Abstract
Phosphoinositide (PI) 3-kinase contributes to a wide variety of biological actions, including insulin stimulation of glucose transport in adipocytes. Both Akt (protein kinase B), a serine-threonine kinase with a pleckstrin homology domain, and atypical isoforms of protein kinase C (PKCζ and PKCλ) have been implicated as downstream effectors of PI 3-kinase. Endogenous or transfected PKCλ in 3T3-L1 adipocytes or CHO cells has now been shown to be activated by insulin in a manner sensitive to inhibitors of PI 3-kinase (wortmannin and a dominant negative mutant of PI 3-kinase). Overexpression of kinase-deficient mutants of PKCλ (λKD or λΔNKD), achieved with the use of adenovirus-mediated gene transfer, resulted in inhibition of insulin activation of PKCλ, indicating that these mutants exert dominant negative effects. Insulin-stimulated glucose uptake and translocation of the glucose transporter GLUT4 to the plasma membrane, but not growth hormone- or hyperosmolarity-induced glucose uptake, were inhibited by λKD or λΔNKD in a dose-dependent manner. The maximal inhibition of insulin-induced glucose uptake achieved by the dominant negative mutants of PKCλ was ∼50 to 60%. These mutants did not inhibit insulin-induced activation of Akt. A PKCλ mutant that lacks the pseudosubstrate domain (λΔPD) exhibited markedly increased kinase activity relative to that of the wild-type enzyme, and expression of λΔPD in quiescent 3T3-L1 adipocytes resulted in the stimulation of glucose uptake and translocation of GLUT4 but not in the activation of Akt. Furthermore, overexpression of an Akt mutant in which the phosphorylation sites targeted by growth factors are replaced by alanine resulted in inhibition of insulin-induced activation of Akt but not of PKCλ. These results suggest that insulin-elicited signals that pass through PI 3-kinase subsequently diverge into at least two independent pathways, an Akt pathway and a PKCλ pathway, and that the latter pathway contributes, at least in part, to insulin stimulation of glucose uptake in 3T3-L1 adipocytes.
Phosphoinositide (PI) 3-kinase, a lipid kinase composed of an SRC homology 2 (SH2) domain-containing regulatory subunit and a 110-kDa catalytic subunit, catalyzes phosphorylation of the D3 position of PIs (46, 48). This enzyme was first identified complexed with SRC kinase and the middle T antigen of polyomavirus and was later found to associate with various tyrosine-phosphorylated proteins in response to stimulation of cells with growth factors or cytokines (46, 48). Activation of PI 3-kinase, either by targeting of the enzyme to the plasma membrane (27) or as a consequence of direct interaction between the SH2 domain of the regulatory subunit and phosphorylated tyrosine residues present within specific motifs (5), results in the triggering of various important biological actions. Thus, with the use of either a dominant negative protein that blocks the interaction between PI 3-kinase and tyrosine-phosphorylated proteins (21, 33, 41) or pharmacological inhibitors of the enzyme, such as wortmannin or LY294002 (12, 47), PI 3-kinase has been shown to participate in intracellular trafficking, organization of the cytoskeleton, cell growth and transformation, prevention of apoptosis, cell differentiation, and several metabolic actions of insulin (11, 17, 23, 24, 33, 41, 46, 48). However, relatively little is known about the downstream effectors of PI 3-kinase that mediate each of these biological effects.
Recently, two types of serine-threonine kinase have been shown to act downstream of PI 3-kinase. One of these kinases is Akt (also known as protein kinase B), which contains a pleckstrin homology domain. Several growth factors induce rapid activation of Akt in intact cells (10, 16, 28). However, the mechanism of Akt activation in cells is not fully understood. Phosphatidylinositol 3,4-bisphosphate, one of the products of PI 3-kinase activity in vivo, binds directly to the pleckstrin homology domain of Akt and stimulates kinase activity in vitro (18, 28), suggesting that Akt may be directly activated by this lipid in intact cells. On the other hand, activation of Akt was shown to parallel its phosphorylation status, and replacement of serine and threonine residues that are sites of ligand-induced phosphorylation in the enzyme abolished its activation (3, 31). These data, together with the identification of a kinase that phosphorylates and activates Akt (4, 44), suggest that Akt activity is regulated mainly by phosphorylation. Whichever mechanism is primarily responsible for regulation of Akt, the observation that pharmacological or molecular biological inhibitors of PI 3-kinase prevent Akt activation in intact cells (10, 16, 26, 29) indicates that Akt acts downstream of PI 3-kinase.
The protein kinase C (PKC) family of serine-threonine kinases comprises at least 11 members (37). One class of PKC isozymes, termed atypical PKC, is distinct from other members of this family in several respects. For example, the atypical PKC enzymes, consisting of PKCλ and PKCζ, are not activated by diacylglycerol or phorbol ester, whereas members of the other two classes, conventional PKC and novel PKC, are activated by these reagents both in vitro and in vivo (1, 2, 36–38, 40, 49). PKCι, also identified as an atypical PKC isozyme, is the human counterpart of mouse PKCλ (42). Evidence suggests that atypical PKC is the second type of serine-threonine kinase that acts downstream of PI 3-kinase. PKCζ is activated in vitro by phosphatidylinositol 3,4,5-trisphosphate (36), another product of PI 3-kinase activity (46). When expressed in 3Y1 fibroblasts or HepG2 hepatoma cells expressing various mutant platelet-derived growth factor receptors, PKCλ contributed to trans activation of the tetradecanoyl phorbol acetate-responsive element in response to platelet-derived growth factor or epidermal growth factor in a PI 3-kinase-dependent manner (2). Furthermore, the activity of atypical PKC stimulated by either insulin or bacterial lipopolysaccharide was shown to be inhibited by either a pharmacological inhibitor or a dominant negative mutant of PI 3-kinase (22, 34, 43). All of these observations indicate that atypical PKC isozymes are downstream effectors of PI 3-kinase.
Stimulation of glucose uptake into skeletal muscle and adipocytes is one of the most important actions of insulin. Insulin-stimulated glucose uptake and translocation of the glucose transporter GLUT4 to the plasma membrane, an essential step for glucose uptake in muscle and adipocytes, were markedly attenuated by pharmacological inhibitors or a dominant negative mutant of PI 3-kinase (13, 39, 41). Furthermore, a constitutively active mutant of PI 3-kinase promoted glucose uptake and translocation of GLUT4 in quiescent adipocytes (20). These observations suggest a central role for PI 3-kinase in regulation of glucose transport. However, a downstream effector of PI 3-kinase that mediates stimulation of glucose uptake has not been identified.
Although Akt and atypical PKC act downstream of PI 3-kinase, it remains unclear which actions of PI 3-kinase are mediated by which protein kinase. It is also not known whether Akt and atypical PKC act in the same signaling pathway or whether they transmit signals through different pathways. To address these important questions, we have investigated the roles of these protein kinases in insulin-stimulated glucose uptake. We recently showed that a mutant Akt in which the sites of ligand-induced phosphorylation were replaced by alanine acts in a dominant negative manner (26). Because this mutant did not affect insulin-induced glucose uptake (26), we have now examined the possible role of atypical PKC as a downstream effector of PI 3-kinase in this process. We have investigated the effects of dominant negative mutants and a constitutively active mutant of PKCλ in order to determine whether this enzyme participates in the regulation of glucose uptake by insulin in 3T3-L1 adipocytes. We have also investigated whether Akt and PKCλ function in the same or different signaling pathways.
MATERIALS AND METHODS
Cells and antibodies.
3T3-L1 preadipocytes were maintained and induced to differentiate into adipocytes as described previously (41). To establish CHO-IR cells that express (in addition to human insulin receptors) tagged PKCλ (CHO-IR/PKCλ cells), we transfected CHO-IR cells with both pSV40-high (which confers resistance to hygromycin) and an SRD vector encoding T7 epitope-tagged mouse PKCλ (2). Transfected cells were selected and cloned as described previously (25). CHO cells that express FLAG epitope-tagged Akt (CHO-Akt cells) have been described previously (26). To prepare a construct encoding mouse PKCζ tagged with the T7 epitope, we performed PCR with a sense primer (5′-AAG GCC ATG GCT AGC ATG ACT GGT GGA CAG CAA ATG GGT CCC AGC AGG ACC GAC), an antisense primer (5′-GAG GTC GAA GTC TTG CAG CCC), and a full-length mouse PKCζ cDNA as the template. The resulting PCR product, containing nucleotides 4 to 762 of PKCζ cDNA fused with a DNA sequence encoding the T7 epitope, was digested with PstI and ligated to the PstI site of cDNA encoding mouse PKCζ. The resulting cDNA construct encoded T7 epitope-tagged mouse PKCζ and was subcloned into an SRα expression vector.
Polyclonal antibodies to PKCλ (αλ190) and to PKCζ (αζ170) were generated against glutathione S-transferase (GST) fusion proteins containing amino acids 190 to 240 of mouse PKCλ or amino acids 170 to 240 of mouse PKCζ, respectively. A monoclonal antibody (MAb) to PKCλ (αλCT), induced by a GST fusion protein containing amino acids 397 to 558 of mouse PKCλ, was obtained from Transduction Laboratories. Polyclonal antibodies to PKCζ (αζCT), generated in response to a peptide corresponding to the COOH terminus of rat PKCζ (amino acids 577 to 592), were obtained from GIBCO BRL. Polyclonal antibodies to PKCλ (αλ197) that were generated in response to a peptide corresponding to amino acids 197 to 213 of mouse PKCλ were as described previously (1). Polyclonal antibodies to Akt, induced by a GST fusion protein containing amino acids 428 to 480 of rat Akt1, as well as a MAb (1F8) and polyclonal antibodies to GLUT4 were as described previously (41). Antibodies to Akt2 and to Akt3 were obtained from Upstate Biotechnology.
RT-PCR.
Transcripts encoding PKCλ or PKCζ were detected by reverse transcription (RT)-PCR analysis. Complementary DNA was synthesized, with the use of a FastTrack 2.0 kit and a cDNA Cycle kit (Invitrogen), from ∼300 μg of polyadenylated RNA extracted from 3T3-L1 adipocytes or total RNA extracted from mouse brain. PCR was then performed with 1/10 of the resulting cDNA as the template and with primers that correspond to nucleotides 247 to 265 and 576 to 595 of mouse PKCλ cDNA or to nucleotides 145 to 164 and 700 to 719 of mouse PKCζ cDNA. The amplification protocol comprised 30 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min.
Construction of and infection with adenovirus vectors.
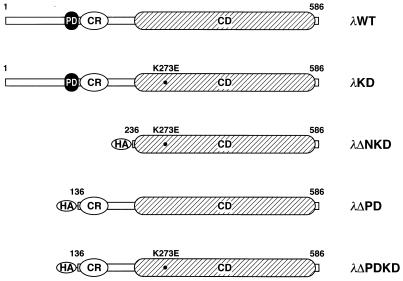
Various PKCλ mutant constructs are shown in Fig. 1. Complementary DNAs encoding wild-type mouse PKCλ (λWT) and a kinase-deficient mutant in which glutamate is substituted for Lys273 in the kinase domain (λKD) were as described previously (2). To prepare constructs encoding λΔPD and λΔPDKD, hemagglutinin (HA) epitope-tagged, NH2-terminal-deletion mutants of PKCλ, we performed PCR with sense (5′-TTA GGT ACC ATG TAC CCA TAC GAT GTT CCG GAT TAC GCT AGC CTC GCC AAA CGT TTC AAT AGG CGC) and antisense (5′-GAT ACC ACT CTC CCT GGT) primers and wild-type mouse PKCλ cDNA as a template. The resulting PCR product, containing nucleotides 442 to 735 of PKCλ cDNA fused with a DNA sequence encoding the HA epitope, was digested with EcoT22I and ligated to the EcoT22I site of cDNA encoding either λWT or λKD. To construct λΔNKD, we performed PCR with sense (5′-TTA GGT ACC ATG TAC CCA TAC GAT GTT CCG GAT TAC GCT AGC CTC TCG TCC AGT CTA GGT CTG CAG) and antisense (5′-GAT AGA ATG CAG CCC GAC) primers and λKD cDNA as a template. The resulting product, containing nucleotides 742 to 966 of PKCλ cDNA fused with a DNA sequence encoding the HA epitope, was digested with ClaI and fused with the ClaI site of cDNA encoding λWT. Complementary DNA encoding the wild-type or various mutant proteins was subcloned into pAxCAwt (35), and adenovirus vectors containing these cDNAs were generated by transfecting 293 cells with the corresponding pAxCAwt plasmid together with DNA-terminal protein complex (35), as described previously (41). The resulting vectors were termed AxCAλWT, AxCAλKD, AxCAλΔPD, AxCAλΔPDKD, and AxCAλΔNKD, respectively.
FIG. 1.
Structures of various mutants of PKCλ, λKD, λΔNKD, and λΔPDKD contain a Lys273-to-Glu mutation (K273E). λΔPD, λΔPDKD, and λΔNKD contain the HA epitope at their truncated NH2 termini. The first and last amino acids of each protein are numbered. PD, CR, and CD, pseudosubstrate, cysteine-rich, and catalytic domains, respectively.
An adenovirus vector encoding a dominant negative mutant of PI 3-kinase (AxCAΔp85) was as described previously (41). An adenovirus vector encoding a mutant Akt (AxCAAkt-AA) in which the phosphorylation sites (Thr308 and Ser473) targeted by growth factors are replaced by alanine was as previously described (26). CHO cells or 3T3-L1 adipocytes were infected with adenovirus vectors at the multiplicities of infection (MOIs) (in PFU per cell) indicated in Results, as described previously (41, 50). The cells were used in experiments 24 to 48 h after infection.
After we submitted this paper, the wild-type mouse PKCλ cDNA used to construct the adenoviruses encoding the various mutant proteins was found to lack the nucleotides encoding the 47 NH2-terminal amino acids. Although the deleted amino acid sequence does not contain any known functional domains, we repeated all of the experiments that used the NH2-terminally deleted λWT or λKD with adenovirus vectors encoding a full-length wild-type or a full-length kinase-deficient PKCλ, and we obtained essentially identical results. This further suggests that the NH2-terminal 47 amino acids are not essential for the signaling of insulin-induced glucose uptake.
Kinase assays.
CHO cells or 3T3-L1 adipocytes were deprived of serum for 16 to 20 h, incubated in the absence or presence of insulin, and then immediately frozen with liquid nitrogen. For assay of the kinase activity of endogenous or T7 epitope-tagged PKCλ, the frozen cells were lysed as described previously (2) and the lysate was centrifuged (15,000 × g for 20 min). The protein concentration in the resulting supernatants was determined with the use of the bicinchoninic acid protein assay reagent (Pierce), and equal amounts of protein were subjected to immunoprecipitation with polyclonal antibodies or MAbs to PKCλ or with antibodies to the T7 epitope tag. After washing twice with buffer A (50 mM MOPS [morpholinepropanesulfonic acid]-HCl [pH 7.5], 0.5% Triton X-100, 10% glycerol, 0.1% bovine serum albumin, 5 mM EDTA, 5 mM EGTA, 20 mM NaF, 50 mM β-glycerophosphate, 2 mM sodium orthovanadate, 2 mM dithiothreitol, 1 μg of leupeptin per ml, and 2 mM phenylmethylsulfonyl fluoride), once with buffer A containing 1 M NaCl, and then once with a solution containing 20 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and 1 mM EGTA, the immunoprecipitates were incubated for 14 min at 30°C with 0.4 μCi of [γ-32P]ATP in a reaction mixture (25 μl) containing 35 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.5 mM EGTA, 0.1 mM CaCl2, 40 μM unlabeled ATP, and 30 μM myelin basic protein (MBP) as a substrate. When indicated, phosphatidylserine (PS) (100 μg/ml) was also present in the reaction mixture.
For assay of the kinase activity of endogenous or FLAG-tagged Akt, the frozen cells were lysed in a solution containing 50 mM HEPES-NaOH (pH 7.6), 150 mM NaCl, 1% Triton X-100, 1 mg of bacitracin per ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 mM sodium orthovanadate, 10 mM NaF, and 30 mM sodium pyrophosphate. The lysates were subjected to immunoprecipitation with antibodies to Akt or to FLAG. After three washes with HEPES-buffered saline (pH 7.5) containing 0.1% Triton X-100, the immunoprecipitates were incubated for 30 min at 30°C with 3.0 μCi of [γ-32P]ATP in a reaction mixture (30 μl) containing 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 25 μM unlabeled ATP, 1 μM protein kinase inhibitor, and 0.2 mg of histone 2B per ml as a substrate.
All kinase reactions were terminated by the addition of sodium dodecyl sulfate (SDS) sample buffer, and the samples were then fractionated by SDS-polyacrylamide gel electrophoresis. The radioactivity incorporated into substrates was determined with a Fuji BAS 2000 image analyzer.
Glucose uptake and translocation of GLUT4.
Glucose uptake was assayed as described previously (41). In brief, 3T3-L1 adipocytes were incubated for 16 h in Dulbecco’s modified Eagle’s medium containing 5.6 mM glucose and 0.5% fetal bovine serum. The cells were washed twice with DB buffer (140 mM NaCl, 2.7 mM KCl, 1 mM CaCl2, 1.5 mM KH2PO4, 8 mM Na2HPO4 [pH 7.4], 0.5 mM MgCl2) and then incubated with 100 nM insulin for 15 min, 0.5 μg of growth hormone (GH) per ml for 10 min, or 300 mM sorbitol for 60 min. DB buffer (1 ml) containing bovine serum albumin (1 mg/ml) and 0.1 mM 2-deoxy-d-[1,2-3H]glucose (1 μCi) was added to each well, and after 5 min, the cells were washed and then solublized with 0.1% SDS. The radioactivity incorporated into the cells was measured with a liquid scintillation counter.
Translocation of GLUT4 to the plasma membrane was measured by the plasma membrane lawn assay as previously described (41). In brief, 3T3-L1 adipocytes cultured on coverslips were incubated in a hypotonic buffer and immediately disrupted by being placed under an ultrasonic microprobe. For antibody labeling, sonicated cells were fixed in 2% paraformaldehyde, and the lawn of plasma membrane fragments was prepared with antibodies to GLUT4 and tetramethyl rhodamine isothiocyanate-labeled secondary antibodies. Samples were then examined with a fluorescence microscope.
RESULTS
Expression of PKCλ in 3T3-L1 adipocytes.
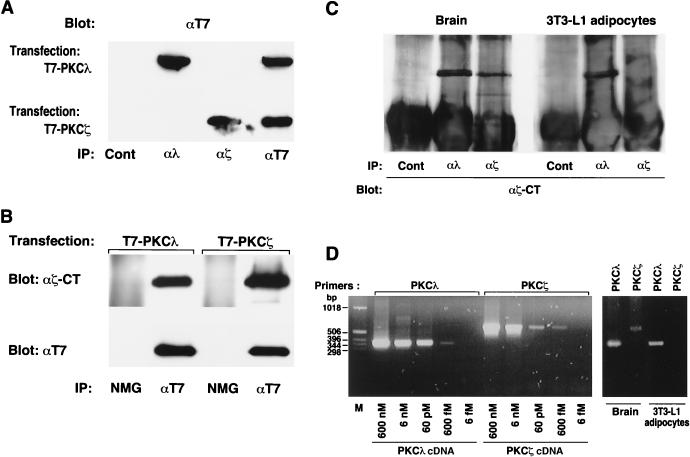
Atypical PKC isozymes comprise PKCλ and PKCζ. To examine the relative abundances of these two isoforms in 3T3-L1 adipocytes, we prepared antibodies specific for each. T7 epitope-tagged PKCλ or PKCζ was transiently expressed in COS7 cells, and cell lysates were subjected to immunoprecipitation with antibodies either to PKCλ (αλ190), to PKCζ (αζ170), or to the T7 epitope or with control serum. The immunoprecipitates were then subjected to immunoblot analysis with antibodies to T7 (Fig. 2A). Proteins of ∼80 kDa reactive with antibodies to T7 were detected in the immunoprecipitates prepared from the lysates expressing T7-tagged PKCλ with αλ190 or with antibodies to T7 and in the immunoprecipitates prepared from the lysates expressing T7-tagged PKCζ with αζ170 or with antibodies to T7. These results indicate that αλ190 and αζ170 specifically recognize PKCλ and PKCζ, respectively. In contrast, polyclonal antibodies generated against a peptide corresponding to the COOH terminus of rat PKCζ (αζCT) detected both T7-tagged PKCλ and PKCζ (Fig. 2B), indicating that these antibodies recognize both PKCλ and PKCζ.
FIG. 2.
Expression of PKCλ, but not PKCζ, in 3T3-L1 adipocytes. (A) Specificity of antibodies to PKCλ and PKCζ. COS7 cells cultured in 6-cm-diameter dishes were transiently transfected, with the use of Lipofectamine (Gibco), with 6 μg of SRD or SRα vectors encoding T7 epitope-tagged PKCλ or PKCζ. Cell lysates were subjected to immunoprecipitation (IP) with antibodies to T7, to PKCλ (αλ190), or to PKCζ (αζ170) or with control serum (Cont), and the resulting immunoprecipitates were subjected to immunoblot analysis with antibodies to T7. (B) Recognition of both PKCλ and PKCζ by antibodies generated in response to a peptide corresponding to the COOH terminus of rat PKCζ (αζCT). COS7 cells transiently transfected as described for panel A were lysed and subjected to immunoprecipitation with either antibodies to T7 or normal mouse globulin (NMG). The resulting immunoprecipitates were then subjected to immunoblot analysis with either antibodies to T7 (lower panel) or αζCT (upper panel). (C) Analysis of PKCλ and PKCζ protein expression in mouse brain and 3T3-L1 adipocytes. Lysates prepared from mouse brain or 3T3-L1 adipocytes were subjected to immunoprecipitation with αλ190, αζ170, or control rabbit serum, and the immunoprecipitates were subjected to immunoblot analysis with αζCT. (D) Analysis of PKCλ and PKCζ transcripts in mouse brain and 3T3-L1 adipocytes. PCR was performed with primers specific for mouse PKCλ or PKCζ cDNA and with either the indicated concentration of full-length PKCλ or PKCζ cDNA subcloned into the SRD vector (left panel) or cDNA synthesized from RNA extracted from either mouse brain or 3T3-L1 adipocytes (right panel). Amplification products were analyzed by agarose gel electrophoresis and ethidium bromide staining. Lane M, molecular size standards. Data are representative of those from three experiments.
When a cell lysate prepared from mouse brain was subjected to immunoprecipitation with either αλ190, αζ170, or control serum and the immunoprecipitates were then subjected to immunoblot analysis with αζCT, proteins of ∼80 kDa were detected in the immunoprecipitates prepared with αλ190 or αζ170 but not in those prepared with control serum, suggesting that brain expresses both PKCλ and PKCζ (Fig. 2C). In contrast, when 3T3-L1 adipocyte lysates were subjected to the same analysis, an ∼80-kDa protein was detected only in the immunoprecipitate prepared with αλ190 (Fig. 2C). When the same membrane was probed with a MAb generated in response to a GST fusion protein containing amino acids 397 to 558 of mouse PKCλ (αλCT), again an ∼80-kDa protein was detected only in the immunoprecipitate prepared with αλ190 (data not shown). These results suggest that 3T3-L1 adipocytes express PKCλ protein but not PKCζ protein.
We also examined the expression of PKCλ and PKCζ at the mRNA level by RT-PCR. PCR performed with specific oligonucleotide primers based on the sequence of either mouse PKCλ or PKCζ cDNA, and with as little as 0.6 pM PKCλ or PKCζ cDNA subcloned into the SRD vector as a template, yielded amplification products of the expected size (∼350 bp for PKCλ and ∼570 bp for PKCζ) (Fig. 2D). When PCR was performed with the same primer pairs and cDNA that was synthesized from RNA extracted from mouse brain, PCR products of the expected size were obtained with each set of primers. However, with cDNA that was synthesized from RNA extracted from 3T3-L1 adipocytes as the template, a PCR product of the expected size was obtained with the primers corresponding to PKCλ but not with those corresponding to PKCζ. Thus, consistent with the results of protein analysis, 3T3-L1 adipocytes contain PKCλ mRNA but not PKCζ mRNA.
Activation of PKCλ by insulin in a PI 3-kinase-dependent manner.
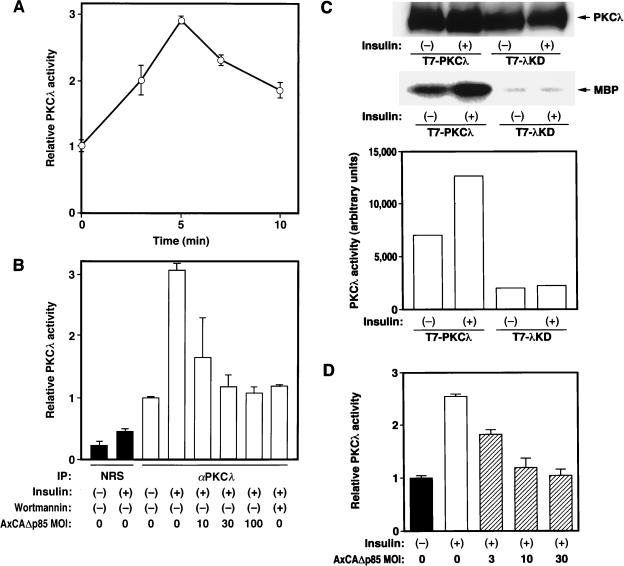
3T3-L1 adipocytes were incubated for various times in the absence or presence of 100 nM insulin, lysed, and subjected to immunoprecipitation with control serum or polyclonal antibodies to PKCλ generated against a peptide corresponding to amino acids 197 to 213 of mouse PKCλ (αλ197). These antibodies were previously shown not to cross-react with PKCζ (1). Kinase activity in the immunoprecipitates was then assayed with MBP as a substrate. The kinase activity precipitated by αλ197 was markedly greater than that precipitated by control serum (Fig. 3B). Activation of PKCλ was evident within 3 min of exposure of cells to insulin; the activity was maximal (about three times that of the basal value) at 5 min and remained increased at 10 min (Fig. 3A). Prior treatment of the cells with 100 nM wortmannin or infection of the cells with AxCAΔp85, an adenovirus vector that encodes a dominant negative mutant of PI 3-kinase (41), prevented insulin-induced activation of PKCλ (Fig. 3B).
FIG. 3.
Stimulation of PKCλ activity by insulin in 3T3-L1 and CHO cells. (A) Time course of insulin stimulation of PKCλ activity in 3T3-L1 adipocytes. 3T3-L1 cells cultured in 6-cm-diameter plates were incubated in the presence of 100 nM insulin for the indicated times, after which the cells were lysed and subjected to immunoprecipitation with polyclonal antibodies to PKCλ (αλ197). The resulting immunoprecipitates were then assayed for kinase activity with MBP as a substrate. Data are expressed relative to the activity at zero time. (B) Effects of inhibitors of PI 3-kinase on insulin stimulation of PKCλ activity in 3T3-L1 adipocytes. 3T3-L1 cells were incubated in the absence or presence of 100 nM wortmannin for 20 min or were infected at the indicated MOI (PFU per cell) with an adenovirus vector encoding Δp85 (AxCAΔp85), and they were then incubated in the absence or presence of 100 nM insulin for 5 min. Cells were then lysed and subjected to immunoprecipitation (IP) with either αλ197 or normal rabbit serum (NRS). Immunoprecipitates were assayed for PKCλ activity as described for panel A. Data are expressed relative to the activity of control αλ197 precipitates. (C) Insulin stimulation of PKCλ activity in transiently transfected CHO-IR cells. CHO-IR cells were transiently transfected with constructs encoding T7-tagged wild-type PKCλ or λKD. After 48 h, the cells were deprived of serum, incubated in the absence or presence of 100 nM insulin for 3 min, and then lysed. The lysates were subjected to immunoprecipitation with antibodies to T7, and the immunoprecipitates were subjected either to immunoblot analysis with antibodies to T7 (upper panel) or to the PKCλ kinase assay (lower two panels). (D) Effect of Δp85 on insulin-stimulated PKCλ activity in CHO-IR/PKCλ cells. CHO-IR/PKCλ cells were infected with AxCAΔp85 at the indicated MOI (PFU per cell). Cells were then incubated in the absence or presence of 100 nM insulin for 3 min, after which cell lysates were subjected to immunoprecipitation with antibodies to the T7 epitope and PKCλ activity was assayed in the resulting precipitates. Data are expressed relative to the activity of precipitates from uninfected cells not exposed to insulin. Quantitative data in panels A, B, and D are means ± standard errors from three experiments, and those in panel C are means from two experiments.
To confirm that insulin activates PKCλ, we transiently transfected CHO-IR cells with constructs encoding T7 epitope-tagged wild-type PKCλ or a mutant PKCλ in which Lys273 in the kinase domain was replaced by glutamate (λKD). The transfected cells were incubated in the absence or presence of insulin, lysed, and subjected to immunoprecipitation with antibodies to T7. Although the amounts of T7-tagged PKCλ and λKD in the immunoprecipitates were similar, the precipitates prepared from the cells expressing PKCλ showed higher kinase activity, and this activity was increased about 1.8-fold in response to insulin (Fig. 3C). These observations suggest that the kinase activity precipitated with antibodies to T7 was attributable to PKCλ and not to some other kinase associated with PKCλ.
To investigate further the effect of insulin on PKCλ activation, we established a CHO cell line that stably expresses both human insulin receptors and T7 epitope-tagged mouse PKCλ (CHO-IR/PKCλ cells). Exposure of CHO-IR/PKCλ cells to insulin for 3 min resulted in an ∼2.5-fold increase in PKCλ activity measured in immunoprecipitates prepared with antibodies to the T7 epitope (Fig. 3D). This stimulation was inhibited by infection of cells with AxCAΔp85. These results suggested that PKCλ is activated by insulin in a PI 3-kinase-dependent manner.
Dominant negative effects of kinase-defective mutants of PKCλ on insulin-induced activation of PKCλ.
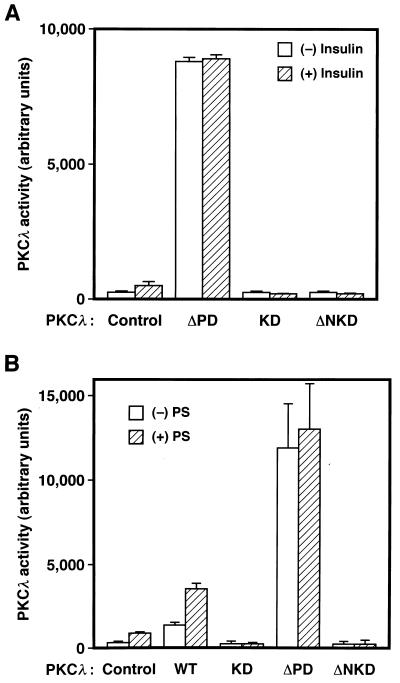
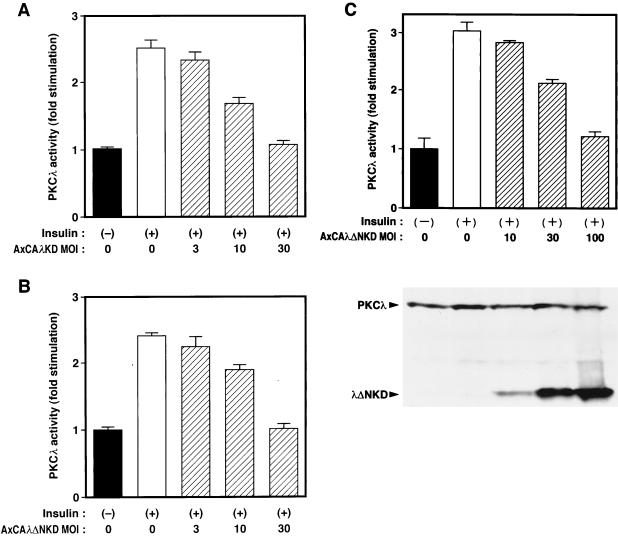
To investigate the roles of PKCλ in cells by specifically inhibiting the activity of the endogenous enzyme, we have constructed adenovirus vectors that encode dominant negative mutants of PKCλ. Infection of cells with an adenovirus (AxCAλKD) encoding λKD revealed that the mutant enzyme was not activated either by insulin treatment in intact cells (Fig. 4A) or by addition of PS to the kinase assay mixture (Fig. 4B); PS stimulated the activity of the wild-type enzyme ∼2.5-fold. Infection of CHO-IR/PKCλ cells with AxCAλKD resulted in inhibition of insulin-induced activation of PKCλ (measured in immunoprecipitates prepared with antibodies to the T7 epitope) in an MOI-dependent manner (Fig. 5A), without an effect on the amount of T7 epitope-tagged PKCλ in the cells (data not shown).
FIG. 4.
Effects of insulin in vivo and PS in vitro on the kinase activities of various PKCλ mutants. (A) 3T3-L1 adipocytes cultured in six-well plates were infected (or not) with adenovirus vectors encoding λΔPD (ΔPD), λKD (KD), or λΔNKD (ΔNKD) at an MOI of 150, 30, or 50 PFU/cell, respectively. The cells were then incubated in the absence or presence of 100 nM insulin for 5 min, lysed, and subjected to immunoprecipitation with a MAb to PKCλ. The immunoprecipitates were assayed for PKCλ activity. (B) KB cells cultured in six-well plates were infected (or not) with adenovirus vectors encoding λWT (WT), λKD (KD), λΔPD (ΔPD), or λΔNKD (ΔNKD) at an MOI of 1, 3, 15, or 5 PFU/cell, respectively; these MOIs resulted in the expression of similar amounts of PKCλ proteins. The cells were lysed, the total lysates were subjected to immunoprecipitation with a MAb to PKCλ, and the immunoprecipitates were assayed for PKCλ activity in the absence or presence of PS (100 μg/ml). Data are means ± standard errors from three experiments.
FIG. 5.
Effects of λKD and λΔNKD on insulin-induced activation of PKCλ. (A and B) CHO-IR/PKCλ cells were infected with AxCAλKD (A) or AxCAλΔNKD (B) at the indicated MOI (PFU per cell), incubated in the absence or presence of 100 nM insulin for 5 min, and lysed. The total lysates were subjected to immunoprecipitation with a MAb to the T7 epitope, and the immunoprecipitates were then assayed for PKCλ activity. (C) 3T3-L1 adipocytes were infected with AxCAλΔNKD at the indicated MOI (PFU per cell), incubated in the absence or presence of 100 nM insulin for 5 min, and lysed. The total lysates were subjected to immunoblot analysis with a MAb to PKCλ (lower panel) or to immunoprecipitation with polyclonal antibodies to PKCλ (αλ197); the immunoprecipitates were then assayed for PKCλ activity (upper panel). Quantitative data are expressed as fold stimulation relative to uninfected cells not exposed to insulin and are means ± standard errors from three experiments.
Although these results indicated that λKD inhibits the activation of transfected PKCλ by insulin, we could not examine the effect of this mutant on endogenous PKCλ activity because the polyclonal antibodies to PKCλ (αλ197) also precipitate λKD. We therefore constructed an adenovirus vector (AxCAλΔNKD) that encodes an NH2-terminally truncated version of λKD (λΔNKD); because this mutant does not possess the region of PKCλ corresponding to the immunogen, it is not recognized by the polyclonal antibodies to PKCλ. λΔNKD was not activated either by insulin in vivo or by PS in vitro (Fig. 4). Infection of CHO-IR/PKCλ cells with AxCAλΔNKD resulted in an MOI-dependent inhibition of insulin-induced activation of T7 epitope-tagged PKCλ (Fig. 5B). The extent of the inhibition paralleled the extent of expression of the mutant protein, and the expression of the mutant protein did not affect the amount of T7 epitope-tagged PKCλ (data not shown).
We then investigated the effect of λΔNKD on endogenous PKCλ activity in 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes were infected with AxCAλΔNKD at various MOIs and incubated in the absence or presence of insulin, after which immunoprecipitates prepared with polyclonal antibodies to PKCλ were assayed for PKCλ activity. Insulin stimulation of endogenous PKCλ activity in 3T3-L1 adipocytes was inhibited by λΔNKD in an MOI-dependent manner, being virtually abolished at an MOI of 100 PFU/cell (Fig. 5C); again, the extent of inhibition paralleled the extent of expression of the mutant protein, and the expression of the mutant protein did not affect the amount of endogenous PKCλ protein (Fig. 5C). Immunoblot analysis revealed that the amount of mutant PKCλ protein in the cells infected with AxCAλΔNKD at an MOI of 100 PFU/cell was 5 to 10 times that of endogenous PKCλ protein. Insulin-induced stimulation of PI 3-kinase activity in 3T3-L1 cells, measured in immunoprecipitates prepared with antibodies to phosphotyrosine, was not inhibited by either λKD or λΔNKD (data not shown). Thus, both of these PKCλ mutants exerted dominant negative effects on insulin-induced activation of PKCλ.
Inhibition of insulin-stimulated glucose uptake and GLUT4 translocation by dominant negative mutants of PKCλ.
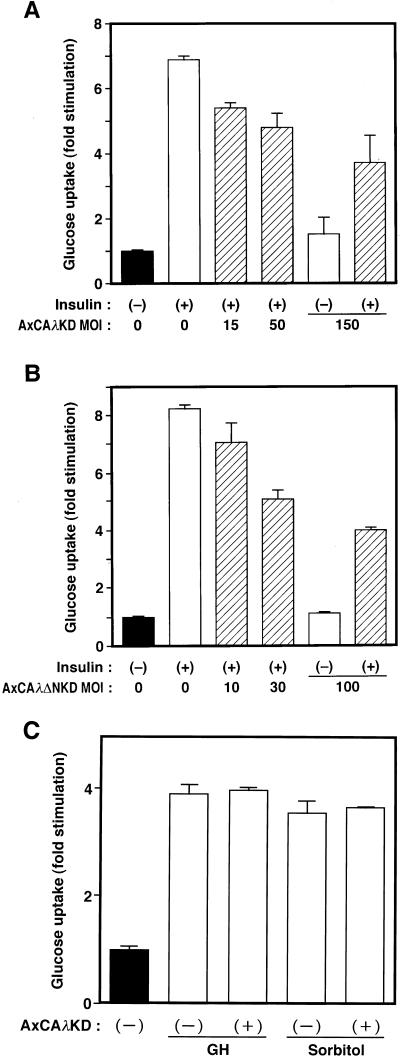
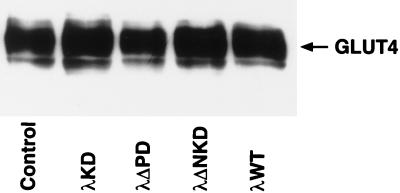
We next examined the effects of the dominant negative mutants of PKCλ on insulin-stimulated glucose uptake and translocation of GLUT4 in 3T3-L1 adipocytes. Cells were infected with either AxCAλKD or AxCAλΔNKD and then assayed for insulin-induced glucose uptake. Overexpression of either λKD or λΔNKD resulted in a dose-dependent inhibition of insulin-stimulated glucose uptake (Fig. 6A and B). Basal glucose transport was not affected by infection of cells with the viruses encoding either of the dominant negative mutants of PKCλ (Fig. 6A and B). The amounts of GLUT4 protein, assessed by immunoblot analysis, in infected and noninfected cells were also similar (Fig. 7). Glucose uptake stimulated by either GH or hyperosmolarity (300 mM sorbitol) was not affected by AxCAλKD (Fig. 6C).
FIG. 6.
Effects of λKD and λΔNKD on glucose uptake in 3T3-L1 adipocytes. (A and B) 3T3-L1 adipocytes were infected with AxCAλKD (A) or AxCAλΔNKD (B) at the indicated MOI (PFU per cell) and then incubated in the absence or presence of 100 nM insulin for 15 min. Cells were then assayed for glucose uptake. (C) 3T3-L1 adipocytes were infected (or not) with AxCAλKD at an MOI of 150 PFU/cell, incubated in the absence or presence of GH (0.5 μg/ml) for 10 min or 300 mM sorbitol for 60 min, and then assayed for glucose uptake. Data are means ± standard errors from three experiments.
FIG. 7.
Effects of various PKCλ mutants on the amount of GLUT4 protein in 3T3-L1 adipocytes. Cells were infected (or not) with AxCAλKD, AxCAλΔPD, AxCAλΔNKD, or AxCAλWT at an MOI of 150 PFU/cell, and total cell lysates were subjected to immunoprecipitation with a MAb to GLUT4. The immunoprecipitates were then subjected to immunoblot analysis with polyclonal antibodies to GLUT4.
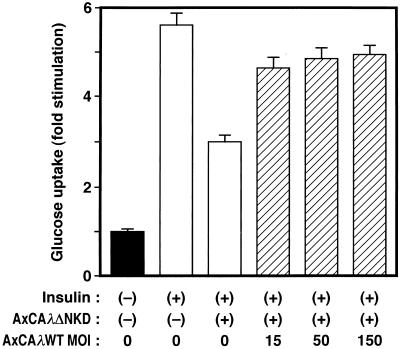
To confirm that the observed inhibition of insulin-induced glucose uptake was due to inhibition of PKCλ activity, we attempted to reverse the effect of the λΔNKD mutant by overexpressing the wild-type enzyme. 3T3-L1 adipocytes were infected first with AxCAλΔNKD at an MOI of 100 PFU/cell, and, after 12 h, they were infected again with AxCAλWT, an adenovirus vector encoding wild-type PKCλ, at different MOIs (Fig. 8). The cells were then assayed for insulin-induced glucose uptake. Infection of the cells with AxCAλWT partially reversed the inhibition of glucose uptake by λΔNKD in an MOI-dependent manner. The expression of λΔNKD protein was not affected by the second infection of the cells with AxCAλWT (data not shown). These results suggested that the inhibition of insulin-induced glucose uptake by λΔNKD is due to inhibition of PKCλ activity.
FIG. 8.
Effect of overexpression of wild-type PKCλ on the inhibition of insulin-stimulated glucose uptake by λΔNKD. 3T3-L1 adipocytes were infected (or not) with AxCAλΔNKD at an MOI of 100 PFU/cell and, after 12 h, with AxCAλWT at the indicated MOI. After an additional 36 h, the cells were assayed for glucose uptake. Data are means ± standard errors from three experiments.
The effects of the dominant negative mutants of PKCλ on insulin-stimulated translocation of GLUT4 were examined by the plasma membrane lawn assay. Exposure of noninfected 3T3-L1 adipocytes to insulin resulted in a marked increase in the amount of GLUT4 immunoreactivity in the plasma membrane, an effect that was substantially inhibited in cells infected with AxCAλKD (Fig. 9). An essentially identical inhibitory effect on insulin-induced translocation of GLUT4 was observed in cells infected with AxCAλΔNKD (data not shown). These results suggested that PKCλ activity is required for insulin-induced glucose uptake and translocation of GLUT4.
FIG. 9.
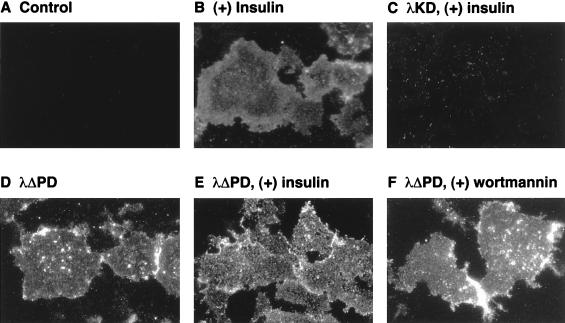
Effects of PKCλ mutants on translocation of GLUT4 to the plasma membrane of 3T3-L1 adipocytes. Uninfected cells (A and B) or cells that were infected with AxCAλKD (C) or AxCAλΔPD (D through F) at an MOI of 150 PFU/cell were incubated in the absence (A through E) or presence (F) of 100 nM wortmannin for 20 min and then in the absence (A, D, and F) or presence (B, C, and E) of 100 nM insulin for 5 min. Plasma membrane fragments were then prepared for immunofluorescence microscopy with antibodies to GLUT4 and tetramethyl rhodamine isothiocyanate-labeled secondary antibodies. Data are representative of those from at least three independent experiments.
Enhancement of glucose transport and GLUT4 translocation by a constitutively active mutant of PKCλ.
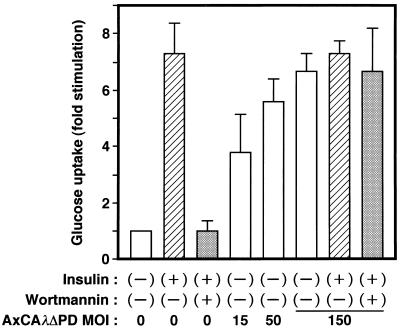
To investigate whether activation of PKCλ is capable of stimulating glucose uptake in 3T3-L1 adipocytes, we prepared an adenovirus vector that encodes a constitutively active mutant of PKCλ. Because the activity of PKC enzymes is negatively regulated by the pseudosubstrate domain, we constructed a mutant PKCλ that lacks this domain (λΔPD). As expected, the kinase activity of λΔPD was markedly greater than that of the wild-type enzyme, and this activity was little affected by insulin treatment in vivo or PS in vitro (Fig. 4). Infection of 3T3-L1 adipocytes with a virus (AxCAλΔPD) that encodes this mutant resulted in stimulation of glucose uptake in an MOI-dependent manner (Fig. 10). The extent of the stimulation paralleled the extent of expression of the mutant protein (data not shown). At an MOI of 150 PFU/cell, the extent of the stimulatory effect was similar to that achieved by 100 nM insulin (Fig. 10). λΔPDKD, a mutant PKCλ that lacks both kinase activity and the pseudosubstrate domain, did not stimulate glucose uptake (data not shown), suggesting that the activation of sugar transport by λΔPD was due to its kinase activity and not to nonspecific effects of viral infection. Furthermore, the amounts of GLUT4 (Fig. 7) and of insulin-stimulated PI 3-kinase activity precipitated with antibodies to phosphotyrosine (data not shown) were not increased by expression of λΔPD. Insulin did not increase glucose uptake further in cells that had been infected with AxCAλΔPD at an MOI of 150 PFU/cell, and treatment of such cells with wortmannin had little effect on glucose uptake (Fig. 10). Moreover, GLUT4 translocation, as assessed by the plasma membrane lawn assay, was stimulated by λΔPD, and this stimulation was not affected by insulin or wortmannin (Fig. 9).
FIG. 10.
Effect of λΔPD on glucose uptake in 3T3-L1 adipocytes. Cells were infected with AxCAλΔPD at the indicated MOI (PFU per cell) and incubated in the absence or presence of 100 nM wortmannin for 20 min and then with or without 100 nM insulin for 10 min. Glucose uptake was then assayed. Data are means ± standard errors from three experiments.
Localization of PKCλ and Akt to different signaling pathways.
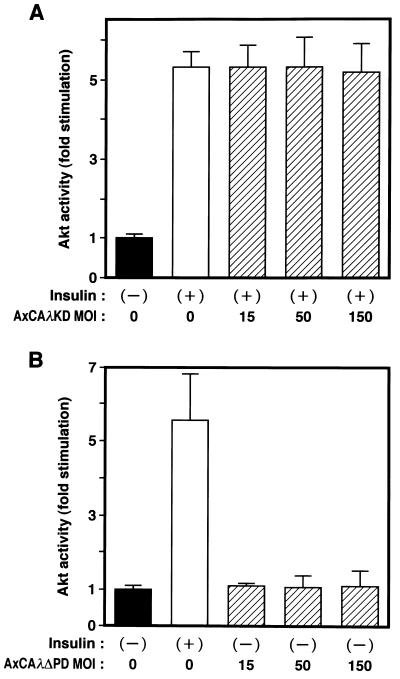
Finally, we examined whether PKCλ and Akt, both of which are downstream effectors of PI 3-kinase, act in the same or different signaling pathways. Insulin induced a fivefold increase in Akt activity in 3T3-L1 adipocytes (Fig. 11A). Infection of cells with AxCAλKD did not affect insulin-induced activation of Akt, even at an MOI of 150 PFU/cell, a dose sufficient to inhibit insulin-induced glucose uptake by ∼50% (Fig. 6A). Infection with AxCAλΔPD did not result in the activation of Akt, even at an MOI of 150 PFU/cell (Fig. 11B), a virus dose sufficient to activate glucose uptake to an extent similar to that achieved by insulin (Fig. 10). Essentially similar results were obtained with CHO cells (data not shown). These observations indicate that Akt does not function downstream of PKCλ.
FIG. 11.
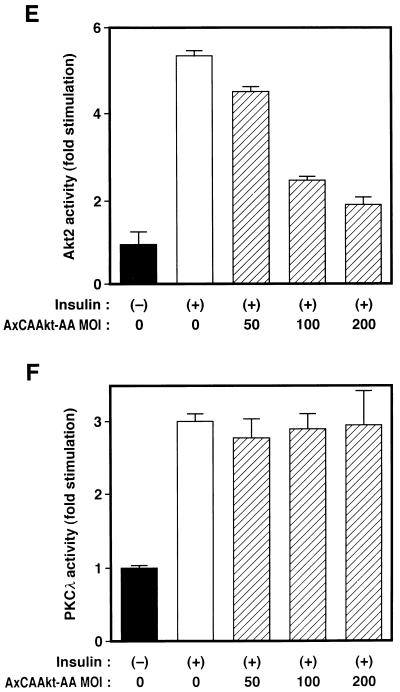
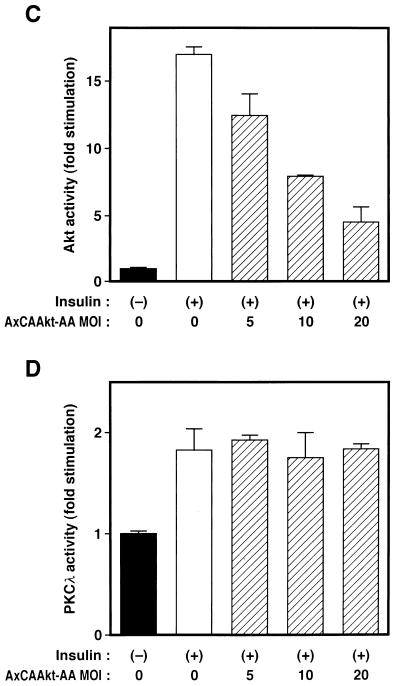
Localization of PKCλ and Akt to different signaling pathways. (A and B) Effects of λKD (A) and λΔPD (B) on Akt activity in 3T3-L1 adipocytes. Cells were infected with AxCAλKD (A) or AxCAλΔPD (B) at the indicated MOI (PFU per cell) and then incubated in the absence or presence of 100 nM insulin for 10 min. Cell lysates were subjected to immunoprecipitation with antibodies to Akt, and the resulting precipitates were assayed for Akt kinase activity. (C and D) Effects of a dominant negative mutant of Akt on insulin-induced activation of Akt (C) and PKCλ (D) in CHO-Akt cells. Cells were infected at the indicated MOI (PFU per cell) with an adenovirus vector (AxCAAkt-AA) encoding Akt-AA, incubated in the absence or presence of 100 nM insulin for 10 min, and lysed. Lysates were subjected to immunoprecipitation with antibodies to either the FLAG epitope (C) or PKCλ (D), and the resulting immunoprecipitates were assayed for Akt or PKCλ activity, respectively. (E and F) Effects of Akt-AA on insulin-induced activation of Akt2 (E) and PKCλ (F) in 3T3-L1 adipocytes. Cells were infected with AxCAAkt-AA at the indicated MOI (PFU/cell), incubated in the absence or presence of 100 nM insulin for 3 min, and lysed. Lysates were subjected to immunoprecipitation with antibodies to Akt2 (E) or to PKCλ (F), and the precipitates were assayed for Akt and PKCλ activity, respectively. Data are means ± standard errors from three experiments.
We next investigated whether Akt contributes to the activation of PKCλ with the use of a dominant negative mutant of Akt. We have recently shown that a mutant Akt (Akt-AA) in which the phosphorylation sites (Thr308 and Ser473) targeted by growth factors are replaced by alanine acts in a dominant negative manner (26). Infection of CHO cells stably expressing FLAG epitope-tagged rat Akt1 (CHO-Akt cells) with an adenovirus encoding Akt-AA (AxCAAkt-AA) resulted in inhibition of insulin-stimulated Akt activity in an MOI-dependent manner; at an MOI of 20 PFU/cell, ∼70% inhibition was achieved (Fig. 11C). Insulin treatment of CHO-Akt cells increased twofold the kinase activity measured in immunoprecipitates prepared with antibodies to PKCλ, and this stimulatory action of insulin on PKCλ activity was not affected by expression of Akt-AA (Fig. 11D).
We also tested the effect of Akt-AA on insulin stimulation of PKCλ activity in 3T3-L1 adipocytes. We have previously shown that infection of these cells with AxCAAkt-AA at an MOI of 200 PFU/cell inhibited insulin-induced activation of endogenous Akt by ∼90% (26). Because the antibodies to Akt used in our previous study recognize Akt1, Akt2, and Akt3 (26), it was suggested that Akt-AA inhibits all three known isoforms of Akt. Because Akt2 is a major isoform of Akt in 3T3-L1 adipocytes (11), we directly tested the effect of Akt-AA on insulin-stimulated Akt2 activity in 3T3-L1 adipocytes with the use of antibodies to Akt2. These antibodies did not recognize Akt-AA (data not shown), which is derived from rat Akt1. Insulin induced an approximately fivefold increase in kinase activity present in immunoprecipitates prepared with the antibodies to Akt2 (Fig. 11E). The amount of kinase activity in immunoprecipitates prepared with antibodies to Akt3 was not substantially greater than that present in precipitates prepared with control antibodies (data not shown). Infection of 3T3-L1 cells with AxCAAkt-AA at an MOI of 200 PFU/cell inhibited insulin stimulation of Akt2 activity by ∼80% (Fig. 11E). However, AxCAAkt-AA had no effect on insulin-stimulated PKCλ activity (Fig. 11F), suggesting that Akt does not contribute to PKCλ activation by insulin.
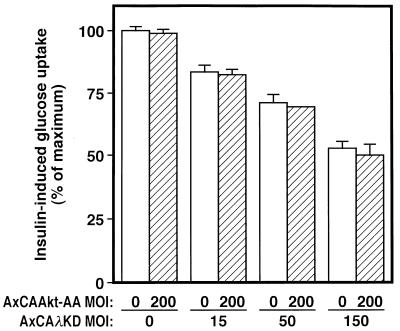
The inhibitory effect of λΔNKD on insulin-stimulated glucose uptake was partial (∼50 to 60%) (Fig. 6B), whereas this mutant almost completely abolished the insulin-induced increase in PKCλ activity (Fig. 5C). These results may suggest the existence of a redundant pathway that mediates insulin stimulation of glucose uptake. To examine whether Akt is responsible for such a redundant pathway, we finally examined the effect of coexpression of λKD and Akt-AA on insulin-induced glucose uptake in 3T3-L1 adipocytes. The inhibitory effect of λKD on insulin stimulation of glucose uptake in cells infected with AxCAAkt-AA at an MOI of 200 PFU/cell was similar to that apparent in cells not infected with AxCAAkt-AA (Fig. 12).
FIG. 12.
Combined effects of Akt-AA and λKD on insulin-induced glucose uptake. 3T3-L1 adipocytes were infected with AxCAλKD at the indicated MOI, and after 8 h, they were infected (or not) with AxCAAkt-AA at an MOI of 200 PFU/cell. After 40 h, the cells were assayed for insulin-induced glucose uptake. Data are expressed as a percentage of maximal insulin-induced glucose uptake and are means ± standard errors from three experiments.
DISCUSSION
Evidence suggests that stimulation of glucose transport by insulin is mediated mainly by a pathway triggered by PI 3-kinase (12, 20, 21, 39, 41). Because both Akt and atypical PKC isozymes act downstream of PI 3-kinase, it has been of interest to determine whether insulin-stimulated glucose uptake is mediated through one of these protein kinases or through an as-yet-unknown effector of the lipid kinase. To address this question, we have now investigated the role of an atypical PKC isoform, PKCλ, in intact cells by specifically inhibiting the activity of the endogenous enzyme. Overexpression of kinase-deficient mutants of atypical PKC has been shown to inhibit various biological actions, including activation of mitogen-activated protein kinase, DNA synthesis, nuclear factor-κB-dependent trans activation, and v-ras-induced transformation (7–9, 15), although it is not clear whether such mutants inhibit the activity of the endogenous enzymes.
We have now shown that expression of kinase-defective mutants of PKCλ (λKD and λΔNKD) inhibited insulin-induced activation of both transfected and endogenous PKCλ. Because the insulin-induced increase in PI 3-kinase activity, measured in immunoprecipitates prepared with antibodies to phosphotyrosine, was not affected by either of these mutant proteins, it appears that they do not inhibit the insulin receptor kinase or subsequent phosphorylation of IRS1; rather, they prevent specific signaling downstream of PI 3-kinase. Normal activation of Akt by insulin in cells expressing λKD also supports this conclusion. These observations thus demonstrated that λKD and λΔNKD act in a dominant negative manner.
Overexpression of either λKD or λΔNKD inhibited insulin stimulation of glucose uptake and GLUT4 translocation in 3T3-L1 adipocytes. Basal glucose transport in cells expressing these mutants was similar to that in uninfected cells. Furthermore, glucose uptake induced by either GH or hyperosmolarity attributable to sorbitol, both of which promote glucose transport through a PI 3-kinase-independent mechanism (41), was not affected by λKD, suggesting that λKD inhibited insulin-induced glucose uptake not by an effect on a component of the transport machinery but by blocking specific signals mediated through PKCλ. Indeed, the amount of GLUT4 protein in cells infected with AxCAλKD or AxCAλΔNKD was unchanged.
To investigate further the role of PKCλ in glucose uptake and translocation of GLUT4, we examined the effects of a constitutively active mutant of PKCλ. The kinase activity of λΔPD, a PKCλ mutant that lacks the pseudosubstrate domain, is markedly greater than that of the wild-type enzyme. Infection of cells with AxCAλΔPD stimulated glucose uptake to an extent similar to that achieved by insulin, without affecting the amount of GLUT4 protein or of insulin-stimulated PI 3-kinase activity precipitated with antibodies to phosphotyrosine. The observation that insulin did not result in further stimulation of glucose uptake in these cells suggests that the effects of insulin and PKCλ are not additive and that PKCλ lies on the insulin signaling pathway responsible for regulating glucose uptake. The inability of wortmannin to inhibit glucose transport stimulated by λΔPD is consistent with the conclusion that PKCλ acts downstream of PI 3-kinase. These results, together with the ability of wild-type PKCλ to compensate for the inhibition of insulin-induced glucose uptake by λΔNKD, indicate that PKCλ contributes to insulin stimulation of glucose uptake.
Expression of Akt fused with a viral Gag protein or tagged with a myristoylation signal sequence induced glucose uptake or translocation of GLUT4 in adipocytes (30, 45). Although these observations do not necessarily indicate that insulin signaling to glucose uptake is mediated by Akt, it is important to determine whether Akt and PKCλ participate in the same signaling pathway or whether they transmit signals through different pathways. We have now shown that λKD did not inhibit insulin-induced activation of Akt and that λΔPD did not increase basal Akt activity. These data indicate that the inhibitory effect of λKD on insulin-stimulated glucose uptake is not mediated by prevention of Akt activation and that λΔPD stimulation of glucose transport is not mediated by activation of Akt. Moreover, we have shown that the inhibition of insulin-induced activation of Akt by a dominant negative mutant of Akt (Akt-AA) did not affect insulin stimulation of PKCλ activity, indicating that insulin activation of PKCλ is independent of Akt activation.
Cong et al. (14) have shown that when isolated adipocytes were transiently transfected with constructs encoding HA-tagged GLUT4 and a kinase-deficient mutant of Akt (Akt-K179A), the amount of HA-tagged GLUT4 on the cell surface, in the absence or presence of insulin, was ∼20% less than that apparent in cells that were transfected with a control plasmid in addition to that encoding HA-tagged GLUT4, suggesting that Akt contributes to insulin stimulation of translocation of GLUT4 in these cells. However, we have recently found that overexpression of a similar kinase-deficient mutant of Akt (Akt-K179D) affected neither insulin-stimulated Akt activity in CHO cells (26) nor insulin-stimulated glucose uptake in 3T3-L1 adipocytes (32). We do not know the reason for this discrepancy. It is possible that the effects of kinase-deficient mutants of Akt may be different in different cells and tissues.
The reason why the dominant negative mutants of PKCλ inhibited insulin-stimulated glucose uptake by only ∼50% in 3T3-L1 adipocytes, whereas λΔNKD almost completely abolished the insulin-induced increase in PKCλ activity precipitated with antibodies to PKCλ, is not clear. One possibility is that there is a redundant pathway that mediates insulin stimulation of glucose uptake and that prevention of signal transduction by the PKCλ pathway is therefore not sufficient to block glucose uptake completely. It is not known which molecules might be responsible for such a redundant pathway; however, the participation of Akt is unlikely, given that expression of Akt-AA did not exert an additive effect on the inhibition of glucose uptake by λKD. Calera et al. (11) have shown that the amount of Akt2 associated with GLUT4-containing vesicles was increased in response to insulin. Although the physiological significance of this observation remains to be elucidated, we cannot exclude the possibility that a small increase in Akt activity in a specific compartment of the cell may be sufficient to activate glucose transport fully. Another possible explanation for the discrepancy between the extents of inhibition of glucose uptake and of PKCλ activity by the dominant negative mutants of PKCλ is that the activity of immunoprecipitated PKCλ does not completely reflect the activity of this enzyme in intact cells. It has been suggested that interaction with lipids produced in response to extracellular stimuli regulates the enzymatic activity of atypical PKC (1, 36–38, 43). Thus, lipids essential for the activation of PKCλ in intact cells may have been removed, at least in part, during immunoprecipitation, possible explaining the relatively small activation of the enzyme observed in the immunoprecipitates. This possibility is supported by our observation that PKCλ immunoprecipitated from insulin-stimulated cells was further activated by PS in vitro (32). Therefore, we cannot exclude the possibility that λKD and λΔNKD may not completely block the activity of PKCλ in intact cells, whereas the insulin-induced increase in the activity of the immunoprecipitated enzyme appears to be abolished.
Two groups have reported that PKCζ is expressed in 3T3-L1 adipocytes on the basis of immunoblot analysis with antibodies generated in response to a peptide corresponding to the COOH-terminal region of rat PKCζ (GFEYINPLLLSAEESV) (6, 19). This amino acid sequence is highly homologous to the corresponding sequence of mouse PKCλ (GFEYINPLLMSAEECV). We have now shown that antibodies induced by the same COOH-terminal peptide of PKCζ (αζCT) recognized mouse PKCλ transiently expressed in COS7 cells. Moreover, αζCT detected an ∼80-kDa protein in immunoprecipitates prepared from 3T3-L1 adipocyte lysates with antibodies to PKCλ (αλ190) but not in those prepared with antibodies to PKCζ (αζ170). These results, together with those of our RT-PCR analysis, suggest that PKCλ, but not PKCζ, is expressed in 3T3-L1 adipocytes and that the protein previously detected in 3T3-L1 adipocytes by the antibodies to the PKCζ peptide is actually PKCλ.
Bandyopadhyay et al. (6) have shown that insulin-stimulated glucose uptake was reduced by ∼30% in 3T3-L1 adipocytes expressing a kinase-deficient mutant of rat PKCζ. We have found that overexpression of a kinase-deficient mutant of PKCζ inhibited the insulin-induced increase in PKCλ activity in 3T3-L1 adipocytes (32), suggesting that the inhibition of glucose transport observed by these investigators may be due to the inhibition of PKCλ. However, it is possible that PKCζ also participates in insulin signal transduction, because the same group recently reported that transient expression of a kinase-defective mutant PKCζ in rat adipocytes, or incubation of the adipocytes with a peptide corresponding to the pseudosubstrate domain of PKCζ, inhibited insulin-induced translocation of transfected GLUT4 or glucose transport, respectively (43). Furthermore, it has been reported that insulin-stimulated protein synthesis in hematopoietic cells was modulated by transfection with a mutant PKCζ cDNA (34). It is thus important to determine which actions of insulin that are dependent on PI 3-kinase are mediated by which isoform of atypical PKC in different cells and tissues.
In summary, we have shown that PKCλ is required for insulin stimulation of glucose uptake, but not for Akt activation, and that a dominant negative Akt mutant did not affect PKCλ activity in 3T3-L1 adipocytes. These results suggest that insulin-elicited signals that pass through PI 3-kinase are subsequently transmitted by at least two independent pathways: an Akt pathway and a PKCλ pathway. The mechanism by which PKCλ is activated by PI 3-kinase remains unclear. Clarification of the mechanism by which PKCλ is regulated should increase our understanding of the mechanism by which signals from PI 3-kinase diverge and are transmitted by various downstream effectors, subsequently resulting in a broad range of biological effects.
ACKNOWLEDGMENTS
This work was supported by a grant from the Ministry of Education, Science, Sports, and Culture of Japan, a Grant-in-Aid for Research for the Future Program from the Japan Society for the Promotion of Science, a grant from the Uehara Memorial Foundation, a grant for studies on the pathophysiology and complications of diabetes from Tsumura Pharma Ltd. (to M.K.), and a grant from Suzuken Memorial Foundation (to W.O.).
REFERENCES
- 1.Akimoto K, Mizuno K, Osada S-I, Hirai S-I, Tanuma S, Suzuki K, Ohno S. A new member of the third class in the protein kinase C family, PKCλ, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem. 1994;269:12677–12683. [PubMed] [Google Scholar]
- 2.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S-I, Mizuno K, Hirai S-I, Kazulauskas A, Ohno S. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 4.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 5.Backer J M, Myer M G, Jr, Shoelsen S E, Chin D J, Sun X J, Miralpleix M, Hu P, Margolis B, Skolnik E Y, Schlessinger J, White M. The phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandyopadhyay G, Standaert M L, Zhao L, Bingzhi Y, Avignon A, Galloway L, Karnam P, Moscat J, Farese R V. Activation of protein kinase C (α, β, and ζ) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-ζ in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 7.Berra E, Diaz-Meco M T, Dominguez I, Municio M M, Sanz L, Lozano J, Chapkin R S, Moscat J. Protein kinase Cζ isoform is critical for mitogenic signal transduction. Cell. 1993;74:555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- 8.Berra E, Diaz-Meco M T, Lozano J, Frutons S, Munico M M, Sánchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase Cζ. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjørkøy G, Perander M, Øvervatn A, Johansen T. Reversion of Ras- and phosphatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of protein kinase Cλ is accompanied by the loss of constitutive nuclear mitogen activated protein kinase/extracellular signal-regulated kinase activity. J Biol Chem. 1997;272:11557–11565. doi: 10.1074/jbc.272.17.11557. [DOI] [PubMed] [Google Scholar]
- 10.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 11.Calera M R, Carmen C, Liu H, Jack A K E, Birnbaum M J, Pilch P F. Insulin increases the association of Akt2 with Glut-4 containing vesicles. J Biol Chem. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- 12.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 13.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cong L-N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Meco M T, Berra E, Municho M M, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera M T, Alcami J, Paya C, Arezana-Seissedod F, Virelizier J-L, Moscat J. A dominant negative protein kinase Cζ subspecies blocks NF-κB activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the AKT protooncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 17.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 18.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct binding of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–667. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 19.Frevert E U, Kahn B B. Protein kinase C isoforms ɛ, η, δ and ζ in murine adipocytes: expression, subcellular localization and tissue-specific regulation in insulin-resistant states. Biochem J. 1996;316:865–871. doi: 10.1042/bj3160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frevert E U, Kahn B B. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol. 1997;17:190–198. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara K, Yonezawa K, Sakaue H, Ando A, Kotani K, Kitamura T, Kitamura Y, Ueda H, Stephens L, Jackson T R, Hawkins P T, Dhand R, Clark A E, Holman G D, Waterfield M D, Kasuga M. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for Ras activation in CHO cells. Proc Natl Acad Sci USA. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera-Velit P, Kuntson K L, Reiner N E. Phosphatidylinositol 3-kinase-dependent activation of protein kinase C-ζ in bacterial lipopolysaccharide-treated human monocytes. J Biol Chem. 1997;272:16445–16452. doi: 10.1074/jbc.272.26.16445. [DOI] [PubMed] [Google Scholar]
- 23.Holman G H, Kasuga M. From receptor to transporter: insulin signaling to glucose transport. Diabetologia. 1997;40:991–1003. doi: 10.1007/s001250050780. [DOI] [PubMed] [Google Scholar]
- 24.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 25.Kitamura Y, Kitamura T, Sakaue H, Maeda T, Ueno H, Nishio S, Ohno S, Osada S-I, Sakaue M, Ogawa W, Kasuga M. Interaction of Nck-associated protein 1 with activated GTP-binding protein Rac. Biochem J. 1997;322:873–878. doi: 10.1042/bj3220873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klippel A, Reinhard C, Kavanaugh M, Apell G, Escobedo M A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn A D, Kovacina K S, Roth R A. Insulin stimulates the kinase-activity of RAC-PK, a pleckstrin homology domain-containing Ser/Thr kinase. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 31.Kohn A D, Takeuchi F, Roth R A. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 32.Kotani, K., W. Ogawa, and M. Kasuga. Unpublished data.
- 33.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, Nishiyama M, Waterfield M D, Kasuga M. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez R, Kollmorgen G, White M F, Rhoads R E. Requirement of protein kinase Cζ for stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA terminal protein complex and a cosmid bearing the full length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi H, Brewer K A, Exton J H. Activation of the ζ isoenzyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 37.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–613. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 38.Ohno S, Akita Y, Hata A, Osada S, Kubo K, Konno Y, Akimoto K, Mizuno K, Saido T, Kuroki T. Structural and functional diversities of a family of signal transducing protein kinases, protein kinase C family; two distinct classes of PKC, conventional cPKC and novel nPKC. Adv Enzyme Regul. 1991;31:287–303. doi: 10.1016/0065-2571(91)90018-h. [DOI] [PubMed] [Google Scholar]
- 39.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 40.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein kinase Cζ subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaue H, Ogawa W, Takata M, Kuroda S, Kotani K, Matsumoto M, Sakaue M, Nishio S, Ueno H, Kasuga M. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol. 1997;11:1552–1562. doi: 10.1210/mend.11.10.9986. [DOI] [PubMed] [Google Scholar]
- 42.Selbie L A, Schmitz-Peiffer C, Sheng Y, Biden T J. Molecular cloning and characterization of PKCι, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 43.Standaert M J, Galloway L, Karnam P, Bandyopathy G, Moscat J, Farese R V. Protein kinase C-ζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 44.Stokes D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 45.Tanti J F, Grillo S, Gremeaux T, Coffer P J, Van-Obberghen E, Le-Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 46.Toker A, Cantley L C. Signaling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 47.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 48.Vanhaesebroeck B, Leevers S J, Panaytou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 49.Ways D K, Cook P P, Webste C, Parker P J. Effect of phorbol esters on protein kinase C-ζ. J Biol Chem. 1992;267:4799–4805. [PubMed] [Google Scholar]
- 50.Welsh G I, Stokes C M, Wang X, Sakaue H, Ogawa W, Kasuga M, Proud C G. Activation of translation initiation factor eIF2B by insulin requires phosphatidylinositol 3-kinase. FEBS Lett. 1997;410:418–422. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]