See Commentary on Page 516
Introduction
CKD is a progressive condition characterized by a loss of kidney function. It is defined as abnormalities of kidney structure and/or function, present for >3 months, with implications for health, and the presence of either estimated glomerular filtration rate (eGFR) of <60 ml/min per 1.73 m2 or markers of kidney damage, including albuminuria.1 CKD is associated with high healthcare costs, poor quality of life, and serious adverse health outcomes (including cardiovascular disease [CVD], kidney failure [KF], infection, and death). As a major contributor to morbidity and mortality due to noncommunicable diseases, CKD is ranked as the 10th leading cause of death worldwide, with an estimated worldwide prevalence ranging from 10% to 16%.2,3 Currently, more than 850 million people have kidney disease, the vast majority of whom are living in low- and middle-income countries with limited access to healthcare resources.4
Africa is witnessing a high and rapidly increasing incidence of cardio-metabolic diseases, which are leading contributors to deaths due to noncommunicable diseases in this region. The significant increase in the prevalence of hypertension; type 2 diabetes mellitus (T2DM); endemic infections such as HIV, hepatitis B and C, tuberculosis, and malaria; as well as the use of herbal medications and other nephrotoxins are projected to drive the burden of CKD in Africa.5
CKD is also a significant risk factor for CVD. Patients with CKD have a higher propensity to develop coronary artery disease, heart failure (HF), arrhythmias, and sudden cardiac death.6 CVD associated with CKD is more likely to cause higher mortality rates than KF alone.7,8 Cardiovascular (CV) events contribute to a large proportion of unfavorable outcomes in patients with CKD.9 Conventional CV risk factors such as hypertension, T2DM, and dyslipidemia are highly prevalent in patients with CKD and contribute to atherosclerotic CVD, particularly in earlier CKD stages. Reducing CV risk in patients with CKD is imperative for any CKD management strategy.
Currently, CKD accounts for 4 million disability-adjusted life years lost annually across the African continent, marginally lower than T2DM (6.4 million disability-adjusted life years).10 It is estimated that by 2030, more than 70% of patients with KF would be living in low-income countries, such as those in Sub-Saharan Africa (SSA).11,12
The true prevalence and disease burden of CKD in Africa remains unknown due to multiple factors ranging from a paucity of epidemiological data to a lack of standardization of diagnostic and staging tests for kidney damage assessment. Accurate estimation of the prevalence and disease burden of CKD is a cost-effective approach to draw government and policymakers’ attention to the exponential rise of CKD in this region, and encourage efforts toward prevention, detection, and treatment of CKD at earlier stages.
Several practical challenges are unique to Africa, which undermine optimal kidney care for patients with CKD. Financial feasibility, clinical and ethical optimization of dialysis services, and lack of uniform and consistent distribution are other significant challenges to KF care.13 Owing to financial constraints, currently existing CKD management strategies include lifestyle modifications (weight control, dietary restrictions, exercise, and smoking cessation), maintaining target levels of blood pressure and blood glucose, avoiding exposure to nephrotoxins, and pharmacological intervention with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. Although newer therapeutic agents such as SGLT2is have demonstrated kidney protective benefits and in associated conditions of T2DM and CVD, their use remains limited due to high cost and accessibility issues in this region.14 Preventing progression to KF is thus of paramount importance. Preventive strategies to reduce the overall risk of CKD development in healthy individuals (primary prevention), early identification of CKD and its risk factors and prompt treatment initiation (secondary prevention), and prevention of precipitating factors in advanced stages of CKD (tertiary prevention) can considerably delay the progression to KF and requirement of kidney replacement therapy.15
This position paper intends to provide an overview of the available region and country-specific prevalence and disease burden of CKD, and the barriers in the management strategies of CKD in the African region. The paper also summarizes the current evidence on the role of SGLT2is and its applicability in different populations with CKD to support their safe implementation in CKD management.
Methods
A steering committee meeting was convened in November 2022 to discuss the current unmet needs and challenges in nephrology practice for CKD management in the region. A panel comprising 13 key external experts in the field of nephrology from 5 African regions: center African region (Cameroon and the Democratic Republic of Congo), west African region (Senegal, Ghana, Cote d’Ivoire, and Nigeria), east African region (Ethiopia and Kenya), Austral African region (Mauritius and South Africa), and north African region (Egypt and Tunisia) congregated to discuss the prevalence and regional burden of CKD, evaluate risk factors on incidence and progression of CKD, and identify gaps in effective screening, diagnosis, and management of CKD in this region. Based on emerging clinical evidence about the effectiveness of SGLT2is in slowing the risk of progression of CKD to KF, the panel also reviewed the current clinical trial evidence of SGLT2is and its implications in the African region. The panel proposed recommendations for their implementation as first-line nephroprotective agents in an effort to reduce the high burden of kidney disease in the region.
The panel is a representation of the key experts and members of the African Association of Nephrology society. The experts were from the 12 countries as mentioned in the methodology; however, the evidence presented on the CKD prevalence, burden, epidemiology, current practices, and challenges can be generalized to the entire African region and the recommendations provided can be adapted across the continent.
The recommendations presented in this position paper are an outcome of open discussion among the experts based on their clinical experience in CKD patient care, supported by published evidence on cardiorenal outcomes of SGLT2is.
Prevelance and Disease Burden of CKD in Africa
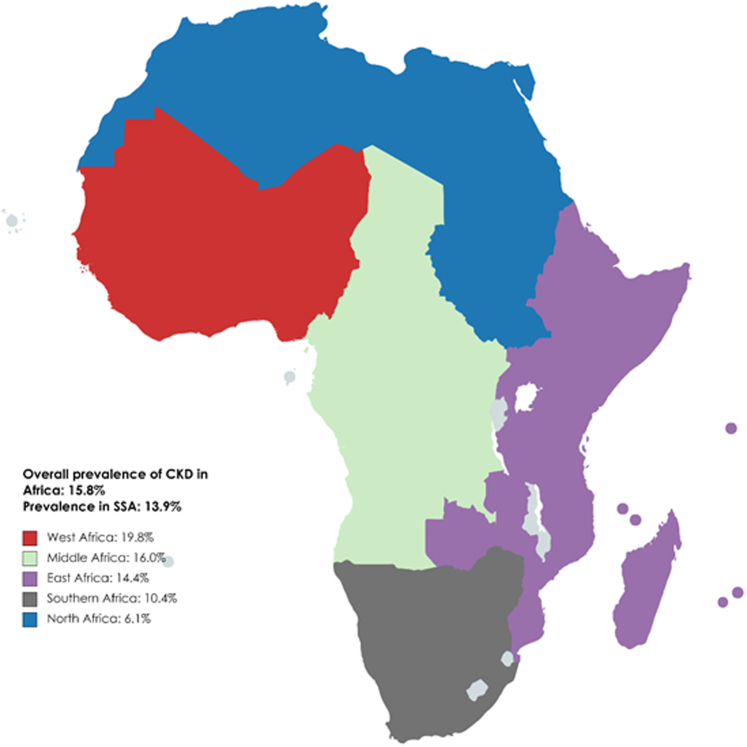
Epidemiological studies on the incidence, prevalence, and disease burden of CKD are sparse across Africa and it is probably underestimated and largely unknown. A systematic review and meta-analysis of 21 medium-quality and high-quality studies in 2014 found the overall prevalence of CKD to be 13.9% (95% confidence interval [CI], 12.2–15.7).16 The International Society of Nephrology Global Kidney Health Atlas - Africa initiative reported the overall prevalence of CKD in Africa to be 6.28%, with wide variability within countries such as Mauritius, an upper-middle income country, reporting the highest prevalence as 17.63%, and Uganda, a low-income country, reporting the lowest prevalence (4.87%).17 A systematic review and meta-analysis published in 2018 reported the prevalence of CKD stages 1 to 5 in the general population of Africa as 15.8% (95% CI, 12.1–19.9), and 4.6% (95% CI, 3.3–6.1) for CKD stages 3 to 5.18 The prevalence was significantly higher in SSA compared to North Africa for both CKD stages 1 to 5 (17.7%; 95% CI, 13.7–22.1 vs. 6.1%; 95% CI, 3.6–9.3) and CKD stages 3 to 5 (4.8%; 95% CI, 3.2–6.6 vs. 2.6%; 95% CI, 2.3–2.9).18 The highest prevalence of CKD (19.8%) was seen in West African countries (Figure 1).19 The overall prevalence of all stages of CKD in the high-risk populations was 32.3%—the highest with hypertension (35.6%), followed by diabetes (32.6%) and HIV (27.3%).18 The mortality rate attributable to CKD ranged from 0.57% in Zambia to 10.36% in Mauritius, and the percentage of disability-adjusted life years ranged from 0.58% in Nigeria to 6.85% in Mauritius.17 Another systematic review assessing CKD burden among the African general population and high-risk groups reported a prevalence that ranged from 2% to 41% (pooled prevalence: 10.1%; 95% CI, 9.8%–10.5%) with higher prevalence in high-risk groups such as HIV, T2DM, and hypertension.20 A systematic review and meta-analysis of 12 African studies that included 5297 participants from 6 countries (Ghana, Nigeria, Uganda, Tanzania, Democratic Republic of Congo, and South Africa) found the pooled prevalence of CKD in hypertensive patients to be approximately 17.8% (95% CI, 13.0–23.3) with the highest prevalence seen in West Africa (21.3%; 95% CI, 16.1–27.0); resulting in almost 4 times higher incidence of KF than in Caucasians.21,22
Figure 1.
Overall prevalence of CKD in Africa. Adapted from Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174-181. doi:10.1016/S2214-109X(14)70002-616; Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):125. doi:10.1186/s12882-018-0930-5.18 CKD, chronic kidney disease; SSA, sub-Saharan Africa.
A recent study in Senegal showed a CKD prevalence of 5.2% with almost 92% of the patients being diagnosed with advanced stages of CKD, and 34.9% with KF.23 Similarly, in another study conducted at a teaching hospital in the southeast of Nigeria, KF cases accounted for 7.96% of all medical admissions and 41.69% of renal admissions.24 The Maremar (Maladies rénales chroniques au Maroc) study conducted in Morocco estimating the prevalence of CKD, hypertension, diabetes, and obesity in a randomized, representative, high response rate (85%) sample found a low prevalence of CKD (2.9% adjusted to the actual adult population of Morocco).25 Another concerning finding of a large community-based study on CKD prevalence in SSA was a CKD prevalence of 6.8% in a community where the mean age of the adult population was 38 years, with a wide variation between regions. The CKD prevalence was primarily estimated by high prevalence of proteinuria with largely preserved kidney function.26
Fabian et al.27 through their population cohort studies from Malawi, Uganda, and South Africa demonstrated that estimating glomerular filtration rate using serum creatinine substantially underestimated the individual and population-level burden of impaired kidney function in Africa, thereby highlighting the need for scalable and affordable ways to accurately identify impaired kidney function in Africa.
Across all 3 countries, creatinine-based GFR-estimating equations were inadequate, and that worsened with declining kidney function. eGFR equations overestimated the proportion of grade 1 CKD compared with measured GFR and underestimated the proportion with grade 2 to 5 stages of kidney function. Creatinine-based equations underestimated stages 2 to 5 by at least 50%. Overestimation of individual GFR and misclassification by GFR stage was exacerbated when using ethnicity coefficients for the Modification of Diet in Renal Disease and CKD-Epidemiology Collaboration equations. GFR estimation using cystatin C alone or in combination with creatinine led to a reduction in the overestimation of measured glomerular filtration rate when compared with creatinine-based estimates; that implies that the estimates of prevalence of impaired kidney function using cystatin C were more than 2 times higher than creatinine-based estimates.27
The cost of nondialysis management and kidney replacement therapies are substantially higher in SSA than in North Africa and is mainly privately funded or out-of-pocket.17 A majority of African countries do not have the provision of kidney transplants and thus rely heavily on dialysis. Although both hemodialysis and peritoneal dialysis are available, hemodialysis is more commonly used and only 41% of countries have peritoneal dialysis facilities.17 The annual costs of hemodialysis and peritoneal dialysis were generally more than US$10,000 higher in SSA than in North Africa. The availability of healthcare resources and workforce in the Africa region are also considerably limited as compared to global estimates with an average of 0.62 nephrologists per million population, compared with 9.95 per million population globally.17 Most patients with KF initiate dialysis in Africa but discontinue soon after due to unsustainable treatment costs and insufficient infrastructure, thus further increasing the disease burden and complications associated with KF.28
CKD Etiology and Risk Factors
The recent epidemiological transition resulting in a surge of noncommunicable diseases such as T2DM, hypertension, and obesity is adding to the growing prevalence rate of CKD in the African region.16,29 CKD progression is also known to be accelerated in patients of African origin due to nonmodifiable risk factors such as age, sex, and genetic susceptibility to hypertension, and higher frequencies of Apolipoprotein L1 (APOL1) G1 and G2 high-risk alleles.30, 31, 32 The prevalence of hypertension in Africa is estimated to be approximately 36% and continues to grow rapidly.33 The pathogenesis of hypertension varies significantly in the African population with a propensity for a more aggressive course of hypertension resulting in early target organ damage. Several factors contribute to this phenomenon, including genetic polymorphisms, low birthweight, aberrant salt sensitivity, and poor socioeconomic conditions.34, 35, 36 Another significant risk factor associated with CKD highly prevalent in the African region is hypertensive disorders of pregnancy, predominantly preeclampsia and eclampsia.37 Coupled with inadequate access and low quality of antenatal care, preeclampsia is one of the leading causes of maternal and perinatal morbidity and mortality in the African region. Early preclinical studies have demonstrated decrease in blood pressure and natriuresis due to SGLT2is thus ameliorating the long term CV risk associated with preeclampsia.38
Autosomal dominant polycystic kidney disease, the most common form of hereditary kidney disease, is noted as another significant cause of CKD in Africa.20 In a 13-year retrospective hospital-based study (2005–2017), autosomal dominant polycystic kidney disease was identified as the fourth leading cause of CKD among Ghanaian adults.39
Approximately 24 million adults were reported to have diabetes in Africa in 2021. This figure is estimated to increase to 33 million by 2030 and 55 million by 2045.40 Uncontrolled T2DM is an established modifiable risk factor for CKD, and also accelerates disease progression. Obesity is not just an initiating risk factor for the development of CKD, but it also leads to CKD progression.19 In a study evaluating the prevalence of undiagnosed CKD in SSA, the prevalence of CKD was found to be 36%, with 16% of them being obese individuals. Obesity ranked as a top risk factor after hypertension (44%) and diabetes (39%).41
Glomerular diseases are one of the most common causes of the progression of CKD. Studies from Africa have shown that glomerular diseases (primary and secondary) account for 10.2% to 52% of patients with KF.42 Chronic glomerulonephritis related to endemic or neglected infectious diseases remains the major cause of CKD in Africa.43 Consequently, the only sign of CKD for those patients at an early stage is urinary abnormalities such as hematuria and proteinuria.
Communicable diseases such as HIV and hepatitis B and C contribute significantly to the large burden of CKD in SSA. The prevalence of CKD in HIV-positive individuals ranges from 22.9% to 51.8% as per some studies conducted in Nigeria with one of the highest prevalences of HIV in Africa.44, 45, 46 The prevalence of CKD in HIV-positive patients, naïve to antiretroviral therapy, was reported to be 13.4% in another Nigerian study.47 A systematic review of published literature from 60 countries reported a prevalence of 7.9% (95% CI, 5.2%–11.1%) in the African region.48 HIV-associated nephropathy is known to be the most severe form of HIV and kidney disease and a leading cause of KF characterized by the presence of focal glomerulosclerosis.49, 50, 51
Hepatitis B prevalence in SSA is also amongst the highest in the world, varying between 5% and 20%.52,53 In a retrospective study of patients with CKD screened for hepatitis B surface antigens and hepatitis C antibodies, the seroprevalence of hepatitis B and hepatitis C, and coinfection was seen in 15.6%, 4.8%, and 0.9% of the patients, respectively.54 Parasitic and protozoal infections such as schistosomiasis, filariasis, leishmaniasis, and malaria are endemic to SSA. Exposure to nephrotoxins also adds to the burden of CKD. The spectrum of kidney disease caused by infectious etiologies varies widely from acute kidney injury, acute and chronic glomerulonephritis, and interstitial nephritis to pyelonephritis. Infections have been implicated in many adverse kidney syndromes, including CKD of unknown etiology.55
Current Management of CKD
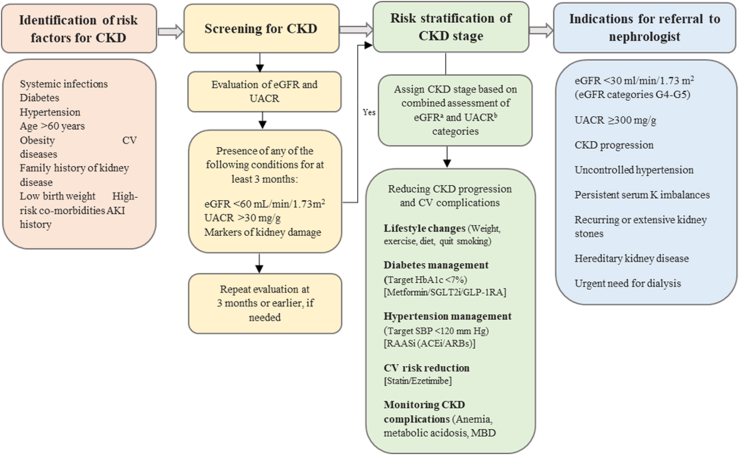
Patients with advanced CKD are more susceptible to developing adverse outcomes, including CKD progression, CV complications, and KF. Early identification of CKD and its accurate staging would enable the implementation of appropriate interventions to delay its progression and reduce the risk of CV complications. For effective CKD management, a comprehensive multidisciplinary approach is recommended to target risk factors for CKD with lifestyle changes (dietary management, physical activity, weight control, and abstinence from smoking) along with optimal management of comorbid T2DM, hypertension, and associated CV risk factors. In addition, patients require regular monitoring for complications of CKD, such as anemia, acid-base and electrolyte imbalances, and mineral bone diseases (Figure 2).1,56,57 Persistently elevated serum creatinine and albuminuria are important diagnostic and prognostic biomarkers of CKD. For initial assessment, measurement of eGFR using serum creatinine is considered the gold standard because of its affordability, easy accessibility, and long history of clinical use. Measurement of albuminuria by a spot urine sample using either albumin-specific dipsticks or urine albumin-to-creatinine ratio (UACR) are most accepted by physicians and nephrologists. The Kidney Disease Improving Global Outcomes (KDIGO) preferentially recommends using UACR, followed by urine protein-to-creatinine ratio over total protein urinalysis strips (either automated or manual) in assessing albuminuria in CKD diagnosis and follow-up. Although quantitative assessment of albuminuria using UACR is favored and recommended by the guidelines, it is unaffordable in African countries and use of urine test strips such as Albustix and dipstick screening is considered acceptable in the absence of more reliable methods and be followed by appropriate confirmatory testing.
Figure 2.
Identification and referral pathway for the management of CKD. ACEi, angiotensin-converting enzyme inhibitors; AKI, acute kidney injury; ARB, angiotensin receptor blockers; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycosylated hemoglobin; MBD, mineral bone diseases; RAASi, renin-angiotensin aldosterone system inhibitors; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter-2 inhibitors; UACR, urine albumin-to-creatinine ratio.
aeGFR categories in CKD classification G1: ≥90 ml/min per 1.73 m2, G2: 60–89 ml/min per 1.73 m2, G3a: 45–59 ml/min per 1.73 m2, G3b: 30–44 ml/min per 1.73 m2, G4: 15–29 ml/min per 1.73 m2 and G5: <15 ml/min per 1.73 m2 (kidney failure)
bUACR categories in CKD classification A1: <30 mg/g, A2: 30–300 mg/g, A3: >300 mg/g. Recreated with permission from Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.01256; permission conveyed through Copyright Clearance Center, Inc.
In the current clinical practice, the use of renin-angiotensin-aldosterone system (RAAS) blockers (including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers) are the mainstay for delaying CKD progression and managing complications. In view of their proven efficacy in terms of improvement in albuminuria and hypertension, the KDIGO 2021 guideline for the management of blood pressure in patients with CKD recommends using angiotensin-converting enzyme inhibitors or angiotensin receptor blockers as first-line therapeutic agents for patients with hypertension, CKD, and moderate to severe albuminuria (G1 [≥90 ml/min per 1.73 m2] to G4 [15–29 ml/min per 1.73 m2], A2 [30–300 mg/g], and A3 [>300 mg/g]), with or without T2DM.57
Despite their established renoprotective efficacy for significant risk reduction (in terms of doubling of serum creatinine, KF, and death) by 16% and 20%, as reported by RENAAL58 and IDNT59 studies, respectively, there is a continued residual risk of progression of CKD to KF or premature death from CV events. These findings highlight the need for novel medications to address this continuing risk.
Emerging Role of SGLT2is in CKD
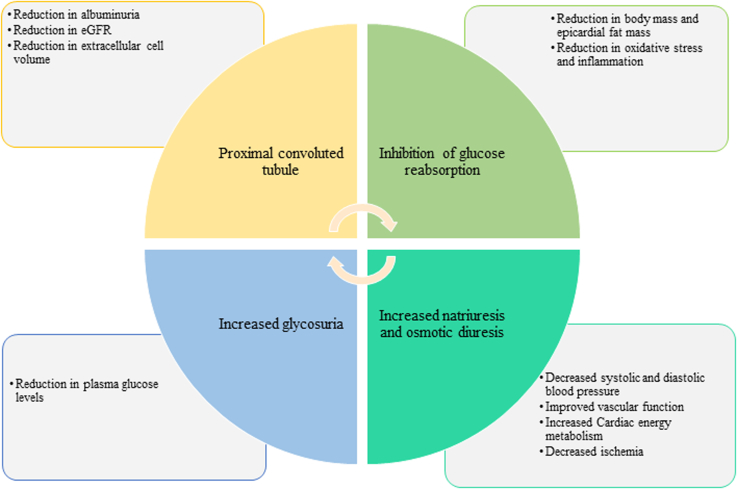
SGLT2is are a novel group of glucose-lowering agents with the mechanism of action based on the inhibition of sodium-glucose cotransporter-2 in the proximal convoluted tubule preventing reabsorption of glucose, thereby causing increased urinary excretion of glucose and sodium.60 This results in a multitude of metabolic benefits and positive clinical outcomes, such as a reduction in glycosylated hemoglobin (i.e., HbA1c), albuminuria, body weight, and blood pressure (Figure 3).
Figure 3.
Mode of action of SGLT2is and beneficial effects. eGFR, estimated glomerular filtration rate.
With emerging evidence, SGLT2is have transformed the management of CKD in patients with and without T2DM. The primary mechanism by which SGLT2is are thought to be nephroprotective is through increasing distal sodium delivery and inhibiting tubuloglomerular feedback resulting in afferent arteriole vasoconstriction and reduction in intraglomerular pressure. Current indications for SGLT2is include T2DM, HF, and CKD (Table 1).61,62
Table 1.
Recommendations for prescribing SGLT2i therapy in CKD
| Indications |
|
| Uncertain indications |
|
| Key practice points: |
|
ACEi, angiotensin-converting enzyme inhibitors; ASCVD, atherosclerotic cardiovascular disease; ARB, angiotensin receptor blockers; CKD, chronic kidney disease; DKD, diabetic kidney disease; DPP-4i, dipeptidyl peptidase-4 inhibitors; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycosylated hemoglobin; LADA, late onset diabetes of adulthood; RAAS, renin-angiotensin aldosterone system; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylureas; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UACR, urine albumin-to-creatinine ratio.
Nondiabetic kidney disease includes ischemic nephropathy, IgA nephropathy, FSGS, chronic pyelonephritis, chronic interstitial nephritis.
Carriazo S, Ortiz A. Stopping kidney protection in the elderly following acute kidney injury: think mortality. Clinical Kidney Journal. 2022 Jun;15(6):1037; Meraz-Muñoz AY, Weinstein J, Wald R. eGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney360. 2021 Jun 24;2(6):1042–7.
Zhuo M, Paik JM, Wexler DJ, Bonventre JV, Kim SC, Patorno E. SGLT2 Inhibitors and the Risk of Acute Kidney Injury in Older Adults with Type 2 Diabetes. Am J Kidney Dis. 2022 Jun;79(6):858-867.e1. doi:10.1053/j.ajkd.2021.09.015.
Summary of Efficacy of SGLT2is
Most CV outcome trials on SGLT2is were not specifically intended to evaluate kidney outcomes. However, they analyzed them as secondary end points; and therefore, were underpowered to validate renoprotective benefits of SGLT2is. Despite insufficient power and a relatively low percentage of patients with advanced-stage CKD in these trials,63 most CV outcome trials demonstrated the renoprotective efficacy of SGLT2is in patients with T2DM. Favorable kidney outcomes from the CV outcome trials paved the way for subsequent clinical studies evaluating the effect of SGLT2is on primary kidney-specific end points in patients with CKD, including the recent landmark EMPA-KIDNEY trial that included a heterogeneous patient cohort with substantial representation of patients with nondiabetic kidney diseases (UACR <30 mg/g; eGFR ≤20 ml/min per 1.73 m2) (Table 264, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75).
Table 2.
Cardiovascular and kidney outcomes with SGLT2is in pivotal randomized clinical trials
| Trial Name | Key eligibility criteria | Sample size (N) | African Race N (%) |
Intervention | Median follow-up period (Years) | CV outcomes | Kidney endpoints | Outcomes |
|---|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME (2015, 2016)64,65 (NCT01131676) |
HbA1c level: 7.0 to 9.0% Established CV disease eGFR: ≥30 ml/min per 1.73m2 |
7020 | Small sample size reported as limitation of the study | Empagliflozin (1:1:1) (either 10 mg or 25 mg or placebo once daily) |
3.1 | CV death, MI, stroke HR, 0.86; 95.02% CI, (0.74–0.99); P = 0.04 (for superiority) |
Worsening of nephropathy | Empagliflozin vs placebo 12.7% vs. 18.8%; HR, 0.61; 95% CI, (0.53–0.70); P < 0.001 |
| Hospitalization for HF HR, 0.65; 95% CI, (0.50–0.85); P = 0.002 |
Doubling of the serum creatinine level accompanied by eGFR of ≤45 ml/min per 1.73 m2 | 1.5% vs. 2.6%; HR, 0.56; 95% CI, (0.39–0.79); P < 0.001 |
||||||
| Initiation of KRT | 0.3% vs. 0.6%; HR, 0.45; 95% CI, (0.21–0.97); P = 0.04 |
|||||||
| Progression to A3 albuminuria | 11.2% vs. 16.2%; HR, 0.62; 95% CI, (0.54–0.72); P < 0.001 |
|||||||
| Rate of incident albuminuria (in patients with normal albumin at baseline) | 51.5% vs. 51.2%; HR, 0.95; 95% CI, (0.87–1.04); P = 0.25 |
|||||||
| Death from kidney cause | Empagliflozin: 0.1% | |||||||
| CANVAS and CANVAS-R (2017)66 (CANVAS: NCT01032629; CANVAS-R: NCT01989754) | T2DM 30 years or older with a prior history of symptomatic ASCVD or 50 years or older with at least 2 CV risk factors eGFR ≥30 ml/min per 1.73 m2 |
10,142 (CANVAS: 4330, CANVAS-R: 5812) |
176 (3) | CANVAS (1:1:1) – canagliflozin (300 mg), canagliflozin (100 mg) or matching placebo CANVAS-R (1:1) – canagliflozin: 100 mg with an optional increase to 300 mg or matched placebo |
2.4 | CV death, MI, stroke Canagliflozin vs. placebo 26.9 vs. 31.5 participants per 1000 patient-years HR, 0.86; 95% CI, (0.75–0.97); P < 0.001 (for non-inferiority); P = 0.02 (for superiority) |
Progression of albuminuria (participants per 1000 patient-years) | 89.4 vs. 128.7 HR, 0.73; 95% CI, (0.67–0.79) |
| Hospitalization for HF 5.5 vs. 8.7; HR, 0.67; 95% CI, (0.52–0.87) |
Regression of albuminuria (participants per 1000 patient-years) | 293.4 vs. 187.5; HR, 1.70; 95% CI, (1.51–1.91) |
||||||
| Progression to A3 albuminuria (participants per 1000 patient-years) | 89.4 vs 128.7; HR, 0.73; 95% CI (0.67–0.79) |
|||||||
| Composite outcome of sustained 40% reduction in eGFR, need for KRT, or death from kidney causes (participants per 1000 patient-years) | 5.5 vs. 9.0; HR, 0.60; 95% CI (0.47–0.77) |
|||||||
| CREDENCE (2019)67 (NCT02065791) |
T2DM patients with CKD eGFR: 30 to <90 ml/min per 1.73 m2 UACR >300 to 5000 mg/g HbA1c level: 6.5% to 12.0% |
4401 | 112 (5.1) | Canagliflozin 100 mg once daily vs. placebo | 2.62 | CV death, MI, stroke HR, 0.80; 95% CI, (0.67–0.95); P = 0.01 |
Relative risk of composite of KF, a doubling of the serum creatinine, or death from kidney or CV causes (participants per 1000 patient-years) | 43.2 vs. 61.2 HR, 0.70; 95% CI, (0.59–0.82); P = 0.00001 |
| Hospitalization for HF HR, 0.61; 95% CI, (0.47–0.80); P < 0.001 |
Kidney-specific composite of KF, a doubling of the serum creatinine level, or death from kidney causes | HR, 0.66; 95% CI, (0.53–0.81); P < 0.001 |
||||||
| Relative risk of KF | HR, 0.68; 95% CI, (0.54–0.86); P = 0.002 |
|||||||
| EMPEROR-Reduced (2020)68 (NCT03057977) |
Chronic HFrEF | 3730 | 123 (6.6) | Empagliflozin 10 mg once daily vs. placebo | 1.3 | CV death or hospitalization for HF 19.4% vs. 24.7% (empagliflozin vs. placebo) HR, 0.75; 95% CI, (0.65–0.86); P < 0.001 |
Annual rate of decline in eGFR | Empagliflozin vs. placebo −0.55 vs. −2.28 ml/min/1.73 m2; P < 0.001 |
| DAPA-CKD (2020)69 (NCT03036150) |
Patients of CKD with or without T2DM eGFR: 25 to 75 ml/min per 1.73 m2 UACR: 200 to 5000 mg/g |
4304 | 104 (4.8) | Dapagliflozin 10 mg vs. placebo once daily | 2.4 | CV death or hospitalization for HF HR, 0.71; 95% CI, (0.55–0.92); P = 0.009 |
Occurrence of the composite of a sustained decline in the eGFR of at least 50%, KF, or death from kidney or CV related causes | Dapagliflozin vs. placebo 9.2% vs. 14.5%; HR, 0.61; 95% CI, (0.51–0.72); P < 0.001 |
| Risk of composite of a sustained decline in the eGFR of at least 50%, KF, or death from kidney-related causes | HR, 0.56; 95% CI, (0.45–0.68); P < 0.001 |
|||||||
| DECLARE-TIMI 58 (2019, 2021)70,71 (NCT01730534) |
Patients with T2DM Established ASCVD or multiple risk factors for ASCVD HbA1c level: (6.5% to 12.0%) Creatinine clearance of ≥60 ml/min |
17,160 | Not mentioned | Dapagliflozin 10 mg once daily vs. matched placebo (1:1) | 4.2 | CV death or hospitalization for HF (among patients with multiple risk factors) HR, 0.84, 95% CI, (0.67–1.04) |
Cardiorenal composite outcome | HR, 0.76: 95% CI, (0.67 to 0.87); P < 0.0001 |
| Kidney-specific outcome | HR, 0.53; 95% CI, (0.43–0.66); P < 0.0001 |
|||||||
| KF or death from kidney causes | HR, 0.41; 95% CI, (0.20–0.82); P = 0.012 |
|||||||
| Sustained decline in eGFR by at least 40% to less than 60 ml/min per 1.73 m2 | HR, 0.54; 95% CI, (0.43–0.67); P < 0.0001 |
|||||||
| eGFR | Dapagliflozin group had higher eGFR compared to placebo P < 0.001 |
|||||||
| UACR | Dapagliflozin group had lower UACR compared to placebo P < 0.001 |
|||||||
| VERTIS CV (2020, 2021)72,73 (NCT01986881) |
T2DM Established ASCVD eGFR ≥30 ml/min per 1.73 m2 |
8246 | 166 (3) | Ertugliflozin 5 mg vs. ertugliflozin 15 mg vs. matched placebo once daily (1:1:1) | 3 | Composite of death from CV causes, MI, or stroke HR, 0.97; 95.6% CI, (0.85–1.11); P < 0.001 (for non-inferiority) |
Composite kidney outcome event of death from kidney causes, KRT, or doubling of the serum creatinine level from baseline | HR, 0.81; 95.8% CI, (0.63–1.04) |
| Death from CV causes or hospitalization for HF 8.1% vs. 9.1% (ertugliflozin vs. placebo) HR, 0.88; 95.8% CI, (0.75–1.03); P = 0.11 (for superiority) |
Composite kidney outcome of sustained 40% reduction from baseline in eGFR, chronic kidney dialysis/transplant or kidney death (events per 1000 person-years) | Ertugliflozin vs. placebo 6.0% vs. 9.0%; HR, 0.66; 95% CI, (0.50–0.88) |
||||||
| Death from CV causes HR, 0.92; 95.8% CI, (0.77–0.11) |
Change in eGFR (relative to baseline) | 2.6 ml/min per 1.73 m2 | ||||||
| change in UACR (relative to baseline) | −16.2% | |||||||
| SCORED (2021)74 (NCT03315143) |
Patients with T2DM and CKD, with or without albuminuria HbA1c level: ≥7%; eGFR: 25 to 60 ml/min per 1.73 m2 Risk for CV disease |
10,584 | 176 (3.3) | Sotagliflozin 200 mg to 400 mg once daily vs. matched placebo (1:1) |
1.3 | CV death, hospitalizations for HF, and urgent visits for HF 5.6 vs. 7.5 (no. of events/100 patient-years) HR, 0.74; 95% CI, (0.63–0.88); P < 0.001 |
First occurrence of sustained decline in eGFR ≥50% from baseline for at least 30 days, long-term dialysis, KRT, or sustained eGFR <15 ml/min per 1.73 m2 for ≥ 30 days | Sotagliflozin vs. placebo 0.5% vs. 0.7%; HR, 0.71; 95% CI, (0.46–1.08) |
| EMPA-KIDNEY (2023)75 (NCT03594110) |
Patients with CKD eGFR: ≥20 to <45 ml/min per 1.73 m2 or ≥45 to <90 ml/min per 1.73 m2 UACR: ≥200 mg/g |
6609 | 128 (4) | Empagliflozin 10 mg daily vs. matched placebo once daily (1:1) | 2 | CV death, hospitalization for HF 4.0% vs. 4.6% (Empagliflozin vs. placebo) |
Progression of kidney disease (KF, sustained decrease in eGFR to <10 ml/min per 1.73 m2, stained decrease in eGFR of ≥40% from baseline, or kidney death) or death from CV events | Empagliflozin group vs placebo 13.1% vs. 16.9%; HR, 0.72; 95% CI, (0.64–0.82); P < 0.001 |
ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; KF, kidney failure; KRT, kidney replacement therapy; MI, myocardial infarction; SGLT2i, sodium-glucose cotransporter-2 inhibitor; T2DM, type 2 diabetes mellitus; UACR, urine albumin-to-creatinine ratio.
Real-World Kidney Outcomes With SGLT2is
Consistent with the results from major clinical trials and CV outcome trials, several real-world effectiveness studies have generated evidence in favor of cardiorenal protective benefits of SGLT2is in patients with T2DM followed-up with under routine clinical care.76,77 In a real-world multinational CVD-REAL 3 observational cohort study involving 65,231 patients with T2DM who received propensity-matched treatment with SGLT2is and other glucose-lowering agents, SGLT2i treatment reduced the annual rate of eGFR decline by 1.53 ml/min per 1.73 m2 (95% CI, 1.34–1.72; P < 0.0001). During the mean follow-up duration of 14.9 months, patients treated with SGLT2is had a lower risk of composite kidney events, compared to other antidiabetic medicines (hazard ratio [HR], 0.49; 95% CI, 0.35–0.67; P < 0.0001).78 Similar findings emerged from another observational study based on the Japanese CKD registry with lower risk of occurrence of composite kidney outcome (50% eGFR decline and KF), regardless of the rate of eGFR decline or presence of proteinuria at baseline.76 Further, the antiproteinuric effect of SGLT2is was first confirmed by the DARWIN-T2D study, which reported a noticeable decline in median urine albumin excretion rate following 6 months of treatment with dapagliflozin, compared to no change in urine albumin excretion rate with the comparator drugs.79
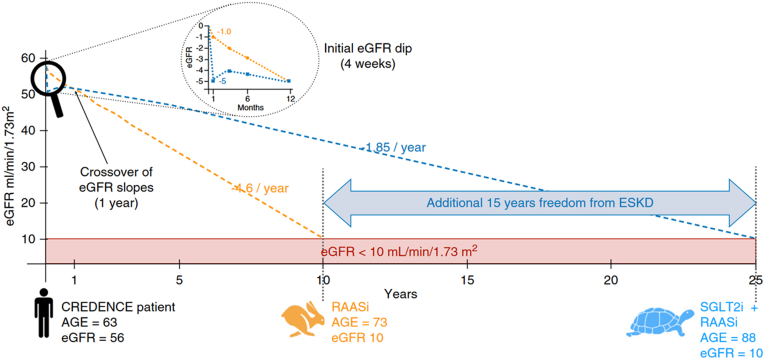
SGLT2is have been shown to prevent decline in kidney function through reduction in glomerular hypertension independent of their effect on glycemic control.61,80 A real-world study analyzed kidney outcomes in 4446 patients with diabetic kidney disease treated with SGLT2is compared to standard anti-diabetic and renoprotective medications. In this study, patients prescribed SGLT2is showed a significant reduction in CKD progression (HR, 0.60; 95% CI, 0.49–0.74) and risk of developing KF (HR, 0.33; 95% CI, 0.17–0.65), with more pronounced beneficial effects seen in moderate to advanced CKD stages.81 A theoretical extrapolation based on CREDENCE trial data is that a hypothetical trial participant aged 63 years with a baseline eGFR of 56 ml/min per 1.73 m2 is likely to develop KF in the next 10 years, with an annual rate of eGFR decline of 4.6 ml/min/year with only RAAS blockade. However, adding SGLT2is to the ongoing RAAS blockers would reduce the rate of eGFR decline to only 1.85 ml/min/year, thus further delaying the risk of developing KF by 15 years, even after adjusting for the initial dipping of eGFR (Figure 4).80 Long-term results from the DAPA-CKD trial extrapolated over a 10-year period found that patients receiving dapagliflozin spent a longer duration in CKD stages 1 to 3 (0.65 [95% CI, 0.41–0.90] years per patient) and shorter period in stages 4 to 5 (−0.23 [95% CI, 0.45–0.00] years per patient) compared with those on placebo. In addition, dapagliflozin treatment prevented 83 deaths and initiation of kidney replacement therapy in 51 cases per 1000 patients over 10 years with a reduction in predicted rates of hospitalizations due to HF and instances of sudden decline in kidney function by 19 and 39 estimated events per 1000 patients, respectively.82 The EMPA-KIDNEY trial included adults with or without T2DM with eGFR of 20 to 45 ml/min per 1.73 m2, regardless of albuminuria or eGFR of 45 to 90 ml/min per 1.73 m2 with UACR ≥200 mg/g on maximally-tolerated RAAS blockade.61,83,84 Unlike the DAPA-CKD trial, the EMPA-KIDNEY encompassed a larger representation of patients within the G2A2 CKD subgroup, thereby extending the SGLT2i intervention to patients with lower risk and higher eGFR.61,83,84 Notably, EMPA-KIDNEY demonstrated lower utilization of RAAS blockade and included a more substantial representation of nondiabetic kidney diseases, encompassing over 1600 participants with glomerular disease, more than 1400 with hypertensive kidney disease, over 450 with tubulointerstitial disease, and more than 600 with an unknown cause.83 The EMPA-KIDNEY trial was stopped early in March 2022 for efficacy, suggesting that patients with CKD without albuminuria also benefitted from SGLT2i, thereby expanding the population eligible for SGLT2i therapy.61,83,84
Figure 4.
Delayed risk of KF with the addition of SGLT2is to RAAS blockers. Reproduced from Meraz-Muñoz AY, Weinstein J, Wald R. eGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney360. 2021;2(6):1042-1047. doi:10.34067/KID.0001172021.80 eGFR, estimated glomerular filtration rate; RAASi, renin-angiotensin aldosterone system inhibitors.
Using the data from CREDENCE and FIDELIO trials, a recent study by Heerspink et al.85 revealed that combined treatment with SGLT2is and mineralocorticoid-receptor antagonists in patients with T2DM and CKD could prolong the event-free survival up to 16.7 years at the age of 50 years, compared with 10 years using only RAAS blockers. These trial data corroborated findings from the CRIC trial that showed an incremental gain in event-free survival of 6.3 years (95% CI, 5.2–7.3) on combined treatment with SGLT2i and mineralocorticoid-receptor antagonists.86
Cost-Effectiveness of SGLT2is
SGLT2is have recently been added to the World Health Organization's Model Essential Medicines List that targets prices to achieve common thresholds for cost-effectiveness or cost-savings achievable with nominal price reductions. An extensive review conducted recently that aimed to estimate price targets to guide negotiations for inclusion in national formularies after the addition of SGLT2is to World Health Organization's Essential Medicines List found that they would need to have a median price of $224 per person per year (a 17·4% cost reduction; IQR $138–359, population-weighted across countries; mean price $257).87 There is currently limited data on cost analysis of SGLT2is on the African continent due to reasons ranging from a delayed introduction of the agents in Africa and limiting costs to inequitable access. Despite the benefit of SGLT2is on primary and secondary prevention of complications and risk factors associated with CKD, the health economic impact of SGLT2is especially in low-and-middle income countries remains unclear. Studies conducted thus far on the socioeconomic impact of initiating with an SGLT2i have shown decreased medical costs and increased the quality-adjusted life year compared with conventional treatment establishing the economic benefit.88 Another significant concern is the availability of resources and copayments within public healthcare systems in Africa, resulting in continued endorsement and listing of older therapies rather than funding newer medications such as SGLT2is.
Use of SGLT2i in Pediatric Population
Although safety and efficacy data of SGLT2is in children with CKD is limited, they are generally well-tolerated and not associated with any unexpected or clinically significant safety findings in the pediatric population with T2DM in the studies conducted so far.89,90 They are proven effective in children with inherited proteinuric CKD with an eGFR >60 ml/min per 1.73 m2.91,92 However further studies in larger populations with long term follow-up are necessary.
Important Practical Considerations for Prescribing SGLT2i Therapy in CKD
Initial Decline in eGFR After SGLT2i Initiation
SGLT2i initiation induces a reduction in eGFR (∼3 to 5 ml/min per 1.73 m2) during the first 2 to 4 weeks of treatment, which is short-lived and starts normalizing by 12 weeks, followed by an attenuation of the slope of eGFR decline.64,67,93,94 This effect is mainly attributed to positive hemodynamic changes and correlated with long-term preservation of kidney function. Evidence from randomized clinical trials suggests that SGLT2i users have a slower annual rate of eGFR decline, compared to the steady decline observed with nonusers, conferring long-term renoprotective benefits (Table 3).64,67,72
Table 3.
Mitigation strategies for adverse events associated with the use of SGLT2is
| Adverse risks | Mitigation strategies |
|---|---|
| Genital infections |
|
| UTI |
|
| DKA |
|
| Volume depletion |
|
| AKI |
|
| Amputations |
|
| Fractures |
|
AKI, acute kidney injury; CKD, chronic kidney disease; DKA, diabetic ketoacidosis; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; SGLT2i, sodium-glucose cotransporter-2 inhibitor; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UTI, urinary tract infection.
Risk of Hypoglycemia
Reduced kidney function may increase the risk of hypoglycemia.95 The glucose-lowering effect of SGLT2is is insulin-independent; their use is associated with a lesser risk of hypoglycemia in patients with CKD and T2DM, compared to other antihyperglycemic agents (sulfonylureas).96 Consistent with these findings, DAPA-CKD, CREDENCE, and EMPA-KIDNEY trials showed no increased risk of hypoglycemia in patients with CKD and T2DM.67,69,75 In the context of dose adjustment of concomitant antidiabetic medications, clinicians must consider that the glucose-lowering efficacy of SGLT2is is reduced at lower eGFR levels (below 30 ml/min per 1.73 m2).97
Risk of Hyperkalemia
Use of RAAS blockers are known to increase the risk of hyperkalemia leading to discontinuation.98 Data from the post hoc analysis of the CREDENCE trial showed that treatment of patients with T2DM and CKD with canagliflozin reduced the incidence of hyperkalemia or initiation of potassium binders compared to placebo (32.7 vs. 41.9 participants per 1000 patient-years; HR, 0.78; 95% CI, 0.64–0.95; P = 0.014) with no effect on the risk of hypokalemia (HR, 0.92; 95% CI, 0.71–1.20; P = 0.53).99 A subsequent meta-analysis involving 6 randomized double-blind trials evaluated the effects of SGLT2i on serum potassium levels in patients with T2DM at high CV risk or with underlying CKD. The findings revealed a reduction in the overall risk of serious hyperkalemia (HR, 0.84; 95% CI, 0.76–0.93; Pheterogeneity = 0.71) with no variations in risk observed across different levels of baseline kidney function, history of HF, and utilization of mineralocorticoid-receptor antagonists and diuretic agents. Furthermore, the use of SGLT2is did not elevate the risk of inducing hypokalemia (HR, 1.04; 95% CI, 0.94–1.15; Pheterogeneity = 0.42).100 These findings align with a meta-analysis of the CANVAS Program, indicating that the use of canagliflozin had no significant impact on serum potassium levels across the general population, regardless of dosage, RAAS inhibitors use, or baseline eGFR levels. Moreover, the frequency of hyperkalemia-related adverse events were similar with canagliflozin and the placebo use.101 In addition, a cross-over, randomized clinical trial investigating the effects of dapagliflozin and the selective mineralocorticoid-receptor antagonists eplerenone in patients with T2DM and CKD, both individually and in combination, showed a higher incidence of hyperkalemia in patients receiving eplerenone alone (n = 8; 17.4%) compared with dapagliflozin and eplerenone combination therapy. Therefore, SGLT2is may present a viable option for patients who are unable to tolerate RAAS blockers because of hyperkalemia.
Patients With Stage 4 CKD
In a prespecified analysis of the DAPA-CKD trial that included 624 patients with stage 4 CKD at baseline (eGFR: 25–30 ml/min per 1.73 m2), the use of dapagliflozin resulted in 27% reduction in primary composite efficacy endpoint (≥50% sustained decline in eGFR, KF, or death due to kidney or CV causes) and significant decrease of eGFR slope decline over time (2.15 ml/min per 1.73 m2 with dapagliflozin vs. 3.38 ml/min per 1.73 m2 with placebo). These findings were in agreement with those reported for the overall dataset comprising a larger population with stages 2 or 3 of CKD. In addition, no increase in the rate of adverse events was observed for this subpopulation. SGLT2i treatment was continued even after eGFR declined to <15 ml/min per 1.73 m2.102 Consistent with these results, the EMPEROR-reduced trial demonstrated similar beneficial effects of empagliflozin on CV and kidney end points across all eGFR categories, extending even in patients with eGFR <30 ml/min per 1.73 m2.68 Therefore, SGLT2is comprise a beneficial addition to the therapeutic armamentarium for patients with CKD, particularly for earlier stages although studies have also shown benefits in case of late stage CKD.103,104
Chronic Glomerulonephritis
Results from a subgroup analysis of DAPA-CKD trial participants with focal segmental glomerulosclerosis105 and IgA nephropathy103 revealed positive outcomes in terms of substantial reduction in annual mean rate of chronic decline of eGFR with dapagliflozin, compared with placebo.
In 104 patients with biopsy-confirmed focal segmental glomerulosclerosis, dapagliflozin reduced the rate of primary composite kidney outcome (sustained eGFR decline ≥50%, KF, or kidney or CV death) compared with placebo (HR, 0.62; 95% CI, 0.17–2.17). The annual rate of eGFR decline was found to be slower in the dapagliflozin group (-1.9 ml/min per 1.73 m2; 95% CI, -3.0 to -0.9) versus placebo (-4.0 ml/min per 1.73 m2; 95% CI, -4.9 to -3.0) resulting in a between-group difference of 2.0 ml/min per 1.73 m2/year (95% CI, 0.6–3.5).105
Among 270 patients with IgA nephropathy, only 6 patients treated with (4%) dapagliflozin reached the same primary composite kidney outcome compared to 20 (15%) in the placebo arm (HR, 0.29; 95% CI, 0.12–0.73) during the median follow-up period of 2.1 years. In addition, the mean rate of eGFR decline with dapagliflozin was −3.5 ml/min per 1.73 m2/year relative to −4.7 ml/min per 1.73 m2/year with placebo. Furthermore, dapagliflozin reduced the UACR by 26% relative to placebo.103 Results from subgroup analysis from the EMPA-CKD trial, including 25% of patients with non-diabetic glomerulonephritis, are awaited.75
Kidney Transplant Recipients
Although there is a scarcity of published evidence, SGLT2i therapy in patients with kidney transplants demonstrated a modest effect on improving glycemic control, and body weight with reassuring safety data, in particular on the risk of urinary tract infection (UTI). There is reported evidence of a physiologic dip in eGFR consistent with an appropriate hemodynamic response and reduction in hyperfiltration, which remains intact in kidney transplant recipients and is likely to translate to long-term renoprotective benefit.106 A very recent retrospective cohort study including 226 kidney transplant SGLT2i users showed a significant reduction in the risk of the composite primary outcome of all-cause mortality, graft loss, and doubling of creatinine in diabetic kidney transplant patients than the control group in the multivariate and propensity score-matched models (adjusted HR, 0.43; 95% CI, 0.24–0.78; P = 0.006 and adjusted HR, 0.45; 95% CI, 0.24–0.85; P = 0.013, respectively).107
Critical Barriers in Effective CKD Care and Optimal Initiation of SGLT2i in Africa
Multiple barriers prove to be hindrances in the implementation of effective screening programs and management of CKD in the African region. Social risk factors, such as limited financial resources and low health literacy are significant patient-level barriers. There are no CKD registries or real-world data from the region. Unavailability of educational materials especially in the rural areas; lack of regional data leading to application of western guidelines in a population that is markedly different in racial, ethnic, and sociocultural profile; inadequate medical reimbursements and insurance schemes; and suboptimal patient adherence to follow-up are major challenges in CKD care pathways.
Despite the clinical practice guideline recommendations and high-quality published evidence supporting the cardiorenal protective effects of SGLT2is, their utilization remains dismally low in real-world clinical scenarios. As is the case with several novel therapies that conventionally take an average of 17 years from research to implementation in clinical practice, SGLT2is are also faced with several barriers to effective adoption to practice.108 Limited insurance coverage, lack of knowledge especially regarding side effects, poor accessibility, high out-of-pocket expenditure, and prescriber inertia were cited by the experts as potential barriers to adopting SGLT2i therapy in the African setting. Changing clinical practice and overcoming “prescriber inertia” requires comprehensive coordination between patients, physicians, and healthcare systems through a targeted approach.109 Despite the fact that patients of African ancestry are genetically predisposed to accelerated CKD progression, most of the SGLT2i trials published to date only enrolled few patients with black ethnicity. The distinct effects of SGLT2i in black and in African populations if any have not been researched. Given the overwhelming racial disparity for CKD risk and progression in black patients, the key experts recommended undertaking future randomized clinical trials to validate clinical outcomes with SGLT2is in African populations.
From 2015 to 2019, despite an increasing trend for SGLT2i use for T2DM treatment (3.8% to 11.9%), their overall use was relatively lower among patients with atherosclerotic CVD, HF with reduced ejection fraction, and CKD. In the same study, the multivariate analysis revealed that black ethnicity, female sex, and poor socioeconomic status were associated with a lower utilization rate of SGLT2is.110 Reportedly, only 32.9% of the eligible population with diabetic kidney disease at risk of disease progression received SGLT2i therapy in real-world clinical practice.111
Adoption of novel therapeutic agents are impacted by critical barriers that include decreased access to quality CKD care, lack of specialists familiar with the benefits of SGLT2i use, provider bias or preconceived notions of physicians that certain groups of patients may be less likely to adhere to treatment with an expensive agent, and prescription abandonment owing to socioeconomic barriers.110 Affordability and out-of-pocket costs of SGLT2is may be prohibitive resulting in prescription abandonment, especially because the cost of older therapies are more affordable.
In addition, it is well-established that overall, hypertension, CVD, HF, and CKD comorbidities each and collectively are more significant in racial or ethnic populations, and thus the potential benefits of SGLT2is may have a greater impact on cardio-renal disease in the Black population.
Racial and ethnic variations in etiology, incidence, prevalence, disease burden, and response to treatment are well-recognized with especially striking disparities between patients of African ancestry and other racial populations.112,113 Patients of African ancestry are known to harbor genetic traits that result in diminished response to key pharmacological therapies. This is further complicated by the lack of a robust assessment of the effect of treatments in Black patients because of their underrepresentation in clinical trials. The underrepresentation of clinical trial participants with African descent in landmark clinical studies conducted worldwide on SGLTis are glaring. Trial participants of Black or African ancestry accounted for approximately 5% of the total trial population (Table 2).114 In addition, more than 99% of Black patients enrolled were from the Americas with considerable differences in the socioeconomic factors, health behavior, and access to healthcare compared to those living on the African continent.
Safety Outcomes with SGLT2i
Although SGLT2is have an acceptable safety profile, some adverse events reported with their use include diabetic ketoacidosis, genital mycotic infection, UTI, risk of lower limb complications, and volume depletion. SGLT2i agents induce glucosuric effects resulting from reduced reabsorption of glucose in kidney tubules, which may favor the growth of pathogenic microbes, thereby suggesting an increased risk of genital infections and UTIs. In 2015, the US Food and Drug Administration warned of a possible risk of severe UTI with the use of SGLT2i agents115; however, subsequent findings from population-based studies and meta-analysis revealed no significant association of SGLT2is with clinically significant UTI, compared with either placebo116,117 or other glucose-lowering agents.67,78,118,119 In the CREDENCE trial, the rate of UTI with canagliflozin was not significantly different from the placebo.69 A meta-analysis of randomized clinical trials reported that SGLT2i use aggravated the risk of genital infections by 3-fold in patients with T2DM and CKD.120 Genital infections occur more frequently among female users of SGLT2is and those with a prior history of such infections.65,121 Of note, such infectious complications resulting from SGLT2i use are generally mild and can be treated by antifungal medications, without necessitating discontinuation of treatment.122
Volume depletion is another concern among SGLT2i users resulting from its diuretic effects. For patients at risk of hypovolemia, decrease in the dose of thiazide or loop diuretics is recommended by the KDIGO 2022 guidelines before initiation of SGLT2i treatment.62,123
In the CANVAS trial, allocation to SGLT2i therapy resulted in 2-fold higher risk of lower limb amputation in comparison to a placebo (6.3 vs. 3.4 events per 1000 patient-years, respectively); however, analysis of other 12 trials revealed no statistically significant association between the use of SGLT2i and instances of lower limb amputation (relative risk of 1.06; 95% CI, 0.93–1.21).124 A meta-analysis concluded that compared with non-SGLT2i users, the risks of amputation and peripheral arterial disease were slightly increased in patients with canagliflozin treatment.125 Monitoring optimal body weight and blood pressure reduction is an important risk precaution action for patients at high risk of lower limb complications during the SGLT2i treatment course. Patients should be educated on examining their foot every day for cuts, redness, swelling, sores, blisters, corns, calluses; and ensure appropriate hygiene.
Considering the hemodynamic effects of SGLT2is, their initiation may be associated with an initial slight decrease in eGFR, in the same way as RAAS blockers. However, initiation of SGLT2i does not require alteration in the frequency of CKD monitoring and the reversible decrease in eGFR does not require discontinuation of SGLT2i therapy.126 In fact, the existence of this initial alteration is correlated with better subsequent nephroprotection.127,128 Therefore, monitoring of renal function beyond what is performed in general clinical practice is not required. Nevertheless, in view of these data and the opinion of key experts, a reduction in eGFR levels by >30% warrants careful assessment of volume status and the temporary discontinuation of SGLT2i therapy.127
SGLT2i-associated diabetic ketoacidosis, although rarely reported in patients with T2DM, is usually accompanied by normal or mildly elevated blood glucose concentrations, which may remain unnoticed, leading to delayed diagnosis and potentially fatal consequences.129 Both patients with type 1 diabetes mellitus and patients T2DM using insulin are at higher risk of ketoacidosis, particularly with type 1 diabetes mellitus and latent autoimmune diabetes in adults phenotype.130 In the CREDENCE67 and EMPA-KIDNEY75 trials, the incidence of diabetic ketoacidosis events was relatively higher with SGLT2i treatment compared to placebo; however, the absolute rates were low, whereas DAPA-CKD reported no diabetic ketoacidosis cases with the use of dapagliflozin (compared to 2 cases with placebo).69
Further, the CANVAS trial identified a significant association between lower extremity amputation and bone fractures with the use of canagliflozin.66 However, the CREDENCE trial reported a similar rate of amputation and fractures in patients with CKD treated with canagliflozin and placebo.67 Likewise, the DAPA-CKD69 and EMPA-KIDNEY75 trials confirmed no increased risk of amputation or fractures with the use of dapagliflozin and empagliflozin, compared with placebo. Reassuringly, no increased risk of adverse events, including acute kidney injury, hyperkalemia, and hypoglycemia were evident with the use of SGLT2is in patients with CKD.67,69,75
Before prescribing SGLT2is, patients should be well-informed of the potential adverse events and their signs and symptoms, thereby facilitating early identification and appropriate management to prevent them from becoming a major barrier to their optimal utilization in the eligible population (Table 3).61,131,132
Prescribing SGLT2i in Patients with CKD
The KDIGO 2022 clinical practice guidelines for T2DM management in CKD recommends a lower eGFR threshold of ≥20 ml/min per 1.73 m2 for prescribing SGLT2is to improve clinical outcomes in patients with CKD and T2DM. Once initiated, SGLT2i therapy is recommended to be continued even at lower eGFR levels until the patient requires dialysis.131 Further, glucagon-like peptide 1 receptor agonist is recommended in patients who do not meet their individualized glycemic target using metformin and/or SGLT2is or when the initiation of these drugs is not possible (for eGFR <20 ml/min per 1.73 m2).131,133
In view of cardioprotective outcomes exhibited by SGLT2i therapy, the UK Kidney Association guidelines recommend their use in albuminuria patients with UACR ≥25 mg/mmol requiring modification of CV risk, with or without T2DM (with eGFR ≥25 ml/min per 1.73 m2).132 Based on the recent EMPA-KIDNEY trial involving patients with CKD irrespective of their albuminuria status, updated recommendations may expand the indications for SGLT2i use to non-albuminuric patients as well.75
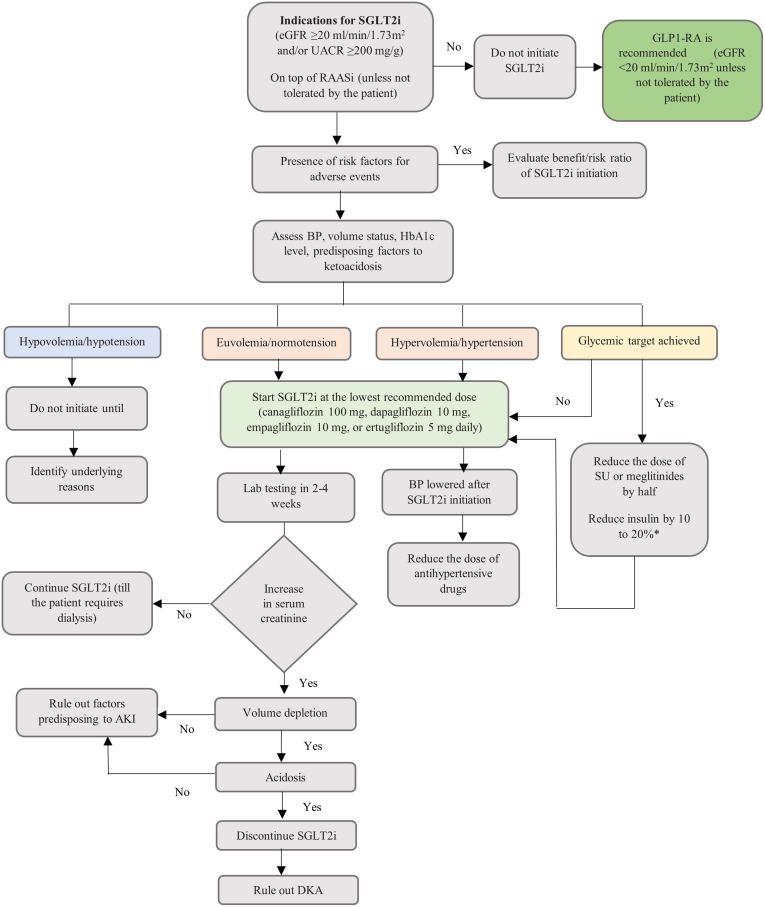
Based on the guideline recommendations for SGLT2i use in CKD management, the key experts have outlined a treatment algorithm to facilitate the safe implementation of SGLT2i therapy into clinical practice by primary care physicians (Table 1 and Figure 5).
Figure 5.
Proposed treatment algorithm for SGLT2i therapy in patients with CKD. AKI, acute kidney injury; BP, blood pressure; CKD, chronic kidney disease; DKA, diabetic ketoacidosis; eGFR, estimated glomerular filtration rate; GLP1-RA, glucagon-like peptide 1 receptor agonist; HbA1c, glycosylated Hemoglobin; RAASi, renin-angiotensin aldosterone system inhibitors; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylureas; UACR, urine albumin-to-creatinine ratio.
∗Refer to Box C for more details. Recreated with permission from Yau K, Dharia A, Alrowiyti I, et al. Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney Int Rep. 2022;7(7):1463-1476. doi:10.1016/j.ekir.2022.04.09461; permission conveyed through Copyright Clearance Center, Inc.
Recommendations from the African Association of Nephrology
Based on the above discussion and the opinion of the experts, the key points related to the CKD burden, contributing risk factors, and barriers and challenges in ensuring optimum kidney care to patients with CKD have been summarized in this manuscript. In addition, the experts have proposed recommendations on the use of SGLT2is in patients with CKD to guide nephrologists and primary care physicians in their decision-making (Table 1).
A few key limitations of this manuscript are the lack of adapting published international guidelines such as the KDIGO into the regional context through the Appraisal of Guidelines Research and Evaluation (i.e., AGREE II) instrument that would enable addressing barriers and challenges associated with identification, management, and use of evidence-based medications in CKD.
Similarly, grading of evidence was not performed as a part of this manuscript’s development and future systematic reviews in the African region on the efficacy and safety of SGLT2is should be envisaged.
Conclusion
The substantial burden of CKD in Africa highlights the need for a concerted effort toward the development of effective prevention and mitigation strategies to overcome the high health and economic burden that this condition entails in a region that has significant resource constraints. There are several barriers to optimal CKD screening, early detection, and timely intervention to delay the progression to KF. SGLT2is have emerged as a potent therapeutic agent with mounting evidence of cardiorenal protective effects with broad indications in T2DM, CKD, and CVDs. SGLT2is play a significant role in CKD management especially in delaying progression to KF in patients who have albuminuria with or without T2DM. However, further research to evaluate their safety and effectiveness in African patients is warranted to further establish these effects in a population that is ethnically and socioeconomically diverse.
Similarly, there is an imminent need for a multidisciplinary consensus guidelines involving other members in the CKD care management system such as primary care physicians, social workers, lateral specializations, nutritionists, pharmacists, and community leaders, to be developed in the African region.
Disclosure
FJ has received a speaker honorarium from Servier and AstraZeneca and travel support for meetings from Servier, Pfizer, and AstraZeneca. IIU has received research support from the International Diabetes Federation and Sanofi Pasteur and speaker honorarium from AstraZeneca and Boehringer Ingelheim. SN has received research support from Newton Fund via Medical Research Council (MRC) (United Kingdom) and MRC (South Africa). HB has received speaker honorarium from Novartis, Boehringer Ingelheim, Sanofi, and AstraZeneca. DIMW has received speaker honorarium from Pfizer and served as President for Renal Association (of Mauritius). All the other authors declared no competing interests.
Acknowledgments
The authors would like to acknowledge Parul Rishi and Dr. Sushma Jayan, of Fortrea Scientific Pvt. Ltd. (formerly Labcorp Scientific Services & Solutions Pvt. Ltd.) for medical writing support, which was done in accordance with Good Publication Practices 2022 guidelines. Medical writing assistance for this manuscript is funded by AstraZeneca International.
References
- 1.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [DOI] [PubMed]
- 2.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The top 10 causes of death. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 4.Jager K.J., Kovesdy C., Langham R., Rosenberg M., Jha V., Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34(11):1803–1805. doi: 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- 5.Jha V., Garcia-Garcia G., Iseki K., et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozzolino M., Mangano M., Stucchi A., Ciceri P., Conte F., Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl_3):iii28–iii34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnak M.J., Amann K., Bangalore S., et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(14):1823–1838. doi: 10.1016/j.jacc.2019.08.1017. [DOI] [PubMed] [Google Scholar]
- 9.Provenzano M., Coppolino G., De Nicola L., et al. Unraveling cardiovascular risk in renal patients: A new take on old tale. Front Cell Dev Biol. 2019;7:314. doi: 10.3389/fcell.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Nahas M., Barsoum R., Dirks J.H., Remuzzi G., editors. Kidney Diseases in the Developing World and Ethnic Minorities. Routledge & CRC Press; 2005. [Google Scholar]
- 12.Ogundele S.B. Chronic kidney disease in Sub-Saharan Africa. Saudi J Kidney Dis Transpl. 2018;29(5):1188–1191. doi: 10.4103/1319-2442.243945. [DOI] [PubMed] [Google Scholar]
- 13.Etheredge H., Paget G. Ethics and rationing access to dialysis in resource-limited settings: the consequences of refusing a renal transplant in the South African state sector. Dev World Bioeth. 2015;15(3):233–240. doi: 10.1111/dewb.12067. [DOI] [PubMed] [Google Scholar]
- 14.Arnold S.V., Tang F., Cooper A., et al. Global use of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. Results from DISCOVER. BMC Endocr Disord. 2022;22(1):111. doi: 10.1186/s12902-022-01026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarraya F. In: Ethnic Diversities, Hypertension and Global Cardiovascular Risk. Updates in Hypertension and Cardiovascular Protection. Modesti P., Cappuccio F., Parati G., editors. Springer; 2018. Chronic kidney disease: global burden and perspectives for Africa; pp. 105–123. [DOI] [Google Scholar]
- 16.Stanifer J.W., Jing B., Tolan S., et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 17.Oguejiofor F., Kiggundu D.S., Bello A.K., et al. International Society of Nephrology Global Kidney Health Atlas: structures, organization, and services for the management of kidney failure in Africa. Kidney Int Suppl (2011) 2021;11(2):e11–e23. doi: 10.1016/j.kisu.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaze A.D., Ilori T., Jaar B.G., Echouffo-Tcheugui J.B. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):125. doi: 10.1186/s12882-018-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oluyombo R., Banjo Oguntade H., Soje M., Obajolowo O., Karim M. Obesity and CKD in sub-Saharan Africa: a narrative review. Kidney Med. 2022;4(2) doi: 10.1016/j.xkme.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abd ElHafeez S., Bolignano D., D’Arrigo G., Dounousi E., Tripepi G., Zoccali C. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2016-015069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajayi S.O., Ekrikpo U.E., Ekanem A.M., et al. Prevalence of chronic kidney disease as a marker of hypertension target organ damage in Africa: a systematic review and meta-analysis. Int J Hypertens. 2021;2021 doi: 10.1155/2021/7243523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp J.B., Nelson G.W., Sampath K., et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour M., Djenaba B., Ahmed L.T., Moustapha C.M., Abdou N. Chronic kidney disease in sub-Saharan Africans: A study of 462 patients. Open J Nephrol. 2021;11(1):114–122. doi: 10.4236/ojneph.2021.111009. [DOI] [Google Scholar]
- 24.Ulasi, Ijoma C.K. The enormity of chronic kidney disease in Nigeria: the situation in a teaching hospital in South-East Nigeria. J Trop Med. 2010;2010 doi: 10.1155/2010/501957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Broe M.E., Gharbi M.B., Elseviers M. Maremar, prevalence of chronic kidney disease, how to avoid over-diagnosis and under-diagnosis. Nephrol Ther. 2016;12(Suppl 1):S57–S63. doi: 10.1016/j.nephro.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Muiru A.N., Charlebois E.D., Balzer L.B., et al. The epidemiology of chronic kidney disease (CKD) in rural East Africa: a population-based study. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0229649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabian J., Kalyesubula R., Mkandawire J., et al. Measurement of kidney function in Malawi, South Africa, and Uganda: a multicentre cohort study. Lancet Glob Health. 2022;10(8):e1159–e1169. doi: 10.1016/S2214-109X(22)00239-X. [DOI] [PubMed] [Google Scholar]
- 28.Ashuntantang G., Osafo C., Olowu W.A., et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5(4):e408–e417. doi: 10.1016/S2214-109X(17)30057-8. [DOI] [PubMed] [Google Scholar]
- 29.Katz I.J., Gerntholtz T., Naicker S. Africa and nephrology: the forgotten continent. Nephron Clin Pract. 2011;117(4):c320–c327. doi: 10.1159/000321524. [DOI] [PubMed] [Google Scholar]
- 30.Peralta C.A., Risch N., Lin F., et al. The Association of African Ancestry and elevated creatinine in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Nephrol. 2010;31(3):202–208. doi: 10.1159/000268955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peralta C.A., Vittinghoff E., Bansal N., et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the coronary artery risk development in young adults (CARDIA) study. Am J Kidney Dis. 2013;62(2):261–266. doi: 10.1053/j.ajkd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasembeli A.N., Duarte R., Ramsay M., Naicker S. African origins and chronic kidney disease susceptibility in the human immunodeficiency virus era. World J Nephrol. 2015;4(2):295–306. doi: 10.5527/wjn.v4.i2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills K.T., Bundy J.D., Kelly T.N., et al. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y., Zhang J.N., Zhao D., et al. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin. 2011;32(6):789–797. doi: 10.1038/aps.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen T.K., Katz R., Estrella M.M., et al. Association of APOL1 genotypes with measures of microvascular and endothelial function, and blood pressure in MESA. J Am Heart Assoc. 2020;9(17) doi: 10.1161/JAHA.120.017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luyckx V.A., Perico N., Somaschini M., et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet. 2017;390(10092):424–428. doi: 10.1016/S0140-6736(17)30576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jikamo B., Adefris M., Azale T., Alemu K. Incidence, trends and risk factors of preeclampsia in sub-Saharan Africa: a systematic review and meta-analysis. PAMJ- One Health. 2023;11:1. doi: 10.11604/pamj-oh.2023.11.1.39297. [DOI] [Google Scholar]
- 38.Zhai R., Liu Y., Tong J., et al. Empagliflozin ameliorates preeclampsia and reduces postpartum susceptibility to adriamycin in a mouse model induced by angiotensin receptor agonistic autoantibodies. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.826792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okyere P., Okyere I., Ephraim R.K.D., et al. Spectrum and clinical characteristics of renal diseases in Ghanaian adults: a 13-year retrospective study. Int J Nephrol. 2020;2020 doi: 10.1155/2020/8967258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diabetes in Africa. International Diabetes Foundation. https://www.idf.org/ournetwork/regions-members/africa/diabetes-in-africa.html
- 41.Sumaili E.K., Cohen E.P., Zinga C.V., Krzesinski J.M., Pakasa N.M., Nseka N.M. High prevalence of undiagnosed chronic kidney disease among at-risk population in Kinshasa, the Democratic Republic of Congo. BMC Nephrol. 2009;10(1):18. doi: 10.1186/1471-2369-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okpechi I.G., Ameh O.I., Bello A.K., Ronco P., Swanepoel C.R., Kengne A.P. Epidemiology of histologically proven glomerulonephritis in Africa: a systematic review and meta-analysis. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0152203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodel N.C., Hamad A., Praehauser C., et al. The epidemiology of chronic kidney disease and the association with non-communicable and communicable disorders in a population of sub-Saharan Africa. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayokunle D.S., Olusegun O.T., Ademola A., Adindu C., Olaitan R.M., Oladimeji A.A. Prevalence of chronic kidney disease in newly diagnosed patients with human immunodeficiency virus in Ilorin, Nigeria. J Bras Nefrol. 2015;37(2):177–184. doi: 10.5935/0101-2800.20150029. [DOI] [PubMed] [Google Scholar]
- 45.Anyabolu E.N., Chukwuonye, Arodiwe E., Ijoma C.K., Ulasi I. Prevalence and predictors of chronic kidney disease in newly diagnosed human immunodeficiency virus patients in Owerri, Nigeria. Indian J Nephrol. 2016;26(1):10–15. doi: 10.4103/0971-4065.156115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agaba E.I., Agaba P.A., Sirisena N.D., Anteyi E.A., Idoko J.A. Renal disease in the acquired immunodeficiency syndrome in north central Nigeria. Niger J Med. 2003;12(3):120–125. [PubMed] [Google Scholar]
- 47.Ekrikpo U.E., Kengne A.P., Akpan E.E., et al. Prevalence and correlates of chronic kidney disease (CKD) among ART-naive HIV patients in the Niger-Delta region of Nigeria. Medicine (Baltimore) 2018;97(16) doi: 10.1097/MD.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekrikpo U.E., Kengne A.P., Bello A.K., et al. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booth J.W., Hamzah L., Jose S., et al. Clinical characteristics and outcomes of HIV-associated immune complex kidney disease. Nephrol Dial Transplant. 2016;31(12):2099–2107. doi: 10.1093/ndt/gfv436. [DOI] [PubMed] [Google Scholar]
- 50.Foy M.C., Estrella M.M., Lucas G.M., et al. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin J Am Soc Nephrol. 2013;8(9):1524–1532. doi: 10.2215/CJN.10991012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Post F.A., Campbell L.J., Hamzah L., et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008;46(8):1282–1289. doi: 10.1086/529385. [DOI] [PubMed] [Google Scholar]
- 52.Kiire C.F. Hepatitis B infection in sub-Saharan Africa. The African Regional Study Group. Vaccine. 1990;8(Suppl):S107–S138. doi: 10.1016/0264-410x(90)90229-f. [DOI] [PubMed] [Google Scholar]
- 53.Spearman C.W., Afihene M., Ally R., et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol. 2017;2(12):900–909. doi: 10.1016/S2468-1253(17)30295-9. [DOI] [PubMed] [Google Scholar]
- 54.Ogunleye A., Oluwafemi T.T., Akinbodewa A.A., Daomi V.O., Adejumo O.A., Omisakin T.C. Seroprevalence of hepatitis B, C and coinfection among patients with chronic kidney disease in a Nigerian hospital. Saudi J Kidney Dis Transplant. 2020;31(3):647–654. doi: 10.4103/1319-2442.289451. [DOI] [PubMed] [Google Scholar]
- 55.Prasad N., Patel M.R. Infection-induced kidney diseases. Front Med (Lausanne) 2018;5:327. doi: 10.3389/fmed.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shlipak M.G., Tummalapalli S.L., Boulware L.E., et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34–47. doi: 10.1016/j.kint.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Brenner B.M., Cooper M.E., de Zeeuw D., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 59.Lewis E.J., Hunsicker L.G., Clarke W.R., et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to Type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 60.Chao E.C., Henry R.R. SGLT2 inhibition --a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 61.Yau K., Dharia A., Alrowiyti I., Cherney D.Z.I. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022;7(7):1463–1476. doi: 10.1016/j.ekir.2022.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127. doi: 10.1016/j.kint.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Cherney D.Z.I., Dagogo-Jack S., McGuire D.K., et al. Kidney outcomes using a sustained ≥40% decline in eGFR: A meta-analysis of SGLT2 inhibitor trials. Clin Cardiol. 2021;44(8):1139–1143. doi: 10.1002/clc.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 65.Wanner C., Inzucchi S.E., Lachin J.M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 66.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 67.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 68.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 69.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 70.Mosenzon O., Wiviott S.D., Cahn A., et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 71.Cahn A., Raz I., Leiter L.A., et al. Cardiovascular, renal, and metabolic outcomes of dapagliflozin versus placebo in a primary cardiovascular prevention cohort: analyses from DECLARE-TIMI 58. Diabetes Care. 2021;44(5):1159–1167. doi: 10.2337/dc20-2492. [DOI] [PubMed] [Google Scholar]
- 72.Cherney D.Z.I., Charbonnel B., Cosentino F., et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64(6):1256–1267. doi: 10.1007/s00125-021-05407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in Type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 74.Bhatt D.L., Szarek M., Pitt B., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 75.The EMPA-KIDNEY Collaborative Group. Herrington W.G., Staplin N., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagasu H., Yano Y., Kanegae H., et al. Kidney outcomes associated with SGLT2 inhibitors versus other glucose-lowering drugs in real-world clinical practice: the Japan chronic kidney disease database. Diabetes Care. 2021;44(11):2542–2551. doi: 10.2337/dc21-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khunti K., Kosiborod M., Kim D.J., et al. Cardiovascular outcomes with sodium–glucose cotransporter-2 inhibitors vs other glucose-lowering drugs in 13 countries across three continents: analysis of CVD-REAL data. Cardiovasc Diabetol. 2021;20(1):159. doi: 10.1186/s12933-021-01345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heerspink H.J.L., Karasik A., Thuresson M., et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]
- 79.Fadini G.P., Solini A., Manca M.L., et al. Effectiveness of dapagliflozin versus comparators on renal endpoints in the real world: a multicentre retrospective study. Diabetes Obes Metab. 2019;21(2):252–260. doi: 10.1111/dom.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meraz-Muñoz A.Y., Weinstein J., Wald R. eGFR decline after SGLT2 inhibitor initiation: the tortoise and the hare reimagined. Kidney360. 2021;2(6):1042–1047. doi: 10.34067/KID.0001172021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu A.Y.L., Low S., Yeoh E., et al. A real-world study on SGLT2 inhibitors and diabetic kidney disease progression. Clin Kidney J. 2022;15(7):1403–1414. doi: 10.1093/ckj/sfac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McEwan P., Boyce R., Sanchez J.J.G., et al. Extrapolated longer-term effects of the DAPA-CKD trial: a modelling analysis. Nephrol Dial Transplant. 2022;38(5):1260–1270. doi: 10.1093/ndt/gfac280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.EMPA-KIDNEY Collaborative Group Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022;37(7):1317–1329. doi: 10.1093/ndt/gfac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herrington W.G., Preiss D., Haynes R., et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749–761. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heerspink H.J.L., Vart P., Jongs N., et al. Estimated lifetime benefit of novel pharmacological therapies in patients with type 2 diabetes and chronic kidney disease: a joint analysis of randomized controlled clinical trials. Diabetes Obes Metab. 2023;25(11):3327–3336. doi: 10.1111/dom.15232. [DOI] [PubMed] [Google Scholar]
- 86.Feldman H.I., Appel L.J., Chertow G.M., et al. The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 87.Global Health & Population Project on Access to Care for Cardiometabolic Diseases (HPACC) Expanding access to newer medicines for people with type 2 diabetes in low-income and middle-income countries: a cost-effectiveness and price target analysis. Lancet Diabetes Endocrinol. 2021;9(12):825–836. doi: 10.1016/S2213-8587(21)00240-0. [DOI] [PubMed] [Google Scholar]
- 88.McEwan P., Darlington O., Miller R., et al. Cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: A health-economic analysis of DAPA-CKD. Clin J Am Soc Nephrol. 2022;17(12):1730–1741. doi: 10.2215/CJN.03790322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tirucherai G.S., LaCreta F., Ismat F.A., Tang W., Boulton D.W. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18(7):678–684. doi: 10.1111/dom.12638. [DOI] [PubMed] [Google Scholar]
- 90.Laffel L.M., Danne T., Klingensmith G.J., et al. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11:169–181. doi: 10.1016/S2213-8587(22)00387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J., Cui J., Fang X., et al. Efficacy and safety of dapagliflozin in children with inherited proteinuric kidney disease: a pilot study. Kidney Int Rep. 2021;7(3):638–641. doi: 10.1016/j.ekir.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grube P.M., Beckett R.D. Clinical studies of dapagliflozin in pediatric patients: a rapid review. Ann Pediatr Endocrinol Metab. 2022;27(4):265–272. doi: 10.6065/apem.2244166.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heerspink H.J.L., Jongs N., Chertow G.M., et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):743–754. doi: 10.1016/S2213-8587(21)00242-4. [DOI] [PubMed] [Google Scholar]
- 94.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 95.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao J.Z., Weinhandl E.D., Carlson A.M., St Peter W.L. Hypoglycemia risk with SGLT2 inhibitors or glucagon-like peptide 1 receptor agonists versus sulfonylureas among medicare insured adults with CKD in the United States. Kidney Med. 2022;4(8) doi: 10.1016/j.xkme.2022.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cherney D.Z.I., Cooper M.E., Tikkanen I., et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93(1):231–244. doi: 10.1016/j.kint.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 98.Oktaviono Y.H., Kusumawardhani N. Hyperkalemia associated with angiotensin converting enzyme inhibitor or angiotensin receptor blockers in chronic kidney disease. Acta Med Indones. 2020;52(1):74–79. [PubMed] [Google Scholar]
- 99.Neuen B.L., Oshima M., Perkovic V., et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42(48):4891–4901. doi: 10.1093/eurheartj/ehab497. [DOI] [PubMed] [Google Scholar]
- 100.Neuen B.L., Oshima M., Agarwal R., et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with Type 2 diabetes: A meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145(19):1460–1470. doi: 10.1161/CIRCULATIONAHA.121.057736. [DOI] [PubMed] [Google Scholar]
- 101.Weir M.R., Slee A., Sun T., et al. Effects of canagliflozin on serum potassium in the CANagliflozin cardioVascular Assessment Study (CANVAS) Program. Clin Kidney J. 2021;14(5):1396–1402. doi: 10.1093/ckj/sfaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chertow G.M., Vart P., Jongs N., et al. Effects of dapagliflozin in Stage 4 chronic kidney disease. J Am Soc Nephrol. 2021;32(9):2352–2361. doi: 10.1681/ASN.2021020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wheeler D.C., Toto R.D., Stefánsson B.V., et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215–224. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 104.Heerspink H.J.L., Cherney D., Postmus D., et al. A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022;101(1):174–184. doi: 10.1016/j.kint.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 105.Wheeler D.C., Jongs N., Stefansson B.V., et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2022;37(9):1647–1656. doi: 10.1093/ndt/gfab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ujjawal A., Schreiber B., Verma A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: what is the evidence? Ther Adv Endocrinol Metab. 2022;13 doi: 10.1177/20420188221090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lim J.H., Kwon S., Jeon Y., et al. The efficacy and safety of SGLT2 inhibitor in diabetic kidney transplant recipients. Transplantation. 2022;106(9):e404–e412. doi: 10.1097/TP.0000000000004228. [DOI] [PubMed] [Google Scholar]
- 108.Committee on Quality of Health Care in America. Crossing the Quality Chasm: (317382004–001) https://www.ncbi.nlm.nih.gov/books/NBK222274/
- 109.Nishi L., Ghossein C., Srivastava A. Increasing sodium-glucose cotransporter 2 inhibitor use in CKD: perspectives and presentation of a clinical pathway. Kidney Med. 2022;4(5) doi: 10.1016/j.xkme.2022.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eberly L.A., Yang L., Eneanya N.D., et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jeong S.J., Lee S.E., Shin D.H., Park I.B., Lee H.S., Kim K.A. Barriers to initiating SGLT2 inhibitors in diabetic kidney disease: a real-world study. BMC Nephrol. 2021;22(1):177. doi: 10.1186/s12882-021-02381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Docherty K.F., Ogunniyi M.O., Anand I.S., et al. Is A. Efficacy of dapagliflozin in Black versus White patients with heart failure and reduced ejection fraction. JACC Heart Fail. 2022;10(1):52–64. doi: 10.1016/j.jchf.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 113.Nasser S.A., Arora N., Ferdinand K.C. Addressing cardiovascular disparities in racial/ethnic populations: the blood pressure-lowering effects of SGLT2 inhibitors. Rev Cardiovasc Med. 2022;23(12):411. doi: 10.31083/j.rcm2312411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clemmer J.S., Ward T.J., Lirette S.T. Retrospective analysis of SGLT2 inhibitors in heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10(30):2010–2018. doi: 10.1002/ehf2.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. Food and drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious
- 116.Puckrin R., Saltiel M.P., Reynier P., Azoulay L., Yu O.H.Y., Filion K.B. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503–514. doi: 10.1007/s00592-018-1116-0. [DOI] [PubMed] [Google Scholar]
- 117.Liu J., Li L., Li S., et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824. doi: 10.1038/s41598-017-02733-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Donnan J.R., Grandy C.A., Chibrikov E., et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dave C.V., Schneeweiss S., Kim D., Fralick M., Tong A., Patorno E. Sodium–glucose cotransporter-2 inhibitors and the risk for severe urinary tract infections: a population-based cohort study. Ann Intern Med. 2019;171(4):248–256. doi: 10.7326/M18-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toyama T., Neuen B.L., Jun M., et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(5):1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 121.Thong K.Y., Yadagiri M., Barnes D.J., et al. Clinical risk factors predicting genital fungal infections with sodium–glucose cotransporter 2 inhibitor treatment: the ABCD nationwide dapagliflozin audit. Prim Care Diabetes. 2018;12(1):45–50. doi: 10.1016/j.pcd.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Engelhardt K., Ferguson M., Rosselli J.L. Prevention and management of genital mycotic infections in the setting of sodium-glucose cotransporter 2 inhibitors. Ann Pharmacother. 2021;55(4):543–548. doi: 10.1177/106008020951928. [DOI] [PubMed] [Google Scholar]
- 123.Tuttle K.R., Brosius F.C., 3rd, Cavender M.A., et al. SGLT2 inhibition for CKD and cardiovascular disease in Type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Diabetes. 2021;70(1):1–16. doi: 10.2337/dbi20-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nuffield Department of Population Health Renal Studies Group SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin C., Zhu X., Cai X., et al. SGLT2 inhibitors and lower limb complications: an updated meta-analysis. Cardiovasc Diabetol. 2021;20(1):91. doi: 10.1186/s12933-021-01276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. KDIGO. https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2023-CKD-Guideline-Public-Review-Draft_5-July-2023.pdf
- 127.Heerspink H.J.L., Cherney D.Z.I. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021;16(8):1278–1280. doi: 10.2215/CJN.02480221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jongs N., Chertow G.M., Greene T., et al. Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor dapagliflozin in patients with CKD. J Am Soc Nephrol. 2022;33(11):2094–2107. doi: 10.1681/ASN.2022030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nasa P., Chaudhary S., Shrivastava P.K., Singh A. Euglycemic diabetic ketoacidosis: a missed diagnosis. World J Diabetes. 2021;12(5):514–523. doi: 10.4239/wjd.v12.i5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buzzetti R., Tuomi T., Mauricio D., et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes. 2020;69(10):2037–2047. doi: 10.2337/dbi20-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Boer I.H., Khunti K., Sadusky T., et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2022;102(5):974–989. doi: 10.1016/j.kint.2022.08.012. [DOI] [PubMed] [Google Scholar]
- 132.Roddick A.J., Wonnacott A., Webb D., et al. UK kidney association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease 2023 UPDATE. BMC Nephrol. 2023;24(1):310. doi: 10.1186/s12882-023-03339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alicic R., Nicholas S.B. Diabetic Kidney Disease Back in Focus: management field guide for health care professionals in the 21st century. Mayo Clin Proc. 2022;97(10):1904–1919. doi: 10.1016/j.mayocp.2022.05.003. [DOI] [PubMed] [Google Scholar]