Abstract
Large-cell anaplastic lymphoma is a subtype of non-Hodgkin’s lymphoma characterized by the expression of CD30. More than half of these lymphomas have a chromosomal translocation, t(2;5), that leads to the expression of a hybrid protein comprised of the nucleolar phosphoprotein nucleophosmin (NPM) and the anaplastic lymphoma kinase (ALK). Here we show that transfection of the constitutively active tyrosine kinase NPM-ALK into Ba/F3 and Rat-1 cells leads to a transformed phenotype. Oncogenic tyrosine kinases transform cells by activating the mitogenic signal transduction pathways, e.g., by binding and activating SH2-containing signaling molecules. We found that NPM-ALK binds most specifically to the SH2 domains of phospholipase C-γ (PLC-γ) in vitro. Furthermore, we showed complex formation of NPM-ALK and PLC-γ in vivo by coimmunoprecipitation experiments in large-cell anaplastic lymphoma cells. This complex formation leads to the tyrosine phosphorylation and activation of PLC-γ, which can be corroborated by enhanced production of inositol phosphates (IPs) in NPM-ALK-expressing cells. By phosphopeptide competition experiments, we were able to identify the tyrosine residue on NPM-ALK responsible for interaction with PLC-γ as Y664. Using site-directed mutagenesis, we constructed a comprehensive panel of tyrosine-to-phenylalanine NPM-ALK mutants, including NPM-ALK(Y664F). NPM-ALK(Y664F), when transfected into Ba/F3 cells, no longer forms complexes with PLC-γ or leads to PLC-γ phosphorylation and activation, as confirmed by low IP levels in these cells. Most interestingly, Ba/F3 and Rat-1 cells expressing NPM-ALK(Y664F) also show a biological phenotype in that they are not stably transformed. Overexpression of PLC-γ can partially rescue the proliferative response of Ba/F3 cells to the NPM-ALK(Y664F) mutant. Thus, PLC-γ is an important downstream target of NPM-ALK that contributes to its mitogenic activity and is likely to be important in the molecular pathogenesis of large-cell anaplastic lymphomas.
Receptor tyrosine kinases (RTKs) play an important role in the control of cell proliferation, differentiation, and malignant transformation. It has been shown that ligand stimulation of RTKs leads to their dimerization and activation, with resultant auto- and cross-phosphorylation (48). Tyrosine autophosphorylation sites on RTKs serve as binding motifs for SH2-containing signaling molecules such as Grb2, SHC, and phospholipase C-γ (PLC-γ) (35). Different signal transducer molecules bind to specific autophosphorylation sites in the cytoplasmic domain of the RTKs via their SH2 domains, thereby undergoing phosphorylation and activation. By recruiting a specific set of signal transducer molecules, a given growth factor receptor is capable of inducing individual, specific cellular responses (35).
In contrast, constitutive activation of an RTK can lead to aberrant stimulation of signal transducing pathways, resulting in cellular transformation and neoplasia (39). It has been shown for several neoplasms, including some lymphomas and leukemias, that specific chromosomal translocations lead to the expression of abnormal fusion proteins that possess unregulated, constitutive tyrosine kinase activity, thus mimicking activated RTKs (37). The best-studied example is the Bcr-Abl fusion protein of chronic myelogenous leukemia, in which the normally nucleus-localized Abl nonreceptor kinase is constitutively activated (3, 15, 26). By mimicking an activated RTK, the cytoplasmic Bcr-Abl protein is able to constitutively activate a whole array of mitogenic signals, thereby inducing cell transformation and leukemia (7, 13, 36, 40). Nucleophosmin (NPM)-anaplastic lymphoma kinase (ALK) is an oncogenic fusion tyrosine kinase which is associated with a specific type of non-Hodgkin’s lymphoma (27, 42). These large-cell anaplastic lymphomas express the membrane antigen CD30, and over half of them display a typical chromosomal translocation, t(2;5), that fuses NPM-encoding sequences on chromosome 5 to ALK-encoding sequences on chromosome 2 (17, 24, 27, 42, 45, 46). NPM is an ubiquitously expressed nucleolar protein responsible for protein shuttling between the cytoplasm and the nucleus (5, 6, 10, 41). ALK is an RTK whose expression is normally restricted to neural tissues (16, 28). The t(2;5) translocation fuses the amino-terminal portion of the NPM protein to the cytoplasmic domain of the ALK RTK (27). It has been shown recently that this fusion protein possesses constitutive tyrosine kinase activity and is able to transform rodent fibroblasts (4, 12). It was further demonstrated that the NPM portion of the molecule is responsible only for dimerization and the resultant activation of the ALK, with no apparent further function for the delivery of a mitogenic signal (4). Although it has been shown that NPM-ALK can associate with and phosphorylate the adapter proteins SHC, Grb2, and insulin receptor substrate 1 (IRS-1), mutational analysis of NPM-ALK that produced mutants unable to activate SHC and IRS-1 could not reveal essential biological functions of these two signal transducers for the oncogenicity of the fusion kinase. The oncogenic importance of Grb2 association and activation has not yet been assessed.
In this paper we show that NPM-ALK is a deregulated and constitutively activated tyrosine kinase that can lead not only to the transformation of fibroblasts but also to growth factor-independent proliferation of lymphocytes, the biological target cells of the lymphoma-associated oncogene. Further, we demonstrate that expression of NPM-ALK in lymphocytes leads to the association of the fusion protein with the signal transducer PLC-γ. PLC-γ has an important function in signal transduction, given that it leads to the generation of diacylglycerol and inositol triphosphate (IP3), which in turn activate protein kinase C and mobilize calcium stores from the endoplasmic reticulum (19, 29). Several investigators have shown that activation of PLC-γ by polypeptide growth factors like epidermal growth factors, platelet-derived growth factor (PDGF), or nerve growth factor is mediated by the interaction of the PLC-γ SH2 domains with one or more autophosphorylation sites on the cytoplasmic tail of the activated RTK (18, 21, 38). This complex formation leads to phosphorylation of PLC-γ on Tyr residues 783 and 1254, which in turn activates the catalytic activity of PLC-γ, leading to hydrolysis of the PLC-γ substrate phosphatidylinositol 4,5-bisphosphate (PIP2) (20). Although the data obtained by different investigators are not completely consistent, PLC-γ activity seems to be important for DNA synthesis and, at least for some RTKs (e.g., the PDGF receptor), for the delivery of a mitogenic signal. By constructing add-back mutants of the autophosphorylation sites of the PDGF receptor, it could be shown that activation of the PLC-γ pathway is sufficient to trigger a mitogenic response (1, 49).
Here we show that association of PLC-γ with NPM-ALK leads to the phosphorylation and activation of PLC-γ, as measured by enhanced PIP2 turnover. In addition, we map the PLC-γ binding site in NPM-ALK to Tyr664, which is located in the C-terminal region of the fusion protein, and we demonstrate by two-dimensional peptide maps that this is an autophosphorylation site in vivo. Site-directed mutagenesis of this single tyrosine residue results in loss of NPM-ALK-mediated stable transformation of lymphocytes and in the return of PIP2 turnover to inositol phosphate (IP) levels observed in parental cells. Thus, NPM-ALK requires the PLC-γ signal transduction pathway to mediate its mitogenicity. Importantly, in contrast to other oncogenic tyrosine kinases, e.g., Bcr-Abl, knockout of this single pathway seems to be sufficient to significantly impair NPM-ALK-mediated oncogenicity in lymphocytes.
MATERIALS AND METHODS
Construction of expression plasmids and site-directed mutagenesis.
The NPM-ALK cDNA (27) was cloned into pCDNA3 (Invitrogen, Leek, The Netherlands) between the HindIII and XbaI sites. Site-directed mutagenesis was performed with the QuickChange site-directed mutagenesis kit with Pfu DNA polymerase (Stratagene, Heidelberg, Germany). All mutated DNAs were sequenced with the ABI PRISM dye terminator cycle sequencing ready reaction kit (Perkin-Elmer, Weiterstadt, Germany). Human PLC-γ2 cDNA was kindly provided by H. Hug, University of Ulm, Ulm, Germany, and S. Ohta, Jichi Medical School, Tochigiken, Japan (31). The cDNA was subcloned into plasmid pCDNA3.1 Zeo(−) (Invitrogen) to select for zeocin resistance.
Cell culture and DNA transfection.
The murine pro-B lymphoid cell line Ba/F3 was maintained in RPMI 1640 with 10% fetal calf serum (FCS) (Seromed, Berlin, Germany) and 1.5 ng of murine recombinant interleukin 3 (IL-3) (R&D Systems, DPC Bierman GmbH, Wiesbaden, Germany) per ml. The human lymphoma cell lines HDLM2 and Karpas299 were grown in RPMI 1640 with 10% FCS. DNA transfections were done by electroporation with the geneZAPPER (IBI, Madison, Wis.) at 250 mV and 950 μF with 25 μg of DNA and 5 × 106 Ba/F3 cells in cold phosphate-buffered saline (PBS). After 48 h, the transfected cells were selected with 1 mg of G418 (Serva, Heidelberg, Germany) per ml for 14 days, and single clones were obtained by limiting dilution in 96-well plates. Rat-1 cells were maintained in Dulbecco modified Eagle medium containing 5% FCS in 10-cm dishes. The transfections of Rat-1 cells with pCDNA3 empty vector, wild-type (wt) NPM-ALK, and the NPM-ALK(Y664F) mutant were performed with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N,-trimethylammonium methylsulfate (DOTAP) transfection reagent (Boehringer, Mannheim, Germany). After undergoing selection with G418 (0.75 mg/ml), the cells were used for Rat-1 soft agar assays.
Rat-1 soft agar assays.
Rat-1 soft agar assays were performed as described previously (25). Rat-1 cells were plated on 6-cm dishes (2 × 104/dish) and then were photographed after 14 days.
Antibodies.
The preparation of the rabbit polyclonal anti-ALK antibody has been previously described (28). Mouse monoclonal antiphosphotyrosine 4G10 antibody was purchased from Upstate Biotechnology, Lake Placid, N.Y.; PY20 was from Transduction Laboratories, Lexington, Ky. Rabbit polyclonal anti-PLC-γ1 and anti-PLC-γ2 antibody were from Santa Cruz Biotechnology, Santa Cruz, Calif.
Expression and purification of GST fusion proteins.
The different SH2-glutathione S-transferase (GST) fusion proteins used in this study were a kind gift of Jean Y. J. Wang, University of California, San Diego, Calif. PLC-γ N- and C-terminal SH2-GST fusion proteins (PLC-γ GST-NSH2 and PLC-γ GST-CSH2) contained amino acids 550 to 669 and 673 to 769 of bovine PLC-γ, respectively. All of the SH2-GST fusion proteins were expressed in Escherichia coli HB101 and affinity purified as described previously (43), with glutathione-Sepharose (Pharmacia Biotech, Freiburg, Germany).
Immunoprecipitation and immunoblotting.
Cells were suspended in lysis buffer (10 mM Tris-HCl [pH 7.5], 130 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mg of bovine serum albumin per ml, 20 mM sodium phosphate [pH 7.5], 10 mM sodium pyrophosphate [pH 7.0], 50 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor cocktail [10 μM benzamidine-HCl and 10 μg each of phenanthroline, aprotinin, leupeptin, and pepstatin per ml]) and kept on ice for 30 min. Lysates were precleared by centrifugation and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, the lysates were incubated for 2 to 12 h with 1 to 2 μg of antibody as indicated at 4°C. Immuno complexes were precipitated with 30 μl of protein A-Sepharose (Pharmacia Biotech) for 1 h, washed three times with lysis buffer, and then boiled in 2% SDS sample buffer, followed by separation on SDS-polyacrylamide gels. Immunoblotting was done with nitrocellulose membranes, and proteins were visualized by chemiluminescence as recommended by the manufacturer (Amersham Life Science, Little Chalfont, United Kingdom).
In vitro translation and protein binding assays.
In vitro translation of NPM-ALK and the various mutants in pCDNA3 was performed with the TNT coupled reticulocyte lysate system with T7 RNA polymerase (Promega, Madison, Wis.) and [35S]methionine (Amersham Life Science). In vitro translated NPM-ALK was diluted in lysis buffer and incubated with various GST fusion proteins for 1 h at 4°C as described previously (11). After being washed four times with lysis buffer, the complexes were boiled with SDS sample buffer and separated on an SDS–7.5% polyacrylamide gel. The gel was dried at 80°C for 2 h, and signals were detected by autoradiography.
Phosphopeptide competition assay.
The following tyrosine-phosphorylated peptides corresponding to the potential autophosphorylation sites of NPM-ALK were synthesized by BioTez GmbH, Berlin, Germany: LRPQNpY17LFGCE, KADKDpY29HFKVD, AEAMNpY67EGSPI, QHLVVpY119RRKHQ, TIMTDpY152NPNYC, DYNPNpY156CFAGK, AFGEVpY191EGQVS, DIACGCQpY299LEENH, MARDIpY338RASYY, IYRASpY342YRKGG, IYRASYpY343RKG, IFSLGpY387MPYPS, LGYMPpY390PSKSN, PGPVpY418RIMTQ, IILERIEpY445CTQDP, LPIEpY461GPLVE, LWNPTpY567GSWFT, NVNYGpY646QQQGL, and PGAGHpY664EDTIL. PLC-γ GST-NSH2 or PLC-γ GST-CSH2 bound on glutathione-Sepharose was first preincubated with 200 μM phosphopeptide in 100 μl of lysis buffer at 4°C for 1 h, and lysates of 106 Karpas299 cells were then added for incubation for another 1 h. Complexes were washed three times, and the binding of NPM-ALK was detected by immunoblotting as described above.
Analysis of IPs.
Ba/F3 cells (prepared for 48 h in RPMI 1640 with 10% FCS and 1.5 ng of murine IL-3 per ml) were incubated for 24 h in RPMI medium (without unlabeled inositol) containing 10% FCS and 2 μCi of myo-[3H]inositol (Amersham Life Science). The media were then removed, and the cells were washed thoroughly and incubated in 0.5 ml of PBS solution containing 10 mM LiCl. After 1 h, the incubation was stopped by adding 250 μl of 3.5% HCl, and the cell lysates were frozen at −80°C. IPs formed were determined by the method described previously (9). Briefly, cell lysates were loaded on an anion-exchange column containing AG 1-X8 (Bio-Rad, Munich, Germany). After the lysates were washed with aqua bidest, inositol and glycerophosphoinositides were removed by eluting with 1.25 mM Na2B3O7 and 15 mM formic acid. Ins(4)P, Ins(1,4)P2, and Ins(1,4,5)P3 were eluted sequentially by the stepwise addition of 10-ml solutions containing 0.1 M formic acid and 0.15 M NH4 formate, 0.4 M NH4 formate, and 2 M NH4 formate, respectively. Fractions of 3 ml were collected and counted for radioactivity.
Two-dimensional peptide mapping.
Ba/F3 cells expressing wt NPM-ALK and the NPM-ALK(Y664F) mutant were maintained in Dulbecco modified Eagle medium without phosphate (Sigma, Deisenhofen, Germany) supplemented with 0.5% FCS for 1 h. [32P]Orthophosphate (Amersham) was added into the cell culture to a final concentration of 0.2 mCi/ml for 4 h. Anti-ALK immunoprecipitation and SDS-PAGE were performed as described above. The gel was stained with Coomassie blue and then destained with buffer containing 10% methanol, 10% acetic acid, and water. An autoradiograph was obtained after 5 h to determine the position of the labeled NPM-ALK proteins. The protein bands were cut out of the gel, cut into small fragments, and washed twice in 50 μl of water and twice in 50 mM NH4HCO3 at 4°C for 30 min. The gel fragments were incubated with 150 ng of trypsin (Promega) in 60 μl of 50 mM NH4HCO3 for 24 h at 37°C. The liquid was first removed into a fresh tube, and the gel fragments were extracted twice with 60 μl of 50 mM NH4HCO3-acetonitrile (50:50). The liquid was dried in a vacuum centrifuge and washed twice with water. The lyophilized pellets were dissolved in 10 μl of electrophoresis running buffer (23 ml of formic acid and 78 ml of acetic acid in 899 ml of water, pH 1.9) and loaded on a thin-layer chromatography (TLC) cellulose plate (Merck, Darmstadt, Germany) which was pretreated with the same buffer. The electrophoresis was performed with a Hunter thin-layer peptide mapping system, model HTLE-7000 (C.B.S. Scientific Co., Del Mar, Calif.), at 1,240 V for 15 min. Ascending chromatography with the buffer (1-butanol–pyridine–acetic acid–water, 375:250:75:300) followed overnight. Radiolabeled phosphopeptides were visualized by autoradiography.
RESULTS
NPM-ALK is a constitutively activated tyrosine kinase.
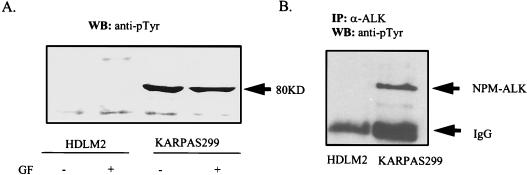
The large-cell anaplastic lymphoma cell line Karpas299 expresses a highly tyrosine-phosphorylated 80-kDa protein (Fig. 1A) which is not expressed in other lymphoma cell lines, like the Hodgkin’s lymphoma line HDLM2. This protein is constitutively activated and autophosphorylated and is not regulated by growth factor stimulation or withdrawal (Fig. 1A). Immunoprecipitation with an ALK-specific antibody identifies this 80-kDa protein as NPM-ALK (Fig. 1B).
FIG. 1.
Growth factor-independent autophosphorylation of NPM-ALK in large-cell anaplastic lymphoma cells. (A) HDLM2 and Karpas299 cells were cultured with 0.5% (−) or 10% (+) FCS for 24 h. Lysates from 107 cells were prepared as described in Materials and Methods and subjected to SDS-PAGE, and immunoblotting was performed with the antiphosphotyrosine antibody PY20. (B) HDLM2 and Karpas299 cells (107 each) were lysed and immunoprecipitated with anti-ALK antibody. Immunoblotting was performed with the antiphosphotyrosine antibody 4G10. GF, growth factor; IP, immunoprecipitation; WB, Western blotting; IgG, immunoglobulin G.
NPM-ALK binds to the SH2 domains of Grb2 and PLC-γ.
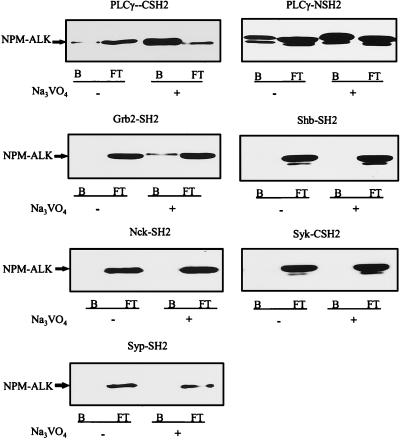
Oncogenic tyrosine kinases exert their transforming capacity through the binding and activation of SH2-containing signaling molecules. To evaluate which SH2-containing signal transducers might bind to NPM-ALK, 14 different GST-SH2 fusion proteins were purified (data not shown). Pull-down assays with these GST-SH2 fusion proteins and cell lysates from large-cell anaplastic lymphoma cells identified the SH2 domains of PLC-γ and Grb2 as binding partners for NPM-ALK (Fig. 2). In our assay, the strongest binding was observed with the N-terminal SH2 domain of PLC-γ, followed by the C-terminal SH2 domain of PLC-γ and the SH2 domain of Grb2 (Fig. 2). Low-level binding was also observed with the C-terminal SH2 domain of p85, whereas no binding was observed with the N-terminal SH2 domain of p85 or the SH2 domains of vav, BTK, p91, SHPTP2, and NCK (data not shown). Grb2 has previously been shown to bind to NPM-ALK in vitro and in vivo (12), but the significance of Grb2 interaction for the biological properties of NPM-ALK and its transforming potential have not yet been assessed and will be described in a forthcoming study. In this report, we have chosen to focus our analysis on the biological importance of PLC-γ interaction with NPM-ALK.
FIG. 2.
The SH2 domains of PLC-γ and Grb2 bind to NPM-ALK from large-cell anaplastic lymphoma cells. For each binding assay, 107 Karpas299 cells were cultured without (−) or with (+) 100 μM Na3VO4 overnight. Cells were washed briefly with ice-cold PBS, lysed in 0.5% Triton X-100-containing lysis buffer, and incubated with different GST-SH2 fusion proteins for 1 h at 4°C. The bound (B) and the flowthrough (FT) fractions were collected with glutathione-Sepharose, subjected to SDS–7.5% PAGE, and analyzed by anti-ALK immunoblotting.
PLC-γ and NPM-ALK form complexes in vivo.
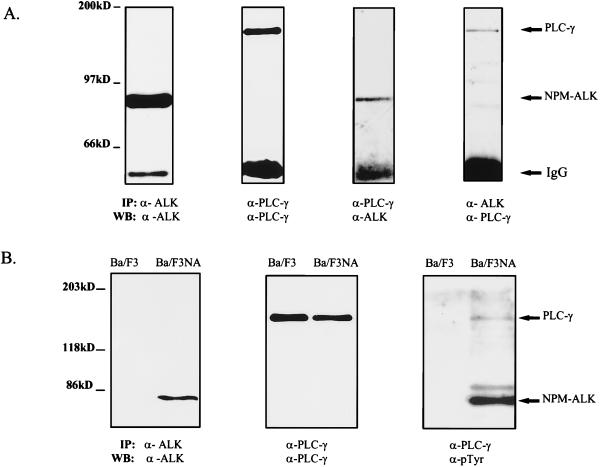
To demonstrate in vivo association of NPM-ALK and PLC-γ coimmunoprecipitation experiments were performed in the human large-cell anaplastic lymphoma cell line Karpas299. These cells express the NPM-ALK fusion protein and PLC-γ (Fig. 3A). Coimmunoprecipitation of NPM-ALK with a PLC-γ-specific antibody, as well as communioprecipitation of PLC-γ with an anti-ALK antibody, demonstrated an in vivo association of the two proteins in Karpas299 cells (Fig. 3A). It has been shown that PLC-γ associates through its SH2 domains with activated growth factor RTKs and that this association leads to tyrosine phosphorylation of PLC-γ at several sites including Tyr771, Tyr783, and Tyr1254. Mutational analysis has demonstrated that phosphorylation of PLC-γ at Tyr783 by activated tyrosine kinase receptors leads to the enzymatic activation of the catalytic domain of PLC-γ (19, 20). To determine whether complex formation of PLC-γ and NPM-ALK in vivo leads to the tyrosine phosphorylation of PLC-γ, NPM-ALK was stably overexpressed in the lymphocyte cell line Ba/F3 (Ba/F3NA) (Fig. 3B). A PLC-γ antibody precipitated PLC-γ from both parental Ba/F3 and Ba/F3NA cells; however, antiphosphotyrosine immunoblotting analysis revealed constitutive tyrosine phosphorylation of PLC-γ only in the NPM-ALK-expressing Ba/F3 cells and not in parental Ba/F3 cells (Fig. 3B). Furthermore, coprecipitation of the tyrosine-phosphorylated PLC-γ with autophosphorylated NPM-ALK was confirmed (Fig. 3B). Thus, complex formation of PLC-γ with the constitutively activated NPM-ALK leads to tyrosine phosphorylation of PLC-γ and, presumably, to activation of the catalytic domain of the protein.
FIG. 3.
NPM-ALK association with PLC-γ in vivo leads to tyrosine phosphorylation of PLC-γ in NPM-ALK-expressing cells. (A) Karpas299 cells (5 × 106 [two left panels] or 2 × 107 [two right panels]) were subjected to immunoprecipitations and immunoblotting with the antibodies indicated. Association of NPM-ALK with PLC-γ was demonstrated by coprecipitation of NPM-ALK with an anti-PLC-γ antibody (second panel from right) and by coprecipitation of PLC-γ with anti-ALK antibody (right panel). (B) The lymphocyte cell line Ba/F3 was transformed with wt NPM-ALK in pCDNA3 by electroporation, and stable clones were established by selection in G418 for 2 weeks, as described in Materials and Methods. Parental Ba/F3 cells and Ba/F3 cells expressing NPM-ALK (Ba/F3NA) (107 each) were analyzed by immunoprecipitation and immunoblotting with anti-ALK antibody to demonstrate the expression of wt NPM-ALK in Ba/F3NA cells (left panel) or with anti-PLC-γ antibody to examine endogenous PLC-γ expression (middle panel). Ba/F3 and Ba/F3NA cells (4 × 107 each) were lysed and immunoprecipitated with anti-PLC-γ antibody, followed by immunoblotting with antiphosphotyrosine antibody (PY20 and 4G10) (right panel). Tyrosine phosphorylation and coprecipitation of PLC-γ with NPM-ALK were detected only in NPM-ALK-expressing cells (Ba/F3NA). IP, immunoprecipitation; WB, Western blotting; IgG, immunoglobulin G.
Tyr664 in NPM-ALK is the binding site for PLC-γ.
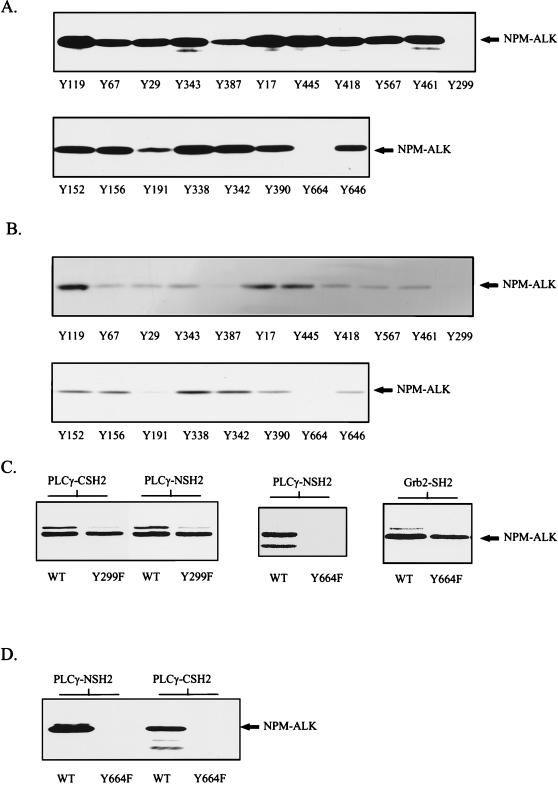
To determine the autophosphorylated tyrosine residue in NPM-ALK responsible for complex formation with PLC-γ, phosphopeptide competition experiments were performed. A series of 11-residue peptides encompassing putative autophosphorylation sites in NPM-ALK were synthesized with 9-fluorenylmethoxycarbonyl-protected phosphotyrosine. Binding studies of the N- and C-terminal SH2 domains of PLC-γ were performed in the presence of 200 μM peptide. As shown in Fig. 4A and B, two peptides (Tyr299 and Tyr664) completely blocked the binding of NPM-ALK to the C- and N-terminal PLC-γ SH2 domains. To more closely examine these results, each of these tyrosine residues was mutated to phenylalanine by site-directed mutagenesis to produce single-point-mutant NPM-ALK cDNAs [encoding NPM-ALK(Y299F) and NPM-ALK(Y664F)]. In vitro binding experiments revealed that the mutant NPM-ALK(Y299F) still formed complexes with the N- and C-terminal SH2 domains of PLC-γ, whereas the mutant NPM-ALK(Y664F) failed to form complexes with the N-terminal SH2 domain (Fig. 4C) and the C-terminal domain of PLC-γ (data not shown). This mutant, however, was still able to form complexes with other SH2 domains, e.g., the SH2 domain of Grb2 (Fig. 4C), indicating that mutation of Tyr664 does not interfere with the autophosphorylation of other tyrosine residues within NPM-ALK.
FIG. 4.
Phosphopeptide competition identifies Tyr664 in NPM-ALK as the binding site for PLC-γ. (A and B) Tyrosine-phosphorylated peptides corresponding to the putative autophosphorylation sites in NPM-ALK were synthesized with an SMPS 350 (Zinsser Analytik, Frankfurt, Germany) according to the method of Atherton and Sheppard (2). Tyrosine-phosphorylated peptides (200 μM) were incubated with ∼3 μg of GST fusion proteins of the PLC-γ N-terminal (A) or C-terminal (B) SH2 domain in lysis buffer for 1 h at 4°C. Cell lysates of 106 Karpas299 cells were then added to each binding reaction mixture, and mixtures were incubated for a further hour. Complexes were finally precipitated with glutathione-Sepharose, and samples were subjected to SDS–7.5% PAGE and analyzed by anti-ALK immunoblotting. Peptides Y299 and Y664 completely blocked in vitro association of NPM-ALK and the N-terminal and C-terminal SH2 domains of PLC-γ. (C) wt NPM-ALK (WT) and NPM-ALK mutants Y299F and Y664F were translated in vitro and labeled with [35S]methionine by the TNT coupled reticulocyte lysate system with T7 RNA polymerase. In vitro binding with the GST-SH2 domains of PLC-γ and Grb2 was performed as described in Material and Methods with ∼3 μg of GST fusion protein. Samples were resolved by SDS–7.5% PAGE and visualized by autoradiography. Y664, but not Y299, is essential for the binding of PLC-γ to NPM-ALK. (D) wt NPM-ALK (WT) and NPM-ALK(Y664F) sequences in pCDNA3 were stably transfected into Ba/F3 cells. Cell lysates were incubated with PLC-γ GST-NSH2 and PLC-γ GST-CSH2 and precipitated with glutathione-Sepharose, and samples were subjected to SDS–7.5% PAGE and analysis by anti-ALK immunoblotting.
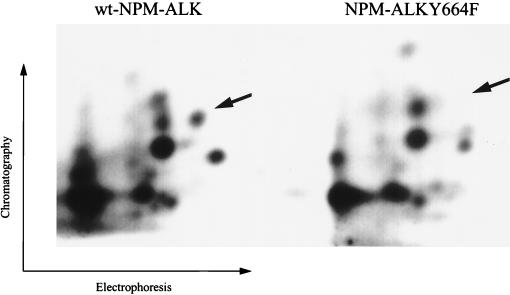
To determine the specificity of Tyr664 for the in vivo association of NPM-ALK and PLC-γ, NPM-ALK(Y664F) was stably transfected into the lymphocyte cell line Ba/F3. Neither the N- nor the C-terminal SH2 domain of PLC-γ precipitated NPM-ALK(Y664F) from lysates of these cells (Fig. 4D). Thus, the PLC-γ binding site in NPM-ALK is tyrosine 664. To verify that Tyr664 is indeed an autophosphorylation site in vivo, two-dimensional tryptic phosphopeptide maps were run on the in vivo-labeled wt NPM-ALK and the NPM-ALK(Y664F) mutant (Fig. 5). These maps show clear differences in the phosphorylation sites with the absence of one phosphorylation site in the digest of NPM-ALK(Y664F) (Fig. 5).
FIG. 5.
Two-dimensional tryptic phosphopeptide maps of wt NPM-ALK and NPM-ALK(Y664F). In vivo-labeled wt NPM-ALK- and NPM-ALK(Y664F)-expressing Ba/F3 cells were prepared as described in Materials and Methods. NPM-ALK proteins were immunoprecipitated and separated by SDS-PAGE. The gel fragments containing NPM-ALK were then extensively digested with trypsin. The resulting digests were washed three times with water and then separated on a TLC cellulose plate electrophoretically at pH 1.9, followed by ascending chromatography (1-butanol–pyridine–acetic acid–water, 375:250:75:300). Radiolabeled phosphopeptides were visualized by autoradiography. The phosphopeptides of wt NPM-ALK and NPM-ALK(Y664F) were loaded on the same TLC plate and separated by a distance of 10 cm. Arrows indicate the position of the phosphopeptide spot missing in the map of the NPM-ALK(Y664F) mutant.
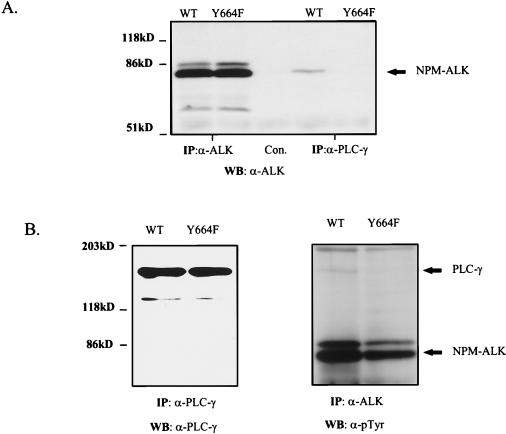
Single clones of NPM-ALK(Y664F)-expressing cells were obtained by serial dilution. As shown by anti-ALK immunoprecipitation, one of these clones expressed the Y664F mutant at levels similar to those in the wt NPM-ALK-expressing Ba/F3 cells (Fig. 6A, lanes 1 and 2). As expected, coimmunoprecipitation of NPM-ALK and PLC-γ could be demonstrated with the wt fusion protein but not with the Y664F mutant (Fig. 6A, lanes 4 and 5). Both cell lines did express PLC-γ at similar levels and exhibited comparable NPM-ALK autophosphorylation activities (Fig. 6B). However, tyrosine phosphorylation of PLC-γ in cells expressing the Y664F mutant seemed to be reduced, compared to that in the wt NPM-ALK-expressing cells (Fig. 6B).
FIG. 6.
In vivo association and tyrosine phosphorylation of PLC-γ by NPM-ALK require Tyr664. (A) Coprecipitation of PLC-γ with NPM-ALK was examined in lysates of 5 × 107 Ba/F3 cells stably transfected with wt NPM-ALK (WT) and NPM-ALK(Y664F). Immunoprecipitation was performed with anti-ALK antibody, rabbit anti-mouse antibody (Con.), or anti-PLC-γ antibody. Samples were resolved by SDS–7.5% PAGE and analyzed by immunoblotting with anti-ALK antibody. (B) Expression levels of PLC-γ (left panel) and tyrosine phosphorylation of NPM-ALK and PLC-γ (right panel) in Ba/F3 cells transfected with wt NPM-ALK and NPM-ALK(Y664F) were determined by immunoprecipitation and immunoblotting with the antibodies indicated. IP, immunoprecipitation; WB, Western blotting.
wt NPM-ALK but not NPM-ALK(Y664F) induces enhanced IP production in Ba/F3 cells.
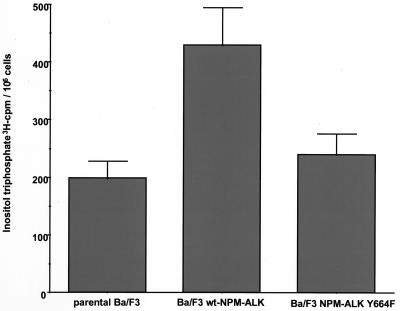
To assess further the functional significance of the interaction of NPM-ALK with PLC-γ, we investigated production of IPs in parental Ba/F3 cells, compared to that in NPM-ALK-expressing Ba/F3 cells and NPM-ALK(Y664F) mutant-expressing cells. As shown in Fig. 7, NPM-ALK expression in Ba/F3 cells led to a twofold increase in IP production compared to that in parental cells. However, cells expressing the NPM-ALK(Y664F) mutant, which is not able to associate with PLC-γ, showed IP levels comparable to those in parental Ba/F3 cells. Thus, complex formation of NPM-ALK and PLC-γ leads to the phosphorylation and enzymatic activation of PLC-γ.
FIG. 7.
IP levels in Ba/F3 cells transfected with wt NPM-ALK and mutant Y664F. Ba/F3 cells (5 × 106) stably expressing wt NPM-ALK or NPM-ALK(Y664F) were incubated for 24 h in RPMI medium containing 10% FCS and 2 μCi of myo-[3H]inositol. The media were then removed, and the cells were washed thoroughly and incubated in 0.5 ml of PBS solution containing 10 mM LiCl. After 1 h the incubation was stopped by adding 250 μl of 3.5% HCl, and the cell lysates were frozen at −80°C. The IPs formed were determined as described in Materials and Methods. Data represent means ± standard deviations from three independent experiments.
Tyr664 is essential for NPM-ALK-induced mitogenicity.
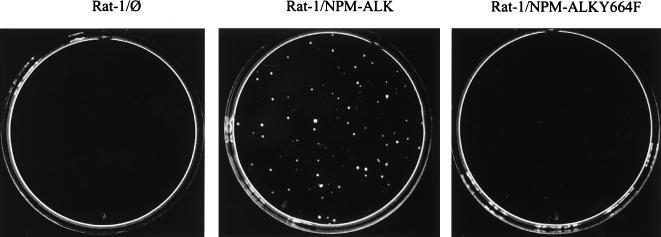
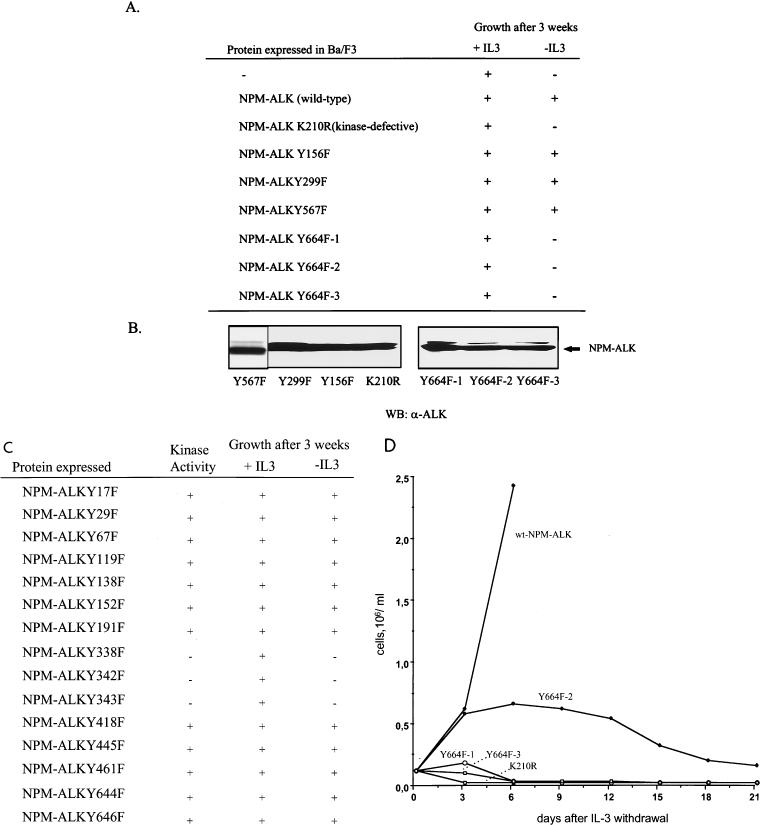
It has been shown previously that NPM-ALK is capable of transforming NIH 3T3 (12) and Fr3T3 (4) rodent fibroblasts. Expression of wt NPM-ALK in Rat-1 fibroblasts also led to transformation and the outgrowth of soft-agar colonies (Fig. 8). In contrast, expression of the NPM-ALK(Y664F) mutant in Rat-1 fibroblasts produced only a few, barely visible colonies (Fig. 8). Thus, the autophosphorylation site Tyr664 is required for the efficient transformation of Rat-1 fibroblasts. However, because NPM-ALK is an oncogene associated with large-cell anaplastic lymphoma, lymphocytes represent the in vivo target of this fusion protein. We therefore chose Ba/F3 cells as a model system to investigate NPM-ALK’s oncogenicity. Ba/F3 is a nontumorigenic murine pro-B lymphocyte cell line dependent on IL-3 for growth (33). wt NPM-ALK expression in these cells was shown to lead to a growth factor-independent phenotype, which is a marker for the transformation of Ba/F3 cells (Fig. 9A). This growth factor independence required an active NPM-ALK tyrosine kinase, since cells expressing a kinase-defective NPM-ALK mutant [NPM-ALK(K210R)] still relied on IL-3 for their growth (Fig. 9A). Of several Tyr-to-Phe mutants tested, only the NPM-ALK(Y664F) mutant lost the ability to induce a growth factor-independent phenotype in Ba/F3 lymphocytes. This result was confirmed by testing three independent single-cell-derived clones of NPM-ALK(Y664F)-expressing Ba/F3 cells [NPM-ALK(Y664F) clones 1, 2, and 3] (Fig. 9A). Neither a mutant which had previously been shown to lack the ability to bind to IRS-1 [NPM-ALK(Y156F)] (12) nor a mutant which lacked the ability to bind to SHC [NPM-ALK(Y567F)] (12) showed any phenotype in Ba/F3 lymphocytes (Fig. 9A).
FIG. 8.
Rat-1 soft-agar assay of wt NPM-ALK and NPM-ALK(Y664F). The transfections of Rat-1 cells with pCDNA3 empty vector, wt NPM-ALK, and NPM-ALK(Y664F) mutant were performed with the DOTAP transfection reagent. After undergoing selection with G418 (0.75 mg/ml), the cells were used for Rat-1 soft-agar assays. On each 6-cm dish 2 × 104 cells were plated, and representative soft-agar plates were photographed after 14 days.
FIG. 9.
The transforming potential of wt NPM-ALK and Tyr-to-Phe NPM-ALK mutants in Ba/F3 cells. (A) Ba/F3 cells were stably transfected with the pCDNA3 empty vector (−) or with vector expressing wt or mutant NPM-ALK by electroporation and selection in RPMI medium containing 1 mg of G418 per ml, 10% FCS, and 1.5 ng of murine IL-3 per ml. Three single-cell-derived clones of Ba/F3 cells transfected with NPM-ALK(Y664F) (Y664F 1, 2, and 3) were obtained by limiting dilution. Growth with or without IL-3 was monitored over a period of 3 weeks, with viable cells determined by using trypan blue staining and a hemacytometer. (B) Expression levels of the different NPM-ALK constructs were determined by SDS–7.5% PAGE and anti-ALK immunoblotting. WB, Western blotting. (C) Tyr-to-Phe mutants were stably expressed in Ba/F3 cells. Protein expression and kinase activity of the mutants were verified by immunoblotting (data not shown). Growth with or without IL-3 was monitored over a period of 3 weeks, with viable cells determined by using trypan blue staining and a hemacytometer. (D) After IL-3 withdrawal, cell numbers in each culture were determined by using trypan blue staining and a hemacytometer. Cell culture media were renewed every 6 days.
In addition we constructed Tyr-to-Phe mutants of all possible autophosphorylation sites in NPM-ALK (Fig. 9C). Expression and autokinase activity of all constructs were confirmed by immunoblotting. Three Tyr-to-Phe mutants lost their kinase activity probably because the mutated tyrosine residues were located within the conserved kinase domain (Y338, Y342, and Y343) of NPM-ALK (Fig. 9C). All other mutants showed no phenotype in Ba/F3 cells (Fig. 9C), underscoring the importance of the single residue tyrosine 664. Thus, in lymphocytes, the association of NPM-ALK and PLC-γ seemed to be indispensable for NPM-ALK-induced mitogenicity. Figure 9D shows the time kinetics of IL-3-independent growth of the different cell clones. Although unable to grow without IL-3 for a prolonged period, the NPM-ALK(Y664F)-expressing cells survived somewhat longer without IL-3 than the NPM-ALK(K210R) kinase-defective cells (Fig. 9D). Interestingly, readdition of IL-3 within the first 2 weeks after IL-3 withdrawal rescued the NPM-ALK(Y664F)-expressing cells but not the NPM-ALK kinase-defective cells (data not shown), suggesting that PLC-γ is important for a mitogenic signal, as opposed to an antiapoptotic signal, delivered by NPM-ALK.
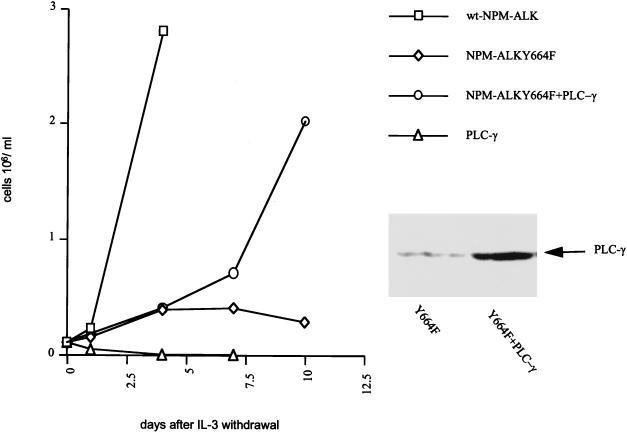
To obtain additional data demonstrating the role of PLC-γ in NPM-ALK-mediated transformation, a rescue experiment for the NPM-ALK(Y664F) mutant in Ba/F3 cells was performed. As shown in Fig. 10, overexpression of wt PLC-γ was able to partially rescue the Y664F mutant for IL-3-independent growth. Expression of wt PLC-γ in parental Ba/F3 cells had no effect on IL-3-independent growth (Fig. 10). Thus, Tyr664 is an important autophosphorylation site in NPM-ALK, allowing its association with and activation of PLC-γ and maintaining a promitogenic signal which is required for the efficient transformation of Rat-1 fibroblasts and the IL-3-independent growth of Ba/F3 lymphocytes.
FIG. 10.
Overexpression of a PLC-γ cDNA rescues Ba/F3 NPM-ALK(Y664F) cells for IL-3-independent growth. The PLC-γ2 sequence in pCDNA3.1 Zeo(−) was transfected into parental Ba/F3 and NPM-ALK(Y664F) 2-expressing Ba/F3 cells, followed by selection in RPMI medium containing 0.25 mg of zeocin per ml, 10% FCS, and 1.5 ng of murine IL-3 per ml. Growth without IL-3 was monitored by determining viable cells by using trypan blue staining and a hemacytometer. The experiment was performed four times with two independent PLC-γ transfections, and results of a representative experiment are shown. The expression level of PLC-γ in the cell lysates was determined by SDS-PAGE and immunoblotting with anti-PLC-γ2 antibody.
DISCUSSION
NPM-ALK is an oncogenic tyrosine kinase associated with large-cell anaplastic lymphomas (27). Earlier studies have shown that NPM-ALK is able to transform fibroblasts (4, 12) and can also induce a lymphoma-like disease when expressed in mice (23). Structural homologies place NPM-ALK in the family of insulin RTKs, with the highest homology to leukocyte tyrosine kinase (LTK) (e.g., 64% amino acid identity in the kinase catalytic domain) (12, 16, 28). LTK has been shown to bind to IRS-1, SHC, PLC-γ, GAP, phosphatidylinositol 3-kinase, and RAF (22, 47). While the importance of the PLC-γ pathway for the biological effects of LTK has not been studied, both IRS-1 and SHC seem to be required for the biological function of LTK. Both of these pathways have been shown to be important for the mitogenic and cell survival signals induced by the activation of LTK (47). Thus, in earlier studies, the importance of these pathways for NPM-ALK-mediated oncogenicity was also investigated. NPM-ALK was shown to bind and phosphorylate both SHC and IRS-1 through their phosphotyrosine-binding domains; however, activation of these signal transducers was demonstrated to be nonessential for the transformation of NIH 3T3 fibroblasts by NPM-ALK (12). In this paper we have demonstrated that NPM-ALK mutants defective in the activation of IRS-1(Y156F) or SHC(Y567F) also do not display any biological phenotypes in lymphocytes (Fig. 9). Thus, although it is highly homologous to LTK, NPM-ALK seems to utilize different signal transduction pathways for its oncogenicity.
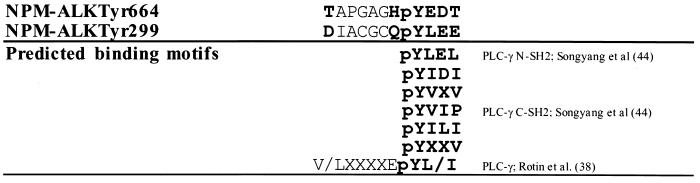
Of an array of proteins used for SH2-GST fusions, PLC-γ was found to be the strongest SH2 binding partner for NPM-ALK in vitro. PLC-γ contains both C-terminal and N-terminal SH2 domains. According to binding affinity tests with a random phosphopeptide library (44) and structural analysis conducted with high-affinity peptides (34), the specific phosphopeptide sequences recognized by the PLC-γ SH2 domains are pYVIP for the C-terminal and pYLEL for the N-terminal SH2 domain (Fig. 11). In addition, the motif (V/L)XXXXEpY(L/I) may constitute a high-affinity binding site for the SH2 domains of PLC-γ (38). Among the 21 tyrosine residues of NPM-ALK, the sequence surrounding Tyr299 (IACGCQpYLEE) best matches the binding motifs suggested for PLC-γ SH2 domains (Fig. 11). Indeed, in a phosphopeptide competition experiment, phosphopeptides surrounding Tyr299 and Tyr664 totally competed for binding to the PLC-γ SH2 domains (Fig. 4A and B). However, mutational analysis revealed that only Tyr664, which does not match any previous binding motif prediction (Fig. 11), was important for binding to both the N- and C-terminal SH2 domains of PLC-γ in vivo (Fig. 4D). This suggests that Tyr299 may not be an autophosphorylation site in vivo or that this residue is not accessible for binding to the PLC-γ SH2 domains. We were able to show that Tyr664 is an autophosphorylation site in vivo and that complex formation of NPM-ALK and PLC-γ is mediated by this single tyrosine residue. This interaction leads to tyrosine phosphorylation and activation of PLC-γ in vivo, evident by the elevated IP levels observed in wt NPM-ALK-expressing cells.
FIG. 11.
Amino acid sequences surrounding Tyr664 and Tyr299 of NPM-ALK and the predicted binding motifs for PLC-γ SH2 domains.
To study the importance of PLC-γ for the mitogenic potential of NPM-ALK we selected Rat-1 fibroblasts and the lymphocyte cell line Ba/F3 as model systems. Since NPM-ALK is associated with lymphomas, this lymphocyte cell line rather than fibroblasts might better represent the in vivo target of this oncogene. In Rat-1 fibroblasts NPM-ALK-induced soft-agar growth was severely impaired by mutation of the PLC-γ binding site. Ba/F3 cells depend on IL-3 for their growth, and this growth factor dependence could be overcome by the expression of the kinase-active wt NPM-ALK. As mentioned above, mutation of the IRS-1(Tyr156) or SHC(Tyr567) binding sites in NPM-ALK did not lead to the loss of growth factor-independent growth by these lymphocytes. Most importantly, however, mutation of the PLC-γ binding site in NPM-ALK(Tyr664) was sufficient to block factor-independent proliferation of these lymphocytes. We obtained mass clones expressing NPM-ALK(Y664F) and three independent single clones by limiting dilution. None of these cell clones retained the ability to grow without IL-3 over a period of more than 2 weeks. Interestingly, the PLC-γ pathway seems to be more significant for the mitogenic signal delivered by NPM-ALK than for a cell survival signal. This suggestion comes from the observation that, although the NPM-ALK(Y664F)-expressing cells did not actively proliferate without IL-3, apoptotic cell death seemed to have been minimized. For example, in contrast to parental Ba/F3 cells, all three single clones could be rescued by the readdition of IL-3 within the first 2 weeks (data not shown). Thus, activation of PLC-γ by NPM-ALK appears to be crucial for the delivery of a mitogenic signal by this lymphoma-associated oncogenic tyrosine kinase.
A question not addressed by our experiments is whether the mutation of Y664F leads to an inability of NPM-ALK to associate with other, unidentified SH2-domain-containing molecules which may be important for mitogenic signaling. Because the family of SH2-containing proteins continues to grow, we cannot rule out the possibility that there are other such substrates that may bind to Tyr664. Indeed, we have found that the recently identified adapter proteins Grb7 and Grb10 seemed to bind Tyr664 in vitro as well (data not shown). The function of these adapter proteins is not established (8, 32). However, neither of these proteins could be detected in large-cell anaplastic lymphoma cell lines by immunoblotting (data not shown); therefore, a role for these two adapters in NPM-ALK-mediated oncogenicity seems unlikely. By contrast, our demonstration that the overexpression of wt PLC-γ is capable of rescuing Ba/F3 cells containing the NPM-ALK(Y664F) mutant for IL-3-independent growth argues strongly for an important role of PLC-γ in NPM-ALK-mediated mitogenic signaling.
By using a truncated NPM-ALK retroviral construct, Bischof et al. showed that, in contrast to Rat-1 fibroblasts and Ba/F3 lymphocytes, the C-terminal 154 amino acids of NPM-ALK, including Tyr664, are dispensable for NPM-ALK-mediated transforming activity in Fr3T3 fibroblasts (4). These data suggest either that PLC-γ activation is cell-type specific and not required for the transformation of Fr3T3 fibroblasts by NPM-ALK or that by deleting the entire C terminus of NPM-ALK, both growth-promoting and -suppressing signals that equalize each other to result in a fully transforming mutant might be lost. In addition, retroviral infection of fibroblasts might lead to a very high level of protein expression, overriding the possible biological defects of the mutant.
The Y664F mutant is the only single tyrosine mutant among 19 tested so far in our laboratory that shows a biological phenotype in lymphocytes. This strongly supports the specific role and indispensability of PLC-γ for the biological function of NPM-ALK. PLC-γ activation leads to IP3 and diacylglycerol production in the cell, which in turn can activate protein kinase C. This pathway has been shown to have a whole array of biological functions, and its essential role for mitogenesis in other cellular systems is well established (29).
The PLC-γ binding tyrosine residue 664 is located in the C-terminal region of NPM-ALK, which is also present in the normal ALK RTK. Therefore, it is reasonable to anticipate that the ALK RTK also utilizes PLC-γ to mediate its biological signals. ALK expression is restricted to central and peripheral nervous tissues (16, 28). Both PLC-γ and SHC have been shown to play an important role in neuronal differentiation signals from other RTKs. For example, mutational analysis of TRK, an RTK activated by nerve growth factor, showed a major function for these signal transducers in neuronal differentiation (30). In PC12 cells, mutational analysis revealed a major function of SHC and a cooperative function of PLC-γ in neuronal outgrowth (30). Although SHC appears not to be crucial for the transformation potential of NPM-ALK, SHC and PLC-γ may be important for unidentified biological functions of the ALK RTK in the nervous system.
The fact that the single phosphotyrosine residue 664 is required to mediate a mitogenic signal by NPM-ALK in lymphocytes opens the possibility of clinical therapeutic interventions at the molecular level. It has been shown for the fibroblast growth factor receptor that fibroblast growth factor-mediated activation of PLC-γ can be completely blocked by cell-permeative peptides representing the PLC-γ-SH2 binding site (14). The peptide sequence surrounding Tyr664 in NPM-ALK is not present on other growth factor receptors. Thus, it might be important to investigate the possibility of treating large-cell anaplastic lymphomas with cell-permeative peptides that specifically block the PLC-γ pathway of NPM-ALK. In vitro studies with NPM-ALK-expressing cell lines have been initiated in our laboratory as a first stage to address this issue.
ACKNOWLEDGMENTS
We thank Jean Y. J. Wang for the GST-SH2 fusion proteins; S. Ohta and H. Hug for the PLC-γ2 cDNA; Josep Lovric, Sascha Dammeier, Gabriela Hübinger, and Thomas Jahn for technical advice; and Sunita Coutinho for careful reading of the manuscript. We thank Ida Rissling, Edith Fitzke, and Xiaoli Cui for technical assistance.
This work was supported in part by grants from the Mildred-Scheel Stiftung and the José-Carreras Stiftung to J.D., grants CA-01702 and CA-69129 and CORE grant CA-21765 to S.W.M., and the American-Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital.
REFERENCES
- 1.Alimandi M, Heidaran M A, Gutkind J S, Zhang J, Ellmore N, Valius M, Kazlauskas A, Pierce J H, Li W. PLC-gamma activation is required for PDGF-betaR-mediated mitogenesis and monocytic differentiation of myeloid progenitor cells. Oncogene. 1997;15:585–593. doi: 10.1038/sj.onc.1201221. [DOI] [PubMed] [Google Scholar]
- 2.Atherton E, Sheppard R C. Solid phase peptide synthesis—a practical approach. Oxford, England: IRL Press; 1989. [Google Scholar]
- 3.Bartram C R, de Klein A, Hagemeijer A, van Agthoven T, van Kessel A D, Bootsma D, Grosveld G, Ferguson-Smith M A, Davies T, Stone M, Heisterkamp N, Stephenson J R, Groffen J. Translocation of c-Abl oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukemia. Nature. 1983;306:277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- 4.Bischof D, Pulford K, Mason D Y, Morris S W. Role of the nucleophosmin (NPM) portion of the non-Hodgkin’s lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997;17:2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borer R A, Lehner C F, Eppenberger H M, Nigg E A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 6.Chan W Y, Liu Q R, Borjigin J, Busch H, Rennert O M, Tease L A, Chan P K. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry. 1989;28:1033–1039. doi: 10.1021/bi00429a017. [DOI] [PubMed] [Google Scholar]
- 7.Cortez D, Kadlec L, Pendergast A M. Structural and signaling requirements for BCR-ABL-mediated transformation and inhibition of apoptosis. Mol Cell Biol. 1995;15:5531–5541. doi: 10.1128/mcb.15.10.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly R J, Sanderson G M, Janes P W, Sutherland R L. Cloning and characterization of GRB14, a novel member of the GRB7 gene family. J Biol Chem. 1996;271:12502–12510. doi: 10.1074/jbc.271.21.12502. [DOI] [PubMed] [Google Scholar]
- 9.Dieter P, Schulze S A, Fitzke E. Activation of phospholipase C is not correlated to the formation of prostaglandins and superoxide in cultured rat liver macrophages. Cell Signalling. 1991;3:65–71. doi: 10.1016/0898-6568(91)90009-j. [DOI] [PubMed] [Google Scholar]
- 10.Dumbar T S, Gentry G A, Olson M O. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry. 1989;28:9495–9501. doi: 10.1021/bi00450a037. [DOI] [PubMed] [Google Scholar]
- 11.Duyster J, Baskaran R, Wang J Y J. Catalytic role for the SH2 domain of Abl tyrosine kinase. Proc Natl Acad Sci USA. 1995;92:1555–1559. doi: 10.1073/pnas.92.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, Yamamoto T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5) Proc Natl Acad Sci USA. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goga A, McLaughlin J, Afar D E, Saffran D C, Witte O N. Alternative signals to RAS for hematopoietic transformation by the BCR-ABL oncogene. Cell. 1995;82:981–988. doi: 10.1016/0092-8674(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 14.Hall H, Williams E J, Moore S E, Walsh F S, Prochiantz A, Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr Biol. 1996;6:580–587. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- 15.Heisterkamp N, Stephenson J R, Groffen J, Hansen P F, de Klein A, Bartram C R, Grosveld G. Localization of the c-abl oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983;315:758–761. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- 16.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 17.Kadin M E. Ki-1/CD30+ (anaplastic) large-cell lymphoma: maturation of a clinicopathologic entity with prospects of effective therapy. J Clin Oncol. 1994;12:884–887. doi: 10.1200/JCO.1994.12.5.884. [DOI] [PubMed] [Google Scholar]
- 18.Kashishian A, Cooper J A. Phosphorylation sites at the C-terminus of the platelet-derived growth factor receptor bind phospholipase C gamma 1. Mol Biol Cell. 1993;4:49–57. doi: 10.1091/mbc.4.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katan M. The control of inositol lipid hydrolysis. Cancer Surv. 1996;27:199–211. [PubMed] [Google Scholar]
- 20.Kim H K, Kim J W, Zilberstein A, Margolis B, Kim J G, Schlessinger J, Rhee S G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 21.Koch C A, Anderson D, Moran M F, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 22.Kozutsumi H, Toyoshima H, Hagiwara K, Yazaki Y, Hirai H. Human ltk receptor tyrosine kinase binds to PLC-gamma 1, PI3-K, GAP and Raf-1 in vivo. Oncogene. 1994;9:2991–2998. [PubMed] [Google Scholar]
- 23.Kuefer M U, Look A T, Pulford K, Behm F G, Pattengale P K, Mason D Y, Morris S W. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood. 1997;90:2901–2910. [PubMed] [Google Scholar]
- 24.Mason D Y, Bastard C, Rimokh R, Dastugue N, Huret J L, Kristoffersson U, Magaud J P, Nezelof C, Tilly H, Vannier J P. CD30-positive large-cell lymphomas (‘Ki-1 lymphoma’) are associated with a chromosomal translocation involving 5q35. Br J Haematol. 1990;74:161–168. doi: 10.1111/j.1365-2141.1990.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 25.McWhirter J R, Wang J Y J. An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 1993;12:1533–1546. doi: 10.1002/j.1460-2075.1993.tb05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mes-Masson A M, McLaughlin J, Daley G Q, Paskind M, Witte O N. Overlapping cDNA clones define the complete coding region for the p210 Bcr-Abl gene product associated with chronic myelogenous leukemia cells containing the Philadelphia chromosome. Proc Natl Acad Sci USA. 1986;83:9768–9772. doi: 10.1073/pnas.83.24.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris S W, Kirstein M N, Valentine M B, Dittmer K G, Shapiro D N, Saltman D L, Look A T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 28.Morris S W, Naeve C, Mathew P, James P L, Kirstein M N, Cui X, Witte D P. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 29.Noh D Y, Shin S H, Rhee S G. Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim Biophys Acta. 1995;1242:99–113. doi: 10.1016/0304-419x(95)00006-0. [DOI] [PubMed] [Google Scholar]
- 30.Obermeier A, Bradshaw R A, Seedorf K, Choidas A, Schlessinger J, Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC gamma. EMBO J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohta S, Matsui A, Nazawa Y, Kagawa Y. Complete cDNA encoding a putative phospholipase C from transformed human lymphocytes. FEBS Lett. 1988;242:31–35. doi: 10.1016/0014-5793(88)80979-7. [DOI] [PubMed] [Google Scholar]
- 32.Ooi J, Yajnik V, Immanuel D, Gordon M, Moskow J J, Buchberg A M, Margolis B. The cloning of Grb10 reveals a new family of SH2 domains proteins. Oncogene. 1995;10:1621–1630. [PubMed] [Google Scholar]
- 33.Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 34.Pascal S M, Singer A U, Gish G, Yamazaki T, Shoelson S E, Pawson T, Kay L E, Forman K J. Nuclear magnetic resonance structure of an SH2 domain of phospholipase C-gamma 1 complexed with a high affinity binding peptide. Cell. 1994;77:461–472. doi: 10.1016/0092-8674(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 35.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 36.Renshaw M W, McWhirter J R, Wang J Y J. The human leukemia oncogene bcr-abl abrogates the anchorage requirement but not the growth factor requirement for proliferation. Mol Cell Biol. 1995;15:1286–1293. doi: 10.1128/mcb.15.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues G A, Park M. Oncogenic activation of tyrosine kinases. Curr Opin Genet Dev. 1994;4:15–24. doi: 10.1016/0959-437x(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 38.Rotin D, Margolis B, Mohammadi M, Daly R J, Daum G, Li N, Fischer E H, Burgess W H, Ullrich A, Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawyers C L, Denny C T. Chronic myelomonocytic leukemia: Tel-a-kinase what Ets all about. Cell. 1994;77:171–173. doi: 10.1016/0092-8674(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 40.Sawyers C L, McLaughlin J, Witte O N. Genetic requirement for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J Exp Med. 1995;181:307–313. doi: 10.1084/jem.181.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt Z M, Franke W W. DNA cloning and amino acid sequence determination of a major constituent protein of mammalian nucleoli. Correspondence of the nucleoplasmin-related protein NO38 to mammalian protein B23. Chromosoma. 1988;96:417–426. doi: 10.1007/BF00303035. [DOI] [PubMed] [Google Scholar]
- 42.Shiota M, Fujimoto J, Semba T, Satoh H, Yamamoto T, Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line AMS3. Oncogene. 1994;9:1567–1574. [PubMed] [Google Scholar]
- 43.Smith D B, Johnson K S. Single-step purification of polypeptide expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 44.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 45.Stein H, Dallenbach F. Neoplastic hematopathology. Baltimore, Md: Williams & Wilkins; 1992. pp. 675–714. [Google Scholar]
- 46.Stein H, Herbst H, Anagnostopoulos I, Niedobitek G, Dallenbach F, Kratzsch H C. The nature of Hodgkin and Reed-Sternberg cells, their association with EBV, and their relationship to anaplastic large-cell lymphoma. Ann Oncol. 1991;2:33–38. doi: 10.1007/978-1-4899-7305-4_5. [DOI] [PubMed] [Google Scholar]
- 47.Ueno H, Sasaki K, Kozutsumi H, Miyagawa K, Mitani K, Yazaki Y, Hirai H. Growth and survival signals transmitted via two distinct NPXY motifs within leukocyte tyrosine kinase, an insulin receptor-related tyrosine kinase. J Biol Chem. 1996;271:27707–27714. doi: 10.1074/jbc.271.44.27707. [DOI] [PubMed] [Google Scholar]
- 48.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 49.Valius M, Bazenet C, Kazlauskas A. Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor β subunit and are required for binding of phospholipase Cγ and a 64-kilodalton protein, respectively. Mol Cell Biol. 1993;13:133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]