Abstract
Background
The eligibility and potential benefit of transcatheter edge-to-edge repair (TEER) in addition to guideline-directed medical therapy to treat moderate-severe or severe secondary mitral regurgitation (MR) has not been reported in a contemporary heart failure (HF) population.
Methods
Eligibility for TEER based on Food and Drug Administration (FDA) labeling: (1) HF symptoms, (2) moderate-severe or severe MR, (3) left ventricular ejection fraction (LVEF) 20% to 50%, (4) left ventricular end-systolic dimension 7.0 cm, and (5) receiving GDMT (blocker + angiotensin-converting enzyme inhibitor/angiotensin receptor blocker). The proportion (%) of patients eligible for TEER. The hypothetical number needed to treat to prevent or postpone adverse outcomes was estimated using relative risk reductions from published hazard ratios in the registration trial and the observed event rates.
Results
We identified 50,841 adults with HF and known LVEF. After applying FDA criteria, 2461 patients (4.8%) were considered eligible for transcatheter mitral valve replacement (FDA+), with the vast majority of patients excluded (FDA-) based on a lack of clinically significant MR (N = 47,279). FDA+ patients had higher natriuretic peptide levels and were more likely to have a prior HF hospitalization compared to FDA- patients. Although FDA+ patients had a more dilated left ventricle and lower LVEF, median (25th-75th) left ventricular end-systolic dimension (cm) was low at 4.4 (3.7-5.1) and only 30.8% had severely reduced LVEF. FDA+ patients were at higher risk of HF-related morbidity and mortality. The estimated number needed to treat to potentially prevent or postpone all-cause hospitalization was 4.4, 8.8 for HF hospitalization, and 5.3 for all-cause death at 24 months in FDA+ patients.
Conclusions
There is a low prevalence of TEER eligibility based on FDA criteria primarily due to absence of moderate-severe or severe MR. FDA+ patients are a high acuity population and may potentially derive a robust clinical benefit from TEER based on pivotal studies. Additional research is necessary to validate the scope of eligibility and comparative effectiveness of TEER in real-world populations.
Keywords: Functional, Heart failure, Mitral regurgitation, Transcatheter edge-to-edge repair
Introduction
Secondary mitral regurgitation (MR) is a common sequela of heart failure (HF) and occurs because of progressive adverse remodeling including left ventricular (LV) chamber dilation and apical displacement of the papillary muscles and chordal apparatus.1 It has been associated with impaired quality of life, functional limitations, increased risk of hospitalization, and reduced survival despite guideline-directed medical therapy (GDMT). Transcatheter edge-to-edge repair (TEER)+ GDMT vs. GDMT alone has been associated with a decreased risk of HF hospitalization and improved survival in adults with HF and moderate-severe or severe secondary MR based on the Cardiovascular Outcomes Assessment of the Mitra Clip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial.2,3 The objective is to evaluate the real-world eligibility and potential clinical benefit of TEER in a diverse, contemporary cohort of adults with HF.
Methods
Kaiser Permanente Northern California (KPNC) is a large integrated health care delivery system with 21 hospitals and >260 freestanding clinics where >4.5 million members receive comprehensive care (i.e., inpatient, emergency department, and ambulatory encounters). KPNC membership is highly representative of the local and statewide population with respect to sociodemographic characteristics. This study was approved by the KPNC Institutional Review Board; a waiver of informed consent was granted as this was a data-only study; and clinical trial registration was N/A. All methods were carried out in accordance with relevant guidelines and regulations. The datasets generated and/or analyzed during the current study are not publicly available due to standard institutional data sharing policies but may be made available from the corresponding author on reasonable request.
All KPNC members aged ≥18 years with a diagnosis of HF between January 1st, 2013 and December 31st, 2018 were included. A diagnosis of HF was based on either having been hospitalized with a primary discharge diagnosis of HF and/or having ≥3 ambulatory visits for HF based on International Classification of Diseases, ninth Edition (ICD-9: 398.91, 402.x1, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.x) and 10th Edition (ICD-10: I09.81, I11.0, I11.9, I13.0, I13.1, I13.10, I13.11, I13.2, I50, I50.1, I50.2, I50.20, I50.21, I50.22, I50.23, I50.3, I50.30, I50.31, I50.32, I50.33, I50.4, I50.40, I50.41, I50.42, I50.43, I50.9, I97.13) codes. These codes have been validated in multiple health care delivery systems and have a positive predictive value ≥95%.4,5 KPNC’s Virtual Data Warehouse was used to ascertain comorbidities, medications, and laboratory values.6,7 Patients with prior heart transplant and/or current LV assist device were excluded. In addition, the electronic health record (EHR) was the primary data source for clinical documentation and echocardiogram reports. All-cause hospitalizations, HF hospitalizations, and all-cause death were obtained from the EHR supplemented by state and national reporting databases.
Echocardiograms are reported using semistructured templates via McKesson Cardiology (Irving, TX) at KPNC. Natural language processing (NLP) queries (available upon request) were developed using Linguamatics i2E software (Cambridge, UK, version 6.2.0) to automate the identification of echocardiographic parameters including LV end-systolic dimension (LVESD), LV ejection fraction (LVEF), MR etiology (degenerative/myxomatous, rheumatic, ischemic, endocarditis, congenital, and/or secondary/functional), and MR severity (none, trace, trace-mild, mild, mild-moderate, moderate, moderate-severe, or severe). All NLP queries were derived and validated using a “gold standard” consisting of manual chart review and validation by two physicians, with final adjudication by a board-certified cardiologist where discrepancies existed. We initially identified a random sample of 25 echocardiogram reports with a diagnostic code for HF and MR to develop the queries. We subsequently identified a random validation set of 100 echocardiogram reports and observed 100% agreement between reviews and a positive predictive value, negative predictive value, and accuracy of 100% for all queries. NLP was also used to identify the presence of signs and symptoms of HF based on clinical documentation.8
Eligibility requirements for TEER based on Food and Drug Administration labeling (i.e., FDA+) included (1) HF symptoms, (2) moderate-severe or severe MR (excluding documented primary etiologies), (3) LVEF 20%-50%, (4) LVESD ≤7.0 cm, and (5) receiving GDMT (i.e., defined as any dose of a β-blocker and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin-receptor neprilysin inhibitor if LVEF ≤40%). Outcomes were ascertained from the EHR as previously described.8 We compared baseline clinical characteristics and outcomes of patients meeting the FDA eligibility criteria for TEER using χ2 tests for categorical variables and analysis of variance or Kruskal-Wallis tests for continuous variables, as appropriate. We calculated outcome rates by dividing the number of events multiplied by 100 and divided by total person-years of follow-up. The hypothetical number needed to treat (NNT) to prevent or postpone adverse outcomes was estimated using relative risk reductions from published hazard ratios in the registration trial and the event rates in this observational study.2,3
Results
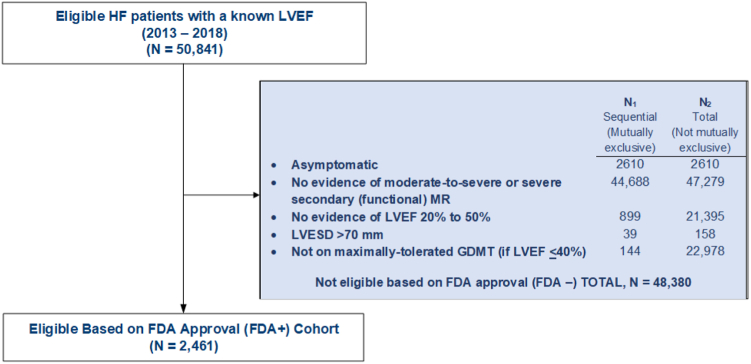
We identified 50,841 adults with HF and a known LVEF including a high proportion of women and racial and/or ethnic minorities. After applying the FDA criteria, 2461 individuals (4.8%) were eligible for TEER (FDA+), with the most common reasons (i.e., not mutually exclusive) for not qualifying (FDA-) being the absence of clinically significant MR (i.e., less than moderate-to-severe) (N = 47,279), a LVEF <20% or >50% (N = 21,398), and/or not receiving GDMT (if LVEF ≤40%) (N = 22,978) (Figure 1). Eligible patients had higher natriuretic peptide levels and were more likely to have a known history of atrial fibrillation/flutter (57.8 vs. 48.8%, p-value < 0.001) prior to 1-year hospitalization for HF compared with noneligible patients (Table 1). Although FDA+ patients had a more dilated LV and lower LVEF, median (25th-75th) LVESD (cm) was low at 4.4 (3.7-5.1), and only 30.8% had a severely reduced LVEF. FDA+ patients were also more likely to have a severely dilated left atrium (46.5 vs. 26.1%, p-value < 0.001). Finally, FDA+ patients experienced higher crude rates of all-cause and HF-specific hospitalizations and all-cause mortality. Based on published hazard ratios and the observed KPNC event rates, the estimated NNT was 4.4 to potentially prevent or postpone all-cause hospitalization, 8.8 for HF hospitalization, and 5.3 for all-cause death at 24 months in FDA+ patients (Table 2).
Figure 1.
Consort diagram showing cohort assembly.
Abbreviations: FDA, Food and Drug Administration; GDMT, guideline-directed medical therapy; HF, heart failure; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
Table 1.
Clinical characteristics and outcomes of patients with heart failure based on Food and Drug Administration eligibility criteria for transcatheter mitral valve repair
| Variable | Overall (N = 50,841) | FDA+ (N = 2461) | FDA- (N = 48,380) | p-Value |
|---|---|---|---|---|
| Age, mean (SD), years | 75.5 (12.7) | 75.3 (12.7) | 75.5 (12.7) | 0.28 |
| Women, n (%) | 22,583 (44.4) | 1157 (47.0) | 21,426 (44.3) | <0.01 |
| Race/ethnicity, n (%) | <0.001 | |||
| Non-Hispanic White | 30,970 (60.9) | 1446 (58.8) | 29,524 (61.0) | |
| Non-Hispanic Black | 5228 (10.3) | 342 (13.9) | 4886 (10.1) | |
| Hispanic | 5839 (11.5) | 295 (12.0) | 5544 (11.5) | |
| Asian/Pacific Islander | 5726 (11.3) | 255 (10.4) | 5471 (11.3) | |
| Missing | 3078 (6.1%) | 123 (5.0) | 2955 (6.1) | |
| Past medical history, n (%) | ||||
| Heart failure hospitalization within prior 1 y | 1355 (2.7) | 1054 (42.8) | 301 (0.6) | <0.001 |
| STS Risk Score, mean (SD), %∗ | 4.6 (3.1) | 4.1 (2.7) | 4.6 (3.1) | |
| Atrial fibrillation and/or flutter | 25,027 (49.2) | 1422 (57.8) | 23,605 (48.8) | <0.001 |
| Coronary artery disease | 9984 (19.6) | 594 (24.1) | 9390 (19.4) | <0.001 |
| Acute myocardial infarction | 5649 (11.1) | 336 (13.7) | 5313 (11.0) | <0.001 |
| Prior coronary artery bypass grafting | 1809 (3.6) | 95 (3.9) | 1714 (3.5) | 0.41 |
| Prior percutaneous coronary intervention | 6820 (13.4) | 394 (16.0) | 6426 (13.3) | <0.001 |
| Implantable cardioverter-defibrillator | 2836 (5.6) | 285 (11.6) | 2551 (5.3) | <0.001 |
| Cardiac resynchronization therapy | 642 (1.3) | 8 (0.3) | 634 (1.3) | <0.001 |
| Ischemic stroke/transient ischemic attack | 7984 (15.7) | 439 (17.8) | 7545 (15.6) | <0.01 |
| Peripheral artery disease | 5167 (10.2) | 295 (12.0) | 4872 (10.1) | <0.01 |
| Chronic kidney disease | 25,948 (51.0) | 1450 (58.9) | 24,498 (50.6) | <0.001 |
| Chronic liver disease | 3281 (6.5) | 144 (5.9) | 3137 (6.5) | 0.21 |
| Chronic lung disease | 14,004 (27.5) | 668 (27.1) | 13,336 (27.6) | 0.65 |
| Anemia | 20,764 (40.8) | 1239 (50.3) | 19,525 (40.4) | <0.001 |
| Labs, median (interquartile range) | ||||
| B-type natriuretic peptide, pg/mL | 495 (231-1043) | 951 (462-1809) | 475 (222-998) | <0.001 |
| eGFR, mL/min/1.73m2, n (%) | <0.001 | |||
| ≥60 | 20,562 (40.4) | 849 (34.5) | 19,713 (40.7) | |
| 45-59 | 10,514 (20.7) | 496 (20.2) | 10,018 (20.7) | |
| 30-44 | 8746 (17.2) | 497 (20.2) | 8249 (17.1) | |
| 15-29 | 4399 (8.7) | 307 (12.5) | 4092 (8,5) | |
| <15 | 845 (1.7) | 46 (1.9) | 808 (1.7) | |
| Dialysis | 1408 (2.8) | 104 (4.2) | 1331 (2.8) | |
| Missing | 4331 (8.5) | 162 (6.6) | 4169 (8.6) | |
| Echocardiogram parameters | ||||
| Left ventricular end-systolic diameter, cm | 3.6 (2.9-4.4) | 4.4 (3.7-5.1) | 3.5 (2.9-4.3) | <0.001 |
| Left ventricular end-diastolic diameter, cm | 5.0 (4.4-5.6) | 5.6 (5.0-6.2) | 5.0 (4.4-5.6) | <0.001 |
| Missing, n (%) | 9530 (18.7) | 178 (7.2) | 9352 (19.3) | |
| Left ventricular ejection fraction, % | 54 (38-60) | 35 (25-45) | 55 (40-60) | <0.001 |
| Qualitative left ventricular systolic function assessment, n (%) | <0.001 | |||
| Preserved | 29,112 (57.3) | 0 (0.0) | 29,112 (60.2) | |
| Mildly reduced | 5826 (11.5) | 736 (29.9) | 5090 (10.5) | |
| Moderately reduced | 9936 (19.5) | 968 (39.3) | 8968 (18.5) | |
| Severely reduced | 5967 (11.7) | 757 (30.8) | 5210 (10.8) | |
| Mitral regurgitation severity, n (%) | <0.001 | |||
| Trace | 231 (0.5) | 0 (0.0) | 231 (0.5) | |
| Mild | 15,451 (30.4) | 0 (0.0) | 15,451 (31.9) | |
| Mild to moderate | 3776 (7.4) | 0 (0.0) | 3776 (7.8) | |
| Moderate | 3920 (7.7) | 0 (0.0) | 3920 (8.1) | |
| Moderate to severe | 2055 (4.0) | 1586 (64.4) | 469 (1.0) | |
| Severe | 1184 (2.3) | 875 (35.6) | 309 (0.6) | |
| None | 24,224 (47.6) | 0 (0.0) | 24,224 (50.1) | |
| Left atrium size, n (%) | <0.001 | |||
| Normal | 11,201 (22.5) | 364 (15.3) | 10,837 (22.9) | |
| Mildly dilated | 10,887 (21.9) | 463 (19.5) | 10,424 (22.0) | |
| Moderately dilated | 7522 (15.1) | 432 (18.2) | 7090 (15.0) | |
| Severe dilated | 13,472 (27.1) | 1104 (46.5) | 12,368 (26.1) | |
| Missing | 6654 (13.4) | 9 (0.4) | 6645 (14.0) | |
| Medications, n (%) | ||||
| ACEi/ARB/ARNi | 30,323 (59.6) | 1751 (71.1) | 28,572 (59.1) | <0.001 |
| β-blocker | 37,800 (74.3) | 2189 (88.9) | 35,611 (73.6) | <0.001 |
| Mineralocorticoid receptor antagonist | 5578 (11.0) | 507 (20.6) | 5071 (10.5) | <0.001 |
| Digoxin | 4615 (9.1) | 420 (17.1) | 4195 (8.7) | <0.001 |
| Nitrates | 8871 (17.4) | 677 (27.5) | 8194 (16.9) | <0.001 |
| Hydralazine | 6829 (13.4) | 484 (19.7) | 6345 (13.1) | <0.001 |
| Loop Diuretic | 30,870 (60.7) | 2008 (81.6) | 28,862 (59.7) | <0.001 |
| Outcomes, per 100 person years (95% confidence) | ||||
| All-cause ED visit | 196.1 (195.1-197.0) | 210.3 (205.1-215.6) | 193.6 (192.6-194.6) | <0.001 |
| HF ED visit | 8.2 (8.0-8.4) | 13.9 (12.6-15.3) | 7.7 (7.5-7.9) | <0.001 |
| All-cause hospitalization | 78.2 (77.5-78.8) | 86.8 (83.5-90.2) | 76.6 (76.0-77.3) | <0.001 |
| HF hospitalization | 3.8 (3.6-3.9) | 12.5 (11.3-13.8) | 3.8 (3.6-3.9) | <0.001 |
| All-cause death | 23.5 (23.1-23.8) | 39.2 (37.0-41.5) | 23.2 (22.9-23.6) | <0.001 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor-neprilysin inhibitor; ED, emergency department; eGFR, estimated glomerular filtration rate; FDA, Food and Drug Administration; HF, heart failure; n, number; SD, standard deviation; STS, Society of Thoracic Surgeons.
Predicted 30-d mortality for isolated mitral valve replacement.
Table 2.
The published relative risk reduction from registration trial, observed event rate in this observational study, and the hypothetical number needed to treat to prevent or postpone individual adverse outcomes
| Outcome | Published relative risk reduction | Observed event rate | NNT |
|---|---|---|---|
| All-cause hospitalization | 0.24 | 94.3 per 100 person-years | 4.4 |
| HF hospitalization | 0.47 | 24.3 per 100 person-years | 8.8 |
| All-cause death | 0.38 | 49.5% | 5.3 |
Abbreviations: HF, heart failure; NNT, number needed to treat.
Discussion
This study applied NLP to EHR data to provide a comprehensive evaluation of the real-world eligibility and potential clinical benefit of TEER in a diverse, contemporary cohort of adults with HF. In summary, we found there is a low prevalence of TEER eligibility, that FDA+ patients experienced higher rates of all-cause and HF-specific morbidity and mortality, and that this high-risk subgroup may potentially derive a robust benefit from TEER.
Our estimate of the prevalence of TEER eligibility is similar to a prior study conducted at two large tertiary HF referral centers, which reported that 6% of patients had moderate-severe or severe MR.9 The FDA+ patients in our study had a BNP level approaching 1000, and more than 40% had a documented HF hospitalization within the last year, suggesting this community-based cohort had a comparable level of acuity to participants enrolled in the approval trial.2,3 This is also reflected in the high rate of HF hospitalizations and death observed in the FDA+ patients.
There continues to be considerable controversy about the optimal approach to selecting ideal candidates for TEER given the discordant findings between the traditional COAPT trial2,3 and the pragmatic MITRA-FR study.10 It has been hypothesized that this discrepancy may be due to the presence of disproportionate (i.e., COAPT) vs. proportionate (i.e., MITRA-FR) MR with respect to the underlying cardiomyopathy. Thus, it is noteworthy that the FDA+ cohort was more similar to COAPT in terms of echocardiographic parameters including the degree of MR, LV dimensions, and systolic function, suggesting the COAPT population may be more representative and generalizable to community-based cohorts.
Finally, the estimated NNT was comparable for all-cause death (i.e., 5.3 vs. 5.9) and somewhat higher for HF hospitalizations (i.e., 8.8 vs. 3.1) in this observational study compared with respective trials. This finding is likely explained by the fact that our study population was older and had a greater burden of comorbidities resulting in a higher competing risk of death, potentially due to noncardiovascular causes, compared to worsening HF events.
The proportion of HF patients eligible for TEER may be lower than reported here, as a systematic review of individual cases may identify further anatomical and/or technical considerations precluding this emerging therapy. The present analysis also did not specifically address atrial SMR, which may also be amenable to treatment with TEER. It is challenging to retrospectively assess for intolerances and/or relative/absolute contraindications to GDMT, which may be undocumented, so an operational definition was adopted for maximally tolerated GDMT. Missingness with respect to LVEF, degree of MR, and other clinical characteristics may have further limited the HF population studied for eligibility. In addition, NLP-based approaches for retrieving semistructured and unstructured EHR data have known limitations. However, the validated NLP-based methods described herein were used to extract echocardiographic parameters for semistructured reporting templates. Finally, there are inherent limitations to translating the treatment effect observed in clinical trials (i.e., efficacy) to more diverse and representative community-based cohorts (i.e., effectiveness), where the relative benefit derived may be diminished.
In conclusion, there is a low prevalence of TEER eligibility, but this high-risk population may derive a robust benefit based on the existing evidence. Additional research is necessary to prospectively validate the scope of eligibility and comparative effectiveness of TEER in real-world populations.
Ethics Statement
This study was approved by the KPNC Institutional Review Board.
Funding
The Kaiser Permanente Cohort of Eligible Patients for Percutaneous Repair of Secondary Mitral Regurgitation (KP CLIP SMR) was an investigator-initiated study (IIS) funded by Abbott Laboratories (Chicago, IL). The funder approved the study in advance but had no formal role in its execution. All data collection and statistical analyses have been performed at KPNC’s Division of Research (Oakland, CA). The first (A.P.A.) and last (A.S.G.) authors take full responsibility for the manuscript’s integrity and had complete control and authority over its preparation and the decision to publish.
Disclosure Statement
A. P. Ambrosy is supported by a Mentored Patient-Oriented Research Career Development Award (K23HL150159) through the National Heart, Lung, and Blood Institute and has received relevant research support through grants to his institution from Abbott, Amarin Pharma, Edwards Lifesciences, Esperion, Lexicon, and Novartis. A. S. Go has received relevant research support through grants to his institution from the National Heart, Lung and Blood Institute; National Institute of Diabetes, Digestive and Kidney Diseases; National Institute on Aging; Amarin Pharma, Inc; Novartis; Janssen Research & Development; and CSL Behring. All other authors have no relevant conflicts of interest to declare. No human studies were carried out by the authors for this article.
References
- 1.Asgar A.W., Mack M.J., Stone G.W. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. 2015;65:1231–1248. doi: 10.1016/j.jacc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Mack M.J., Abraham W.T., Lindenfeld J., et al. Cardiovascular outcomes assessment of the MitraClip in patients with heart failure and secondary mitral regurgitation: design and rationale of the COAPT trial. Am Heart J. 2018;205:1–11. doi: 10.1016/j.ahj.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 4.Smith D.H., Thorp M.L., Gurwitz J.H., et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go A.S., Lee W.Y., Yang J., Lo J.C., Gurwitz J.H. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 6.Go A.S., Magid D.J., Wells B., et al. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 7.Magid D.J., Gurwitz J.H., Rumsfeld J.S., Go A.S. Creating a research data network for cardiovascular disease: the CVRN. Expert Rev Cardiovasc Ther. 2008;6:1043–1045. doi: 10.1586/14779072.6.8.1043. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosy A.P., Parikh R.V., Sung S.H., et al. A natural language processing-based approach for identifying hospitalizations for worsening heart failure within an integrated health care delivery system. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.35152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine N.M., McAlister F.A., Howlett J.G., Youngson E., Ezekowitz J.A. Low prevalence of transcatheter mitral valve repair eligibility in a community heart failure population. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.006952. [DOI] [PubMed] [Google Scholar]
- 10.Obadia J.F., Messika-Zeitoun D., Leurent G., et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]