Abstract
Background
While steerable sheaths are widely used to improve catheter stability and contact force during radiofrequency (RF) catheter ablation in patients with atrial fibrillation (AF), steerable sheaths are less commonly used during transseptal puncture. This study evaluated whether left atrial catheterization efficiency can be improved using the VersaCross combined steerable sheath and transseptal system compared to previous standard workflow.
Methods
This study retrospectively analyzed AF ablation performed using the VersaCross Workflow consisting of VersaCross steerable sheath and RF wire for transseptal puncture and catheter ablation (VCW) to the standard workflow using a fixed curve sheath with RF needle followed by exchange for an Agilis steerable sheath for catheter ablation (STW).
Results
Thirty patients underwent RF ablation for paroxysmal or persistent AF, 15 using the VCW and 15 using the STW. Transseptal puncture time was 10.8 mins faster with the VCW compared to the standard workflow (20.9 ± 5.9 min vs. 31.7 ± 15.1 min; p = 0.024). Time to left atrial catheterization was 40% faster with the VCW compared to the STW (21.3 ± 5.8 min vs. 35.2 ± 14.4 min; p = 0.003). Overall procedure time was 14.2 min faster in the VCW compared to the STW (86.3 ± 16.1 min vs. 100.5 ± 19.3 min; p = 0.044).
Conclusions
Use of the VersaCross steerable system significantly reduced time to transseptal puncture, time to left atrial catheterization, and overall RF ablation time.
Keywords: Catheter ablation, Radiofrequency ablation, Transseptal puncture, Atrial fibrillation
1. Introduction
Catheter ablation with energy sources such as radiofrequency (RF) has been established as the standard of care for patients with symptomatic atrial fibrillation (AF) [1]. Catheter ablation is associated with a reduction in mortality in patients with coexisting heart failure with reduced ejection fraction; patients with symptomatic AF undergoing catheter ablation exhibit improved quality of life and improved freedom from atrial arrhythmias compared to medical therapy [[1], [2], [3]]. Pulmonary vein isolation requires safe and efficient transseptal puncture for left atrial (LA) catheterization. With the widespread use of RF ablation and transseptal puncture procedures, many developments have been made to assist operators, reduce procedural and fluoroscopy times, and reduce complications. Among these developments is the use of steerable sheaths to facilitate catheter manipulation, contact force and catheter stability [4].
Transseptal puncture is technically demanding and requires adequate operator experience to avoid complications. While knowledge of atrial anatomy as well as the adjacent structures is crucial for transseptal catheterization, new tools have been developed to facilitate transseptal catheterization and improve safety [5]. Apart from imaging advancements, tools such as the use of purpose-built RF puncture technology have been shown to result in shorter instrumentation time, greater efficacy in transseptal crossing, and fewer episodes of pericardial tamponade [6]. More recently, the RF wire system was shown to achieve completely fluoroless transseptal puncture safely and effectively while improving procedural efficiency in RF ablations [7]. Several studies have shown the benefits of a steerable sheath for RF ablations [4,[8], [9], [10]], however, the use of steerable sheath for transseptal puncture remains uncommon. Here we evaluate whether LA catheterization efficiency and ablation time can be improved using a RF-wire based steerable transseptal system compared to the current needle-based workflow.
2. Methods
2.1. Study design
A retrospective analysis was conducted involving 30 patients who were treated with low fluoro/fluoroless RF ablation for AF at a single center. All ablations were performed by a single operator performing approximately 150–200 catheter ablation procedures yearly. The workflow of fifteen consecutive patients who underwent RF ablation between July 2020 and July 2021 using a standard radiofrequency-needle based transseptal puncture (NRG, Baylis Medical, Montreal, QC, Canada) for transseptal puncture followed by over-wire exchange to an Agilis steerable sheath (Abbott, St. Paul, MN; STW group) was compared to the workflow of 15 consecutive patients who underwent RF ablation between October 2021 and December 2021 using a combined RF transseptal wire and steerable system (Baylis Medical, Montreal, QC, Canada; VCW group). Radiofrequency catheter ablations were performed by a single operator (A.J.), with standard informed consent obtained prior to each procedure. The primary endpoints were time to transseptal puncture and LA catheterization time (time to steerable sheath across the septum). Secondary endpoints were RF ablation start time after venous access, acute procedural success, procedure time, procedural complications, fluoroscopy use, and LA dwell time. LA dwell time was defined as time determined by time from steerable sheath across atrial septum to procedure end.
2.2. Catheter placement and mapping
All antiarrhythmic drugs were discontinued depending on patient characteristics and at the discretion of the operator. All patients underwent general endotracheal intubation and low volume, high frequency ventilation. Transeptal puncture was guided by intracardiac echocardiography (ICE) and 3D electroanatomic mapping (EAM; Biosense Webster, Diamond Bar, CA, USA). Fluoroscopy was only used to locate the esophageal temperature probe and in patients with tortuous venous anatomy. Using ultrasound guidance and a modified Seldinger technique, three sheaths were placed into the right femoral vein (RFV). A weight-based heparin bolus was administered prior to transseptal puncture. Activated clotting time (ACT) was monitored throughout the procedure, and intravenous heparin bolus was administered to target ACT >350 seconds prior to and after transseptal puncture. A 10F ICE catheter (SoundStar; Biosense Webster, Diamond Bar, CA, USA), a 3.5mm irrigated ablation catheter (SmartTouch STSF; Biosense Webster, Diamond Bar, CA, USA) were introduced into the right atrium (RA). EAM was used to create geometry of the RA, superior and inferior vena cavae, coronary sinus (CS), transseptal puncture location and LA. A decapolar diagnostic catheter (Inquiry; Abbott) was inserted into the coronary sinus using EAM guidance (CARTO 3; Biosense Webster).
2.3. Transseptal puncture and radiofrequency ablation
In both groups, a single transseptal puncture was performed using ICE and EAM guidance. A dedicated DuoMode extension cable (Baylis Medical) was used to connect the RF transseptal needle (STD group) or RF-wire (VC group) to the CARTO 3 system for direct visualization using EAM.
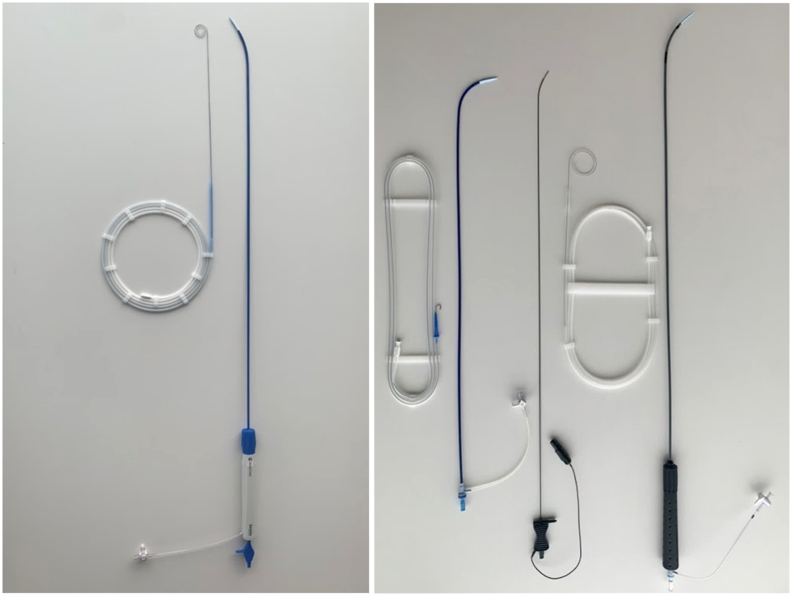
In the STD group (Fig. 1, Fig. 2B), an 8.5F TorFlex transseptal guiding sheath and dilator (Baylis Medical) were advanced to the SVC using ICE guidance over a J-tipped guidewire. The guidewire was exchanged for an RF NRG Transseptal needle (Baylis Medical), and the entire assembly was dragged to the transseptal puncture location under EAM and ICE guidance. A 1-s RF pulse was delivered using an RFP-100A generator (Baylis Medical). After obtaining LA access, a stiff ProTrack Pigtail wire (Baylis Medical) was positioned in the mid LA and used to exchange for an 8.5F Agilis steerable sheath (Abbott), through which RF ablation was performed with continuous heparinized saline flush.
Fig. 1.
Streamlined workflow using VersaCross steerable system. One right-sided device exchange is needed for LA access and RF ablation using VersaCross steerable system, compared four right-sided exchanges with the standard workflow. TSP = Transseptal puncture, SVC = Superior vena cava, RF = radiofrequency, LA = Left atrium.
Fig. 2.
Catheter setup for RF catheter ablation. A) VersaCross steerable workflow consisting of VersaCross steerable sheath with shapeable dilator and RF wire used as both a guidewire and for RF puncture. B) Standard workflow consisting of J-tip guidewire, 8.5F TorFlex fixed curve sheath, NRG RF transseptal needle, ProTrack pigtail wire, and Agilis steerable sheath.
In the VCW group (Figs. 1 and 2A), a pigtail RF wire (VersaCross RF Wire, Baylis Medical) was advanced into the SVC using ICE and EAM guidance. Over this wire, a VersaCross steerable sheath and dilator was advanced to the SVC. The entire assembly was dragged to the transseptal puncture location under ICE and EAM guidance. Transseptal puncture was performed using the VersaCross RF wire and steerable sheath using 1-s RF pulse delivered through the RFP-100A generator. After LA access was obtained, the VersaCross steerable sheath was used for subsequent ablation steps and flushed continuously with heparinized saline. All ablations in the STW and VCW groups were performed using the SmartTouch STSF ablation catheter.
2.4. Statistical analysis
Procedural parameters were compared using descriptive statistics and reported as mean ± standard deviation as indicated. Categorical variables were summarized as frequency and percentages. Statistical analysis was performed using a two-tailed t-test, whereby significance was defined using α = 0.05. Data variability was assessed using a two-tailed F-test, with significance assessed at α = 0.05.
3. Results
3.1. Patient characteristics
A total of 30 patients underwent RF catheter ablation for paroxysmal or persistent atrial fibrillation; 15 using STW and 15 using VCW. The mean patient age was 70 ± 9.5 years, and 60% of the total study population was male. Average patient body mass index (BMI) was 29.4 kg/m2 ± 5.6 kg/m2. Baseline characteristics are shown in Table 1; a greater percentage of males and patients with persistent AF were observed in the VCW (p = 0.03, Table 1).
Table 1.
Baseline characteristics.
| Characteristics | VersaCross Steerable Workflow (n = 15) | Standard Workflow (n = 15) | p-value |
|---|---|---|---|
| Age (years) | 66.6 ± 8.8 | 73.4 ± 9.1 | 0.05 |
| Gender (male, %) | 80 | 40 | 0.03 |
| BMI | 30.5 ± 6.4 | 28.3 ± 4.5 | 0.30 |
| AF classification (%) | |||
| Paroxysmal | 27 | 67 | 0.03 |
| Persistent | 73 | 27 | 0.03 |
| Long standing persistent | 0 | 6 | 0.33 |
| Hypertension (%) | 80 | 73 | 0.68 |
| Diabetes (%) | 27 | 27 | 1.00 |
| Heart Failure (%) | 27 | 47 | 0.27 |
| Ejection Fraction (%) | |||
| Normal (>60%) | 13 | 20 | 0.64 |
| Borderline (50%–60%) | 73 | 53 | 0.27 |
| Low (<50%) | 13 | 27 | 0.64 |
| Coronary artery disease (%) | 33 | 40 | 0.72 |
| Cardiac defect (%) | 7 | 0 | 0.33 |
| Implant/Pacemaker (%) | 13 | 33 | 0.21 |
| Prior ablation (%) | 7 | 20 | 0.30 |
| OAC (%) | 100 | 93 | 0.31 |
| AAD (%) | 47 | 44 | 0.47 |
BMI; Body mass index, OAC; oral anticoagulants, AAD; antiarrhythmic drug.
3.2. Primary endpoint
Transseptal puncture was successful in 100% of the cases without any intraoperative complications (Table 2). The average transseptal time was 3.6 ± 2.2 min after RF wire insertion to SVC in VCW group and 3.5 ± 3.6 min in the STW group (p = 0.978). Time to transseptal puncture after femoral access was 10.8 min faster in the VCW group compared to STW group (20.9 ± 5.8 min vs. 31.7 ± 15.1 min p = 0.024; Fig. 3A). Time to transseptal puncture was also more consistent between the patients in the VCW group (F-test; p < 0.05). Time to LA catheterization, defined as steerable sheath across the septum, was 40% faster in the VCW group versus STW group (21.3 ± 5.8 min vs. 35.2 ± 14.4 min, p = 0.003; Fig. 3B).
Table 2.
Procedural information.
| Parameter | VersaCross Steerable (n = 15) | Standard workflow (n = 15) | p-value |
|---|---|---|---|
| LA catheterization | |||
| TSP time (RF-wire/guidewire insertion) (avg; min) | 3.6 ± 2.2 | 3.5 ± 3.6 | 0.98 |
| TSP time (femoral access) | 20.9 ± 5.8 | 31.7 ± 15.1 | 0.02 |
| Number of punctures (avg no./case) | 1 | 1 | 1.00 |
| Time to steerable sheath in LA (avg; min) | 21.3 ± 5.8 | 35.2 ± 14.4 | 0.003 |
| Fluoroscopy use | |||
| Time (avg; min) | 0.8 ± 1.0 | 0.3 ± 0.4 | 0.10 |
| Dose (avg; mGy) | 42.1 ± 63.3 | 12.3 ± 17.5 | 0.10 |
| AF ablation | |||
| Time to ablation start (avg; min) | 42.1 ± 7.6 | 56.8 ± 13.9 | 0.003 |
| LA dwell time (avg; min) | 61.4 ± 12.8 | 65.2 ± 10.8 | 0.40 |
| Overall procedure time (avg; min) | 86.3 ± 16.1 | 100.5 ± 19.3 | 0.04 |
| Acute Success, PVI (%) | 100 | 100 | 1.00 |
| Complications (%) | 0 | 0 | 1.00 |
Fig. 3.
Workflow efficiency evaluated in terms of procedure times. A) Time to Transseptal puncture after femoral access and B) Time to LA catheterization using the VersaCross steerable workflow compared to the standard workflow C) Total RF ablation procedure time using VersaCross steerable workflow compared to standard workflow (Mean ± SD; *p ≤ 0.05).
3.3. Secondary endpoints
Acute procedural success, defined as PVI with entrance and exit block, was achieved in all patients (Table 2). PVI was performed in 100% of the patients, and additional ablation was performed in 80% of patients (87% of patients in the VCW group, and 93% of patients in the STW group; p = 0.559). The average RF ablation start time from initial vascular access was 42.1 ± 7.6 min in the VCW group compared to 56.8 ± 13.9 min in the STW group (p = 0.003; Table 2). The average procedure time from initial venous access to final sheath removal was 86.3 ± 16.1 min and 100.5 ± 19.3 min in the VCW and STW groups, respectively (p = 0.044; Fig. 3C). There were no significant differences in LA dwell time between the VersaCross steerable and the standard workflow (61.4 ± 12.8 min vs. 65.2 ± 10.8 min, respectively, p = 0.40; Table 2). There was no difference in fluoroscopy time (0.8 ± 1.0 min vs. 0.3 ± 0.4 min; p = 0.103) or dose (42.1 ± 63.3 mGy vs. 12.3 ± 17.5 mGy; p = 0.100) between the two workflows (Table 2). All procedures were performed without any intraprocedural or postprocedural complications.
4. Discussion
Use of steerable sheaths during catheter ablation for AF has been shown to have several advantages over a fixed curved sheath including better catheter manipulation and contact force stability [4,[8], [9], [10]]; however, the use of steerable sheath for transseptal access is not widely adopted due to potential risks of tissue injury, perforation, and heart block. Our standard RF ablation workflow involved using an RF needle with fixed curve sheath for transseptal access, then exchanging for an Agilis steerable sheath for RF ablation. Here we compared a new RF ablation procedural workflow using the VersaCross steerable system consisting of an RF wire and dedicated steerable sheath to our standard workflow.
Both the RF wire and RF needle used in these cases allowed for effective transseptal puncture without any intraprocedural complications regardless of septal anatomy or prior history of transseptal puncture. Findings from previous studies suggest that purpose-built RF puncture devices are similar in effectiveness and time to transseptal puncture (Table 2), allowing for more efficient and safer transseptal access compared to conventional mechanical needles [6]. By eliminating the RF needle and additional sheath exchange, time to transseptal puncture from femoral access was 34% (10.8 min) faster using the VersaCross steerable system compared to the standard workflow (Fig. 3B). The transseptal puncture times in our study are comparable to those seen in previous fluoroless transseptal punctures [7,11], and fluoroscopy-guided transseptal puncture [6,12] using RF wire or RF needle.
In our study, interestingly, time to transseptal puncture was more consistent between patients in the VCW group compared to the STW group, which we attribute to being able to easily reposition the RF wire back into the SVC if optimal transseptal puncture location was not achieved at first pass. This is a major advantage in patients with less than standard anatomy or patient with pacing leads, in that it the VersaCross workflow does not require removing the RF needle, reintroducing a wire, and then exchanging the wire for the RF needle each time another transseptal attempt is made (Fig. 3A). In the standard workflow, the 8.5F fixed transseptal sheath is exchanged for an Agilis sheath to perform the ablation; in the VersaCross workflow, a more rounded dilator tip and smoother sheath-dilator transition allowed the same steerable sheath to be used for both transseptal puncture and ablation without any increased risks of complications, leading to 40% faster time to steerable sheath across the septum (Fig. 3B). This time saving was sustained throughout the remainder of the procedure. Importantly, LA dwell time was similar between both steerable sheaths, suggesting similar maneuverability during RF catheter ablation. Finally, the use of the VersaCross steerable system led to an average of 14.2 min time savings in the overall RF ablation procedure compared to the standard workflow (Fig. 3C). The improvements in procedure efficiency may result in economic benefits, as indicated in recent cost-effectiveness analysis on transseptal devices, demonstrating that time savings in RF catheter ablation procedures can result in incremental cost-savings [13].
The workflow using the RF needle and fixed curve sheath for transseptal access, followed by the Agilis steerable sheath for RF ablation is the standard practice for many electrophysiologists. In our experience, the VersaCross steerable system improved procedural efficiency by eliminating several steps and device exchanges (Fig. 1), while allowing fluoroless visualization and maintaining procedural safety. The RF wire is the distal portion of the same pigtail wire that is placed in the SVC prior to transseptal puncture, which eliminates the need to exchange it for a transseptal needle. Similar to the RF needle, the RF wire uses RF energy to facilitate passage across the septum with minimal mechanical force. The RF wire was also integrated into the fluoroless visualization workflow, enabling visualization of the RF wire tip on ICE and EAM, similar to the standard RF needle. The RF wire eliminates the need for wire/needle exchanges, reducing the risk of air embolism and tissue injury [12,14]. The complete removal of a sheath exchange step in the VersaCross workflow effectively eliminates another step wherein air entrapped in a sheath could be transmitted to the left atrium.
Though generally depicted as being in the middle of the septal wall, the location of the fossa ovalis varies widely in different patient anatomies, and the proximity of the margins of the fossa to other important structures may be diverse [5]. Consequently, precise transseptal puncture location is important. The use of a steerable sheath for transseptal puncture can be used to optimize location on the fossa; however, because steerable sheaths are generally more stiff, there are concerns of perforation and other complications with their use during transseptal puncture. A recent report has also been published on injury to the atrioventricular node with consequent complete heart block, which was attributed to the use of the Agilis sheath for transseptal puncture [15]. With the VersaCross system, the dedicated RF pigtail wire can be fully exposed from the sheath to provide a safe bumper during drop down and sheath manipulations. Additional differences in geometry of the steerable sheaths, including tip roundness and sheath-dilator transition, may translate to less traumatic dropdown, force to cross the septum, and may lead to a safer experience.
4.1. Limitations
This is a single-center non-randomized retrospective analysis that is subject to several limitations. A single operator performed all procedures in a small patient population, and was not blinded to the workflow. Although there were more females in the standard workflow group, this did not seem to impact the catheter ablation step in the overall procedure. Larger studies are required to compare safety and effectiveness of conducting RF ablations using the standard workflow to the VersaCross steerable workflow. While no difference in ablation times were observed between the two groups, additional studies are needed to further evaluate performance of each steerable sheath. Finally, some operators may prefer to forgo a steerable transseptal sheath altogether, in favor of a fixed transseptal sheath. For such operators, the VersaCross system would add unnecessary complexity and cost to the procedure.
5. Conclusions
While steerable sheaths have been shown to have several advantages over fixed-curve sheaths during catheter ablation, there are concerns of injury and/or perforation associated with their use during transseptal puncture. These complications can be potentially avoided using sheaths with optimized stiffness and rounded tip geometry, along with an atraumatic pigtail wire. Our previous workflow required several device exchanges, including guidewires, RF needle, fixed curve sheath, and steerable sheath for ablation. This study showed that the use of the RF wire based VersaCross steerable system eliminated four steps in RF ablation workflow, leading to a 14.2 min reduction in the overall procedure time without compromising safety.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
To facilitate open access to scientific knowledge, a preprint of this manuscript has previously been published [Janardhan A et al., 2022] [16].
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Hindricks G., Potpara T., Dagres N., et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;2021(42):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Cosedis Nielsen J., Johannessen A., Raatikainen P., et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 3.Asad Z.U.A., Yousif A., Khan M.S., Al-Khatib S.M., Stavrakis S. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007414. [DOI] [PubMed] [Google Scholar]
- 4.Ullah W., Hunter R.J., McLean A., et al. Impact of steerable sheaths on contact forces and reconnection sites in ablation for persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26:266–273. doi: 10.1111/jce.12573. [DOI] [PubMed] [Google Scholar]
- 5.Tzeis S., Andrikopoulos G., Deisenhofer I., Ho S.Y., Theodorakis G. Transseptal catheterization: considerations and caveats. Pacing Clin Electrophysiol. 2010;33:231–242. doi: 10.1111/j.1540-8159.2009.02598.x. [DOI] [PubMed] [Google Scholar]
- 6.Winkle R.A., Mead R.H., Engel G., Patrawala R.A. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrillation ablation. Heart Rhythm. 2011;8:1411–1415. doi: 10.1016/j.hrthm.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Demo H., Aranda C., Razminia M. Fluoroless left atrial access for radiofrequency and cryoballoon ablations using a novel radiofrequency transseptal wire. J Intervent Card Electrophysiol. 2022 doi: 10.1007/s10840-022-01157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali A., Plettenburg D.H., Breedveld P. Steerable catheters in cardiology: classifying steerability and assessing future challenges. IEEE Trans Biomed Eng. 2016;63:679–693. doi: 10.1109/TBME.2016.2525785. [DOI] [PubMed] [Google Scholar]
- 9.Piorkowski C., Eitel C., Rolf S., et al. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol. 2011;4:157–165. doi: 10.1161/CIRCEP.110.957761. [DOI] [PubMed] [Google Scholar]
- 10.Deyell M.W., Wen G., Laksman Z., et al. The impact of steerable sheaths on unblinded contact force during catheter ablation for atrial fibrillation. J Intervent Card Electrophysiol. 2020;57:417–424. doi: 10.1007/s10840-019-00514-1. [DOI] [PubMed] [Google Scholar]
- 11.Salam T., Wilson L., Bohannan S., Morin M. Safety and effectiveness of a novel fluoroless transseptal puncture technique for lead-free catheter ablation: a case series. J Innov Card Rhythm Manag. 2020;11:4079–4085. doi: 10.19102/icrm.2020.110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayah N., Simon F., Garceau P., et al. Initial clinical experience with VersaCross transseptal system for transcatheter mitral valve repair. Cathet Cardiovasc Interv. 2021;97:1230–1234. doi: 10.1002/ccd.29365. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez J.M., Shah R., Kouassi Y., Chronowic M., Wilson L., Marcus G.M. A cost-effectiveness analysis comparing a conventional mechanical needle to a radiofrequency device for transseptal punctures. J Cardiovasc Electrophysiol. 2020;31:1672–1677. doi: 10.1111/jce.14500. [DOI] [PubMed] [Google Scholar]
- 14.Anselmino M., Matta M., Toso E., et al. Silent cerebral embolism during atrial fibrillation ablation:pathophysiology, prevention and management. J Atr Fibrillation. 2013;6:796. doi: 10.4022/jafib.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siroky G.P., Bisht D., Huynh H., et al. Reversible mechanical atrioventricular block caused by A steerable introducer sheath during transseptal catheterization. J Atr Fibrillation. 2021;13 doi: 10.4022/jafib.20200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janardhan A.H., Berggren K., Lampert T. Improved Left Atrial Catheterization Efficiency and Consistency using a novel steerable transseptal puncture sheath. Author. 2022 doi: 10.22541/au.166472643.34650005/v1. [DOI] [PubMed] [Google Scholar]