See Guidelines on Page 526
Health and healthcare are important issues for Africa, as elsewhere. Healthcare delivery has been challenging; however, Africa has made great strides in recent years. This is happening because economic development is picking up in many parts of the continent; and in kidney care, in part thanks to the International Society of Nephrology, which supports knowledge sharing and advocacy. Rapid economic strides, however, bring newer healthcare concerns.1 Once a continent portrayed as home to “dangerous” infections, Africa is now catching up with the rest of the world in terms of life-style associated noncommunicable diseases. The position paper by 13 senior leaders from the African Association of Nephrology in this issue of Kidney International Reports highlights the important concern of the increasing prevalence of chronic kidney disease (CKD) in Africa, driven by the triple burdens of rising noncommunicable diseases, infections and social determinants of health, superimposed on genetic predisposition in some regions.2 The authors are to be congratulated for a comprehensive and sober representation of the burden of CKD across the continent and the emphasis on the potential value of sodium-glucose cotransporter-2 inhibitors (SGLT2i) in addition to standard care to reduce the burden and morbidities associated with CKD in Africa. As a position paper, the authors also consider important gaps in screening and challenges in delivery of quality care for CKD across the continent.
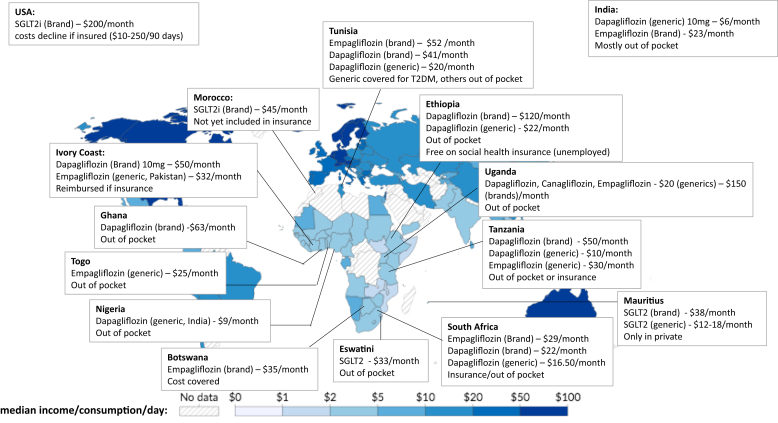
Africa comprises 54 countries and is not a uniform continent.3 It is home to 18% of the world’s population and hosts the greatest proportion of people living under the poverty line of $2.15 per day globally (Figure 1).3 The strength, quality, and financing of health systems varies across the continent, with most kidney services being covered by the health system in North Africa and most services being paid for out of pocket in many countries in Sub-Saharan Africa.1 The challenges experienced in diagnosis and access to care for CKD vary with local contexts, being generally greater in Sub-Saharan Africa than in North Africa. Despite this diversity, the recommendations put forward in this position paper are largely generalizable across Africa and beyond and must urgently be implemented everywhere.
Figure 1.
Map depicting the global distribution of median income or consumption per day (2019), and examples of monthly costs of SGLT2 inhibitors (converted to current US dollars) across Africa. It is clear that many patients across Africa would struggle to afford SGLT2i out of pocket. Cost data obtained through personal communication as of November 2023 with the following: Dr. Gordana Cavric (Botswana), Dr. Yewondwossen Tadasse Mengistu (Ethiopia), Dr. Thandiwe Dlamini (Eswatini), Dr Elliot K. Tannor (Ghana), Dr. Tea Weu Mélanie (Ivory Coast), Dr. Tarik Sqalli (Morocco), Dr. Ogo Egbuna (Nigeria), Dr. Claudia L. Do Vale (South Africa), Dr. Mahzar Amirali (Tanzania), Dr. Kossi Sabi (Togo), Prof Faical Jarraya (Tunisia), and Dr. Grace Kansiime (Uganda). Costs in USA (Prof Rasheed A. Balogun) and India (Prof Manisha Sahay) are included for comparison. Map reproduced from Joe Hasell, Max Roser, Esteban Ortiz-Ospina, and Pablo Arriagada (2022) - “Poverty” Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/poverty'
Across Africa, the major risk factors for CKD include hypertension, diabetes, and HIV.2 As highlighted by the authors, other important risk factors include pregnancy-associated complications, preeclampsia, hepatitis B and C, the use of traditional remedies, and low birth weight. In addition, it is likely that acute kidney injury is an important contributor to the CKD burden in Africa.4 Better understanding of CKD risk factors in Africa is required, to define high-risk groups and to inform screening strategies, which based on data from elsewhere, is cost-effective in high-risk groups.5 Exact data on the prevalence of CKD in Africa are however not available given the lack of kidney disease registries across the continent. This important lack of data is highlighted by the over 3-fold difference in CKD prevalence reported in the position paper across the 5 regions of the continent (from 6%–20%).2 The diversity of these ranges likely reflect the absence of systematic screening programs, challenges in access to diagnosis, and the lack of robust health information systems to capture data, rather than or in addition to, true differences in CKD prevalence. This lack of robust data has hampered advocacy for kidney diseases and recognition of its importance across the continent. Data from the Global Burden of Disease study shows that CKD is among the top 5 causes of death in North Africa (Egypt, Tunisia, Libya, and Morocco), Mauritius, Sao Tome and Principe, and the Seychelles.6 CKD falls within the top 10 leading causes of death in other 6 countries, namely Algeria, Senegal, Mauritania, Eswatini, Cape Verde, and Sudan. Given the limitations of these data and the high global prevalence of undiagnosed CKD, even in high income settings, it is likely that the true burden of CKD in Africa remains underestimated.
Importantly, the position paper highlights that CKD is often diagnosed at a younger age in Africa compared to their counterparts with kidney failure (KF) in higher resource settings. Concerningly, the relatively consistent observation that many people with CKD in Sub-Saharan Africa are first diagnosed with KF reflects the severe impact of resource constraints and nonavailability or unaffordability of primary care services and appropriate screening. For many, especially in Sub-Saharan Africa, any intervention at this stage comes too late. Insufficient and prohibitively expensive out of pocket costs of medications, not to speak of dialysis and transplant services, mean that most patients die within weeks to months of the diagnosis of KF.7
The position paper builds the case around the critical importance of appropriate screening, early diagnosis, and optimal treatment of CKD in Africa to prevent KF and to save lives. The suggestion to use dipstick detection of proteinuria in place of unaffordable and unavailable albumin-to-creatinine ratio assessment is a reasonable compromise. The global discussion about including race in equations to estimate glomerular filtration rate extends to black Africans as well; however, thus far no equation has proven to be ideal.5 Diagnosis however is not enough, and must be followed by equitable, sustainable, and quality care. In Africa, as elsewhere, the use of renin-angiotensin system blockade has been the mainstay of CKD care; and there is growing recognition that this first line therapy does not mitigate all the adverse consequences of CKD. Therefore, the need for medication, which not only retards the progression but also improves outcomes in people living with CKD. In this context, there has been a profusion of “real-world” literature, which has demonstrated cardiorenoprotective effects of SGLT2i. A major problem in these groundbreaking studies, which is highlighted in the position paper, is the fact that inclusion of black subjects has been unacceptably low, especially considering the greater burden of kidney disease in people of African origin. This omission is even more concerning, given the fact that no studies have been conducted in Africa. This “blind spot” of many global researchers and funders toward Africa is illustrated by a study that concluded that SGLT2i were safe and effective for heart failure across “global” regions, although glaringly there was no data from Africa.8 A cynical interpretation here would be that funders may acknowledge upfront that the market will be small based on reliance on out of pocket payments in Sub-Saharan Africa, or a presumption that the research infrastructure is insufficient and countries are corrupt. These paradigms must change to ensure that if studies are to be “global,” Africa must be included. In Africa, studies to understand effectiveness in local contexts and potential safety concerns would be very important; especially given the potential higher risks of infections and amputations reported in some studies with SGLT2i, which may become more real concerns in settings where monitoring and access to care if problems arise may be delayed.
With Africa’s unique challenges and CKD demographics and the immediately life-threatening risk of KF when dialysis and transplantation are inaccessible, despite lack of data from Africa, the authors make a strong case for inclusion of SGLT2i as a cornerstone of CKD care. The authors highlight the globally poor uptake of SGLT2i and outline the barriers in implementation of kidney care and specifically SGLT2i treatment in Africa. Out of pocket costs are a major barrier. As shown in Figure 1, prices for SGLT2i vary significantly across the continent, with generics imported from India and Pakistan being more affordable; however, in many cases, these prices are higher than the median daily income. Prices of SGLT2i are high everywhere, and lack of insurance and the cost of the medication were reported barriers even in high-income settings.9 This status quo should not be acceptable.
An important issue not discussed in detail in the position paper is physician behavior impacting prescribing of SGLT2i in CKD. Studies from many parts of the world have consistently shown poor uptake of SGLT2i by primary care physicians and nephrologists. A recent survey found that 36% of nephrologists did not know the indications of SGLT2i and 20% were not familiar with the class of drugs.9 This is likely to be a major constraint in Africa, and given reliance on out of pocket payment, clinician confidence or experience with these medications will only grow slowly because the number of patients using them will be small. In addition, physicians may be hesitant to prescribe the medications and the necessary follow-up laboratory testing, which they know their patients cannot afford. Improving implementation of comprehensive quality CKD care in primary care and at higher levels of care requires understanding of local challenges and barriers and development of strategies to get around these. Solutions may include simplification and alignment of guidelines such that they are less overwhelming to clinicians on the ground (e.g., merging hypertension, diabetes, cardiac, and kidney guidelines), integration of kidney care into other disease programs (e.g., HIV and tuberculosis clinics), offer continuing medical education support online and in-person, empowering patients to demand and expect quality care, and reducing drug prices.
People living with CKD in Africa cannot afford to wait any longer until the SGLT2i trickle down to them. This position paper outlines a simple and feasible clinical approach to screening for and managing CKD and aligns well with the theme of World Kidney Day in 2024, to advance equitable access to care and optimal medication practice to achieve kidney health for all. This requires political will, capacity building of healthcare workers and patients, affordable pricing, and availability of medication. The time is now to scale up quality kidney care in Africa and beyond.
Disclosure
VAL reports royalties as editor of The Kidney. All the other authors declared no competing interests.
References
- 1.Okpechi I., Niang A., Hafez M., et al. A roadmap for kidney care in Africa: an analysis of International Society of Nephrology–Global Kidney Health Atlas Africa data describing current gaps and opportunities. Afr J Nephrol. 2022;25 doi: 10.21804/25-1-5100. [DOI] [Google Scholar]
- 2.Jarraya F., Niang A., Bagha H., et al. The role of sodium-glucose cotransporter 2 inhibitors in the treatment paradigm of CKD in Africa: an African association of nephrology panel position paper. Kidney Int Rep. 2024;9:526–548. doi: 10.1016/j.ekir.2023.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer. African population. 2023. https://www.worldometers.info/world-population/africa-population/
- 4.Yao K.H., Konan S.D., Tia W.M., Diopoh S.P., Moh R., Sanogo S. Outcomes of acute kidney injury in a department of internal medicine in ABIDJAN (cote D'IVOIRE) Nephrology (Carlton) 2018;23:653–660. doi: 10.1111/nep.13064. [DOI] [PubMed] [Google Scholar]
- 5.Kalyesubula R., Conroy A.L., Calice-Silva V., et al. Screening for kidney disease in low- and middle-income countries. Semin Nephrol. 2022;42 doi: 10.1016/j.semnephrol.2023.151315. [DOI] [PubMed] [Google Scholar]
- 6.Institute for Health Metrics and Evaluation (IHME) Institution; Seattle, WA: 2020. GBD compare data visualization.http://vizhub.healthdata.org/gbd-compare [Google Scholar]
- 7.Ashuntantang G., Osafo C., Olowu W.A., et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5:e408–e417. doi: 10.1016/S2214-109X(17)30057-8. [DOI] [PubMed] [Google Scholar]
- 8.Kondo T., Wang X., Yang M., et al. Efficacy of dapagliflozin according to geographic location of patients with heart failure. J Am Coll Cardiol. 2023;82:1014–1026. doi: 10.1016/j.jacc.2023.05.056. [DOI] [PubMed] [Google Scholar]
- 9.Singh T., Li T., Mandelbrot D., Astor B.C., Mehr A.P. Prescribing patterns for sodium-glucose cotransporter 2 inhibitors: a survey of nephrologists. Kidney Int Rep. 2023;8:1669–1671. doi: 10.1016/j.ekir.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]