Abstract
INTRODUCTION
Alzheimer's disease (AD) affecting adults with Down syndrome (DS‐AD), like late‐onset AD (LOAD) in the neurotypical population, has preclinical, prodromal, and more advanced stages. Only tasks placing high demands on cognition are expected to be affected during the prodromal stage, with activities of daily living (ADLs) typically being spared. However, cognitive demands of ADLs could be high for adults with DS and may be affected during prodromal DS‐AD.
METHODS
Cognitively stable cases that subsequently developed prodromal DS‐AD were identified within a set of archived data from a previous longitudinal study. Measures of ADLs and multiple cognitive domains were examined over time.
RESULTS
Clear declines in ADLs accompanied cognitive declines with prodromal DS‐AD while stability in all measures was verified during preclinical DS‐AD.
DISCUSSION
Operationally defining prodromal DS‐AD is essential to disease staging in this high‐risk population and for informing treatment options and timing as new disease‐modifying drugs become available.
Highlights
Cognitive and functional stability were demonstrated prior to the onset of prodromal DS‐AD.
ADL declines accompanied cognitive declines as adults with DS transitioned to prodromal AD.
Declines in ADLs should be a defining feature of prodromal AD for adults with DS.
Better characterization of prodromal DS‐AD can improve AD diagnosis and disease staging.
Improvements in DS‐AD diagnosis and staging could also inform the timing of interventions.
Keywords: activities of daily living, Alzheimer's disease, Down syndrome, mild cognitive impairment, prodromal Alzheimer's disease
1. BACKGROUND
Down syndrome (DS), the most common genetic cause of intellectual disability, has a distinct phenotype that includes an exceptionally high risk for Alzheimer's disease (AD). 1 This “DS‐AD” risk is strongly associated with an increased expression of the gene coding for amyloid precursor protein, which in the case of DS is triplicated along with other genes located on human chromosome 21.
AD, the most prevalent cause of old age‐associated dementia, is a slowly progressing disease with an extended preclinical period beginning some 20 to 30 years prior to clinically significant impacts on abilities. 2 The earliest indications of decline are limited to subtle changes in tasks that place high demands on cognition, with tasks involving the greatest mental effort being the most vulnerable. Thus, the clinical progression of AD is described as having an insidious onset, with its prodromal stage commonly referred to as mild cognitive impairment (MCI).
Operationally, MCI is characterized by declines in memory or other domains of cognitive processing, with routine activities of daily living (ADLs) remaining essentially unaffected. 3 While difficulty with more complex routine tasks, referred to as instrumental activities of daily living (IADLs), can be experienced (e.g., managing finances), clinical practice guidelines recommend a shift to a diagnosis of dementia once ADLs declines are evident. One way to place the staging of AD clinical progression in broader context is offered by models like that proposed by Reisberg et al., 4 where abilities are affected in the reverse order that they develop with normal maturation and education. Reisberg et al. referred to this as a form of retrogenesis, but it might be better conceptualized along a continuum of the degree to which a task place demands on cognition. In this framework, tasks requiring the least allocation of effortful cognitive processing would be most resistant to the progression of AD while those requiring the most effort would be most vulnerable.
For adults without a history of intellectual or developmental disability (IDD), ADLs place low demands on cognition, being highly overlearned and completed with little cognitive effort. This aligns nicely with models suggesting that these domains are only affected once AD has progressed beyond its prodromal stage. However, the higher demands of IADLs make them more vulnerable as AD transitions from the preclinical to prodromal stage. 5 Which ADLs or IADLs place high demands on underlying cognitive capabilities depends not only on the inherent nature of each task and the stage of AD progression, but also on the presence/absence of an individual history of IDD and, when present, its severity.
In the case of adults with DS, histories of IDD typically include cognitive impairments varying in type and severity but always originating during early development. 6 While the severity of developmental impairments can vary substantially and some adults with DS are able to master IADLs, this is the exception rather than the rule. The vast majority of adults with DS have not achieved mastery of tasks falling into the IADL category and it is very possible that some functional domains traditionally classified as ADLs place high demands on cognition for them. If that proves to be the case, then these domains might be affected during prodromal AD and the defining features of prodromal DS‐AD cannot exclude declines in ADLs.
With promising disease‐altering treatments currently available and more on the horizon, it is critically important to understand the differences between preclinical, prodromal, and more advanced stages of the DS‐AD progression. In fact, the pending revised clinical criteria (Revised Criteria for Diagnosis and Staging of Alzheimer's Disease: Alzheimer's Association Workgroup) 7 suggest that individuals with DS might be considered to have DS‐AD from birth, with a recommendation of considering this as Stage 0 and corresponding to a pre‐preclinical status. Thus, each stage of underlying DS‐AD progression must be carefully studied to discover the key biomarkers and clinical characteristics that define it. Perhaps more important, consensus is needed on explicit criteria for identifying when treatments are appropriate and for whom. 8 , 9 The distinction between prodromal and more advanced DS‐AD has become a concern of immediate importance, and the present study utilized archived data from a large longitudinal study of aging and DS‐AD to determine, one way or the other, if declines in ADLs were present at the time of initial transition from preclinical to prodromal DS‐AD.
RESEARCH IN CONTEXT
Systematic review: Literature on adaptive functioning, mild cognitive impairment (MCI), and Alzheimer's disease (AD) progression in adults with and without Down syndrome (DS) was reviewed using traditional sources (e.g., PubMed and related citations).
Interpretation: Our findings demonstrate that activities of daily living (ADLs) can be affected when adults with DS experience a transition from preclinical to prodromal AD.
Future directions: The diagnosis of early AD‐related change is complicated for adults with DS given the substantial variation in lifelong impairments that characterize this phenotype and that predate the onset of AD. Further research is needed to determine how prodromal AD impacts ADLs across all levels of intellectual disability, and which measures are most informative for (1) initial diagnosis and (2) tracking further AD progression. Thus, diagnostic precision can be enhanced. With improved diagnosis, disease‐altering treatments can be introduced at those timepoints that will produce the greatest benefit.
2. METHODS
Archived data from a previously conducted longitudinal study of aging and AD in 613 adults with DS were searched to identify cases meeting the inclusion criteria for the present analyses (see Krinsky‐McHale et al. 10 ). All aspects of that research were reviewed and approved by the Institutional Review Boards (IRBs) of all collaborating institutions, informed consent was obtained from all participants or their legally authorized representatives (LARs), and all testing was conducted with participants' assent. Participants, recruited through contacts with community‐based agencies providing direct care within a 200‐mile radius of New York City, were assessed at approximately 18‐month intervals for up to a total of nine times (baseline plus eight follow‐ups covering a maximum span of approximately 12 to 15 years). Data were collected between the years 1987 and 2017. Detailed procedures and assessment measures have been described in multiple previous publications (e.g., Krinsky‐McHale et al. 10 ). These procedures included (1) in‐depth review of clinical records maintained at agencies providing direct services, (2) structured interviews with informants having direct day‐to‐day knowledge of the individual's behavioral and functional characteristics, and (3) approximately two hours of direct one‐on‐one testing employing procedures appropriate for adults with pre‐existing IDD and focused on cognitive domains likely to be affected as AD progressed from its preclinical to prodromal stage (although not all participants completed all tests). 11 Following each assessment cycle, an overall dementia status was determined for each individual in a consensus case conference that considered all available clinical data, past and present. Overall ratings comprised the following: (1) cognitive stability, corresponding to preclinical DS‐AD; (2) MCI‐DS, corresponding to prodromal DS‐AD; (3) probable or definite dementia, corresponding to DS‐AD in its more advanced stages; and (4) uncertain due to complications, i.e., conditions unrelated to AD that might have impacts on test results, for example, personal traumatic event or medical illness.
To be included in the present analyses, an individual had to have (1) a Stanford–Binet IQ ≥ 25 documented in medical records and established prior to risk for prodromal DS‐AD, (2) two or three assessment cycles indicating cognitive stability prior to a determination of MCI‐DS, and (3) development of prodromal AD as indicated by a consensus determination of MCI‐DS at the time of a subsequent follow‐up. Note that IQ estimates based on the various older versions of the Wechsler Adult Intelligence Scale (WAIS) are significantly higher for this population compared to the Stanford‐Binet and even the Wechsler Intelligence Scale for Children (WISC), 12 and in cases where only WAIS IQ data were available in clinical records, a Stanford‐Binet equivalent was calculated.
2.1. Participants
Table 1 provides an overview of the two samples meeting the inclusion criteria, which were selected based on the duration of tracking prior to MCI‐DS onset. Across the two samples, IQs ranged from 25 to 68, and the ages at the time of initial determination of incident MCI‐DS ranged from 46.1 to 70.2 years. The first sample included data from 62 adults with DS determined to be cognitively stable over the course of three assessment cycles (54 months) prior to developing MCI‐DS. A second separate sample served as a replication and included data from 43 adults with DS determined to be cognitively stable over the course of two assessment cycles (36 months) prior to developing MCI‐DS.
TABLE 1.
Selected demographic characteristics (means with SDs in parentheses) of the adults with Down syndrome included in the two samples.
| Sample 1 | Sample 2 | |
|---|---|---|
| (followed for 54 months) | (followed for 36 months) | |
| N | 62 | 43 |
| Age, years a | 57.9 (4.89) | 54.8 (4.84) |
| % male | 33.9 | 53.5 |
| IQ | 37.3 (7.70) | 36.7 (4.66) |
Values reflect the age at the cycle of mild cognitive impairment‐Down syndrome (MCI‐DS) onset.
2.2. Measures
Eight summary measures were selected to provide indications of changes over time, including the transition from preclinical to prodromal AD, that is, from cognitive stability to MCI‐DS. With one exception, these methods were developed explicitly for use with individuals with IDD at risk for AD. Multiple studies have established their utility for quantifying stability in cognition and functional abilities over time during preclinical DS‐AD and in detecting the transition from preclinical to prodromal to more advanced DS‐AD. 10 , 13
For the present set of analyses, the total score from the American Association on Mental Deficiency (AAMD) Adaptive Behavior Scale, Part I (ABSI), 14 developed as an informant interview specifically to evaluate ADLs of individuals with IDD, provided a quantitative measure of ADLs. The ABSI assesses functional abilities across ten domains (Independent Function, Physical Development, Economic Activity, Language, Time and Numbers, Domestic Activities, Vocational Activities, Self‐Direction, Responsibility, and Social Abilities). The sum across all domains was calculated with a maximum possible score of 280.
Various domains of cognition likely to be affected with the transition from preclinical to prodromal AD 15 , 16 , 17 were assessed with direct testing. A modified Selective Reminding Test (MSRT) 18 examined episodic memory, requiring free recall of a list of eight items within a single category, repeated for six trials. The total number of items correctly recalled served as the summary score (maximum = 48). Three tests provided broader‐based indications of mental status. An expanded version of the Down Syndrome Mental Status Examination (DSMSE) 19 was used and included six subtests (Personal Information (including orientation), Memory, Apraxia, Language, Visuospatial Abilities, and Knowledge of the Examiner). For the Language subtest, two scoring methods were available. The archived dataset utilized one of the scoring options, while more recent studies have shifted toward use of the other method. 10 DSMSE subtest scores were summed, and a total summary score was employed for the present analyses (maximum = 81). A modified version of the Mini‐Mental Status Examination (MMMSE) 20 , 21 included subtests in the areas of Orientation to Person, Place and Time, Knowledge of Colors, Anomia, Concentration, and Fine Motor Skill. Subtest totals were summed for an overall summary score (maximum = 74). The Test for Severe Impairment (TSI) 22 was also administered, with subtest scores (Language Comprehension and Production, General Knowledge, Conceptualization, Memory, and Motor Performance) summed for an overall summary score (maximum = 24).
More focused assessments comprised the following: (1) Verbal Fluency, 23 with the total number of correct responses serving as a single summary score; (2) the Beery Buktenica Developmental Test of Visual–Motor Integration (VMI), 24 where the number of figures correctly copied served as the single summary score (maximum = 27); and (3) the Block Design subtest of the WISC‐R 25 supplemented by simpler items taken from the original version of the DSMSE, with the total number of designs correctly copied serving as the single summary score (maximum = 78).
2.3. Statistical analyses
The descriptive statistics, correlation, and the analysis of variance for repeated measures subroutines of Statistica, Version 13.2, were used for all analyses. Each of the eight measures of performance/ability were initially analyzed separately to verify stability in performance preceding incident MCI‐DS and to determine the significance of declines associated with its onset. Two sets of analyses were conducted, one for the sample with three cognitively stable assessment cycles prior to determination of incident MCI‐DS and one for the sample with only two such assessment cycles preceding MCI‐DS onset.
These analyses were followed by combining the two samples to examine the relationship between individual change in cognition and change in ADLs over the two assessment cycles (36‐month period) preceding the onset of MCI‐DS. These descriptive analyses focused on the prediction, by current operational definitions of AD‐related MCI, that ADLs should be relatively spared while declines in tests of episodic memory should be evident. Therefore, changes in the total score on the ABSI were related to changes on the DSMSE and MSRT, the two components of the assessment battery assumed to place the greatest demands on episodic memory.
Additional analyses were conducted to examine the relationship between severity of IDD and the likelihood of change in ADLs. First, a correlation test between IQ and change in ABSI total scores was performed. Second, the overall sample was divided into two groups on the basis of their IQ score. Using a median split, the high IQ group was operationalized as IQ 37 to 68 and the low IQ group was operationalized as IQ 25 to 36. These groupings, high versus low IQ, were compared with respect to change in the ABSI total scores.
3. RESULTS
Table 2 provides a summary of findings from the eight separate repeated measures analyses of variance for the sample with three cognitively stable assessment cycles prior to the onset of MCI‐DS. Only partial data were available for some participants for some assessment cycles, accounting for differences in the degrees of freedom reported. All showed essentially the same profile of change over time, indicating minor changes when participants were judged to be in the preclinical stage of DS‐AD (cognitively stable) and then showing clear decline with the transition to prodromal DS‐AD. The only exceptions were observed for the DSMSE, which showed a small but statistically significant decline for the cycle preceding the transition in overall clinical status, and the VMI, which failed to show any statistically significant decline.
TABLE 2.
Results of repeated measures multivariate analyses of variance along with means and standard deviations (in parentheses) for each time of assessment for the sample of adults with Down syndrome having three cycles of assessment prior to developing MCI‐DS.
| Months prior to onset of MCI‐DS | |||||
|---|---|---|---|---|---|
| Assessment measure total scores | 54 months | 36 months | 18 months | 0 months | |
| ABSI | 208.1 (29.6) | 204.5 (30.1) | 204.0 (30.0) | 187.5 (34.3) a | F(3,58) = 23.0, p < 0.00001 |
| DSMSE | 54.6 (11.8) | 53.6 (11.2) | 51.4 (12.1) a | 44.7 (11.6) a | F(3,54) = 37.1, p < 0.00001 |
| MSRT (Recall) | 30.0 (9.6) | 28.5 (9.8) | 27.5 (9.8) | 18.1 (9.6) a | F(3,50) = 41.9, p < 0.00001 |
| Block Design | 15.0 (8.4) | 14.2 (8.4) | 13.5 (8.3) | 9.4 (7.6) a | F(3,54) = 12.5, p < 0.0001 |
| Verbal Fluency | 7.1 (3.6) | 7.4 (3.8) | 6.9 (3.8) | 5.0 (3.7) a | F(3,52) = 8.5, p < 0.0001 |
| MMMSE | 58.1 (13.7) | 57.5 (13.0) | 56.6 (13.7) | 52.1 (16.4) a | F(3,48) = 6.0, p < 0.002 |
| TSI | 20.1 (2.8) | 19.7 (4.4) | 20.3 (3.3) | 18.4 (4.8) a | F(3,46) = 3.7, p < 0.02 |
| VMI | 10.7 (3.0) | 10.7 (3.3) | 10.6 (2.9) | 10.4 (3.5) | F(3,53) = 0.4, p > 0.75 |
Abbreviations: ABSI, American Association on Mental Deficiency Adaptive Behavior Scale, Part I; DSMSE, Down Syndrome Mental Status Examination; MCI‐DS, mild cognitive impairment‐Down syndrome; MMMSE, Modified Mini‐Mental Status Examination; MSRT, Modified Selective Reminding Test; TSI, Test for Severe Impairment; VMI, Beery Buktenica Developmental Test of Visual‐Motor Integration.
Indicates a statistically significant change from the previous assessment cycle. Note that only the DSMSE showed a significant decline prior to onset of MCI‐DS.
The small trends toward cognitive decline seen prior to the initial determination of MCI‐DS suggest that some cases may have been false negatives at their prior assessment cycle. To address this possibility, the ABSI analysis was repeated with a subset of cases excluded based on evidence of decline of 10% or more for the DSMSE prior to incident MCI‐DS. For the remaining sample of 35 individuals, the mean DSMSE score now increased slightly from 54.4 to 55.3 over the three cycles preceding incident MCI‐DS, then dropping to 46.3. ABSI total scores followed the same pattern, increasing slightly from 206.8 to 207.2 prior to MCI‐DS onset and then declining to 191.4 (p < 0.0003), contributing to an overall multivariate F(3,32) = 9.53, p < 0.0002.
Table 3 provides a comparable summary of results for the replication sample, where only two assessment cycles were available indicating cognitive stability prior to MCI‐DS onset. The same profiles were found, the only exception being that the decline following MCI‐DS for the VMI, although still small, now reached statistical significance.
TABLE 3.
Results of repeated measures multivariate analyses of variance along with means and standard deviations (in parentheses) for each time of assessment for the sample of adults with Down syndrome having two cycles of assessment prior to developing MCI‐DS.
| Months prior to onset of MCI‐DS | ||||
|---|---|---|---|---|
| Assessment measure total scores | 36 months | 18 months | 0 months | |
| ABSI | 203.2 (30.3) | 200.1 (29.3) | 179.2 (27.8) a | F(2,40) = 29.4, p < 0.00001 |
| DSMSE | 49.2 (11.3) | 46.0 (10.3) a | 39.1 (10.9) a | F(2,35) = 30.3, p < 0.00001 |
| MSRT (Recall) | 18.6 (9.4) | 19.7 (9.5) | 12.3 (7.8) a | F(2,29) = 20.1, p < 0.00001 |
| Block Design | 11.1 (8.6) | 10.3 (9.7) | 6.5 (6.5) a | F(2.35) = 12.8, p < 0.0001 |
| Verbal Fluency | 5.6 (3.2) | 5.8 (3.6) | 4.3 (2.8) a | F(2,36) = 4.53, p < 0.02 |
| MMMSE | 53.2 (16.0) | 53.4 (10.6) | 47.2 (11.0) a | F(2,34) = 14.2, p < 0.0001 |
| TSI | 19.4 (3.2) | 18.6 (3.8) | 18.3 (3.5) a | F(2,32) = 4.49, p < 0.02 |
| VMI | 10.6 (3.7) | 10.1 (3.7) | 9.2 (3.7) a | F(2,38) = 4.59, p < 0.02 |
Abbreviations: ABSI, American Association on Mental Deficiency Adaptive Behavior Scale, Part I; DSMSE, Down Syndrome Mental Status Examination; MCI‐DS, mild cognitive impairment‐Down syndrome; MMMSE, Modified Mini‐Mental Status Examination; MSRT, Modified Selective Reminding Test; TSI, Test for Severe Impairment; VMI, Beery Buktenica Developmental Test of Visual‐Motor Integration.
Indicates a statistically significant change from the previous assessment cycle. Note that only the DSMSE showed a significant decline prior to onset of MCI‐DS.
The two panels of Figure 1 provide a more detailed picture of the relationship between individual change in cognition and adaptive behavior associated with the preclinical to prodromal transition. For these plots, data from the two samples were combined, with DSMSE and MSRT scores selected as illustrative of changes in cognition. Each point indicates the change in performance from the time of assessment 36 months prior to the time MCI‐DS was first detected. As clearly illustrated, decline in cognition is most often accompanied by decline in ADLs (lower left quadrant). In fact, of cases showing at least a 5% decline in the DSMSE or MSRT with onset of MCI‐DS, 67% also showed at least a 5% decline on the ABSI (data not shown).
FIGURE 1.
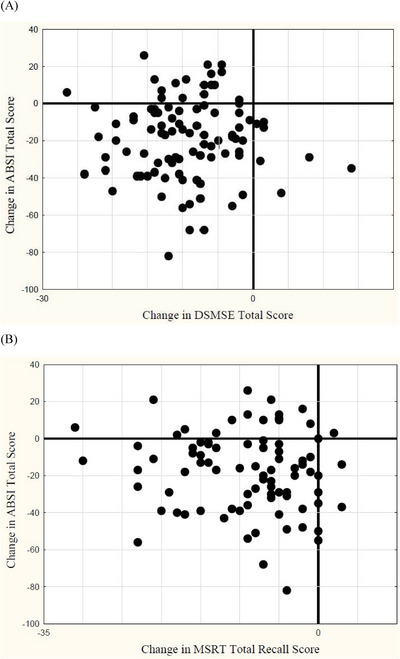
Scatterplots relating changes in activities of daily living (ADLs) as assessed with the Adaptive Behavior Scale (ABSI) to changes in the expanded Down Syndrome Mental Status Examination (DSMSE), a broadly based assessment of cognition (A), or to changes in the modified Selective Reminding Test (MSRT), a specific test of episodic memory (B), for adults with Down syndrome transitioning from preclinical to prodromal Alzheimer's disease (mild cognitive impairment [MCI]‐DS).
The relationship between IQ and change in ABSI total scores was not significant (r = 0.106, p > 0.1). When the sample was divided into two groups, high IQ versus low IQ, there was no statistically significant difference between the groups with regard to change in ABSI total scores (t = 0.253, p > 0.8). To explore the degree of ABSI decline, the correlation between the last preclinical ABSI total scores and the change in ABSI total scores with MCI‐DS onset was also examined. The relationship was small, but statistically significant (r = −0.234, p < 0.03). Individuals with higher preclinical ABSI total scores showed a slight tendency toward larger decline. This finding could reflect the structure of the AAMD ABSI, where higher scores are only possible when individuals are able to perform the more challenging tasks that are most closely aligned with IADLs. However, this correlation accounted for only 5.5% of the variance in ABSI total score change.
4. DISCUSSION
The present analyses provide convincing evidence that ADLs can be affected as adults with DS experience a transition from preclinical to prodromal AD as reflected by a diagnosis of MCI‐DS. Results showed a consistent profile of cognitive and functional stability preceding an initial determination of MCI‐DS. Results also showed clear evidence of functional declines accompanying cognitive declines analogous to those seen with the transition from preclinical to prodromal AD in affected elderly adults without a history of IDD. Strength was added by analyses of performance for a second sample, replicating initial findings in all critical respects.
The present findings have parallels with impacts on IADLs seen with prodromal AD more generally. Distinctions between IADLs and ADLs are somewhat arbitrary to begin with and rest on the degree to which effortful cognitive processing is needed for successful task engagement. Underlying task‐specific demands must vary with global intelligence and history of experience. For example, prodromal AD might have impacts on balancing a checkbook for some elderly adults but only at a later stage of disease progression for most certified public accountants. For the vast majority of adults with DS, simple arithmetic could be as cognitively demanding for them and as vulnerable to prodromal DS‐AD as checkbook balancing is for older adults without a history of IDD.
With promising disease‐altering treatments now available, it is critically important to be able to understand the differences between preclinical, prodromal, and more advanced stages of AD progression for all adults at risk. Our findings have direct implications for diagnostic practice for this high‐risk population. Current consensus statements requiring a diagnosis of dementia with evidence of declining ADLs need to have an exception made for adults with DS, and most likely for all adults with longstanding histories of comparable impairments. Recognition that ADLs can be affected with the transition from preclinical to prodromal DS‐AD not only advances our understanding of disease progression, but also directs our next steps to further characterize the impact upon ADLs. In addition to determining the impact of prodromal AD on ADLs across all levels of IDD, it is essential to identify which specific measures are most informative for initial diagnosis and for tracking further progression of underlying disease. This is especially true for those individuals with more severe forms of IDD for whom direct measures of cognition are uninformative. With this knowledge, we can enhance diagnostic precision for this high‐risk population, and with improved diagnosis and staging, disease‐altering treatments can be introduced at timepoints that will yield the greatest benefit.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Recruitment, informed consent, and study procedures were approved by the Institutional Review Boards of the New York State Institute for Basic Research in Developmental Disabilities, New York State Psychiatric Institute, and Columbia University Irving Medical Center. Informed consent was obtained from those participants with capacity. Surrogate informed consent was obtained from legally authorized representatives for those participants who lacked capacity.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Drs. Elizabeth Lennon and Phyllis Kittler for their expertise and valuable comments and suggestions on this manuscript. As always, we are grateful to all our participants, their families, and the agencies serving the needs of individuals with intellectual and developmental disabilities. This work was supported by grants from the National Institutes of Health, P01 HD035897, U01 AG051412 and U19 AG068054, as well as funds from the New York State Office for People with Developmental Disabilities.
Listwan TA, Krinsky‐McHale SJ, Kovacs CM, et al. Prodromal Alzheimer's disease can affect activities of daily living for adults with Down syndrome. Alzheimer's Dement. 2024;16:e12562. 10.1002/dad2.12562
REFERENCES
- 1. Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019;15(3):135‐147. doi: 10.1038/s41582-018-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):257‐262. doi: 10.1016/j.jalz.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reisberg B, Franssen EH, Souren LE, Auer SR, Akram I, Kenowsky S. Evidence and mechanisms of retrogenesis in Alzheimer's and other dementias: management and treatment import. Am J Alzheimers Dis Other Dement. 2002;17(4):202‐212. doi: 10.1177/153331750201700411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jekel K, Damian M, Wattmo C, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7(1):17. doi: 10.1186/s13195-015-0099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silverman W. Down syndrome: cognitive phenotype. Ment Retard Dev Disabil Res Rev. 2007;13(3):228‐236. doi: 10.1002/mrdd.20156 [DOI] [PubMed] [Google Scholar]
- 7. Alzheimer's Association Workgroup (2023, October). Revised Criteria for Diagnosis and Staging of Alzheimer's Disease: Alzheimer's Association Workgroup. https://alz.org/diagnostic‐criteria [DOI] [PMC free article] [PubMed]
- 8. Hillerstrom H, Fisher R, Janicki MP, the Working Group on Criteria for Access to Alzheimer's Therapeutics for Adults with Down Syndrome . (May 30, 2023). Adapting eligibility criteria for prescribing FDA approved anti‐amyloid immunotherapeutics for adults with Down syndrome with early‐stage Alzheimer's dementia. Lumind IDSC and the National Task Group. www.lumind.org and www.the‐ntg.org
- 9. Rafii MS, Fortea J. Down Syndrome in a new era for Alzheimer disease. JAMA. 2023;330(22):2157‐2158. doi: 10.1001/jama.2023.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krinsky‐McHale SJ, Zigman WB, Lee JH, et al. Promising outcome measures of early Alzheimer's dementia in adults with Down syndrome. Alzheimers Dement. 2020;12(1):e12044. doi: 10.1002/dad2.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverman W, Schupf N, Zigman W, et al. Dementia in adults with mental retardation: assessment at a single point in time. Am J Ment Retard. 2004;109(2):111‐125. doi: 10.1352/0895-8017(2004)109<111:DIAWMR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 12. Silverman W, Miezejeski C, Ryan R, Zigman W, Krinsky‐McHale S, Urv T. Stanford‐Binet & WAIS IQ differences and their implications for adults with intellectual disability (aka mental retardation). Intelligence. 2010;38(2):242‐248. doi: 10.1016/j.intell.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esbensen AJ, Hooper SR, Fidler D, et al. Outcome measures for clinical trials in Down syndrome. Am J Intellect Dev Disabil. 2017;122(3):247‐281. doi: 10.1352/1944-7558-122.3.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nihira K, Foster R, Shellhaas M, Leland H. AAMD Adaptive Behavior Scale. American Association on Mental Deficiency; 1974. [Google Scholar]
- 15. Benejam B, Videla L, Vilaplana E, et al. Diagnosis of prodromal and Alzheimer's disease dementia in adults with Down syndrome using neuropsychological tests. Alzheimers Dement. 2020;12(1):e12047. Published 2020 Jun 28. doi: 10.1002/dad2.12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartley SL, Handen BL, Devenny D, et al. Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer's disease in Down syndrome. Alzheimers Dement. 2020;12(1):e12096. Published 2020 Sep 13. doi: 10.1002/dad2.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer's disease in Down syndrome. Alzheimers Dement. 2019;15(2):245‐257. doi: 10.1016/j.jalz.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buschke H. Selective Reminding for analysis of memory and learning. J Verbal Learning Verbal Behav. 1973;12(5):534‐550. [Google Scholar]
- 19. Haxby JV. Neuropsychological evaluation of adults with Down's syndrome: patterns of selective impairment in non‐demented old adults. J Intellect Disabil Res. 1989;33(3):193‐210. [DOI] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state, a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 21. Wisniewski KE, Hill AL. Clinical aspects of dementia in mental retardation and developmental disabilities. In: Wisniewski H, Janicki M, eds. Aging and Developmental Disabilities: Issues and Approaches. Brookes; 1985:p195‐210. [Google Scholar]
- 22. Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40(5):449‐453. [DOI] [PubMed] [Google Scholar]
- 23. McCarthy D. Scales of Children's Abilities. Psychological Corp; 1972. [Google Scholar]
- 24. Beery KE, Buktenica NA. Developmental Test of Visual‐Motor Integration. Modern Curriculum Press; 1989. [Google Scholar]
- 25. Wechsler D. Wechsler Intelligence Scale for Children‐Revised . Psychological Corp; 1974. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


