Abstract
Introduction
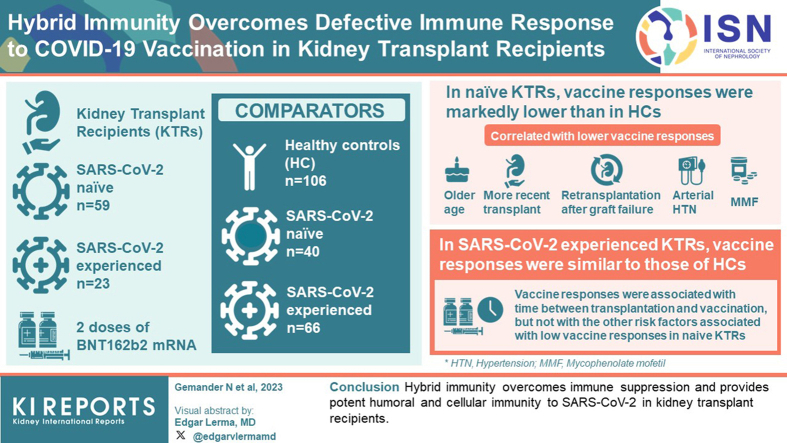
Comorbidities and immunosuppressive therapies are associated with reduced immune responses to primary COVID-19 mRNA vaccination in kidney transplant recipients (KTRs). In healthy individuals, prior SARS-COV-2 infection is associated with increased vaccine responses, a phenotype called hybrid immunity. In this study, we explored the potential influence of immune suppression on hybrid immunity in KTRs.
Methods
Eighty-two KTRs, including 59 SARS-CoV-2-naïve (naïve KTRs [N-KTRs]) and 23 SARS-CoV-2-experienced (experienced KTRs [E-KTRs]) patients, were prospectively studied and compared to 106 healthy controls (HCs), including 40 SARS-CoV-2-naïve (N-HCs) and 66 SARS-CoV-2-experienced (E-HCs) subjects. Polyfunctional antibody and T cell responses were measured following 2 doses of BNT162b2 mRNA vaccine. Associations between vaccine responses and clinical characteristics were studied by univariate and multivariate analyses.
Results
In naïve KTRs, vaccine responses were markedly lower than in HCs and were correlated with older age, more recent transplantation, kidney retransplantation after graft failure, arterial hypertension, and treatment with mycophenolate mofetil (MMF). In contrast, vaccine responses of E-KTRs were similar to those of HCs and were associated with time between transplantation and vaccination, but not with the other risk factors associated with low vaccine responses in naïve KTRs.
Conclusion
In conclusion, hybrid immunity overcomes immune suppression and provides potent humoral and cellular immunity to SARS-CoV-2 in KTRs.
Keywords: COVID-19, hybrid immunity, kidney transplantation, mRNA vaccination, nonneutralizing antibodies, systems immunology
Graphical abstract
Compared to the general population, KTRs were at much higher risk of coronavirus disease 2019 (COVID-19) related severe disease and death during the first waves of the pandemic.1,2 This higher risk has been attributed to immunosuppressive therapies required to prevent allograft rejection, a higher prevalence of comorbidities, and increased healthcare-associated exposure to the virus.3,4 KTRs also developed lower humoral and cellular immune responses to primary COVID-19 vaccination as compared to healthy individuals and have been at higher risk of breakthrough infections.5, 6, 7, 8, 9, 10, 11, 12 Reduced vaccine responses were associated with recent induction therapy, cumulative immunosuppressive treatments particularly with MMF or belatacept, and specific comorbidities such as lower kidney function.8,13, 14, 15, 16 Multiple doses of vaccines have been proposed as a mean to induce higher levels of immunity among KTRs.17, 18, 19, 20, 21 However, about 50%, 40%, and 20% of patients remained low responders even following 3, 4, and 5 vaccinations, respectively.19,20,22, 23, 24 Therefore, optimal vaccination strategies to protect this vulnerable population in the context of a pandemic remain uncertain and require further investigations.
In healthy adults, previous SARS-CoV-2 infection markedly modifies immunity induced by COVID-19 vaccination. This hybrid immunity is characterized by higher levels of neutralizing antibodies (nAbs) and antibody Fc-dependent effector functions as well as enhanced effector CD4 T cell responses as compared to COVID-19 vaccination alone.25, 26, 27, 28, 29, 30 In contrast, recent studies suggest a reduction of CD8 T cell responses to vaccination in subjects with previous SARS-CoV-2 infection.31 The basis for this differential regulation of vaccine-induced immune response components after SARS-CoV-2 infection remains incompletely understood.
We and others have previously reported that KTRs preinfected with SARS-CoV-2 infection have higher binding and nAb responses to mRNA vaccination than patients who were SARS-CoV-2-naïve before vaccination.32, 33, 34, 35 This observation suggests that the factors reducing vaccine responses in naïve KTRs may have a limited impact on the response of previously infected patients. In this study, we provide a detailed characterization of hybrid immunity in KTRs. Beyond nAbs, we explored key components of humoral immunity to COVID-19, including SARS-CoV-2 spike (S) protein-specific antibody isotypes and subclasses, as well as IgG binding to Fcγ receptors and Fc-dependent effector functions, including antibody-dependent complement deposition (ADCD) and antibody-dependent cellular phagocytosis (ADCP).36, 37, 38, 39 In addition, we assessed S protein-specific T cell responses that are a key component of COVID-19 vaccine-induced immunity in KTRs.9 We then explored how clinical factors underlying immune suppression influence vaccine-induced immunity in KTRs who were either naïve or infected with SARS-CoV-2 before vaccination.
Methods
Study Design and Participant Characteristics
The study was designed to evaluate associations between clinical characteristics and immune responses to BioNTech/Pfizer BNT162b2 mRNA (Comirnaty) vaccination in COVID-19 naïve (N) and experienced (E) KTRs and HCs. KTRs were recruited from the department of nephrology, dialysis, and transplantation of the Hôpital Erasme, Belgium; and HCs were healthcare workers recruited from 2 Belgian nursing homes. Patients transplanted with multiple organs or with active invasive cancer were excluded from the study. All participants were adults of at least 18 years of age and provided written informed consent. Participants were enrolled before COVID-19 vaccination and then received 2 doses of the BNT162b2 vaccine (30 μg) 21 days apart, according to the Belgian national vaccination program. Patients recruited in this study were also included in our previously published report.32
The ethics committee of the Hôpital Erasme, Brussels, Belgium (references P2020/284 and A2021/131) and the Belgian Federal Agency for Medicines and Health Products (FAMHP, EudraCT 2021-000-412-28) approved the monocentric prospective phase IV investigator-initiated study of the immunogenicity of the BNT161b2 vaccine (Pfizer-BioNTech) in KTRs. HCs were included from a prospective cohort study named PICOV-VAC.40,41 This latter study was approved by the Ethics Committee of the Hôpital Erasme, Brussels, Belgium (reference B4062020000134), the Federal Agency for Medicines and Health Products (2021-000401-24) and is registered on ClinicalTrials.gov (NCT04527614).
Previous SARS-CoV-2 infection status was established according to the following criteria. Participants with a previous laboratory-confirmed SARS-CoV-2 infection were considered previously infected irrespective of prevaccination serology. All other participants with a baseline antireceptor binding domain (anti-RBD) IgG level <5 binding antibody units (BAU)/ml were considered infection naïve, and those with a level >20 BAU/ml were considered previously infected. Participants with a level >5 and <20 BAU/ml were further tested with a multiplexed Luminex assay, as previously described,42 detecting IgG specific for 4 antigens, including SARS-CoV-2 RBD, spike subunit 1 (S1), spike subunit 2 (S2) and nucleocapsid. Participants with detectable IgG to ≥3 out of 4 antigens were considered previously infected.
The first vaccine dose was administered to HCs between January 21 and January 28, 2021, and to KTRs between March 2 and March 18, 2021. The second vaccine dose was administered to HCs between February 11 and February 18, 2021, and to KTRs between March 23 and April 8, 2021. Blood was collected to assess humoral and cellular immunity to SARS-CoV-2 just before the first vaccine dose (baseline, or day 0) and 4 weeks after the second dose (day 49).
Binding Antibodies
Levels of total IgG specific for the Wuhan SARS-CoV-2 RBD were measured using an enzyme-linked immunosorbent assay (Wantai SARS-CoV-2 IgG ELISA [Quantitative]; CE-marked; WS-1396; Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, China), as previously described.41,43 Levels lower than the limit of detection of 5 BAU/ml were attributed the value 2.5 BAU/ml. Detailed methods are provided in the Supplementary Methods.
Antibody Avidity
Avidity of Wuhan SARS-CoV-2 RBD-specific IgG was measured with biolayer interferometry, as previously described.44 Biolayer interferometry measurements were performed using the Fortebio HTX Octet instrument and Fortebio AR2G biosensors. Of note, IgG avidity was only calculated in KTRs and HCs who had detectable levels of RBD-binding IgG. Detailed methods are provided in the Supplementary Methods.
nAbs
Titers of antibodies neutralizing Wuhan SARS-CoV-2 were measured using a live virus neutralization assay, as previously described.45 The Reed-Muench method was used to calculate the nAb titer that reduced the number of infected wells by 50% or 90%. Titers lower than the limit of detection of 50 IU/ml for 50% neutralization titer were attributed the value of 25 IU/ml. Detailed methods are provided in Supplementary Methods.
IgG Subclasses and Fc-Dependent Functions
Levels of SARS-CoV-2-specific antibody isotypes, subclasses, FcγR-binding profiles and ADCD were measured using a 96-well-based customized multiplexed immunoassay, as previously described.42,46,47 Antigens used for multiplex assays included Wuhan SARS-CoV-2 S1 protein (Sanyou Biopharmaceuticals #PNA002), SARS-CoV-2 S2 protein (Sinobiological #40590-V08H1), SARS-CoV-2 RBD (Proteogenix #PX-COV-P046) and SARS-CoV-2 N protein (Sanyou Biopharmaceuticals #PNA006). Data were acquired with a BioPlex-200 (Bio-Rad, CA) and measured as median fluorescence intensity. Of note, systems serology analysis was done in all N-KTRs, E-KTRs, N-HCs and in a subset of 40 E-HCs. In addition, IgA, IgG2, and IgG4 were measured only in a subset of the cohort, including 14 N-KTRs, 22 E-KTRs, 10 N-HCs, and 18 E-HCs. ADCP was assessed using the human monocyte cell line THP-1 (ATCC #TIB-202), as previously described.48 Bead phagocytosis was measured by flow cytometry with an LSR Fortessa Flow Cytometer (BD), and analysis was performed using FlowJo V10.8.1. A phagocytosis score was calculated as follows: Percentage of cells that phagocytosed beads × median fluorescence intensity of bead positive cells/10,000. Detailed methods are provided in Supplementary Methods.
Cellular Immune Responses
SARS-CoV-2 S1 and S2 of Wuhan strain-specific T cell frequencies were measured in peripheral blood mononuclear cells by flow cytometry following intracellular cytokine staining (BD Fastimmune, BD-Beckton Dickinson and Company-Biosciences, San Jose, CA), as previously described,49,50 and analysis was performed using FlowJo V10.8.1. Percentages of CD4 and CD8 T cells expressing CD154 (only in CD4), interferon-γ, and IL-2 were measured. Gating strategies are shown in Supplementary Figure S1. The lower limit of quantitation was set at 0.0001% (after background subtraction). Detailed methods are provided in Supplementary Methods.
Statistical Analyses
Demographic characteristics of KTRs and HCs are presented as median (first quartile Q1 − third quartile Q3) for continuous variables and n (%) for categorical variables. The comparison of categorical variables was done using the χ2 test, or Fisher’s exact test when appropriate. Single comparisons between other metrics were done using the 2-tailed Mann-Whitney U test. Simple comparisons between groups of RBD-binding IgG, IgG avidity, nAb, subclasses and isotypes, FcγR-binding, ADCD, ADCP, CD4, and CD8 T cells responses were performed using the 2-tailed Mann-Whitney U test and multiple comparisons were done using the analysis of variance Kruskal-Wallis test with Dunn’s correction. Spearman correlation analysis was used for single continuous variate correlation analyses. Associations between immune variables (RBD IgG, IgG avidity, wild type 50% neutralization titer, S1 CD154+CD4, S1 IFNγ+CD4, and S1 IL2+CD4) and continuous clinical characteristics (age, body mass index, time between transplantation and vaccination, absolute lymphocyte count, plasmatic creatinine, estimated glomerular filtration rate, time between infection and vaccination) and categorical variables (sex, kidney retransplantation after graft failure described as transplantation rank with the number of transplantations, induction treatment, number of chronic immunosuppressive treatments, corticosteroids, MMF, azathioprine, tacrolimus, cyclosporine A, everolimus, donor-specific antibodies, arterial hypertension, diabetes, cardiovascular disease, chronic respiratory disease, chronic kidney insufficiency, noninvasive skin cancer or cancer remission, oxygen dependance, and intensive care unit hospitalization during SARS-CoV-2 infection) were explored by univariate and multivariate linear regressions. All variables with univariate P < 0.1 were included in the multivariate model and the best models after multicollinearity assumption are shown in Supplementary Methods. RBD IgG avidity, levels of RBD-binding IgG, wild type 50% neutralization titer, S1 IgG1, S2 IgG1, RBD IgG1, S1 IgG3, S2 IgG3, RBD IgG3, S1 FcγRIIa, S2 FcγRIIa, RBD FcγRIIa, S1 FcγRIIIa, S2 FcγRIIIa, RBD FcγRIIIa, S1 ADCD, S2 ADCD, RBD ADCD, RBD ADCP, S1 CD154+CD4, S1 IFNγ+CD4, S1 IL2+CD4, S1 IFNγ+CD8, S1 IL2+CD8, S2 CD154+CD4, S2 IFNγ+CD4, S2 IL2+CD4, S2 IFNγ+CD8, and S2 IL2+CD8 responses after mRNA vaccination were selected for principal component analysis (PCA). Levels of IgG2, IgG4, and IgA were not selected for PCA because of missing data. PCA was applied to reduce the immunological features to a minimal set of features to the entire cohort on the one hand, and in N-KTRs and E-KTRs separately on the other hand. R packages stats51 and ggplot252 were used to perform and visualize PCA in scaled and centered data. A 2-sided P-value less than 0.05 was considered statistically significant. Statistical analyses were done using GraphPad Prism 9.5.0 (GraphPad Software, San Diego, CA), R version 4.2.0 and Rstudio version 1.3.1073 with R version 4.2.1.51
Results
Cohorts and Participant Characteristics
Eighty-six KTRs were enrolled in the study, including 63 N-KTRs and 23 E-KTRs. Four N-KTRs were excluded because a SARS-CoV-2 infection was diagnosed between the 2 doses of COVID-19 vaccine. No SARS-CoV-2 infection was diagnosed in E-KTRs between enrolment and day 49 postvaccination. Demographic and clinical characteristics were similar between N-KTRs and E-KTRs except for age, everolimus treatment, and estimated glomerular filtration rate (Table 1). A total of 106 healthcare workers enrolled in the parallel PICOV-VAC trial were included in our analysis as HCs, with 40 N-HCs and 66 E-HCs. Detailed characteristics of N-KTRs, E-KTRs, N-HCs, and E-HCs are summarized in Supplementary Table S1. HCs were younger than KTRs. As previously described for patients with chronic kidney disease,53 most KTRs were male. All KTRs were taking immunosuppressive treatment and had a higher proportion of comorbidities as compared to HCs. SARS-CoV-2 infections among experienced participants were more commonly symptomatic among E-KTRs, who required more oxygen than E-HCs.
Table 1.
Comparison of baseline characteristics between SARS-CoV-2-naïve and SARS-CoV-2-experienced KTRs
| N (%) or Median (Q1–Q3) | N-KTRs |
E-KTRs |
P -valuei |
|---|---|---|---|
| N = 59 | N = 23 | ||
| Age, yr | 63 (54–70) | 51 (45–63) | 0.011 |
| Female sex | 27 (45.8) | 6 (26.1) | 0.167 |
| Body mass index, KG/M2 | 25.5 (21.4–29.5) | 26.0 (22.6–27.6) | 0.942 |
| Time between KT and RNA vaccination, yr | 9.7 (3.5–15.7) | 8.1 (2.9–12.2) | 0.227 |
| Transplantation rank >1 | 7 (11.9) | 4 (17.4) | 0.493 |
| Immunosuppression | |||
| Induction (BX/ATG/MUROMONAB-CD3) | 35/17/5 (61.4/29.8/8.8)a | 14/8/2 (60.9/34.8/8.7)b | 0.553 |
| CS | 45 (76.3) | 19 (82.6) | 0.744 |
| MMF | 30 (50.8) | 10 (43.5) | 0.723 |
| AZA | 15 (25.4) | 7 (30.4) | 0.855 |
| TAC | 39 (66.1) | 14 (60.9) | 0.851 |
| CyA | 8 (13.6) | 0 | 0.098 |
| EVE | 14 (23.7) | 12 (52.2) | 0.026 |
| Triple is chronic therapy | 33 (55.9) | 16 (69.6) | 0.379 |
| DSA prior vaccination | 4 (6.9)c | 1 (5)d | 1 |
| Comorbidities | |||
| Arterial hypertension | 50 (84.7) | 18 (78.3) | 0.522 |
| Diabetes | 22 (37.3) | 10 (43.5) | 0.792 |
| Cardiovascular disease | 16 (27.1) | 8 (34.8) | 0.678 |
| Chronic respiratory disease | 5 (8.5) | 0 | 0.315 |
| Chronic kidney insufficiency (EGFR <30 ml/min per 1.73 m2) | 8 (13.6) | 1 (4.4) | 0.231 |
| Cancer | 16 (27.1) | 3 (13) | 0.287 |
| Biological data | |||
| Absolute lymphocyte count,/mm3 | 1390 (770–2108)e | 1340 (1015–1970)f | 0.493 |
| EGFR, ml/min per 1.73 M2 | 48 (36–61) | 62 (43–73) | 0.051 |
| Plasmatic creatinine, mg/dl | 1.40 (1.08–1.72) | 1.17 (1.04–1.43) | 0.213 |
| SARS-CoV-2 infection | |||
| Time between SARS-CoV-2 infection and RNA vaccination, D | NA | 149 (126–335.5)g | NA |
| Asymptomatic | NA | 6 (26.1) | NA |
| Need for supplemental oxygen | NA | 3 (13.6)h | NA |
| Intensive care requirement | NA | 1 (4.5)h | NA |
ATG, antithymocyte globulin; AZA, azathioprine; BX, basiliximab; CS, corticosteroids; CyA, cyclosporine A; DSA, donor-specific antibodies; eGFR, estimated glomerular filtration rate; EVE, everolimus; IS, immunosuppressive; KT, kidney transplantation; KTR, kidney transplant recipient; MMF, mycophenolate mofetil; NA, not applicable or not available; RNA, ribonucleic acid; TAC, tacrolimus.
Continuous variables are expressed as median (Q1–Q3) and categorical variables as frequency (%).
57 values.
One immune KTR received BX and ATG.
58 values.
20 values.
44 values.
21 values.
17 values.
22 values.
Qualitative variables were compared using a Fisher’s Exact or chi-square test and quantitative variables were compared using a Mann-Whitney U test.
E-KTRs Have Higher Antibody Responses to COVID-19 mRNA Vaccination Than N-KTRs
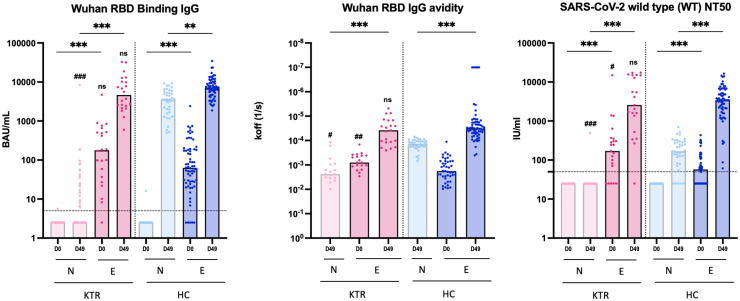
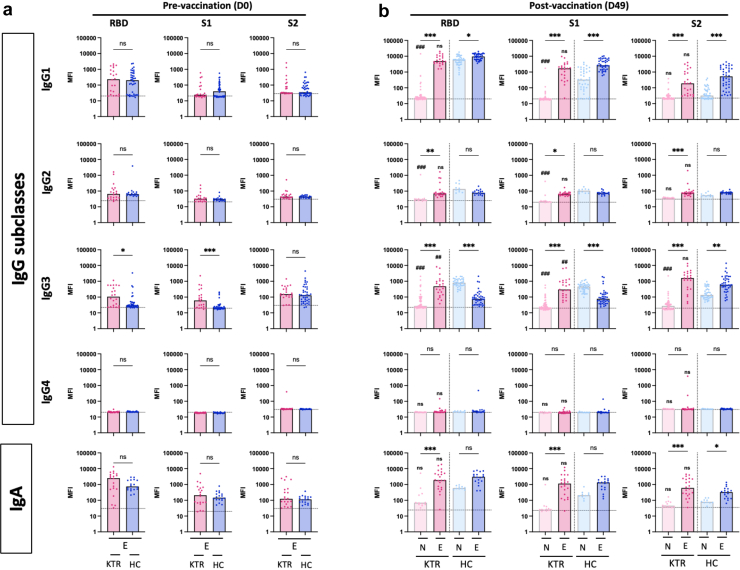
At baseline, SARS-CoV-2 specific antibodies were not detected in naïve KTRs and HCs, whereas most previously infected participants had detectable antibodies (Figure 1). Intriguingly, E-KTRs had significantly higher levels of RBD IgG avidity and higher titers of nAb (Figure 1) as well as higher levels of RBD and S1 specific IgG3 (Figure 2a) than E-HCs. Following vaccination, N-KTRs had markedly lower levels of binding and nAbs as compared to N-HCs (Figure 1 and Figure 2b), as previously described.5,6 In contrast, high antibody responses to vaccination were detected in both E-HCs and E-KTRs. On day 49, E-KTRs had markedly higher levels of SARS-CoV-2 specific IgG, IgG avidity and nAb (Figure 1) as well as IgG subclasses and IgA (Figure 2b) than N-KTRs. Antibody levels in vaccinated E-KTRs reached similar levels as those detected in E-HCs, and the higher levels of SARS-CoV-2 specific IgG3 detected at baseline in E-KTRs than in E-HCs were also detected after vaccination (Figure 2b).
Figure 1.
Binding IgG, RBD IgG avidity, and neutralizing antibody responses to SARS-CoV-2 mRNA vaccination in naïve and experienced KTRs. Serum levels of SARS-CoV-2 RBD specific binding IgG (BAU: binding antibody units), RBD specific IgG avidity (koff: dissociation rate constant) and titers of neutralizing antibodies (NT50: 50% neutralization titer) were measured before vaccination (D0) and 1 month after 2 doses of mRNA vaccine (D49) in naïve KTRs (N-KTRs, light pink), experienced KTRs (E-KTRs, dark pink), naïve HCs (N-HCs, light blue) and experienced HCs (E-HCs, dark blue). Bars indicate median values. Horizontal grid lines indicate a technical negative signal (blank). Groups were compared using the analysis of variance Kruskal-Wallis test with Dunn’s correction. For within HC or KTR comparisons, ns; aP < 0.05; bP < 0.01; cP < 0.001. For comparisons between HC and KTR, ns; dP < 0.05; eP < 0.01; fP < 0.001. HC, healthy control; KTR, kidney transplant recipient; ns, not significant; RBD, receptor binding domain.
Figure 2.
IgG Subclasses and IgA responses to SARS-CoV-2 mRNA vaccination in naïve and experienced KTRs. Serum levels of SARS-CoV-2 RBD specific, spike S1 subunit specific, and spike S2 subunit specific IgG1, IgG2, IgG3, IgG4 and IgA were measured before vaccination (D0, panel a) and 1 month after vaccination (D49, panel b) in naïve KTRs (N-KTRs, light pink), experienced KTRs (E-KTRs, dark pink), naïve HCs (N-HCs, light blue) and experienced HCs (E-HC, dark blue). Bars indicate median values. Horizontal grid lines indicate a technical negative signal (blank). Groups were compared using the analysis of variance Kruskal-Wallis test with Dunn’s correction. For within HC or KTR comparisons, aP < 0.05; bP < 0.01; cP < 0.001. For comparisons between HC and KTR, ns; dP < 0.05; eP < 0.01; fP < 0.001. HC, healthy control; KTR, kidney transplant recipient; MFI, median fluorescent intensity; ns, not significant; RBD, receptor binding domain.
E-KTRs Develop Higher IgG Fc-Dependent Effector Responses to mRNA Vaccination Than N-KTRs
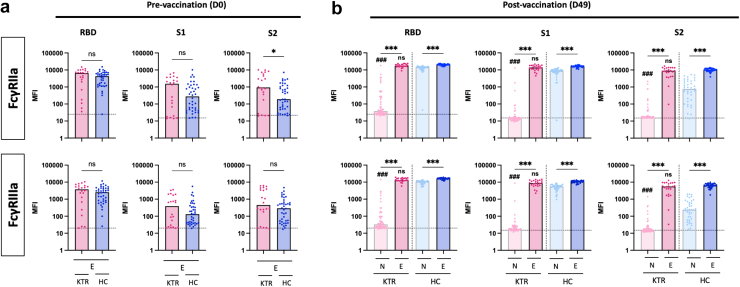
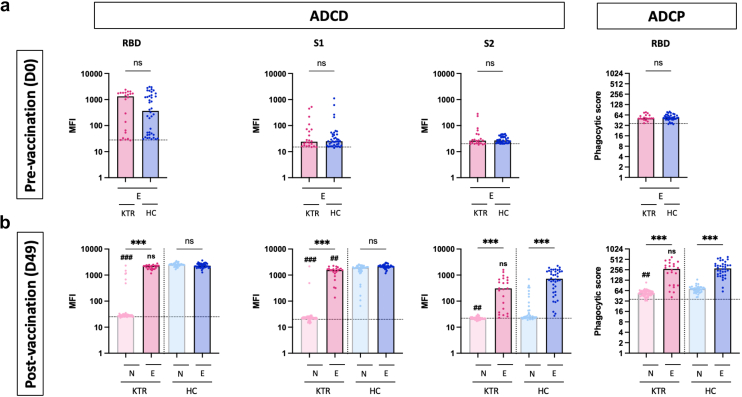
To understand how prior SARS-CoV-2 infection influences vaccine-induced antibody Fc-dependent effector functions among KTRs, we measured the binding capacity of IgG to human Fc-receptors. At baseline, levels of spike-specific antibody binding to FcγRIIa and FcγRIIIa levels were similar in E-KTRs and E-HCs, except for S2-specific antibody binding to FcγRIIa that were significantly higher in E-KTRs. Consistent with binding IgG responses to vaccination, spike-specific antibody binding to Fcγ receptors were higher in E-KTRs than in N-KTRs and were comparable to those detected in E-HCs (Figure 3). To determine whether these FcγR-binding profiles translated to increased antibody effector functions, we measured ADCD and ADCP activities. At baseline and postvaccination, E-KTRs had similar levels of ADCD and ADCP as E-HCs, thus supporting a role for Fc-dependent effector functions in hybrid immunity acquired by KTRs (Figure 4).
Figure 3.
Fcγ receptors binding antibody responses to SARS-CoV-2 mRNA vaccination in naïve and experienced KTRs. Serum levels of SARS-CoV-2 RBD specific, spike S1 subunit specific, and spike S2 subunit specific antibodies binding the Fcγ receptors, FcγRIIa and FcγRIIIa were measured before vaccination (D0, panel a) and 1 month after vaccination (D49, panel b) in naïve KTRs (N-KTRs, light pink), experienced KTRs (E-KTRs, dark pink), naïve HCs (N-HCs, light blue) and experienced HCs (E-HC, dark blue). Bars indicate median values. Horizontal grid lines indicate a technical negative signal (blank). Groups were compared using the analysis of variance Kruskal-Wallis test with Dunn’s correction. For within HC or KTR comparisons, ns; aP < 0.05; bP < 0.01; cP < 0.001. For comparisons between HC and KTR, ns; dP < 0.05; eP < 0.01; fP < 0.001. HC, healthy control; KTR, kidney transplant recipient; MFI, median fluorescent intensity; ns, not significant; RBD, receptor binding domain
Figure 4.
IgG-dependent complement deposition and phagocytosis responses to SARS-CoV-2 mRNA vaccination in naïve and experienced KTRs. Serum levels of SARS-CoV-2 RBD specific, spike S1 subunit specific, and spike S2 subunit specific IgG promoting complement deposition (ADCD) and cellular phagocytosis (ADCP) were measured before vaccination (D0, panel a) and 1 month after vaccination (D49, panel b) in naïve KTRs (N-KTRs, light pink), experienced KTRs (E-KTRs, dark pink) naïve HCs (N-HCs, light blue) and experienced HCs (E-HCs, dark blue). Levels of ADCD are expressed as MFI. Levels of ADCP are expressed as phagocytic score (see methods). Bars indicate median values. Horizontal grid lines indicate a technical negative signal (blank). Groups were compared using the analysis of variance Kruskal-Wallis test with Dunn’s correction. For within HC or KTR comparisons, ns; aP <0.05; bP < 0.01; cP < 0.001. For comparisons between HC and KTR, ns; dP < 0.05; eP < 0.01; fP < 0.001. HC, healthy control; KTR, kidney transplant recipient; MFI, median fluorescent intensity; ns, not significant; RBD, receptor binding domain.
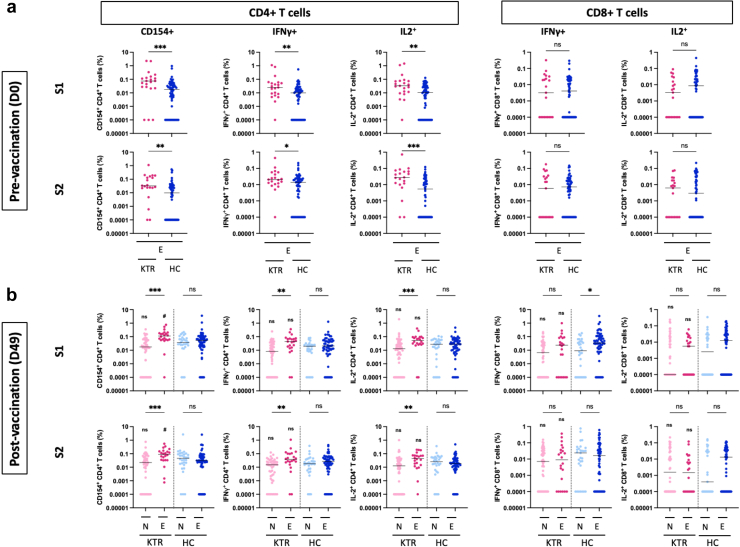
E-KTRs Have Higher CD4 T Cell and Similar CD8 T Cell Responses to mRNA Vaccination Compared to N-KTRs
To assess the impact of previous SARS-CoV-2 infection on cellular immunity of KTRs, we explored T cell responses to the spike protein by flow cytometry. Prevaccination, E-KTRS had higher frequencies of CD4 T cell expressing CD154, IFNγ, and IL2 in response to both S1 and S2 than E-HCs (Figure 5a). Postvaccination, E-KTRs had significantly higher frequencies of CD4 T cells expressing CD154, IFNγ, and IL2 in response to both S1 and S2 than N-KTRs (Figure 5b). Frequencies of S1- and S2-specific CD4 T cells expressing CD154 were higher in E-KTRs than in E-HCs. In contrast, CD8 T cell responses to S1 and S2 were similar in SARS-CoV-2-experienced groups at baseline and postvaccination across study groups, except for S1-specific CD8 T cells expressing IFNγ that were significantly higher in E-HCs than in N-HCs.
Figure 5.
CD4 and CD8 T cell responses to SARS-CoV-2 mRNA vaccination in naïve and experienced KTRs. Percentage of SARS-CoV-1 spike S1 subunit and spike S2 subunit specific CD4 T cells expressing CD154, IFNγ and IL2, and of CD8 T cells expressing IFNγ and IL-2 were measured in peripheral blood before vaccination (D0, panel a) and 1 month after vaccination (D49, panel b) in naïve KTRs (N-KTRs, light pink), experienced KTRs (E-KTRs, dark pink), naïve HCs (N-HCs, light blue) and experienced HCs (E-HC, dark blue). Bars indicate median values. Groups were compared using the analysis of variance Kruskal-Wallis test with Dunn’s correction. HC, healthy control; KTR, kidney transplant recipient; ns, not significant.
For within HC or KTR comparisons, ns; aP < 0.05; bP < 0.01; cP < 0.001.
For comparisons between HC and KTR, ns; dP < 0.05; eP < 0.01; fP < 0.001.
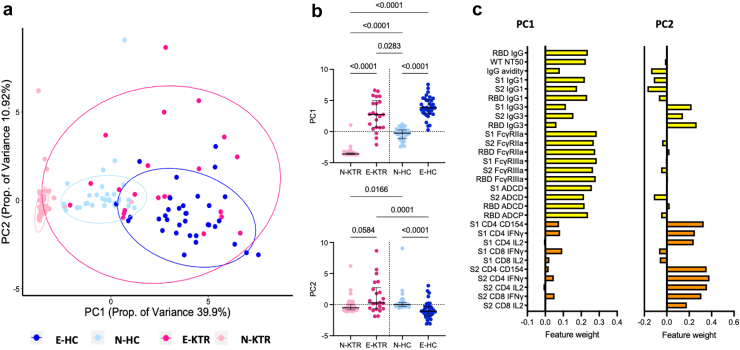
Integrated Analysis of Immune Response to mRNA Vaccination in Naïve and SARS-CoV-2 Experienced KTRs and HCs
To visualize and characterize differences in immune response features across individuals and groups, dimensionality reduction was performed using PCA.54 PCA resulted in 2 components with eigenvalues >1 that described relationships between immunological parameters following mRNA vaccination. The 2 major components, PC1 and PC2, accounted for 50.8% of the total variance (Figure 6a). N-KTRs, N-HCs, and E-HCs formed distinct clusters whereas responses among E-KTRs were more diffuse, encompassing N-HCs and E-HCs. Within KTRs and HCs, PC1 and PC2 were distinct between SARS-CoV-2-naïve and experienced subjects (Figure 6b). As shown in Figure 6c, humoral immune response features dominated the main principal component, PC1, where T cell response features as well as IgG3 responses, contributed most to PC2. Together, these analyses indicate that N-KTRs and E-KTRs have unique vaccine response profiles across many different immune effectors. The potential contribution of clinical factors to these different profiles was assessed by multivariate analyses. As shown in Table 1, N-KTRs were older, included fewer patients taking everolimus and had poorer kidney function than E-KTRs. Multivariate linear regression showed that previous SARS-CoV-2 infection was the only significant variable determining the differences of immune response features between N-KTRs and E-KTRs (Supplementary Table S2).
Figure 6.
Principal component analysis of immune responses to SARS-CoV-2 mRNA vaccination in naïve and experienced KTRs. (a) Scatter plot of PCA including all immune response parameters except IgG2, IgG4 and IgA measured 1 month after vaccination (D49) in naïve KTRs (N-KTRs, light pink), experienced KTRs (E-KTRs, dark pink), naïve HCs (N-HCs, light blue) and experienced HCs (E-HC, dark blue). (b) Comparison of PC1 and PC2 values between groups by analysis of variance Kruskal-Wallis test with Dunn’s correction with P < 0.1. (c) Relative weighting of individual immune response parameters in PC1 and PC2. HC, healthy control; KTR, kidney transplant recipient, PC, principal component; PCA, principal component analysis.
Immune Response to mRNA Vaccination in N-KTRs and E-KTRs Do Not Correlate With the Same Clinical Characteristics
The contrast between the low responses to mRNA vaccination in N-KTRs and the potent hybrid immunity acquired in E-KTRs suggests a different role for immunosuppression-related factors in the 2 study groups. Univariate and multivariate linear regressions were used to explore correlations between demographic and clinical factors and the immune response features that differed most between N-KTRs and E-KTRs.
In N-KTRs, levels of RBD binding IgG were negatively correlated with age, arterial hypertension, kidney retransplantation after graft failure and MMF treatment; and were positively correlated with azathioprine treatment in univariate analyses (Table 2 and Supplementary Table S3). Significant correlations were also observed in multivariate analyses, except for azathioprine treatment (Table 2 and Supplementary Table S3). RBD IgG avidity and titers of nAb were not included in these analyses because of their very low values in N-KTRs. Several clinical parameters were correlated with S1-specific CD4 T cell responses in univariate analyses (Supplementary Tables S4, S5, and S6). In multivariate analyses, only absolute lymphocyte counts were negatively correlated with frequencies of SI-specific IFNγ+ CD4 T cells (Supplementary Table S5). Together, these results indicate that clinical factors associated with immune suppression negatively correlate with humoral immune responses in N-KTRs, with limited correlations with cellular immune responses.
Table 2.
Univariate and multivariate linear regressions related to humoral response after mRNA vaccination in N-KTRs and E-KTRs
| RBD binding IgG | Univariate linear regression |
Multivariate linear regression |
||||
|---|---|---|---|---|---|---|
| Estimate (B) | 95% CI | P-value | Estimate | Standard error | P-value | |
| N-KTRS | ||||||
| Age | −0.02 | −0.03, 0.00 | 0.037 | −0.014452 | 0.005833 | 0.016 |
| Transplantation rank | ||||||
| 1 | 0.39 | −0.17, 0.96 | 0.2 | |||
| 2 | −0.51 | −1.1, 0.09 | 0.093 | −0.641834 | 0.231526 | 0.008 |
| Time between KT and RNA vaccination | 0.01 | −0.01, 0.03 | 0.3 | |||
| Arterial hypertension | −0.75 | −1.2, −0.28 | 0.002 | −0.611656 | 0.197785 | 0.003 |
| MMF | −0.61 | −0.94, −0.28 | <0.001 | −0.664619 | 0.141061 | <0.001 |
| AZA | 0.75 | 0.38, 1.1 | <0.001 | |||
| E−KTRS | ||||||
| Age | 0.01 | −0.01, 0.02 | 0.2 | |||
| Transplantation rank | ||||||
| 1 | −0.26 | −0.78, 0.26 | 0.3 | |||
| 2 | 0.01 | −0.71, 0.72 | >0.9 | |||
| Time between KT and RNA vaccination | 0.03 | 0.00, 0.05 | 0.063 | 0.02502 | 0.01270 | 0.063 |
| Arterial hypertension | 0.30 | −0.17, 0.77 | 0.2 | |||
| MMF | 0.08 | −0.34, 0.49 | 0.7 | |||
| AZA | 0.34 | −0.09, 0.78 | 0.12 | |||
AZA, azathioprine; CI, confidence interval; E-KTRs, SARS-CoV-2-experienced kidney transplant recipients; KT, kidney transplantation MMF: mycophenolate mofetil; N-KTRs: SARS-CoV-2-naïve kidney transplant recipients; RBD, receptor binding domain.
Significant univariate (P < 0.1) and multivariate (P < 0.05) linear regressions with log(10) RBD binding IgG after vaccination in N-KTRs and E-KTRs, respectively. For the univariate analyses, all variables that were significant (P < 0.1) in N-KTRs and/or E-KTRs are reported for both groups. A multivariate analysis per group was then done, including only the variables (P < 0.1) significant in its own group.
In E-KTRs, levels of RBD binding IgG were positively correlated with time between transplantation and vaccination, and nAb titers were positively correlated with absolute lymphocyte count in univariate analyses but no significant correlation was observed in multivariate analyses (Table 2, Supplementary Tables S7 and S8). RBD IgG avidity was positively correlated with time between transplantation and vaccination, and with muromonab-CD3 treatment in univariate analysis; and only time between transplantation and vaccination was significantly correlated in multivariate analyses (Supplementary Table S9). Univariate analyses indicated significant correlations between demographic and clinical factors and S1-specific CD4 T cell responses (Supplementary Tables S10, S11, and S12). In multivariate analyses, frequencies of S1-specific IFNγ+ CD4 T cells and IL2+ CD4 T cells were negatively correlated with time between SARS-CoV-2 infection and vaccination (Supplementary Tables S11 and S12). To further explore the role of demographic and clinical factors in immune response features in N-KTRs and E-KTRs, we performed PCA and analyzed their correlations with dominant PCs (Supplementary Figures S2 and S3). In line with multivariate regression analyses, in N-KTRs, PC1 was correlated with time between transplantation and vaccination, kidney retransplantation after graft failure, arterial hypertension, and azathioprine treatment (Supplementary Table S13). In E-KTRs, PC1 was correlated with time between transplantation and vaccination and PC2 was correlated with time between infection and vaccination (Supplementary Table S14). Together, these data indicate that, except for time between transplantation and vaccination, factors associated with low vaccine responses in N-KTRs were not correlated with vaccine responses in E-KTRs.
Discussion
The defective immune response of KTRs to mRNA vaccination remains a concern for the protection of this vulnerable population against emerging infectious diseases. The acquisition of hybrid immunity to SARS-CoV-2 provides proof-of-principle that KTRs can develop high immune responses to viruses despite their state of immune suppression. This study provides a comprehensive analysis of hybrid immunity in KTRs as compared to HCs and provides evidence that vaccine responses in E-KTRs are not correlated with most factors associated with decreased responses in N-KTRs.
Although the mechanisms underlying hybrid immunity remain incompletely understood, priming of B and T cell responses by natural infection probably plays a central role. Before vaccination, polyfunctional antibody and T cell responses to SARS-CoV-2 spike protein were detected in E-KTRs. Whereas the levels of RBD binding antibodies were similar in E-KTRS and E-HCs before vaccination, E-KTRs had higher RBD IgG avidity and higher titers of nAbs. This observation suggests higher germinal center reactions following SARS-CoV-2 infection in E-KTRs, a possibility supported by the higher frequency of CD4 T cells expressing CD154, with potential B cell help capacity, in E-KTRs than in E-HCs. At baseline, E-KTRs also had higher frequencies of CD4 T cells expressing IFNγ and IL2 than E-HCs. In contrast, frequencies of spike specific CD8 T cells were similar in the 2 groups. The higher levels of immune effectors in E-KTRs as compared to E-HCs following SARS-CoV-2 infection could be related to several factors. Symptomatic COVID-19 was associated with more intense immune responses.55 This factor is unlikely to play a dominant role because most E-KTRs had experienced mild COVID-19 and because RBD binding antibody levels were similar in the 2 groups. Previous studies have shown defective antibody and T cell responses to SARS-CoV-2 in KTRs than in controls during the early phase of the infection.56,57 In contrast, KTRs and controls were shown to have similar levels of SARS-CoV-2 specific antibodies and T cells during the convalescent phase,58 in line with our observations. The delayed acquisition of antibody and T cell responses in KTRs as compared to controls may be related to the duration of SARS-CoV-2 antigen exposure that may have further stimulated B cells and CD4 T cells. In support of this hypothesis, prolonged viral excretion was observed in KTRs following COVID-19.59 Prolonged antigen stimulation may have also played a role in the higher levels of the IgG3 observed in E-KTRs at baseline, because this subclass is typically produced following recent antigen exposure. Alternatively, the high levels of antibodies and T cells detected in convalescent KTRs may also involve a better survival of patients with highest immune responses in the early phase of the infection. This possibility is not supported by longitudinal studies showing delayed acquisition of antibodies in KTRs compared to controls.
Postvaccination, N-KTRs had lower humoral responses to the spike protein than N-HCs, as previously reported.5, 6, 7, 8,60, 61, 62 Levels of RBD binding IgG were negatively correlated with age, arterial hypertension, kidney retransplantation after graft failure, and MMF treatment, confirming previous reports.8,13, 14, 15,34 Humoral and cellular responses to vaccination were markedly higher in E-KTRs than in N-KTRs. E-KTRs produced high levels of high avidity binding IgG and IgA and high titers of nAbs in response to mRNA vaccination. They also displayed high binding to Fcγ receptors, including FcγRIIa, a receptor promoting antibody-dependent phagocytosis by myeloid cells, and FcγRIIIa, a receptor promoting activation of natural killer cells by IgG. These high levels of FcγR binding IgG were associated with high levels of ADCP and ADCD. E-KTRs also had high frequencies of CD4 T cells expressing CD154, IFNγ and IL2. This higher level of vaccine-induced immunity in E-KTRs than in N-KTRs is likely to provide increased protection against breakthrough infections. Indeed, we recently reported that both antibody and T cell responses to vaccination correlated with the risk of breakthrough infection in N-KTRs.9 In contrast to CD4 T cells, E-KTRs did not show higher frequencies of spike-specific CD8 T cells than N-KTRs. A dissociation between CD4 and CD8 T cell responses to mRNA vaccination was also observed in healthy adults who acquired hybrid immunity to SARS-CoV-2.31 However, Gao et al.31 reported lower CD8 T cell responses to vaccination in SARS-CoV-2-experienced than in naïve subjects, a phenomenon that was not observed in our study. The factors associated with decreased vaccine responses in N-KTRs did not correlate with either antibody or T cell responses to vaccination in E-KTRs. Time between transplantation and vaccination were correlated with RBD IgG avidity, but not with the other immune vaccine response features, suggesting the immune suppression induced at the time of transplantation has some impact on vaccine response in E-KTRs. A negative correlation was also observed between T cell responses and time between SARS-CoV-2 infection and vaccination, suggesting waning of cellular immune response priming.
An important strength of this study is that KTRs and HCs were recruited before the administration of the first dose of COVID-19 vaccine and were included in parallel studies with standardized protocols and procedures. A limitation of the study is its relatively small sample size, in relation to the monocentric recruitment of KTRs. However, differences in vaccine responses between groups and risk factors associated with low vaccine responses in N-KTRs that were previously reported in larger studies could be confirmed in our study. Further studies of hybrid immunity in other KTR populations are needed to validate our observations. Other limitations are the lack of follow-up with the study population and the focus of the analysis on the immune response to the vaccine strain. The study was focused on the response to primary immunization because it offered the best model to compare vaccine-induced immunity and their determinants independently of postvaccination exposure to SARS-CoV-2 variants.
In conclusion, this study shows that KTRs can acquire potent humoral and cellular immune responses to COVID-19 mRNA vaccination when they have been primed by natural infection and that these responses are not correlated with factors associated with low vaccine responses in SARS-CoV-2-naïve patients. Understanding the cellular and molecular bases of hybrid immunity in KTRs should help the development of optimized vaccination strategies against emerging pathogens for this vulnerable population.
Disclosure
MEA declared support from NIH NIAID, NIH NIGMS, Bill and Melinda Gates Foundation, and SD Ireland Foundation to her institution; from NIAID/Johns Hopkins Universities for materials; and from Elsevier (book royalty), Seromyx systems, Keystone Conferences, Bill and Melinda Gates Foundation, and NIH to her. All the other authors declared no conflicting interests.
Acknowledgments
The authors thank all patients for their generous participation in the study. The authors thank Sorya Fagnoul and Emilie Navaux for their help in the recruitment of patients. Maria Spanu and Anne Gillardin for the sampling and the follow-up of patients. Inès Vu Duc, Fatima Gjemajli, Sara Cuman, Robin Van Naemen, Florian Ballieu, Vianney Nana Tchouta, Marie-Annick Ottou Eyenga, and Caroline Rodeghiero for technical assistance. The authors thank the Robotein platform of the BE Instruct-ERIC Centre for providing access to the Octet HTX and the Microlab STAR liquid handling workstation. Graphical abstract was created with BioRender.com. This study was cofunded by the Belgian Federal Government through Sciensano, Belgium, by the Fonds de la Recherche Scientifique, F.R.S.-FNRS, Belgium, and by the Fonds Erasme, Belgium. NG received a PhD studentship from the Fonds Erasme and F.R.S- FNRS. DK received a PhD studentship from the F.R.S- FNRS. AM is Research Director of the F.R.S.-FNRS. This study was supported in part by the US NIAID (grants R56AI165448 and P01AI162242).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request, following material transfer agreement.
Author Contributions
NG, DK, ALM, and AM conceptualized the study. DK and PP wrote the clinical study protocol. DK obtained permission from the ethics committee and A.F.M.P.S. MEG, ALM, and AM secured the funding of the study. NG, DK and AL conducted the clinical study. ALM overviewed the clinical study. NG, DK, SS, AW, VO, DG, ED, MB, LH, JM, and MV conducted the laboratory analyses. PP, SS, AT, AMat, ID, KKA, MEA, and AM overviewed the laboratory analyses. NG, DK, SD, NK, DG, and LH contributed to data analysis and interpretation. NG, DK, SD, MEA, and AM interpreted the data. NG, PP, and AM drafted the manuscript. All coauthors reviewed, edited, and approved the manuscript.
Footnotes
Supplementary Methods.
Figure S1. Representative gating of CD3 T cells, CD4 T cells and CD8 T cells.
Figure S2. Principal component analysis of immune responses to SARS-CoV-2 mRNA vaccination in N-KTRs.
Figure S3. Principal component analysis of immune responses to SARS-CoV-2 mRNA vaccination in E-KTRs.
Table S1. Baseline characteristics of the study population.
Table S2. Multivariate linear regression analysis of factors associated with the immune response to mRNA vaccination in N-KTRs compared to E-KTRs.
Table S3. Univariate and multivariate linear regression analyses of spike RBD specific binding IgG responses to mRNA vaccination in N-KTRs.
Table S4. Univariate and multivariate linear regression analyses of spike S1 specific CD154+CD4 T cell responses to mRNA vaccination in N-KTRs.
Table S5. Univariate and multivariate linear regression analyses of spike S1 specific IFNγ+CD4 T cell responses to mRNA vaccination in N-KTRs.
Table S6. Univariate and multivariate linear regression analyses of spike S1 specific IL2+CD4 T cell responses to mRNA vaccination in N-KTRs.
Table S7. Univariate and multivariate linear regression analyses of spike RBD specific binding IgG responses to mRNA vaccination in E-KTRs.
Table S8. Univariate and multivariate linear regression analyses of SARS-CoV-2 neutralizing antibody responses to mRNA vaccination in E-KTRs.
Table S9. Univariate and multivariate linear regression analyses of spike RBD specific IgG avidity responses to mRNA vaccination in E-KTRs.
Table S10. Univariate and multivariate linear regression analyses of spike S1 specific CD154+CD4 T cell responses to mRNA vaccination in E-KTRs.
Table S11. Univariate and multivariate linear regression analyses of spike S1 specific IFNγ+CD4 T cell responses to mRNA vaccination in E-KTRs.
Table S12. Univariate and multivariate linear regression analyses of spike S1 specific IL2+CD4 T cell responses to mRNA vaccination in E-KTRs.
Table S13. Association between clinical parameters and principal components of immunological parameters in N-KTRs.
Table S14. Association between clinical parameters and principal components of immunological parameters in E-KTRs.
Supplementary Material
Supplementary Methods
Figure S1. Representative gating of CD3 T cells, CD4 T cells and CD8 T cells.
Figure S2. Principal component analysis of immune responses to SARS-CoV-2 mRNA vaccination in N-KTRs.
Figure S3. Principal component analysis of immune responses to SARS-CoV-2 mRNA vaccination in E-KTRs.
Table S1. Baseline characteristics of the study population.
Table S2. Multivariate linear regression analysis of factors associated with the immune response to mRNA vaccination in N-KTRs compared to E-KTRs.
Table S3. Univariate and multivariate linear regression analyses of spike RBD specific binding IgG responses to mRNA vaccination in N-KTRs.
Table S4. Univariate and multivariate linear regression analyses of spike S1 specific CD154+CD4 T cell responses to mRNA vaccination in N-KTRs.
Table S5. Univariate and multivariate linear regression analyses of spike S1 specific IFNγ+CD4 T cell responses to mRNA vaccination in N-KTRs.
Table S6. Univariate and multivariate linear regression analyses of spike S1 specific IL2+CD4 T cell responses to mRNA vaccination in N-KTRs.
Table S7. Univariate and multivariate linear regression analyses of spike RBD specific binding IgG responses to mRNA vaccination in E-KTRs.
Table S8. Univariate and multivariate linear regression analyses of SARS-CoV-2 neutralizing antibody responses to mRNA vaccination in E-KTRs.
Table S9. Univariate and multivariate linear regression analyses of spike RBD specific IgG avidity responses to mRNA vaccination in E-KTRs.
Table S10. Univariate and multivariate linear regression analyses of spike S1 specific CD154+CD4 T cell responses to mRNA vaccination in E-KTRs.
Table S11. Univariate and multivariate linear regression analyses of spike S1 specific IFNγ+CD4 T cell responses to mRNA vaccination in E-KTRs.
Table S12. Univariate and multivariate linear regression analyses of spike S1 specific IL2+CD4 T cell responses to mRNA vaccination in E-KTRs.
Table S13. Association between clinical parameters and principal components of immunological parameters in N-KTRs.
Table S14. Association between clinical parameters and principal components of immunological parameters in E-KTRs.
References
- 1.Jager K.J., Kramer A., Chesnaye N.C., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilbrands L.B., Duivenvoorden R., Vart P., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caillard S., Chavarot N., Francois H., et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattler A., Schrezenmeier E., Weber U.A., et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131 doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon-Arevalo H., Choi M., Stefanski A.L., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 7.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stumpf J., Siepmann T., Lindner T., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemlin D., Gemander N., Depickère S., et al. Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am J Transplant. 2023;23:649–658. doi: 10.1016/j.ajt.2023.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yetmar Z.A., Bhaimia E., Bierle D.M., Ganesh R., Razonable R.R. Breakthrough COVID-19 after SARS-CoV-2 vaccination in solid organ transplant recipients: an analysis of symptomatic cases and monoclonal antibody therapy. Transpl Infect Dis. 2022;24 doi: 10.1111/tid.13779. [DOI] [PubMed] [Google Scholar]
- 11.Sun J., Zheng Q., Madhira V., et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anjan S., Natori Y., Fernandez Betances A.A., et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation. 2021;105:e139–e141. doi: 10.1097/TP.0000000000003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grupper A., Rabinowich L., Schwartz D., et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meshram H.S., Kute V., Rane H., et al. Humoral and cellular response of COVID-19 vaccine among solid organ transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis Off J Transplant Soc. 2022;24 doi: 10.1111/tid.13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang K., Wu X., Luo Y., Wei Z., Feng L., Wu L. Meta-analysis of immunologic response after COVID-19 mRNA vaccination in solid organ transplant recipients. J Infectol. 2022;84:e73–e75. doi: 10.1016/j.jinf.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cucchiari D., Egri N., Bodro M., et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaba A.H., Zhu X., Liang T., et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22:1253–1260. doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benotmane I., Gautier G., Perrin P., et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuniduzi Y., Chen B., Zeng J., et al. Efficacy and safety of a fourth dose of the COVID-19 vaccine in kidney transplant recipients: a systematic review and meta-analysis. Transpl Immunol. 2023;79 doi: 10.1016/j.trim.2023.101864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abedon A.T., Teles M.S., Alejo J.L., et al. Improved antibody response after a fifth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2022;106:e262–e263. doi: 10.1097/TP.0000000000004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efros O., Anteby R., Halfon M., Meisel E., Klang E., Soffer S. Efficacy and safety of third dose of the COVID-19 vaccine among solid organ transplant recipients: a systemic review and meta-analysis. Vaccines. 2022;10:95. doi: 10.3390/vaccines10010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson T., Prendecki M., Gleeson S., et al. Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients. EClinicalmedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osmanodja B., Ronicke S., Budde K., et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J Clin Med. 2022;11:2565. doi: 10.3390/jcm11092565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel R.R., Apostolidis S.A., Painter M.M., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samanovic M.I., Cornelius A.R., Gray-Gaillard S.L., et al. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. Sci Transl Med. 2021;14 doi: 10.1126/scitranslmed.abi8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatatos L., Czartoski J., Wan Y.H., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372:1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds C.J., Pade C., Gibbons J.M., et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates T.A., McBride S.K., Leier H.C., et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abn8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebinger J.E., Fert-Bober J., Printsev I., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao F., Mallajoysula V., Arunachalam P.S., et al. Spheromers reveal robust T cell responses to the Pfizer/BioNTech vaccine and attenuated peripheral CD8+ T cell responses post SARS-CoV-2 infection. Immunity. 2023;56:864–878.e4. doi: 10.1016/j.immuni.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemlin D., Lemy A., Pannus P., et al. Hybrid immunity to SARS-CoV-2 in kidney transplant recipients and hemodialysis patients. Am J Transplant. 2022;22:994–995. doi: 10.1111/ajt.16853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firket L., Descy J., Seidel L., et al. Serological response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients depends on prior exposure to SARS-CoV-2. Am J Transplant. 2021;21:3806–3807. doi: 10.1111/ajt.16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magicova M., Zahradka I., Fialova M., et al. Determinants of immune response to anti-SARS-CoV-2 mRNA vaccines in kidney transplant recipients: a prospective cohort study. Transplantation. 2022;106:842–852. doi: 10.1097/TP.0000000000004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassaniti I., Bergami F., Arena F., et al. Immune response to BNT162b2 in solid organ transplant recipients: negative impact of mycophenolate and high responsiveness of SARS-CoV-2 recovered subjects against delta variant. Microorganisms. 2021;9:2622. doi: 10.3390/microorganisms9122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atyeo C., Fischinger S., Zohar T., et al. Distinct early serological signatures track with SARS-CoV-2 survival. Immunity. 2020;53:524–532.e4. doi: 10.1016/j.immuni.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zohar T., Loos C., Fischinger S., et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183:1508–1519.e12. doi: 10.1016/j.cell.2020.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorman M.J., Patel N., Guebre-Xabier M., et al. Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler E.S., Gilchuk P., Yu J., et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021;184:1804–1820.e16. doi: 10.1016/j.cell.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pannus P., Neven K.Y., De Craeye S., et al. Poor antibody response to BioNTech/Pfizer coronavirus disease 2019 vaccination in severe acute respiratory syndrome coronavirus 2-naïve residents of nursing homes. Clin Infect Dis. 2022;75:e695–e704. doi: 10.1093/cid/ciab998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goossens M.E., Neven K.Y., Pannus P., et al. The prior infection with SARS-CoV-2 study (PICOV) in nursing home residents and staff - study protocol description and presentation of preliminary findings on symptoms. Arch Public Health. 2021;79:195. doi: 10.1186/s13690-021-00715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown E.P., Licht A.F., Dugast A.S., et al. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods. 2012;386:117–123. doi: 10.1016/j.jim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canti L., Humblet-Baron S., Desombere I., et al. Predictors of neutralizing antibody response to BNT162b2 vaccination in allogeneic hematopoietic stem cell transplant recipients. J Hematol Oncol. 2021;14:174. doi: 10.1186/s13045-021-01190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasi L., Thiriard A., Heyndrickx L., et al. Younger children develop higher effector antibody responses to SARS-CoV-2 infection. Open Forum Infect Dis. 2022;9:ofac554. doi: 10.1093/ofid/ofac554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariën J., Ceulemans A., Michiels J., et al. Evaluating SARS-CoV-2 spike and nucleocapsid proteins as targets for antibody detection in severe and mild COVID-19 cases using a Luminex bead-based assay. J Virol Methods. 2021;288 doi: 10.1016/j.jviromet.2020.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown E.P., Dowell K.G., Boesch A.W., et al. Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. J Immunol Methods. 2017;443:33–44. doi: 10.1016/j.jim.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischinger S., Fallon J.K., Michell A.R., et al. A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J Immunol Methods. 2019;473 doi: 10.1016/j.jim.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman M.E., Moldt B., Wyatt R.T., et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres I., Albert E., Giménez E., et al. B- and T-cell immune responses elicited by the Comirnaty® COVID-19 vaccine in nursing-home residents. Clin Microbiol Infect. 2021;27:1672–1677. doi: 10.1016/j.cmi.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giménez E., Albert E., Torres I., et al. SARS-CoV-2-reactive interferon-γ-producing CD8+ T cells in patients hospitalized with coronavirus disease 2019. J Med Virol. 2021;93:375–382. doi: 10.1002/jmv.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R Core Team . R Foundation for Statistical Computing; 2013. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ [Google Scholar]
- 52.Ggplot . Springer-Verlag New York; 2016. Elegant Graphics for Data Analysis.https://ggplot2.tidyverse.org [Google Scholar]
- 53.Bikbov B., Perico N., Remuzzi G. on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in chronic kidney disease prevalence among males and females in 195 countries: analysis of the global burden of disease 2016 study. Nephron. 2018;139:313–318. doi: 10.1159/000489897. [DOI] [PubMed] [Google Scholar]
- 54.Jolliffe I.T., Cadima J. Principal component analysis: a review and recent developments. Philos Transact A Math Phys Eng Sci. 2016;374(2065) doi: 10.1098/rsta.2015.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyton R.J., Altmann D.M. The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? Nat Rev Immunol. 2021;21:762–768. doi: 10.1038/s41577-021-00631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cravedi P., Ahearn P., Wang L., et al. Delayed Kinetics of IgG, but Not IgA, Antispike antibodies in Transplant Recipients following SARS-CoV-2 Infection. J Am Soc Nephrol. 2021;32:3221–3230. doi: 10.1681/ASN.2021040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favà A., Donadeu L., Sabé N., et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21:2749–2761. doi: 10.1111/ajt.16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantarelli C., Angeletti A., Perin L., et al. Immune responses to SARS-CoV-2 in dialysis and kidney transplantation. Clin Kidney J. 2022;15:1816–1828. doi: 10.1093/ckj/sfac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caillard S., Benotmane I., Gautier Vargas G., Perrin P., Fafi-Kremer S. SARS-CoV-2 viral dynamics in immunocompromised patients. Am J Transplant. 2021;21:1667–1669. doi: 10.1111/ajt.16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozen-Zvi B., Yahav D., Agur T., et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertrand D., Hamzaoui M., Lemée V., et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benotmane I., Gautier-Vargas G., Cognard N., et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request, following material transfer agreement.