Introduction
Tricuspid transcatheter edge-to-edge repair (T-TEER) has emerged as a safe and effective therapy for patients with severe tricuspid regurgitation (TR).1 In The Trial to Evaluate Cardiovascular Outcomes in Patients Treated with the Tricuspid Valve Repair System (TRILUMINATE), T-TEER reduced TR severity and improved quality of life.1 Severe TR is associated with an increased risk of mortality and morbidity secondary to right ventricle (RV) failure and venous congestion.2 Despite T-TEER being an appealing therapy for patients with severe TR, not all patients screened are suitable candidates for T-TEER. The outcomes of patients who screen failed T-TEER have not been previously reported. We aim to describe the clinical, echocardiographic, hemodynamic characteristics, and outcomes of patients who failed T-TEER screening at our institution.
Methods
Between March 2020 and October 2022, 44 of 53 consecutive patients with severe or greater TR evaluated for T-TEER at our institution failed screening, and the remaining 9 subsequently underwent T-TEER. Baseline clinical, anatomic, echocardiographic, and invasive hemodynamic characteristics were recorded. The primary outcome of interest was all-cause mortality at 1 year. Large coaptation gap was characterized by the presence of severe coaptation gap (>2cm) of the tricuspid leaflets.1 RV dysfunction and enlargement were based on visual inspection. RV was labeled as moderately enlarged when the RV chamber was roughly equal to the size of the left ventricle. Patients who survived at 1 year (N = 30) were compared to those who did not (N = 14). The two groups were compared using chi-square or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. The study was approved by the Houston Methodist Institutional Review Board.
Results
Of the 44 screen failures managed medically, 68.2% were female, and the mean age was 72.2 ± 12.0 years. Baseline clinical characteristics and screen fail causes are summarized in Supplemental Table 1.
Median time to decision (from consent to screen failure) was 2.3 months (interquartile range: 1-3.5). Primary reasons for screen failure for T-TEER were clinical (68.2%), anatomic (large coaptation gap; 25%), and presence of pacemaker or defibrillator wire in the grasping zone (6.8%). Clinical reasons for exclusion included fixed precapillary pulmonary hypertension (PH) or pulmonary artery systolic pressure (PASP) > 70 mmHg (27.2%), participation in another trial (15.9%), chronic dialysis (9.1%), death before completing screening (9.1%), left-sided heart failure with severe mitral regurgitation (6.8%), active gastrointestinal bleeding (4.6%), and active endocarditis (2.3%). Five patients (11.3%) had improved TR severity throughout the screening process due to medical optimization and thus were also excluded from the trial. When comparing the patients who met the primary endpoint (death at 1 year) and those who did not, the screen failure causes were not statistically different.
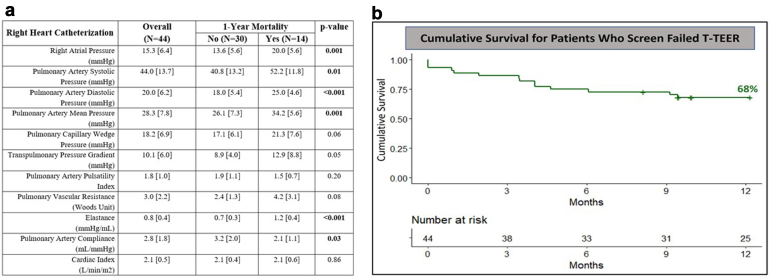
The median follow-up was 11 months (interquartile range: 6-15). Cumulative survival was 68% at 1 year (Figure 1). Patients who died within 1 year were older (77.0 ± 13.3 vs. 69.9 ± 10.9 years, p = 0.04), more frail (64.3 vs. 30.0%, p = 0.03) with greater RV dysfunction (85.7 vs. 56.7%, p = 0.04) compared to those survived. In terms of invasive hemodynamics, these patients had higher mean PAP (34.2 ± 5.6 vs. 26.1 ± 7.3 mmHg, p = 0.001), higher pulmonary artery (PA) elastance [defined as pulmonary artery systolic pressure (PASP)/stroke volume] (1.2 ± 0.4 vs. 0.7 ± 0.3 mmHg/mL, p < 0.001), and lower PA compliance [defined as stroke volume/PASP-pulmonary artery diastolic pressure] (2.1 ± 1.1 vs. 3.2 ± 2.0 mL/mmHg, p = 0.03) (Figure 1).
Figure 1.
(a) Invasive hemodynamics. (b) Survival analysis.
Abbreviation: T-TEER, tricuspid transcatheter edge-to-edge repair.
Discussion
The present study examined the characteristics and outcomes of patients who screen failed T-TEER. The key findings are as follows: First, the screen failure rate was 83%. Second, the primary reasons for screen failure were anatomic with large coaptation gap and fixed precapillary PH or PASP>70 mmHg. Third, patients who screen failed had high 1-year mortality of 32%. Finally, 1-year mortality after screen failure was associated with greater comorbidities and worse invasive hemodynamics.
To our knowledge, this is the first study examining patients who screen failed T-TEER. The ineligibility rate was high in our population (in comparison to around 50% in the TRILUMINATE trial) mainly due to severe PH and large coaptation gap. These parameters are indicative of advanced disease, which highlights the importance of serial monitoring to prevent TR progression and perhaps earlier intervention. Unlike left heart disease, TR symptoms are usually more tolerated, making this population challenging to follow up and treat in a timely manner.3
The mortality was particularly high in the year following screen failure (32% at 1 year). This is in striking contrast to the 1-year mortality of around 8% of the device and control arm of the recently published TRILUMINATE trial.1 This emphasizes the fact that the population screened constituted a very morbid and high-risk cohort of patients. Mortality was associated with higher mean PAP, higher PA elastance, and lower PA compliance. Elevated pulmonary pressures trigger irreversible remodeling of the pulmonary vasculature and lead to RV dysfunction and failure.4,5 Therefore, the timing of intervention and patient selection constitute major considerations in patients with advanced right-sided heart failure. Limitations of this study include small sample size, short follow-up, and single-center retrospective nature of the study.
Conclusion
Patients ineligible for T-TEER constituted a high-risk population with significant 1-year mortality following screen failure that is associated with higher comorbidity burden and worse invasive hemodynamics. Studies of newer generation devices are needed in this population to address the anatomical limitations prohibiting a TEER procedure. Earlier identification of significant TR and measures to decrease the severity of PH and optimize left-sided heart failure may lead to optimization of this patient population.
Ethics Statement
All study procedures were conducted in accordance with the Declaration of Helsinki. This observational study was approved by the Houston Methodist Institutional Review Board.
Funding
The authors have no funding to report.
Disclosure Statement
Michael J Reardon is a consultant for Medtronic, Boston Scientific, Abbott, W L Gore & Associates. Neal S Kleiman is a local principal investigator in trials sponsored by Boston Scientific, Medtronic, Abbott, and Edwards Lifesciences. Sachin S Goel is a consultant for Medtronic, W L Gore & Associates, and on the Speakers Bureau for Abbott Structural Heart. The other authors had no conflicts to declare.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Sorajja P., Whisenant B., Hamid N., et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388:1833–1842. doi: 10.1056/NEJMoa2300525. [DOI] [PubMed] [Google Scholar]
- 2.Topilsky Y., Maltais S., Medina Inojosa J., et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. 2019;12:433–442. doi: 10.1016/j.jcmg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Mangieri A., Montalto C., Pagnesi M., et al. Mechanism and implications of the tricuspid regurgitation: from the pathophysiology to the current and future therapeutic options. Circ Cardiovasc Interv. 2017;10(7) doi: 10.1161/CIRCINTERVENTIONS.117.005043. [DOI] [PubMed] [Google Scholar]
- 4.Kuehne T., Yilmaz S., Steendijk P., et al. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation. 2004;110:2010–2016. doi: 10.1161/01.CIR.0000143138.02493.DD. [DOI] [PubMed] [Google Scholar]
- 5.Konstam M.A., Kiernan M.S., Bernstein D., et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:e578–e622. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.