7,8]. Regardless of the cause of PM rupture, PM necrosis and friability represent potential barriers to durable repair. Inadequate chordal preservation due to chordal excision may result in post-operative left ventricular enlargement and failure [9]. While prolonged antibiotic therapy beyond the peri-operative period is recommended in the setting of PM endocarditis, the possibility of infection of prosthetic material still remains an important concern [10]. Surgical strategies employed in patients with PM rupture due to endocarditis have included both valve repair [2] and replacement [6].
The hemodynamic insult from acute mitral regurgitation will be similar with PM ischemia and infection as the common mechanism is the varying degrees of pulmonary edema and/or cardiogenic shock. In ischemic PM ruputure, shock can be compounded by ventricular dysfunction from acute myocardial infarction whereas sepsis may complicate endocarditis. Surgical mortality from PM rupture remains fairly high. While there are no outcomes data for rupture due to PM endocarditis, mortality due to ischemic PM rupture has been reported to be as high as 21 % [8]. The degree of shock, timeliness of intervention, and preoperative co-morbidities directly impact perioperative morbidity and mortality [7]. In ischemic PM rupture, long term prognosis for patients who survive the perioperative period is reportedly identical to that of matched cohorts who had acute myocardial infarction but did not have mechanical complications from the infarction [7]. One can only speculate whether patients with PM rupture due to endocarditis have a long-term prognosis comparable to that of matched cohorts who undergo isolated mitral valve replacement.
The following are the supplementary data related to this article.
Transesophageal echocardiographic image demonstrating to-and-fro motion of the ruptured PM head between the left ventricle and left atrium.
Transesophageal echocardiographic image demonstrating severe mitral regurgitation.
Informed consent
Written informed consent has been obtained for this manuscript. Local Institutional Review Board approval was not required.
Declaration of competing interest
There are no conflicts of interest to report.
References
- 1.Gouda P., Weilovitch L., Kanani R., Har B. Case report and review of nonischemic spontaneous papillary muscle rupture reports between 2000 and 2015. Echocardiography. 2017;34:786–790. doi: 10.1111/echo.13498. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead N.J., Li S., Lai K. Anterolateral papillary muscle rupture in Staphylococcus aureus endocarditis due to direct bacterial invasion of papillary muscle. Echocardiography. 2017;34:1382–1384. doi: 10.1111/echo.13640. [DOI] [PubMed] [Google Scholar]
- 3.Marumoto A., Shijo T., Hasegawa S. Acute posteromedial papillary muscle rupture secondary to aortic valve endocarditis: a case report. Eur Heart J Case Rep. 2022;6:1–7. doi: 10.1093/ehjcr/ytab374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najib M.Q., Lee H.R., DeValeria P.A., Vinale K.L., Surapaneni P., Chaliki H.P. Anterolateral papillary muscle rupture: an unusual complication of septic coronary embolism. Eur J Echocardiogr. 2011;12:E10. doi: 10.1093/ejechocard/jeq117. [DOI] [PubMed] [Google Scholar]
- 5.Charfeddene S., Triki S., Gueldiche M., Ellouze T., Bahloul A., Triki F., et al. Anterolateral papillary muscle rupture revealing infective endocarditis. J Cardiovasc Echogr. 2022;32:60–62. doi: 10.4103/jcecho.jcecho_57_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nurkalem Z., Gorgulu S., Orhan A.I., Demirci D.E., Sargin M., Gumrukcu G. Papillary muscle rupure secondary to infective endocarditis. Echocardiography. 2008;25:901–903. doi: 10.1111/j.1540-8175.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 7.Russo A., Suri R.M., Grigioni F., Roger V.L., Oh J.K., Mahoney D.W., et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation. 2008;118:1528–1534. doi: 10.1161/CIRCULATIONAHA.107.747949. [DOI] [PubMed] [Google Scholar]
- 8.Massimi G., Matteuci M., Kowalewski M., Ronco D., Jiritano F., Beghi C., et al. Surgical treatment of post-infarction papillary muscle rupture: Sysytemic review and meta-analysis. Ann Cardiothorac Surg. 2022;11:252. doi: 10.21037/acs-2021-ami-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma W., Wijdh-den Hamer I., Koene B.M., Kuijpers M., Natour E., Erasmus M.E., et al. Long-term survival after mitral valve surgery for post-myocardial infarction papillary muscle rupture. J Cardiothorac Surg. 2015;10:11. doi: 10.1186/s13019-015-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilic A., Sultan I., Chu D., Wang Y., Gleason T.G. Mitral valve surgery for papillary muscle rupture: outcomes in 1,342 patients from the Society of Thoracic Surgeons’ database. Ann Thorac Surg. 2020;110:1975–1982. doi: 10.1016/j.athoracsur.2020.03.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal echocardiographic image demonstrating to-and-fro motion of the ruptured PM head between the left ventricle and left atrium.
Transesophageal echocardiographic image demonstrating severe mitral regurgitation.