Introduction
The incidence of diabetes mellitus is dramatically increasing worldwide, and diabetic nephropathy now represents the cause of 48% of newly diagnosed cases of end-stage renal disease in the US and approximately 20% of new entrances on dialysis in Italy.1
In kidney transplanted patients (KTRs), the occurrence of new-onset diabetes after transplant (NODAT) determined an increased risk of adverse cardiovascular events and may also result in progressive diabetic kidney disease.2 In addition, few data are available about the recurrence of diabetic nephropathy in KTRs, with a limited characterization of the studied population (e.g., no kidney biopsy at diagnosis, incomplete data about glycemic or lipidic profile and antidiabetic therapy) and sample biases (i.e., no differentiation or contemporary inclusion of patients with type 1 diabetes mellitus, type 2 diabetes mellitus [T2DM], or NODAT).3,4 Indeed, the association between poor glycemic control and lower graft survival is still debated.5,6
However, some concerns remained about risk factors and predictor factors for both conditions (e.g., weight gain after transplant), thus limiting the possibility of prophylactic intervention.
Results
We, analyzed 1061 consecutive KTRs, identified 190 KTRs who developed NODAT (17.9%) and 71 subjects with a pretransplant diagnosis of T2DM and diabetic nephropathy (Supplementary Methods). Demographics, clinical characteristics, and immunosuppressive therapies are included in Supplementary Table S1 and discussed in Supplementary discussion.
Similar to the literature,2 higher age at transplant, tacrolimus at discharge, pretransplant hypertension, and cardiovascular disease are related to NODAT in our population. The observed increased steroid withdrawal at 1 and 5 years in the NODAT group seems to reflect the modulation of immunosuppressive medication according to NODAT occurrence. Interestingly, weight gain after transplant does not determine an additional risk, as observed in other case series.7 In contrast, NODAT showed a strong correlation with acute steroid-induced transient hyperglycemia after steroid induction (P < 0.001). When analyzed in the multivariate logistic regression model (Table 1) and Cox regression analysis with the other significant variables at transplant, only age ≥55 years (based on receiver operating characteristic curve analysis) and steroid-induced transient hyperglycemia maintained their significance but with a substantial impact of steroid-induced hyperglycemia (odds ratio: 9.75 [6.83–13.94]). In the time-to-event analysis considering disease-free survival (Supplementary Figure S1), the impact of acute steroid-induced hyperglycemia was graphically emphasized, with an expected significant importance in the first month after transplant.
Table 1.
Multivariate analysis of clinical determinants of NODAT
| Characteristics | P-value | Odds ratio (95% confidence interval) |
|---|---|---|
| Acute transient hyperglycemia after steroid induction at transplant | <0.001 | 9.75 (6.83–13.94) |
| Tacrolimus at discharge | 0.110 | 2.28 (0.83–6.27) |
| Pretransplant arterial hypertension | 0.122 | 1.52 (0.89–2.6) |
| Age ≥55 years at transplant | 0.024 | 1.52 (1.06–2.18) |
Patients who experience NODAT have significantly more posttransplant vascular complications (P < 0.001) without differences in death-censored graft survival (P = 0.138). We therefore, separately analyzed the 71 subjects with a pretransplant diagnosis of T2DM and diabetic nephropathy (Supplementary Table S2); among them, 19 of 71 (26.8%) developed a clinically significant and biopsy-proven recurrence of diabetic nephropathy. Similar to NODAT, the risk of recurrence was deeply influenced by acute transient hyperglycemia after steroid induction at transplant with the need to start or boost insulin therapy. Interestingly, patients with recurrence have similar variations in HBA1c (Supplementary Figure S2) and body mass index at 5 years, duration of T2DM, and rejection episodes than patients without recurrence.
In the multivariate logistic regression model (Supplementary Tables S3 and S5), only acute transient hyperglycemia was significantly associated with the recurrence of clinically significant and biopsy-proven diabetic nephropathy (odds ratio: 57.51, 95% confidence interval: 7.68–430.96).
Regarding risk factors for glycemic decompensation after steroid induction at transplant in patients with T2DM (Supplementary Table S4), the women was prevalent in this group (36.8% vs. 17.6% in patients without decompensation, P = 0.005), and both pretransplant total cholesterol and triglycerides were higher in this subset of patients (P = 0.013 and P = 0.005, respectively). Other potential conditions, including insulin therapy and Hb1Ac before transplant, did not reach statistical significance.
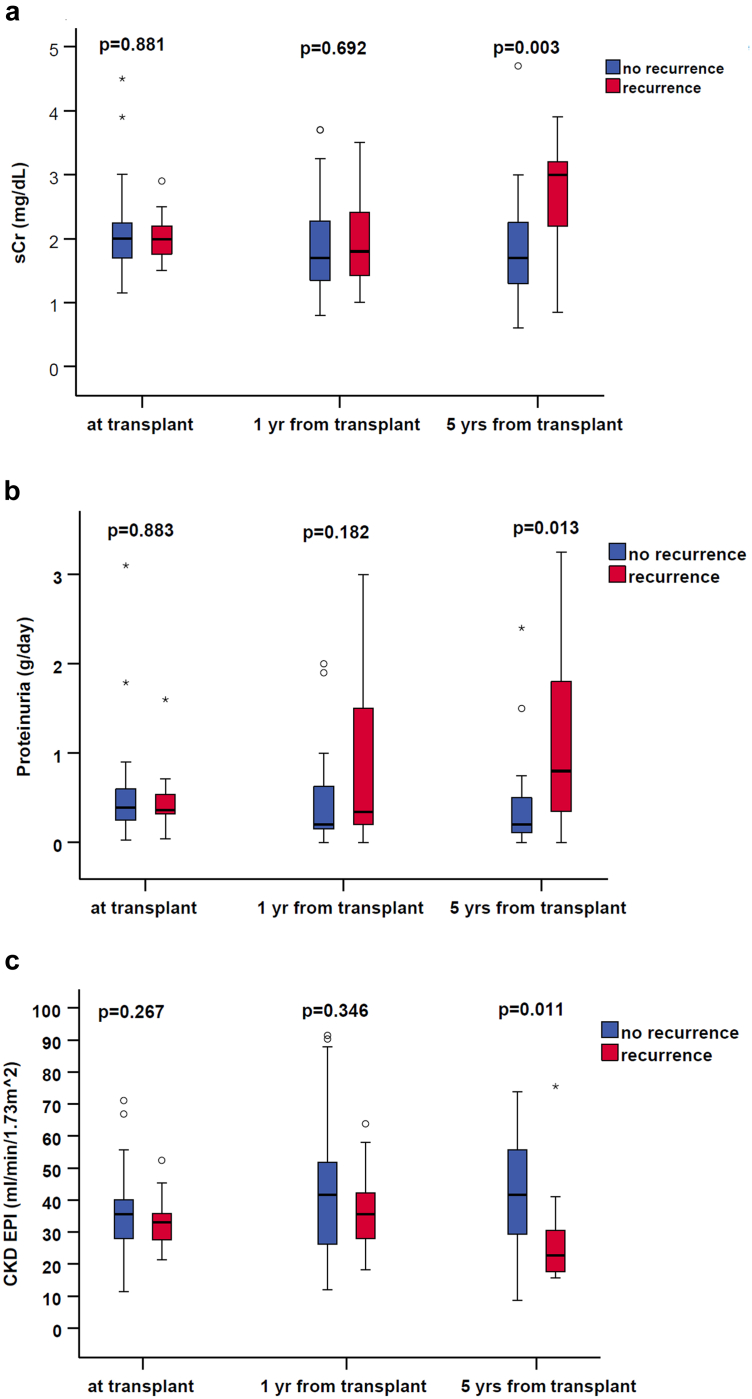
The recurrence of diabetic nephropathy was associated with progressive worsening of serum creatinine, estimated glomerular filtration rate, and proteinuria; and was statistically significant 5 years after transplant (Figure 1).
Figure 1.
Recurrence of diabetic nephropathy was associated with progressive worsening of serum creatinine, estimated glomerular filtration rate, and proteinuria, statistically significant 5 years after transplant. CKD EPI, chronic kidney disease epidemiology collaboration; sCr, serum creatinine.
The median time between transplant and biopsy diagnosis of recurrence was 57.7 months (17.1–81.3). KTRs with histological diagnosis of recurrence of diabetic nephropathy have significantly higher graft loss (P = 0.003, odds ratio: 3.39, 95% confidence interval: 1.14–10.09) and reduced death-censored graft survival (Supplementary Figure S3), without differences for patient survival (Supplementary Figure S4).
Discussion
Our study confirmed the importance of well-known conditions in the development of NODAT and recurrence of diabetic nephropathy; however, we highlighted that acute transient steroid hyperglycemia after steroid induction has a profound impact and represents the critical determinant of NODAT and recurrence of diabetic nephropathy in T2DM patients.
Regarding NODAT, we documented that weight gain after transplant does not affect NODAT development. Marrero et al.7 previously reported similar findings, noting that recipient age, tacrolimus, triglycerides, positive hepatitis C virus status, and pretransplant body mass index, but not weight gain, are associated with developing NODAT. The authors speculated that a lower weight gain in patients with NODAT might be related to underlying metabolic disorders that prevent weight increase or strict dietary control and a more rapid reduction of steroids in the NODAT group, as observed in our population. The absence of correlation in our analysis between hepatitis C virus and weight at transplant may depend on the low number of hepatitis C virus-positive patients, the role of newly available antiviral therapies, and the strict indication for weight control before transplant, which is reflected by the median weight observed in our KTRs.
Regarding the recurrence of diabetic nephropathy in T2DM, we observed a similar frequency and median relapse time than in the literature,3,4 despite some differences in the population distribution, especially for the Sub-Saharan heritage. The present series better reflects the characteristics of most European cohorts with similar distribution; however, it requires additional confirmation for different geographical areas (e.g., the US).
Previous studies, including heterogeneous populations (patients with type 1 diabetes mellitus, T2DM, and other particular types of diabetes; repeated KTRs; donations after cardiac death together with donations after brainstem death and living donors) have proposed controversial results about the link between hyperglycemia, high Hb1Ac values, and recurrence of diabetic nephropathy on KTRs.8 In addition, considering insulin use, a functioning kidney allograft increases insulin clearance in the transplanted individual, thereby increasing insulin needs posttransplant, independent of other factors.
In our analysis, only for-cause and no protocol biopsies were performed in diabetic patients; therefore, the absolute number of relapses could be underestimated: the existence of subclinical forms of diabetic nephropathy is well-known. It is becoming evident in native kidneys how diabetic nephropathy could manifest itself with different patterns in addition to the classic one, such as nonproteinuric diabetic nephropathy, in which the beginning and progression of loss of renal function may occur independently from albuminuria. This last form of diabetic nephropathy has a better renal prognosis, considering the degree of proteinuria as a strong predictor of the risk of progression, even though it is a significant risk factor for death and cardiovascular disease.
Despite all these considerations, a recurrence of diabetic nephropathy has a statistically significant correlation with the loss of function and transplant survival in our experience, similar to the literature (55% at 5 years and 22% at 10 years in Hariharan et al.)9
In conclusion, the present study identified acute transient steroid hyperglycemia after steroid induction as an early predictor factor of NODAT in the T2DM population and of the recurrence of diabetic nephropathy in KTRs with pretransplant T2DM.
During the early period after transplant, glycemic control is challenging, particularly in patients with pre-existing diabetic nephropathy; and the negative impact of immunosuppressive agents (e.g., tacrolimus, corticosteroids) is well-known. The potential impact of acute transient steroid hyperglycemia at transplant may suggest further studies to assess the proper immunosuppression characterized by less metabolic implications (Supplementary References).
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Luca Besso, MD (Clinical Department of Nephrology Santa Croce e Carle Cuneo Hospital Cuneo Italy); Stefano Maffei, MD (Unit of Nephrology and Dialysis, Cardinal Massaia Hospital, Asti, Italy); Natalia Rossi, MD (Unit of Nephrology and Dialysis, Casale Monferrato and Novi Ligure Hospitals, Alessandria, Italy); Colombano Salvatore Martino Sacco, MD (Nephrology and Dialysis Unit, ASL Biella, Biella, Italy); Marita Marengo, MD (Unit of Nephrology and Dialysis, Savigliano, Mondovi' and Ceva Hospitals, Cuneo, Italy); Loris Neri, MD (Department of Nephrology and Dialysis, "Michele E Pietro Ferrero" Hospital-ASLCN2); Giulio Cesano, MD (Nephrology and Dialysis, Martini Hospital, ASL Città di Torino, Turin, Italy); Dario Roccatello, MD, Prof. (Nephrology and Dialysis Unit, San Giovanni Bosco Hub Hospital, Turin, Italy); Giuliana Tognarelli, MD (Unit of Dialysis, San Luigi Hospital, Orbassano, Turin, Italy); Gianluca Leonardi, MD (Unit of Nephrology and Dialysis, Chieri and Moncalieri Hospitals, ASL TO5, Italy); and Massimo Manes, MD (Unit of Nephrology and Dialysis, Umberto Parini Hospital, Aosta, Italy) for their expert support on the clinical management and patients' follow-up. This study was supported by TGT study funding (Department of Medical Sciences, University of Turin).
Author Contributions
LB conceptualized the study; CFZ, CD, and FF were responsible for data curation; LB was responsible for investigation; CFZ, CD, FF, MS, and AM were responsible for formal analysis; CFZ, CD, FF, AM, and LB were responsible for methodology; LB was responsible for validation; AM and FF were responsible for visualization; LB was responsible for funding acquisition; LB, EG, and FV provided supervision; CFZ and CD wrote the original draft; AM, MS, SB, FB, EG, AB, GB, FV, AM, AL, RG, and LB reviewed and edited the manuscript. All the authors approved the final version of the manuscript.
Footnotes
Supplementary Methods.
Population Characteristics.
Figure S1. Disease-free survival (DFS) for NODAT in the overall population without T2DM, according to acute steroid-induced hyperglycemia occurrence.
Figure S2. Changes in HbA1c at 5 years in patients with or without recurrence.
Figure S3. Death-censored graft survival in the overall cohort of kidney transplanted patients and in patients with pre-existing diabetes mellitus with or without recurrence of diabetic nephropathy.
Figure S4. Patient survival in kidney transplanted patients with pre-existing diabetes mellitus with or without recurrence of diabetic nephropathy.
Table S1. Demographic and clinical characteristics of kidney-transplant patients according to NODAT occurrence. Data are expressed as percentages or medians with IQR according to their distribution.
Table S2. Demographic and clinical characteristics of kidney-transplant patients with pre-existing T2DM with or without clinically relevant and biopsy-proven recurrence of diabetic nephropathy. Data are expressed as percentages or medians with IQR according to their distribution.
Table S3. Multivariate analysis of clinical determinants of clinically relevant and biopsy-proven recurrence of diabetic nephropathy.
Table S4. Risk factors for glycemic decompensation after steroid induction at transplant. Data are expressed as percentages or medians with IQR according to their distribution.
Table S5. Pretransplant and posttransplant insulin therapy, glycemic decompensation after steroid induction at transplant, and pretransplant glycolipid profiles.
Supplementary Discussion.
Supplementary Reference.
STROBE Statement
Supplementary Material
Supplementary Methods.
Population Characteristics.
Figure S1. Disease-free survival (DFS) for NODAT in the overall population without T2DM, according to acute steroid-induced hyperglycemia occurrence.
Figure S2. Changes in HbA1c at 5 years in patients with or without recurrence.
Figure S3. Death-censored graft survival in the overall cohort of kidney transplanted patients and in patients with pre-existing diabetes mellitus with or without recurrence of diabetic nephropathy.
Figure S4. Patient survival in kidney transplanted patients with pre-existing diabetes mellitus with or without recurrence of diabetic nephropathy.
Table S1. Demographic and clinical characteristics of kidney-transplant patients according to NODAT occurrence. Data are expressed as percentages or medians with IQR according to their distribution.
Table S2. Demographic and clinical characteristics of kidney-transplant patients with pre-existing T2DM with or without clinically relevant and biopsy-proven recurrence of diabetic nephropathy. Data are expressed as percentages or medians with IQR according to their distribution.
Table S3. Multivariate analysis of clinical determinants of clinically relevant and biopsy-proven recurrence of diabetic nephropathy.
Table S4. Risk factors for glycemic decompensation after steroid induction at transplant. Data are expressed as percentages or medians with IQR according to their distribution.
Table S5. Pretransplant and posttransplant insulin therapy, glycemic decompensation after steroid induction at transplant, and pretransplant glycolipid profiles.
Supplementary Discussion
Supplementary Reference
STROBE Statement
References
- 1.Giorda C.B., Carnà P., Salomone M., et al. Ten-year comparative analysis of incidence, prognosis, and associated factors for dialysis and renal transplantation in type 1 and type 2 diabetes versus non-diabetes. Acta Diabetol. 2018;55:733–740. doi: 10.1007/s00592-018-1142-y. [DOI] [PubMed] [Google Scholar]
- 2.Ponticelli C., Favi E., Ferraresso M. New-onset diabetes after kidney transplantation. Medicina (Kaunas) 2021;57:1–9. doi: 10.3390/medicina57030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariharan S., Peddi V.R., Savin V.J., et al. Recurrent and de Novo renal diseases after renal transplantation: a report from the renal allograft disease registry. Am J Kidney Dis. 1998;31:928–931. doi: 10.1053/ajkd.1998.v31.pm9631835. [DOI] [PubMed] [Google Scholar]
- 4.Cimeno A., Munley J., Drachenberg C., et al. Diabetic nephropathy after kidney transplantation in patients with pretransplantation type II diabetes: a retrospective case series study from a high-volume center in the United States. Clin Transpl. 2018;32 doi: 10.1111/ctr.13425. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.C., Shin N., Lee S., et al. Effect of post-transplant glycemic control on long-term clinical outcomes in kidney transplant recipients with diabetic nephropathy: a multicenter cohort study in Korea. PLoS One. 2018;13:1–13. doi: 10.1371/journal.pone.0195566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez S.C., Maaske J., Kim Y., et al. The association between glycemic control and clinical outcomes after kidney transplantation. Endocr Pract. 2014;20:894–900. doi: 10.4158/ep13463.or. [DOI] [PubMed] [Google Scholar]
- 7.Marrero D., Hernandez D., Tamajón L.P., et al. Pre-transplant weight but not weight gain is associated with new-onset diabetes after transplantation: a multi-centre cohort Spanish study. NDT Plus. 2010;3(suppl 2):ii5–ii20. doi: 10.1093/ndtplus/sfq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coemans M., Van Loon E., Lerut E., et al. Occurrence of diabetic nephropathy after renal transplantation despite intensive glycemic control: an observational cohort study. Diabetes Care. 2019;42:625–634. doi: 10.2337/dc18-1936. [DOI] [PubMed] [Google Scholar]
- 9.Hariharan S., Smith R.D., Viero R., First M.R. Diabetic nephropathy after renal transplantation: clinical and pathologic features. Transplantation. 1996;62:632–635. doi: 10.1097/00007890-199609150-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.